Abstract
Remdesivir is an antiviral drug that results in clinical improvement after five days of treatment and accelerates recovery by 31%. No studies have discussed the pharmacokinetic analysis of remdesivir in patients with severe COVID-19 requiring extracorporeal membrane oxygenation (ECMO). A 63-year-old American man who underwent mechanical ventilation and ECMO for severe COVID-19 was administered remdesivir for ten days. The loading dosage was 200 mg at 7 PM on day 12 and 100 mg daily at 0:00 PM from day 13–21, administered within 1 h. The pharmacokinetic analysis was performed. The serum creatinine concentration was within the normal range of 0.5–0.7 mg/dL during treatment. According to the pharmacokinetic analysis, the plasma concentrations of remdesivir and GS-441524 4 h after administration (C4) were 662 ng/mL and 58 ng/mL, respectively, and the concentrations 18 h after administration (C18) were 32 ng/mL and 44 ng/mL, respectively. Therefore, the half-life of remdesivir and GS-441524 was 3.2 and 35.1 h, respectively. Monitoring the plasma concentrations of remdesivir and GS-441524 in patients undergoing ECMO may be necessary.
Keywords: Remdesivir, Severe COVID-19, Extracorporeal membrane oxygenation, Pharmacokinetics analysis
Highlights
-
•
A COVID-19 patient undergoing ECMO was administered remdesivir.
-
•
The loading dosage was 200 mg at 7:00 PM on day 12.
-
•
From days 13–21, it was 100 mg daily at 0:00 PM administered within 1 h.
-
•
The half-lives of remdesivir and GS-441524 were 3.2 and 35.1 h, respectively.
-
•
Plasma concentrations of remdesivir and GS-441524 must be monitored during ECMO.
Introduction
Remdesivir, a nucleotide analog prodrug administered under compassionate use, resulted in clinical improvements in 68% of coronavirus disease (COVID-19) patients [1]. A phase Ⅲ trial in critically ill hospitalized COVID-19 patients also showed clinical improvements in 64.5% of patients after five days of treatment and accelerated the time to recovery by 31% [2]. Remdesivir has a very short blood half-life of approximately 1 h. However, when hydrolyzed in the body, remdesivir becomes a circulating metabolite, GS-441524, with a half-life of approximately 27 h [3]. We report the pharmacokinetic data on remdesivir in a severe COVID-19 patient who required invasive ventilator support and extracorporeal membrane oxygenation (ECMO).
Case report
A 63-year-old American man with a medical history of obesity embarked on a cruise ship. He developed fever, nasal discharge, sore throat, and cough six days before admission. The result of the polymerase chain reaction test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was positive, and he was transferred to our hospital. He had no history of smoking. Upon admission, his vital signs revealed a temperature of 102.3°F and arterial oxygen saturation of 99% on 2 L of oxygen administration. Chest computed tomography revealed multiple pan-lobular consolidations just below the pleura of the bilateral lower lungs. The result of the blood tests is shown in Table 1.
Table 1.
Blood test results of the patient on admission.
| WBC | 3980 | /mm3 | Alb | 3.7 | g/dL | CRP | 10.41 | mg/dL |
|---|---|---|---|---|---|---|---|---|
| Neutrophil | 71.1 | % | T-bil | 0.4 | mg/dL | BNP | 12.6 | pg/mL |
| Lymphophil | 23.6 | % | AST | 44 | IU/L | Troponin I | <0.010 | ng/mL |
| RBC | 421 × 104 | /mm3 | ALT | 53 | IU/L | HBs Ag | (-) | |
| Hb | 13.3 | g/dL | LDH | 251 | IU/L | HCV Ab | (-) | |
| Ht | 37.8 | % | ALP | 741 | U/L | HIV Ag/Ab | (-) | |
| PLT | 8.1 × 104 | /μL | GGT | 287 | ng/mL | |||
| PT | 11.4 | s | CK | 34 | mg/dL | Arterial blood gas | ||
| APTT | 25 | s | BUN | 11.6 | mg/dL | pH | 7.53 | |
| FDP | 4.7 | mg/dL | Cre | 0.85 | mg/dL | pCO2 | 27 | mmHg |
| Na | 133 | mEq/L | pO2 | 94 | mmHg | |||
| K | 4.1 | mEq/L | HCO3- | 22.6 | mmol/L | |||
| Cl | 101 | mEq/L | Lactate | 0.9 | mg/dL | |||
Abbreviations
WBC: white blood cell; RBC: red blood cell; Hb: hemoglobin; Ht: hematocrit; PLT: platelet; PT: prothrombin time; APTT: activated partial thrombin time; FDP: fibrin degradation product; Alb: albumin; T-bil: total-bilirubin; AST: asparate transaminase; ALT: alanine transaminase; LDH: lactate dehydrogenase; ALP: alkaline phosphatase; GGT: gamma-glutamyl transferase; CK: creatine kinase; BUN: blood urea nitrogen; Cre: creatinine; Na: sodium; K: serum potassium; Cl: serum chloride; CRP: C-reactive protein; BNP: brain natriuretic peptide
The patient was diagnosed with COVID-19 pneumonia, and lopinavir–ritonavir (LPV/r) 400 mg/100 mg twice daily was administered. On day 4 of hospitalization, the patient was intubated, and ventilator support was initiated owing to the exacerbation of respiratory failure. On day 6, he was subcutaneously injected with 180 μg interferon-alpha. Despite a high fraction of inspired oxygen and positive end-expiratory pressure, his oxygenation status worsened, and subcutaneous emphysema developed. Veno-venous ECMO with silicone-coated polypropylene membranes was implemented on day 8 via the right femoral vein for blood drainage and the right internal jugular vein for blood return.(Fig. 1).
