Abstract
Aim
The aim of this study was to describe the long‐term safety and efficacy of lanreotide in Japanese patients with neuroendocrine tumors.
Methods
The final analyses of a 48‐week open‐label phase II study (n = 32) and its extension study (n = 17) were conducted. Patients received 4‐weekly subcutaneous injections of lanreotide autogel 120 mg. Safety was evaluated by adverse events. Efficacy endpoints included tumor response by RECIST and change in tumor size. Post hoc analyses including tumor growth rate were performed.
Results
The median (range) of lanreotide exposure in the safety analysis set (n = 17) and efficacy analysis set (n = 28) were 151.4 (52–181) and 52.7 (12–181) weeks, respectively. Sixteen patients developed adverse drug reaction; of these, upper abdominal pain and urticaria were not reported before 48 weeks. No patient discontinued lanreotide or died from an adverse event. Two serious events of bile duct stones in one patient were drug‐related. Partial response was observed in 2 patients (7.1%; at 60 and 108 weeks), stable disease in 20 (71.4%) and progressive disease in 6 (21.4%). The mean of the greatest change from baseline in the sum of diameters of target lesions was −5.5%. The mean (standard deviation) tumor growth rate before treatment and from baseline to last observation was 25.3% (35.7%)/month and 6.4% (9.6%)/month, respectively.
Conclusion
Lanreotide treatment had an acceptable safety profile and was effective over long‐term treatment in Japanese patients with neuroendocrine tumors. No unexpected serious adverse events developed during prolonged use of lanreotide.
Keywords: clinical trial, Japanese, lanreotide, neuroendocrine tumor, somatostatin
1. INTRODUCTION
Neuroendocrine tumors (NETs) are rare and slow‐growing tumors, originating from various organs including the gastrointestinal tract, pancreas and lung. 1 , 2 , 3 The aims of pharmacotherapy for inoperable or metastatic NETs are to suppress tumor growth, relieve symptoms and prolong survival. Lanreotide, a long‐acting somatostatin analog, has both direct (cellular growth inhibition via somatostatin receptor activation 4 and apoptosis 5 ) and indirect (inhibition of growth factor secretion 6 ) effects on tumors. The CLARINET, 96‐week, randomized, controlled phase III study conducted in Europe, the United States and India, demonstrated that lanreotide autogel 120 mg prolonged progression‐free survival (PFS) in patients with NETs. 7 , 8 Based on the study results, lanreotide autogel 120 mg has been approved for the treatment of gastroenteropancreatic (GEP)‐NETs in Europe and the United States. Subsequently, the clinical guidelines now recommend the use of lanreotide autogel 120 mg for first‐line treatment of metastatic GEP‐NETs. 9 , 10
Since Japanese patients were not included in the CLARINET study, a multicenter, single‐arm, open‐label phase II, study was conducted to evaluate the efficacy, safety and pharmacokinetics of lanreotide autogel in Japanese patients with NETs. 11 Based on the results from these studies, 7 , 8 , 11 lanreotide autogel 120 mg for the treatment of GEP‐NETs was approved in Japan in July 2017 and is recommended by recent published guideline. 12 To evaluate the long‐term safety and efficacy of lanreotide autogel in Japanese patients with NETs, an open‐label extension study was conducted following the phase II study. The initial results of this study were published in the primary publication (up to 60 weeks of treatment; cut‐off date 7 December 2015). 11 Here, we report the final results of this study.
2. MATERIALS AND METHODS
2.1. Study design and patients
This single‐arm, open‐label, phase II study of lanreotide autogel in Japanese patients with NETs consists of two parts: a 48‐week phase II study (Study 001) and its extension study until lanreotide autogel was approved for NETs in Japan (Study 002). The detailed design of this study has been described previously. 11 Study 001 and Study 002 were conducted in accordance with the Declaration of Helsinki, Good Clinical Practice and applicable regulatory requirements. The protocols were approved by the institutional review boards, and all patients provided written informed consent prior to participation in Study 001 and Study 002.
Patients who completed Study 001 were eligible to enroll in Study 002, and they continued to receive lanreotide treatment. The eligibility criteria of Study 001 were described in detail previously, 11 but, in brief, this study included patients who were aged ≥20 years and had grade 1 or 2 NETs according to the World Health Organization (WHO) 2004 classification for the lungs, bronchi or thymus and WHO 2010 classification for other locations. Patients who enrolled in Study 001 were also required to have target lesions based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, a WHO performance status of 0–2, and metastatic disease and/or a locally advanced tumor that was unresectable. Patients were excluded from Study 002 if they had a malignant tumor other than a NET.
