Abstract
Purpose
To describe the treatment outcomes and recurrence risk of chronic central serous chorioretinopathy (cCSC) in patients who had complete resolution of subretinal fluid (SRF) after either primary half‐dose photodynamic therapy (PDT) or high‐density subthreshold micropulse laser (HSML) in the PLACE trial.
Methods
This multicentre prospective follow‐up study evaluated cCSC patients at 1 year after completion of the PLACE trial. Outcomes included: complete resolution of SRF on OCT, best‐corrected visual acuity (BCVA) in Early Treatment of Diabetic Retinopathy Study (ETDRS) letters, retinal sensitivity on microperimetry and a visual function questionnaire (NEI‐VFQ25).
Results
Twenty‐nine out of 37 patients who received half‐dose PDT and 15 out of 17 patients who received HSML could be evaluated at final visit. At final visit, 93% of the patients treated with half‐dose PDT had complete resolution of SRF, compared with 53% of HSML‐treated patients (p = 0.006). At final visit, the mean estimate increase in the PDT group compared with the HSML group was + 2.1 ETDRS letters, +0.15 dB for the retinal sensitivity and + 5.1 NEI‐VFQ25 points (p = 0.103, p = 0.784 and p = 0.071, respectively). The mean estimated central retinal thickness in the half‐dose PDT group was −7.0 µm compared with the HSML group (p = 0.566). The mean estimated subfoveal choroidal thickness in the half‐dose PDT group was −16.6 µm compared with the HSML group (p = 0.359).
Conclusion
At 20 months after treatment, cCSC patients successfully treated with half‐dose PDT are less likely to have recurrences of SRF compared with those successfully treated with HSML. However, functional outcomes did not differ.
Keywords: central serous chorioretinopathy, long‐term follow‐up, micropulse laser, photodynamic therapy
Introduction
Central serous chorioretinopathy (CSC) is a chorioretinal disease, in which an accumulation of serous subretinal fluid (SRF) causes a detachment of the neurosensory retina. When the macula is affected, this may result in decrease of visual acuity, diminished contrast vision and metamorphopsia. Dysfunction of the choroid is presumed to be the primary cause of damage to the retinal pigment epithelium (RPE) outer blood‐retina barrier and subsequent SRF leakage, but the exact pathophysiological mechanism remains unclear (Prunte & Flammer 1996; Daruich et al. 2015). The disease usually occurs in men between 20 to 60 years of age. The most important exogenous risk factor for developing CSC is the use of steroids (Haimovici et al. 1997). There is no universally accepted classification of CSC, but a general distinction is often made between acute CSC and chronic CSC (cCSC) (van Rijssen et al. 2019c). Patients with acute CSC are likely to have a spontaneous SRF resolution within a few months after the onset of symptoms (Yannuzzi 2010). In contrast, those with cCSC tend to have persistent SRF and accompanying atrophic RPE, which may lead to permanent damage to the photoreceptors and a decreased quality of life (Breukink et al. 2017; Mrejen et al. 2019). Treatment should be recommended to cCSC patients, because of these possible permanent harmful effects of a long‐standing serous detachment (Loo et al. 2002; Breukink et al. 2017; Mrejen et al. 2019; van Rijssen et al. 2019c). Half‐dose photodynamic therapy (PDT) and high‐density subthreshold micropulse laser (HSML) are commonly used treatments for cCSC (Yannuzzi et al. 2003; Chen et al. 2008; Wood et al. 2017; Roca et al. 2018; van Rijssen et al. 2019c). The effect of PDT lies in remodelling of the choroid, leading to a cessation of SRF leakage by reducing choroidal congestion, hyperpermeability and leakage (Chan et al. 2003). HSML has been postulated to work through inducing a cascade of reactions in the chromophores in the RPE, including the possible production of heat shock proteins, which may stimulate the process of SRF absorption (Sramek et al. 2011).
The PLACE trial, the first large multicentre investigator‐initiated randomized controlled trial for cCSC, compared the outcome of indocyanine green (ICGA)‐guided treatment with either half‐dose PDT or HSML. In this trial, 67% of half‐dose PDT treated patients had a complete resolution of SRF at final visit (7–8 months after first treatment), which was significantly higher than the 29% of patients with complete SRF resolution in the HSML group. The best‐corrected visual acuity (BCVA) also showed a significantly higher increase in the half‐dose PDT group at the first evaluation visit, while retinal sensitivity on microperimetry showed a significantly higher increase in the PDT group as compared with the HSML group both at the first and final evaluation visit. In a subsequent study, this superiority in outcome of PDT over HSML has been found to be regardless of the presence of focal or diffuse leakage on FA (van Rijssen et al. 2019a; van Rijssen et al. 2019b). However, until now, the long‐term outcomes of half‐dose PDT or HSML as a treatment for cCSC have not been reported in the context of a large prospective trial. In this follow‐up study, we describe the outcome in terms of recurrence rates and functional parameters in cCSC patients who had a complete resolution of SRF at the final evaluation visit (7–8 months after half‐dose PDT or HSML treatment) in the PLACE trial, at 1 year after this final visit.
