Abstract
Background
This is the first large‐scale Russian study describing semen quality and reproductive hormone levels among young men.
Objectives
The aim of the study was to compare semen quality and reproductive hormone levels in young men of four cities and three ethnic groups living in the Siberian region of Russia and to find out ethnic or environmental reasons for regional differences.
Materials and methods
The study population consisted of 1291 young men from Novosibirsk, Kemerovo, Ulan‐Ude, and Yakutsk, including 1013 men of three most numerous ethnic groups: Slavs, Buryats, and Yakuts. Each participant provided one sperm and blood sample, information about lifestyle and ethnicity. Anthropometric parameters, semen quality and reproductive hormone levels, were evaluated.
Results
Significant regional and ethnic differences were detected for semen and reproductive hormone parameters. Median sperm concentrations in Novosibirsk, Kemerovo, Ulan‐Ude, and Yakutsk were 54.6, 39.9, 34.7, 33.1 × 106/ml; total sperm counts—202.5, 138.7, 97.9, 93.4 × 106; percentages of morphologically normal spermatozoa—7.8%, 6.5%, 6.3%, 5.0%, respectively. Median sperm concentrations in Slavs, Buryats, and Yakuts were 43.7, 37.0, 30.6 × 106/ml; total sperm counts—150.0, 102.3 and 74.8 × 106; percentages of morphologically normal spermatozoa—6.8%, 6.8%, 4.8%, respectively.
Discussion
The young men in Novosibirsk and Kemerovo, populated by Slavs, had a higher semen quality compared to Ulan‐Ude and Yakutsk, populated by Buryats and Yakuts, apparently due to the higher testicular function in Slavic compared to Asian ethnicity. Impaired spermatogenesis in young men in Kemerovo compared to Novosibirsk, located in the same climatic zone and having a socio‐cultural and ethnic identity, may be due to the influence of a polluted environment.
Conclusion
The findings suggest that ethnic composition and environment may be responsible for regional differences in semen and reproductive hormone parameters.
Keywords: semen quality, reproductive hormones, regional and ethnic differences
1. INTRODUCTION
At present, there is a considerable rising of interest in male reproductive health. A number of important new discoveries have stimulated increased interest in the problem of male fertility. Recent studies and comprehensive meta‐analyses have provided significant evidence of a temporary trend of declining semen quality and increasing prevalence of male infertility, as well as similar adverse trends for other male urological diseases including hypospadias, cryptorchidism, and testicular cancer. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 In some populations, semen quality reached a level where a significant proportion of men of reproductive age was at risk of subfertility or infertility. Moreover, it has been shown that impaired semen quality or male infertility may be associated with shorter life expectancy and increased morbidity. 11 , 12 , 13
A number of studies in the United States and Europe have shown regional differences in semen quality, reproductive hormone levels, and the prevalence of certain diseases associated with the male reproductive system. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 Despite a large number of studies, the reasons for the regional variability of male fertility parameters are not fully clear due to their multiplicity and interaction with each other. The results of comparative epidemiological studies of semen quality in many countries and regions of the world indicate a multi‐factor reason for regional differences, suggesting that environmental, climatic, genetic, and individual lifestyle factors can determine spermatogenic potential and reproductive neuroendocrine system, 5 , 15 , 26 , 27 but there are limited data to identify specific causes of regional differences already recorded.
Most epidemiological studies of semen quality included participants with proven fertility (male partners of pregnant women), sperm donors, and male partners from infertile couples or young men recruited during military conscription. 17 , 28 , 29 , 30 , 31 , 32 , 33 , 34 Although these studies did not provide information about the semen quality of the general population, since subfertile men were underrepresented and infertile men were not included, they were very useful in demonstrating regional differences; now, they can serve as reference or starting points for research of other male populations around the world. 18 Because in the studies on conscripts, 15 , 16 , 28 , 34 participants were young, looked generally healthy, and had virtually no prior information about their own fertility or semen quality, the conscript population can be considered partially representative of the general population.
From the point of view of regional differences, the study of semen quality and reproductive hormones based on random samples from the general population deserves more attention, since it more accurately reflects the male fertility potential of the population. In recent years, a number of studies have studied semen quality and reproductive hormones in men from the general population, which also confirmed the existence of regional differences both between countries and between regions in the same country. 19 , 20 , 35 , 36 , 37 The authors noted that the study groups of young men from the general population were usually characterized by lower semen quality than men with proven fertility, since some of the young men were subfertile or infertile, but such men were not included in the studies of fertile men. 18
Some authors suggest that the ethnicity of men living in different geographical locations may contribute to regional differences in spermatozoa and hormonal parameters, but very few studies have focused on the influence of ethnic or race factors on regional differences in semen quality and hormonal profile. 23 , 38 , 39 , 40 , 41 , 42 Several European and USA studies were carried out on participants of different ethnicities, but ethnic differences were not discussed rigorously, often because too small number of participants in ethnic groups. 18 , 24 , 25 , 43 , 44
Systematic population‐based studies of semen quality were conducted mainly in the Scandinavian countries or in the United States, but the geography of research was constantly expanding. Regional studies of semen quality have a long tradition in both Europe and North America, but there is a substantial gap in our knowledge of male fertility potential in different regions of the Russian Federation. In particular, there are very scarce data on semen quality and reproductive hormone patterns in men residing in the Siberian region of Russia. The population living within this area is ethnically diverse and preserves its national socio‐cultural traditions and customs, but it is affected by various factors such as climate, urbanization, level of industrialization, and environmental pollution. The Siberian region of the Russian Federation is inhabited by many indigenous peoples and immigrants of Slavic origin, as well as descendants of a mixture of indigenous and Slavic ethnic groups, presenting an interesting model for studying the relative impact of ethnicity, lifestyle, and environment on male fertility, including semen quality and endocrine status.
Although previous studies have established regional differences in semen quality and reproductive hormone levels in men from Baltic countries, the United States and China, this is the first large‐scale Russian study to compare semen quality and reproductive hormone levels in young men from different Siberian regions and to clarify the ethnic and environmental reasons for the observed regional differences. Here, we present the results of prospective population‐based study of men from four Siberian cities of the Russian Federation. The first aim of this study was to examine regional differences in semen parameters (semen volume, sperm concentration, total sperm count, sperm motility, and normal morphology) and reproductive hormone levels (testosterone, estradiol, follicle‐stimulating hormone, luteinizing hormone, inhibin B) in young males from the general population. Since the regional variability of semen and hormonal parameters may be due to the different ethnicity (genetic background) of citizens or the unfavorable environmental situation in the compared regions, we aimed to find out the ethnic or environmental reasons for regional differences. The last aim was to establish ethnic influences on semen and endocrine parameters by comparing ethnically homogenous groups living compactly in different geographic locations of Siberia. Additionally, we compared our semen data with the normal values recommended World Health Organization's fifth edition guidelines, 45 and with similar data obtained in other countries.
2. MATERIALS AND METHODS
2.1. Geographical areas
For the present study, we selected four cities located in the Siberian region. The cities of Novosibirsk and Kemerovo are located in Western Siberia at a distance of 360 km from each other; they have a predominantly Slavic population (approximately 94 and 95%, respectively) and do not differ significantly in climate conditions and lifestyle, but they are characterized by significant differences in the environmental situation. The cities of Ulan‐Ude and Yakutsk are located in Eastern Siberia, while Yakutsk, located near the Arctic Circle, is the coldest city in Russia and the world. Buryats make up 32% of the total population of Ulan‐Ude, and Yakuts make up 43% of the total population of Yakutsk. More detailed climatic, geographical, and ethnic characteristics of these cities are provided in the Appendix S1. The study in Novosibirsk covered period: May 2009–May 2014, in Kemerovo October 2009–March 2013, in Ulan‐Ude October 2009–April 2012; and in Yakutsk October 2010–June 2011.