Lanreotide autogel 120 mg was administered by deep subcutaneous injection in the buttocks every 4 weeks. Treatment was continued until disease progression, the occurrence of an intolerable adverse event (AE), or patient withdrawal from the study. Treatment exposure of lanreotide was defined as “from the first treatment day until the last treatment day + 4 weeks” because lanreotide autogel is a prolonged‐release formulation given at 4‐week intervals.
2.2. Endpoints and assessments
Safety was evaluated by AEs and adverse drug reactions (ADRs), including abnormal changes in vital signs, electrocardiography, abdominal ultrasound, or clinical laboratory tests. Efficacy endpoints included PFS, overall survival (OS), objective response rate (ORR) and the percent change from baseline in the sum of the diameter of target lesions.
During the study treatment with lanreotide autogel, patients visited to the clinic every 4 weeks for drug administration and assessment of AEs and ADRs. Assessment of disease progression was performed every 12 weeks after the start of drug administration. At each visit, patients underwent a thorough physical examination, in which vital signs were measured and blood and urine samples were taken. The names of the AEs and ADRs were coded using the terms of the Medical Dictionary for Regulatory Activities, version 16.0, and their severity was assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Tumor size was measured by each investigator before initiating treatment and by a centralized review process at each subsequent 12‐week visit, using computed tomography or magnetic resonance imaging of the neck, chest, abdomen and pelvis. Patients with suspected brain lesions also underwent computed tomography or magnetic resonance imaging of the head. Patients with suspected bone lesions underwent bone scintigraphy or fluorodeoxyglucose positron emission tomography. The centralized review used RECIST version 1.1 to categorize tumor responses as a complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD). Patients underwent electrocardiography and abdominal ultrasound for gallbladder assessment every 24 weeks.
PFS was defined as the time from the first dose of lanreotide to PD or death from any cause, and OS was defined as the time from the first dose of lanreotide to death from any cause. ORR was defined as the proportion of patients with best overall response of confirmed CR or PR.
2.3. Statistical analysis
The sample size of Study 001 was determined as described hereafter. The primary endpoint of Study 001 was clinical benefit rate (CBR; proportion of patients with CR, PR, and SD ≥24 weeks). 11 With regard to the patient population similar to the CLARINET study, 7 , 8 we set the threshold of CBR as 40% and the expected CBR as 70%. To achieve 80% power at a one‐sided α level of 0.025, the required number of patients was calculated to be 22. Additionally, considering the enrollment of eight patients with “primary lesions other than the pancreas, midgut, or hindgut” or “endocrine symptoms associated with NETs,” who were not included in the CLARINET study, 7 , 8 the sample size of Study 001 was planned to be 30 patients. 11
The safety analysis set included all patients who enrolled in Study 002 and received at least one dose of lanreotide. The efficacy analysis set included all patients who enrolled in Study 001, received at least one dose of lanreotide and were evaluated for efficacy at least once. Descriptive analysis for baseline patient characteristics was performed by using mean, median and standard deviation for continuous variables and frequency and proportions for categorical variables. PFS and OS were summarized using a Kaplan–Meier plot and medians with 95% confidence intervals (CIs). For ORR, point estimates and exact 95% CIs were calculated based on the F‐distribution. The proportions of patients with AEs and ADRs were summarized. All statistical analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC, USA).
Post hoc analyses were performed as follows. The percent change in the sum of tumor diameters of each target lesion over time was calculated and depicted in a spider plot. Tumor growth rate (TGR), which estimates the change in tumor volume over 1 month, 13 was also computed (details are in the Supplementary Method). For subgroup analyses of PFS by baseline characteristics, the Cox proportional hazard model was used in a univariate regression analysis with a significance level of 0.05.