Materials and Methods
This prospective, multicentre study included cCSC patients who had complete resolution of SRF at the final visit of the PLACE trial. The protocol and primary results of the PLACE trial have been published previously (Breukink et al. 2015; van Dijk et al. 2018). For the current study, a new research protocol was developed. Patients from four academic medical centres located in Cologne (Germany), Leiden (the Netherlands), Nijmegen (the Netherlands), and Oxford (the United Kingdom) were included in the present study. This study adhered to the tenets of the Declaration of Helsinki, and written informed consent was obtained from all participants. Approval was received from all medical ethics committees and review boards in the participating centres (NL‐number: NL50642.091.14).
Patients who had complete resolution of SRF on spectral‐domain optical coherence tomography (SD‐OCT) at the final visit of the PLACE trial (7–8 months after first treatment), after either 1 or 2 treatments with half‐dose PDT or HSML, were eligible for inclusion into the current study. The exact treatment procedure and exclusion criteria have been described previously in the context of the PLACE trial (van Dijk et al. 2018).
The final visit of the PLACE trial was identical to the baseline visit of the current follow‐up study. Patients were scheduled for a visit to the outpatient clinic 1 year after baseline visit. The following clinical data were obtained at baseline and final visit of the current study: BCVA in ETDRS letters, retinal sensitivity on microperimetry, National Eye Institute Visual Functioning Questionnaire 25‐items (NEI‐VFQ25) questionnaire score, central retinal thickness (CRT; measured as distance from inner limiting membrane to ellipsoid zone (EZ) in the foveal pit on SD‐OCT) (van Rijssen et al. 2018a), subfoveal choroidal thickness (SFCT; measured underneath the foveal pit on SD‐OCT), continuity of the external limiting membrane (ELM) and EZ on SD‐OCT, and degree of hyperautofluorescence on fundus autofluorescence (FAF). The NEI‐VFQ25 responses were converted to a score from 0 to 100, according to a previously published method (Mangione et al., 2001). Discontinuity of either the ELM or the EZ was defined as a decrease in signal intensity or an absence of signal intensity on SD‐OCT within a 500 μm distance of the foveal pit (van Rijssen et al. 2018a). FAF imaging was categorized as either predominantly hyperautofluorescent or predominantly hypoautofluorescent, based on the degree of autofluorescence that was most apparent within the vascular arcades (van Rijssen et al. 2019b).
Statistics
Statistical analyses were performed using spss statistics (IBM Corp. version 23.0. Armonk, New York, USA). Binary outcomes were analysed with mixed effects logistic regression using the GLMMadaptive package in R statistics (R Foundation for Statistical Computing, Vienna, Austria). For the continuous outcomes, marginal multivariate regression models were used to model the mean progression for each treatment group between baseline and final visit. The mean outcome was modelled as a function of the main effect of treatment, time (taken as categorical) and their interaction. Marginal multivariate regression models were used to contrast mean progression between the treatment groups. For testing hypotheses of interest regarding treatment effects or changes in time, the F‐test has been used.
Results
Data of 133 patients were available at final visit of the PLACE trial, of whom, 54 patients could be included in this long‐term follow‐up after PLACE study. (van Dijk et al. 2018) The 79 patients who could not be included in this study either did not have a complete resolution of SRF at the final visit of the PLACE trial (42 patients), did not sign informed consent for the long‐term follow‐up study (20 patients), or because the patient already had a PLACE trial final visit before the start of this study (17 patients). This study of 54 patients included 43 men (80%) and 11 women (20%), with a mean baseline age of 48.0 ± 8.1 years. At baseline, the mean BCVA was 86.5 ± 9.4 ETDRS letters (Snellen equivalent of 6/6), the mean retinal sensitivity on microperimetry was 25.0 ± 4.6 dB (n = 53), and the mean NEI‐VFQ25 was 80.7 ± 12.7 points. There were 37 patients who had received half‐dose PDT in the PLACE trial (including six patients who received PDT twice), and 17 patients who had received HSML in the PLACE trial (including 12 patients received who received HSML twice). Ten of these 54 patients did not attend their final visit (8 in PDT, 2 in HSML group), because their health care provide did not reimburse the costs of the final visit, and their symptoms had disappeared (6), unspecified loss to follow‐up (3), and the occurrence of leukaemia (1). Choroidal neovascularization was not observed during follow‐up. The baseline characteristics are reported in Table 1.
Table 1.
