Graphical abstract
Keywords: COVID-19, Coagulopathy, Immunothrombosis, Pathophysiology, Venous thromboembolism
Abstract
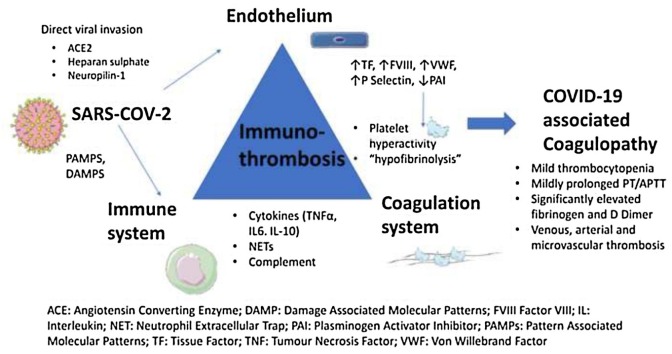
The coagulopathy of COVID-19 is characterised by significantly elevated D Dimer and fibrinogen, mild thrombocytopenia and a mildly prolonged PT/APTT. A high incidence of thrombotic complications occurs despite standard thromboprophylaxis. The evidence to date supports immunothrombosis as the underlying mechanism for this coagulopathy which is triggered by a hyperinflammatory response and endotheliopathy. A hypercoagulable state results from endothelial damage/activation, complement activation, platelet hyperactivity, release of Extracellular Neutrophil Traps, activation of the coagulation system and a “hypofibrinolytic” state. Significant cross-talk occurs between the innate/adaptive immune system, endothelium and the coagulation system. D dimer has been shown to be the most reliable predictor of disease severity, thrombosis, and overall survival. In this context, targeting pathways upstream of coagulation using novel or repurposed drugs alone or in combination with other anti-thrombotic agents may be a rational approach to prevent the mortality/morbidity due to COVID-19 associated coagulopathy.
1. Introduction
The emergence of coronavirus 2019 (COVID-19), a new disease entity caused by the severe acute respiratory syndrome coronavirus 2 (SARS−COV2) virus has led to an unprecedented global health crisis. It was first declared a global pandemic by the World Health Organisation on 11 March 2020 and as of 10 September 2021, over 223 million cases have been reported worldwide, with 4.6 million deaths attributed to this devastating disease. The clinical spectrum ranges from asymptomatic carriers to a critical illness manifested by acute respiratory distress syndrome which occurs in about 5% of patients typically around day 10 of the onset of illness and can progress to respiratory failure, multiorgan failure and death (Berlin et al., 2020). While the reasons that predispose some individuals to a more severe illness are poorly understood, severe disease has been associated with a hypercoagulable state. Indeed, the coagulopathy associated with COVID-19 characterised by elevated d-dimer and fibrin degradation products (FDPs) has been shown to correlate with disease severity and increased mortality (Tang et al., 2020a). In addition, COVID-19 is associated with an increased rate of thrombotic complications including microvascular, venous thromboembolism and arterial thrombosis (McFadyen et al., 2020). Further, the incidence of thrombotic complications associated with COVID-19 appears to be higher in intensive care unit (ICU) patients with COVID-19 than Non−COVID-19 ICU patients and other respiratory viruses such as Middle East respiratory syndrome (MERS) coronavirus and influenza viruses (Nopp et al., 2020). Various mechanisms have been proposed to explain the coagulopathy caused by COVID-19, although immunothrombosis, an interplay between the immune system and the coagulation pathway is believed to be the primary underlying mechanism. In this narrative review we discuss the pathophysiology, clinical, laboratory and therapeutic implications of COVID-19 coagulopathy. A summary of the pathophysiology is shown in Fig. 1 .
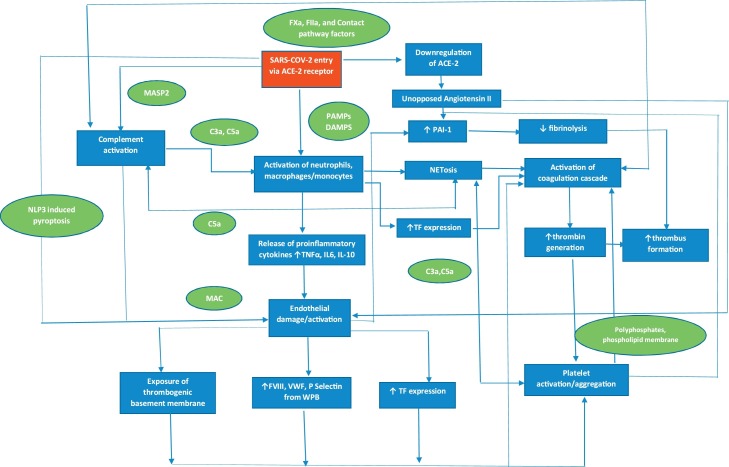
Fig. 1.
Pathophysiology of COVID-19 associated coagulopathy. SARS-CoV2 triggers the release of cytokines from monocytes, macrophages and neutrophils leading to a cytokine storm. This results in activation of monocytes, macrophages and neutrophils with upregulation of tissue factor and release of NETs. The endothelium is damaged/activated due to pyroptosis induced by direct viral invasion, release of cytokines, complement activation and downregulation of ACE2. This leads to exposure of the thrombogenic basement membrane, upregulation of tissue factor and release of factor VIII, VWF and P-Selectin from WPB resulting in activation of platelets and coagulation factors. Fibrinolysis is also suppressed due to inhibition of PAI-I further contributing to the procoagulant state. In addition, there is significant cross talk between the immune, complement, and coagulation systems leading to a positive feedback loop, thus amplifying this response. ACE-2, angiotensin converting enzyme 2; C, complement; COVID-19, coronavirus disease 2019; IL, interleukin; NET, neutrophil extracellular trap; MASP2, Mannan-binding lectin serine protease 2; MAC; membrane attack complex; NLP3, NLR pyrin domain containing 3; PAI-1, plasminogen activator inhibitor 1; SARS-CoV2, severe acute respiratory syndrome coronavirus 2; TF, tissue factor; TNF, tumour necrosis factor; WPB, Weibel Palade body.
2. Pathophysiology of COVID-19 coagulopathy
2.1. Entry of SARS-Cov-2 via ACE2
SARS−COv-2 enters the host cell by binding to the transmembrane Angiotensin Converting Enzyme 2 (ACE2) receptor via the S1 subunit of its spike protein. This receptor is widely expressed in a variety of cell types and organs throughout the body including the lungs, cardiovascular system, gut, kidneys, central nervous system, and adipose tissue. In addition to ACE2, SARS−COV-2 also binds to heparan sulphate, a cell surface glycosaminoglycan via the receptor binding domain on S1 which induces a conformational change in of S1, thus enhancing its interaction with ACE2 (Clausen et al., 2020). Fusion of the S2 subunit with the host membrane is facilitated by a serine protease, Transmembrane protease serine 2 (TMPRSS2) which primes this step by proteolytic cleavage of the S2 binding site. Binding of SARs−COV-2 to ACE2 leads to subsequent downregulation of surface ACE2 expression which may be mediated by proteolysis and ectodomain shedding of ACE-2 by A Disintegrin And Metalloproteinase-17 (ADAM-17) (Gheblawi et al., 2020). ACE-2 is an important mediator of the Renin-Angiotensin System, converting Angiotensin II to Angiotensin 1–7. Angiotensin II is a potent vasoconstrictor which has pro-inflammatory and pro-fibrotic effects while Angiotensin 1–7 is a vasodilator which negatively regulates the Renin Angiotensin system and has cardioprotective effects. Downregulation of ACE-2 therefore leads to unopposed angiotensin II effects, including proinflammatory, prothrombotic, and pro-oxidant risks. In addition, differences in tissue expression of ACE-2 receptor and activating proteases may contribute to unique aspects of the pathophysiology of different coronaviruses.
2.2. Systemic inflammatory response versus “cytokine storm”
Severe COVID-19, is characterised by a hyperinflammatory response with elevated levels of ferritin, CRP, and cytokines such as interleukin-2R (IL-2R), IL-6, IL-8, IL-10, and Tumour Necrosis Factor alpha (TNF-α) (Henry et al., 2020; Chen et al., 2020). In particular, IL-6 has been shown to be selectively induced by SARS-CoV-2 and circulating IL-6 levels are closely associated with the severity of COVID-19 (Tang et al., 2020b). The release of proinflammatory cytokines is believed to be triggered by pathogen-associated molecular patterns (PAMPs) and host derived damage-associated molecular patterns (DAMPs) leading to activation of the immune system (Iba et al., 2020a) of which macrophages and monocytes are believed to play a key role (Merad and Martin, 2020). This is supported by the detection of macrophage/monocyte attracting chemokines such as monocyte chemotactic protein 1 (MCP-1), interferon-inducible protein-10, macrophage inflammatory protein-1α in the Bronchial Alveolar Lavage Fluid (BALF) obtained from COVID-19 patients (Xiong et al., 2020) and similarities in the cytokine profile of patients with severe COVID-19 pneumonia and other hyperinflammatory syndromes such as macrophage activation syndrome or secondary haemophagocytosis lymphohistiocytosis (McGonagle et al., 2020a; Mehta et al., 2020).