3. RESULTS
3.1. Patients
Between December 2013 and December 2014, 32 patients were enrolled in Study 001 at 10 institutions in Japan (Figure 1); of these, 28 patients were included in the efficacy analysis set (duration of exposure 12–181 weeks, median 52.7 weeks). Study 002 was conducted at seven institutions from December 2014 to July 2017, and all the 17 patients who completed 48 weeks of lanreotide treatment in Study 001 were enrolled in and continued lanreotide treatment (safety analysis set; duration of exposure 52–181 weeks, median 151.4 weeks). Thereafter, eight patients completed lanreotide treatment in Study 002 (duration of exposure 156–176 weeks). Among the nine patients who did not complete Study 002, the reasons for discontinuation were insufficient efficacy (seven patients) and consent withdrawal (one patient). The reason for discontinuation in the remaining patient was initially classified as an AE, but finally the investigators determined that the AE did not warrant treatment discontinuation. Patient baseline characteristics are shown in Supplementary Table S1.
FIGURE 1.
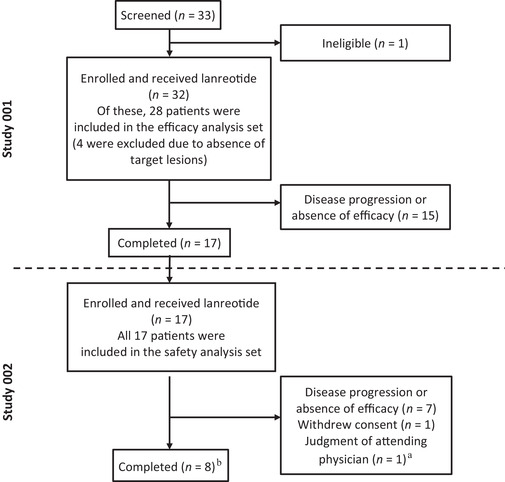
Study design and patient disposition. This open‐label, phase II study of lanreotide in Japanese patients with NETs consisted of two parts: a 48‐week phase II study (Study 001) and its extension study (Study 002). The efficacy analysis set comprised all patients who enrolled in Study 001, received at least one dose of lanreotide and underwent efficacy evaluation at least once. The safety analysis set comprised all patients who completed Study 001 and enrolled in Study 002 and received at least one dose of lanreotide. aThe investigators originally classified one patient as discontinuing treatment because of an adverse event, but finally determined that the adverse event did not warrant discontinuation of the study. bOne patient who completed treatment was not included in the efficacy analysis set as the target tumor site was not evaluable
3.2. Safety
A total of 268 AEs occurred in all 17 patients in the safety analysis set (Table 2), with 128 of these events in 16 patients (94.1%) considered as ADRs. Seven patients (41.2%) developed a total of 16 AEs of grade ≥3 severity. These events included decreased blood glucose and hypertension (both n = 2; 11.8%); diabetes mellitus; impaired glucose tolerance; pancreatitis; road traffic accident; parathyroidectomy; liver abscess; bile duct stone; and increases in alanine aminotransferase (ALT), amylase, aspartate aminotransferase (AST), blood glucose and gamma‐glutamyltransferase (GGT) levels (each n = 1; 5.9%). Of these, diabetes mellitus, impaired glucose tolerance, pancreatitis, bile duct stone and increases in ALT and AST levels were considered ADRs.
TABLE 2.
Adverse drug reactions by time of onset in the safety analysis set (n = 17)
| Adverse drug reaction | Onset of adverse drug reaction (weeks) | |||||||
|---|---|---|---|---|---|---|---|---|
| Study 001 | Study 002 | |||||||
| 0–24 | 25–48 | 49–72 | 73–96 | 97–120 | 121–144 | 145–168 | ≥169 | |
| N | 17 | 17 | 17 | 14 | 13 | 13 | 10 | 4 |
| Pale feces | 4 (23.5) | 2 (11.8) | 1 (5.9) | 0 | 0 | 0 | 0 | 0 |
| Flatulence | 3 (17.6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Injection site induration | 2 (11.8) | 4 (23.5) | 4 (23.5) | 1 (7.1) | 1 (7.7) | 1 (7.7) | 1 (10.0) | 1 (25.0) |
| Malaise | 2 (11.8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Abdominal distension | 2 (11.8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diabetes mellitus | 2 (11.8) | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 |
| Injection site pruritus | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pyrexia | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Abdominal pain | 1 (5.9) | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 1 (5.9) | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 |
| Dental caries | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gingival bleeding | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stomatitis | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Impaired glucose tolerance | 1 (5.9) | 0 | 0 | 1 (7.1) | 0 | 0 | 0 | 0 |
| T2DM | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cholelithiasis | 1 (5.9) | 0 | 0 | 0 | 0 | 1 (7.7) | 0 | 0 |
| Hepatic steatosis | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Increased ALT levels | 1 (5.9) | 1 (5.9) | 1 (5.9) | 0 | 0 | 0 | 0 | 0 |
| Increased AST levels | 1 (5.9) | 1 (5.9) | 1 (5.9) | 0 | 0 | 0 | 0 | 0 |
| Bronchopneumonia | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Herpes zoster infection | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pharyngitis | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dizziness | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Injection site pain | 0 | 1 (5.9) | 1 (5.9) | 0 | 0 | 0 | 0 | 0 |
| Upper abdominal pain | 0 | 0 | 0 | 0 | 1 (7.7) | 0 | 0 | 0 |
| Pancreatitis | 0 | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperglycemia | 0 | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 |
| Bile duct stone | 0 | 1 (5.9) | 0 | 0 | 1 (7.7) | 0 | 0 | 0 |
| Urticaria | 0 | 0 | 1 (5.9) | 0 | 0 | 0 | 0 | 0 |
Data are shown as n (%).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; T2DM, type 2 diabetes mellitus.