Baseline characteristics of chronic central serous chorioretinopathy patients in the current study who received treatment with either half‐dose photodynamic therapy or high‐density subthreshold micropulse laser treatment within the PLACE trial.
| Half‐dose PDT (n = 37) | HSML (n = 17) | p‐value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 46.9 ± 8.3 | 48.4 ± 7.7 | 0.543 |
| Best‐corrected visual acuity (ETDRS letters) | 87.9 ± 4.3 | 83.4 ± 15.4 | 0.099 |
| NEI‐VFQ25 composite score (points) | 90.0 ± 7.9 | 89.5 ± 15.4 | 0.824 |
| Retinal sensitivity (dB) | 25.6 ± 3.1 | 23.6 ± 6.9 | 0.136 |
| Central foveal thickness (µm) | 128.5 ± 26.8 | 110.3 ± 30.5 | 0.031 |
| Subfoveal choroidal thickness (µm) | 354.2 ± 115.5 | 379.6 ± 98.3 | 0.471 |
| Number (%) | Number (%) | p‐value | |
|---|---|---|---|
| Sex | |||
| Male | 29 (78%) | 14 (82%) | 0.736 |
| Female | 8 (22%) | 3 (18%) | |
| Continuous external limiting membrane | |||
| Yes | 36 (97%) | 16 (94%) | 0.566 |
| Continuous ellipsoid zone | |||
| Yes | 30 (81%) | 12 (71%) | 0.389 |
| Degree of autofluorescence on FAF | |||
| Predominantly hyperautofluorescent | 7 (41%) | 25 (68%) | 0.067 |
ETDRS = Early Treatment of Diabetic Retinopathy Study; HSML = high‐density subthreshold micropulse laser; NEI‐VFQ25 = National Eye Institute Visual Functioning Questionnaire 25‐items; PDT = photodynamic therapy; SD = standard deviation.
Half‐dose photodynamic therapy (PDT) group
A total of 37 patients who had received half‐dose PDT and showed complete SRF resolution at the final visit of the PLACE trial (corresponding to the baseline visit of the current study) were enrolled in the long‐term follow‐up study. Multimodal imaging of a patient included in the half‐dose PDT group is depicted in Figure 1. Nine out of these 37 patients (24%) had received 2 half‐dose PDT treatments in the PLACE trial, because there was no complete resolution of SRF on OCT at the first evaluation visit of the PLACE trial, necessitating another ICGA‐guided half‐dose PDT treatment according to the trial protocol. Twenty‐nine out of these 37 patients (78%) who received half‐dose PDT were available at final follow‐up visit and could be included in the analyses. No treatment‐related adverse events occurred in the half‐dose PDT group.
Fig. 1.
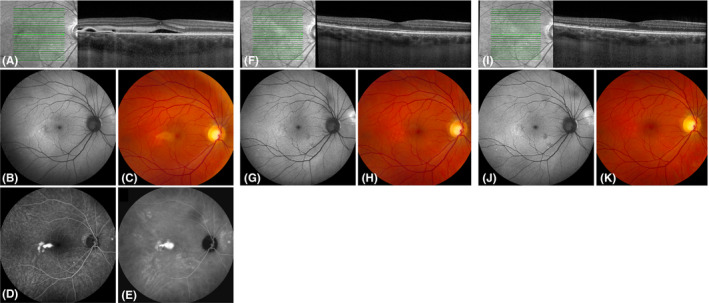
Multimodal imaging of a 47‐year‐old man with chronic central serous chorioretinopathy who was treated twice with half‐dose photodynamic therapy (PDT) in the PLACE trial. At baseline visit of the PLACE trial (before PDT; A–E), subretinal fluid and discontinuity of the external limiting membrane (ELM) and ellipsoid zone (EZ) were visible on optical coherence tomography (OCT; A). In addition, a focal leakage point was visible on fluorescein angiography (FA; D) and hyperfluorescent abnormalities were present on indocyanine green angiography (ICGA; E). Both at baseline visit of the current study (8 months after treatment within the PLACE trial; F–H) and at final visit of the current study (20 months after treatment; I–K), subretinal fluid had resolved and the ELM and EZ were no longer discontinuous on OCT (F, I). Fundus autofluorescence and fundus photography did not markedly change between baseline visit and final visit of this study (G, J and H, K, respectively). FA and ICGA were not obtained at baseline visit and final visit of this study due to the absence of subretinal fluid.
High‐density subthreshold micropulse laser (HSML) group
Seventeen HSML‐treated patients who showed complete SRF resolution at the final visit of the PLACE trial (corresponding to the baseline visit of the current study) could be included in the HSML group of the long‐term follow‐up study. Multimodal imaging of a patient treated with HSML is depicted in Fig. 2. Out of these 17 patients, 12 (71%) had received HSML treatment twice in the PLACE trial, because there was no complete resolution of SRF on OCT at the first evaluation visit of the PLACE trial, necessitating another ICGA‐guided HSML treatment according to the trial protocol. Fifteen out of 17 patients (88%) who previously received HSML treatment in the PLACE trial were available at final follow‐up, since 2 patients were lost to follow‐up. No treatment‐related adverse events occurred in the HSML group.
