Abstract
Background
Scrotal ultrasonography is an essential diagnostic tool in daily clinical practice. The availability of new‐generation ultrasound machines characterized by clearly improved image quality, low health cost, and higher patient safety, represents only some characteristics of ultrasound investigation. The usefulness of scrotal ultrasonography is particularly evident in the period of life from infancy to puberty, during which males undergo important morphofunctional changes, and several pathological conditions may occur.
Objectives
This pictorial review primarily aimed to investigate the aspects of ultrasonography related to the normal physiological development of the gonads from mini‐puberty to pubertal onset. This study also aimed to provide an update on the use of ultrasonography in main andrological pathologies that may occur during this period. The conditions that are discussed in depth are: cryptorchidism, inguinoscrotal hernias, and hydrocele in the neonatal phase; acute scrotum, epididymo‐orchitis, and testicular cancers in childhood; and hypogonadism, varicoceles, testicular microlithiasis, and oncohematological pathology in puberty.
Discussion
We provided an ultrasound slant for all the above‐mentioned pathologies while purposely avoiding excessive deepening of the pathogenetic, clinical, and therapeutic aspects. Studying the ultrasound aspects of the gonads also facilitates differential diagnosis between various conditions and represents a good aid in evaluating therapeutic success (e.g., in hypogonadism or postsurgical evaluation of varicoceles and cryptorchidism).
Conclusion
Scrotal ultrasonography is now globally recognized as the necessary completion of clinical–laboratory overview in gonads evaluation. This diagnostic procedure is even more indispensable in the infancy–childhood–puberty period for the evaluation of normal gonadal development as well as diagnosis of other possible diseases.
Keywords: andrological pathologies, childhood, infancy, mini‐puberty, puberty, scrotal ultrasonography
1. INTRODUCTION
Given the ease of use and total absence of radiation, resulting in patient safety and low cost to public health, scrotal ultrasonography (US) has presently become a key diagnostic tool in clinical practice. Moreover, endocrinologists and andrologists cannot disregard the use of echography given the large amount of information it can provide.
The term “infancy” literally corresponds to early childhood. Furthermore, infancy (or early childhood), that is, from birth to 2 years of life, includes mini‐puberty, which plays an important role in reaching a gonadal maturative step after birth, due to its physiological implications. 1 , 2 Finally, childhood ends with the beginning of puberty, which usually occurs at ages between 9.5 and 13.5 years (11.5 years on average) and leads to the achievement of complete sexual maturity. 3
This phase of life from birth to the end of puberty can last approximately 13–14 or 17–18 years, depending on the variable initiation of male puberty. During this phase, males undergo important macroscopic changes. 4 Scrotal US could play a decisive diagnostic role in multiple, and sometimes dangerous, pathological conditions that may present themselves during infancy, childhood, and puberty. 5 , 6
This study conducted a review of the literature to analyze the role of scrotal US in normal gonadal development and in the common pathological conditions of the infantile–adolescent period, with the aim of emphasizing the diagnostic advantages and possible limitations of its use.
2. ACTIVATION OF THE HYPOTHALAMIC–PITUITARY–GONADAL AXIS AND THE ROLE OF SCROTAL US IN THE STUDY OF GONADAL MATURATION STAGES
The hypothalamic–pituitary–gonadal (HPG) axis is activated at three stages in life, the first of which is the intrauterine period, when changes in the concentrations of luteinizing hormone (LH) and follicle‐stimulating hormone (FSH) seem to be crucial for the activation of the peripheral hormones involved in gonadal maturation. At 9 weeks of gestation, FSH and LH become detectable in the anterior pituitary gland and general circulation. 7 , 8 At this stage, the increase in gonadotropins is probably related to the gonadotropin‐releasing hormone (GnRH) neuron activity, whereas certain GnRH‐induced stimulus is seen only at 30–31 weeks of gestation. 9 Furthermore, during the first trimester of pregnancy, placental human chorionic gonadotropin detects an increase in gonadal testosterone production in male fetuses because of its LH‐like effect, allowing the male differentiation of the fetus. Gonadotropin concentration decreases markedly toward the end of pregnancy due to the increase in placental estrogen secretion, 10 which suppresses HPG axis activity. This suppression is maintained for some days after birth (usually 7–10 days) due to the persistence of a high concentration of placental hormones. After the concentration of these placental hormones reduces, the HPG axis activity is initiated again, resulting in mini‐puberty. 11 , 12
Compared to females, males not only have a higher concentration of LH than FSH that peaks between the 2nd and 10th week of life, but also have an earlier decrease in the levels of these hormones, which usually return to suppression values at approximately 4–6 months. 13 The trend of testosterone levels is exactly the same as that of LH. During mini‐puberty, there is also an increase in the values of anti‐Müllerian hormone (AMH), 12 which reflects the initial maturation of Sertoli cells, accompanied by germ cell (spermatogonia) proliferation, that remains at the immature stage. 14 Finally, initial maturation of Leydig cells also occurs, which produces testosterone and induces a trophic effect on the genitalia. 13 During mini‐puberty, the testicles increase in volume; this increase is not easily detected using simple palpation, but requires the use of scrotal US, that allows for the determination of the normal US characteristics during this period. At this phase, the testes frequently appear symmetrical, equal in size (approximately 0.5–1.5 mL), and homogeneous, but are more hypoechoic than adult testes due to immature seminiferous tubules (Figure 1).
FIGURE 1.
Ultrasound image of the normal male testicle during mini‐puberty using high‐frequency (12 MHz) linear probe. During this phase of life, the testes appear symmetrical, equal in size (approximately 0.5–1.5 mL), and homogeneous, but also more hypoechoic than adult testes. Note that the mediastinum appears evident and tendentially hyperechogenic
After mini‐puberty, the HPG axis remains quiescent throughout childhood through an as‐yet unknown mechanism, and the only hormones that remain detectable are AMH and inhibin B; this indicates that the Sertoli cells do not stop their secretive activity during this period. 15 Scrotal US provides important information regarding the gonads even during childhood when its echographic aspect is quite similar to that of mini‐puberty, with the only difference being the slightly larger volume (1.5–2 mL on average) (Figure 2).
FIGURE 2.
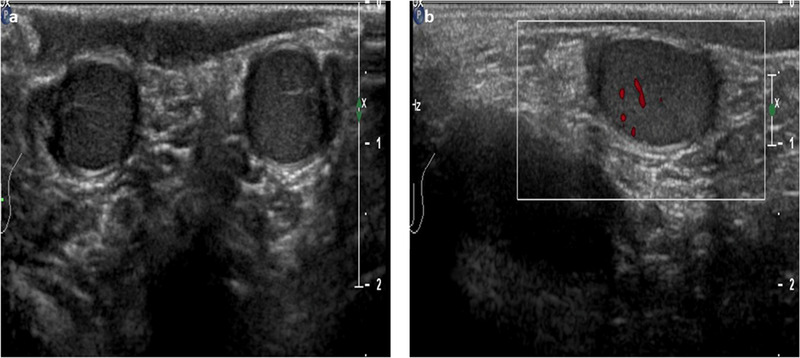
Ultrasound images of the normal male testicle during childhood using high‐frequency (12 MHz) linear probe. (A)The testes appear symmetrical, equal in size (approximately 1.5–2 mL) and homogeneous. (B) An initial increase of the color signal is possible
After this quiescent period, the HPG axis appears to be reactivated by the Kisspeptin stimulation on the hypothalamic neurons secreting GnRH, 4 thus promoting the onset of pulsatile FSH and LH secretion. In males, the first sign of pubertal onset is the attainment of a testicular volume of 4 mL because of the trophic effect of FSH on the immature Sertolian compartment. 16 In contrast, LH stimulates the Leydigian compartment, resulting in an increase in intratesticular testosterone, which causes the maturation of Sertoli cells. This maturation is characterized by the loss of mitotic capacity, development of the blood–testis barrier, and downregulation of AMH production. 17 Finally, all these hormonal changes induce the onset of germ cell meiosis, with increased seminiferous tubules and consequently, testicular volume. During puberty, secondary sexual characteristics appear, and their progression over time is represented by Tanner's pubertal stages (G1–G5). 3 During the progression of Tanner's stages, the testicles undergo profound modifications that can be highlighted through US. Beside the increase in volume, the greatest echographic change is represented by the increase in the echogenicity of the parenchyma, which tends to acquire greater reflectance with the increase in number of seminiferous tubules. Moreover, with each passing stage, the echo distribution becomes more homogeneous with a pattern of medium amplitude. 18
To the best of our knowledge, color Doppler US allows for the assessment of blood flow in normal testes and surrounding structures. Gonadal flow is slower during pre‐puberty than during post‐puberty, and gradually tends to increase and arborize from the periphery within the gonads with the progression of pubertal stages. In this perspective, the flow increase is an additional indication of gonadal maturation. 18
Figure 3 highlights the clinical progression of pubertal stages, from G1 to G5, with related US modifications.