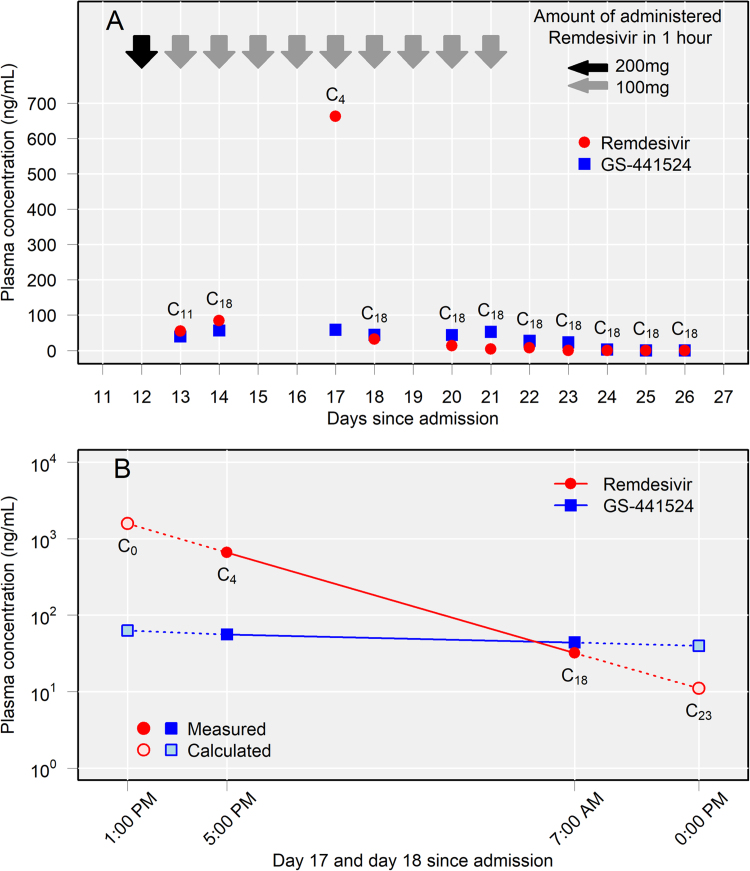
Fig. 1.
Plasma concentrations of remdesivir and GS-441524. (A) Plasma concentrations of remdesivir and GS-441524 during the remdesivir administration period. ( ) Remdesivir 200 mg administered in 1 h, (
) Remdesivir 200 mg administered in 1 h, ( ) Remdesivir 100 mg administered in 1 h. (B) The plasma concentrations of remdesivir and GS-441524 4 h after administration (C4) on day 17 were 662 ng/mL and 58 ng/mL, respectively. However, the concentrations of remdesivir and GS-441524 18 h after administration (C18) were 32 ng/mL and 44 ng/mL, respectively. The peak values of remdesivir and GS-441524 were calculated to be 1576 ng/mL and 63 ng/mL, respectively, and the trough values were calculated to be 11 ng/mL and 40 ng/mL, respectively. The following formulas were used to calculate the values. The half-life (hr) = T1/2 = ln2 × (1/kel) = ln2 × (TC18-TC4)/(lnC4-lnC18). The peak value (ng/mL) = C0 = C4 × 2^{(TC4-TC0)/(T1/2)}. The trough value (ng/mL) = C23 = C18 × (1/2)^{(TC23-TC18)/(T1/2)}. Kel: elimination rate constant (hr-1), T: time of blood sampling.