Fig. 2.
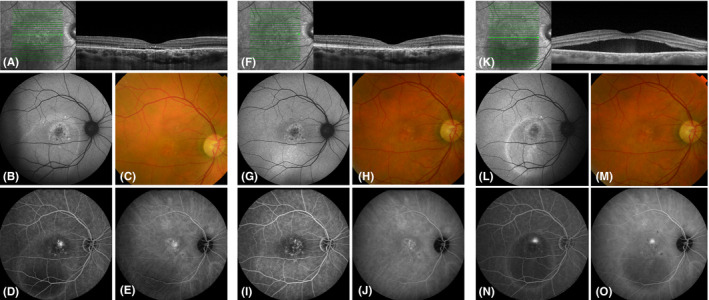
Multimodal imaging of a 45‐year‐old man diagnosed with chronic central serous chorioretinopathy who received 2 high‐density subthreshold micropulse laser (HSML) treatments in the PLACE trial. At the baseline visit of the PLACE trial (before HSML treatment; A–E), subretinal fluid was present along with discontinuity of both the external limiting membrane (ELM) and ellipsoid zone (EZ) on optical coherence tomography (OCT; A). Some degree of retinal pigment epithelium alterations is visible inferior to the fovea on fundus autofluorescence (B, G, L) and fundus photography (C, H, M). A focal leakage point with retinal pigment epithelium alterations are present on fluorescein angiography (FA; D), and hyperfluorescent abnormalities are visible on indocyanine green angiography (ICGA; E). At baseline visit of this study (8 months after treatment within the PLACE trial; F–J), subretinal fluid had resolved, however, the ELM and EZ remain discontinuous (F), and the focal leakage point had disappeared (I). At final visit of the current study (20 months after treatment; K–O), there was a recurrence of subretinal fluid directly underneath the fovea on OCT (K), with a focal leakage point on FA (N).
Half‐dose photodynamic therapy versus high‐density subthreshold micropulse laser
At final visit, 27 out of 29 patients (93%) treated with half‐dose PDT had complete resolution of SRF, compared with seven out of 15 patients (53%) in the HSML group (p = 0.006). The mean estimate increase in the PDT group compared with the HSML group was + 2.1 ETDRS letters (p = 0.103), +0.15 dB for the retinal sensitivity on microperimetry (p = 0.784), and + 5.1 NEI‐VFQ25 points (p = 0.071). At final visit, the mean estimated CRT in the half‐dose PDT group was −7.0 µm compared with the HSML group (p = 0.566). The mean estimated SFCT in the half‐dose PDT group was −16.6 µm compared with the HSML group (p = 0.359). There was 1 patient in the half‐dose PDT group and 3 patients in the HSML group who had to be excluded from the EZ and ELM analyses because of insufficient imaging quality. The ELM at final visit was continuous in 27 out of 28 patients (96%) treated with half‐dose PDT and in 8 out of 12 patients (67%) in the HSML group. The EZ at final visit was continuous in 26 out of 28 patients (93%) in the half‐dose PDT group, and in 7 out of 12 patients (58%) in the HSML group. In the half‐dose PDT group, 9 out of 27 patients (33%) had predominant hypoautofluorescent abnormalities within the vascular arcades, as compared with 8 out of 14 patients in the HSML group (p = 0.142). FAF imaging was unavailable in 2 patients in the half‐dose PDT group and 1 patient in the HSML group. The outcomes of the PDT and HSML group are summarized in Table 2.
Table 2.
Treatment outcome in chronic central serous chorioretinopathy patients who received either half‐dose photodynamic therapy or high‐density subthreshold micropulse laser treatment within the PLACE trial.
| Half‐dose PDT (n = 37) | HSML (n = 17) | p‐value | |
|---|---|---|---|
| Mean (SE) | Mean (SD) | ||
| Change in best‐corrected visual acuity (ETDRS letters)* | +1.01 (0.718) | −1.05 (1.004) | 0.103 |
| Change in retinal sensitivity (dB)* | +0.85 (0.307) | +0.70 (0.462) | 0.784 |
| Change in NEI‐VFQ25 composite score (points)* | +0.66 (1.618) | −4.47 (2.252) | 0.071 |
| Change in central retinal thickness (µm)* | −14.8 (6.8) | −7.8 (10.0) | 0.566 |
| Change in subfoveal choroidal thickness (µm)* | −37.8 (30.4) | −21.2 (55.7) | 0.359 |
| Number (%) | Number (%) | p‐value | |
|---|---|---|---|
| Continuity of the external limiting membrane on OCT | 27 (96%) | 8 (67%) | 0.022 |
| Continuity of the ellipsoid zone on OCT | 26 (93%) | 7 (58%) | 0.017 |
The mean change estimated by the mixed model between baseline and final visit. ETDRS, Early Treatment of Diabetic Retinopathy Study; HSML, high‐density subthreshold micropulse laser; NEI‐VFQ25, National Eye Institute Visual Functioning Questionnaire 25‐items; OCT, optical coherence tomography; PDT, photodynamic therapy; SE, standard error.