FIGURE 3.
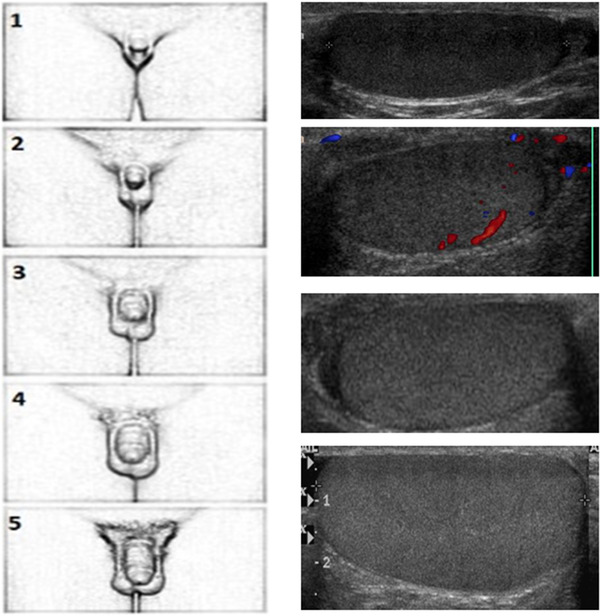
Clinical progression of pubertal stages from G1 to G5 with related ultrasound modifications. It is possible to note the increase in volume and echogenicity as pubertal stages and seminiferous tubule maturation advance
3. US ASPECTS IN SCROTAL DISEASE FROM INFANCY TO PUBERTY
Scrotal US is indispensable for the diagnosis of many andrological diseases of the infancy–childhood–puberty period. This section provides an excursus of the main pathological conditions during this span of time, focusing on the US aspects and subdividing the pathologies based on the period in which they manifest most frequently: infancy, childhood, and adolescence (puberty).
Table 1 synthesizes the original studies that investigated infancy, childhood, and adolescence US aspects of main scrotal pathologies.
TABLE 1.
List of original studies that investigated infancy, childhood and adolescence US aspects of main scrotal pathologies
| Author, year of publication and journal | Caseload | Study populations’ characteristics | Scrotal disease | US findings |
|---|---|---|---|---|
| Cain, 1996, J Urol. | 64 patients | Mean age 4.5 years (0.5 to 17 years) | Cryptorchidism | Scrotal‐inguinal ultrasound correctly identified 40 of the 42 inguinal testes (95% sensitivity), 7 of the 21 atrophic inguinal testes (33% sensitivity) and 1 of the 11 intra‐abdominal testes (9% sensitivity). |
| Kollin, 2012, J Clin Endocrinol Metab. | 225 patients | Mean age 1.87 years (0.7 to 3 years) | Cryptorchidism | Correlation between US‐obtained testicular volume and number of germ and Sertoli cells in cryptorchid patients who underwent testicular biopsies. This randomized controlled study compared the outcome of surgery for congenital cryptorchidism at 9 months or 3 years of age: at both ages, testicular volume correlated to the number of germ and Sertoli cells. |
| Jedrzejewski, 2012, Early Hum Dev. | 1448 patients | Mean age 1.4 years (0.7 to 3 years) | Cyptorchidism and hydrocele | In this scrotal ultrasound screening program performed on boys up to 3 years old, abnormalities in scrotal ultrasound were found in 20.1% of boys. Undescended, cryptorchid testes were found in 4.8% of patients, mobile testicle in 7.6% and hydrocele in 2.8%. |
| Cuervo, 2009, J Pediatr Surg. | 9 patients | Newborns | Hydrocele | Ultrasound was useful to confirm the initial suspicion of the presence of an abdomen‐scrotal hydrocele. Hydrocele appeared as an hourglass‐shaped cystic mass with homogeneous anechoic content. |
| Chmelnik, 2010, Pediatr Surg Int. | 25 patients | Mean age 9.8 (0.3 to 15 years) | Testicular torsion | TT was detected in 16 out of 25 patients. The Authors, in addition to the absence of blood flow, detected focal hyper‐/hypoechogenicity in 12.5% of patients, diffuse hyper‐/hypoechogenicity in 31.3% of patients and a normal echogenicity in 56.3%. US features changed over time: the time span since torsion developed determined testicular viability. No testis with focal hyper/ipoechogenicity could be saved. The mean volume of the affected testes was 6.0 ± 6.9 mL, significantly higher than the mean volume of the non‐affected ones (3.3 ± 3.7 mL). |
| Liang, 2012, Am J Roentgenol. | 266 patients | Mean age 12.2 years (from 1 month to 17 years) | Testicular torsion |
TT was detected in 29 out of 266 patients, with absent blood flow seen by color Doppler. The affected testes were found to have a significantly higher incidence of decreased flow and heterogeneous echogenicity. Similarly, a higher incidence of decreased flow also in the epididymis and enlarged scrotal size were found in testicular torsion patients. |
| Lev, 2015, Eur J Endocrinol. | 19 patients | Mean age 9.4 years (3 to 14 years) | Torsion of the testicular appendix |
It appeared as an oval, avascular mass with increased, low, or mixed echogenicity, which could be located between the head of the epididymis and the upper pole of the testis. Torsion of the testicular appendix is often accompanied by hydrocele, enlargement of the head of the epididymis and surrounding hyperemia. The lesions had a maximal diameter ranging from 5.8 to 15.6 mm (mean 9.5 mm). |
| Karmazyn, 2009, Pediatr Radiol. | 47 patients | Mean age 9.6 years (from 1 month to 17 years) | Epididymitis | Increased epididymal blood flow was seen in all children. Epididymal enlargement was bilateral in 4 cases, right‐sided in 23 cases, and left‐sided in 20 children. Epididymal enlargement was marked in 14 children, moderate in 26 children, and mild in 7 children. Testicular swelling and hydrocele could also occur. |
| Caballero Mora, 2012, An Pediatr (Barc). | 15 patients | Mean age of the patients was 9.7 years (0.6 months to 16 years) | Testicular cancer | US detects testicular neoplasms in almost 100% of cases. Benign tumours appeared as well‐defined masses with demarcated borders and poor vascularity. Epidermoid cysts appeared as well‐defined intratesticular lesions with a central hypoechogenic area surrounded by a hyperechogenic ring. Yolk sac tumours have a more solid, hypoechogenic, homogeneous appearance. |
| Song, 2018, J Ultrasound Med. | 21 patients | Mean age 16.2 months (0.8 to 5 years) | Yolk‐sac tumor | Grayscale US images showed focal lesions in 14 out of 21 cases. The focal lesions were purely solid in 10 patients or solid with cystic components in 4 patients. The mixed focal lesions (both solid and with cystic components) were ovoid, with randomly distributed multiple anechoic spaces, which were variable in number, size, and shape. In most of cases homogeneous echo texture (43%) and increased blood flow (85.7%) were found. |
| Epifanio, 2014, Urology | 7 patients | Mean age 8.1 months (from the date of birth to 4 years) | Teratoma | The US findings were heterogeneous: cystic (1 case), multi‐cystic (2 cases), solid‐cystic (1 case), solid containing larger or smaller calcifications (2 cases), and focal calcification (1 case). All the solid lesions had few vessels in the interior of the lesions. Testicular teratoma could be single, multiseptated, small, or large, and could contain diffuse or localized calcifications. Finally, the gonad could also have an increased or a normal volume. |
| Posey, 2010, J Urol. | 1765 patients | Patients were grouped into 5‐year age intervals, including less than 5, 5 to 10, 10 to 15 and greater than 15 years | Epididymal cysts | This retrospective study on the occurrence of epididymal cysts showed an increased incidence with age and a correlation between epididymal cysts and testicular size, which resulted larger in boys with cysts. |
| Christman, 2014, J Urol. | 73 patients | Mean age 15.5 years (13.2 to 17.7 years) | Varicocele in adolescence | In this retrospective study on adolescents with varicocele evaluated by serial scrotal ultrasound, testicular volume was shown to predict total motile count at the end of adolescence but not throughout. |
| Zampieri, 2008, J Urol. | 2107 patients | Mean age 13 years (10 to 16 years) | Varicocele in adolescence | This longitudinal follow‐up observational study determined the prognostic value of clinical examination and US in predicting the risk of progression, time to worsening and the final outcome of varicocele in adolescence. |
| Cooper, 2014, Radiology | 3370 patients | Mean age 11.0 years (0.6 to 17.9 years) | Testicular microlithiasis | In this large US study, TM had a prevalence of 2% in boys who underwent scrotal US. It was most commonly bilateral, classic type, and stable at follow‐up. There was a significant association of TM and testicular tumors. Malignant tumors were seen only in adolescent boys. |
| Trout, 2017, Radiology | 37863 patients | Mean age 11.1 years (6.4 to 15.8 years) | Testicular microlithiasis |
In this retrospective study on a large pediatric population the strong association between TM and testicular neoplasia was assessed. Primary testicular tumors of any type were present in 4.64% of boys with TM. Malignant germ cell tumors were present in 2.83% of boys with TM and sex cord–stromal tumors were identified in 0.46% of boys with TM. |
Abbreviations: TM, testicular microlithiasis; TT, testicular torsion; US, ultrasound
3.1. Infancy
Two different scenarios can occur during the examination of neonatal scrotum: absence of one or, less frequently, both testes inside the scrotal sac (cryptorchidism), or the discovery of an accessory and palpable scrotal mass. Both situations require comprehensive diagnostic assessment, and US imaging procedures are important to enable the clinician to decide between future medical or surgical management.