TABLE 1.
Adverse events/adverse drug reactions in the safety analysis set (n = 17)
| Events, n (%) | Safety analysis set (n = 17) | |
|---|---|---|
| AE | ADR | |
| Any AE/ADR | 17 (100.0) | 16 (94.1) |
| Any serious AE/ADR | 4 (23.5) | 1 (5.9) |
| Treatment discontinuation due to AE/ADR | 0 | 0 |
| Treatment interruption due to AE/ADR | 4 (23.5) | 1 (5.9) |
| AE/ADR of severity grade ≥3 | 7 (41.2) | 4 (23.5) |
| Specific AEs/ADRs of any grade occurring in >2 patients | ||
| Nasopharyngitis | 10 (58.8) | 0 |
| Pale feces | 5 (29.4) | 5 (29.4) |
| Dental caries | 4 (23.5) | 1 (5.9) |
| Diarrhea | 4 (23.5) | 2 (11.8) |
| Injection site induration | 4 (23.5) | 4 (23.5) |
| Flatulence | 3 (17.6) | 3 (17.6) |
| Nausea | 3 (17.6) | 1 (5.9) |
| Decrease in blood glucose levels | 3 (17.6) | 0 |
| Diabetes mellitus | 3 (17.6) | 3 (17.6) |
| Vertigo | 3 (17.6) | 0 |
Abbreviations: ADR, adverse drug reaction; AE, adverse event.
No AE‐related deaths occurred. Four patients had a total of eight serious AEs. One patient had bile duct stones (two separate events), one patient had a road traffic accident and underwent parathyroidectomy, one patient had a fractured pubis and sacrum, and another developed cholangitis and a liver abscess. Of these, the two bile duct stone events in the same patient were considered to be drug‐related. No patient permanently discontinued treatment because of AEs. In four patients, lanreotide treatment was temporarily interrupted because of AEs; one serious AE (cholangitis) and three nonserious AEs (hot flush, increased GGT levels and increased ALT levels, each in one patient). Of these events, the investigators assessed that the increase in ALT was possibly related to lanreotide treatment.
The majority of ADRs were reported in the first 24 weeks of lanreotide exposure (Table 2). ADRs that developed at any time over the 181 weeks of lanreotide exposure, but after the first 48 weeks of treatment, included injection site induration (n = 9; 52.9% of patients), pale feces, impaired glucose tolerance, cholelithiasis, increases in ALT and AST, injection site pain, upper abdominal pain, bile duct stone and urticaria (each n = 1; 5.9%). Of these, upper abdominal pain and urticaria were not reported before 48 weeks of treatment.
3.3. Efficacy
PFS and OS in the phase II extension study are shown in Figure 2. As previously shown, 11 median PFS with lanreotide treatment was 36.3 (95% CI, 24.1–53.1) weeks. Median OS was not reached. A CR was not achieved by any patient, but two patients achieved a PR; therefore, the ORR was 7.1% (95% CI, 0.9–23.5). The two patients who achieved a PR both had pancreatic NETs. One of these PRs was reported at week 6011 and the other was identified at week 108. In addition, SD was achieved in 20 patients (71.4%), whereas six patients (21.4%) had PD.
FIGURE 2.