In children, a delayed hyperinflammatory syndrome has also been recognised which typically occurs 2–6 weeks after SARS-CoV-2 infection. This rare but serious condition has been termed multisystem inflammatory syndrome in children associated with COVID-19 (MIS-C) or paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) although it has also been reported in adolescents (Jiang et al., 2020). It shares similar clinical features with Kawasaki disease, but affects older children and more often presents with intestinal involvement, myocardial failure and shock (Brodin, 2021).
Although the hyperinflammatory response has been referred widely in the literature as “cytokine storm syndrome”, use of this term in COVID-19 is controversial. Critics have argued that to date there has been no standard definition for the “cytokine storm syndrome” and that compared to non−COVID-19 acute respiratory distress syndrome (ARDS), levels of IL-6 and other cytokines in severe COVID-19 patients are significantly lower (typically 10–200 fold) (Sinha et al., 2020; Kox et al., 2020). Further, distinguishing an appropriate from a dysregulated inflammatory response in the pathophysiology of critical illness is challenging and this has implications for the use of anti-cytokine therapies such as IL-6 inhibitors and high dose corticosteroids which may block pathways critical to host immune response. This has led to the proposal to use the alternative term “systemic inflammatory response syndrome” which has historically been described in patients with sepsis (Sinha et al., 2020).
While severe COVID-19 is associated with an excessive inflammatory response, it should be noted that immunosuppression or immune-paralysis occurs concurrently in patients with severe COVID-19. This manifests as progressive lymphopenia, with a decrease in lymphocytes, predominantly CD4+ and CD8 + T cells, resulting in a decrease in numbers as well as IFN-γ production by CD4 + T cells which may correlate with disease severity (Chen et al., 2020; Kiselevskiy et al., 2020; Triggle et al., 2021).
An association between hyperinflammation characterised by elevated cytokine levels and coagulopathy is supported by work by Ranucci et al. who demonstrated a correlation between IL-6 and fibrinogen levels in patients with COVID-19 acute respiratory distress syndrome (Ranucci et al., 2020). A few mechanisms have been postulated to explain how this hyperinflammatory response leads to thrombosis (Iba et al., 2020b) which include: (1) increased expression of tissue factor (TF) on monocytes/macrophages and vascular endothelial cells stimulated by pro-inflammatory cytokines such as Tumour necrosis factor-α, IL-1β, IL-6 thus promoting coagulation via the extrinsic pathway (MacKman et al., 2020) (2) suppression of the fibrinolytic system by decreased activity of urokinase-type plasminogen activator and increased release of plasminogen activator inhibitor-1 (3) platelet activation by various proinflammatory cytokines and (4) endothelial damage by the inflammatory reaction. Conversely, thrombin generation enhances the release of pro-inflammatory cytokines such as IL-6 and TNF-a by monocytes through protease-activated receptors (PARs). Thus there is significant cross-talk between inflammation and coagulation which leads to a vicious cycle. (Levi and Hunt, 2020).
2.3. Endotheliopathy/endotheliitis
The intact endothelium plays an important role in maintaining haemostasis under physiological conditions by providing a protective glycocalyx barrier to the thrombogenic subendothelial basement membrane, as well as regulating tight junctions, adhesion molecules and vascular tone. It also has antithrombotic properties through the expression of antiplatelet (e.g. nitric oxide and prostacyclin) and anticoagulant (e.g. antithrombin, thrombomodulin, Endothelial protein C receptor (EPCR), and heparin-like proteoglycans) mediators (Yau et al., 2015). SARs-Cov-2 infection results in endothelial damage/activation which can be mediated via direct or indirect mechanisms. Evidence for direct viral infection of the endothelial cell is supported by a study by (Varga et al. (2020)) who showed that direct viral infection was associated with diffuse endothelial inflammation and apoptosis. Several mechanisms for this have been described of which activation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome (Freeman and Swartz, 2020), leading to pyroptosis, an inflammatory form of programmed cell death usually seen in infections by intracellular pathogens is believed to play a major role (Manolis et al., 2020; Price et al., 2020). The endothelium can also be activated by indirect mechanisms including pro-inflammatory cytokines, downregulation of ACE2, hypoxia, complement activation, and Neutrophil Extracellular Traps (NET)osis (O’Sullivan et al., 2020). This in turn leads to activation of the coagulation system through a number of mechanisms (Teuwen et al., 2020) such as (1) exposure of the thrombogenic basement membrane (2) release of factor VIII (FVIII), von Willebrand factor (VWF) and P-selectin from Weibel-Palade bodies (WPB) and (3) release of Vascular Endothelial Growth factor (VEGF), from activated platelets which causes upregulated expression of TF.
Evidence for endothelial activation/dysfunction leading to coagulopathy is supported by biomarker and postmortem studies in COVID-19 patients. Goshua et al. showed that markers of endothelial cell activation, including VWF antigen/antibody, FVIII activity, and thrombin antithrombin (TAT) complexes were significantly higher in intensive care unit (ICU) patients with severe COVID-19 compared to non-ICU patients (Goshua et al., 2020). In addition, a post-mortem study by Ackermann et al. demonstrated that alveolar capillary microthrombi were nine times as prevalent in patients who died from respiratory failure caused by SARS-CoV-2 infection as in patients with influenza, thus supporting the hypothesis of endothelial injury-mediated microvascular thrombosis in COVID-19 (Ackermann et al., 2020).
2.4. Platelet hyperactivity
While platelets have an established role in haemostasis and thrombosis, their role in the immune response to pathogens is also increasingly being recognised (Koupenova et al., 2018). Thrombocytopenia occurs in 5–40 % of patients with COVID-19(Larsen et al., 2020) and is associated with severe disease and increased mortality.(Lippi et al., 2020) Recent studies have also demonstrated that COVID-19 is associated with platelet hyperreactivity, (Manne et al., 2020; Hottz et al., 2020; Zaid et al., 2020) thus suggesting that platelets may contribute to COVID 19 coagulopathy. Unlike sepsis induced coagulopathy/disseminated intravascular coagulopathy (SIC/DIC) however, the coagulopathy associated with COVID-19 is characterised by significantly elevated D Dimer levels and fibrinogen and only mild thrombocytopenia. The exact mechanisms for how platelets may contribute to coagulopathy in COVID-19 have yet to be elucidated although several possible mechanisms have been discussed in recent reviews (McFadyen et al., 2020; Koupenova, 2020). One leading hypothesis is internalisation of the SARs-CoV2 virus by platelets which has been previously described in studies of patients infected with influenza, a single stranded RNA virus similar to SARs-CoV-2. This leads to activation of the Toll-like receptor TLR-7 resulting in the release of complement C3 from platelet alpha granules which in turn stimulates the release of NETs, a scaffold and potent activator of coagulation via multiple mechanisms. While there is ongoing debate as to whether internalisation of SARS-CoV-2 by platelets occurs and whether ACE2 the main receptor for SARS-CoV2 is expressed on platelets, platelets can also be activated by other mechanisms such as the release of tissue factor, platelet activating factor (PAF) and binding of platelet GP (Glycoprotein) VI to collagen. Coagulation is subsequently triggered by platelet degranulation and aggregation, chemokine induced leucocyte-platelet aggregates, as well as release of platelet derived inorganic polyphosphate and tissue bearing microvesicles.
2.5. Neutrophil extracellular traps: scavenger and scaffold
As a first-line host defence against foreign microbes, neutrophils release NETs, web-like structures composed of Deoxyribonucleic acid (DNA) filaments coated with histones and granule proteins. However, in addition to its immune function, NETs also provide a scaffold for platelet, red blood cells, TF bearing extracellular vesicles and pro-coagulant molecules (e.g. VWF, fibronectin, fibrinogen, FXII, and TF), thus leading to activation of platelets and the coagulation system. The pro-coagulant effect of NETs is perpetuated by cross-talk between the endothelium, platelets and neutrophils/NETs resulting in a vicious circle which leads to further thrombus formation (Laridan et al., 2019). Of note, studies on respiratory viral infections have demonstrated a key role for neutrophils in clearing viruses in the lung by phagocytosing viral particles and by releasing NETs (Tate et al., 2009; Camp and Jonsson, 2017). In keeping with this, high levels of markers of NETs (cell-free DNA, myeloperoxidase-DNA complexes, and citrullinated histone H3) are found in the plasma of patients with COVID-19 which have been shown to corelate with disease severity (Middleton et al., 2020; Leppkes et al., 2020; Zuo et al., 2020a). Further, in lung tissue obtained from autopsies of COVID-19 patients, NETs were associated with microthrombi and platelet deposition (Middleton et al., 2020; Leppkes et al., 2020; Nicolai et al., 2020). Interestingly, NETosis can be induced by plasma from COVID-19 patients (Middleton et al., 2020; Nicolai et al., 2020) which can be successfully blocked by a NET-inhibitory peptide (Middleton et al., 2020). In clinical studies, markers of NETs were associated with higher risk of morbid thrombotic events despite prophylactic anticoagulation in hospitalised COVID-19 patients (Zuo et al., 2020b). Taken together, this suggests that NETosis plays an important role in COVID-19 associated coagulopathy/thrombosis.