Besides cryptorchidism, other frequent inguinoscrotal pathologies during infancy are congenital hydrocele and inguinal and inguinoscrotal hernias, which represent a spectrum of embriogenetic disorders related to the persistence of the peritoneal‐derived processus vaginalis, with an alteration taking place during the migration of the testes to the scrotal sac. 19
3.1.1. Cryptorchidism
Cryptorchidism is one of the most common congenital abnormalities observed in males. This condition is present in approximately 1–4.5% of newborns, with higher incidence in pre‐terms (30–45%). 20
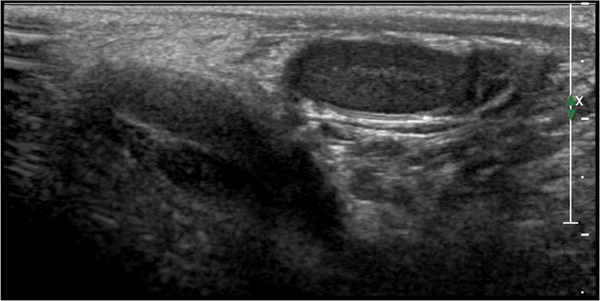
The undescended testis may complete its migration during the first few months of infancy; therefore, the incidence can decrease to 0.8–1.2% at 1 year of age. Nevertheless, given the impact of future complications, such as reduced fertility, higher risk of developing testicular malignancies, and susceptibility to testicular torsion, this condition needs prompt diagnosis. 21 US is a useful tool for the localization of the undescended testes, which can be detected in the inguinal canal (70% of cases), pre‐scrotal area (20%), and, less frequently, across the internal inguinal ring 22 (Figure 4). The testes located higher in the abdominal region are difficult to distinguish using US due to sonographic interference from surrounding tissues and bowel gas. In such cases, magnetic resonance imaging (MRI) offers greater sensitivity and should be considered. 23 If US as well as MRI fail to localize the undescended testis, laparoscopic surgery is required.
FIGURE 4.
Ultrasound images of an inguinal cryptorchid testes using high‐frequency (12 MHz) linear probe. The testicle appears hypoechoic
US detects cryptorchid testes usually as a soft, oval mass, smaller and frequently hypoechoic with respect to the contralateral descended testis with a dishomogeneous echotexture and detectable mediastinum. Sometimes, the margins may appear poorly defined. Color Doppler US can show irregular vascularization. Differential diagnosis should be made on the basis of inguinal lymph nodes (absence of mobility and no mediastinum testes), inguinal hernias, and retractile testes. 24 Scrotal US is also useful after orchidopexy and hormonal treatment for cryptorchidism, and in long‐term monitoring of patients with previous cryptorchidism who are at a higher risk of malignancies.
3.1.2. Hydrocele
In neonatal hydrocele, the patency of the processus vaginalis allows serous peritoneal fluid to reach and occupy the space between the visceral and parietal layers of the tunica vaginalis (communicating hydrocele), where normally, only 1–2 mL of fluid is present. On US evaluation, this appears as an anechoic fluid collection surrounding the anterolateral margins of the testis, with the posterior margin firmly adhered to the epididymis and scrotal walls (Figure 5). Given the presence of unobstructed communication, the anechoic fluid can sometimes extend to the inguinal canal. 25 The condition resolves spontaneously in 63–89% of the cases within 12–24 months after birth and rarely requires intervention. 26 Two less common forms of congenital hydrocele are spermatic cord cyst, which can be visualized as a small, localized, collection of fluid along the spermatic cord, and abdominoscrotal hydrocele, a large protruding communicating hydrocele that often requires surgical intervention. 27
FIGURE 5.
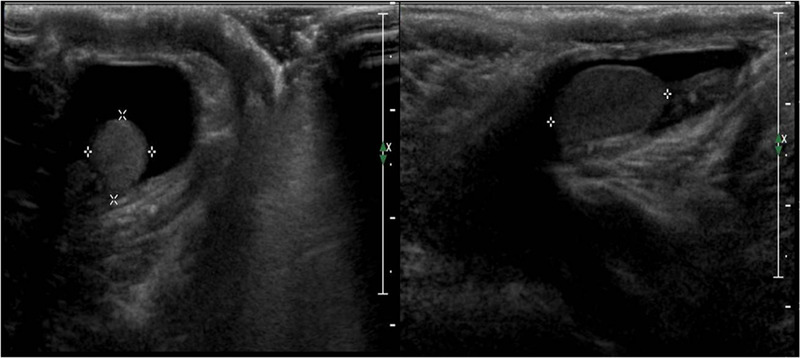
Ultrasound images of a neonatal hydrocele using high‐frequency (12 MHz) linear probe. The testes are surrounded by anechoic fluid collection, with the posterior margin firmly adherent to epididymis and scrotal walls
During childhood or puberty, acquired hydrocele can be associated with inflammatory processes, testicular trauma, cancer, or torsion of the testis or appendages. This form of hydrocele is often non‐communicating, heterogeneous in fluid content, and can present septa. 28
3.1.3. Inguinoscrotal hernia
Inguinal hernia is defined as the protrusion of intra‐abdominal tissues, such as intestinal loops, omentum, or vermiform appendix, into the groin area. The incidence ranges from 0.8% to 4% and is more common in premature newborns. 29 Inguinoscrotal hernias are located most commonly on the right side 22 and are bilateral in 15–20% of the cases. 30 Most hernias increase in size with crying or straining, and usually manifest as an intermittent bulge in the groin or scrotum. In addition to physical examination, US can be effective for the diagnosis and management of the condition. The appearance on US varies with respect to the content of the hernial sac. Dynamic imaging can detect fluid‐ or air‐filled bowel loops within the funiculus or in the scrotum associated with intestinal peristalsis. In contrast, hyperechoic areas often indicate omental herniation. 23 The contralateral inguinal canal should also be scanned. Color Doppler US may provide clues for differential diagnosis between two potential complications: incarcerated and strangulated hernias. In the latter, vascularization is not evident by color Doppler US, and emergency surgery is indicated. 31
3.2. Childhood
At this stage of life, several pathological conditions may occur, which may seriously impair gonadal function and sometimes endanger the patient's life. In fact, the most frequent diseases of childhood are acute scrotum and some gonadal neoplasms.