2.2. Study population
The study design and standardized recruitment protocol were the same in the four cities. Male volunteers (n = 1291) from the general population were enrolled in the study. Participants were informed about the study by advertising on the Internet, on television, or at special lectures on men's health, giving detailed information about the purpose and objectives of the study. All participants were born or lived for at least 3–5 years in cities where physical examination was performed. Inclusion criteria for participation in the study were absence of acute general diseases or chronic illness in an acute phase, and genial tract infections. Each participant was asked about necessity of sexual abstinence for 2–3 days before the examination; the abstinence time was recorded according to the information given by the participant before the time of the semen sample delivery. Some young people had a period of abstinence less or longer than recommended, but their semen data were included in our data set. All participants gave informed consent to participation in the examination. Each participant filled in a standardized questionnaire including information on age, place of birth, self‐identified nationality and nationality of his parents and grandparents, family status, alcohol consumption, tobacco smoking, profession, previous urological diseases. The data of participants were stored anonymously. All study subjects were voluntaries and did not receive any financial compensation. The motivation for participating volunteers was based on a desire to learn more about their fertility status and the incentive to get the semen and hormonal results free of charge. The ethics committee of the Federal Research Center “Institute of Cytology and Genetics,” the Siberian Branch of the Russian Academy of Sciences approved the study.
2.3. Physical examination
In each city, all participants were examined by the same experienced andrologist and the results of examination were recorded. Current urogenital disorders diagnosed at the examination included clinical varicocele (grade I, II, and III), chronic prostatitis, testicular and epididymal cysts, hydrocele, epididymitis, hypospadias and cryptorchidism or consequences of surgical operation regarding cryptorchidism. Body weight (kg), height, waist, and hip circumference (cm) were also determined. Body mass index (BMI, kg/m2) was calculated. Testicular volume (ml) was estimated by a Prader orchidometer and was presented as bitesticular volume (paired testicular volume). Age calculated as the difference between year of attendance in study and self‐reported year of birth.
2.4. Blood and sperm collection
Each participant provided both blood and semen sample. A fasting blood sample from each participant was drawn from the cubital vein immediately after the physical examination, but before the semen sample was collected, between eight and eleven a.m., to reduce the effect of diurnal variation in hormone levels, and centrifuged. The serum samples were stored at −40°C until an analysis. Semen samples were collected by masturbation into disposable sterile plastic containers in the special private room.
2.5. Semen analysis
The semen samples were analyzed for semen volume (ml), sperm concentration (×106/ml), and morphology (percentage) according to the WHO laboratory manual for the examination and processing of human semen 45 ; sperm progressive motility was determined using the automatic sperm analyzer SFA‐500 (Biola). The semen samples were kept at 37°C for 1 h. for liquefaction. Ejaculate volume was estimated by weighing the collection container and subtracting the weight of the empty preweighed container, assuming that 1 ml of ejaculate weighs 1 g. To determine the sperm concentration, 100 μl of well‐mixed ejaculate was diluted in 400 μl of a solution (5% NaHCO3; 0.35% formaldehyde; 0.025% trypan blue in distilled water). Staining was carried out for 1 h. at room temperature, after which the samples were stored in the refrigerator at a temperature of +4°C for subsequent counting. Sperm concentration was assessed using the Goryaev's hemocytometer under light microscope (magnification ×400). The Goryaev's hemocytometer is similar to the improved Neubauer hemocytometer recommended by the WHO guidelines, 45 and it is widely used in reproductive centers of Russia. Total sperm count was calculated by multiplying the individual's sperm concentration by the ejaculate volume.
The principle of estimating the percentage of spermatozoa with progressive motility in the native ejaculate using the SFA‐500 automatic sperm analyzer is based on the measurement of optical density fluctuations as a result of sperm movement through the optical channel illuminated by a laser beam. Optical fluctuations are registered by a photodetector; the number of spermatozoa with rapid progressive motility (velocity ≥25 µm/sec, the WHO class A) and with slow progressive motility (velocity = 5–25 µm/sec, the WHO class B) is calculated by special software. Sperm motility measurements were carried out three times for each sample, and mean value was calculated. More information on this device is provided in the Appendix S1.
To assess the sperm morphology, ejaculate smears were prepared, fixed with methanol, and stained using commercially available Diff‐Quick kits (Abris plus, Russia) according to the manufacturer's manual. More information on using the Diff‐Quick for staining ejaculate smears for morphology evaluation is provided in the Appendix S1. Two hundred spermatozoa were examined for morphology with an optical microscope (Axio Skop.A1, Carl Zeiss) at ×1000 magnification with oil immersion, and the sperm anomalies were listed according to the WHO guidelines. 45 Sperm morphology evaluations were done in duplicates in random and blinded order by a single trained junior researcher, one of the authors (M.K.). Here, we report the percentage of morphologically normal spermatozoa (%).
2.6. Hormone assay
Serum hormone concentrations were determined by enzyme immunoassay using commercially available kits Steroid IFA‐Testosterone‐01, Gonadotropin IFA‐LH, Gonadotropin IFA‐FSH (Alkor Bio), Estradiol‐IFA (Xema Medica), and Inhibin B Gen II ELISA (Beckman Coulter) according to manufacturer manuals. The ranges of evaluated concentrations for total testosterone (T), estradiol (E2), follicle‐stimulating hormone (FSH), luteinizing hormone (LH), and inhibin B (InhB) were 0.2–50, 0.1–20 nmol/L, 2.0–100, 20–90 IU/L, and 12–105 pg/ml, respectively. The sensitivities for T, E2, FSH, LH, and InhB were 0.2, 0.025 nmol/L, 0.25, 0.25 IU/L, and 2.6 pg/ml, respectively. The intra‐ and interassay coefficients of variation were as follows: for total T < 8.0%; for E 2 < 8.0%; FSH < 8.0%; LH < 8.0%, InhB ≤ 6.8%, respectively.
2.7. Ethnic composition of study groups from each city
We investigated ethnic differences in semen, hormonal, and anthropometrical parameters between Slavs, Buryats, and Yakuts, as they are the three most common ethnicities in the Siberian region of Russia. Brief description of Buryats and Yakuts is provided in the Appendix S1. Participants were selected to these groups from our study population according to information obtained from the self‐reporting questionnaires, taking into account self‐identified ethnicity and ethnicities of their mothers, fathers, and grandparents. The participant of the Slavic, Buryat, or Yakut ethnicity was eligible if the ethnicity of the man himself, his mother, father, and both grandparents was the same. Our research cohort consisted of Slavs living in all four cities, Buryats and Yakuts living compactly in the cities of Ulan‐Ude and Yakutsk, respectively.
2.8. Statistical analysis
A statistical analysis of the obtained data was performed using the statistical package “Statistica” (version 8.0). The results are presented as means (SD) as well as medians with 5th and 95th percentiles. The Kolmogorov‐Smirnov test was used to confirm the normal distribution of quantitative variables. The parameters, which were not normally distributed, were transformed by a cubic root transformation (semen volume and total sperm count), square root transformation (sperm concentration, progressive motility, and normal morphology), or natural logarithm (hormonal levels) before analysis. Descriptive statistics are presented using untransformed data. Analysis of covariance (ANCOVA) was applied to compare regional and ethnic groups. The period of sexual abstinence had an increasing effect on semen parameters and entered the statistical model as a covariate. Age tended to associate with FSH, testosterone, estradiol, and inhibin B levels and was included in the model as a covariate. Therefore, semen variables were adjusted for the period of sexual abstinence, and hormonal variables were adjusted for age. Regional and ethnic differences for categorical variables were tested using Chi‐square analysis. Spearman's correlation coefficients were calculated to evaluate the relationships between the all parameters studied. A p‐value of <0.05 was regarded as statistically significant.
3. RESULTS
3.1. Anthropometric and socio‐demographic characteristics of the entire study population and regional differences
Of the 1291 participants, Slavs (Russians, Belarusians, and Ukrainians) represented 642 (49.7%), Buryats—223 (17.3%), Yakuts—148 (11.5%), other ethnic groups—23 (1.8%), and descendants from ethnic mixed marriages—251 (19.4%); data were missing for four men (0.3%). According to questionnaire information, 884 (68.6%) participants had professions, which were not related to physical labor, 194 (15.0%) were engaged in physical labor, and 180 (13.9%) indicated a combination of physical and mental labor; data were missing for 32 men (2.5%).
