Abstract
Here we report a palladium‐catalysed difunctionalisation of unsaturated C−C bonds with acid chlorides. Formally, the C−COCl bond of an acid chloride is cleaved and added, with complete atom economy, across either strained alkenes or a tethered alkyne to generate new acid chlorides. The transformation does not require exogenous carbon monoxide, operates under mild conditions, shows a good functional group tolerance, and gives the isolated products with excellent stereoselectivity. The intermolecular reaction tolerates both aryl‐ and alkenyl‐substituted acid chlorides and is successful when carboxylic acids are transformed to the acid chloride in situ. The reaction also shows an example of temperature‐dependent stereodivergence which, together with plausible mechanistic pathways, is investigated by DFT calculations. Moreover, we show that benzofurans can be formed in an intramolecular variant of the reaction. Finally, derivatisation of the products from the intermolecular reaction provides a highly stereoselective approach for the synthesis of tetrasubstituted cyclopentanes.
Keywords: Acid chlorides, Carbonylation, CO-free, Difunctionalisation, Palladium
Using a catalytic system comprised of Pd and Xantphos, acid chlorides can be added across strained alkenes or tethered alkynes to form two new C−C bonds via formal C−COCl cleavage. This reaction allows for a dicarbofunctionalisation in which an acid chloride group is retained in the product. DFT studies are used to rationalise a plausible pathway, while product derivatisation highlights the synthetic utility of the method.
Introduction
The concept of atom economy is a cornerstone of sustainable organic synthesis. [1] Yet, achieving this goal in synthetic transformations which generate multiple C−C or C−X bonds, and therefore drastically increase molecular complexity, is challenging. Given their unique reactivity, transition metals can mediate remarkable transformations that can form new bonds in a highly selective manner. [2] One area of research which has attracted significant attention in catalysis over recent decades is hydrofunctionalisation (Scheme 1 A). [3] These reactions have been developed into transformations which are often completely atom economic and can be performed with excellent control of selectivity. However, these reactions are limited by the addition of one functional group across the unsaturated C−C bond.
Scheme 1.
Context of the research.
By contrast, difunctionalisation reactions, including dicarbofunctionalisations, rapidly increase molecular complexity (Scheme 1 B).[ 4 , 5 ] This has emerged as a creative platform for the installation of two new C−C or C−X bonds across an unsaturated bond in a single step to generate a diverse array of compounds. [6] While this line of research has resulted in several novel transformations, they commonly rely on reactions which take a “single‐use” approach to synthetic handles. For example, a reaction can add an electrophile, for example, an aryl halide, across an alkene or alkyne with a nucleophilic partner, such as an aryl boronic ester, in the presence of a metal catalyst. This delivers the difunctionalised product, but the synthetic handle, for example, the halide or boronic ester, is not retained in the final product. As such, the overall atom economy is reduced and the synthetic handle is lost after a single transformation.
In pursuit of realising new atom economical transformations, we considered if a coupling partner, an electrophile or nucleophile, itself could be added across an alkene or alkyne (Scheme 1 C). Overall, the synthetic handle would be retained while building molecular complexity.
Previous work that has focused on retaining the synthetic handle while forming two new C−C bonds across alkenes or alkynes is limited, highlighting the demanding nature of such transformations.[ 7 , 8 , 9 , 10 , 11 ] So far, research has focused on cleaving C−C(acyl) bonds. For example, a directing group can be used with a metal catalyst to cleave the C−C(acyl) bond of diaryl ketones. An alkene can then undergo migratory insertion into the product of oxidative addition to give the difunctionalised product (Scheme 1 D). [11] Related work by Hiyama, Nakao, and co‐workers demonstrated the nickel‐catalysed arylcyanation of unsaturated C−C bonds. They showed that an organonitrile such as benzonitrile could be used, via activation of the C−CN bond, for the difunctionalisation of alkenes (Scheme 1 E). [7c]
We considered whether acid chlorides could be utilised in a similar manner to achieve a carbochlorocarbonylation reaction. Previously, Tsuji,[ 12a , 12b ] Nomura, [12c] and Tanaka[ 12d , 12e ] demonstrated that acid chlorides can react with terminal alkynes in the presence of an iridium or rhodium catalyst for acyl chlorination or decarbonylative carbochlorination (Scheme 1 F). While this results in a difunctionalisation reaction, the acid chloride synthetic handle is lost, and, in addition, only one new C−C bond is formed.
Recently, our group reported a carboformylation reaction where an acid chloride served as both the carbon unit and carbon monoxide source (Scheme 1 G). [13a] Formally, this allowed for addition of the C−CO bond of an acid chloride across an alkyne. Given this precedent, we questioned whether it would be possible to add both the carbon unit and an acyl chloride unit of an acid chloride across an unsaturated C−C bond. The acid chloride would enable the transformation to occur and be conserved in the product, generating new acid chlorides which are highly reactive electrophiles. [14] Moreover, this precludes the requirement to handle carbon monoxide, a toxic gas requiring specialised equipment on a laboratory scale.[ 15 , 16 ]
A key challenge to realise this transformation is finding a catalyst system capable of both the selective and sequential elementary steps over undesired pathways.[ 12 , 17 , 18 ] Here, we present the realisation of this concept, in which the C−COCl bond of an acid chloride is formally cleaved and added across either a strained alkene or a tethered alkyne to generate the difunctionalised products (Scheme 1 H). Notably, the retention of the highly electrophilic acid chloride moiety allows a wide variety of synthetic modifications of the product.[ 14 , 16 , 19 ]
Results and Discussion
Intermolecular Reaction
We initiated our studies using Pd2dba3 in combination with 4‐methylbenzoyl chloride (1) and norbornadiene (NBD, 2) (Table 1). Here, the release of strain energy would provide a thermodynamic driving force to favour migratory insertion under mild conditions. [20] As sterically bulky ligands favour reductive elimination by relieving steric crowding of the metal centre,[ 16 , 17 , 21 ] we tested a selection of ligands which have been used for similarly challenging reductive eliminations.[ 16 , 22 ] We found that Xantphos provided the acid chloride product 3 in 88 % yield (Table 1, Entry 1). Other ligands, including electron‐poor phosphines, gave little, if any, of the desired product (Table 1, Entries 2–5). Whilst an alternative palladium source, [Pd(allyl)Cl]2, only gave 3 % yield of the product even at elevated temperature (Table 1, Entry 6), a reduced loading of Pd2dba3 could be used, albeit with a slightly reduced yield of 72 % (Table 1, Entry 7). Notably, a 1:1 ratio of the two starting materials reacted to give the product in 83 % yield (Table 1, Entry 8). The palladium catalyst is essential for the reaction to proceed as reactions in the absence of palladium (Table 1, Entry 9) or in the presence of other metal sources (Table 1, Entry 10) failed to form the desired product. The stereochemistry of the product was determined by NOESY NMR analysis and by X‐ray crystallography of derivatives of the acid chloride product. It was found that the substituents undergo syn addition to the alkene, and both are exo relative to the bicycle (see SI). [7c]
Table 1.