3.2.1. Acute scrotum
In children, the most frequent causes of acute scrotal pain and swelling are testicular and appendicular torsion, which together account for more than half of the total cases; one‐third are caused by epididymo‐orchitis.
Testicular torsion
In testicular torsion, the rotation of the testis around its longitudinal axis results in impaired arterial supply to the gonads, especially in the case of a complete and persistent rotation. Testicular torsions can be divided into intravaginal and extravaginal, the latter almost exclusively observed in infants.
The most common underlying cause in children is an anatomical variant, also known as “bell clapper” deformity. It is characterized by the lack of normal attachment of the testis to the scrotal wall with the spermatic cord, which can rotate together with the tunica vaginalis. In view of the possible chronic and irreversible complications due to severe ischemia, whose likelihood of occurrence increases with any delay in diagnosis, prompt evaluation and immediate intervention are mandatory for testis salvage.
The salvage rate for twisted testes is 80–100% if surgery is performed within 5–6 h after the pain onset and 70% if surgery is performed within 6–12 h after torsion. If surgery is delayed by >12 h, the salvage rate is reduced to 20%. 32
Color Doppler US is essential for the confirmation or exclusion of testicular torsion. A twisted spermatic cord is normally seen together with an absence of detectable blood flow in the testis and increased flow in the paratesticular tissue. When this US evidence is present, a sensitivity of 100% and specificity of 76% are usually obtained. 33 , 34
The US features of testicular torsion vary depending on the duration of the ischemic problem. Therefore, at least two US evaluations are usually necessary, the second one after a minimum gap of 2 h, to prevent a false‐negative diagnosis.
In the first 4 h, when arterial perfusion is not yet compromised, the testis may appear normal; subsequently, the affected testis appears enlarged and diffusely hypoechogenic. With time, heterogeneous gonads are observed, due to focal hemorrhage and necrosis. 35 In younger children, the gonads normally appear more hypoechogenic; therefore, the sign of hypoechogenicity of the parenchyma may not be visible. In some instances, the scan may even show a diffuse increased echogenicity. 36 In later stages, hyperechogenic areas show intraparenchymal bleeding. The epididymis is also involved; it may appear enlarged and heterogeneous with hyperechogenic areas due to hemorrhage. 37
Torsion of the testicular appendix
Another common cause of acute scrotum is torsion of the testicular and epididymal appendages (Figure 6), which in children, unlike in adults, can occur almost with the same frequency.
FIGURE 6.
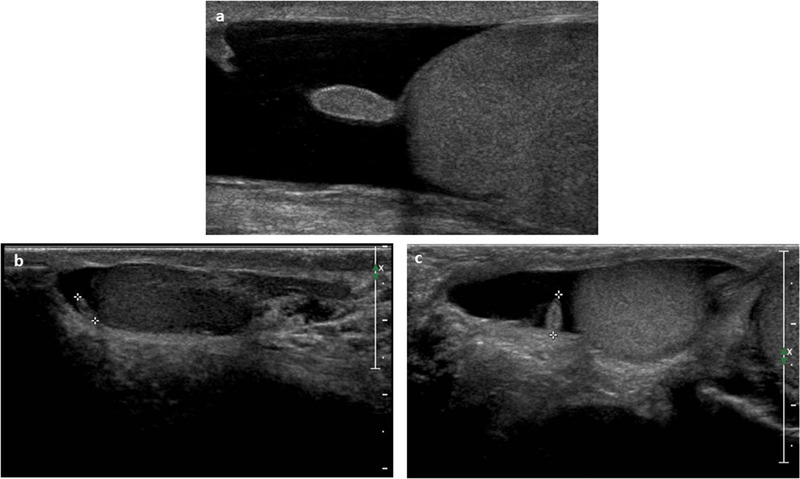
Ultrasound images of (A, B) testicular and (C, D) epididymal appendages using high‐frequency (12 MHz) linear probe. They appear oval in shape and isohypoechoic; visualization may be aided by the presence of a reactive hydrocele
On US, the twisted testicular appendix is observed as an oval, avascular mass, with increased, low, or mixed echogenicity, surrounded by an area of increased vascularity adjacent to a normally perfused testis. The mass can be located between the upper pole of the testis and the head of the epididymis. A reactive hydrocele, detectable on US, may often occur. 38
Epididymo‐orchitis
Epididymitis is a common inflammation of the epididymis seen in both children and adults, which clinically manifests as testicular pain, fever, and urinary symptoms. 39 When the testis is involved, the condition is defined as epididymo‐orchitis. 40 Isolated orchitis rarely occurs, and is mostly seen in adolescents with mumps orchitis. 24
In post‐pubertal boys, epididymo‐orchitis is often a consequence of a sexually transmitted disease, whereas, in younger children, it is usually idiopathic. 5 In pediatric patients, one of the causes may also be an anatomic abnormality, which determines urinary reflux into the ejaculatory ducts. 41
On US examination, the epididymis appears hypoechogenic and enlarged with a heterogeneous parenchyma, and is often accompanied by concomitant hydrocele. 42 If orchitis is present, the testis may appear enlarged with initially increased echogenicity. Subsequently, hypoechogenicity may develop due to edema, venous infarction, and hemorrhage. 43 , 44
In color Doppler scans, epididymo‐orchitis presents with increased vascularity in both the epididymis and testis. 45 Color Doppler US is also decisive in differential diagnosis between other causes of acute scrotum, for example, in distinguishing epididymitis from testicular or testicular appendage torsion. 18
3.2.2. Testicular tumors
In children, germ cell and gonadal tumors are rare, accounting for only 3–4% of cases of all testicular tumors (TTs). 46 Teratomas (50%) and yolk sac tumors (15%) are the most common types found during childhood. Other possible TTs are epidermoid cysts (15%) and Sertoli cell stromal tumors (approximately 3%). 47 Finally, leukemic infiltration can also be detected in the testes of children.
TTs usually present as painless scrotal masses. Less than 10% of patients report testicular pain, and when it does appear, it is usually related to hemorrhage or testicular necrosis. 48
With its 100% sensitivity, US is the imaging modality of choice for studying TT. Despite its low specificity when correlated with clinical data and biochemical markers (e.g., alpha‐fetoprotein, human chorionic beta gonadotropin, lactate dehydrogenase, or testosterone), US can be highly reliable in the differential diagnosis of different histological types. 49
Generally, TTs appear homogeneous and hypoechogenic compared to the surrounding parenchyma, but they may also be heterogeneous with areas of increased echogenicity, calcifications, or cystic formations. 21 Both color and power Doppler show high blood perfusion in most malignant tumors, whereas benign masses usually have reduced blood flow and absent ischemic lesions. 50
Yolk sac tumor
Yolk sac tumor, also known as endodermal sinus tumor, deserves special mention since it is the most frequent malignant tumor in children aged <2 years. Pre‐pubertal yolk sac tumor develops primarily in a pure form. 51 Alpha‐fetoprotein levels are increased in >90% of patients and therefore, are extremely useful markers for tumor diagnosis and follow‐up. 52 The US findings are nonspecific: the tumor may be detected as a large, focal hypoechogenic solid lesion, round or oval, of homogeneous or heterogeneous echogenicity. Occasionally, the tumor may also be detected as a diffuse mass that can occupy the entire testis so that the only US finding may be diffuse testicular enlargement. 22 , 50 , 53 , 54 Yolk sac tumor is often hypervascularized on color Doppler examination. 50
Teratoma
Teratoma is the most common TT in children. 55 They are complex tumors derived from all three germ layers: endoderm, mesoderm, and ectoderm. Pre‐pubertal teratomas are usually benign and not associated with germ cell neoplasia in situ. They do not have significant cellular atypia and do not metastasize. Mature teratomas contain only adult cells and are mainly cystic. Immature teratomas may contain embryonic or fetal cells, and the content is highly variable, with solid areas having a neuro‐ectodermal component and therefore an extremely heterogeneous US appearance. 54 On US, they typically appear as well‐circumscribed, heterogeneous masses. They can be solid, but usually have a predominantly cystic component. Cysts may be simple (anechogenic) or complex, with echogenicity, depending on their contents (e.g., mucoid, keratinous fluid, and serous). Focal calcification, cartilaginous residues, fibrous material, or immature bone (which appears as dense echogenic foci causing acoustic shadowing) can also be found. 52
Epidermoid cysts
Dermoid and epidermoid cysts are included in the category of pre‐pubertal teratomas. 50 Epidermoid cyst of the testis is a rare and benign TT. On US, the cyst appears as a well‐circumscribed mass with heterogeneous echogenic features. A typical US presentation is a solid mass surrounded by concentric rings of hypo‐ and hyperechogenicity (onion ring appearance) due to keratin layers and internal cystic component (Figure 7). 54
FIGURE 7.