Characteristics of the study subjects are summarized in Table 1. The vast majority of participants (84.4%) were between the ages of 18 and 30. The entire study population was characterized by a normal average BMI, 309 (23.9%) participants were overweight and 97 (7.5%) participants suffered from obesity, 57 (4.4%) men categorized as underweight, and 824 (63.8%) participants had a normal BMI. Data were missing for four men (0.4%). Age was positively correlated with BMI, waist, and hip circumference (r = 0.377; r = 0.462; r = 0.275, p < 0.001 in all cases). Just over one‐third of men were smokers (mean ± SEM: 10.9 ± 0.3 cigarettes per day). Near two‐third of men reported alcohol consumption as beer, wine, or vodka (mean ± SEM: 0.8 ± 0.03 times per week). In the entire study population, 182 (14.3%) men had children (mean ± SEM: 1.4 ± 0.05 child). One quarter of men suffered from urogenital diseases, among them 174 (13.5%) subjects had clinical varicocele, including 84 (6.5%) with grade I, 61 (4.8%) with grade II, 29 (2.3%) with grade III. In addition, 60 (4.7%) subjects had prostatitis; 52 (4.0%) testicular or epididymal cysts; 23 (1.8%) epididymitis; 19 (1.5%) congenital abnormalities of the reproductive system; 10 (0.8%) hydrocele; seven (0.5%) other urological diseases, between them 36 (2.8%) men suffered from multiple urological diseases.
TABLE 1.
Physical appearance and self‐reported information of men from four Siberian cities in the Russian Federation
| Entire study population (n = 1291) | Novosibirsk (n = 419) | Kemerovo (n = 268) | Ulan‐Ude (n = 331) | Yakutsk (n = 273) | p (between regions) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | ||
| Age (years) | 24.5 (6.2) | 22 (18–37) | 24.7 (6.2)a | 23 (18–37) | 23.8 (5.1)ab | 22 (18–36) | 23.4 (6.2)b | 21 (18–37) | 26.0 (6.9)c | 24 (18–39) | <0.001 |
| Weight (kg) | 74.7 (13.8) | 73 (57–101) | 77.7 (14.4)a | 75 (59–105) | 76.1 (12.4)a | 74 (60–101) | 71.5 (13.2)b | 70 (56–97) | 72.7 (14.0)b | 70 (55–101) | <0.001 |
| Height (cm) | 177.0 (7.3) | 177 (165–189) | 179.3 (7.1)a | 179 (168–191) | 178.7 (6.8)a | 180 (168–190) | 175.7 (6.5)b | 176 (166–187) | 173.4 (7.0)c | 173 (162–186) | <0.001 |
| Waist circumference (cm) | 83.6 (10.9) | 82 (69–104) | 83.9 (11.0)a | 82.0 (70–106) | 83.8 (8.8)a | 83 (71–101) | 81.7 (11.5)b | 79 (68–102) | 85.2 (11.7)a | 83 (70–108) | <0.001 |
| Hip circumference (cm) | 97.4 (8.1) | 96 (86–112) | 99.2 (8.2)a | 98 (88–114) | 98.1 (8.0)a | 98 (86–112) | 94.1 (7.2)b | 93 (84–106) | 98.2 (7.7)a | 97 (87–113) | <0.001 |
| BMI (kg/m2) | 23.8 (3.9) | 23.2 (19–31) | 24.1 (3.9)a | 23 (19–31) | 23.8 (3.3)a | 23 (19–30) | 23.2 (4.1)b | 22 (18–31) | 24.1 (4.2)a | 24 (18–32) | <0.01 |
| BTV (ml) | 38.1 (8.4) | 38.3 (24–50) | 37.5 (8.1)a | 36 (24–50) | 41.1 (8.0)b | 40 (29–54) | 37.3 (8.0)a | 40 (24–50) | 37.1 (9.0)a | 36 (26–54) | <0.001 |
| Age of sexual debut (years) | 17.1 (2.2) | 17 (14–21) | 17.4 (2.4)a | 17 (14–21) | 16.8 (1.9)b | 17 (14–20) | 16.9 (2.0)b | 17 (14–20) | 17.2 (2.1)ab | 17 (14–21) | <0.01 |
| Sexual abstinence (days) | 4.3 (4.7) | 3 (2–7) | 5.3 (4.8)a | 4 (3–9) | 4.3 (5.5)b | 3 (2–7) | 4.0 (5.1)b,c | 3 (1–7) | 3.4 (2.6)c | 3 (1–7) | <0.001 |
| Frequency, % | Frequency, % | Frequency, % | Frequency, % | Frequency, % | |||||||
| Cigarette smokers | 35.0 | 26.3a | 32.8a | 42.6b | 41.4b | <0.001 | |||||
| Drinkers | 71.5 | 75.4a | 75.4a | 73.4a | 59.3b | <0.001 | |||||
| Men having children | 14.3 | 9.8a | 10.1ab | 14.5ab | 24.9c | <0.001 | |||||
| Current urogenital disorders | 25.0 | 22.3a | 22.8ab | 25.7ab | 30.6b | =0.074 | |||||
Results based on raw data. Analysis of variance was applied to compare anthropometric and demographic parameters and Chi‐square analysis was performed to compare the frequency parameters between four cities. a, b, c, d comparisons with different superscripts within variables were significant (p < 0.05).
Abbreviations: (5–95), 5‐95th percentile; BMI, body mass index; BTV, bitesticular volume (paired testicular volume); SD, standard deviation.
Table 1 shows the differences in the physical appearance and socio‐demographic characteristics of men in four Siberian cities. Men from Yakutsk were older compared to men from other cities (p < 0.05–0.001 in all pairwise comparisons). The body mass and height of men from Novosibirsk and Kemerovo were higher compared to men from Ulan‐Ude or Yakutsk (p < 0.001 in pairwise comparisons). Waist and hip circumference, as well as BMI, were the lowest in Ulan‐Ude compared to men from other cities (p < 0.05–0.001). The bitesticular volume (BTV) was the largest in Kemerovo compared to men from other cities (p < 0.001 in all pairwise comparisons). Age of sexual debut was lower in men from Kemerovo and Ulan‐Ude compared to men from Novosibirsk (p < 0.05–0.001). The period of sexual abstinence was the longest in Novosibirsk and the shortest in Yakutsk (p < 0.001 in pairwise comparisons). The proportion of smokers was higher in Ulan‐Ude and Yakutsk than in Novosibirsk or Kemerovo (p < 0.05–0.001 in pairwise comparisons). Participants from Kemerovo smoked more often than from Novosibirsk, Ulan‐Ude, Yakutsk (mean ± SEM: 13.3 ± 0.7; 11.3 ± 0.6; 9.5 ± 0.5; 10.3 ± 0.6 cigarettes per day, respectively, p < 0.05–0.001). The proportion of drinkers was the lowest in Yakutsk than in other cities (p < 0.001 in pairwise comparisons). Men in Novosibirsk or Kemerovo drank more often than in Ulan‐Ude or Yakutsk (mean ± SEM: 0.9 ± 0.05; 0.8 ± 0.06; 0.6 ± 0.05; 0.7 ± 0.05 times per week, respectively, p < 0.05–0.01). The proportion of men having children was the highest in Yakutsk compared to other cities (p < 0.001 in pairwise comparisons). The number of children per men was also the highest in Yakutsk compared to Novosibirsk, Kemerovo, Ulan‐Ude (mean ± SEM: 1.7 ± 0.10, 1.2 ± 0.06; 1.2 ± 0.08; 1.4 ± 0.09 children per men, respectively, p < 0.05–0.005). The proportion of men with urogenital disorders did not differ between Novosibirsk, Kemerovo, and Ulan‐Ude and was higher in Yakutsk compared to Novosibirsk (p < 0.05).
3.2. Semen parameters of the entire study population and regional differences
Semen parameters are summarized in Figure 1 and Table 2. Of the 1291 participants, some men refused to donate ejaculate, so 1236 semen samples were collected. According to the WHO reference limits, 45 semen quality is considered as “normal” if sperm concentration is ≥15 mill/ml, progressive motility ≥32%, normal morphology ≥4% and as “lowered” if sperm concentration is <15 mill/ml, and/or progressive motility <32%, and/or normal morphology <4%. The average semen parameters for the entire study population were within the “normal” values, although a significant proportion of men (42.2%) fell into the group with “lowered” semen quality (Figure 1).
FIGURE 1.
