Abstract
Background
Hazelnut allergy, which is characterized by symptoms that range from mild to severe, is one of the most common allergies in children throughout Europe, and an accurate diagnosis of this allergy is therefore essential. However, lipophilic allergens, such as oleosins, are generally underrepresented in diagnostic tests. We therefore sought to characterize the IgE reactivity of raw and roasted hazelnut oleosins, using the sera of hazelnut‐allergic pediatric patients.
Methods
Raw and roasted hazelnut oil body–associated proteins were analyzed by means of 1D and 2D electrophoresis and MS. Oleosin IgE reactivity was assessed by immunoblotting with the sera of 27 children who have confirmed hazelnut allergies and from 10 tolerant subjects. A molecular characterization of the oleosins was performed by interrogating the C. avellana cv. Jefferson and cv. TGL genomes, and through expression and purification of the recombinant new allergen.
Results
A proteomic and genomic investigation allowed two new oleosins to be identified, in addition to Cor a 12 and Cor a 13, in hazelnut oil bodies. One of the new oleosins was registered as a new allergen, according to the WHO/IUIS Allergen Nomenclature Subcommittee criteria, and termed Cor a 15. Cor a 15 was the most frequently immunorecognized oleosin in our cohort. Oleosins resulted to be the only immunorecognized allergens in a subgroup of allergic patients who showed low ImmunoCAP assay IgE values and positive OFC and PbP. Hazelnut roasting resulted in an increase in oleosin immunoreactivity.
Conclusion
A novel hazelnut oleosin, named Cor a 15, has been discovered. Cor a 15 could play a role in eliciting an allergic reaction in a subgroup of pediatric patients that exclusively immunorecognize oleosins. The high prevalence of hazelnut oleosin sensitization here reported further confirms the need to include oleosins in routine diagnostic procedures.
Keywords: Cor a 15, hazelnut allergy, oleosins, pediatric population, roasting
Key Message.
The manuscript deals with the discovery of a new hazelnut allergen, Cor a 15. It also provides novel evidence concerning the role of oleosins in hazelnut allergy, the most common tree nut allergy in Europe. The research findings are relevant for clinical practice, since they confirm the need to include lipophilic allergens, such as oleosins, in the protein extracts used for routine diagnostic tests, as already found for peanuts.
1. INTRODUCTION
Hazelnut (Corylus avellana L.) allergy is the most common tree nut allergy in Europe 1 , with a different prevalence being observed among European countries. 2 Associated oil body (OB) proteins have been receiving increasing interest, since oleosins have been described as major allergens in peanuts 3 , 4 and in sesame. 5 , 6 Moreover, they have been associated with severe symptoms as a result of the consumption of hazelnuts. 7 , 8 In 2015, the EuroPrevall project 2 established that hazelnut oleosin sensitization is quite common in Europe (which has from 10 to 25% of hazelnut‐allergic patients), with a higher prevalence in children than in adults (34% vs 11.4%). Akkerdaas et al. 9 reported the two first allergenic oleosins in hazelnuts, Cor a 12 and Cor a 13.
Oleosins are usually underrepresented in skin prick test (SPT) extracts and in vitro reagents, thereby giving rise to false negatives in hazelnut allergy diagnosis. 7 , 10 Their absence in test reagents is due to their hydrophobic structure, which results in their eluding the standard protein purification procedures. Zuidmeer‐Jongejan et al. 7 pointed out the absence of Cor a 12 and Cor a 13 in nine of the most commonly used SPT extracts.
The IgE binding potential of peanut oleosins has recently been found to be increased by in‐shell roasting. 4 However, the effect of roasting on hazelnut oleosin immunorecognition remains unknown.
Our aim was to investigate the contribution of oleosins to hazelnut pediatric allergies and to assess the effect of roasting on hazelnut oleosin immunogenicity.
2. METHODS
Details about the methods used for most of the conducted experiments are available in the Online Repository.
2.1. Study population and clinical methods
Pediatric subjects with a convincing history of hazelnut allergy (HA patients, no. = 27) and pediatric subjects sensitized but tolerant to hazelnuts (HS patients, no. = 10) were retrospectively recruited from the Paediatric Allergy Unit, Regina Margherita Children’s Hospital (Città della Salute e della Scienza, Turin, Italy). Hazelnut allergy was defined as a convincing history of allergic symptoms (oral allergy syndrome (OAS), urticaria, angioedema, respiratory, cardiovascular, and/or gastrointestinal symptoms) appearing within 1 h after hazelnut ingestion. The allergy diagnosis was confirmed by means of an oral food challenge (OFC) (except for patients with severe anaphylaxis), by prick by prick (PbP), and by hazelnut sIgE dosed with ImmunoCAP (Thermo Fisher Scientific, Uppsala, Sweden) (Table 1).
TABLE 1.
Clinical characterization of the patient cohort (N=37)
| Panel A: first patient recruitment | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Patient classification | Sex | Age (y) | FHA | AD | FA | PA | NA | Hazelnut PbP (ø mm) | IgE ImmunoCAP (KU/l) | Roasted OFC Symptoms | OFC symptoms Severity grading | Reduced immunoblotting to RAW hazelnut OB | Reduced immunoblotting to ROASTED hazelnut OB | ||||||||
| Raw | Roasted | Hazelnut | rCor a 1 | rCor a 8 | rCor a 9 | rCor a 14 | rBet v 1 | 14‐kDa band | 17‐kDa band | 14‐kDa band | 17‐kDa band | |||||||||||
| 1 | HA | M | 15 | Yes | Yes | Yes | No | No | 6 | 6 | 75.80 | 58.80 | 1.02 | 19.20 | 67.80 | 55.80 | OS, NS, V, ER, AP, LE, H | Severe | No | No | Yes | Yes |