2.6. Complement activation: friend or foe
The complement system is a key mediator of the innate immune response that can be activated by three pathways (classical, lectin, and alternative) of which binding of the lectin pathway of complement component mannan-binding lectin (MBL) via the SARS CoV-1 spike protein is believed to be the likely mechanism of systemic activation of complement in COVID-19 (Gralinski et al., 2018). C3a and C5a anaphylatoxins recruit and/or activate neutrophils, monocytes, endothelial cells and platelets resulting in the release of proinflammatory cytokines which promotes coagulation as previously described. Significant interaction occurs between the complement and coagulation systems. C5a upregulates TF expression on neutrophils and endothelial cells and stimulates secretion of VWF from endothelial cells. In addition, C5a also impairs fibrinolysis by increasing the release of plasminogen activator inhibitor 1 (PAI-1) from mast cells and basophils (Foley and Conway, 2016). Coagulation factors FXa, FXIa and plasmin in turn cleave both C5 and C3, to generate C5a and C3a while thrombin cleaves C5 to generate C5a (Amara et al., 2008). The complement system is also activated by Contact pathway factors, such as FXIIa which activates complement C1, leading to activation of the classical pathway of complement (Ghebrehiwet et al., 1983), and kallikrein which cleaves both C3 and factor B (Irmscher et al., 2018). In addition, indirect activation of coagulation by the complement system occurs via C5a induced NETosis while neutrophils themselves can activate the complement system. Finally, there is also reciprocal activation between platelets and the complement system (Peerschke et al., 2006).
Excessive complement activation leading to widespread microvascular thrombosis is known to occur in a number of pathological settings. The classic example is atypical haemolytic-uremic syndrome (aHUS), a rare disorder of uncontrolled complement activation characterised by microangiopathic haemolytic anaemia, thrombocytopenia, and acute renal failure. (Sakari Jokiranta, 2017). Evidence that the complement system may play a role in the coagulopathy of COVID-19 in at least a subset of patients was demonstrated in a case series of 5 patients with severe COVID-19. Autopsies of these patients showed evidence of deposition of C5b-9 (membrane attack complex), C4d, and mannose binding lectin (MBL)-associated serine protease (MASP)2 in the microvasculature of their lungs and skin. In addition, two of these patients also had co-localization of COVID-19 spike glycoproteins with C4d and C5b-9 in the interalveolar septa and the cutaneous microvasculature (Magro et al., 2020). In this context, studies of complement inhibition in murine models of SARS−COV and MERS−COV, coronaviruses similar to SARS−COV-2, have demonstrated improved outcomes (Gralinski et al., 2018; Jiang et al., 2018) suggesting a role for complement inhibitors in COVID-19.
2.7. Antiphospholipid antibodies epiphenomenon or pathogenic
Antiphospholipid syndrome is an acquired thrombophilia characterised by thrombotic events or pregnancy morbidity and the presence of persistently positive antiphospholipid antibodies. Evidence for an association between antiphospholipid antibodies and COVID-19 coagulopathy/thrombosis emerged from an early report of three COVID-19 ICU patients with multiple cerebral infarcts who tested positive for IgA anticardiolipin antibodies and IgA/G anti-Beta 2-glcoprotein antibodies (Zhang et al., 2020a). This was followed by reports of a high incidence of positive Lupus anticoagulant (45–87 %) among non-ICU and ICU patients with COVID-19 (Harzallah et al., 2020; Helms et al., 2020). Subsequently, the presence of antiphospholipid antibodies and/or lupus anticoagulant in COVID-19 patients was confirmed in other studies (Xiao et al., 2020; Zhang et al., 2020b; Amezcua-Guerra et al., 2020; Bertin et al., 2020) with one study demonstrating a correlation between IgG anticardiolipin antibodies and severity of disease (Bertin et al., 2020). This has been tempered by counter-arguments that antiphospholipid antibodies often appear transiently during critical illness and infections, the use of heparin and marked elevation of C-Reactive Protein (CRP) in most of these patients which can cause false positive lupus anticoagulant tests, the inclusion of non-criteria antiphospholipid antibodies and the baseline high incidence of thrombosis in the patient populations studied (Connell et al., 2020). Indeed, several subsequent studies have shown that the detection of lupus anticoagulant was not associated with an increased risk of thrombosis. In addition, cases of triple positive antibodies were rare, and the antibodies were transient in most cases (negative on repeat testing) (Devreese et al., 2020; Siguret et al., 2020; Gatto et al., 2020) suggesting that antiphospholipid antibodies are an epiphenomenon rather than pathogenic in COVID-19 coagulopathy. Interestingly, however, a recent Ex-vivo study by (Zuo et al. (2020c)) demonstrated that IgG fractions purified from COVID-19 patient serum positive for antiphospholipid antibodies (anti-beta2-glycoprotein, anti-phosphatidylserine/prothrombin) promoted NET release similar to Immunoglobulin G (IgG) isolated from individuals with established antiphospholipid syndrome. Further, when injected into mice, thrombosis was stimulated, suggesting that these auto-antibodies are potentially pro-thrombotic. Further studies are therefore required to clarify the presence, temporal association and role of antiphospholipid antibodies in COVID-19 patients.
2.8. Fibrinolytic system shutdown or consumption
Suppressed fibrinolysis due to elevated levels of PAI-1 has been described in acute lung injury and SIC/DIC (Idell, 2003; Iba and Levy, 2020). This has led to the suggestion that abnormalities in the fibrinolytic system may play an important role in acute lung injury caused by COVID-19 associated coagulopathy. Indeed, in a mouse model of acute lung injury by SARS-CoV infection, levels of Serpine1, a urokinase inhibitor were elevated, while Serpine1 knockout mice demonstrated increased fibrinolytic activity and haemorrhage in the lungs thus highlighting the role of the urokinase pathway in the pathogenesis of SARS-CoV induced acute lung injury (Gralinski et al., 2013). In humans, impaired fibrinolysis has also been reported in SARS-CoV2 infected patients which was mainly associated with high PAI-1 levels (Nougier et al., 2020). In addition, fibrinolysis shutdown was reported in a significant proportion of COVID-19 ICU patients using viscoelastic testing and this was associated with a higher incidence of thrombotic complications (Wright et al., 2020). While the exact mechanisms for suppressed fibrinolysis in Covid‐19 are uncertain, plausible mechanisms include activation of Thrombin Activable Fibrinolysis Inhibitor (TAFI) by high concentrations of thrombin due to a hypercoagulable state and increased release of PAI-1 from endothelial cells and activated platelets secondary to inflammation (Nougier et al., 2020). Another possible mechanism is overactivation of the Renin-Angiotensin system due to SARS-CoV-2 mediated downregulation of ACE2, leading to unopposed Angiotensin II effects which increases the expression of PAI-1 (Lazzaroni et al., 2020; Hanff et al., 2020). Of note, however, significantly elevated D dimer, a breakdown product of fibrin is a common feature of late, severe disease, suggesting that the fibrinolytic system is still functional. This has led to the alternative hypothesis that “consumptive fibrinolysis”, a failing attempt of the fibrinolytic system to remove fibrin and necrotic tissue from the lung parenchyma, with the fibrinolytic system being consumed or overwhelmed in the process occurs rather than “fibrinolytic shutdown” per se (Medcalf et al., 2020). The same authors propose a “plasmin paradox” in which anti-fibrinolytics may be protective in the early stages of COVID-19 through an anti-viral effect, whereas exogenous plasmin(ogen) or plasminogen activators may be useful in later stages to enhance fibrinolysis (Medcalf et al., 2020).