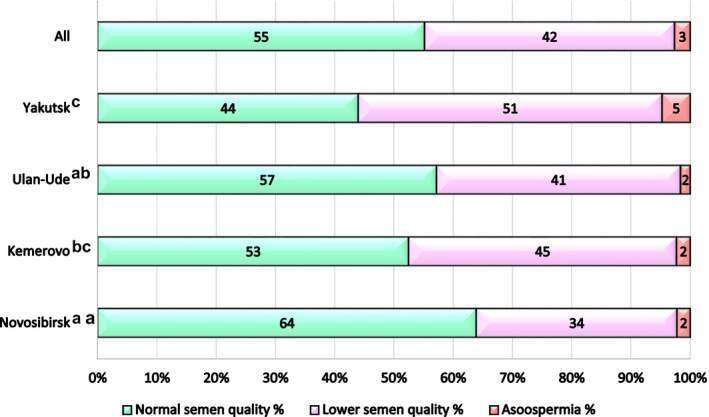
Distribution of men in different semen quality groups from four Siberian cities using sperm concentration, motility, and morphology. According to the WHO reference limits, 45 men were categorized into “normal” semen quality group if sperm concentration was ≥15 mill/ml, progressive motility ≥32%, normal morphology ≥4%, and “lowered” semen quality group if sperm concentration was <15 mill/ml, and/or progressive motility <32%, and/or normal morphology <4%. a, b, c comparisons with different superscripts between “normal” semen quality groups are significant (p < 0.01)
TABLE 2.
Semen and hormonal parameters of men from four Siberian cities in the Russian Federation
| Entire study population (n = 1291) | Novosibirsk | Kemerovo | Ulan‐Ude | Yakutsk | p (between cities) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | ||
| Semen volume (ml) | 3.5 (1.6) | 3.3 (1.4–6.4) | 3.9 (1.8)a | 3.7 (1.4–7.0) | 3.7 (1.7)a | 3.5 (1.3–6.6) | 3.2 (1.3)b | 3.0 (1.3–5.7) | 3.2 (1.3)b | 3.0 (1.5–5.6) | <0.001 |
| Total sperm count/ejaculate (×106) | 169 (172) | 130 (6–439) | 228 (229)a | 203 (12–526) | 176 (150)b | 139 (4–438) | 134 (122)c | 98 (10–371) | 113 (96)d | 93 (1–298) | <0.001 |
| Sperm concentration, (×106/ml) | 48.7 (39.3) | 40.2 (2.9–125.7) | 60.3 (45.0)a | 54.6 (4.4–143.1) | 49.2 (38.6)b | 39.9 (2.0–129.5) | 43.7 (36.4)b | 34.7 (5.0–120.0) | 36.1 (27.0)c | 33.1 (0.5–89.3) | <0.001 |
| Progressive motility (%) | 43 (27) | 41 (3–89) | 46 (27)a | 44 (4–91) | 41 (27)b | 40 (2–86) | 44 (27)ab | 42 (3–90) | 39 (25)b | 37 (5–86) | <0.05 |
| Normal morphology (%) | 6.6 (3.1) | 6.5 (1.9–11.8) | 7.7 (3.3)a | 7.8 (2.5–13.8) | 6.4 (3.0)b | 6.5 (1.5–11.3) | 6.5 (2.7)b | 6.3 (2.3–11.2) | 5.0 (2.3)c | 5.0 (1.3–8.9) | <0.001 |
| LH (IU/L) | 3.6 (1.6) | 3.3 (1.5–6.4) | 3.4 (1.3)a | 3.1 (1.5–5.8) | 3.9 (1.6)c | 3.7 (1.7–6.6) | 3.8 (1.6)bc | 3.4 (1.6–6.4) | 3.7 (2.0)ab | 3.3 (1.3–7.0) | <0.001 |
| FSH (IU/L) | 4.3 (3.1) | 3.7 (1.5–8.7) | 3.9 (2.3)a | 3.5 (1.4–7.6) | 3.5 (2.0)b | 3.0 (1.3–7.8) | 4.5 (2.9)c | 3.9 (1.6–9.1) | 5.4 (4.6)d | 4.5 (1.9–10.8) | <0.001 |
| Testosterone (nmol/L) | 21.0 (7.5) | 19.9 (11.1–34.0) | 23.0 (8.3)a | 22.2 (11.5–37.8) | 21.4 (7.5)b | 20.5 (11.5–33.5) | 18.7 (6.2)c | 17.7 (9.9–30.5) | 20.1 (6.8)d | 18.9 (11.1–32.1) | <0.001 |
| Estradiol (nmol/L) | 0.21 (0.07) | 0.20 (0.12–0.32) | 0.19 (0.08)a | 0.18 (0.10–0.32) | 0.19 (0.06)a | 0.19 (0.13–0.29) | 0.23 (0.08)b | 0.22 (0.16–0.33) | 0.22 (0.05)b | 0.21 (0.16–0.32) | <0.001 |
| Inhibin B (pg/ml) | 174 (67) | 167 (73–294) | 204 (66)a | 196 (111–323) | 162 (68)bc | 160 (56–280) | 154 (65)b | 154 (41–272) | 162 (53)c | 161 (87–257) | <0.001 |
Results based on raw data. Of 1291 participants, semen parameters were determined in 1236 (403 in Novosibirsk; 266 in Kemerovo; 310 in Ulan‐Ude; 257 in Yakutsk). Analysis of covariance was used to test between‐city differences in semen and hormonal parameters. Semen parameters were adjusted for the period of sexual abstinence, and hormonal parameters were adjusted for age. Semen volume and total sperm count were cubic root transformed; sperm concentration, progressive motility, and normal morphology were square root transformed. Hormonal parameters were transformed by the natural logarithm. a, b, c, d comparisons with different superscripts within variables were significant (p < 0.05).
Abbreviations: (5–95), 5–95th percentile; FSH, follicle‐stimulating hormone; LH, luteinizing hormone; SD, standard deviation.
Significant upward trends in all semen parameters were observed with increasing abstinence period: The positive correlations were observed between duration of sexual abstinence and semen volume, total sperm count, sperm concentration, progressive motility, normal morphology (r = 0.291, r = 0.381, r = 0.279, r = 0.141, r = 0.146, p < 0.001 in all cases). Sperm concentration was positively correlated with progressive motility and normal morphology (r = 0.811, r = 0.586, p < 0.001 in both cases). Total sperm count was positively correlated with progressive motility and normal morphology (r = 0.690, r = 0.514, p < 0.001 in both cases). There was positive correlation between progressive motility and normal morphology (r = 0.638, p < 0.001).
Semen parameters differed between the four regional groups (Table 2). The semen volume was higher in the participants from Novosibirsk and Kemerovo compared to Ulan‐Ude or Yakutsk. The total sperm count, sperm concentration, and percentage of morphologically normal spermatozoa varied with the highest values in Novosibirsk and the lowest in Yakutsk (p < 0.001 in pairwise comparisons). The proportion of participants with “normal” semen quality was the highest in Novosibirsk and the lowest in Yakutsk (p < 0.005–0.001 in pairwise comparisons, Figure 1).
3.3. Hormonal parameters of the entire study population and regional differences
The hormonal data of the participants are presented in Table 2. Of the 1289 participants who had a blood sample taken, 100 (7.8%) participants had testosterone levels below the normal reference value of 12.1 nmol/L as the lower limit, 46 of them 10 participants had sperm concentrations below 15 ×106/ml. In the entire study population, out of 110 (8.5%) participants having the inhibin B levels below the normal reference values of 92 pg/mL as the lower limit, 47 only 78 (6.1%) had “lowered” semen quality.
Significant positive relationships were found between LH and FSH, LH and T concentrations (r = 0.335; r = 0.100, respectively, p < 0.001). Negative relationships were observed between LH and inhibin B, FSH, and inhibin B concentrations (r = −0.226, r = −0.447; respectively, p < 0.001), and positive—between T and inhibin B concentration (r = 0.220, p < 0.001). Significant negative relationships were revealed between LH and FSH level, and sperm concentration, total sperm count, progressive sperm motility, and normal morphology (LH: r = −0.151, r = −0.139, r = −0.117, r = −0.063, respectively; FSH: r = −0.226, r = −0.247, r = −0.130, r = −0.106, respectively, p < 0.001 in all cases). Significant positive relationships were revealed between inhibin B level and sperm concentration, total sperm count, progressive sperm motility, and normal morphology (r = 0.271, r = 0.306, r = 0.151, r = 0.129, respectively, p < 0.001 in all cases).