Optimisation.

|
Entry |
Deviations from standard conditions |
Yield of 3 [%][a,b] |
|---|---|---|
|
1 |
none |
88 |
|
2 |
tBuXantPhos |
0 |
|
3 |
P(tBu)3 |
0 |
|
4 |
DPEPhos |
1 |
|
5 |
dAr F pe |
0 |
|
6[c] |
[Pd(allyl)Cl]2 |
3 |
|
7[d] |
Pd2dba3 (2.5 mol %) |
72 |
|
8 |
NBD (2) (1.0 equiv) |
83 |
|
9 |
Without [Pd] |
0 |
|
10[e] |
[Ni], [Rh], [Ir] |
0 |
|
| ||
[a] Reactions on a 0.25 mmol scale in 1 mL of toluene. [b] The GC yields are based on moles of 1 versus n‐dodecane. The products are derivatised to the corresponding methyl esters. [c] 100 °C. [d] 5.0 mol % Xantphos. [e] See SI for details.
With the optimised conditions in hand, we explored the scope of the reaction (Scheme 2). 4‐Methylbenzoyl chloride and benzoyl chloride were successful in the reaction giving the products 3 and 4 in 80 % and 85 % yields, respectively. The reaction of a 13C‐labelled benzoyl chloride also gave the product 5 in 85 % yield with excellent 13C incorporation (>95 %), highlighting the potential of the present chemistry to be used as a platform for isotopic labelling. 2‐Naphthoyl chloride reacted to give the product 6 in 83 % yield while the more sterically encumbered 2‐methylbenzoyl chloride formed the product 7 in 50 % yield at an elevated temperature of 80 °C. We observed that 4‐fluoro‐, 4‐chloro‐, and 4‐bromobenzoyl chloride successfully reacted to give the products 8, 9, 10 in 63 %, 66 %, and 65 % yields, respectively, while 3‐Bromobenzoyl chloride gave the product 11 in 24 % yield. Notably, these functional groups offer parallel reactivity to the acid chloride, demonstrating the chemoselectivity of this reaction. Acid chlorides containing benzothiophene, benzofuran, ether, or azo functional groups gave the products 12, 13, 14, and 15 in good yields of 71 %, 75 %, 68 %, and 54 %, respectively. When a benzoyl chloride bearing an electron‐withdrawing cyanide group was used, we found that an increase of the temperature to 80 °C was required to obtain the product 16 in a synthetically useful yield of 69 %. In this instance, the product was isolated with a 93:7 ratio of cis exo:trans endo isomers. Other very electron poor acid chlorides such as 4‐nitrobenzoyl chloride and pentafluorobenzoyl chloride did not afford the desired product. Excitingly, we could show that benzoic acids could be used directly with Ghosez's reagent [13b] to generate the acid chloride in situ, as demonstrated by the reaction of 4‐(trimethylsilyl ethynyl) benzoic acid which provided the product 17 in a very good yield of 82 %. An acid chloride containing a boronic ester gave the product 18 in 54 % yield, highlighting the tolerance of an orthogonal functional handle to the acid chloride. We were also able to expand the acid chloride scope beyond that of aromatic systems to α,β‐unsaturated acid chlorides as demonstrated with 3,3‐dimethyl acryloyl chloride and trans‐4‐phenylcinnamic acid, with Ghosez's reagent, which gave the products 19 and 20 in 57 % and 27 % yield, respectively. Further, two reactions were performed on a 10 mmol scale, showing that the reaction is insensitive to scale. Trapping with methanol gave the product 3 a in 83 % yield (2.0 g). A second run trapping with 2,6‐diisopropyl aniline also gave the product 3 b in 83 % yield (3.2 g). [17f] While only 2 different nucleophiles are shown here, our previous work has highlighted the synthetic versatility of acid chlorides. [16]
Scheme 2.
Scope of the reaction with different acid chlorides. All products were isolated as the corresponding methyl esters unless noted otherwise. See SI for details. [a] All isolated products gave a>95:5 cis exo:trans endo selectivity unless noted otherwise. [b] 80 °C for 21 h. [c] 21 h. [d] 93:7 cis exo:trans endo ratio of isomers. [e] The acid chloride was generated in situ from the corresponding acid with Ghosez's reagent (1 equiv). [f] Reactions performed on a 10 mmol scale.
Beyond NBD, we could successfully react norbornene (NBE, 21) at 80 °C to obtain the product with the same selectivity observed for NBD (2), (cis,exo)‐22 in 53 % yield. Notably, by increasing the reaction temperature to 100 °C we could obtain the product (trans,endo)‐22 in 67 % yield, providing an example of temperature‐dependent stereodivergence. [23]
Computational Studies
In order to study the feasibility of different mechanistic pathways and gain an understanding of the temperature‐dependent stereodivergence, we performed a computational analysis of the transformations.