Discussion
In this study, the long‐term outcomes after successful treatment for cCSC with either half‐dose PDT or HSML treatment were evaluated. In the half‐dose PDT group, there were significantly less recurrences of SRF compared with HSML‐treated patients at final visit (one year after the final follow‐up visit of the PLACE trial), with almost half of the HSML‐treated patients having recurrence of SRF on OCT at the final visit, in contrast to only 7% of the half‐dose PDT treated cCSC patients. Moreover, the percentage of patients with continuity of the ELM and EZ at final visit was significantly higher in the half‐dose PDT group compared with the HSML group, with no significant differences between the half‐dose PDT and HSML group at baseline (PLACE trial final visit; Table 1). The Retinal sensitivity on microperimetry and the CRT only increased significantly at final visit in the half‐dose PDT group. There were no significant differences in changes in BCVA, retinal sensitivity on microperimetry, and NEI‐VFQ25 score when comparing the half‐dose PDT and HSML group. Nevertheless, half‐dose PDT still showed statistically significant superiority over HSML with regard to important anatomical (SRF resolution and continuity of the EZ) outcomes.
A recurrence of SRF occurred in 7% of the half‐dose PDT treated patients at 20 months after initial treatment in this long‐term follow‐up study, which is in line with the reported recurrence rate of 6–25% within a mean follow‐up of 12–60 months in previous studies on the outcome of PDT in cCSC (Fujita et al. 2015; Lai et al. 2016; Park et al. 2019). Previous retrospective follow‐up studies have found that the rate of recurrence of SRF after half‐dose PDT is significantly lower compared to untreated cCSC patients, but no prospective studies are available on this topic (Lai et al. 2015; Lai et al. 2016). Additionally, it has been reported that persistence of subretinal fluid may lead to irreversible damage to the neuroretina and to subsequent vision loss (Laatikainen 1994; Loo et al. 2002). In the VICI trial, 30% of placebo‐treated patients and 16% of eplerenone‐treated cCSC patients had a complete resolution of SRF after 12 months (Lotery et al. 2020). Although a direct comparison is impossible, these data and our current data suggests that half‐dose PDT may superior in terms of SRF resolution, as 67% of cCSC patients in the PLACE trial had a complete resolution of fluid at 7–8 months post‐PDT, and 93% of patients at 19–20 months post‐PDT in the current study (van Dijk et al. 2018). The baseline visual acuity in cCSC is relatively high, and it may thus be difficult to reach a statistically significant increase after treatment. In addition, visual acuity may change more gradually compared with other (anatomical) parameters after treatment such as resolution of subretinal fluid. Therefore, the short‐term (and long‐term) aim of treatment in cCSC should probably be a complete resolution of subretinal fluid and prevention of recurrences, so that visual acuity and other functional outcomes can be preserved (van Rijssen et al. 2019c). In the literature, male sex, absence of hyperfluorescence on ICGA, posterior cystoid retinal degeneration or bilateral cCSC have been found to be indicative for a higher chance of recurrence of SRF, and absence of intense hyperfluorescence on ICGA is correlated with persistent SRF after PDT treatment (Inoue et al. 2010; Nicolo et al. 2012; Lai et al. 2016; van Rijssen et al. 2018b; Park et al. 2019). Potential subtle, low‐grade type‐1 subretinal neovascularization should also be considered as a possible cause of recurrence of SRF (Cheung et al. 2018; van Rijssen et al. 2019c). In this study, the recurrence rate was significantly higher in cCSC patients treated with HSML (47%) compared with those treated with half‐dose PDT (7%). Previous studies have provided information on the recurrence of SRF in cCSC patients successfully treated with HSML, with a reported recurrence rate of 0–23% (Chen et al. 2008; Ricci et al., 2009; Scholz et al. 2015; van Rijssen et al. 2019c). The lower recurrences rates reported in these studies compared to our study may be explained by differences in inclusion criteria, as well as their short follow‐up compared to the relatively long follow‐up (20 months) in our study, which makes it more likely to observe recurrences of SRF. Variability in HSML treatment settings may also lead to differences in study outcomes (Wood et al. 2017; van Rijssen et al. 2019c). However, in the PLACE trial, HSML treatment settings used were at the upper limit of subthreshold, and the whole hyperfluorescent area on ICGA was treated with adjacent HSML spots using a fixed protocol by experienced micropulse laser surgeons. This makes it very unlikely that the cCSC patients in this study were under‐treated with HSML.