High‐frequency (12 MHz) Doppler ultrasound images of testicular epidermoid cysts from two 17‐year‐old pubertal boys. (A) The typical onion ring appearance presentation of a solid mass surrounded by concentric rings of hypo‐ and hyperechogenicity. (B) Another epidermoid cyst characterized by a particularly calcific margin that generates an evident posterior shadow cone. Scattered microlithiasis is also visible
Sertoli cell tumor
Sertoli cell tumors account for <1% of all TTs. There are three types: classic, intratubular large‐cell hyalinizing, and large‐cell calcifying tumors. 56 The calcifying large‐cell Sertoli tumor subtype, the most frequent in children, has been associated with Peutz–Jeghers and Carney syndrome and may occur as multiple and bilateral masses, especially if associated with the Carney complex. US can identify multiple hyperechoic, partially calcified lesions. 52 , 57
Leukemic infiltration
Primary testicular leukemia is rare, but the testis is a common site of leukemia recurrence in children. 54 During chemotherapy, leukemic cells remain hidden inside the testes because of the blood–testis barrier. Testicular recurrence of leukemia can manifest with various US appearances, as it can be unilateral or bilateral, hypoechogenic or hyperechogenic, and diffuse or focal (Figure 8). Increased testicular blood flow is seen on color Doppler images. 52 In view of the heterogeneity of presentation, clinical history is very helpful in the diagnosis.
FIGURE 8.
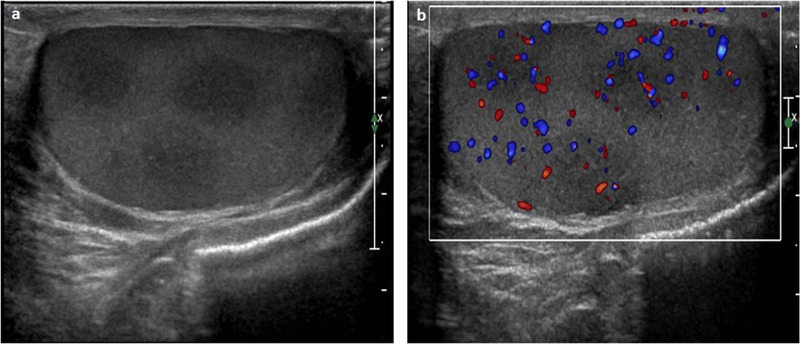
Ultrasound images of testicular recurrence of leukemia using high‐frequency (12 MHz) linear probe. (A) Several hypoechogenic foci of leukemic infiltration (recurrence of acute lymphatic leukemia). (B) The color Doppler US results, which shows an increased blood flow in each lesion
3.3. Adolescence (puberty)
Among the most common conditions investigated using US during adolescence, there are hypogonadisms like Klinefelter syndrome, varicocele, and, less frequently, epididymal cysts and testicular microlithiasis. US is also a reliable tool for the evaluation of pubertal development. Assessing the testicular volume increase, change in echogenicity, structure, and vascularization allows for the early detection of pubertal development disorders. 58
3.3.1. Hypogonadism
Male hypogonadism is the result of reduced testicular function in its production of testosterone and/or spermatozoa. US testicular appearances of hypogonadism show a wide range of differences in volumes, echogenicity, and echotexture, reflecting the variety in etiology of this condition. Recently, a novel US score, known as “testis unit” (TU) score, based on the correlation between US testicular characteristic and hormonal and seminal fluid parameters, has been proposed. The TU score can predict both impaired spermatogenesis (area under the curve [AUC], 0.73; sensitivity, 72%; specificity, 61%; p < 0.001) and hypogonadism (AUC, 0.71; sensitivity, 71%; specificity, 53%; p < 0.001), giving clinicians important hints for further interventions. 59
Primary (due to testicular failure) and secondary (due to gonadotropin deficiency or peripheral insensitivity) hypogonadism often present different US color and power Doppler features, which can help in the differential diagnosis. In primary testicular failure, the testes are usually small in size, hypoechoic, with a dishomogeneous echotexture, ranging from mild to severe. Areas of focal or diffuse Leydig cell hyperplasia can be observed. Moreover, in secondary hypogonadism, hypoechogenicity and heterogeneous echotexture can be found. 60 In adult hypogonadism, the primitive form, different from the secondary form, may sometimes show a disorganized and increased vascularity. This sign is missing in cases with children, due to the normal findings of low blood flow. Hypogonadotropic hypogonadism often shows good clinical response to exogenous gonadotropic treatment (increase in size and change in echotexture and echogenicity), which can be monitored with the aid of US.
3.3.2. Klinefelter syndrome
Among hypergonadotropic hypogonadism, 47, XXY‐Klinefelter syndrome (KS) deserves a separate section, as it represents the most frequent chromosome disorder in men, affecting 1:650 newborns. From a phenotypic point of view, it is an extremely heterogeneous syndrome, with large differences in manifestation between individuals. Gonadal function seems to be maintained until the onset of puberty, 61 when the classical phenotype usually becomes overt: it includes tall stature, relatively long legs and arms, gynecomastia, small and firm testes, azoospermia, and hypogonadism associated with several other metabolic, cardiovascular, and systemic alterations. 62 In gonadal US, the testes appear reduced in volume (2–6 mL), with hypoechogenicity that varies according to the severity of the disorder (less pronounced in some form of mosaicism), and have a coarse or nodular echotexture characterized by scattered foci of hyper‐ and hypoechogenic parenchyma. Hypervascularization, with a vascular pattern of high resistance and microlithiasis, can also be observed. 63 Under chronic LH stimulus, there is possible evidence of Leydig cell hyperplasia, which may echographically appear through the formation of commonly multiple hypoechoic round lesions, with regular but frequently blurred margins (Figure 9A). These lesions, which often have a certain degree of vascularity, should not be confused with Leydig cell tumors. 64
FIGURE 9.
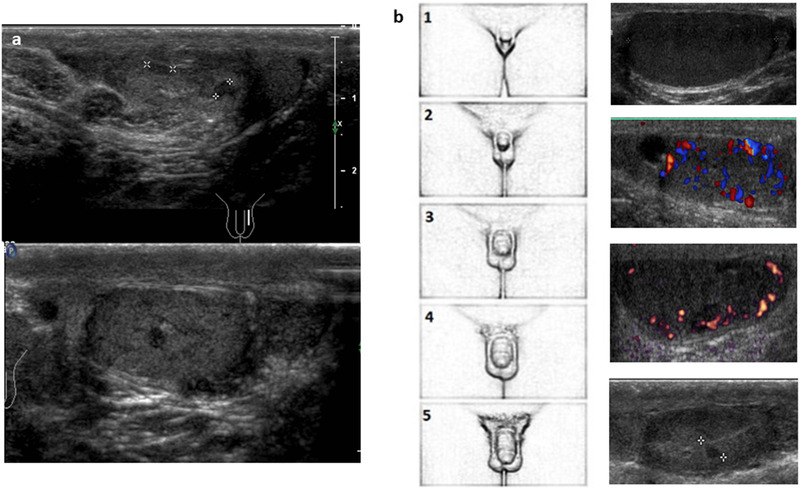
Ultrasound images of testis appearance in Klinefelter syndrome using high‐frequency (12 MHz) linear probe. (A) Some foci of Leydig cell hyperplasia as hypoechoic round lesion with regular but blurred margins; (B) the different ultrasound histological damage aspects of the gonads during the succession of pubertal stages. The parenchymal irregular hypoechogenicity and hypervascularization are particularly evaluable. The last image reports a focus of Leydig cell hyperplasia
Both mono‐ and bilateral cryptorchidisms are frequently found in KS, particularly in patients with earlier hypogonadism. In cases of a long‐standing cryptorchidism and as a consequence of the orchidopexy operation, our US experience has allowed us to note that the testicle develops a typical US pattern: greater echostructure irregularity, reduced echogenicity, and presence of ectasic venous vessels.