Conformer searches were conducted with CREST using default settings. [24] The obtained conformers were subjected to additional density functional theory (DFT) calculations, using the Gaussian09 suite of programs. [25] The keyword integral(grid=ultrafine) was used in all calculations to limit grid‐based errors. [26] Optimisations were conducted at the PBE0 level of theory[ 27 , 28 , 29 , 30 ] including Grimme's dispersion correction (D3) with Becke–Johnson damping[ 31 , 32 ] and thermal correction to 333 K. Palladium was modelled with the def2tzvp basis set and ECP.[ 33 , 34 ] All other atoms were modelled with the def2svp basis set. Thermochemical corrections were calculated at the same level of theory as the optimisations, and quasi‐harmonic vibrational corrections [35] were applied using GoodVibes. [36] Single point energies were calculated at the PBE0D3‐(BJ) level of theory including the SMD solvent model [37] for toluene. All atoms were modelled with the def2tzvp basis set. The corresponding ECP was used for palladium. Structures were visualised with CYLview.[ 38 , 39 ]
The reaction of NBD (2) and benzoyl chloride was selected as a model system (Figure 1) to evaluate different plausible mechanistic pathways. Based on previous results by our group,[ 13 , 16 ] it is likely that the reaction is initiated by the molecular deconstruction of benzoyl chloride to form a palladium phenyl species. This process proceeds through a low barrier oxidative addition of palladium species A into the C−Cl bond of benzoyl chloride (TS1) and a subsequent decarbonylation step (TS2), delivering the palladium phenyl complex D.
Figure 1.
Gibbs free energy profile for the palladium‐catalysed formation of acyl chloride 4 from benzoyl chloride and NBD (2) at the PBE0‐D3(BJ)/SMD(toluene)‐def2tzvp//PBE0‐D3(BJ)/def2svp‐def2tzvp(Pd) level of theory. The optimised geometries of the transition states TS3 and TS4‐exo are depicted. Hydrogen atoms are omitted for clarity.
We examined different pathways for the carbopalladation of NBD (2, see Figure S13 for full overview). One possibility is that NBD (2) could directly insert into the Pd−Ph bond of the carbonyl‐containing complex D. The lowest energy transition state we found for this process (TS3) features an elongated Pd−Cl bond distance of 3.0 Å and is therefore best described as a contact‐ion pair rather than a neutral structure with a bound chlorido ligand.[ 40 , 41 , 42 ] The dissociation of the chlorido ligand is presumably due to the coordinative saturation of the palladium centre. This ion pair formation is associated with a large energy penalty in the non‐polar toluene solvent, resulting in a very high energy barrier of 38.9 kcal mol−1 for TS3. This pathway is therefore unlikely to operate under the experimental conditions. The most favourable pathway involves carbon monoxide dissociation from the carbonyl‐containing complex D to form the tetracoordinated complex E from which the migratory insertion of NBD (2) can take place via transition state TS4‐exo. Notably, the computational model correctly predicts the experimentally observed high exo selectivity (TS4‐exo: 25.9 kcal mol−1; TS4‐endo: 29.6 kcal mol−1).
The migratory insertion process of NBD (2) to generate the substituted NBE fragment in complex G is exergonic by 29.2 kcal mol−1 and the main driving force of the reaction. Interestingly, the thermodynamics of this step is only partially accounted for by the release of ring strain as the difference in ring strain energy between NBD (2) and NBE (21) is only 10.7 kcal mol−1. [43] Re‐coordination of the second phosphine moiety of the Xantphos ligand from F, the structure preceding the insertion event, to G and favourable interactions of the palladium centre with the hydrocarbyl fragment of complex G are likely to also play a significant role.
Complex G can recapture CO in a nearly thermoneutral fashion (complex H). Carbonylation of the norbornyl fragment (TS5) and subsequent reductive elimination (TS6) complete the formation of the acyl chloride product 4.
During the experimental part of this work, it was found that NBE (21) did not react at 60 °C, but required a reaction temperature of 80 °C to form the corresponding product. To probe the origin of this observation, we also calculated the proposed reaction mechanism with NBE (21) as the substrate (see Figure S14). Overall, the energy profiles are very similar, with the exception of the migratory insertion of the bicyclic alkene into the Pd−Ph bond of complex E. A comparison between the reactions of NBE (21) and NBD (2) shows that this step has a higher energy barrier (NBE: 27.3 kcal mol−1; NBD: 25.9 kcal mol−1) for the reaction of NBE (21). This higher reaction barrier for NBE (21) compared to NBD (2) is likely the reason why the reaction of NBE (21) requires a higher temperature.
A possible explanation for the observed temperature‐dependent stereodivergence could be that β‐hydride elimination occurs from acyl complex I‐NBE through transition state TS7 (Figure 2). [44] The resulting palladium hydride species K can reinsert into the formed ketene 24 from the other face of the molecule, leading to isomerisation at this position. The energy barrier of 33.1 kcal mol−1 for the β‐hydride elimination process is too high for this to operate to a significant extent at 80 °C. However, this step becomes kinetically accessible at elevated temperature. Through this pathway, the kinetically formed (cis,exo)‐product 23 could equilibrate to the thermodynamically favoured (trans,endo)‐product 23 (−2.9 kcal mol−1 relative to (cis,exo)‐23).
Figure 2.
DFT rationalisation for the observed temperature‐dependent stereodivergence.