Since choroidal dysfunction is presumed to be the most important factor in CSC pathophysiology, stimulation of the RPE by HSML laser may induce only temporary resolution of SRF by solely targeting the RPE without addressing choroidal dysfunction (Daruich et al. 2015; van Rijssen et al. 2019c). Therefore, multiple HSML treatments may be required in order to retain a complete and prolonged resolution of SRF, in particular in cCSC patients with extensive RPE involvement (Chen et al. 2008; Lanzetta et al. 2008). It is still unclear if there is indeed a treatment effect of HSML in cCSC, or if the resolution rate is largely due to the natural course of the disease, which tends to wax and wane even in long‐standing cases with signs of chronicity. Since half‐dose PDT has been shown to have its main effect on the choroidal tissue, this treatment may directly address the primary disease mechanism of CSC (Maruko et al. 2010). By directly targeting the affected choroid, half‐dose PDT may lead to a more durable remodelling of the choroid which is returned to a state that is more comparable to the normal situation, resulting in a prolonged absence of SRF in the great majority of patients, in contrast to HSML.
During the study, the retinal sensitivity on microperimetry significantly improved, and the CRT on OCT significantly increased only within the half‐dose PDT group. It has been shown previously that a slight CRT increase should be expected and is desired after SRF resolution in successfully treated CSC patients (van Rijssen et al. 2018a). The increased CRT and improved retinal sensitivity at final visit after successful cCSC treatment may imply a gradual improvement of normal foveal photoreceptor structure and function, for instance, because the complete resolution of SRF allows the gradual reconstitution of the normal close physical and functional interaction of the photoreceptors with the RPE, resulting in further improvement even between 7 to 20 months after successful treatment. This reconstitution of the normal photoreceptor‐RPE anatomy is also reflected in our finding that half‐dose PDT was also superior to HSML treatment with regard to restoring the continuity of the ELM and EZ in the long term. Our finding that almost half of the cCSC patients treated with HSML had presence of SRF at final follow‐up is of concern, because this situation precludes normal photoreceptor‐RPE interaction, while persistent SRF in CSC has been previously associated with reduced long‐term BCVA and decreased quality of life (Loo et al. 2002; Breukink et al. 2017; Mrejen et al. 2019).
Limitations of this study include the limited number of patients eligible for inclusion, since only patients who adhered to the PLACE trial protocol and with no SRF at the final visit of the PLACE trial could be included in the current study. Moreover, some patients did not attend the final visit, because their health care provider did not reimburse the costs of final visit and their symptoms had disappeared or because they were lost to follow‐up. Consequently, we are unable to assume with certainty that patients who did not attend final visit were missing in a randomly fashion. Due to the limited number of available patients, a possible significant difference in SFCT between the half‐dose PDT and HSML groups may not have been detected. Finally, this study did not include a cost‐effectiveness analysis of both treatments.
In conclusion, only a small fraction of cCSC patients who have received successful treatment with half‐dose PDT experienced a recurrence of SRF between 7 and 20 months after the first treatment, while almost half of cCSC patients treated with HSML developed a recurrence. Although functional parameters did not significantly differ between both treatment groups at final visit, previous studies have indicated that persistence of subretinal fluid may lead to visual loss in the long term. At the final visit of the PLACE trial, the increase in retinal sensitivity on microperimetry was significantly higher in the half‐dose PDT group compared with the HSML group (van Dijk et al. 2018). Additionally, in the REPLACE trial, there was a significant increase in retinal sensitivity in the half‐dose PDT group, as opposed to the HSML group (van Rijssen et al. 2020). This indicates that half‐dose PDT may be preferred over HSML treatment for the treatment of cCSC. When half‐dose PDT is not available or cost‐prohibitive, HSML can be considered. If patients are treated with HSML, follow‐up visits may be planned more often compared to patients treated with PDT, since persistence and recurrence of SRF are more likely to happen.
This study was supported by Stichting Macula Fonds; Retina Nederland Onderzoek Fonds; Stichting Blinden‐Penning; Algemene Nederlandse Vereniging ter Voorkoming van Blindheid; Landelijke Stichting voor Blinden en Slechtzienden, which contributed through UitZicht (Delft, the Netherlands); Rotterdamse Stichting Blindenbelangen (Rotterdam, the Netherlands); Stichting Leids Oogheelkundig Ondersteuningsfonds (Leiden, the Netherlands); Haagse Stichting Blindenhulp (The Hague, the Netherlands); Stichting Ooglijders (Rotterdam, the Netherlands); Stichting Blindenhulp; Oxford NIHR Biomedical Research Centre (Oxford, United Kingdom); the Gisela Thier Fellowship of Leiden University, Leiden, the Netherlands (C.J.F.B.); and the Netherlands Organization for Scientific Research (VENI grant to C.J.F.B.). These funding organizations provided unrestricted grants and had no role in the design or conduct of this research. This investigator‐initiated study received funding from Novartis Pharma B.V. (Arnhem, the Netherlands) solely for the purchase of verteporfin (Visudyne) to enable photodynamic therapy treatment at the Oxford site, because photodynamic therapy currently is not reimbursed routinely by the United Kingdom National Health Service for treating central serous chorioretinopathy. Novartis Pharma B.V. had no role in funding, designing, conducting or evaluating the study, nor in the writing of this manuscript.