3. Role of biomarkers in COVID-19 coagulopathy
COVID-19 associated coagulopathy is characterised by significantly elevated D Dimer and fibrinogen, mild thrombocytopenia and mildly prolonged prothrombin (PT) and activated partial thrombin time (APTT). This laboratory pattern is distinct from DIC/SIC and other coagulopathies (Iba et al., 2020a) although progression to DIC can also occur. DIC typically occurs between 7–10 days after admission, but can occur as early as 4 days and may be due to secondary causes independent of COVID-19 effects, such as prolonged hospitalization, mechanical ventilation, superinfection, and other typical ICU aetiologies (Connors and Levy, 2020). D Dimer is the most well-studied biomarker and consistent predictor of outcome in COVID-19 patients. Studies have shown that levels of D Dimer correlate with disease severity (Lippi and Favaloro, 2020) and can be used to predict the risk of venous thromboembolism, (Nopp et al., 2020) need for ventilatory support, (Berger et al., 2020) and mortality.(Gungor et al., 2020) Interim guidelines from the International Society on Thrombosis and Haemostasis (ISTH) recommend performing D Dimer, prothrombin (PT), platelet count and fibrinogen on admission as a risk stratification tool (Thachil et al., 2020). It has also been suggested that D Dimer, PT, and platelet count should be repeated every 2–3 days to monitor for the development of coagulopathy (Levi et al., 2020).
4. Thrombotic complications
A high incidence of thrombotic complications occurs in COVID-19 patients with venous or arterial thrombotic complications reported in one-third of ICU patients despite pharmacological thromboprophylaxis (Klok et al., 2020). VTE accounts for the majority of thrombotic events although arterial thrombosis such stroke and acute myocardial infarction also occurs (Cheruiyot et al., 2020). Diagnosis of VTE is challenging and may be underestimated for various reasons including the need to limit patient contact with health personnel, limited resources in hospitals overwhelmed by high caseloads, and inability to perform diagnostic imaging in unstable patients (e.g. inability to perform Computer Tomography Pulmonary Angiogram (CTPA) in ventilated patients lying prone). The reported incidence of venous thromboembolism varies depending of disease severity (ICU versus Non-ICU patients) and whether screening of asymptomatic DVT cases was performed. A meta-analysis reported an overall VTE prevalence estimate of 14.1 % (95 % confidence interval [CI], 11.6–16.9) with a higher incidence in those who underwent ultrasound screening (40.3 % 95 % CI, 27.0–54.3) compared to those without screening (9.5 % 95 % CI, 7.5–11.7). The VTE prevalence was also higher in ICU 22.7 % (95 % CI, 18.1–27.6) versus non-ICU patients 7.9 % (95 % CI, 5.1–11.2) (Nopp et al., 2020). In another meta-analysis, VTE occurred in 24 % (95 % PI, 5%–66 %), PE in 19 % (95 % PI, 6%–47 %), and DVT alone in 7% (95 % PI, 0%–69 %) of ICU patients (Porfidia et al., 2020). Taken together, this suggests a higher incidence of VTE in patients with severe COVID-19 compared to historical cohorts of ICU patients (5%–15%) (Nopp et al., 2020). Interestingly, the prevalence of pulmonary embolism (PE) appears to be disproportionately high relative to deep venous thrombosis (DVT) with one study reporting PE accounting for up to 80 % of all thrombotic events in ICU patients (Klok et al., 2020) suggesting that the underlying mechanism is primary in-situ thrombosis (pulmonary arterial thrombosis) rather than embolism. This has led to the use of new terminology such as ‘diffuse pulmonary intravascular coagulation’ or ‘microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome’ (MicroCLOTS) to describe this phenomenon (McGonagle et al., 2020b; Ciceri et al., 2021). In keeping with this theory, an autopsy series of 11 patients with COVID-19 found thrombosis predominantly affecting small and mid-sized (subsegmental/segmental) pulmonary arteries (Lax et al., 2020).
5. Therapeutic implications
5.1. Institutional guidelines
Most societal guidelines recommend thromboprophylaxis with low molecular weight heparin (LMWH) or unfractionated heparin (UFH) in all hospitalised patients unless contraindicated (Thachil et al., 2020; Bikdeli et al., 2020a; Spyropoulos et al., 2020; Moores et al., 2020). However, there is no consensus regarding the optimal risk stratification, intensity and duration of anticoagulation or whether post-hospital discharge should be given (Flaczyk et al., 2020). In brief, whereas guidelines by the American College of Chest physicians (ACCP) suggest standard dose thromboprophylaxis only in hospitalised patients with COVID-19 (Moores et al., 2020), ISTH Scientific and Standardisation Committee (SSC) goes beyond this to suggest intermediate dose low molecular weight heparin be considered in severe or high risk patients (Spyropoulos et al., 2020). In addition, ISTH SCC and the American College of Cardiology (ACC) also suggest consideration of post hospital discharge thromboprophylaxis for approximately 14 days in patients at high risk of thrombosis with a low risk of bleeding (Bikdeli et al., 2020a; Spyropoulos et al., 2020). Therapeutic anticoagulation is not currently recommended for primary prevention although ISTH SSC suggest consideration of increased intensity of anticoagulation (i.e., from standard or intermediate intensity to therapeutic intensity) in patients without confirmed VTE or PE but with deteriorating pulmonary status or acute respiratory distress syndrome (ARDS). In this context, a number of randomised controlled trials comparing intermediate/therapeutic vs standard prophylactic dose UFH/LMWH in hospitalised COVID-19 patients are currently underway/have been completed which will further inform guidelines (see Table 1 ) (Tritschler et al., 2020). Recently, results from the ACTIV-4 clinical trial (NCT04505774), part of an international collaboration of 3 linked studies (ACTIV-4, ATTAC and REMAP-CAP) using a multi-platform trial design have been published. This demonstrated that therapeutic anticoagulation is superior to standard care thromboprophylaxis in terms of reduced need for organ-support for moderate but not severe COVID-19 without a significant rate of bleeding (Investigators et al., 2021a, b). A recent randomised trial comparing standard to intermediate dose enoxaparin for thromboprophylaxis in COVID-19 patients admitted to ICU also did not show any significant difference in the composite outcome of arterial or venous thrombosis, need for extracorporeal membrane oxygenation or 30-day mortality (INSPIRATION Investigators et al., 2021). Taken together, these preliminary results do not support the use of higher intensity anticoagulation in unselected ICU patients with COVID-19. In terms of confirmed PE, most societies suggest the use of established guidelines. However, LMWH may be preferred in the inpatient setting over vitamin K antagonists (VKA), unfractionated heparin or direct oral anticoagulants (DOACs) for a number of reasons. For example, DOACs and warfarin may be limited by drug-drug interactions (e.g. interaction with antiviral agents such as lopinavir/ritonavir and other immunomodulatory investigational COVID-19 therapies). The limited experience and availability of specific reversal agents for DOACs may be also be a concern. Another concern is the need for monitoring with warfarin and unfractionated heparin which may increase health care worker exposure due to frequent blood draws. Finally, heparin resistance due to acute phase reactants may be an issue with unfractionated heparin.
Table 1.
Clinical trials of potential therapeutic agents targeting immunothrombosis in COVID-19.