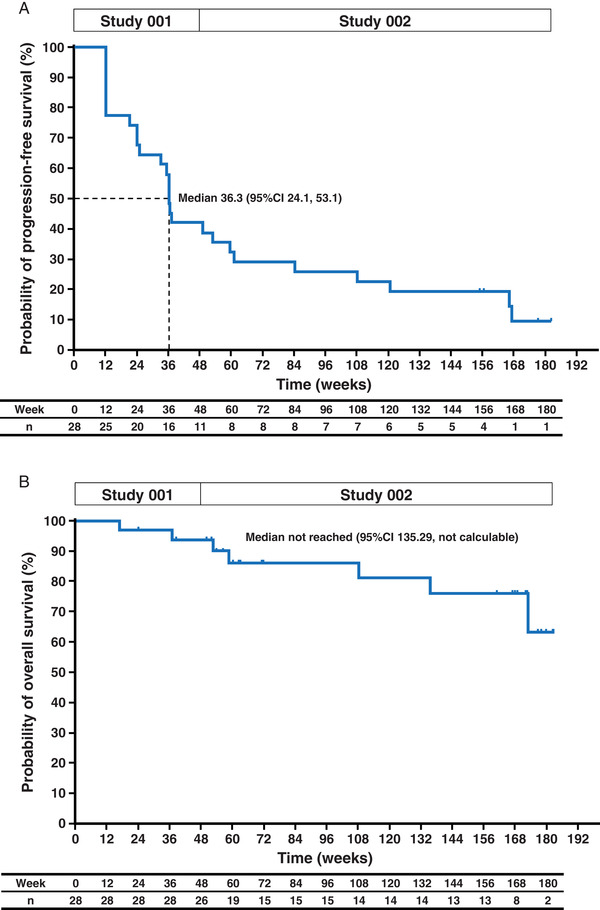
Progression‐free survival (A) and overall survival (B) in the efficacy analysis set (n = 28). CI, confidence interval [Colour figure can be viewed at wileyonlinelibrary.com]
Three patients (including two patients who achieved a PR) had a ≥30% decrease from baseline in the sum of tumor diameters of target lesions at the time point of the last observation (at 156, 166.4 and 182.1 weeks; Figure 3A). The mean (standard deviation) of percent change in the sum of diameters of target lesions at the time point of the last observation was +15.9% (28.4%), and at the time point of the greatest change was −5.5% (22.8%). Target lesions were found to be more likely to increase in patients with primary hindgut NETs than in patients with NETs in other locations. In post hoc analyses, the mean (standard deviation) TGR before the first injection was 25.3% (35.7%)/month and the TGR from baseline to the last available value was 6.4% (9.6%)/month (Figure 3B).
FIGURE 3.
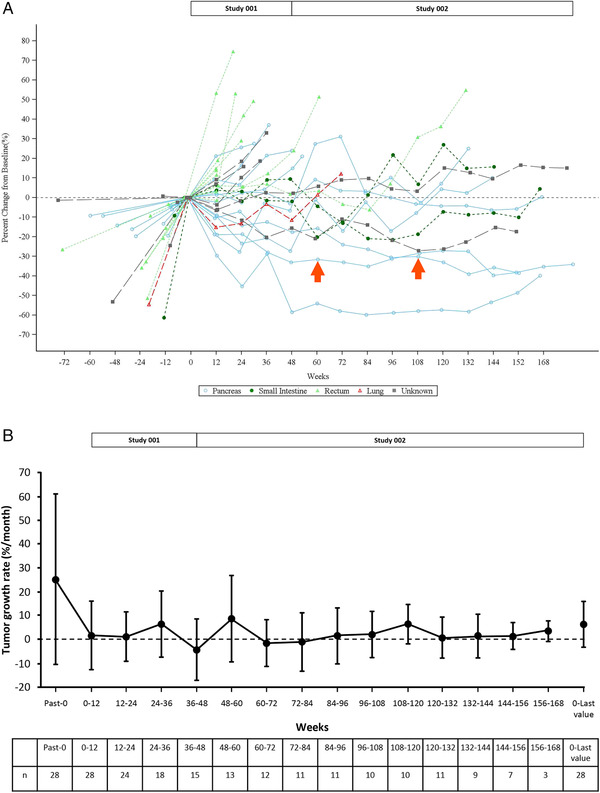
Changes in tumor size during lanreotide treatment in the efficacy analysis set (n = 28). Data, based on RECIST (ver. 1.1), were evaluated by each investigator before treatment and via central assessment by an independent third party at every subsequent evaluation. Spider plot of the percent change in the sum of tumor diameters of each target lesion from the baseline measurement (A). Arrows indicate partial responses achieved in two patients (one was at 60 weeks and the other was at 108 weeks). Tumor growth rate as a percent change (± standard deviation) in tumor volume per month (B). The calculation of the tumor growth rate is described in the “Supplementary Method” [Colour figure can be viewed at wileyonlinelibrary.com]
Post hoc subgroup analyses of PFS showed that at baseline PD, Ki67 index, and baseline TGR were statistically significant in the univariate Cox proportional hazard model (Supplementary Table S2). The hazard ratio for PFS was 2.94 (95% CI, 1.09–7.94) in patients with a hindgut versus pancreatic NET (median 22.7 weeks vs 36.8 weeks).