| 2 | HA | M | 5 | Yes | Yes | No | Yes | No | 13 | 5 | 13.70 | 0.10 | 0.25 | 9.80 | 4.50 | <0.10 | OAS, LAE | Mild | Yes | Yes | Yes | Yes |
| 3 | HA | M | 11 | Yes | No | Yes | No | No | 12 | 6 | 51.70 | 72.30 | <0.10 | 4.95 | 5.43 | <0.10 | OAS, PI, AP | Moderate | No | Yes | No | Yes |
| 4 | HA | F | 7 | Yes | Yes | Yes | Yes | Yes | 10 | 10 | 28.90 | <0.10 | <0.10 | 27.40 | 39.30 | <0.10 | V, OAS, PI, ER | Moderate | No | No | Yes | Yes |
| 5 | HA | M | 8 | Yes | Yes | Yes | No | Yes | 9 | 8 | 73.00 | 64.30 | 19.20 | 11.20 | 26.10 | <0.10 | V, AS, LE | Severe | No | No | Yes | Yes |
| 6 | HA | F | 12 | Yes | Yes | Yes | Yes | Yes | 9 | 8 | 84.20 | <0.10 | <0.10 | 73.40 | 41.20 | n.d. | AN | Severe | No | No | Yes | Yes |
| 7 | HA | M | 7 | Yes | No | Yes | Yes | Yes | 6 | 4 | 55.90 | <0.10 | 0.54 | 39.30 | 4.16 | <0.10 | V, AP, ER | Moderate | Yes | No | Yes | Yes |
| 8 | HA | F | 7 | Yes | Yes | Yes | No | Yes | 5 | 5 | 9.55 | <0.10 | <0.10 | 19.90 | 3.94 | <0.10 | OAS, AP, NS, C, H | Severe | Yes | No | Yes | Yes |
| 9 | HA | M | 8 | Yes | Yes | Yes | Yes | Yes | 8 | 5 | 16.60 | 11.50 | <0.10 | 6.85 | 4.76 | 9.80 | OAS, C, ER | Moderate | Yes | Yes | Yes | Yes |
| 10 | HA | M | 12 | Yes | No | No | Yes | Yes | 10 | 14 | >100 | 29.10 | 0.11 | >100 | >100 | 36.50 | V, AP, U, LE, AS | Severe | No | Yes | Yes | Yes |
| 11 | HA | M | 11 | No | No | Yes | Yes | No | 3 | 0 | 30.70 | 0.42 | 36.80 | 0.69 | 0.27 | 4.44 | ER | Mild | No | Yes | No | Yes |
| 12 | HA | F | 16 | Yes | Yes | Yes | No | Yes | 10 | 8 | 41.90 | <0.10 | <0.10 | 34.70 | 19.90 | <0.10 | OAS | Mild | Yes | Yes | Yes | Yes |
| 13 | HA | M | 10 | Yes | Yes | Yes | Yes | Yes | 10 | 8 | 7.45 | 4.33 | <0.10 | 1.92 | 1.49 | 4.40 | OAS, PI, ER | Mild | No | Yes | Yes | Yes |
| 14 | HA | M | 6 | Yes | Yes | No | No | No | 6 | 7.5 | 3.75 | <0.10 | <0.10 | <0.10 | 4.67 | <0.10 | ER, U, AP, LAE | Moderate | No | Yes | Yes | Yes |
| 15 | HA | M | 10 | Yes | Yes | No | Yes | Yes | 7 | 6.5 | 38.20 | 59.70 | 0.75 | 0.22 | <0.10 | >100 | U, PI | Mild | No | Yes | No | Yes |
| 16 | HA | M | 10 | Yes | Yes | No | Yes | Yes | 8 | 4.5 | 69.00 | 0.31 | >100 | 29.10 | 81.50 | 0.52 | U, PI | Mild | No | Yes | No | Yes |
| 17 | HA | M | 14 | Yes | Yes | Yes | Yes | Yes | 7 | 8 | >100 | 17.40 | 2.30 | 56.90 | 74.50 | 57.60 | U, PI | Mild | No | Yes | Yes | Yes |
| 18 | HA | M | 4 | Yes | Yes | No | No | No | 5 | 15 | 20.10 | <0.10 | 0.73 | 8.57 | 26.30 | <0.10 | AS, C, R | Severe | No | Yes | Yes | Yes |
| 19 | HA | F | 13 | n.d. | Yes | Yes | Yes | Yes | 11 | 7.5 | >100 | 0.12 | <0.10 | >100 | >100 | <0.10 | U | Mild | No | Yes | No | Yes |
| 20 | HA | M | 14 | Yes | Yes | Yes | Yes | Yes | 10 | 7 | 3.62 | <0.10 | 0.49 | 9.18 | 4.94 | <0.10 | LE, AP | Severe | No | Yes | No | Yes |
| 21 | HA | F | 9 | Yes | Yes | Yes | Yes | No | 3 | 6 | 48.00 | 2.73 | 29.80 | 9.68 | 34.30 | n.d. | OAS | Mild | Yes | Yes | Yes | Yes |
| 22 | HA | M | 2 | Yes | Yes | Yes | No | Yes | 3 | 0 | 2.75 | 0.40 | <0.10 | n.d. | 4.98 | <0.10 | LAE, U, LE | Severe | No | No | No | No |
| 23 | LIHA | M | 11 | Yes | No | Yes | No | Yes | 5 | 5 | 0.76 | <0.10 | 1.78 | 0.13 | 0.47 | <0.10 | OAS, PI, H | Severe | No | Yes | No | Yes |
| 24 | LIHA | M | 12 | Yes | Yes | Yes | Yes | Yes | 12 | 7 | 1.29 | 0.55 | 0.13 | <0.10 | <0.10 | <0.10 | LAE, A, U | Mild | No | Yes | Yes | Yes |
| 25 | LIHA | M | 16 | Yes | Yes | Yes | Yes | No | 7 | 5 | 0.71 | <0.10 | <0.10 | 0.36 | 0.76 | <0.10 | LAE | Mild | No | Yes | Yes | Yes |
| 26 | LIHA | M | 5 | n.d. | n.d. | Yes | No | No | 5 | 6 | 1.59 | 1.88 | <0.10 | <0.10 | 0.70 | 4.98 | LAE | Mild | No | Yes | No | Yes |
| 27 | LIHA | F | 5 | n.d. | n.d. | No | Yes | Yes | 5 | 0 | 0.45 | <0.10 | <0.10 | 1.13 | 0.19 | <0.10 | OS, U, C | Moderate | No | Yes | No | Yes |
| 28 | HS | F | 7 | Yes | Yes | Yes | No | Yes | 3 | 5 | 7.76 | <0.10 | 6.50 | 0.32 | <0.10 | <0.10 | None | No | Yes | Yes | Yes | |
| 29 | HS | M | 6 | Yes | Yes | Yes | No | No | 5 | 5 | 24.70 | 38.10 | 0.85 | <0.10 | <0.10 | <0.10 | None | Yes | Yes | Yes | Yes | |
| 30 | HS | F | 6 | No | Yes | No | Yes | Yes | 4.5 | 0 | 78.00 | >100 | 1.37 | 1.74 | 0.69 | >100 | None | No | Yes | Yes | Yes | |
| 31 | HS | M | 17 | Yes | Yes | Yes | Yes | No | 10 | 10 | 7.42 | 7.53 | <0.10 | 0.71 | 0.14 | 15.80 | None | No | Yes | Yes | Yes | |
| 32 | HS | F | 12 | Yes | No | Yes | Yes | No | 4.5 | 0 | 47.40 | 58.70 | <0.10 | 0.18 | <0.10 | 67.30 | None | No | Yes | Yes | Yes | |
| 33 | HS | M | 11 | Yes | Yes | Yes | Yes | Yes | 3 | 0 | 0.65 | <0.10 | <0.10 | <0.10 | 0.30 | <0.10 | None | No | Yes | Yes | Yes | |
| 34 | HS | M | 11 | Yes | No | No | Yes | Yes | 5 | 0 | 15.20 | <0.10 | 14.60 | <0.10 | <0.10 | <0.10 | None | No | Yes | Yes | Yes | |
| 35 | HS | M | 9 | Yes | Yes | Yes | No | Yes | 5 | 4 | 6.44 | <0.10 | 0.18 | 1.00 | 0.91 | <0.10 | None | No | Yes | Yes | Yes | |
| 36 | HS | M | 7 | Yes | No | Yes | No | No | 12 | 9 | 6.14 | <0.10 | <0.10 | 0.65 | 1.37 | <0.10 | None | No | No | No | Yes | |
| 37 | HS | M | 7 | Yes | Yes | No | Yes | Yes | 5 | 0 | 54.90 | <0.10 | <0.10 | 79.00 | 6.40 | n.d. | None | No | Yes | no | Yes | |
Abbreviations: A, angioedema; AD, atopic dermatitis; AN, anaphylaxis; AP, abdominal pains; AS, asthma; C, cough; ER, erythema; F, female; FA, other food allergy; FHA, family history of atopy; H, hoarseness; HA, hazelnut‐allergic patient; HS, hazelnut‐sensitized patient; HY, hypotension; LAE, labial edema; LE, laryngeal edema; LIHA, low ImmunoCAP hazelnut‐allergic patient; M, male; NA, other nut allergy; NS, nasal symptoms; OAS, oral allergy syndrome; OS, ocular symptoms; PA, pollen allergy; PI, pharyngeal itching; R, respiratory problems; U, urticaria; V, vomiting.