) Remdesivir 100 mg administered in 1 h. (B) The plasma concentrations of remdesivir and GS-441524 4 h after administration (C4) on day 17 were 662 ng/mL and 58 ng/mL, respectively. However, the concentrations of remdesivir and GS-441524 18 h after administration (C18) were 32 ng/mL and 44 ng/mL, respectively. The peak values of remdesivir and GS-441524 were calculated to be 1576 ng/mL and 63 ng/mL, respectively, and the trough values were calculated to be 11 ng/mL and 40 ng/mL, respectively. The following formulas were used to calculate the values. The half-life (hr) = T1/2 = ln2 × (1/kel) = ln2 × (TC18-TC4)/(lnC4-lnC18). The peak value (ng/mL) = C0 = C4 × 2^{(TC4-TC0)/(T1/2)}. The trough value (ng/mL) = C23 = C18 × (1/2)^{(TC23-TC18)/(T1/2)}. Kel: elimination rate constant (hr-1), T: time of blood sampling.
With his wife’s consent, we administered remdesivir on compassionate use from day 12–21 (10 days) after LPV/r was discontinued. The loading dosage was 200 mg at 7:00 PM on day 12, then 100 mg daily at 0:00 PM from day 13–21 administered within 1 h. His serum creatinine concentration was 0.53 mg/dL (estimated glomerular filtration rate, 118.3 mL/min/1.73 m2) on day 12 and was within the normal range of 0.5–0.7 mg/dL during remdesivir treatment. Administration of extracellular fluid increased his body weight from 89 kg at admission to 108 kg on day 12. Lung permeability and respiratory status improved with treatment, and ECMO was liberated on day 27. The patient was extubated on day 34 with no re-exacerbation of pneumonia. After rehabilitation, he was discharged on day 87.
For pharmacokinetic analysis, the patient’s blood was collected from a central venous catheter placed in the left internal jugular vein at 7:00 AM (5:00 PM on day 17 only), and plasma concentrations were measured in the remaining samples on days 13, 14, 17, 18, and 20–26. Therefore, values of days 13, 14, 18, and 20–26 revealed 18 h after administration (C18), and that of day 17 was revealed 4 h after administration (C4). Each sample was measured using liquid chromatography-mass spectrometry, calibrated, and evaluated using the internal standard method. Coefficients of determination (R2) of remdesivir and GS-441524 were 0.997 and 0.993, respectively. All values were measured twice, and the mean concentrations were graphed.
Plasma concentrations of remdesivir and GS-441524 peaked on the third day of treatment. The C4 values on day 17 of remdesivir and GS-441524 were 662 ng/mL and 58 ng/mL, respectively, and the C18 values on day 18 were 32 and 44 ng/mL, respectively.
Discussion
Since COVID-19 treatment methods with antiviral drugs were not established in March 2020 [4] and the remdesivir was one of the candidate treatments for severe COVID-19, we have evaluated the pharmacokinetic data of remdesivir. Remdesivir inhibits SARS-CoV-2 in human cells in vitro [5]. The half-life of remdesivir is short, and when hydrolyzed to GS-441524, a nucleoside analog, in the plasma, its half-life is approximately 27 h [3]. A previous study reported pharmacokinetic data of remdesivir and GS-441524 within the first 24 h of administration in a patient with normal renal function. The plasma concentration of remdesivir immediately after administration (C0) was 3317 ng/mL, with a sharp decrease to 171 ng/mL at 1 h (C1) and below the quantification limit at 24 h (C24). The concentrations of plasma GS-441524 were 113 ng/mL, 184 ng/mL, and 93 ng/mL at C0, C1, and C24, respectively [6]. Another study in 23 patients with normal renal function (creatinine clearance>90 mL/min) revealed that the half-lives of remdesivir and GS-441524 on Days 7 and 14 combined were 0.92–1.09 h and 23.3–35.5 h, respectively. The peak concentrations of remdesivir and GS-441524 were 3220 and 231 ng/mL, respectively [7]. In our patients, the plasma concentrations of both remdesivir and GS-441524 were lower than those in previous studies [6], [7]. Additionally, the plasma concentration of remdesivir was not within the quantification limit, although blood samples were obtained 18 h after administration. Moreover, the half-life of remdesivir was three times longer than that reported in the manufacturer’s sheet. Despite insufficient knowledge regarding the pharmacokinetics of remdesivir, we hypothesized that using ECMO might significantly affect its pharmacokinetics via these mechanisms: sequestration into and release from the circuit, increase in the volume of distribution (Vd), and decrease in the excretion rates [8].