Intramolecular Reaction
Further investigation of the reaction led to the discovery that it can be successful in an intramolecular setting (Scheme 3).[ 8a , 8b , 8e ] Tethered alkynes could successfully react with the acid chlorides to generate benzofuran products with retention of the acid chloride moiety. Again, a palladium catalyst was used in combination with Xantphos at 125 °C to deliver the product. In this instance, the double bond migrated into the ring which can be rationalised through the thermodynamic preference to form an extended aromatic system. The tethered alkynes reacted to generate benzofuran products 25, 26, 27, and 28 in 43 %, 75 %, 56 % and 41 % yield, respectively. The reaction showed no significant difference in reactivity between electron‐donating and electron‐withdrawing groups at the 4‐position relative to the acid chloride.
Scheme 3.
The intramolecular addition of acid chlorides across alkynes. The products are isolated as the corresponding methyl ester.
Norbornene Product Derivatisation
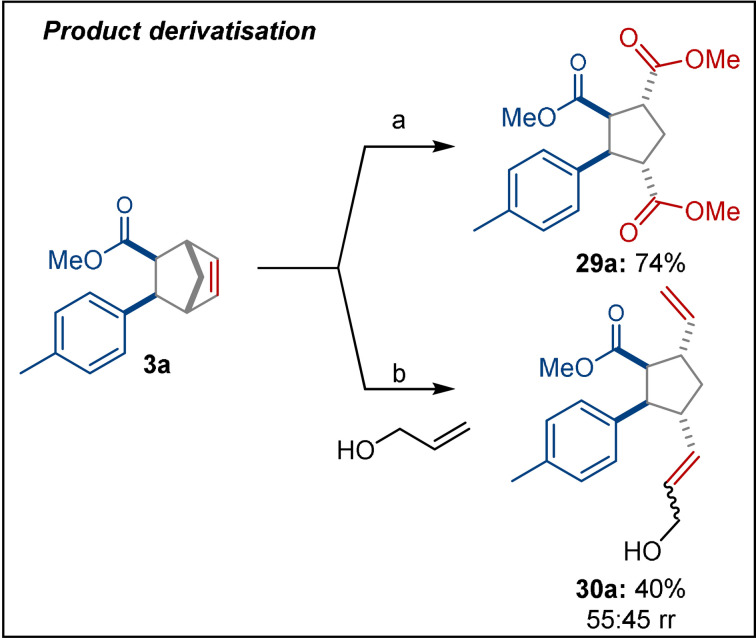
To highlight the utility of the products from the intermolecular reaction, we transformed them by two different ring‐opening reactions to stereoselectively obtain the corresponding 1,2,3,4‐tetrasubstituted cyclopentanes. Notably, the tolerance of alkenes as well as the orthogonal selectivity compared to related approaches such as the Diels–Alder reaction, [45] makes this a useful entry for their synthesis. First, we performed an oxidative cleavage of the alkene 3 a, followed by an esterification to form 29 a in 74 % yield (Scheme 4). [7c] Next, we subjected 3 a to ring‐opening cross‐metathesis with allyl alcohol to afford 30 a as a separable mixture of regioisomers (55:45 rr) with a combined yield of 40 %, without optimisation. [46]
Scheme 4.
Derivatisation of the products from the intermolecular reaction. Abbreviated reaction conditions are given, see SI for full details. a) RuCl3⋅n H2O (5 mol %), NaIO4 (4.5 equiv), then trimethylsilyl diazomethane (2.5 equiv). b) Hoveyda–Grubbs 2nd generation catalyst (5 mol %), allyl alcohol (10 equiv). rr=regiomeric ratio.
Conclusion
We have developed a reaction which uses a palladium catalyst to add an acid chloride across a strained unsaturated C−C bond. This reaction uses the acid chloride as the limiting reagent to generate substituted norbornenes decorated with aryl or alkenyl carbon units, and an acid chloride. The reaction showed a good functional group tolerance, and with NBE we observed a temperature‐dependent stereodivergence. A DFT study provides support for a plausible reaction pathway as well as a rationale, consistent with the observed experimental results, for the reactivity and selectivity of the intermolecular reaction with NBD and NBE. We also show that an intramolecular version of this reaction is possible, generating substituted benzofurans. Further, the opportunity, after derivatisation, to stereoselectively access 1,2,3,4‐tetrasubtituted cyclopentanes from the products of the intermolecular reaction will streamline synthesis of these important synthetic cores.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
The financial support by the European Research Council under the European Union's Horizon 2020 research and innovation program (Shuttle Cat, Project ID: 757608), the ETH Zürich, the Max‐Planck‐Society, the MPI für Kohlenforschung, LG Chem (fellowship to Y.H.L.), the FCI (scholarships to P.B. and S.R.) and both the Cusanuswerk and Swiss–European Mobility Programme (scholarships to M.F.) are gratefully acknowledged. We thank the NMR, MS (MoBiAS), and X‐ray (SMoCC) service departments at ETH Zürich for technical assistance. We acknowledge Laura Gürtler for reproducing results. We are also grateful to our group for critical proofreading of the manuscript. Open access funding provided by Eidgenossische Technische Hochschule Zurich.
E. H. Denton, Y. H. Lee, S. Roediger, P. Boehm, M. Fellert, B. Morandi, Angew. Chem. Int. Ed. 2021, 60, 23435.
In memory of Ei‐ichi Negishi
A previous version of this manuscript has been deposited on a preprint server (https://chemrxiv.org/engage/api‐gateway/chemrxiv/assets/orp/resource/item/60f1599fe0c60f6feee11ac3/original/catalytic‐carbochlorocarbonylation‐of‐unsaturated‐hydrocarbons‐via‐c‐co‐cl‐bond‐cleavage.pdf).
References
- 1.