US follow‐up during pubertal development is crucial, as it allows for monitoring and early detection of testicular damage: the US aspect, together with hormonal testing and any somatic changes, offers the possibility to identify and eventually exploit the most appropriate time window for a successful microscopic testicular retrieval of sperm and cryoconservation of eventual foci of spermatogenesis within the testes. 65 Figure 9B shows the different US aspects of the gonads as the pubertal stages progress.
3.3.3. Varicocele
In adolescents with varicocele, which usually first manifest during early puberty or less frequently in pre‐pubertal age, US evaluation is the only objective method to quantify and monitor the extent of testicular damage. 66 In fact, gonadal spermatogenesis maturation requires several years to reach its final stage of development, and spermiogram analysis could give only limited information on gonadal health status compared to adults. A prospective short series of follow‐up scans is the most appropriate way of assessing the evolution of the pathology, selecting proper candidates for varicocele interventions early on. 67 Relevant US features that must be considered are as follows: grade of varicocele, change in parenchymal echotexture, testicular growth arrest, or size and volume discrepancy between testes (Figure 10). The latter assumes clinical significance when a >20% of variation is registered. 68
FIGURE 10.
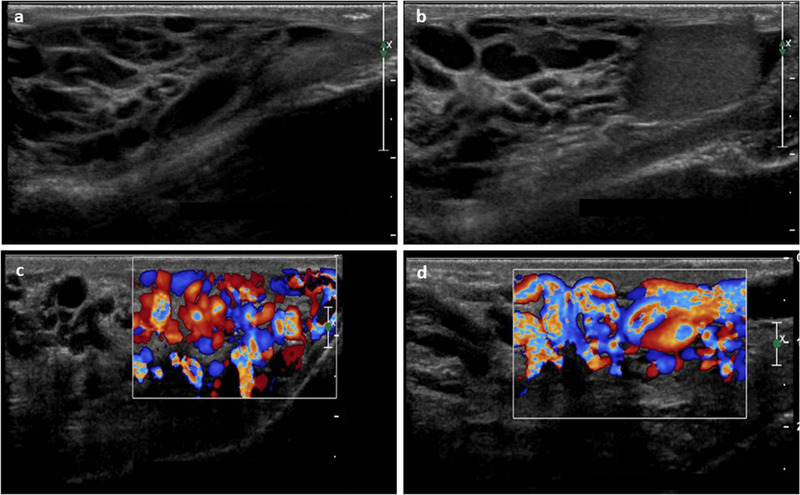
High‐frequency (12 MHz) linear probe ultrasound images of testicular varicocele. (A, B) Normal grayscale images, (C, D) color Doppler analysis
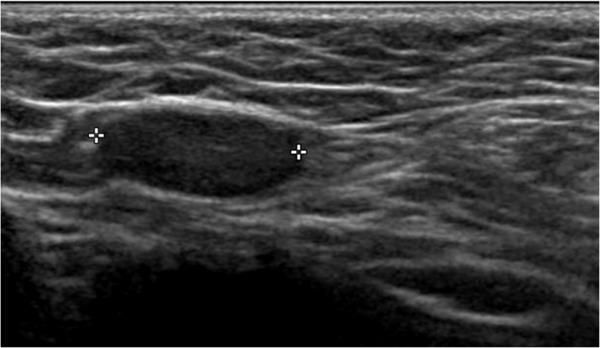
3.3.4. Epididymal cysts
A round, painless, anechoic cyst with acoustic posterior enhancement can be found in the head or body of the epididymis of pubertal boys. The cyst can be single or multiple, simple or present septa, and varies in size from small to >4 cm in diameter. 24 Usually, small, simple cysts do not require further interventions, unless associated with pain, inflammation, and concomitant epididymitis. Color Doppler examination is important in distinguishing the latter condition, which can require prompt medical intervention. 69
3.3.5. Testicular microlithiasis
Testicular microlithiasis is referred to as a punctate 1–3‐mm hyperechoic foci in the absence of acoustic shadowing, which reflects the presence of lamellated calcifications within the seminiferous tubules. It is often an incidental US finding and becomes significant when at least five of such foci are present in a single US scan 18 (Figure 11). The distribution can be variable, from limited hyperechoic clusters to a diffuse starry sky‐appearance of the testis. When associated with other risk factors, the discovery of testicular microlithiasis suggests the need for a periodic US monitoring of the testes, given its association with some forms of testicular germ cell neoplasia in situ. 70
FIGURE 11.

High‐frequency (12 MHz) ultrasound images of testicular microlithiasis. (A, B) Infant patients, (C) a pubertal boy with a classic “starry sky” appearance
3.3.6. Pubertal disorders
Progression of puberty through G1–G5 stages of development can be monitored with the aid of scrotal US. 71 The increase in testicular volume (>4 mL), acquisition of a medium echogenicity, and modification of the echotexture of the testes, reflect germ cell and seminiferous tubule maturation. The vascularization of the organ changes in pre‐pubertal stages from a peripheral pattern, observed in neonates and infants, to an increase in blood inflow from the capsular artery beneath the tunica albuginea, with the identification of pulsatile vascular spots referred to as intratesticular arteries. The increase in blood supply, visualized as higher peaked systolic velocities, and growth in vascular bed architecture inside the testes, leads to changes in volume and echogenicity and is thought to be the first hormonal‐driven step of puberty. 72 Discrepancies among chronological age, testicular volumetric increase, US modifications, and acquisition of other secondary sexual characteristics represent important issues for the andrologist in the study of boys with precocious or delayed puberty.
3.3.7. Oncohematological surveillance
Testicular US evaluation is also recommended in patients with a medical history of oncohematological malignancies (e.g., acute lymphocytic leukemia and chronic lymphocytic leukemia), given the possibility of testicular relapses during clinical remission. 73 As discussed above, the testes remain a privileged site for pathological recurrence due to the blood–testis barrier, which allows escape from chemotherapeutic agents.
At the same time, scrotal US is also useful for the assessment of gonadal health status in adolescent patients with oncohematological diseases who received high doses of chemotherapeutic agents or autologous bone marrow transplant during childhood and monitoring the evolution of pubertal development.
4. CONCLUSION
The long life span from birth to the end of puberty represents a crucial phase, and the most striking changes occur during this phase. Using US to follow these changes and observe the physiological evolution of gonadal maturation is essential. Moreover, the US study of the main andrological diseases, typical of this period, provides important details on the nature and degree of danger of various diseases, in addition to being a guide in resolving differential diagnoses. Furthermore, scrotal US also allows us to reduce the need to resort to level II instrumental investigations, which are often expensive and not always readily available. Finally, inguinoscrotal US guides the clinician in the therapeutic management and evaluation of the effectiveness of medical and surgical therapies.
In conclusion, the present study clearly highlights the strategic importance of scrotal US, especially during the first 15–18 years of life, when it represents a really essential diagnostic aid for endocrinologists and andrologists in daily clinical practice.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design of the paper; acquisition, analysis, and interpretation of data; and drafting of the paper. They all approved the final version to be submitted and verified its accuracy and integrity.
Spaziani M, Lecis C, Tarantino C, Sbardella E, Pozza C, Gianfrilli D. The role of scrotal ultrasonography from infancy to puberty. Andrology. 2021;9:1306–1321. 10.1111/andr.13056
Equal contribution.