4. DISCUSSION
The results of this final analysis of the phase II study and its extension study in Japan were mostly consistent with those of previous studies 8 , 14 and confirmed the long‐term safety and efficacy of lanreotide autogel in Japanese patients with NETs. This phase II study is, to our knowledge, the first clinical trial of lanreotide autogel conducted in Asia. The overall safety profile of lanreotide autogel in this long‐term study was similar to that observed in the CLARINET study. 8 , 14 As seen in the CLARINET study, 8 , 14 the incidence of ADRs in our study did not increase over time, with the majority of events reported during the first 24 weeks of lanreotide exposure. The ADRs first reported during administration after 48 weeks were upper abdominal pain and urticaria. Of the serious AEs, only two of bile duct stones in same patient were drug‐related. Furthermore, no patient discontinued lanreotide treatment because of AEs. These results suggested that the risk of ADRs during long‐term lanreotide treatment is low in Japanese patients.
Although this study included patients with baseline factors associated with a relatively poor prognosis, a favorable outcome under lanreotide treatment was observed. In our study, almost 80% of the patients achieved PR or SD, whereas the remaining 20% had PD. In the efficacy analysis set, in which the median PFS was 36.3 weeks, baseline characteristics were 68% of patients with tumor grade 2, 39% with disease progression, and primary tumor site of pancreas 43%, hindgut 29% and midgut 7%. In contrast, those of the lanreotide group of the CLARINET extension study, where the median PFS was 32.8 months, were 27% with tumor grade 2, 0% with disease progression, and 27%, 12% and 41% with primary tumor site of pancreas, hindgut and midgut, respectively. 8 Ethnic differences between the locations of the primary tumor in NETs are known to exist. Midgut NETs are more common in western countries; in contrast, pancreas or hindgut NETs are more common than midgut NETs in Asian countries including Japan. 1 , 2 In this study of a Japanese patient population, hindgut NETs, which are relatively aggressive, were four times more frequent than midgut NETs.
A ≥30% reduction in tumor size was achieved in three patients (including two with PRs); this fact indicates a clear antitumor effect of lanreotide. Particularly, a late‐onset effect as a second PR at week 108, that is over 2 years, was observed in our study. Only one clinical trial, Spanish phase II study, of lanreotide autogel 120 mg in NETs has previously shown that one patient (1/27, 4%) with PR was observed during 92 weeks of follow‐up. 15 Furthermore, the change in tumor diameter over time in the patient who achieved PR at 108 weeks showed a time‐dependent decrease with lanreotide treatment.
The spider plot showed that some of the tumors were increasing in size before treatment was initiated, but that growth stabilized during lanreotide treatment. We have previously reported the TGR in NETs until 60 weeks of lanreotide treatment, 11 and this final analysis updated the results of TGR with longer‐term lanreotide treatment over 3 years. In contrast to using RECIST criteria to analyze tumor response, calculating TGR is thought to provide a more dynamic evaluation of treatment effects, as it evaluates monthly tumor growth. 13 , 16 , 17 Post hoc analysis of our study showed that TGR was reduced as early as 12 weeks after initiating lanreotide administration and remained stable over the study period. This is consistent with the recently reported TGR over a period of within 2 years of lanreotide treatment observed in the CLARINET study. 16 Taken together, our results demonstrated that the antitumor effects of lanreotide are seen early after initiating treatment and are maintained in patients who continue to receive lanreotide treatment for more than 60 weeks.