All the HA patients reported having had a reaction to foods containing roasted hazelnuts (cocoa, hazelnut spreads, chocolate, hazelnut ice cream, chocolate pralines with whole roasted hazelnuts, or hazelnut nougat). For this reason, the OFC was performed with finely ground roasted hazelnuts.
The OFC was initiated using one drop of physiological solution mixed with the hazelnut powder and placing the solution on the inner side of the lower lip. The challenge was continued with increasing doses of hazelnut powder (0.2 g, 0.4 g, 1 g, 2 g, 3 g, 6 g, 8 g, and 10 g) administered in 20‐min intervals, unless symptoms occurred or until a total cumulative dose of 30 g of hazelnut powder (equivalent to 15 hazelnuts). The OFC procedure was stopped after observation of the first objective symptom. In the case of mild objective or subjective symptoms, it was decided to wait longer for the subsequent dose and/or to repeat the same dose, and the OFC procedure was stopped in the case of persistent symptoms (lasting longer than 30 minutes) and/or worsening symptoms and in the case of repeated elicitation (over 3 doses). The scoring of the severity of the reaction to the OFC was evaluated independently by two allergy specialists (G.M. and R.C.), and the results were coded as mild, moderate, or severe, according to the Integrated Grading System for Scoring Reaction Severity of Oral Food Challenge Reactions of Zhu et al. 11
All the patients underwent PbP, using both raw and roasted hazelnuts. Histamine (10 mg/ml) was used as a positive control, and a saline solution was used as the negative control. PbP was considered positive if the wheal was higher than 3 mm, and there was no reaction to the negative control.
The IgEs sera were assayed against a hazelnut extract, rCor a 1, rCor a 8, rCor a 9, rCor a 14, and rBet v 1 using ImmunoCAP. The cutoff for positivity was set at 0.10 KU/l. rCor a 1, rCor a 8, rCor a 9, and rCor a 14 sIgE concentrations were standardized for the statistical analysis by subtracting the mean values of each variable and then dividing by the corresponding standard deviations. The sIgE values below the limit of detection (<0.10 KU/L) were assigned a value of 0.01; the sIgE values above the limit of detection (>100 KU/L) were assigned a value of 100. Missing data (codified in Table 1 with n.d.) were not considered in the statistical analysis. The standardized data of the patients classified as HA versus the patients classified as HS were analyzed by means of nonparametric Kruskal‐Wallis one‐way ANOVA, because of the non‐normal distribution. The correlation between the standardized sIgE concentrations of the different allergens was calculated by means of Kendall’s tau. All the analyses were performed using PAST software, version 2.17. 12
A subgroup of the HA patients (patient nos. 23, 24, 25, 26, and 27), who were characterized by low rCor a 1, rCor a 8, rCor a 9, and rCor a 14 ImmunoCAP IgE values, ranging from 0.10 kU/L to 1.88 kU/L, were defined as low ImmunoCAP hazelnut‐allergic (LIHA) patients.
The HS patients were characterized according to the presence of atopic dermatitis (AD) and/or other food allergy (FA) and/or other nut allergies (NA), with a positive PbP to hazelnut and/or positive sIgE to hazelnut, but negative to OFC.
The experimental controls were the sera of non‐nut‐allergic pediatric subjects (NNA) and of non‐food‐allergic pediatric subjects (NFA), used individually or pooled.
The study was approved by the local ethics committee of the Città della Salute e della Scienza (Turin, Italy) (approval no. 312, prot. no. 22050). The parents of all the patients gave written informed consent.
2.2. Hazelnut water‐soluble and OB‐associated protein extraction
Raw hazelnuts (Corylus avellana cv. Tonda Gentile delle Langhe, TGL) were obtained from experimental fields, roasted at a laboratory scale (120°C for 30 min), and used for the OFC, PbP, and immunoblotting experiments. The water‐soluble proteins were extracted according to Platteau et al. 13 The hazelnut OBs were isolated according to Cao et al. 14 and the OB‐associated proteins were extracted according to Lamberti et al., 2020. 15
2.3. Protein separation by means of LDS‐PAGE, 2‐dimensional electrophoresis (2‐DE), and immunoblotting analysis
The hazelnut water‐soluble proteins were separated by means of LDS‐PAGE. The OB‐associated proteins were separated by means of both LDS‐PAGE and 2‐DE. Protein bands and spots were electro‐transferred to PVDF membranes and incubated with the patient’s serum. Immunoblotting was developed using an Alkaline Phosphatase Substrate Kit (Bio‐Rad).
2.4. Use of sera
Individual serum was used for 1D immunoblotting of the OB protein extracts, ELISA, and an immunoblotting inhibition assay with a raw OB protein extract. Pooled sera were used for the remaining experiments: 2D immunoblotting of the OB protein extracts, an immunoblotting inhibition assay with rCor a 15, and immunoblotting of the total water‐soluble hazelnut protein extracts, due to the paucity of the available sera.
2.5. Protein identification by means of MALDI‐TOF/TOF and ESI‐Q‐TOF analyses
In‐gel digestion was performed to identify the allergens contained in the bands and spots from the gel electrophoresis, as in Nebbia et al., 16 and protein identification was performed by coupling the MALDI‐TOF/TOF 17 and ESI‐Q‐TOF‐MS techniques. 15 The spectra were searched against the NCBI C. avellana database implemented with two new oleosin sequences derived from both the C. avellana cv Jefferson genome 18 and C. avellana cv TGL. 19
2.6. Expression and purification of recombinant Cor a 15
In order to establish whether the newly discovered oleosin was an allergen, it was produced as a recombinant protein. Its complete coding sequence, obtained by means of RNA retrotranscription, was cloned in a pGEM‐T Easy vector (Promega, Madison, WI, The USA) and sent to PRIMM (Milan, Italy) for protein expression and purification. The full‐length allergen (rCor a 15) and the C‐terminal fragment (rCor a 15 C‐term, from aa 102 to aa 169) were used for the blot inhibition studies, and only the full‐length rCor a 15 was used for ELISA.