Funding information
This work was supported by Ministry of Research, MIUR Grant PRIN: 2017 2017TK7Z8L
REFERENCES
- 1. Becker M, Hesse V. Minipuberty: why does it happen? Horm Res Paediatr. 2020;93(2):76–84. [DOI] [PubMed] [Google Scholar]
- 2. Kuiri‐Hänninen T, Sankilampi U, Dunkel L. Activation of the hypothalamic‐pituitary‐gonadal axis in infancy: minipuberty. Horm Res Paediatr. 2014;82(2):73–80. [DOI] [PubMed] [Google Scholar]
- 3. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spaziani M, Tarantino C, Tahani N, et al. Hypothalamo‐Pituitary axis and puberty. Mol Cell Endocrinol. 2021;520:111094. [DOI] [PubMed] [Google Scholar]
- 5. Delaney LR, Karmazyn B. Ultrasound of the pediatric scrotum. Semin Ultrasound CT MR. 2013;34(3):248–256. 10.1053/j.sult.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 6. Karmazyn B. Scrotal ultrasound. Ultrasound Clin. 2010;5:61–74. [Google Scholar]
- 7. Kaplan SL, Grumbach MM. The ontogenesis of human foetal hormones. II. Luteinizing hormone (LH) and follicle stimulating hormone (FSH). Acta Endocrinol. 1976;81:808–829. [DOI] [PubMed] [Google Scholar]
- 8. Clements JA, Reyes FI, Winter JS, Faiman C. Studies on human sexual development. III. Foetal pituitary and serum, and amniotic fluid concentrations of LH, CG, and FSH. J Clin Endocrinol Metab. 1976;42:9–19. [DOI] [PubMed] [Google Scholar]
- 9. Guimiot F, Chevrier L, Dreux S, et al. Negative foetal FSH/LH regulation in late pregnancy is associated with declined kisspeptin/KISS1R expression in the tuberal hypothalamus. J Clin Endocrinol Metab. 2012;97:E2221–E2229. [DOI] [PubMed] [Google Scholar]
- 10. Kuijper EAM, Ket JCF, Caanen MR, Lambalk CB. Reproductive hormone concentrations in pregnancy and neonates: a systematic review. Reprod Biomed Online. 2013;27(1):33–63. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt H, Schwarz HP. Serum concentrations of LH and FSH in the healthy newborn. Eur J Endocrinol. 2000;143:213–215. [DOI] [PubMed] [Google Scholar]
- 12. Bergadá I, Milani C, Bedecarrás P, et al. Time course of the serum gonadotropin surge, inhibins, and anti‐Müllerian hormone in normal newborn males during the first month of life. J Clin Endocrinol Metab. 2006;91:4092–4098. [DOI] [PubMed] [Google Scholar]
- 13. Kuiri‐Hänninen T, Kallio S, Seuri R, et al. Postnatal developmental changes in the pituitaryovarian axis in preterm and term infant girls. J Clin Endocrinol Metab. 2011;96:3432–3439. [DOI] [PubMed] [Google Scholar]
- 14. Rey RA. Mini‐puberty and true puberty: differences in testicular function. Ann Endocrinol. 2014;75(2):58–63. [DOI] [PubMed] [Google Scholar]
- 15. Rodprasert W, Virtanen HE, Mäkelä JA, Toppari J. Hypogonadism and cryptorchidism. Front Endocrinol. 2020;10:906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ankarberg‐Lindgren C, Norjavaara E. Changes of diurnal rhythm and levels of total and free testosterone secretion from pre to late puberty in boys: testis size of 3 ml is a transition stage to puberty. Eur J Endocrinol. 2004;151(6):747–757. [DOI] [PubMed] [Google Scholar]
- 17. Rey RA, Musse M, Venara M, Chemes HE. Ontogeny of the androgen receptor expression in the fetal and postnatal testis: its relevance on Sertoli cell maturation and the onset of adult spermatogenesis. Microsc Res Tech. 2009;72:787–795. [DOI] [PubMed] [Google Scholar]
- 18. Alkhori NA, Barth RA. Pediatric scrotal ultrasound: review and update. Pediatr Radiol. 2017;47(9):1125–1133. [DOI] [PubMed] [Google Scholar]
- 19. Sameshima T, Yamanari MGI, Funari MBG. The challenging sonographic inguinal canal evaluation in neonates and children: an update of differential diagnoses. Pediatr Radiol. 2017;47:461–472. [DOI] [PubMed] [Google Scholar]
- 20. Ashley RA, Barthold JS, Kolon TF. Cryptorchidism: pathogenesis, diagnosis, treatment and prognosis. Urol Clin North Am. 2010;37(2):183–193. [DOI] [PubMed] [Google Scholar]
- 21. Ferguson L, Agoulnik AI. Testicular cancer and cryptorchidism. Front Endocrinol. 2013;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aso C, Enríquez G, Fité M, et al. Gray‐scale and color Doppler sonography of scrotal disorders in children: an update. Radiographics. 2005;25:1197–1214. [DOI] [PubMed] [Google Scholar]
- 23. Hörmann M, Balassy C, Philipp MO, Pumberger W. Imaging of the scrotum in children. Eur Radiol. 2004;14(6):974–983. [DOI] [PubMed] [Google Scholar]
- 24. Dogra VS, Gottlieb RH, Oka M, Rubens DJ. Sonography of the scrotum. Radiology. 2003;227(1):18–36. [DOI] [PubMed] [Google Scholar]
- 25. Patil V, Shetty SMC, Das S. Common and uncommon presentation of fluid within the scrotal spaces. Ultrasound Int Open. 2015;1(2):E34–E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koski ME, Makari JH, Adams MC, et al. Infant communicating hydroceles — do they need immediate repair or might some clinically resolve? J Pediatr Surg. 2010;45(3):590–593. [DOI] [PubMed] [Google Scholar]
- 27. Doudt DA, Kehoe JE, Ignacio RC, Christman MS. Abdominoscrotal hydrocele: a systematic review. J Pediatr Surg. 2016;51(9):1561–1564. [DOI] [PubMed] [Google Scholar]
- 28. Dagur G, Gandhi J, Suh Y, et al. Classifying hydroceles of the pelvis and groin: an overview of etiology, secondary complications, evaluation, and management. Curr Urol. 2017;10(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brandt ML. Pediatric hernias. Surg Clin North Am. 2008;88(1):27–43, vii–viii. [DOI] [PubMed] [Google Scholar]
- 30. Ein SH, Njere I, Ein A. Six thousand three hundred sixty‐one pediatric inguinal hernias: a 35‐year review. J Pediatr Surg. 2006;41(5):980–986. [DOI] [PubMed] [Google Scholar]
- 31. Lao BO, Fitzgibbons RJ Jr, Cusick RA. Pediatric inguinal hernias, hydroceles, and undescended testicles. Surg Clin North Am. 2012;92(3):487–504, vii. [DOI] [PubMed] [Google Scholar]
- 32. Hricak H, Lue T, Filly RA, Alpers CE, Zeineh SJ, Tanagho EA. Experimental study of the sonographic diagnosis of testicular torsion. J Ultrasound Med. 1983;2(8):349–356. [DOI] [PubMed] [Google Scholar]
- 33. Altinkilic B, Pilatz A, Weidner W. Detection of normal intratesticular perfusion using color coded duplex sonography obviates need for scrotal exploration in patients with suspected testicular torsion. J Urol. 2013;189(5):1853–1858. [DOI] [PubMed] [Google Scholar]
- 34. Avery LL, Scheinfeld MH. Imaging of penile and scrotal emergencies. Radiographics. 2013;33(3):721–740. [DOI] [PubMed] [Google Scholar]
- 35. Muttarak M, Lojanapiwat B. The painful scrotum: an ultrasonographical approach to diagnosis. Singapore Med J. 2005;46(7):352–357. [PubMed] [Google Scholar]
- 36. Prando D, Décio MD. Torsion of the spermatic cord: sonographic diagnosis. Ultrasound Q. 2002;18(1):41–57. [DOI] [PubMed] [Google Scholar]
- 37. Liang T, Metcalfe P, Sevcik W, Noga M. Retrospective review of diagnosis and treatment in children presenting to the pediatric department with acute scrotum. Am J Roentgenol. 2013;200(5):W444–W449. [DOI] [PubMed] [Google Scholar]
- 38. Lev M, Ramon J, Mor Y, Jacobson JM, Soudack M. Sonographic appearances of torsion of the appendix testis and appendix epididymis in children. J Clin Ultrasound. 2015;43(8):485–489. [DOI] [PubMed] [Google Scholar]
- 39. McConaghy JR, Panchal B. Epididymitis: an overview. Am Fam Physician. 2016;94:723–726. [PubMed] [Google Scholar]