Reproductive hormone levels varied between regional groups (Table 2). The LH level in participants from Novosibirsk was lower compared to men from Kemerovo and Ulan‐Ude (p < 0.01 in pairwise comparisons), but did not differ from Yakutsk. FSH and testosterone levels differed between all four regional groups with the lowest FSH level in Kemerovo and the highest FSH level in Yakutsk, as well as with the lowest testosterone level in Ulan‐Ude and the highest testosterone level in Novosibirsk (p < 0.05–0.001 in pairwise comparisons). Estradiol levels did not differ between men from Novosibirsk and Kemerovo, as well as between men from Ulan‐Ude and Yakutsk, but were lower in the first two cities compared to the last two (p < 0.001 in pairwise comparisons). The inhibin B level in participants from Novosibirsk was higher than in men from other cities (p < 0.001 in all pairwise comparisons).
3.4. Ethnic differences in socio‐demographic and anthropometric parameters
The ethnic composition of the four regional study groups was different. The study groups from Novosibirsk and Kemerovo consisted predominantly of Slavs (79.5 and 77.2%, respectively); the study group from Ulan‐Ude‐Buryats (66.2%) and Slavs (23.3%), from Yakutsk‐Yakuts (54.2%) and Slavs (9.2%), other ethnic groups and descendants from mixed marriages filled in the remainder proportions. Since only Slavs, Buryats, and Yakuts represented significant cohorts, we limited our comparative analysis to these three ethnic groups.
The socio‐demographic and anthropometric data of Slavs, Buryats, and Yakuts are summarized in Table 3. Yakuts were older than Buryats (p < 0.05) and did not differ from Slavs. Slavs were heavier than Buryats and Yakuts (p < 0.001 in pairwise comparisons), but the latter two did not differ. Slavs had the highest height, Yakuts had the lowest, and Buryats ranked middle (p < 0.001 in pairwise comparisons). Buryats had the lowest waist and hip circumference, as well as BMI, and Yakuts did not differ from Slavs (p < 0.05–0.001 in pairwise comparisons). The age of sexual debut was higher in Yakuts compared to Buryats (p < 0.05) and did not differ from Slavs. The period of sexual abstinence was higher in Slavs compared to Yakuts (p < 0.05), and Buryats ranked middle.
TABLE 3.
Physical appearance and self‐reported information for men of three different ethnic groups
| Slavs (n = 642) | Buryats (n = 223) | Yakuts (n = 148) | p (between groups) | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | ||
| Age (years) | 24.4 (5.9)ab | 23 (18–37) | 23.7 (6.5)a | 21 (18–37) | 25.5 (7.4)b | 23 (18–40) | <0.05 |
| Weight (kg) | 77.2 (13.9)a | 75.0 (59.8–103.2) | 70.9 (13.2)b | 68 (55.5–94.0) | 70.6 (13.8)b | 68 (54–96) | <0.001 |
| Height (cm) | 179.1 (16.8)a | 179 (168–190) | 174.8 (6.3)b | 175 (165–184) | 172.2 (7.2)c | 172 (162–182) | <0.001 |
| Waist circumference (cm) | 84.0 (10.5)ab | 82 (70–103) | 82.0 (12.1)a | 80 (67–101) | 84.7 (12.6)b | 82 (68–110) | <0.05 |
| Hip circumference (cm) | 98.5 (8.2)a | 98 (87–113) | 94.2 (7.3)b | 94 (84–106) | 97.3 (7.9)a | 96 (86–112) | <0.001 |
| BMI (kg/m2) | 24.0 (3.8)a | 23.4 (19.0–30.8) | 23.2 (4.3)b | 22.5 (18.0–30.8) | 23.8 (4.4)ab | 23.2 (17.6–32.0) | <0.05 |
| BTV (ml) | 39.4 (8.2)a | 40 (26–52) | 35.5 (7.0)b | 40 (24–50) | 36.1 (8.5)b | 36 (26–50) | <0.001 |
| Age of sexual debut (years) | 17.2 (2.2)ab | 17 (14–21) | 16.9 (2.0)a | 17 (14–20) | 17.4 (2.1)b | 17 (14–21) | =0.161 |
| Sexual abstinence (days) | 4.7 (5.1)a | 4 (2–8) | 4.3 (5.8)ab | 3 (2–14) | 3.4 (3.0)b | 3 (1–7) | <0.05 |
| Frequency (%) | Frequency (%) | Frequency (%) | |||||
| Cigarette smokers | 31.0a | 44.8b | 37.8ab | <0.001 | |||
| Drinkers | 74.8a | 74.9a | 60.1b | <0.01 | |||
| Men having children | 10.1a | 16.0b | 27.0c | <0.001 | |||
| Current urogenital disorders | 24.5a | 23.8a | 28.6a | =0.530 | |||
Results based on raw data. Analysis of variance was applied to compare anthropometric and demographic parameters, and Chi‐square analysis was performed to compare the frequency parameters. a, b, c comparisons with different superscripts within variables were significant (p < 0.05).
Abbreviations: (5–95), 5–95th percentile; BMI, body mass index; BTV, bitesticular volume (paired testicular volume); SD, standard deviation.
The proportion of smokers among Buryats was higher than among Slavs (p < 0.001), Yakuts ranked middle, although Slavs smoked more cigarettes than Buryats or Yakuts (mean ± SEM: 12.6 ± 0.5; 9.0 ± 0.5 and 8.1 ± 0.7 cigarettes per day, respectively, p < 0.001 in both cases). The proportion of drinkers was the lowest in Yakuts (p < 0.001 in both comparisons), while Slavs and Buryats did not differ. Slavs drank significantly more often than Buryats or Yakuts (mean ± SEM: 0.8 ± 0.04; 0.6 ± 0.05; 0.6 ± 0.04 times per week; p < 0.05 in both comparisons), while Buryats and Yakuts did not differ. The proportion of men having children differed between all the ethnic groups: It was the highest in Yakuts and the lowest in Slavs, Buryats ranked middle. Slavs had fewer children than Buryats or Yakuts, while Buryats and Yakuts were no different (mean ± SEM: 1.2 ± 0.05; 1.5 ± 0.1; 1.6 ± 0.2 child, respectively, p < 0.05 in both comparisons). The presence of urogenital disorders did not differ between the ethnic groups.
3.5. Ethnic differences in semen and hormonal parameters
Semen data for the Slavic, Buryat, and Yakut ethnic groups are summarized in Figure 2. Slavs had a higher semen volume than Buryats or Yakuts (p < 0.01), while Yakuts and Buryats did not differ. The sperm concentration and percentage of morphologically normal spermatozoa were the lowest in Yakuts compared to Slavs or Buryats (p < 0.001), while Slavs and Buryats did not differ. Total sperm counts varied between ethnic groups (p < 0.01 in pairwise comparisons), and it was the highest in Slavs, the smallest in Yakuts, Buryats ranked middle. The proportion of participants with “normal” semen quality varied between ethnic groups (p < 0.01), and it was the lowest in Yakuts (59 men, 43.7%), higher in Buryats (131 men, 62.7%) and Slavs (353 men, 56.5%) (p < 0.01 in both comparisons), while Slavs and Buryats did not differ.
FIGURE 2.
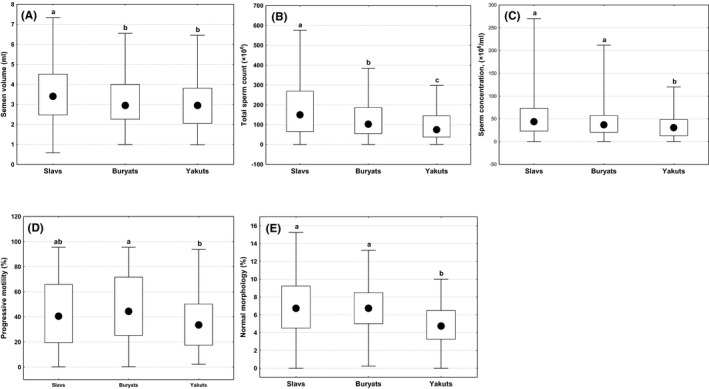
Differences in semen parameters between men of three ethnic groups in (A) semen volume; (B) total sperm count; (C) sperm concentration; (D) progressive motility; (E) normal morphology. Boxes indicate interquartile range, circles—median, whiskers—non‐outlier range. a, b, c, d comparisons with different superscripts are significant (p < 0.05)
Hormonal data for the Slavic, Buryat, and Yakut ethnic groups are summarized in Figure 3. LH concentrations were higher in Buryats than in Slavs or Yakuts (p < 0.001 in both comparisons). FSH levels differed between all ethnic groups (p < 0.001) and were lower for Slavs than for Buryats or Yakuts (p < 0.001 in both comparisons), and lower for Buryats than for Yakuts (p < 0.05). The testosterone level was the lowest in Buryats compared to Slavs or Yakuts (p < 0.05–0.001 in both comparisons), but Slavs and Yakuts did not differ. The estradiol level was lower for Slavs than for Buryats or Yakuts (p < 0.001 in both comparisons), but Buryats and Yakuts did not differ. Inhibin B levels differed between all the ethnic groups (p < 0.001) with the highest level in Slavs, the lowest level in Buryats, Yakuts ranked middle (p < 0.01).