The prognosis of patients with hindgut NETs was worse than that of other primary origins. 2 , 18 This was also observed in our study; the hazard ratio for PFS of hindgut versus pancreatic NETs was 2.94 in the subgroup analyses, and the summed diameters of target lesions were more likely to increase in hindgut NETs than in pancreatic NETs. However, seven of the eight patients with hindgut NETs (all rectal NETs) had PD at baseline, which may have influenced the prognosis. These results are not consistent with the recent report; a study in Japan showed that median PFS on somatostatin analogs in patients with foregut/hindgut NETs was similar to that with midgut NETs. 19 A clinical trial of lanreotide is currently ongoing in South Korea in patients with hindgut NETs and this may help clarify the efficacy of lanreotide for hindgut NETs; 20 however, additional research into the response of patients with hindgut NETs is also warranted.
This study has some limitations: these include the single‐arm design, with no control arm and a small sample size because of the low incidence of NETs. The numbers of patients in each subgroup were too small to allow statistically meaningful results. Also, somatostatin receptor scintigraphy was not performed before study enrollment because it had not been approved by the Japanese regulatory authority at the time of the study. Notwithstanding these limitations, this study described the extended follow‐up data of the long‐term use of lanreotide autogel in a Japanese patient population.
In conclusion, this final analysis showed an acceptable safety profile and effectiveness of long‐term lanreotide treatment for Japanese patients with NETs; therefore, this treatment could be a suitable option. No unexpected serious AEs developed during prolonged use of lanreotide. Future investigation is needed to identify more suitable target populations for lanreotide treatment and to evaluate the clinical effectiveness of lanreotide in combination with other antitumor drugs.
DATA SHARING AND DATA ACCESSIBILITY
No additional data are available.
AUTHORS’ CONTRIBUTIONS
All authors contributed equally to conception or design of the study, analysis or interpretation of data and writing of the manuscript. All authors but SH and AN performed the study and acquired data. All authors also contributed critical revision for important intellectual content and final approval of the manuscript. AS supervised this study.
CONFLICTS OF INTEREST
Tetsuhide Ito has received personal fees and nonfinancial support from Teijin Pharma; Nao Fujimori has received personal fees from Teijin Pharma; Susumu Hijioka has received personal fees from Teijin Pharma, Novel Pharma, Novartis, FUJIFILM Toyama Chemical and Pfizer; Yasutoshi Kimura has received grants from Bayer academic support, Asahi Kasei Pharma academic support and Shionogi academic support; Akira Fukutomi has received personal fees from Teijin Pharma; Akira Shimatsu has received personal fees and nonfinancial support from Teijin Pharma; Seiichi Hisamatsu and Akihiro Nakajima are employees of Teijin Pharma and hold Teijin Pharma stock; Yoshitaka Honma, Atsushi Kudo and Shinji Katsushima have no conflicts of interest to declare.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The study was presented in part at the American Society for Clinical Oncology Gastrointestinal Cancers Symposium (ASCO‐GI), San Francisco, CA, January 23–25, 2020.
This work was supported by Teijin Pharma Limited and IPSEN Pharma.
We would like to thank all patients and investigators who participated in these studies (Kyushu University Hospital, National Cancer Center Hospital, Aichi Cancer Center Hospital, Tokyo Medical and Dental University Hospital, Shizuoka Cancer Center, National Hospital Organization Kyoto Medical Center, Sapporo Medical College Hospital, Tohoku University Hospital, The University of Tokyo Hospital and Kansai Electric Power Hospital). We also thank medical writers of the Medical Science Department, Teijin Pharma Limited for providing medical writing support during the preparation of the manuscript. Simone Tait of inScience Communications, Springer Healthcare provided medical writing support of drafting the manuscript funded by Teijin Pharma Limited. Kae Uetani of Statcom Co., Ltd., Tokyo, Japan, provided writing assistance for revising the manuscript funded by Teijin Pharma Limited.
Ito T, Fujimori N, Honma Y, et al. Long‐term safety and efficacy of lanreotide autogel in Japanese patients with neuroendocrine tumors: Final results of a phase II open‐label extension study. Asia-Pac J Clin Oncol. 2021;17:e153–e161. 10.1111/ajco.13371
Current address for S. Hijioka, Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital, Tokyo, Japan; current address for A. Shimatsu, Advanced Medical Care Center, Kusatsu General Hospital, Kusatsu, Japan.