2.7. Quantification of Cor a 15‐specific IgEs by means of direct ELISA test
IgEs specific to rCor a 15 were quantified as described in Hilger et al., 20 with some modifications. Briefly, patient sera (10 HA, 3 LIHA, 2 HS, 9 NNA, and 9 NFA patients) were transferred to 96‐well plates previously coated with 0.1 µg/ml of rCor a 15. In order to obtain a semi‐quantification of hazelnut‐allergic patient IgE against rCor a 15, the serum of a patient with a known titer of IgE specific to α‐lactalbumin (as determined by ImmunoCAP) was used as the reference standard. The reference serum was chosen since it contained IgE that was completely unrelated to the allergy that was the subject of this study. The reference serum was serially diluted in duplicate and added to α‐lactalbumin‐coated wells. The obtained standard curve allowed us to calculate the IgE titers specific to rCor a 15 for each patient’s serum.
3. RESULTS
A flowchart of the experiments and a summary of the main results are given in Figure 1.
FIGURE 1.

Workflow of the experiments
3.1. Study population
The main characteristics of the pediatric subjects included in the study are summarized in Table 1. All the patients tested with PbP resulted positive to raw hazelnuts: 24/27 HA patients and 6/10 HS patients showed a wheal diameter ≥ 5 mm. Three HA patients out of 27 and 5/10 HS patients showed negative PbP results when tested for roasted hazelnuts.
The median scores of the sIgE of rCor a 1, rCor a 8, rCor a 9, and rCor a 14 for the HA patients were 0.31 (IQR 0.01–11.50), 0.11 (IQR 0.01–1.02), 9.18 (IQR 0.36–29.10), and 4.94 (IQR 0.76–39.30), respectively. The median scores of the sIgE of rCor a 1, rCor a 8, rCor a 9, and rCor a 14 for the HS patients were 0.01 (IQR 0.01–38.10), 0.10 (IQR 0.01–1.37), 0.49 (IQR 0.01–1.00), and 0.22 (IQR 0.01–0.91), respectively. The HA and HS patients differed significantly as far as the rCor a 9 and rCor a 14 values are concerned (pCora9 = 0.004, pCora14 = 0.002), while Cor a 1 and Cor a 8 did not discriminate the HA from the HS patients (pCora1 = 0.75, pCora8=0.98). Furthermore, the rCor a 9 and rCor a 14 values were directly correlated (r2 = 0.67; p < .001). Seventeen patients (12/27 HA patients and 5/10 HS patients) also suffered from a pollen allergy (PA). Finally, 17/27 HA patients and 6/10 HS patients were allergic to other tree nuts (NA). It was not possible to establish a correlation between the severity grading and oleosin immunorecognition because of the limited number of LIHA patients.
3.2. Discovery of two new hazelnut oleosins
The OB‐associated proteins were separated (Figures 2 and 3) and identified (Table 2, Table S1, and Table S2), and two new oleosin‐like proteins were found (Figure S1). Their sequences were submitted to GenBank under accession numbers MK737923 and MK737924. The MK737923 sequence showed 56% similarity with Cor a 12 and 41% with Cor a 13. The MK737924 sequence showed 63% similarity with Cor a 12 and 76% with Cor a 13 (Figure S2).
FIGURE 2.

For each panel: first lane, stained LDS‐PAGE of raw (A) and roasted (B) OB‐associated protein extracts; lanes 1 to 37: immunoblotting with individual patient’s serum. C, a pool of five sera of non‐nut‐allergic (NNA) pediatric subjects was used as a control. CII, secondary antibody control. MW, molecular weight; HA, hazelnut‐allergic patients; LIHA, low ImmunoCAP hazelnut‐allergic patients; HS, hazelnut‐sensitized patients
FIGURE 3.

2‐DE and immunoblotting of the OB‐associated protein extracts. 2‐DE of raw (A) and roasted (B) hazelnut OB protein extracts. Immunoblotting of raw (C) and roasted (D) hazelnut OB protein extracts with the pool of sera of the LIHA patients (nos. 23, 24, and 25). Immunoblotting of raw (E) and roasted (F) hazelnut OB protein extracts with a pool of five sera of non‐nut allergic (NNA) pediatric subjects. MW, molecular weight; pI, isoelectric point; NL, non‐linear
TABLE 2.
1‐DE and 2‐DE protein identification
| No band | Entry (NCBI) | Protein name | MWexp/MWtheor | Protein score | No of matching peptides | Protein coverage (%) | Molar fraction (%) |
|---|---|---|---|---|---|---|---|
| 1‐DE protein identification | |||||||
| Raw hazelnut | |||||||
| 1A | AAO65960 | Cor a 13 | 14,000/14,723 | 776 | 7 | 36.6 | 78.4 |
| AAO67349 | Cor a 12 | 14,000/16,745 | 455 | 4 | 26.9 | 21.6 | |
| 2A | MK737923 | Cor a 15 | 17,000/17,695 | 1045 | 7 | 45.9 | 100 |
| Roasted hazelnut | |||||||
| 1B | AAO65960 | Cor a 13 | 14,000/14,723 | 1231 | 6 | 38.9 | 64.4 |
| AAO67349 | Cor a 12 | 14,000/16,745 | 624 | 6 | 34.8 | 35.6 | |
| 2B | MK737923 | Cor a 15 | 17,000/17,695 | 1080 | 8 | 45.9 | 89.5 |
| AAO67349 | Cor a 12 | 17,000/16,745 | 140 | 3 | 21.7 | 10.5 | |
| 3B | AHA36627 | Cor a 9 | 22,000/59,200 | 1853 | 14 | 31.2 | 69.5 |
| AAO65960 | Cor a 13 | 22,000/14,723 | 203 | 4 | 26.9 | 30.6 | |
| 4B | AHA36627 | Cor a 9 | 23,000/59,200 | 2268 | 17 | 28.4 | 100 |
| 5B | AAO65960 | Cor a 13 | 27,000/14,723 | 456 | 4 | 29.1 | 47 |
| AAO67349 | Cor a 12 | 27,000/16,745 | 378 | 4 | 26.9 | 38.3 | |
| AHA36627 | Cor a 9 | 27,000/59,200 | 433 | 7 | 16.2 | 14.7 | |
| 6B | MK737923 | Cor a 15 | 29,000/17,695 | 293 | 5 | 36.6 | 40.6 |
| AAO65960 | Cor a 13 | 29,000/14,723 | 401 | 4 | 29.1 | 27.2 | |