| Study | Design | Target sample size |
Population | Intervention | Control | Primary Outcome |
Established/postulated mechanisms for investigational agent |
|---|---|---|---|---|---|---|---|
| Systemic heparin/low molecular weight heparin | |||||||
| COVID-HEP NCT04345848 |
Randomised, open-label, multicentre, clinical trial | 200 | 1) Non-ICU patients with d-dimer >1000 μg/L or 2) ICU patients | Therapeutic LMWH or UFH | Prophylactic LMWH or UFH (augmented dose for ICU patients) | Composite outcome of arterial or venous thrombosis, DIC, and all-cause mortality (30 days) | Anticoagulant Anti-inflammatory Antiviral |
| HEP-COVID NCT04367831 |
Randomised, open-label, multicentre, clinical trial | 308 | Patients with d-dimer >4 × ULN or SIC score ≥4 stratified by ICU vs non- ICU stay | Therapeutic LMWH | Prophylactic or intermediate dose LWMH or UFH | Composite outcome of arterial thromboembolic events, venous thromboembolic events, and all-cause mortality (30 days) | Anticoagulant Anti-inflammatory Antiviral |
| IMPACT NCT04406389 |
Randomised, open-label, clinical trial | 186 | Non-ICU or ICU patients requiring supplemental oxygen and d-dimer >3× ULN | Therapeutic LMWH, UFH, fondaparinux, or argatroban | Intermediate dose LMWH, UFH, or fondaparinux |
Mortality (30 days) | Anticoagulant Anti-inflammatory Antiviral |
| X-Covid 19 NCT04366960 |
Randomised, open-label, multicentre, clinical trial |
2712 | Non-ICU patients | Intermediate-dose LMWH | Prophylactic LMWH | Objectively confirmed venous thromboembolism (30 days) |
Anticoagulant Anti-inflammatory Antiviral |
| IMPROVE-COVID NCT04367831 |
Cluster randomised, open-label, single-centre, adaptive trial |
100 | ICU patients | Intermediate-dose LMWH or UFH |
BMI- and weight-adjusted prophylactic dose LMWH |
Clinically relevant venous or arterial thrombotic events in ICU (30 days) |
Anticoagulant Anti-inflammatory Antiviral |
| ACTIV-4 NCT04505774 |
Randomized, open label, adaptive platform trial | 2000 | Hospitalised patients with confirmed COVID-19 | Therapeutic dose UFH or LMWH | Prophylactic dose UFH or LMWH | Organ Support (respiratory or vasopressor) Free Days | Anticoagulant Anti-inflammatory Antiviral |
| ATTACC NCT04372589 |
Randomized, open-label, multicentre, adaptive clinical trial |
Adaptive with maximum of 3000 | Patients with COVID-19 requiring hospitalisation or hospitalised not on mechanical ventilation | Therapeutic dose UFH or LMWH | Local standard care thromboprophylaxis | Mortality and days free of organ support | Anticoagulant Anti-inflammatory Antiviral |
| REMPA-CAP NCT02735707 |
Randomised, embedded, multifactorial, adaptive platform trial | Estimated enrolment 7100 | Patients admitted to an ICU for severe CAP within 48 h of hospital admission | Therapeutic dose UFH or LMWH | Local standard care thromboprophylaxis | Mortality and days free of organ support | Anticoagulant Anti-inflammatory Antiviral |
| RAPID COVID COAG NCT04362085 |
Randomised, pragmatic, open-label, multicentre, adaptive clinical trial | 462 | Hospitalised, Non-ICU patients with D Dimer ≥2 times ULN or above ULN and Oxygen saturation ≤93 % | Therapeutic dose UFH or LMWH | Local standard care thromboprophylaxis | Composite outcome of ICU admission, non-invasive positive pressure ventilation, invasive mechanical ventilation, or all-cause death up to 28 days | Anticoagulant Anti-inflammatory Antiviral |
| Nebulised heparin | |||||||
| NEBUHEPA NCT04530578 |
Randomised, open-label, clinical trial | 200 | Patients with suspected COVID-19 and severe acute respiratory syndrome | Nebulised unfractionated heparin and prophylactic dose LMWH | Prophylactic LMWH | Requirement for mechanical ventilation | Anticoagulant Anti-inflammatory Antiviral |
| CHARTER-MT NCT04545541 |
Randomised, open label and blinded placebo controlled, multicentre, clinical trials (Meta-trial) |
202 | Mechanically ventilated COVID-19 patients | Nebulised unfractionated heparin and prophylactic dose LMWH | Standard care and nnebulised 0.9 % sodium chloride (5 mL) in placebo-controlled studies | Alive and Ventilator Free Score | Anticoagulant Anti-inflammatory Antiviral |
| Fibrinolytic therapy | |||||||
| NCT04357730 | Randomised, open-label, multicentre, clinical trial (phase 2a) | 60 | Patients with known/suspected COVID-19 and ARDS | IV Alteplase 50 mg +/- re-bolus in patients who shown initial transient response | Standard care | PaO2/FiO2 improvement from pre-to-post intervention | Fibrinolytic |
| PACA NCT04356833 |
Non-randomised, open-label, single-centre clinical trial (phase 2) |
24 | Patients with COVID-19 and ARDS | Nebulised recombinant tissue-Plasminogen Activator (rt-PA) every 6 h for 66 h | Standard care | Percentage change in PaO2/FiO2 ratio from baseline and to day 5 (96 h ± 2 h) post treatment and day 7 (144 h ± 4 h) in the groups receiving rt-PA | Fibrinolytic |
| Dipyridamole | |||||||
| TOLD NCT04424901 |
Randomised, open-label, single-centre clinical trial |
100 | Hospitalized patients with moderate to severe COVID-19 | Dipyridamole 100 mg, 3 times daily for 7 days. | Standard care | D-dimer and platelet count | Anti-platelet Antiviral Inhibition of NETs |
| ATTAC-19 NCT04410328 |
Randomised, open-label, single-centre clinical trial |
132 | Patients with SARS-CoV-2 infection and symptoms consistent with COVID-19. | Dipyridamole ER 200 mg/ Aspirin 25 mg orally/enterally, 2 times daily starting on the day of enrolment for a total of 2 weeks. | Standard care | Change in composite COVID ordinal scale at day 15. | Anti-platelet Antiviral Inhibition of NETs |
| DICER NCT04391179 |
Randomised, Placebo-controlled, clinical trial |
160 | Non-severe hospitalised COVID-19 patients | Dipyridamole 100 mg 4 times a day for 14 days while in hospital | Placebo 4 times a day for 14 days while in hospital | Change in D Dimer | Anti-platelet Antiviral Inhibition of NETs |
| Complement inhibitors | |||||||
| CORIMUNO19-ECU NCT04346797 |
Randomised, open-label, clinical trial (cohort multiple RCT design) | 120 | 1) Non-ICU patients with moderate or severe COVID-19 pneumonia 2) ICU patients | Eculizumab | Standard care | 1) Survival without intubation at day 14 2) Change in organ failure at day 3, defined by the Sequential Organ Failure Assessment score |
C5a inhibition |
| NCT04570397 | Randomised, open-label, single-centre clinical trial |
120 | COVID-19 patients with acute kidney injury and clinical diagnosis of TMA (D dimer >100 % of upper limit and >25 % increase in Cr above normal range or baseline) | Ravulizumab | Standard care | 50 % improvement in eGFR compared to conventional therapy within 30 days of treatment | C5a inhibition |
| NCT04369469 | Randomised, open-label, multicentre, clinical trial (phase 3) | 270 | Patients With COVID-19 Severe Pneumonia, Acute Lung Injury, or ARDS | Ravulizumab | Standard Care | Survival (based on all-cause mortality) at Day 29 | C5a inhibition |
| TACTIC-R NCT04390464 |
Randomised, parallel arm, open-label platform trial | 1167 | Pre-ICU Patients admitted with Covid-19 who are at risk as defined by specific risk count criteria | Patients randomised in a 1:1:1 ratio to Ravulizumab, Baricrintinb or standard care | NA | Time to incidence of the composite endpoint of: Death, Mechanical ventilation, ECMO, Cardiovascular organ support, or Renal failure | C5a inhibition |
| SAVE NCT04395456 |
Randomized, placebo-controlled, single-blind clinical trial (phase 2) | 144 | Patients with ARDS due to COVID-19 | AMY-101 | Placebo | 1) Survival without evidence of ARDS. 2) COVID-19 ordinal scale |
C3 inhibition |
| NCT04402060 | Phase 1 Single arm, open label Phase 2 Randomized, Double-Blinded, Vehicle-Controlled, Multicentre, Parallel-Group Study | 66 | Adults with mild to moderate ARDS Due to COVID-19 | APL-9 | No comparator (phase 1) Vehicle control and standard of care (phase 2) |
Cumulative incidence of treatment-emergent serious adverse events and treatment-emergent adverse events | C3 inhibitor |
| ZILU-COV NCT04382755 |
Randomized controlled, open-label, multicentre clinical trial (phase 2) | 81 | Patients with suspected/confirmed COVID-19 with acute hypoxic respiratory Failure | Zilucoplan® for 14 days | Placebo and standard of care | Mean change in oxygen as defined by Pa02/FiO2 at room air, P(Aa)O2 gradient and a/A pO2 ratio |
C5 inhibitor |
Aa, alveolar-arterial; ARDS, acute respiratory distress syndrome; BMI, body-mass index; C, complement; CAP, Community Acquired Pneumonia; COVID-19, coronavirus disease 2019; Cr, creatinine; DIC, disseminated intravascular coagulation; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation; ER, extended release; IV, intravenous; eGFR, estimated glomerular filtration rate; FiO2, fraction of inspired oxygen; NET, neutrophil extracellular trap; LMWH, Low Molecular Weight Heparin; PaO2, partial pressure of oxygen; SARS-CoV2, severe acute respiratory syndrome coronavirus 2; SIC, sepsis-induced coagulopathy; TMA, thrombotic microangiopathy; UFH, unfractionated heparin, ULN; upper limit of normal.
5.2. Selected potential/experimental therapies
The high incidence of thrombosis in COVID-19 patients despite standard prophylaxis and association of thrombosis with increased mortality (Malas et al., 2020) has led to the search for novel approaches to prevent thrombosis driven by an overactive immune response beyond traditional anticoagulation strategies alone. For example, immune modulators or immunosuppressive therapy such as hydroxychloroquine, corticosteroids and IL-6 inhibitors have been used in clinical trials to target pathways upstream of the coagulation cascade. In addition, a number of antithrombotics have been repurposed which in addition to their direct antithrombotic effects may also have other potential mechanisms of action such as antiviral or anti-inflammatory effects. An extensive discussion of potential pharmacological agents targeting immunothrombosis is beyond the scope of this review and for this the reader is referred to a recent review article prepared by the Global COVID-19 Thrombosis Collaborative Group (Bikdeli et al., 2020b). However, a few selected candidate therapies are briefly discussed here. In addition, Table 1 gives a summary of a number of clinical trials investigating the use of these potential therapeutic agents in COVID-19 associated immunothrombosis.