An association has been reported between the adsorption to ECMO and log P values, which indicates the compound’s liposolubility and could lead to increased Vd. Midazolam and propofol have high adsorption, with log P values of 3.9 and 4.0, respectively [9]. The log P of remdesivir is 3.2, and it may be adsorbed to the circuit, although the log P of GS-441524 is − 1.79 [10]. Lipophilic drugs and highly protein-bound drugs are significantly sequestered in the circuit [8]. The protein binding rate of remdesivir is as high as 87.9% [11], and drugs with higher protein binding were found to have higher losses despite similar lipophilicity [12]. The prolonged remdesivir half-life in our patient was possibly influenced by the increase in extracellular fluid during ECMO, which could increase Vd. Furthermore, although no interactions have been reported, lopinavir/ritonavir might have affected the pharmacodynamics or adsorption to ECMO.
This study was limited by the lack of data for days 15, 16, and 19. To our knowledge, this is the first case report to investigate the plasma concentrations of remdesivir and GS-441524 in patients undergoing ECMO. Remdesivir is one of the few effective drugs against COVID-19; however, further investigation of the pharmacokinetics of remdesivir during ECMO is necessary. Further studies are warranted as there are limits to the generalizability of a single case study compared to a broader observational cohort study.
Ethical approval
Informed consent was obtained from the patient for publication.
Informed consent
Informed consent was obtained from the patient for publication.
Funding
This work was supported by MHLW “Research on Emerging and Re-emerging Infectious Diseases and Immunization” [grant number 19HA1003].
CrediT authorship contribution statement
Satoshi Ide: Conceptualization, Data curation, Formal analysis, Writing – original draft. Sho Saito: Conceptualization, Data curation, Formal analysis, Writing – review & editing. Tsubasa Akazawa: Conceptualization, Data curation, Formal analysis, Writing – review & editing. Takahito Furuya: Conceptualization, Data curation, Formal analysis, Writing – review & editing. Junichi Masuda: Data curation, Writing – review & editing. Maki Nagashima: Data curation, Writing – review & editing. Tatsunori Ogawa: Writing – review & editing. Ryohei Yamamoto: Writing – review & editing. Haruhiko Ishioka: Writing – review & editing. Kohei Kanda: Writing – review & editing. Ayako Okuhama: Writing – review & editing. Yuji Wakimoto: Writing – review & editing. Tetsuya Suzuki: Writing – review & editing. Yutaro Akiyama: Writing – review & editing. Yusuke Miyazato: Writing – review & editing. Keiji Nakamura: Writing – review & editing. Takato Nakamoto: Writing – review & editing. Hidetoshi Nomoto: Writing – review & editing. Yuki Moriyama: Writing – review & editing. Masayuki Ota: Writing – review & editing. Shinichiro Morioka: Writing – review & editing. Wataru Matsuda: Writing – review & editing. Tatsuki Uemura: Writing – review & editing. Kentaro Kobayashi: Writing – review & editing. Ryo Sasaki: Writing – review & editing. Daisuke Katagiri: Writing – review & editing. Satoshi Kutsuna: Writing – review & editing. Kayoko Hayakawa: Writing – review & editing. Norio Ohmagari: Writing – review & editing.
Declaration of interest
The authors report no conflict of interest.
Acknowledgement
We thank Dr. Ryohei Oda, Dr. Akira Shimomura, and Dr. Takeshi Inagaki for patient care and all clinical staff at the National Center for Global Health and Medicine for their support.
Contributor Information
Satoshi Ide, Email: side@hosp.ncgm.go.jp.
Sho Saito, Email: ssaito@hosp.ncgm.go.jp.
Tsubasa Akazawa, Email: takazawa@hosp.ncgm.go.jp.
Takahito Furuya, Email: tfuruya@hosp.ncgm.go.jp.
Junichi Masuda, Email: jmasuda@hosp.ncgm.go.jp.
Maki Nagashima, Email: mnagamatsu@hosp.ncgm.go.jp.
Yusuke Asai, Email: yuasai@ri.ncgm.go.jp.
Tatsunori Ogawa, Email: togawa@hosp.ncgm.go.jp.
Ryohei Yamamoto, Email: ryoheiyamamoto11@gmail.com.
Haruhiko Ishioka, Email: ha7503@hotmail.com.
Kohei Kanda, Email: kkanda@hosp.ncgm.go.jp.
Ayako Okuhama, Email: ayokuhama@hosp.ncgm.go.jp.