| AAO67349 | Cor a 12 | 29,000/16,745 | 410 | 4 | 26.9 | 22.2 | |
| AHA36627 | Cor a 9 | 29,000/59,200 | 539 | 8 | 18.6 | 10.1 | |
| 7B | MK737923 | Cor a 15 | 34,000/17,695 | 78 | 4 | 36.6 | 53.6 |
| AHA36627 | Cor a 9 | 34,000/59,200 | 181 | 3 | 21.7 | 46.4 | |
| 8B | AHA36627 | Cor a 9 | 38,000/59,200 | 524 | 8 | 19.9 | 68.8 |
| AAO67349 | Cor a 12 | 38,000/16,745 | 178 | 4 | 26.9 | 31.2 | |
| No band | Entry (NCBI) | Protein name | MWexp/MWtheor ‐PIexp/PItheor | Protein score | No of matching peptides | Protein coverage (%) | Molar fraction (%) |
|---|---|---|---|---|---|---|---|
| 2‐DE protein identification | |||||||
| Raw hazelnut | |||||||
| 1A2DE | AAO67349 | Cor a 12 | 14,000/16,745‐10.00/10.54 | 4430 | 8 | 38.8 | 64.1 |
| AAO65960 | Cor a 13 | 14,000/14,723‐10.00/9.98 | 3846 | 8 | 41.1 | 35.9 | |
| 2A2DE | MK737923 | Cor a 15 | 17,000/17,695‐10.00/9.56 | 3709 | 13 | 55.8 | 96.5 |
| AAO67349 | Cor a 12 | 17,000/16,745‐10.00/10.54 | 666 | 5 | 28.9 | 3.5 | |
| 3A2DE | MK737923 | Cor a 15 | 29,000/17,695‐10.00/9.56 | 889 | 8 | 49.6 | 62.2 |
| AAO67349 | Cor a 12 | 29,000/16,745‐10.00/9.98 | 404 | 4 | 26.9 | 16.2 | |
| AAO65960 | Cor a 13 | 29,000/14,723‐10.00/10.54 | 563 | 4 | 29.1 | 13.7 | |
| MK737924 | Oleosin | 29,000/15,790‐10.00/9.43 | 93 | 3 | 18.7 | 7.9 | |
| 4A2DE | MK737923 | Cor a 15 | 34,000/17,695‐10.00/9.56 | 1298 | 8 | 45.9 | 65.8 |
| AAO65960 | Cor a 13 | 34,000/14,723‐10.00/9.98 | 524 | 4 | 29.1 | 18.8 | |
| AAO67349 | Cor a 12 | 34,000/16,745‐10.00/10.54 | 312 | 3 | 21.7 | 15.4 | |
| 5A2DE | Not identified | ||||||
| 6A2DE | Not identified | ||||||
| 7A2DE | Not identified | ||||||
| Roasted hazelnut | |||||||
| 1B2DE | AAO67349 | Cor a 12 | 14,000/16,745‐10.00/10.54 | 1679 | 7 | 35.5 | 81 |
| AAO65960 | Cor a 13 | 14,000/14,723‐10.00/9.98 | 1051 | 5 | 31.4 | 19.0 | |
| 2B2DE | AAO67349 | Cor a 12 | 15,000/16,745‐10.00/10.54 | 2155 | 7 | 39.4 | 72.6 |
| AAO65960 | Cor a 13 | 15,000/14,723‐10.00/9.98 | 1001 | 5 | 31.4 | 22.2 | |
| MK737923 | Cor a 15 | 15,000/17,695‐10.00/9.56 | 134 | 3 | 24.8 | 5.1 | |
| 3B2DE | AAO67349 | Cor a 12 | 17,000/16,745‐10.00/9.56 | 920 | 6 | 34.2 | 45.3 |
| MK737923 | Cor a 15 | 17,000/17,695‐10.00/9.98 | 1054 | 7 | 45.3 | 39.8 | |
| AAO65960 | Cor a 13 | 17,000/14,723‐10.00/10.54 | 962 | 4 | 29.1 | 14.9 | |
| 4B2DE | AHA36627 | Cor a 9 | 22,000/59,200‐10.50/8.93 | 1406 | 13 | 27.8 | 100 |
| 5B2DE | Not identified | ||||||
| 6B2DE | AHA36627 | Cor a 9 | 23,000/59,200‐10.00/8.93 | 4799 | 14 | 30.2 | 57.1 |
| MK737923 | Cor a 15 | 23,000/17,695‐10.00/9.10 | 132 | 5 | 36.6 | 27.0 | |
| AAO65960 | Cor a 13 | 23,000/14,723‐10.00/9.56 | 273 | 3 | 34.3 | 15.9 | |
| 7B2DE | AHA36627 | Cor a 9 | 23,000/59,200‐9.00/8.93 | 6112 | 14 | 32.3 | 81.1 |
| MK737923 | Cor a 15 | 23,000/17,695‐9.00/9.56 | 124 | 4 | 36.6 | 18.9 | |
| 8B2DE | AHA36627 | Cor a 9 | 23,000/59,200‐8.50/8.93 | 3519 | 14 | 29 | 100 |
| 9B2DE | AHA36627 | Cor a 9 | 22,000/59,200‐10.00/9.56 | 3333 | 18 | 29.4 | 84.5 |
| MK737923 | Cor a 15 | 22,000/17,695‐10.00/10.54 | 56 | 3 | 30.4 | 15.5 | |
| 10B2DE | AHA36627 | Cor a 9 | 22,000/59,200‐9.00/8.93 | 2197 | 13 | 27.4 | 75.1 |
| MK737923 | Cor a 15 | 22,000/17,695‐9.00/9.56 | 96 | 3 | 31 | 24.9 | |
| 11B2DE | Not identified | ||||||
| 12B2DE | Not identified | ||||||
| 13B2DE | Not identified | ||||||
| 14B2DE | Not identified | ||||||
| 15B2DE | AAL86739 | Cor a 11 | 27,000/51,110‐5.00/6.12 | 473 | 8 | 23.2 | 100 |
| 16B2DE | AAL86739 | Cor a 11 | 24,000/51,110‐5.00/6.15 | 536 | 8 | 22.4 | 75.2 |
| AHA36627 | Cor a 9 | 24,000/59,200‐5.00/6.12 | 616 | 5 | 15.2 | 24.8 | |
| 17B2DE | AHA36627 | Cor a 9 | 30,000/59,200‐5.00/6.15 | 296 | 5 | 14.4 | 60,6 |
| AAL86739 | Cor a 11 | 30,000/51,110‐5.00/6.12 | 145 | 3 | 9.2 | 39,4 | |
| 18B2DE | AHA36627 | Cor a 9 | 38,000/59,200‐5.00/6.15 | 189 | 5 | 15.7 | 51.4 |
| AAL86739 | Cora 11 | 38,000/51,110‐5.00/6.12 | 112 | 5 | 9.4 | 48.6 | |
| 19B2DE | AHA36627 | Cor a 9 | 38,000/59,200‐5.70/6.15 | 1562 | 16 | 45.1 | 100 |
| 20B2DE | AHA36627 | Cor a 9 | 38,000/59,200‐6.00/6.46 | 1702 | 17 | 40.1 | 90.8 |
| AAL86739 | Cor a 11 | 38,000/51,110‐6.00/6.12 | 122 | 5 | 13.2 | 9.2 | |
| 21B2DE | AAL86739 | Cor a 11 | 45,000/51,110‐6.20/6.12 | 4659 | 24 | 55.3 | 91.4 |
| AHA36627 | Cor a 9 | 45,000/59,200‐6.20/5.69 | 985 | 15 | 39 | 8.6 | |
| 22B2DE | MK737923 | Cor a 15 | 17,000/17,695‐8.00/9.56 | 803 | 6 | 45.3 | 80.6 |
| AAO65960 | Cor a 13 | 17,000/14,723‐8.00/9.98 | 174 | 3 | 28.4 | 19.4 | |
| 23B2DE | MK737923 | Cor a 15 | 17,000/17,695‐6.70/9.56 | 17741 | 7 | 44.6 | 83.3 |
| AAO67349 | Cor a 12 | 17,000/16,745‐6.70/10.54 | 16745 | 3 | 21.7 | 16.7 | |
| 24B2DE | MK737923 | Cor a 15 | 36,000/17,695‐10.00/9.56 | 1055 | 8 | 45.9 | 74.8 |
| AAO65960 | Cor a 13 | 36,000/14,723‐10.00/9.98 | 517 | 4 | 29.1 | 16.4 | |
| AAO67349 | Cor a 12 | 36,000/16,745‐10.00/10.54 | 350 | 3 | 28.2 | 8.8 | |
3.3. IgE reactivity to hazelnut OB‐associated proteins and discovery of Cor a 15
We hypothesized that other allergens, not included in the ImmunoCAP assay, could account for the positivity of the LIHA patients to OFC. We thus performed immunoblotting to evaluate the immunoreactivity of the single patients to the OB‐associated proteins. Two oleosin‐containing bands were found at 14 and 17 kDa (Figure 2).