5.2.1. Nebulised heparin
Nebulised heparin has been proposed as one of the potential therapeutic agents for COVID-19 induced ARDS and pneumonia. Besides its anticoagulant properties, unfractionated heparin may also have other advantages through off-target effects. Heparan sulphate is used as a co-receptor for some coronaviruses (including SARS−COV-2) for host cell attachment. Nebulised heparin may thus have an anti-viral effect by competitive binding to the SAR−COV-2 surface protein S1 thus inhibiting viral entry. In addition, there is evidence that heparin also has anti-inflammatory and mucolytic properties. The benefits of nebulised heparin in acute lung injury have been demonstrated in previous pre-clinical and clinical studies (Van Haren et al., 2020) and a number of clinical trials in COVID-19 patients are also currently underway (Table 1).
5.2.2. Fibrinolytic (thrombolytic) agents
Fibrinolytic therapy is another potential treatment for thrombotic complications and ARDS in COVID-19 patients. ARDS develops in about 5% of COVID-19 patients and is characterised by a diffuse inflammatory reaction, progressing through 3 phases – exudative, proliferative and fibrosis. The hallmark of ARDS is diffuse alveolar damage and fibrin deposition, leading to hyaline membrane formation and subsequent alveolar fibrosis. This, coupled with association of a hypofibrinolytic state in ARDS due to upregulation of PAI-1 has provided a rationale for the use of fibrinolytic therapy in COVID-19 patients. Indeed, use of fibrinolytics such as urokinase or tissue plasminogen activator has been shown to prevent ARDS in porcine models (Hardaway et al., 1990) and results of a phase 1 clinical trial to treat ARDS in critically ill patients has been promising with improvements in oxygenation (Hardaway et al., 2001). To date, only a small case series for the use of fibrinolytic therapy in COVID-19 patients has been reported (Wang et al., 2020). The main concern is the risk of bleeding, especially intracranial bleeding which is reported to occur in 1–3 % of patients given systemic thrombolysis (Chatterjee et al., 2014; Goldhaber et al., 1999). In this context, viscoelastic testing may be of value to identify patients with fibrinolytic shutdown who may benefit most from fibrinolytic therapy (Wright et al., 2020). The use of nebulised plasminogen activator to promote local fibrinolysis in the lungs while minimising the risk of systemic bleeding has also been proposed and the results of an ongoing phase 2 clinical trial using nebulised fibrinolysis in COVID-19 patients (NCT04356833) are eagerly awaited.
5.2.3. Dipyridamole
Dipyridamole is a phosphodiesterase inhibitor that inhibits platelet aggregation by increasing the intracellular concentration of cyclic adenosine monophosphate. Pre-clinical studies have shown that dipyridamole is able to inhibit NETosis and thrombosis in antiphospholipid syndrome by activation of adenosine A2A receptors (Ali et al., 2019). In addition, Dipyridamole has a broad-spectrum activity to a wide range of viruses and is able to inhibit positive-stranded RNA viruses in vitro (Fata-Hartley and Palmenberg, 2005). A recent study showed that dipyridamole has specific affinity to the human coronavirus (HCoV-19) protease Mpro using in silico data and suppresses HCoV-19 replication in vitro. Further, Dipyridamole was shown to induce type 1 interferon response and prolong survival in a mouse model of viral pneumonia. The study also provided early data suggesting a clinical benefit of dipyridamole in a small group of severely ill COVID-19 patients (n = 31) who were treated with concomitant antiviral drugs. Compared to controls, patients treated with Dipyridamole had decreased d-dimer levels, improvements in platelet and lymphocyte counts and a trend towards improved clinical outcomes including clinical cure and discharge rates (Liu et al., 2020). While this data is encouraging, further high-quality studies are needed for confirmation.
5.2.4. Complement inhibitors
A potential role for complement inhibition in COVID-19 is supported by studies showing efficacy of this approach in murine models of SARS-CoV (Gralinski et al., 2018) and MERSCoV (Jiang et al., 2018), as well as autopsy evidence of complement deposition in organs of patients with severe COVID-19 (Magro et al., 2020). Eculizumab, a C5 inhibitor has already been used successfully in the treatment of atypical haemolytic uraemic syndrome, a complement mediated disease. The use of eculizumab (Diurno et al., 2020) and IFX-1, another anti-C5a monoclonal antibody (Vlaar et al., 2020) has recently been reported in a small number of patients with COVID-19 although further studies are currently underway. Another promising target is C3 which is positioned at the convergence of all three activation pathways, upstream of C5. Inhibition of C3 simultaneously blocks both C3a and C5a thus leading to more potent inhibition of the complement cascade. In addition, inhibition of C3 can also prevent IL-6 release from cells such as macrophages expressing the C3aR (Mastellos et al., 2019). One such drug compound is Compstatin (AMY-101), a C3 inhibitor which is currently undergoing investigation in phase 2 clinical trials of COVID-19 patients with ARDS (NCT04395456). A third potential target is the lectin pathway. MASP-2 an activator of the lectin pathway has been shown to bind to the nucleocapsid protein of coronaviruses such as MERSCoV, SARS-CoV, and SARS-CoV2 (Gao et al., 2020). Suppression of this pathway can be achieved using the anti‐MASP2 antibody narsoplimab (Elhadad et al., 2020).
6. Summary and conclusions
In summary, the coagulopathy associated with COVID-19 is the consequence of a complex interplay between the immune, endothelium and coagulation system, with bidirectional interactions, resulting in a procoagulant milieu. The observation of a high incidence of thrombotic event despite routine thromboprophylaxis suggests a different approach is required. In particular, targeting pathways upstream of coagulation using novel or repurposed drugs alone or in combination with other anti-thrombotic agents may be a rational approach to reduce the mortality and morbidity associated with Covid-19 coagulopathy. Further studies are required to determine the optimal agent or combinations, dose, timing of intervention, efficacy and safety of such an approach.
Contributions
M. S. Lim wrote the first draft of the manuscript. S. Mcrae contributed to critical revision of the manuscript. Both authors approved the final version of the manuscript.
Ethical standards
The authors declare no conflict of interests.
Funding
None
Declaration of Competing Interest
The authors declare no conflict of interests.
Acknowledgements
None
Biographies
Dr Ming Sheng Lim is a clinical and Laboratory Haematologist and is a fellow of both the Royal Australasian College of Physicians (RACP) and Royal College of Pathologists of Australasia (RCPA).Dr Lim undertook his undergraduate training at the University of Melbourne where he obtained degrees in Medicine (MBBS) and Biomedical Science (BMedSci). He then completed haematology training at Calvary Mater Hospital and John Hunter Hospital (Newcastle) before undertaking a 2-year Thrombosis Research Fellowship at Monash Health (Melbourne).His interests are in both malignant and non-malignant haematology with a specific focus on thrombosis and haemostasis as well as obstetric haematology.
Dr Simon McRae is a clinical and laboratory consultant haematologist, with an interest in benign haematology including bleeding and thrombotic disorders. Dr McRae graduated from the University of Tasmania in 1992. He underwent haematology training in Edinburgh, Hobart, and Newcastle, followed by a 3-year fellowship at McMaster University focussing on management of patients with venous thrombosis.He has worked as a consultant haematologist at the Royal Adelaide Hospital for the last 14 years, including 2 years as clinical director of haematology. He has over 50 peer reviewed publications and has authored book chapters in his specialist area. He is a previous chair of the Australian Haemophilia Centre Director’s organization, and currently is the chair of the steering committee of the Australian Bleeding Disorder Registry.