- 40. Kaver I, Matzkin H, Braf ZF. Epididymo‐orchitis: a retrospective study of 121 patients. J Fam Pract. 1990;30:548–552. [PubMed] [Google Scholar]
- 41. Redshaw JD, Tran TL, Wallis MC, deVries CR. Epididymitis: a 21‐year retrospective review of presentations to an outpatient urology clinic. J Urol. 2014;192:1203–1207. [DOI] [PubMed] [Google Scholar]
- 42. Siegel MJ. The acute scrotum. Radiol Clin North Am. 1997;35:959–976. [PubMed] [Google Scholar]
- 43. Wittenberg AF, Tobias T, Rzeszotarski M, Minotti AJ. Sonography of the acute scrotum: the four T's of testicular imaging. Curr Probl Diagn Radiol. 2006;35:12–21. [DOI] [PubMed] [Google Scholar]
- 44. Gorman B, Caroll BA. The scrotum. In: Rumack CM, Wilson SR, Charboneau JW, eds. Diagnostic Ultrasound. 3rd ed. St Louis, MO: Elsevier Mosby; 2005:849–888. [Google Scholar]
- 45. Jee WH, Choe BY, Byun JY, Shinn KS, Hwang TK. Resistive index of the intrascrotal artery in scrotal inflammatory disease. Acta Radiol. 1997;38:1026–1030. [DOI] [PubMed] [Google Scholar]
- 46. Steliarova‐Foucher E, Colombet M, Ries LAG, et al. International incidence of childhood cancer, 2001–10: a population‐based registry study. Lancet Oncol. 2017;18(6):719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pohl HG, Shukla AR, Metcalf PD, et al. Prepubertal testis tumors: actual prevalence rate of histological types. J Urol. 2004;172(6 Pt 1):2370–2372. [DOI] [PubMed] [Google Scholar]
- 48. Caballero Mora FJ, Muñoz Calvo MT, García Ros M, et al. Testicular and paratesticular tumors during childhood and adolescence. An Pediatr (Barc). 2013;78:6–13. [DOI] [PubMed] [Google Scholar]
- 49. Tallen G, Hernáiz Driever P, Degenhardt P, Henze G, Riebel T. High reliability of scrotal ultrasonography in the management of childhood primary testicular neoplasms. Klin Padiatr. 2011;223(3):131–137. [DOI] [PubMed] [Google Scholar]
- 50. Woodward PJ, Sohaey R, O'Donoghue MJ, Green D. From the archives of the AFIP: tumors and tumorlike lesions of the testis: radiologic pathologic correlation. Radiographics. 2002;22:189–216. [DOI] [PubMed] [Google Scholar]
- 51. Williamson SR, Delahunt B, Magi‐Galluzzi C, Algaba F, Egevad L, Ulbright TM, et al. The World Health Organization 2016 classification of testicular germ cell tumours: a review and update from the International Society of Urological Pathology Testis Consultation Panel. Histopathology. 2017;70:335–346. [DOI] [PubMed] [Google Scholar]
- 52. Song Q‐D. Ultrasound appearances of pediatric testicular yolk sac tumors: twenty‐one cases in a single institution. J Ultrasound Med. 2018;37:2457–2463. [DOI] [PubMed] [Google Scholar]
- 53. Sangüesa C, Veiga D, Llavador M, Serrano A. Testicular tumours in children: an approach to diagnosis and management with pathologic correlation. Insights Imaging. 2020;11(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fujino J, Yamamoto H, Kisaki Y, et al. Epidermoid cyst: rare testicular tumor in children. Pediatr Radiol. 2004;34:172–174. [DOI] [PubMed] [Google Scholar]
- 55. Taskinen S, Fagerholm R, Aronniemi J, Rintala R, Taskinen M. Testicular tumors in children and adolescents. J Pediatr Urol. 2008;4(2):134–137. [DOI] [PubMed] [Google Scholar]
- 56. Dogra VS, Gottlieb RH, Rubens DJ, Liao L. Benign intratesticular cystic lesions: US features. Radiographics. 2001;21:S273–S281. [DOI] [PubMed] [Google Scholar]
- 57. Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs‐part A: renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93–105. [DOI] [PubMed] [Google Scholar]
- 58. Ocal O, Baydar D, Idilman I, Serkan H, Tekgul S, Ozmen M. Sonographic diagnosis of large‐cell calcifying Sertoli cell tumor. J Ultrason. 2019;19:161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tyloch JF, Wieczorek AP. Standards for scrotal ultrasonography. J Ultrason. 2016;16(67):391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pozza C, Kanakis G, Carlomagno F, et al. Testicular ultrasound score: a new proposal for a scoring system to predict testicular function. Andrology. 2020;8(5):1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Isidori A, Lenzi A. Ultrasound of the Testis for the Andrologist. Cham, Switzerland: Springer International Publishing; 2017. [Google Scholar]
- 62. Spaziani M, Granato S, Liberati N, et al. From mini‐puberty to pre‐puberty: early impairment of the hypothalamus‐pituitary‐gonadal axis with normal testicular function in children with non‐mosaic Klinefelter syndrome. J Endocrinol Invest. 2021;44(1):127–138. [DOI] [PubMed] [Google Scholar]
- 63. Spaziani M, Radicioni AF. Metabolic and cardiovascular risk factors in Klinefelter syndrome. Am J Med Genet C Semin Med Genet. 2020;184(2):334–343. [DOI] [PubMed] [Google Scholar]
- 64. Rocher L, Moya L, Correas JM, et al. Testis ultrasound in Klinefelter syndrome infertile men: making the diagnosis and avoiding inappropriate management. Abdom Radiol (NY). 2016;41(8):1596–1603. [DOI] [PubMed] [Google Scholar]
- 65. Pozza C, Pofi R, Tenuta M, et al. Clinical presentation, management and follow‐up of 83 patients with Leydig cell tumors of the testis: a prospective case‐cohort study. Hum Reprod. 2019;34(8):1389–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shiraishi K, Matsuyama H. Klinefelter syndrome: from pediatrics to geriatrics. Reprod Med Biol. 2018;18(2):140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bertolotto M, Freeman S, Richenberg J, et al. Ultrasound evaluation of varicoceles: systematic literature review and rationale of the ESUR‐SPIWG guidelines and recommendations. J Ultrasound. 2020;23(4):487–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Światłowski Ł, Pyra K, Kuczyńska M, et al. Selecting patients for embolization of varicoceles based on ultrasonography. J Ultrason. 2018;18(73):90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sansone A, Fegatelli DA, Pozza C, et al. Effects of percutaneous varicocele repair on testicular volume: results from a 12‐month follow‐up. Asian J Androl. 2019;21(4):408–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mes sina M, Fusi G, Ferrara F, et al. A rare cause of acute scrotum in a child: torsion of an epididymal cyst. Case report and review of the literature. Pediatr Med Chir. 2019;41(1).22–23. [DOI] [PubMed] [Google Scholar]
- 71. Barbonetti A, Martorella A, Minaldi E, et al. Testicular cancer in infertile men with and without testicular microlithiasis: a systematic review and meta‐analysis of case‐control studies. Front Endocrinol. 2019;10:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. De Sanctis V, Soliman AT, Di Maio S, Millimaggi G, Kattamis C. For debate: testicular volume development along ages: evaluation by different methods. Pediatr Endocrinol Rev. 2019;16(4):421–430. [DOI] [PubMed] [Google Scholar]
- 73. Isido ri AM, Lenzi A. Scrotal Ultrasound: Morphological and Functional Atlas. Forum Service Editore s.r.l., via Martin Piaggio 17/6, 16122 Genova, Italy: Accademia Nazionale di Medicina; 2008. [Google Scholar]
- 74. Gaynon PS, Qu RP, Chappell RJ, et al. Survival after relapse in childhood acute lymphoblastic leukemia: impact of site and time to first relapse–the Children's Cancer Group Experience. Cancer. 1998;82(7):1387–1395. [DOI] [PubMed] [Google Scholar]