- 1a. Trost B. M., Li C.-J., Modern Alkyne Chemistry: Catalytic and Atom-Economic Transformations, Wiley-VCH, Weinheim, 2015; [Google Scholar]
- 1b. Trost B. M., Acc. Chem. Res. 2002, 35, 695–705; [DOI] [PubMed] [Google Scholar]
- 1c. Trost B. M., Angew. Chem. Int. Ed. Engl. 1995, 34, 259–281; [Google Scholar]; Angew. Chem. 1995, 107, 285–307; [Google Scholar]
- 1d. Dunn P. J., Chem. Soc. Rev. 2012, 41, 1452–1461; [DOI] [PubMed] [Google Scholar]
- 1e. Anastas P., Eghbali N., Chem. Soc. Rev. 2010, 39, 301–312. [DOI] [PubMed] [Google Scholar]
- 2. Transition Metals for Organic Synthesis: Building Blocks and Fine Chemicals, Vol. 1 (Eds.: Beller M., Bolm C.), Wiley-VCH, Weinheim, 2004. [Google Scholar]
- 3. Ananikov V. P. in Hydrofunctionalisation; Topics in Organometallic Chemistry, Springer, Berlin, Heidelberg, 2013. [Google Scholar]
- 4.
- 4a. Derosa J., Apolinar O., Kang T., Tran V. T., Engle K. M., Chem. Sci. 2020, 11, 4287–4296; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4b. Derosa J., Tran V. T., van der Puyl V. A., Engle K. M., Aldrichim. Acta 2018, 51, 21–32; [Google Scholar]
- 4c. Catellani M., Chiusoli G. P., Tetrahedron Lett. 1982, 23, 4517–4520; [Google Scholar]
- 4d. Kosugi M., Tamura H., Sano H., Magita T., Chem. Lett. 1987, 16, 193–194; [Google Scholar]
- 4e. Yamane M., Kubota Y., Naraksaka K., Bull. Chem. Soc. Jpn. 2005, 78, 331–340; [Google Scholar]
- 4f. Oguma K., Miura M., Satoh T., Nomura M., J. Organomet. Chem. 2002, 648, 297–301; [Google Scholar]
- 4g. Tatsumi K., Fujihara T., Terao J., Tsuji Y., Chem. Commun. 2014, 50, 8476–8479; [DOI] [PubMed] [Google Scholar]
- 4h. Zhao X., Tu H.-Y., Guo L., Zhu S., Qing F.-L., Chu L., Nat. Commun. 2018, 9, 3488–3494; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4i. Kadam A. A., Mertz T. L., Qian Y., Stanley L. M., ACS Catal. 2019, 9, 5651–5656; [Google Scholar]
- 4j. Xue F., Zhao J., Hor T. S., Hayashi T., J. Am. Chem. Soc. 2015, 137, 3189–3192; [DOI] [PubMed] [Google Scholar]
- 4k. Zhou C. X., Emrich D. E., Larock R. C., Org. Lett. 2003, 5, 1579–1582; [DOI] [PubMed] [Google Scholar]
- 4l. Zhou C. X., Larock R. C., Org. Lett. 2005, 7, 259–262; [DOI] [PubMed] [Google Scholar]
- 4m. Zhou C. X., Larock R. C., J. Org. Chem. 2005, 70, 3765–3777; [DOI] [PubMed] [Google Scholar]
- 4n. Lv W., Liu S., Chen Y., Wen S., Lan Y., Cheng G., ACS Catal. 2020, 10, 10516–10522; [Google Scholar]
- 4o. Giri R., KC S., J. Org. Chem. 2018, 83, 3013–3022; [DOI] [PubMed] [Google Scholar]
- 4p. Zhang J.-S., Liu L., Chen T., Han L.-B., Chem. Asian J. 2018, 13, 2277–2291; [DOI] [PubMed] [Google Scholar]
- 4q. Breitwieser K., Chen P., Organometallics 2021, 40, 776–782; [Google Scholar]
- 4r. Dhungana R. K., KC S., Basent P., Giri R., Chem. Rec. 2018, 18, 1314–1340; [DOI] [PubMed] [Google Scholar]
- 4s. Shibuya M., Matsuda M., Yamamoto Y., Chem. Eur. J. 2021, 27, 8822–8831. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. Namirembe S., Morken J. P., Chem. Soc. Rev. 2019, 48, 3464–3474; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5b. Lovinger G. J., Morken J. P., Eur. J. Org. Chem. 2020, 2362–2368; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5c. Jiang Z., Niu S.-L., Zeng Q., Ouyang Q., Chen Y.-C., Xiao Q., Angew. Chem. Int. Ed. 2021, 60, 297–303; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 301–307; [Google Scholar]
- 5d. Luo Y.-C., Xu C., Zhang X., Chin. J. Chem. 2020, 38, 1371–1394. [Google Scholar]
- 6.