References
- Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali R.A., Gandhi A.A., Meng H., Yalavarthi S., Vreede A.P., Estes S.K., et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-09801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara U., Rittirsch D., Flierl M., Bruckner U., Klos A., Gebhard F., et al. Interaction between the coagulation and complement system. Adv. Exp. Med. Biol. 2008;632:71–79. doi: 10.1007/978-0-387-78952-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amezcua-Guerra L.M., Rojas-Velasco G., Brianza-Padilla M., Vázquez-Rangel A., Márquez-Velasco R., Baranda-Tovar F., et al. Presence of antiphospholipid antibodies in COVID-19: case series study. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-218100. annrheumdis-2020-218100. [DOI] [PubMed] [Google Scholar]
- Berger J.S., Kunichoff D., Adhikari S., Ahuja T., Amoroso N., Aphinyanaphongs Y., et al. Prevalence and outcomes of D-Dimer elevation in hospitalized patients with COVID-19. Arterioscler. Thromb. Vasc. Biol. 2020;40:2539–2547. doi: 10.1161/ATVBAHA.120.314872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N. Engl. J. Med. 2020;383:2451–2460. doi: 10.1056/nejmcp2009575. [DOI] [PubMed] [Google Scholar]
- Bertin D., Brodovitch A., Beziane A., Hug S., Bouamri A., Mege J.L., et al. Anticardiolipin IgG autoantibody level is an independent risk factor for COVID-19 severity . 2020;72:1953–1955. doi: 10.1002/art.41409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-Art review. J. Am. Coll. Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli B., Madhavan M.V., Gupta A., Jimenez D., Burton J.R., Der Nigoghossian C., et al. Pharmacological agents targeting thromboinflammation in COVID-19: review and implications for future research. Thromb. Haemost. 2020;120:1004–1024. doi: 10.1055/s-0040-1713152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- Camp J.V., Jonsson C.B. A role for neutrophils in viral respiratory disease. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.00550. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Chakraborty A., Weinberg I., Kadakia M., Wilensky R.L., Sardar P., D.J, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage. JAMA. 2014;311 doi: 10.1001/jama.2014.5990. 2414. [DOI] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019, J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheruiyot I., Kipkorir V., Ngure B., Misiani M., Munguti J., Ogeng’o J., et al. Arterial Thrombosis in Coronavirus Disease 2019 Patients: A Rapid Systematic Review. Ann. Vasc. Surg. 2020;70:273. doi: 10.1016/j.avsg.2020.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciceri F., Beretta L., Scandroglio A.M. Microvascular COVID-19 Lung Vessels Obstructive Thromboinflammatory Syndrome (MicroCLOTS): an Atypical Acute Respiratory Distress Syndrome Working Hypothesis - PubMed, Crit. Care Resusc. (n.d.) https://pubmed.ncbi.nlm.nih.gov/32294809/ [DOI] [PMC free article] [PubMed]
- Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell. 2020;183:1043–1057. doi: 10.1016/j.cell.2020.09.033. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell N.T., Battinelli E.M., Connors J.M. Coagulopathy of COVID-19 and antiphospholipid antibodies. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/BLOOD.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreese K.M.J., Linskens E.A., Benoit D., Peperstraete H. Antiphospholipid antibodies in patients with COVID-19: A relevant observation? J. Thromb. Haemost. 2020;18:2191–2201. doi: 10.1111/jth.14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diurno F., Numis F.G., Porta G., Cirillo F., Maddaluno S., Ragozzino A., Negri P.D.E., et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur. Rev. Med. Pharmacol. Sci. 2020;24:4040–4047. doi: 10.26355/EURREV_202004_20875. [DOI] [PubMed] [Google Scholar]
- Elhadad S., Chapin J., Copertino D., Van Besien K., Ahamed J., Laurence J. MASP2 levels are elevated in thrombotic microangiopathies: association with microvascular endothelial cell injury and suppression by anti-MASP2 antibody narsoplimab. Clin. Exp. Immunol. 2020;203 doi: 10.1111/cei.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata-Hartley C.L., Palmenberg A.C. Dipyridamole reversibly inhibits mengovirus RNA replication. J. Virol. 2005;79:11062–11070. doi: 10.1128/jvi.79.17.11062-11070.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaczyk A., Rosovsky R.P., Reed C.T., Bankhead-Kendall B.K., Bittner E.A., Chang M.G. Comparison of published guidelines for management of coagulopathy and thrombosis in critically ill patients with COVID 19: implications for clinical practice and future investigations. Crit. Care. 2020;24 doi: 10.1186/s13054-020-03273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J.H., Conway E.M. Cross talk pathways between coagulation and inflammation. Circ. Res. 2016;118:1392–1408. doi: 10.1161/CIRCRESAHA.116.306853. [DOI] [PubMed] [Google Scholar]
- Freeman T.L., Swartz T.H. Targeting the NLRP3 inflammasome in severe COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01518. 1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T., Hu M., Zhang X., Li H., Zhu L., Liu H., et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. MedRxiv. 2020 doi: 10.1101/2020.03.29.20041962. 2020.03.29.20041962. [DOI] [Google Scholar]
- Gatto M., Perricone C., Tonello M., Bistoni O., Cattelan A.M., Bursi R., et al. Frequency and clinical correlates of antiphospholipid antibodies arising in patients with SARS-CoV-2 infection: findings from a multicentre study on 122 cases. Clin. Exp. Rheumatol. 2020;38:754–759. [PubMed] [Google Scholar]
- Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebrehiwet B., Randazzo B.P., Dunn J.T., Silverberg M., Kaplan A.P. Mechanisms of activation of the classical pathway of complement by Hageman factor fragment. J. Clin. Invest. 1983;71:1450–1456. doi: 10.1172/JCI110898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhaber S.Z., Visani L., De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet (London, England). 1999;353:1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H., Bahel P., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski L.E., Bankhead A., Jeng S., Menachery V.D., Proll S., Belisle S.E., et al. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. MBio. 2013;4 doi: 10.1128/mBio.00271-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski L.E., Sheahan T.P., Morrison T.E., Menachery V.D., Jensen K., Leist S.R., et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio. 2018;9 doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor B., Atici A., Baycan O.F., Alici G., Ozturk F., Tugrul S., et al. Elevated D-dimer levels on admission are associated with severity and increased risk of mortality in COVID-19: a systematic review and meta-analysis. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff T.C., Mohareb A.M., Giri J., Cohen J.B., Chirinos J.A. Thrombosis in COVID-19. Am. J. Hematol. 2020;95:1578–1589. doi: 10.1002/ajh.25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway R.M., Williams C.H., Marvasti M., Farias M., Tseng A., Pinon I., et al. Prevention of adult respiratory distress syndrome with plasminogen activator in pigs. Crit. Care Med. 1990;18:1413–1418. doi: 10.1097/00003246-199012000-00021. [DOI] [PubMed] [Google Scholar]
- Hardaway R.M., Harke H., Tyroch A.H. Treatment of severe acute respiratory distress syndrome: a final report on a phase I study - PubMed. Am. Surg. 2001;67(April(4)):377–382. (n.d.) [PubMed] [Google Scholar]
- Harzallah I., Debliquis A., Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J. Thromb. Haemost. 2020;18:2064–2065. doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., De Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., Teixeira L., Barreto E.A., Pão C.R.R., et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136:1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba T., Levy J.H. Sepsis-induced Coagulopathy and Disseminated Intravascular Coagulation. Anesthesiology. 2020;132:1238–1245. doi: 10.1097/ALN.0000000000003122. [DOI] [PubMed] [Google Scholar]
- Iba T., Levy J.H., Connors J.M., Warkentin T.E., Thachil J., Levi M., et al. The unique characteristics of COVID-19 coagulopathy. Crit. Care. 2020;24 doi: 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba T., Levy J.H., Levi M., Connors J.M., Thachil J. Coagulopathy of coronavirus disease 2019. Crit. Care Med. 2020;48:1358–1364. doi: 10.1097/CCM.0000000000004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idell S. Crit. Care Med., Lippincott Williams and Wilkins. 2003. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. [DOI] [PubMed] [Google Scholar]
- INSPIRATION Investigators, Sadeghipour P., Talasaz A.H., Rashidi F., Sharif-Kashani B., Beigmohammadi M.T., Farrokhpour M., et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021 doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Investigators T.R.-C., Investigators T.A.-4a, Investigators T.A. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N. Engl. J. Med. 2021;385:777–789. doi: 10.1056/NEJMOA2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Investigators T.R.-C., Investigators T.A.-4a, Investigators T.A. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N. Engl. J. Med. 2021;385:790–802. doi: 10.1056/NEJMOA2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmscher S., Döring N., Halder L.D., Jo E.A.H., Kopka I., Dunker C., et al. Kallikrein Cleaves C3 and activates complement. J. Innate Immun. 2018;10:94–105. doi: 10.1159/000484257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Zhao G., Song N., Li P., Chen Y., Guo Y., et al. Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV article. Emerg. Microbes Infect. 2018;7 doi: 10.1038/s41426-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Tang K., Levin M., Irfan O., Morris S.K., Wilson K., et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents, Lancet Infect. Dis. 2020;20:e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselevskiy M., Shubina I., Chikileva I., Sitdikova S., Samoylenko I., Anisimova N., et al. Immune pathogenesis of covid-19 intoxication: Storm or silence? Pharmaceuticals. 2020;13:1–17. doi: 10.3390/ph13080166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koupenova M. Potential role of platelets in COVID‐19: implications for thrombosis. Res. Pract. Thromb. Haemost. 2020;4:737–740. doi: 10.1002/rth2.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koupenova M., Clancy L., Corkrey H.A., Freedman J.E. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ. Res. 2018;122:337–351. doi: 10.1161/CIRCRESAHA.117.310795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kox M., Waalders N.J.B., Kooistra E.J., Gerretsen J., Pickkers P. Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA - J. Am. Med. Assoc. 2020;324:1565–1567. doi: 10.1001/jama.2020.17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laridan E., Martinod K., De Meyer S.F. Neutrophil extracellular traps in arterial and venous thrombosis. Semin. Thromb. Hemost. 2019;45:86–93. doi: 10.1055/s-0038-1677040. [DOI] [PubMed] [Google Scholar]