FIGURE 3.
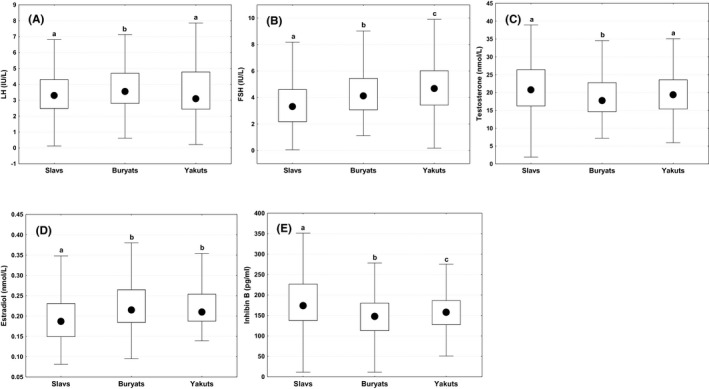
Differences in hormonal parameters between men of three ethnic groups in (A) LH, luteinizing hormone; (B) FSH, follicle‐stimulating hormone; (C) testosterone; (D) estradiol; (E) inhibin B. Boxes indicate interquartile range; circles—median, whiskers—non‐outlier range. a, b, c, d comparisons with different superscripts are significant (p < 0.05)
Correlation coefficients for relationships between age, anthropometric and hormonal parameters, and between semen quality variables and anthropometric and hormonal parameters (Spearman's test) for men of different ethnic groups are provided in the Appendix S1. Age was positively related to FSH concentrations and negatively related to testosterone and estradiol concentrations in Slavs and Buryats. Significant negative relationships between body weight, waist and hip circumference, BMI, and testosterone were observed in all ethnic groups. Sperm concentration, total sperm count, and progressive motility were positively correlated with inhibin B concentration; sperm concentration was negatively correlated with LH and FSH concentration in all ethnic groups.
4. DISCUSSION
Our population‐based cohort study of semen quality and reproductive hormone levels in young men from different Siberian regions of Russia showed that the ethnic composition of the populations and anthropogenic environmental pollution may be involved in regional differences in semen and endocrine parameters. The male populations in Russia were previously rarely investigated for semen quality and endocrine profile. Thus, this study seems to be the first large‐scale Russian study to describe regional and ethnic variability in sperm output and reproductive hormones of men from the general population.
Epidemiological studies in many countries have included regional comparisons of semen parameters in men from the general population. 15 , 16 , 20 , 21 , 24 , 25 , 35 , 36 , 37 One of the earliest published studies 15 reported that Finnish, Estonian, Norwegian, and Danish young men from the general population had median sperm concentrations of 61, 62, 53, and 44 × 106/ml and normal morphology of 9.0%, 9.0%, 7.0%, and 5.0%, respectively. Semen parameters obtained for our whole study population seem lower than reported for young men from above‐mentioned European countries except Danish results, which were close to our findings. Similar median sperm concentration and percentage of spermatozoa with normal morphology (40 × 106/ml and 6.5%, respectively) were seen in Faroese young men (Denmark) from the general population; however, total sperm count and percentage of motile spermatozoa (medians 159 mill and 64%) were higher. 24 The results of a Spanish study 20 demonstrated that healthy young volunteers had higher semen quality (median sperm concentration 60 × 106/ml; progressive motility 51%) than young men from our whole study population. In young students living in Rochester, the United States, 48 median sperm concentration and total sperm count (52 × 10 6 /ml and 159 mill, respectively) were also higher than our semen results. A comparison of semen parameters in Russian men living in Estonia 25 and Slavs living in Siberia (our study) who have the same ethnic identity but live in different climatic environment zones seems to indicate a lower semen quality in Siberian men. In Russians from Estonia, median sperm concentration was 68 × 106/ml, total sperm count 208 × 106, and progressive motility 57%, while in Slavs from Siberia, the corresponding values were 43.7 × 106/ml, 150.0 × 106, and 40.6%. It can be concluded that the possible reasons for differences between two Slavic populations may be due to the influence of unfavorable climate or/and environmental conditions. Thus, the semen quality in men from the Siberian region of Russia was worse, although sometimes similar to that in man from other European countries or the United States.
To highlight a role of environment as a possible cause of regional differences, we compared the semen and hormonal indicators of young men living in Siberian cities of Novosibirsk and Kemerovo, which have a very similar climate and socio‐cultural and ethnic identity, but differ in the degree of industrial pollution. A lower spermatogenic potential of men from Kemerovo compared to men from Novosibirsk was detected, which may due to the highly polluted environment associated with ferrous metallurgy, coal mining and processing, mechanical engineering, metalworking and chemistry in this city, weakening spermatogenesis, and negatively affecting male fertility. 27 , 49 , 50 , 51 , 52 Thus, we have shown that regional differences in semen and hormonal parameters may be due to different environmental exposures in the compared regions.
We found that young men from two cities of the West Siberian region populated mainly by Slavs (Novosibirsk and Kemerovo) had higher semen parameters and inhibin B levels compared to men from two cities of the East Siberian region populated mainly by Asians (Ulan‐Ude and Yakutsk). Additional comparison of three most common Siberian ethnic groups Slavs, Buryats, and Yakuts showed ethnic differences in semen quality and reproductive hormone levels, indicating lower testicular function in Asians (Buryats and Yakuts) compared to Caucasians (Slavs). Thus, it can be concluded that the ethnic composition of the compared populations may influence regional differences in semen and endocrine parameters, although ethnic differences may include genetic background, lifestyle, and environmental influences.
To date, comparative studies of reproductive parameters in Asian and Caucasian men are very rare and contradictory. Early studies have shown smaller testicular volume in Hong Kong Chinese men than in Danes 53 ; lower testicular parenchymal weight, the diameter of seminiferous tubules, the daily sperm production, the number of Sertoli cells per man in Chinese men from China compared with White men from the United States. 54 The authors suggested that the smaller testes coupled with reduced Sertoli cell number and function and reduced daily sperm production could contribute to the inherently lower spermatogenic potential of Asian men. These conclusions coincide with our own, but not consistent with the results of recent population studies in the United States. 39 , 40 The first study showed racial differences in semen parameters between large cohorts of White and Asian men living in the same geographic area and evaluated at a single center, 39 while the second study characterized men of Asian and White ethnicity who were geographically diverse within the United States. 40 The results of both studies were similar and indicated a higher semen quality and a lower prevalence of men with suboptimal semen parameters in Asians compared to Whites. Comparisons of semen parameters of Asians from these two American studies with our results showed a higher sperm concentration and total sperm count in Asian Americans compared to Asian Russians. On the other hand, the term “Asian” used in the US studies is vague and does not lend itself to a precise ethnic definition. The median sperm concentration and total sperm count in young Japanese men from the general population 36 or sperm donors and healthy Chinese young men with proven or unknown fertility 35 , 38 , 55 were higher compared to the corresponding values in Buryats and Yakuts from our study. A comparative analysis of above semen results for Asians from the United States, Japan, China, and the Siberian region of Russia shows wide variability of values, revealing the multi‐factor combination effect of ethnic heterogeneity, environmental, and lifestyle factors on semen quality of Asians.
Interestingly, the semen data in Siberian Slavs and American White men 39 , 40 were comparable (means of sperm concentration 53.7, 51.3, 39 and 56.5 40 mill/ml; total sperm counts 187.7, 146.2, 39 and 160.1 40 mill; motility 43.1, 39.0, 39 and 55.4 40 %, respectively). In addition, semen parameters in young Siberian Slavs were similar to corresponding semen data in young German, 21 Norwegian, Danish, 15 Spanish, 56 and American men 48 from the general populations.