- 6a. Burke M. D., Schreiber S. L., Angew. Chem. Int. Ed. 2004, 43, 46–58; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2004, 116, 48–60; [Google Scholar]
- 6b. Sun A. W., Lackner S., Stoltz B. M., Trends Chem. 2019, 1, 630–643; [Google Scholar]
- 6c. Gallaway W. R. J. D., Isidro-Llober A., Spring D. R., Nat. Commun. 2010, 1, 80. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a. Murakami M., Ishida N., J. Am. Chem. Soc. 2016, 138, 13759–13769; [DOI] [PubMed] [Google Scholar]
- 7b. Nakao Y., Chem. Rev. 2021, 121, 327–344; [DOI] [PubMed] [Google Scholar]
- 7c. Nakao Y., Yada A., Satoh J., Ebata S., Oda S., Hiyama T., Chem. Lett. 2006, 35, 790–791; [Google Scholar]
- 7d. Nakao Y., Bull. Chem. Soc. Jpn. 2012, 85, 731–745; [Google Scholar]
- 7e. Nakao Y., Oda S., Hiyama T., J. Am. Chem. Soc. 2004, 126, 13904–13905; [DOI] [PubMed] [Google Scholar]
- 7f. Nakao Y., Yukawa T., Hirata Y., Oda S., Satoh J., Hiyama T., J. Am. Chem. Soc. 2006, 128, 7116–7117; [DOI] [PubMed] [Google Scholar]
- 7g. Hirata Y., Yukawa T., Kashihara N., Nakao Y., Hiyama T., J. Am. Chem. Soc. 2009, 131, 10964–10973; [DOI] [PubMed] [Google Scholar]
- 7h. Nakao Y., Yada A., Ebata S., Hiyama T., J. Am. Chem. Soc. 2007, 129, 2428–2429; [DOI] [PubMed] [Google Scholar]
- 7i. Nakao Y., Yada A., Hiyama T., J. Am. Chem. Soc. 2010, 132, 10024–10026; [DOI] [PubMed] [Google Scholar]
- 7j. Nakao Y., Hirata Y., Tanaka M., Hiyama T., Angew. Chem. Int. Ed. 2008, 47, 385–387; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 391–393; [Google Scholar]
- 7k. Tobisu M., Chatani N., Chem. Soc. Rev. 2008, 37, 300–307; [DOI] [PubMed] [Google Scholar]
- 7l. Hirata Y., Yada A., Morita E., Nakao Y., Hiyama T., Ohashi M., Ogoshi S., J. Am. Chem. Soc. 2010, 132, 10070–10077; [DOI] [PubMed] [Google Scholar]
- 7m. Frost G. B., Serratore N. A., Ogilvie J. M., Douglas C. J., J. Org. Chem. 2017, 82, 3721–3726; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7n. Zhu B., Du G.-F., Ren H., Yan L.-K., Guan W., Su Z.-M., Organometallics 2017, 36, 4713–4720. [Google Scholar]
- 8.Selected examples of carbohalogenation:
- 8a. Marchese A. D., Larin E. M., Mirabi B., Lautens M., Acc. Chem. Res. 2020, 53, 1605–1619; [DOI] [PubMed] [Google Scholar]
- 8b. Marchese A. D., Adrianov T., Lautens M., Angew. Chem. Int. Ed. 2021, 60, 16750–16762; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 16888–16900; [Google Scholar]
- 8c. Liu H., Chen C., Wang L., Tong X., Org. Lett. 2011, 13, 5072–5075; [DOI] [PubMed] [Google Scholar]
- 8d. Petrone D. A., Ye J., Lautens M., Chem. Rev. 2016, 116, 8003–8104; [DOI] [PubMed] [Google Scholar]
- 8e. Le C. M., Hou X., Sperger T., Schoenenbeck F., Lautens M., Angew. Chem. Int. Ed. 2015, 54, 15897–15900; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 16127–16131; [Google Scholar]
- 8f. Lee Y. H., Morandi B., Angew. Chem. Int. Ed. 2019, 58, 6444–6448; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 6510–6515; [Google Scholar]
- 8g. Takahashi T., Kuroda D., Kumano T., Yoshida Y., Kurahashi T., Matsubara S., Chem. Commun. 2018, 54, 12750–12753. [DOI] [PubMed] [Google Scholar]
- 9.Selected examples of carbothiolation:
- 9a. Sugoh K., Kuniyasu H., Sugae T., Ohtaka A., Takai Y., Tanaka A., Machino C., Kambe N., Kurosawa H., J. Am. Chem. Soc. 2001, 123, 5108–5109; [DOI] [PubMed] [Google Scholar]
- 9b. Hua R., Takeda H., Onozawa S.-y., Abe Y., Tanaka M., Org. Lett. 2007, 9, 263–266; [DOI] [PubMed] [Google Scholar]
- 9c. Iwasaki M., Fujino D., Wada T., Kondoh A., Yorimitsu H., Oshima K., Chem. Asian J. 2011, 6, 3190–3194; [DOI] [PubMed] [Google Scholar]
- 9d. Hooper J. F., Chaplin A. B., González-Rodríguez C., Thompson A. L., Weller A. S., Willis M. C., J. Am. Chem. Soc. 2012, 134, 2906–2909; [DOI] [PubMed] [Google Scholar]
- 9e. Arisawa M., Tanii S., Yamada T., Yamaguchi M., Tetrahedron 2015, 71, 6449–6458; [Google Scholar]
- 9f. Sun F., Li M., He C., Wang B., Li B., Sui X., Gui Z., J. Am. Chem. Soc. 2016, 138, 7456–7459. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Rao B., Tang J., Zeng X., Org. Lett. 2016, 18, 1678–1681; [DOI] [PubMed] [Google Scholar]
- 10b. Dreis A. M., Douglas C. J., J. Am. Chem. Soc. 2009, 131, 412–413; [DOI] [PubMed] [Google Scholar]
- 10c. Rathbun C. M., Johnson J. B., J. Am. Chem. Soc. 2011, 133, 2031–2033; [DOI] [PubMed] [Google Scholar]
- 10d. Lutz J. P., Rathbun C. M., Stevenson S. M., Powell B. M., Boman T. S., Baxter C. E., Zona J. M., Johnson J. B., J. Am. Chem. Soc. 2012, 134, 715–722; [DOI] [PubMed] [Google Scholar]
- 10e. Rong Z.-Q., Lim H. N., Dong G., Angew. Chem. Int. Ed. 2018, 57, 475–479; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 484–488. [Google Scholar]
- 11. Wentzel M. T., Reddy V. J., Hyster T. K., Douglas C. J., Angew. Chem. Int. Ed. 2009, 48, 6121–6123; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 6237–6239. [Google Scholar]
- 12.