- Larsen J.B., Pasalic L., Hvas A.M. Platelets in coronavirus disease 2019. Semin. Thromb. Hemost. 2020;46:823–825. doi: 10.1055/s-0040-1710006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koelblinger C., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome : results from a prospective, single-center, clinicopathologic case series. Ann. Intern. Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaroni M.G., Piantoni S., Masneri S., Garrafa E., Martini G., Tincani A., et al. Coagulation dysfunction in COVID-19: the interplay between inflammation, viral infection and the coagulation system. Blood Rev. 2020 doi: 10.1016/j.blre.2020.100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppkes M., Knopf J., Naschberger E., Lindemann A., Singh J. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58 doi: 10.1016/j.ebiom.2020.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M., Hunt B.J. Thrombosis and coagulopathy in COVID‐19: an illustrated review. Res. Pract. Thromb. Haemost. 2020;4:744–751. doi: 10.1002/rth2.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S23523026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Favaloro E.J. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb. Haemost. 2020;120:876–877. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Li Z., Liu S., Sun J., Chen Z., Jiang M., et al. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm. Sin. B. 2020;10:1205–1215. doi: 10.1016/j.apsb.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKman N., Antoniak S., Wolberg A.S., Kasthuri R., Key N.S. Coagulation abnormalities and thrombosis in patients infected with SARS-CoV-2 and other pandemic viruses. Arterioscler. Thromb. Vasc. Biol. 2020;40:2033–2044. doi: 10.1161/ATVBAHA.120.314514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malas M.B., Naazie I.N., Elsayed N., Mathlouthi A., Marmor R., Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020:29–30. doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne B.K., Denorme F., Middleton E.A., Portier I., Rowley J.W., Stubben C., et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136:1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolis A.S., Manolis T.A., Manolis A.A., Papatheou D., Melita H. COVID-19 infection: viral macro- and micro-vascular coagulopathy and Thromboembolism/Prophylactic and therapeutic management. J. Cardiovasc. Pharmacol. Ther. 2020;26 doi: 10.1177/1074248420958973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastellos D.C., Ricklin D., Lambris J.D. Clinical promise of next-generation complement therapeutics. Nat. Rev. Drug Discov. 2019;18:707–729. doi: 10.1038/s41573-019-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadyen J.D., Stevens H., Peter K. The emerging threat of (Micro)Thrombosis in COVID-19 and its therapeutic implications. Circ. Res. 2020;127:571–587. doi: 10.1161/CIRCRESAHA.120.317447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle D., O’Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2:e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medcalf R.L., Keragala C.B., Myles P.S. Fibrinolysis and COVID-19: A plasmin paradox. J. Thromb. Haemost. 2020;18:2118–2122. doi: 10.1111/jth.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moores L.K., Tritschler T., Brosnahan S., Carrier M., Collen J.F., Doerschug K., et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158:1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolai L., Leunig A., Brambs S., Kaiser R., Weinberger T., Weigand M., et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopp S., Moik F., Jilma B., Pabinger I., Ay C. Risk of venous thromboembolism in patients with COVID‐19: a systematic review and meta‐analysis. Res. Pract. Thromb. Haemost. 2020;4:1178–1191. doi: 10.1002/rth2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nougier C., Benoit R., Simon M., Desmurs-Clavel H., Marcotte G., Argaud L., et al. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J. Thromb. Haemost. 2020;18:2215–2219. doi: 10.1111/jth.15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan J.M., Gonagle D.M., Ward S.E., Preston R.J.S., O’Donnell J.S. Endothelial cells orchestrate COVID-19 coagulopathy. Lancet Haematol. 2020;7:e553–e555. doi: 10.1016/S2352-3026(20)30215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerschke E.I.B., Yin W., Grigg S.E., Ghebrehiwet B. Blood platelets activate the classical pathway of human complement. J. Thromb. Haemost. 2006;4:2035–2042. doi: 10.1111/j.1538-7836.2006.02065.x. [DOI] [PubMed] [Google Scholar]
- Porfidia A., Valeriani E., Pola R., Porreca E., Rutjes A.W.S., Di Nisio M., et al. Venous thromboembolism in patients with COVID-19: systematic review and meta-analysis. Thromb. Res. 2020;196:67–74. doi: 10.1016/j.thromres.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price L.C., McCabe C., Garfield B., Wort S.J. Thrombosis and COVID-19 pneumonia: The clot thickens! Eur. Respir. J. 2020;56 doi: 10.1183/13993003.01608-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M., et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome, J. Thromb. Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakari Jokiranta T. HUS and atypical H.U.S. Blood. 2017;129:2847–2856. doi: 10.1182/blood-2016-11-709865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siguret V., Voicu S., Neuwirth M., Delrue M., Gayat E., Stépanian A., et al. Are antiphospholipid antibodies associated with thrombotic complications in critically ill COVID-19 patients? Thromb. Res. 2020;195:74–76. doi: 10.1016/j.thromres.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha P., Matthay M.A., Calfee C.S. Is a “cytokine storm” relevant to COVID-19? JAMA Intern. Med. 2020;180:1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T., et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C., et al. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01708. 1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate M.D., Deng Y.-M., Jones J.E., Anderson G.P., Brooks A.G., Reading P.C., et al. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J. Immunol. 2009;183:7441–7450. doi: 10.4049/jimmunol.0902497. [DOI] [PubMed] [Google Scholar]
- Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggle C.R., Bansal D., Ding H., Islam M.M., Farag E.A.B.A., Hadi H.A., et al. A comprehensive review of viral characteristics, transmission, pathophysiology, immune response, and management of SARS-CoV-2 and COVID-19 as a basis for controlling the pandemic. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.631139. 631139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritschler T., Mathieu M.E., Skeith L., Rodger M., Middeldorp S., Brighton T., et al. Anticoagulant interventions in hospitalized patients with COVID-19: a scoping review of randomized controlled trials and call for international collaboration. J. Thromb. Haemost. 2020;18:2958–2967. doi: 10.1111/jth.15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haren F.M.P., Page C., Laffey J.G., Artigas A., Camprubi-Rimblas M., Nunes Q., et al. Nebulised heparin as a treatment for COVID-19: scientific rationale and a call for randomised evidence. Crit. Care. 2020;24 doi: 10.1186/s13054-020-03148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaar A.P.J., de Bruin S., Busch M., Timmermans S.A.M.E.G., van Zeggeren I.E., et al. Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial. Lancet Rheumatol. 2020;2:e764–e773. doi: 10.1016/S2665-9913(20)30341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Hajizadeh N., Moore E.E., McIntyre R.C., Moore P.K., Veress L.A., et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J. Thromb. Haemost. 2020;18:1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright F.L., Vogler T.O., Moore E.E., Moore H.B., Wohlauer M.V., Urban S., et al. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J. Am. Coll. Surg. 2020;231:193–203. doi: 10.1016/j.jamcollsurg.2020.05.007. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M., Zhang Y., Zhang S., Qin X., Xia P., Cao W., et al. Antiphospholipid antibodies in critically ill patients with COVID-19. Arthritis Rheumatol. 2020;72:1998–2004. doi: 10.1002/art.41425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau J.W., Teoh H., Verma S. Endothelial cell control of thrombosis, BMC Cardiovasc. Disord. 2015;15 doi: 10.1186/s12872-015-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid Y., Puhm F., Allaeys I., Naya A., Oudghiri M., Khalki L., et al. Platelets can associate with SARS-CoV-2 RNA and are hyperactivated in COVID-19. Circ. Res. 2020;127:1404–1418. doi: 10.1161/CIRCRESAHA.120.317703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382:e38. doi: 10.1056/nejmc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Cao W., Jiang W., Xiao M., Li Y., Tang N., et al. Profile of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients. J. Thromb. Thrombolysis. 2020;50:580–586. doi: 10.1007/s11239-020-02182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y., Zuo M., Yalavarthi S., Gockman K., Madison J.A., Shi H., et al. Neutrophil extracellular traps and thrombosis in COVID-19. J. Thromb. Thrombolysis. 2020 doi: 10.1007/s11239-020-02324-z. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y., Estes S.K., Ali R.A., Gandhi A.A., Yalavarthi S., Shi H., et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]