The funding organizations had no role in the design or conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. They provided unrestricted grants.
References
- Breukink MB, Dingemans AJ, den Hollander AI et al. (2017): Chronic central serous chorioretinopathy: long‐term follow‐up and vision‐related quality of life. Clin Ophthalmol 11: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breukink MB, Downes SM, Querques G et al. (2015): Comparing half‐dose photodynamic therapy with high‐density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy (the PLACE trial): study protocol for a randomized controlled trial. Trials 16: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WM, Lam DS, Lai TY, Tam BS, Liu DT & Chan CK (2003): Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol 87: 1453–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SN, Hwang JF, Tseng LF & Lin CJ (2008): Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology 115: 2229–2234. [DOI] [PubMed] [Google Scholar]
- Cheung CMG, Lee WK, Koizumi H, Dansingani K, Lai TYY & Freund KB (2018): Pachychoroid disease. Eye (London, England) 33: 14–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daruich A, Matet A, Dirani A, Bousquet E, Zhao M, Farman N, Jaisser F & Behar‐Cohen F (2015): Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res 48: 82–118. [DOI] [PubMed] [Google Scholar]
- Fujita K, Imamura Y, Shinoda K, Matsumoto CS, Mizutani Y, Hashizume K, Mizota A & Yuzawa M (2015): One‐year outcomes with half‐dose verteporfin photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology 122: 555–561. [DOI] [PubMed] [Google Scholar]
- Haimovici R, Gragoudas ES, Duker JS, Sjaarda RN & Eliott D (1997): Central serous chorioretinopathy associated with inhaled or intranasal corticosteroids. Ophthalmology 104: 1653–1660. [DOI] [PubMed] [Google Scholar]
- Inoue R, Sawa M, Tsujikawa M & Gomi F (2010): Association between the efficacy of photodynamic therapy and indocyanine green angiography findings for central serous chorioretinopathy. Am J Ophthalmol 149: 441–446 e441–442. [DOI] [PubMed] [Google Scholar]
- Laatikainen L (1994): Diffuse chronic retinal pigment epitheliopathy and exudative retinal detachment. Acta Ophthalmol (Copenh) 72: 533–536. [DOI] [PubMed] [Google Scholar]
- Lai FH, Ng DS, Bakthavatsalam M, Chan VC, Young AL, Luk FO, Tsang CW & Brelen ME (2016): A multicenter study on the long‐term outcomes of half‐dose photodynamic therapy in chronic central serous chorioretinopathy. Am J Ophthalmol 170: 91–99. [DOI] [PubMed] [Google Scholar]
- Lai TY, Wong RL & Chan WM (2015): Long‐term outcome of half‐dose verteporfin photodynamic therapy for the treatment of central serous chorioretinopathy (an American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc 113: T8. [PMC free article] [PubMed] [Google Scholar]
- Lanzetta P, Furlan F, Morgante L, Veritti D & Bandello F (2008): Nonvisible subthreshold micropulse diode laser (810 nm) treatment of central serous chorioretinopathy. A pilot study. Eur J Ophthalmol 18: 934–940. [DOI] [PubMed] [Google Scholar]
- Loo RH, Scott IU, Flynn HW Jr, Gass JD, Murray TG, Lewis ML, Rosenfeld PJ & Smiddy WE (2002): Factors associated with reduced visual acuity during long‐term follow‐up of patients with idiopathic central serous chorioretinopathy. Retina 22: 19–24. [DOI] [PubMed] [Google Scholar]
- Lotery A, Sivaprasad S, O'Connell A et al. (2020): Eplerenone for chronic central serous chorioretinopathy in patients with active, previously untreated disease for more than 4 months (VICI): a randomised, double‐blind, placebo‐controlled trial. Lancet (London, England) 395: 294–303. [DOI] [PubMed] [Google Scholar]
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD & National Eye Institute Visual Function Questionnaire Field Test Investigators (2001): Development of the 25‐item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 119: 1050–1058. [DOI] [PubMed] [Google Scholar]
- Maruko I, Iida T, Sugano Y, Ojima A, Ogasawara M & Spaide RF (2010): Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology 117: 1792–1799. [DOI] [PubMed] [Google Scholar]
- Mrejen S, Balaratnasingam C, Kaden TR et al. (2019): Long‐term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology 126: 576–588. [DOI] [PubMed] [Google Scholar]