- 12a. Iwai T., Fujihara T., Terao J., Tsuji Y., J. Am. Chem. Soc. 2012, 134, 1268–1274; [DOI] [PubMed] [Google Scholar]
- 12b. Iwai T., Fujihara T., Terao J., Tsuji Y., J. Am. Chem. Soc. 2009, 131, 6668–6669; [DOI] [PubMed] [Google Scholar]
- 12c. Yasukawa T., Satoh T., Miura M., Nomura M., J. Am. Chem. Soc. 2002, 124, 12680–12681; [DOI] [PubMed] [Google Scholar]
- 12d. Hua R., Shimada S., Tanaka M., J. Am. Chem. Soc. 1998, 120, 12365–12366; [Google Scholar]
- 12e. Hua R., Onozawa S., Tanaka M., Chem. Eur. J. 2005, 11, 3621–3630. [DOI] [PubMed] [Google Scholar]
- 13.
- 13a. Lee Y. H., Denton E. H., Morandi B., Nat. Chem. 2021, 13, 123–130; [DOI] [PubMed] [Google Scholar]
- 13b. Lee Y. H., Denton E. H., Morandi B., J. Am. Chem. Soc. 2020, 142, 20948–20955 and references therein. [DOI] [PubMed] [Google Scholar]
- 14.
- 14a. Patai S., The Chemistry of Acyl Halides, Interscience, London, 1972; [Google Scholar]
- 14b. Dieter R. K., Tetrahedron 1999, 55, 4177–4236; [Google Scholar]
- 14c. Montalbetti C. A. G. N., Falque V., Tetrahedron 2005, 61, 10827–10852. [Google Scholar]
- 15.
- 15a. Wu L., Liu Q., Jackstell R., Beller M., Angew. Chem. Int. Ed. 2014, 53, 6310–6320; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 6426–6436; [Google Scholar]
- 15b. Friis S. D., Lindhardt A. T., Skrydstrup T., Acc. Chem. Res. 2016, 49, 594–605; [DOI] [PubMed] [Google Scholar]
- 15c. Hermange P., Lindhardt A. T., Taaning R. H., Bjerglund K., Lupp D., Skrydstrup T., J. Am. Chem. Soc. 2011, 133, 6061–6071; [DOI] [PubMed] [Google Scholar]
- 15d. Morimoto T., Kakiuchi K., Angew. Chem. Int. Ed. 2004, 43, 5580–5588; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2004, 116, 5698–5706; [Google Scholar]
- 15e. Wang X., Nakajima M., Serrano E., Martin R., J. Am. Chem. Soc. 2016, 138, 15531–15534. [DOI] [PubMed] [Google Scholar]
- 16.
- 16a. Fang X., Cacherat B., Morandi B., Nat. Chem. 2017, 9, 1105–1109; [DOI] [PubMed] [Google Scholar]
- 16b. Lee Y. H., Morandi B., Nat. Chem. 2018, 10, 1016–1022; [DOI] [PubMed] [Google Scholar]
- 16c. Boehm P., Roediger S., Bismuto A., Morandi B., Angew. Chem. Int. Ed. 2020, 59, 17887–17896; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 18043–18052. [Google Scholar]
- 17.
- 17a. Sugihara T., Satoh T., Miura M., Nomura M., Adv. Synth. Catal. 2004, 346, 1765–1772; [Google Scholar]
- 17b. Malapit C. A., Ichiishi N., Sanford M. S., Org. Lett. 2017, 19, 4142–4145; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17c. Kokubo K., Matsumasa K., Miura M., Nomura M., J. Org. Chem. 1996, 61, 6941–6946; [DOI] [PubMed] [Google Scholar]
- 17d. Schoenberg A., Heck R. F., J. Am. Chem. Soc. 1974, 96, 7761–7764; [Google Scholar]
- 17e. Della Cá N., Fontana M., Motti E., Catellani M., Acc. Chem. Res. 2016, 49, 1389–1400; [DOI] [PubMed] [Google Scholar]
- 17f. Wang J., Dong G., Chem. Rev. 2019, 119, 7478–7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.
- 18a. Coperet C., Sugihara T., Wu G., Shimoyama I., Negishi E., J. Am. Chem. Soc. 1995, 117, 3422–3431; [Google Scholar]
- 18b. Tour J. M., Negishi E., J. Am. Chem. Soc. 1985, 107, 8289–8291; [Google Scholar]
- 18c. Sugihara T., Coperet C., Owczarczyk Z., Harring L. S., Negishi E., J. Am. Chem. Soc. 1994, 116, 7923–7924; [Google Scholar]
- 18d. Tsuji J., Morikawa M., Kiji J., J. Am. Chem. Soc. 1964, 86, 4851–4853; [Google Scholar]
- 18e. Bénard N., Bonnet M. C., Lécolier S., Tkatchenko I., J. Chem. Soc. Chem. Commun. 1993, 1448–1450. [Google Scholar]
- 19.
- 19a. De La Higuera Macias M., Arndtsen B. A., J. Am. Chem. Soc. 2018, 140, 10140–10144; [DOI] [PubMed] [Google Scholar]
- 19b. Torres G. M., Liu Y., Arndtsen B. A., Science 2020, 368, 318–323; [DOI] [PubMed] [Google Scholar]
- 19c. Quesnel J. S., Arndtsen B. A., J. Am. Chem. Soc. 2013, 135, 16841–16844; [DOI] [PubMed] [Google Scholar]
- 19d. Quesnel J. S., Moncho S., Ylijoki K. E. I., Torres G. M., Nrothers E. N., Bengali A. A., Arndtsen B. A., Chem. Eur. J. 2016, 22, 15107–15118; [DOI] [PubMed] [Google Scholar]
- 19e. Quesnel J. S., Kayser L. V., Fabrikant, Arndtsen B. A., Chem. Eur. J. 2015, 21, 9550–9555. [DOI] [PubMed] [Google Scholar]
- 20.
- 20a. Li C.-S., Cheng C.-H., Liao F.-L., Wang S.-L., J. Chem. Soc. Chem. Commun. 1991, 710–712; [Google Scholar]
- 20b. Larock R. C., Hershberger S. S., Takagi K., Mitchel M. A., J. Org. Chem. 1986, 51, 2450–2457. [Google Scholar]
- 21. Hartwig J. F., Organotransition Metal Chemistry, from Bonding to Catalysis, University Science Books, New York, 2009. [Google Scholar]
- 22.