One hundred percent of the allergic patients recognized at least one oleosin‐containing band (Table 1). Thirty‐three percent recognized the 14‐kDa band, containing both Cor a 12 and Cor a 13 (band 1A in Figure 2, Table 2). The 17‐kDa band, which exclusively contains the MK737923 oleosin (band 2A in Figure 2 and Table 2), was the most recognized one and was immunoreactive for 88% of the allergic patients. The MK737923 oleosin was therefore submitted as an allergen candidate to the WHO/IUIS Allergen Nomenclature Subcommittee, who approved it as a new hazelnut allergen and termed it Cor a 15. The three hazelnut oleosins were also immunorecognized by the HS patients, with a similar frequency to that of the allergic patients (Table 1 and Figure 2).
In order to verify that the LIHA patients did not recognize any other water‐soluble allergens than those found negative by ImmunoCAP, a total hazelnut water‐soluble protein extract was tested by immunoblotting against the pooled sera of the LIHA patients (nos. 23, 24, and 25) and against the pooled sera of the HA patients (nos. 1 to 14). The IgEs of the LIHA patients did not bind with any protein band, while several protein bands were recognized by the HA pool (Figure S3).
3.4. Effect of roasting on the hazelnut oleosin profile
We further assessed whether the hazelnut OB protein profile was affected by processing, since hazelnuts are usually consumed roasted. In addition to the 14‐kDa and 17‐kDa bands, higher MW bands appeared (Figure 2, panel B). The 14‐kDa band, containing a mixture of Cor a 12 and Cor a 13 (band 1B in Figure 2 and Table 2), was recognized by 62% of the allergic patients (Table 1). The 17‐kDa band, containing Cor a 15 with traces of Cor a 12 (band 2B in Figure 2 and Table 2), was again the most recognized band (96% of the allergic children) (Table 1). The bands around 20–40 kDa, generated by roasting, were recognized by almost all the patients. In addition to oleosins, these bands also contained Cor a 9 and/or Cor a 11 soluble hazelnut allergens (bands 3B to 8B in Figure 2, Table 2). To discriminate whether the LIHA patients’ sera immunoreacted to Cor a 9 and Cor a 11 or against oleosins, a 2DE separation of the OB‐associated proteins was performed, exploiting the different isoelectric points of these allergens (Figure 3, panels A and B). Most of the spots in the alkaline zone (right part) of the 2DE map were identified as oleosins, or as the basic subunit of Cor a 9, while those in the acidic zone were found to contain acidic subunits of Cor a 9 and Cor a 11 (Table 2).
In spite of the different 2DE protein profiles of the raw and roasted OB proteins (Figure 3, Panel A and B), the immunoreactivity profiles were very similar (Figure 3, Panels C and D) and exclusively localized in the alkaline region.
3.5. Hazelnut oleosins are major allergens for LIHA patients and Cor a 15 is the most frequently recognized oleosin
Most reactive spots on 2DE separation of raw and roasted extracts contained co‐migrating oleosins exclusively: Cor a 12, Cor a 13, and Cor a 15 (Figure 3, Table 2). Interestingly, the 1A2DE and 2A2DE spots, which showed a similar densitometric intensity in the 2DE gel, resulted in a highly different immunoreactivity. Spot 2A2DE, which contained 96.5% of Cor a 15, was much more immunoreactive than the 1A2DE spot, which exclusively contained Cor a 12 and 13 (Figure 3, Panels A and C, and Table 2).
In order to verify whether Cor a 15 was the most frequently recognized oleosin, full‐length Cor a 15 and its C‐term fragment (from aa 102 to aa 168) were expressed and purified (Figure 4, panels A and C). The C‐terminal fragment was expressed because it contains a similar peptide (11 aa out of 14 aa) to the most immunoreactive IgE epitope in Ara h 15 4 (Figure S4). The addition of full‐length rCor a 15 to both the LIHA and HA pooled patients’ sera completely inhibited the recognition of the 17‐kDa band exclusively containing Cor a 15 (Figure 4, panel B). The same amount of rCor a 15 C‐terminal did not result in the complete inhibition of the immunoreactivity of the sera (Figure 4, panel D). Moreover, the reverse was also true: the hazelnut oleosins present in the OB extract were able to inhibit the Cor a 15 immunorecognition by the patients’ sera (Figure 4, panel E).
FIGURE 4.