- Nicolo M, Zoli D, Musolino M & Traverso CE (2012): Association between the efficacy of half‐dose photodynamic therapy with indocyanine green angiography and optical coherence tomography findings in the treatment of central serous chorioretinopathy. Am J Ophthalmol 153: 474–480.e471. [DOI] [PubMed] [Google Scholar]
- Park YJ, Kim YK, Park KH & Woo SJ (2019): Long‐term efficacy and safety of photodynamic therapy in patients with chronic central serous chorioretinopathy. Ophthalmic Surg Lasers Imaging Retina 50: 760–770. [DOI] [PubMed] [Google Scholar]
- Prunte C & Flammer J (1996): Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol 121: 26–34. [DOI] [PubMed] [Google Scholar]
- Ricci F, Missiroli F, Regine F, Grossi M & Dorin G (2009): Indocyanine green enhanced subthreshold diode‐laser micropulse photocoagulation treatment of chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 247: 597–607. [DOI] [PubMed] [Google Scholar]
- Roca JA, Wu L, Fromow‐Guerra J et al. (2018): Yellow (577 nm) micropulse laser versus half‐dose verteporfin photodynamic therapy in eyes with chronic central serous chorioretinopathy: results of the Pan‐American Collaborative Retina Study (PACORES) Group. Br J Ophthalmol 102: 1696–1700. [DOI] [PubMed] [Google Scholar]
- Scholz P, Ersoy L, Boon CJ & Fauser S (2015): Subthreshold micropulse laser (577 nm) treatment in chronic central serous chorioretinopathy. Ophthalmologica 234: 189–194. [DOI] [PubMed] [Google Scholar]
- Sramek C, Mackanos M, Spitler R, Leung LS, Nomoto H, Contag CH & Palanker D (2011): Non‐damaging retinal phototherapy: dynamic range of heat shock protein expression. Invest Ophthalmol Vis Sci 52: 1780–1787. [DOI] [PubMed] [Google Scholar]
- van Dijk EHC, Fauser S, Breukink MB et al. (2018): Half‐dose photodynamic therapy versus high‐density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: the PLACE trial. Ophthalmology 125: 1547–1555. [DOI] [PubMed] [Google Scholar]
- van Rijssen TJ, Mohabati D, Dijkman G, Theelen T, de Jong EK, van Dijk EHC & Boon CJF (2018a): Correlation between redefined optical coherence tomography parameters and best‐corrected visual acuity in non‐resolving central serous chorioretinopathy treated with half‐dose photodynamic therapy. PLoS One 13: e0202549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijssen TJ, van Dijk EHC, Dijkman G & Boon CJF. (2018b): Clinical characteristics of chronic central serous chorioretinopathy patients with insufficient response to reduced‐settings photodynamic therapy. Graefes Arch Clin Exp Ophthalmol 256: 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijssen TJ, van Dijk EHC, Scholz P et al. (2019a): Focal and diffuse chronic central serous chorioretinopathy treated with half‐dose photodynamic therapy or subthreshold micropulse laser: PLACE trial report no. 3. Am J Ophthalmol 205: 1–10. [DOI] [PubMed] [Google Scholar]
- van Rijssen TJ, van Dijk EHC, Scholz P et al. (2019b): Patient characteristics of untreated chronic central serous chorioretinopathy patients with focal versus diffuse leakage. Graefes Arch Clin Exp Ophthalmol 257: 1419–1425. [DOI] [PubMed] [Google Scholar]
- van Rijssen TJ, van Dijk EHC, Scholz P et al. (2020): Cross‐over to photodynamic therapy or micropulse laser after failure of primary treatment of chronic central serous chorioretinopathy. Am J Ophthalmol 216: 80–89. [DOI] [PubMed] [Google Scholar]
- van Rijssen TJ, van Dijk EHC, Yzer S et al. (2019c): Central serous chorioretinopathy: towards an evidence‐based treatment guideline. Prog Retin Eye Res 73: 100770. [DOI] [PubMed] [Google Scholar]
- Wood EH, Karth PA, Sanislo SR, Moshfeghi DM & Palanker DV (2017): Nondamaging laser therapy for treatment of central serous chorioretinopathy: what is the evidence? Retina 37: 1021–1033. [DOI] [PubMed] [Google Scholar]
- Yannuzzi LA (2010): Central serous chorioretinopathy: a personal perspective. Am J Ophthalmol 149: 361–363. [DOI] [PubMed] [Google Scholar]
- Yannuzzi LA, Slakter JS, Gross NE, Spaide RF, Costa D, Huang SJ, Klancnik JM Jr & Aizman A (2003): Indocyanine green angiography‐guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina 23: 288–298. [DOI] [PubMed] [Google Scholar]