Androgens and estrogens play an important role in men's general health and physical performance, muscle strength, body growth, mass, and composition. 57 , 58 , 59 In our study, we found higher estradiol levels in both Asian groups and lower testosterone levels in Buryats compared to Slavs, and the negative relations between testosterone levels and body weight, waist, and hip circumference, BMI in all ethnic groups. It is assumed that ethnic differences in circulating testosterone and estradiol levels might be a cause of anthropometric differences, but given the powerful influence of sex hormones on the musculoskeletal system mainly during pubertal development, a future comparative study might provide important information about the extent to which the anthropometric parameters are related to sex steroids in different ethnic groups.
Few studies have examined ethnic differences in circulating levels of androgens or estrogens, which have pointed to the influence of genetic and environmental/dietary factors as the cause of the differences found. 38 , 60 , 61 , 62 One British study found that Pakistani men of Pakistani origin had lower both total and free testosterone in comparison with White men of European origin. 60 In a nationally representative sample of Americans, it was found that the estradiol level was significantly higher in Blacks than in Whites. 61 To distinguish genetic from environmental factors of ethnic differences, Chinese men living in Pennsylvania and a similar group living in Beijing were compared. 62 The reduction in testosterone production rates, total plasma testosterone, and sex hormone‐binding levels in Beijing Chinese as an opposed to those living in Pennsylvania were detected indicating dietary or other environmental factors appeared to alter serum levels and production rates of testosterone of Chinese men living in China.
There are few strengths and limitations of our study. The subjects of our study were young volunteers who were not selected for fertility or sperm parameters, so our study population was completely representative of the young part of the general population. Our participants were permanent residents of the cities, where the recruitment was performed, and have been exposed to the same environmental and socio‐cultural factors. A standardized recruitment protocol and questionnaire were used at all four cities to minimize between‐city differences; the study groups were quite similar in social status. All samples were collected in the morning, processed by the same scientific team using standardized laboratory methods with the same equipment and supplies. We believe that our semen and hormonal results are representative for the general population, especially given the difficulty of obtaining semen and hormone samples in population‐based studies. The limitations of our research are as follows. Since we recruited different ethnic groups in different cities, it was difficult to separate influence of a person's ethnicity from environmental and lifestyle factors. The sperm quality and hormone levels in Buryats and Yakuts identified in our study reflect the simultaneous influence of genetic, socio‐cultural, and climatic factors on these ethnic groups, so we should be careful to generalize our results to other Asian ethnic groups living in the Russian Federation.
5. CONCLUSION
The semen and hormonal results obtained in this study may serve as the point of departure for further studies of other Russian populations taken into account a wide diversity of climatic conditions and nationalities in Russia. In the population‐based study of young men living in four Siberian cities in Russia, we found regional and ethnic differences in semen quality and reproductive hormone levels that could be explained by the ethnic composition of the population or environmental pollution. Differences in sperm parameters and hormone levels between the studied Asian groups (Buryats and Yakuts) indicated ethnic heterogeneity of Asians, which may be due to genetic factors and/or ethnic cultural features, since the historical roots and ethnic history of these two peoples were different. Further work is warranted to understand genetic basis of such differences. Further research perspectives are aimed at genome/exome sequencing in representatives of various Siberian ethnic groups, providing an understanding of the differential expression of genes that regulate spermatogenesis.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interests.
AUTHOR CONTRIBUTIONS
LO provided overall supervision of the project, collected questionnaires and samples, involved in hormonal analysis, interpreted the data, and wrote the manuscript; LS and IT organized and carried out the experiments, and involved in recruiting young males from the general population; MK and AO performed the semen and statistical analysis. All authors read and approved the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We thank physicians Andrei Erkovich, Natalia Voroschilova, Natalia Kuznezova, Bair Daschiev, and Vasily Ostobunaev for coordinating the recruitment and performing a physical examination of participants, Ms. Natalia Gutorova for help in collection of questionnaires and performing of hormonal analysis. All the volunteers participating in the study are thanked.
Funding information
This study was funded by grant from the Russian Science Foundation, No. 19‐15‐00075.
REFERENCES
- 1. Giwercman A, Carlsen E, Keiding N, Skakkebaek NE. Evidence for increasing incidence of abnormalities of the human testis: a review. Environ Health Perspect. 1993;101(Suppl 2):65‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: An analysis of 101 studies published 1934–1996. Environ Health Perspect. 2000;108(10):961‐966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rolland M, Le Moal J, Wagner V, Royère D, De Mouzon J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod. 2013;28(2):462‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: A review of literature. J Hum Reprod Sci. 2015;8(4):191‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skakkebaek NE, Rajpert‐De Meyts E, Buck Louis GM, et al. Male reproductive disorders and fertility trends: Influences of environment and genetic susceptibility. Physiol Rev. 2016;96(1):55‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levine H, Jørgensen N, Martino‐Andrade A, et al. Temporal trends in sperm count: A systematic review and meta‐regression analysis. Hum Reprod Update. 2017;23(6):646‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sengupta P, Dutta S, Krajewska‐Kulak E. The disappearing sperms: analysis of reports published between 1980 and 2015. Am J Mens Health. 2017;11(4):1279‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mínguez‐Alarcón L, Williams PL, Chiu Y‐H, et al. Secular trends in semen parameters among men attending a fertility center between 2000 and 2017: identifying potential predictors. Environ Int. 2018;121(Pt 2):1297‐1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mishra P, Negi MPS, Srivastava M, Singh K, Rajender S. Decline in seminal quality in Indian men over the last 37 years. Reprod Biol Endocrinol. 2018;16(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siqueira S, Ropelle AC, Nascimento JAA, et al. Changes in seminal parameters among Brazilian men between 1995 and 2018. Sci Rep. 2020;10(1):6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eisenberg ML, Li S, Behr B, et al. Semen quality, infertility and mortality in the USA. Hum Reprod. 2014;29(7):1567‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Latif T, Kold Jensen T, Mehlsen J, et al. Semen quality as a predictor of subsequent morbidity: A Danish cohort study of 4,712 men with long‐term follow‐up. Am J Epidemiol. 2017;186(8):910‐917. [DOI] [PubMed] [Google Scholar]
- 13. Glazer CH, Eisenberg ML, Tøttenborg SS, et al. Male factor infertility and risk of death: a nationwide record‐linkage study. Hum Reprod. 2019;34(11):2266‐2273. [DOI] [PubMed] [Google Scholar]
- 14. Jørgensen N, Andersen A‐G, Eustache F, et al. Regional differences in semen quality in Europe. Hum Reprod. 2001;16(5):1012‐1019. [DOI] [PubMed] [Google Scholar]
- 15. Jørgensen N, Carlsen E, Nermoen I, et al. East‐West gradient in semen quality in the Nordic‐Baltic area: A study of men from the general population in Denmark, Norway, Estonia and Finland. Hum Reprod. 2002;17(8):2199‐2208. [DOI] [PubMed] [Google Scholar]
- 16. Punab M, Zilaitiene B, Jorgensen N, et al. Regional differences in semen qualities in the Baltic region. Int J Androl. 2002;25(4):243‐252. [DOI] [PubMed] [Google Scholar]
- 17. Swan SH, Brazil C, Drobnis EZ, et al. Geographic differences in semen quality of fertile US males. Environ Health Perspect. 2003;111(4):414‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jørgensen N, Asklund C, Carlsen E, Skakkebaek NE. Coordinated European investigations of semen quality: results from studies of Scandinavian young men is a matter of concern. Int J Androl. 2006;29(1):54‐108. [DOI] [PubMed] [Google Scholar]