Immunoblotting inhibition assay. Panels A and C: LDS‐PAGE of full‐length rCor a 15 (rCor a 15) and rCor a 15 C‐terminal (rC‐term), respectively. Immunoblotting with pooled sera of the LIHA patients (25, 26 and 27) and HA patients (2, 10, and 13). CII: secondary antibody control; MW: molecular weight. Panels B and D: LDS‐PAGE of raw hazelnut OB protein extracts (raw OB). Immunoblotting with the pooled sera of the LIHA patients (25, 26, and 27) and HA patients (2, 10, and 13), without (Tq) and with the addition of 0.2 and 2 µg of rCor a 15 and rCor a 15 C‐terminal to the patients’ sera, respectively. Panel E: Immunoblotting with the sera of 10 patients (nos. 4, 5, 9, 16, 17, 18, 19, 27, 36, and 37) without (Tq) and with the addition of 3 and 6 µg of raw OB protein extract to the patients’ sera. C1: pool of five sera of non‐nut‐allergic (NNA) pediatric subjects; C2: pool of five sera of non‐food‐allergic (NFA) pediatric subjects
3.6. All the patients showed specific IgEs toward Cor a 15
In order to obtain a semi‐quantification of the patients’ IgEs that were specific to Cor a 15 in the different subgroups of patients (HA, LIHA, and HS) and in the negative controls (NNA and NFA), the patients’ sera were tested by means of an in‐house direct ELISA test. The patients’ sera all showed specific IgEs for rCor a 15 (Figure 5); however, the ELISA test did not differentiate between the HA, LIHA, and HS patients on the basis of Cor a 15 immunorecognition, as already demonstrated by the immunoblotting experiments. An aspecific reaction was found in 3/18 of the control sera.
FIGURE 5.
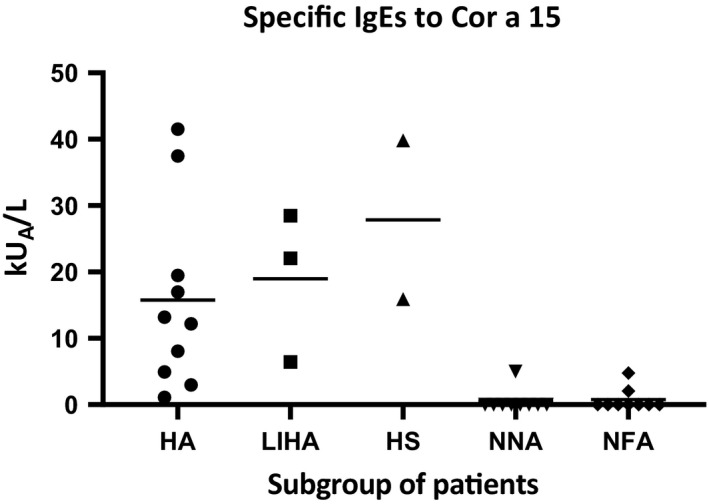
ELISA quantification of Cor a 15 IgEs in three subgroups of patients’ sera (10 HA, 3 LIHA, and 2 HS). A line marks the mean kUA/L for each subgroup of patients. NNA control: 10 sera of non‐nut allergic pediatric subjects; NFA control: 10 sera of non‐food‐allergic pediatric subjects
4. DISCUSSION
With the aim of conducting an immune‐allergological characterization of hazelnut oleosins in pediatric patients, we performed the isolation and identification of hazelnut OB‐associated proteins. We were able to separate oleosins from soluble allergens and to identify, for the first time, a new allergen belonging to the oleosin family, termed Cor a 15 by WHO‐IUIS. We then demonstrated the immunogenic power of the newly reported Cor a 15 oleosin for most of our hazelnut‐allergic pediatric patients. In addition, we succeeded in unambiguously ascribing the immunoreactivity of LIHA patients (characterized by low ImmunoCAP assay IgEs values and positive OFC and PbP) only to oleosins, thereby excluding the contribution of other allergens.
Regarding the IgE levels, the Cor a 9 and Cor a 14 IgE levels were clearly associated with the elicitation of the allergic reaction, according to Datema et al., 21 and were useful to discriminate between the hazelnut‐allergic and hazelnut‐sensitized but tolerant patient groups. This was not found for the Cor a 1 and Cor a 8 IgE levels, for which a moderate diagnostic predictive value has been demonstrated. 22 The IgE levels of the newly discovered Cor a 15 seemed to have a similar behavior, as they were unable to discriminate between allergic and sensitized patients.
The evaluation of oleosin immunoreactivity for hazelnut‐allergic patients was assessed by 1D and 2D immunoblotting, coupled with protein identification by means of mass spectrometry. The results indicated that all the HA patients immunorecognized at least one oleosin, although it was not possible to assess the role of the oleosins in eliciting the allergic reaction, as they were co‐sensitized to genuine hazelnut allergens. On the contrary, we found that a subset of LIHA patients exclusively immunorecognized oleosins and, among them, mainly Cor a 15. Cor a 15 appeared to be the best candidate for the allergic reaction in this subgroup of patients. This evidence highlights the relevance of dosing IgEs against Cor a 15 in hazelnut allergy diagnosis. We also observed IgE reactivity to hazelnut oleosins in the HS patients, which could be ascribed to a cross‐reactivity with other oleosins, at least for those patients with a confirmed allergy to other nuts. 23
Cor a 15, which we exclusively identified in the 17‐kDa band, was the most immunorecognized oleosin (88% of the allergic patients). This result was different from the results of Zuidmeer‐Jongejan, who detected both Cor a 12 and Cor a 13 in a band at the same MW. 7 This is probably due to the lack of Cor a 15 gene sequence in the database at the time of the protein identification. We also identified Cor a 12 and 13 in a band at 14 KDa, with a recognition frequency of 33%, similar to what was reported in the EuroPrevall project for Cor a 12 in children (34%). 2
As already demonstrated by Schwager et al. 4 for Ara h 15, we hypothesized that the epitope responsible the most for Cor a 15 immunorecognition is located at the C‐terminal, since both the full‐length rCor a 15 and rCor a 15 C‐terminal were able to inhibit IgEs against the 17‐kDa band containing only Cor a 15.
Our findings also show a resistance of oleosins to high temperatures, which resulted in an apparent enhancement of oleosin immunoreactivity after roasting, with a more evident effect on Cor a 12 and Cor a 13. 15 The immunorecognition of roasted Cor a 12 and Cor a 13 exceeded 60% for the allergic patients, while Cor a 15 reached 96%. Similarly, Schwager et al. 4 demonstrated an increase in oleosin immunoreactivity after roasting in‐shell peanuts. Glycation after roasting may play a role in increasing the affinity and/or the accessibility of oleosins to IgEs. 24
In conclusion, we have discovered a new hazelnut oleosin allergen, Cor a 15, which resulted to play a role in eliciting an allergic reaction in a subgroup of our pediatric patients that exclusively immunorecognized oleosins, thus confirming the relevance of including lipophilic allergens in the protein extracts used for diagnostic tests.
CONFLICT OF INTEREST
The authors have no conflict of interest.