Yuji Wakimoto, Email: yujiwakimoto.stat@gmail.com.
Tetsuya Suzuki, Email: tesuzuki@hosp.ncgm.go.jp.
Yutaro Akiyama, Email: yakiyama@hosp.ncgm.go.jp.
Yusuke Miyazato, Email: ymiyazato@hosp.ncgm.go.jp.
Keiji Nakamura, Email: keinakamura@hosp.ncgm.go.jp.
Takato Nakamoto, Email: tnakamoto@hosp.ncgm.go.jp.
Hidetoshi Nomoto, Email: hnomoto@hosp.ncgm.go.jp.
Yuki Moriyama, Email: moriyama.yuki1986@gmail.com.
Masayuki Ota, Email: maota@niid.go.jp.
Shinichiro Morioka, Email: shmorioka@hosp.ncgm.go.jp.
Wataru Matsuda, Email: wmatsuda@hosp.ncgm.go.jp.
Tatsuki Uemura, Email: tuemura@hosp.ncgm.go.jp.
Kentaro Kobayashi, Email: kkentaro@hosp.ncgm.go.jp.
Ryo Sasaki, Email: rysasaki@hosp.ncgm.go.jp.
Daisuke Katagiri, Email: dkatagiri@hosp.ncgm.go.jp.
Satoshi Kutsuna, Email: skutsuna@hosp.ncgm.go.jp.
Kayoko Hayakawa, Email: khayakawa@hosp.ncgm.go.jp.
Norio Ohmagari, Email: nohmagari@hosp.ncgm.go.jp.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., et al. Compassionate use of remdesivir for patients with severe covid-19. New Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldman J.D., Lye D.C.B., Hui D.S., Marks K.M., Bruno R., Montejano R., et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. New Engl J Med. 2020;383:1827–1837. doi: 10.1056/NEJMoa2015301. 〈https://www.nejm.org/doi/full/10.1056/NEJMoa2015301〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fact Sheet for health care providers – Emergency use authorization (EUA) of VEKLURY (remdesivir), 〈https://www.fda.gov/media/137566/download〉;2020.
- 4.Saito S., Hayakawa K., Mikami A., Izumi S., Funazaki H., Ashida S., Sugiura W., Sugiyama H., Kokudo N., Ohmagari N. Investigator initiated clinical trial of remdesivir for the treatment of COVID-19 in Japan. Glob Health Med. 2021;3:62–66. doi: 10.35772/ghm.2020.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tempestilli M., Caputi P., Avataneo V., Notari S., Forini O., Scorzolini L., Marchioni L., Ascoli Bartoli T., Castilletti C., Lalle E., Capobianchi M.R., Nicastri E., D'Avolio A., Ippolito G., Agrati C., COVID INMI Study G. Pharmacokinetics of remdesivir and GS-441524 in two critically ill patients who recovered from COVID-19. J Antimicrob Chemother. 2020;75:2977–2980. doi: 10.1093/jac/dkaa239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humeniuk R., Mathias A., Cao H., Osinusi A., Shen G., Chng E., Ling J., Vu A., German P. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects. Clin Transl Sci. 2020;13:896–906. doi: 10.1111/cts.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shekar K., Fraser J.F., Smith M.T., Roberts J.A. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care. 2012;27(741):e9–e18. doi: 10.1016/j.jcrc.2012.02.013. e7.41E18s. [DOI] [PubMed] [Google Scholar]
- 9.Lemaitre F., Hasni N., Leprince P., Corvol E., Belhabib G., Fillâtre P., Luyt C.E., Leven C., Farinotti R., Fernandez C., Combes A. Propofol, midazolam, vancomycin and cyclosporine therapeutic drug monitoring in extracorporeal membrane oxygenation circuits primed with whole human blood. Crit Care. 2015;19:40. doi: 10.1186/s13054-015-0772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Medicines Agency. Assessment report- Veklury., 〈https://www.ema.europa.eu/en/documents/assessment-report/veklury-epar-public-assessment-report_en.pdf〉;2020.
- 11.Jorgensen S.C.J., Kebriaei R., Dresser L.D. Remdesivir: review of pharmacology, pre-clinical data, and emerging clinical experience for COVID-19. Pharmacotherapy. 2020;40:659–671. doi: 10.1002/phar.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ha M.A., Sieg A.C. Evaluation of altered drug pharmacokinetics in critically ill adults receiving extracorporeal membrane oxygenation. Pharmacotherapy. 2017;37:221–235. doi: 10.1002/phar.1882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.