- 22a. Petrone D. A., Lischka M., Lautens M. L., Angew. Chem. Int. Ed. 2013, 52, 10635–10638; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 10829–10832; [Google Scholar]
- 22b. Shen X., Hyde A. M., Buchwald S. L., J. Am. Chem. Soc. 2010, 132, 14076–14078; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22c. Grushin V. V., Marshall W. J., J. Am. Chem. Soc. 2006, 128, 12644–12645; [DOI] [PubMed] [Google Scholar]
- 22d. Nielsen M. C., Bonney K. J., Schoenebeck F., Angew. Chem. Int. Ed. 2014, 53, 5903–5906; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 6013–6016; [Google Scholar]
- 22e. Cho E. J., Senecal T. D., Kinzel T., Zhang Y., Watson D. A., Buchwald S. L., Science 2010, 328, 1679–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The fluorinated ligand dAr F pe also reacted with NBE at 125 °C to give (trans,endo)-22 in 82 % yield. See ref. [8f, 22d].
- 24. Pracht P., Bohle F., Grimme S., Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [DOI] [PubMed] [Google Scholar]
- 25.Gaussian 09, Revision D.01, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, et al., Gaussian Inc., Wallington, CT, 2016. See SI for full reference.
- 26.“Popular Integration Grids Can Result in Large Errros in DFT-Computed Free Energies”: Bootsma A. N., Wheeler S., ChemRxiv 2019, 10.26434/chemrxiv.8864204.v5. [DOI] [Google Scholar]
- 27. Perdew J. P., Burke K., Ernzerhof M., Phys. Rev. Lett. 1996, 77, 3865–3868. [DOI] [PubMed] [Google Scholar]
- 28. Perdew J. P., Burke K., Ernzerhof M., Phys. Rev. Lett. 1997, 78, 1396. [DOI] [PubMed] [Google Scholar]
- 29. Adamo C., Barone V., J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar]
- 30. Ernzerhof M., Scuseria G. E., J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar]
- 31. Grimme S., Antony J., Ehrlich S., Krieg H., J. Chem. Phys. 2010, 132, 154104. [DOI] [PubMed] [Google Scholar]
- 32. Grimme S., Ehrlich S., Goerigk L., J. Comput. Chem. 2011, 32, 1456–1465. [DOI] [PubMed] [Google Scholar]
- 33. Weigend F., Ahlrichs R., Phys. Chem. Chem. Phys. 2005, 7, 3297. [DOI] [PubMed] [Google Scholar]
- 34. Weigend F., Phys. Chem. Chem. Phys. 2006, 8, 1057. [DOI] [PubMed] [Google Scholar]
- 35. Ribeiro R. F., Marenich A. V., Cramer C. J., Truhlar D. G., J. Phys. Chem. B 2011, 115, 14556–14562. [DOI] [PubMed] [Google Scholar]
- 36. Luchini G., Alegre-Requena J. V., Funes-Ardoiz I., Paton R. S., F1000Research 2020, 9, 291. [Google Scholar]
- 37. Marenich A. V., Cramer C. J., Truhlar D. G., J. Phys. Chem. B 2009, 113, 6378–6396. [DOI] [PubMed] [Google Scholar]
- 38.C. Y. Legault, CYLview20, Université de Sherbrooke, 2020 (http://cylview.org).
- 39.See SI for further computational details.
- 40. Zuideveld M. A., Swennenhuis B. H. G., Boele M. D. K., Guari Y., van Strijdonck G. P. F., Reek J. N. H., Kamer P. C. J., Goubitz K., Fraanje J., Lutz M., Speck A. L., van Leeuwen P. W. N. M., J. Chem. Soc. Dalton Trans. 2002, 2308. [Google Scholar]
- 41. Fu X.-P., Xue X.-S., Zhang X.-Y., Xiao Y.-L., Zhang S., Guo Y.-L., Leng X., Houk K. N., Zhang X., Nat. Chem. 2019, 11, 948–956. [DOI] [PubMed] [Google Scholar]
- 42. Johns A. M., Utsunomiya M., Incarvito C. D., Hartwig J. F., J. Am. Chem. Soc. 2006, 128, 1828–1839. [DOI] [PubMed] [Google Scholar]
- 43. Khoury P. R., Goddard J. D., Tam W., Tetrahedron 2004, 60, 8103–8112. [Google Scholar]
- 44.
- 44a. Gauthier D., Lindhardt A. T., Olsen E. P. K., Overgaard J., Skrydstrup T., J. Am. Chem. Soc. 2010, 132, 7998–8009; [DOI] [PubMed] [Google Scholar]
- 44b. Jean M., Renault J., Uriac P., Capet M., van de Weghe P., Org. Lett. 2007, 9, 3623–3625. [DOI] [PubMed] [Google Scholar]
- 45. Martin J. G., Hill R. K., Chem. Rev. 1961, 61, 537–562. [Google Scholar]
- 46.
- 46a. Koh M. J., Khan K. M., Torker S., Hoveyda A. H., Angew. Chem. Int. Ed. 2014, 53, 1968–1972; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 1999–2003; [Google Scholar]
- 46b. Liu Z., Rainer J. D., Org. Lett. 2005, 7, 131–133; [DOI] [PubMed] [Google Scholar]
- 46c. Weeresakare G. M., Liu Z., Rainer J. D., Org. Lett. 2004, 6, 1625–1627; [DOI] [PubMed] [Google Scholar]; Berlin J. M., Goldberg S. D., Grubbs R. H., Angew. Chem. Int. Ed. 2006, 45, 7591–7595; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2006, 118, 7753–7757. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information