- 19. Osadchuk L, Tipisova E, Kleshchev M, Gorenko I, Osadchuk A. Study of semen quality, reproductive hormone levels, and lipid levels in men from Arkhangelsk, a city in North of European Russia. Am J Mens Health. 2020;14(4):1557988320939714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. López‐Teijón M, Elbaile M, Alvarez JG. Geographical differences in semen quality in a population of young healthy volunteers from the different regions of Spain. Andrologia. 2008;40(5):318‐328. [DOI] [PubMed] [Google Scholar]
- 21. Paasch U, Salzbrunn A, Glander HJ, et al. Semen quality in sub‐fertile range for a significant proportion of young men from the general German population: A coordinated controlled study of 791 men from Hamburg and Leipzig. Int J Androl. 2008;31(2):93‐102. [DOI] [PubMed] [Google Scholar]
- 22. Fernandez MF, Duran I, Olea N, et al. Semen quality and reproductive hormone levels in men from southern Spain. Int J Androl. 2012;35(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Redmon JB, Thomas W, Ma W, et al. Semen parameters in fertile US men: the Study for Future Families. Andrology. 2013;1(6):806‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Halling J, Petersen MS, Jørgensen N, et al. Semen quality and reproductive hormones in Faroese men: A cross‐sectional population‐based study of 481 men. BMJ Open. 2013;3(3):e001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Erenpreiss J, Punab M, Zilaitiene B, et al. Semen quality of young men from the general population in Baltic countries. Hum Reprod. 2017;32(6):1334‐1340. [DOI] [PubMed] [Google Scholar]
- 26. Giwercman A, Giwercman YL. Environmental factors and testicular function. Best Pract Res Clin Endocrinol Metab. 2011;25(5):391‐402. [DOI] [PubMed] [Google Scholar]
- 27. Nordkap L, Joensen UN, Blomberg Jensen M, Jørgensen N. Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol Cell Endocrinol. 2012;355(2):221‐230. [DOI] [PubMed] [Google Scholar]
- 28. Andersen AG, Jensen TK, Carlsen E, et al. High frequency of sub‐optimal semen quality in an unselected population of young men. Hum Reprod. 2000;15(2):366‐372. [DOI] [PubMed] [Google Scholar]
- 29. Crazzolara S, Wunder D, Nägeli E, Bodmer C, Graf S, Birkhäuser MH. Semen parameters in a fertile Swiss population. Swiss Med Wkly. 2007;137(11–12):166‐172. [DOI] [PubMed] [Google Scholar]
- 30. Stewart TM, Liu DY, Garrett C, Jørgensen N, Brown EH, Baker HW. Associations between andrological measures, hormones and semen quality in fertile Australian men: inverse relationship between obesity and sperm output. Hum Reprod. 2009;24(7):1561‐1568. [DOI] [PubMed] [Google Scholar]
- 31. Iwamoto T, Nozawa S, Yoshiike M, et al. Semen quality of fertile Japanese men: a cross‐sectional population‐based study of 792 men. BMJ Open. 2013;3(1):e002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xia W, Zhu C‐H, Xiong C‐L, et al. Evaluation of semen quality in 1808 university students, from Wuhan, Central China. Asian J Androl. 2015;17(1):111‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang Y‐G, Qin W‐B, Tang L‐X, et al. The reference values for semen parameters of 1213 fertile men in Guangdong province in China. Asian J Androl. 2015;17(2):298‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahban R, Priskorn L, Senn A, et al. Semen quality of young men in Switzerland: a nationwide cross‐sectional population‐based study. Andrology. 2019;7(6):818‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gao J, Gao ES, Yang Q, et al. Semen quality in a residential, geographic and age representative sample of healthy Chinese men. Hum Reprod. 2007;22(2):477‐484. [DOI] [PubMed] [Google Scholar]
- 36. Iwamoto T, Nozawa S, Mieno MN, et al. Semen quality of 1559 young men from four cities in Japan: a cross‐sectional population‐based study. BMJ Open. 2013;3(4):e002222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kamieniczna M, Fraczek M, Malcher A, et al. Semen quality, hormonal levels and androgen receptor gene polymorphisms in a population of young male volunteers from two different regions of Poland. Med Sci Monit. 2015;21:2494‐2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Houten ME, Gooren JG. Differences in reproductive endocrinology between Asian men and Caucasian men – a literature review. Asian J Androl. 2000;2(1):13‐20. [PubMed] [Google Scholar]
- 39. Khandwala YS, Zhang CA, Li S, Behr B, Guo D, Eisenberg ML. Racial variation in semen quality at fertility evaluation. Urology. 2017;106:96‐102. [DOI] [PubMed] [Google Scholar]
- 40. Glazer CH, Li S, Zhang CA, Giwercman A, Bonde JP, Eisenberg ML. Racial and sociodemographic differences of semen parameters among US men undergoing a semen analysis. Urology. 2019;123:126‐132. [DOI] [PubMed] [Google Scholar]
- 41. Punjani N, Nayan M, Jarvi K, Lo K, Lau S, Grober ED. The effect of ethnicity on semen analysis and hormones in the infertile patient. Can Urol Assoc J. 2020;14(2):31‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McCray NL, Young HA, Irwig MS, et al. The association between race, obesity, and sperm quality among men attending a University physician practice in Washington, DC. Am J Mens Health. 2020;14(3):1557988320925985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Giwercman A, Rylander L, Hagmar L, Giwercman YL. Ethnic differences in occurrence of TDS – genetics and/or environment? Int J Androl. 2006;29(1):291‐297. [DOI] [PubMed] [Google Scholar]
- 44. Povey AC, Clyma J‐A, McNamee R, et al. Modifiable and non‐modifiable risk factors for poor semen quality: a case‐referent study. Hum Reprod. 2012;27(9):2799‐2806. [DOI] [PubMed] [Google Scholar]
- 45. World Health Organization . WHO laboratory manual for the examination and processing of human semen, 5th edn. Geneva: World Health Organization; 2010. [Google Scholar]
- 46. Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015;18(1):5‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barbotin A‐L, Ballot C, Sigala J, et al. The serum inhibin B concentration and reference ranges in normozoospermia. Eur J Endocrinol. 2015;172(6):669‐676. [DOI] [PubMed] [Google Scholar]
- 48. Mendiola J, Jørgensen N, Andersson A‐M, et al. Reproductive parameters in young men living in Rochester, New York. Fertil Steril. 2014;101(4):1064‐1071. [DOI] [PubMed] [Google Scholar]
- 49. Tavares RS, Escada‐Rebelo S, Correia M, Mota PC, Ramalho‐Santos J. The non‐genomic effects of endocrine‐disrupting chemicals on mammalian sperm. Reproduction. 2016;151(1):R1‐R13. [DOI] [PubMed] [Google Scholar]
- 50. Lafuente R, García‐Blàquez N, Jacquemin B, Checa MA. Outdoor air pollution and sperm quality. Fertil Steril. 2016;106(4):880‐896. [DOI] [PubMed] [Google Scholar]
- 51. Jurewicz J, Dziewirska E, Radwan M, Hanke W. Air pollution from natural and anthropic sources and male fertility. Reprod Biol Endocrinol. 2018;16(1): 10.1186/s12958-018-0430-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Di Nisio A, Foresta C. Water and soil pollution as determinant of water and food quality/contamination and its impact on male fertility. Reprod Biol Endocrinol. 2019;17(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Diamond JM. Variation in human testis size. Nature. 1989;320(6062):488‐489. [DOI] [PubMed] [Google Scholar]
- 54. Johnson L, Barnard JJ, Rodriguez L, et al. Ethnic differences in testicular structure and spermatogenic potential may predispose testes of Asian men to a heightened sensitivity to steroidal contraceptives. J Androl. 1998;19(3):348‐357. [PubMed] [Google Scholar]
- 55. Junqing WU, Qiuying Y, Jianguo T, et al. Reference value of semen quality in Chinese young men. Contraception. 2002;65(5):365‐368. [DOI] [PubMed] [Google Scholar]
- 56. Mendiola J, Jørgensen N, Mínguez‐Alarcón L, et al. Sperm counts may have declined in young university students in Southern Spain. Andrology. 2013;1(3):408‐413. [DOI] [PubMed] [Google Scholar]
- 57. Rochira V, Kara E, Carani C. The endocrine role of estrogens on human male skeleton. Int J Endocrinol. 2015;2015:165215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Trabert B, Graubard BI, Nyante SJ, et al. Relationship of sex steroid hormones with body size and with body composition measured by dual‐energy X‐ray absorptiometry in US men. Cancer Causes Control. 2012;23(12):1881‐1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Watts EL, Appleby PN, Albanes D, et al. Circulating sex hormones in relation to anthropometric, sociodemographic and behavioural factors in an international data set of 12,300 men. PLoS One. 2017;12(12):e0187741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Heald AH, Ivison F, Anderson SG, et al. Significant ethnic variation in total and free testosterone concentration. Clin Endocrinol (Oxf). 2003;58(3):262‐266. [DOI] [PubMed] [Google Scholar]
- 61. Rohrmann S, Nelson WG, Rifai N, et al. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J Clin Endocrinol Metab. 2007;92(7):2519‐2525. [DOI] [PubMed] [Google Scholar]
- 62. Santner SJ, Albertson B, Zhang GY, et al. Comparative rates of androgen production and metabolism in Caucasian and Chinese subjects. J Clin Endocrinol Metab. 1998;83(6):2104‐2109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