AUTHOR CONTRIBUTION
Stefano Nebbia: Data curation (equal); Formal analysis (equal); Investigation (lead); Writing‐original draft (equal). Cristina Lamberti: Investigation (lead); Methodology (lead); Supervision (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Simona Cirrincione: Data curation (equal); Investigation (equal); Methodology (equal). Alberto Acquadro: Investigation (equal); Writing‐review & editing (equal). Simona Abbà: Investigation (equal); Resources (equal). Marina Ciuffo: Investigation (equal); Writing‐review & editing (equal). Daniela Torello Marinoni: Resources (equal); Writing‐review & editing (equal). Marcello Manfredi: Investigation (equal); Writing‐review & editing (equal). Emilio Marengo: Investigation (equal); Writing‐review & editing (equal). Roberta Calzedda: Resources (equal); Writing‐review & editing (equal). Giovanna none Monti: Conceptualization (equal); Methodology (equal); Resources (lead); Writing‐review & editing (equal). laura cavallarin: Conceptualization (lead); Methodology (lead); Project administration (lead); Supervision (equal); Writing‐original draft (equal); Writing‐review & editing (lead). Maria Gabriella Giuffrida: Conceptualization (lead); Funding acquisition (equal); Methodology (lead); Supervision (lead); Writing‐original draft (equal); Writing‐review & editing (equal).
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pai.13579.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Table S1
Table S2
Nebbia S, Lamberti C, Cirrincione S, et al. Oleosin Cor a 15 is a novel allergen for Italian hazelnut allergic children. Pediatr Allergy Immunol. 2021;32:1743–1755. 10.1111/pai.13579
Nebbia and Lamberti contributed equally to this work.
Laura Cavallarin and Maria Gabriella Giuffrida share co‐seniorship.
Funding information
This study was supported by the CRT Foundation, Turin, Italy (grant no. 2014.2693).
REFERENCES
- 1. McWilliam V, Koplin J, Lodge C, et al. The prevalence of tree nut allergy: a systematic review. Curr Allergy Asthma Rep. 2015;15(9). 10.1007/s11882-015-0555-8. [DOI] [PubMed] [Google Scholar]
- 2. Datema MR, Zuidmeer‐Jongejan L, Asero R, et al. Hazelnut allergy across Europe dissected molecularly: a EuroPrevall outpatient clinic survey. J Allergy Clin Immunol. 2015;136:382–391. [DOI] [PubMed] [Google Scholar]
- 3. Schwager C, Kull S, Krause S, et al. Development of a novel strategy to isolate lipophilic allergens (oleosins) from peanuts. PLoS One. 2015;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwager C, Kull S, Behrends J, et al. Peanut oleosins associated with severe peanut allergy—importance of lipophilic allergens for comprehensive allergy diagnostics. J Allergy Clin Immunol. 2017;140:1331–1338.e8. [DOI] [PubMed] [Google Scholar]
- 5. Leduc V, Moneret‐Vautrin DA, Tzen JTC, et al. Identification of oleosins as major allergens in sesame seed allergic patients. Allergy Eur J Allergy Clin Immunol. 2006;61:349–356. [DOI] [PubMed] [Google Scholar]
- 6. Teodorowicz M, Terlouw RJ, Jansen A, et al. Immunological characterization of Dutch sesame seed‐allergic patients. Int Arch Allergy Immunol. 2016;169:13–22. [DOI] [PubMed] [Google Scholar]
- 7. Zuidmeer‐Jongejan L, Fernández‐Rivas M, Winter MGT, et al. Oil body‐associated hazelnut allergens including oleosins are underrepresented in diagnostic extracts but associated with severe symptoms. Clin Transl Allergy. 2014;4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang AHC. Plant lipid droplets and their associated proteins: potential for rapid advances. Plant Physiol. 2018;176:1894–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akkerdaas JH, Schocker F, Vieths S, et al. Cloning of oleosin, a putative new hazelnut allergen, using a hazelnut cDNA library. Mol Nutr Food Res. 2006;50:18–23. [DOI] [PubMed] [Google Scholar]
- 10. Jappe U, Schwager C. Relevance of lipophilic allergens in food allergy diagnosis. Curr Allergy Asthma Rep. 2017;17:1–9. [DOI] [PubMed] [Google Scholar]
- 11. Zhu J, Pouillot R, Kwegyir‐Afful EK, et al. A retrospective analysis of allergic reaction severities and minimal eliciting doses for peanut, milk, egg, and soy oral food challenges. Food Chem Toxicol. 2015;80:92–100. [DOI] [PubMed] [Google Scholar]
- 12. Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron; 2001:4.
- 13. Platteau CMF, Bridts CH, Daeseleire EA, et al. Comparison and functional evaluation of the allergenicity of different hazelnut (Corylus avellana) protein extracts. Food Anal Methods. 2010;3:382–388. [Google Scholar]
- 14. Cao Y, Zhao L, Ying Y, et al. The characterization of soybean oil body integral oleosin isoforms and the effects of alkaline pH on them. Food Chem. 2015;177:288–294. [DOI] [PubMed] [Google Scholar]
- 15. Lamberti C, Nebbia S, Antoniazzi S, et al. Effect of hot air and infrared roasting on hazelnut allergenicity. Food Chem. 2021;342:128174. [DOI] [PubMed] [Google Scholar]
- 16. Nebbia S, Lamberti C, Giorgis V, et al. The Cockroach Allergen‐like protein is involved in primary respiratory and food allergy to yellow mealworm (Tenebrio molitor). Clin Exp Allergy. 2019;1:4. [DOI] [PubMed] [Google Scholar]
- 17. Zava S, Barello C, Pessione A, et al. Mare’s colostrum globules stimulate fibroblast growth in vitro: a biochemical study. J Med Food. 2009;12:836–845. [DOI] [PubMed] [Google Scholar]
- 18. Rowley ER, VanBuren R, Bryant DW, et al. A draft genome and high‐density genetic map of European hazelnut (Corylus avellana L.). bioRxiv. 2018;1:469015. 10.1101/469015. [DOI] [Google Scholar]
- 19. Pavese V, Cavalet‐Giorsa E, Barchi L, et al. Whole‐genome assembly of Corylus avellana cv “Tonda Gentile delle Langhe” using linked‐reads (10X Genomics). G3‐Genes Genom Genet. 2021. 10.1093/g3journal/jkab152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hilger C, Bessot JC, Hutt N, et al. IgE‐mediated anaphylaxis caused by bites of the pigeon tick Argas reflexus: Cloning and expression of the major allergen Arg r 1. J Allergy Clin Immunol. 2005;115:617–622. [DOI] [PubMed] [Google Scholar]
- 21. Datema MR, van Ree R , Asero R, et al. Component‐resolved diagnosis and beyond: Multivariable regression models to predict severity of hazelnut allergy. Allergy Eur J Allergy Clin Immunol. 2018;73:549–559. [DOI] [PubMed] [Google Scholar]
- 22. Caffarelli C, Mastrorilli C, Santoro A, et al. Component‐resolved diagnosis of hazelnut allergy in children. Nutrients. 2021;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smeekens JM, Bagley K, Kulis M. Tree nut allergies: Allergen homology, cross‐reactivity, and implications for therapy. Clin Exp Allergy. 2018;48:762–772. [DOI] [PubMed] [Google Scholar]
- 24. Toda M, Hellwig M, Henle T, et al. Influence of the Maillard reaction on the allergenicity of food proteins and the development of allergic inflammation. Curr Allergy Asthma Rep. 2019;19:1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Table S1
Table S2