Trial registration: JapicCTI‐132375, JapicCTI‐142698
REFERENCES
- 1. Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50:58‐64. [DOI] [PubMed] [Google Scholar]
- 2. Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063‐3072. [DOI] [PubMed] [Google Scholar]
- 3. Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61‐72. [DOI] [PubMed] [Google Scholar]
- 4. Hasskarl J, Kaufmann M, Schmid HA. Somatostatin receptors in non‐neuroendocrine malignancies: the potential role of somatostatin analogs in solid tumors. Future Oncol. 2011;7:895‐913. [DOI] [PubMed] [Google Scholar]
- 5. Florio T. Molecular mechanisms of the antiproliferative activity of somatostatin receptors (SSTRs) in neuroendocrine tumors. Front Biosci. 2008;13:822‐840. [DOI] [PubMed] [Google Scholar]
- 6. Strosberg J, Kvols L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World J Gastroenterol. 2010;16:2963‐2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224‐233. [DOI] [PubMed] [Google Scholar]
- 8. Caplin ME, Pavel M, Ćwikła JB, et al. Anti‐tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open‐label extension study. Endocr Relat Cancer. 2016;23:191‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Neuroendocrine and Adrenal Tumors. Version 3, 2018. Available from: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. Accessed 19 November 2018. [DOI] [PubMed]
- 10. Pavel M, O'Toole D, Costa F, et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology. 2016;103:172‐185. [DOI] [PubMed] [Google Scholar]
- 11. Ito T, Honma Y, Hijioka S, et al. Phase II study of lanreotide autogel in Japanese patients with unresectable or metastatic well‐differentiated neuroendocrine tumors. Invest New Drugs. 2017;35:499‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Japan NeuroEndocrine Tumor Society (ed) . Clinical Practice Guidelines for Gastroenteropancreatic Neuroendocrine Neoplasms (GEP‐NEN) 2019. Kanehara, Tokyo; 2019. Japanese. [Google Scholar]
- 13. Ferté C, Koscielny S, Albiges L, et al. Tumor growth rate provides useful information to evaluate sorafenib and everolimus treatment in metastatic renal cell carcinoma patients: an integrated analysis of the TARGET and RECORD phase 3 trial data. Eur Urol. 2014;65:713‐720. [DOI] [PubMed] [Google Scholar]
- 14. Khan MS, El‐Khouly F, Davies P, Toumpanakis C, Caplin ME. Long‐term results of treatment of malignant carcinoid syndrome with prolonged release lanreotide (Somatuline Autogel). Aliment Pharmacol Ther. 2011;34:235‐242. [DOI] [PubMed] [Google Scholar]
- 15. Martín‐Richard M, Massutí B, Pineda E, et al. Antiproliferative effects of lanreotide autogel in patients with progressive, well‐differentiated neuroendocrine tumours: a Spanish, multicentre, open‐label, single arm phase II study. BMC Cancer. 2013;13:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dromain C, Pavel ME, Ruszniewski P, et al. Tumor growth rate as a metric of progression, response, and prognosis in pancreatic and intestinal neuroendocrine tumors. BMC Cancer. 2019;19(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamarca A, Crona J, Ronot M, et al. Value of Tumor Growth Rate (TGR) as an Early Biomarker Predictor of Patients' Outcome in Neuroendocrine Tumors (NET)—The GREPONET Study. Oncologist. 2019;24:e1082‐e1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nuñez‐Valdovinos B, Carmona‐Bayonas A, Jimenez‐Fonseca P, et al. Neuroendocrine tumor heterogeneity adds uncertainty to the World Health Organization 2010 Classification: real‐world data from the Spanish Tumor Registry (R‐GETNE). Oncologist. 2018;23:422‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ida H, Honma Y, Hirano H, et al. Clinical outcomes of patients with G1/G2 neuroendocrine tumors arising from foregut or hindgut treated with somatostatin analogs: a retrospective study. Invest New Drugs. 2019;37:573‐578. [DOI] [PubMed] [Google Scholar]
- 20.Samsung Medical Center Pilot study of lanreotide in metastatic or recurrent Grade I‐II hindgut NET [ClinicalTrial.gov record: NCT03083210]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT03083210. Accessed 19 November 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
No additional data are available.


