Abstract
Introduction
Persistent symptoms have recently emerged as a clinical issue in COVID-19. We aimed to assess the prevalence and risk factors in symptomatic non-hospitalized individuals with mild COVID-19.
Methods
We performed a prospective cohort study of symptomatic COVID-19 outpatients, from March to May 2020, with weekly phone calls from clinical onset until day 30 and up to day 60 in case of persistent symptoms. The main outcomes were the proportion of patients with complete recovery at day 30 and day 60 and factors associated with persistent symptoms.
Results
We enrolled 429 individuals mostly women (72.5%) and healthcare workers (72.5%), with a median age of 41.6 years [IQR 30–51.5]. Symptoms included: cough (69.7%), asthenia (68.8%), anosmia (64.8%), headaches (64.6%), myalgia (62.7%), gastrointestinal symptoms (61.8%), fever (61.5%), and ageusia (60.8%). Mean duration of disease was 27 days (95%CI: 25–29). The rate of persistent symptoms was 46.8% at day 30 and 6.5% at day 60 consisting in asthenia (32.6%), anosmia (32.6%), and ageusia (30.4%). The probability of complete recovery was 56.3% (95%CI: 51.7–61.1) at day 30 and 85.6% (95%CI: 81.2–89.4) at day 60. Factors associated with persistent symptoms were age > 40 (HR 0.61), female sex (HR 0.70), low cycle threshold (HR 0.78), and ageusia (HR 0.59).
Conclusions
COVID-19 – even in its mild presentation – led to persistent symptoms (up to one month) in nearly half of individuals. Identification of risk factors such as age, gender, ageusia and viral load is crucial for clinical management and argues for the development of antiviral agents.
Keywords: COVID-19, Mild COVID-19, Persistent COVID-19 symptoms, SARS-CoV-2 RT-PCR
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by the novel coronavirus SARS-CoV-2, is a highly heterogeneous disease in terms of clinical manifestations, disease severity and evolution [1], [2]. While the severe acute respiratory distress had revealed COVID-19 to the world with initially high mortality rates in critically ill high-risk patients (up to 62% mortality rate in patients managed in intensive care units) [2], [3], [4], the mild-to-moderate form is by far the most frequent (around 80%) [1]. The clinical picture of mild-to-moderate COVID-19 includes flu-like symptoms [1], [5] and some less common symptoms in a context of respiratory viral infection such as cutaneous manifestations [6], anosmia [7], dysgeusia [7], neurological [8] and cardiovascular [9] symptoms. A median time to recovery of approximately 2 weeks for mild cases was reported in 2020 [10], but recent studies reported persistence of symptoms after several months in a large proportion of patients [11], [12], [13], [14], [15], [16].
While the first wave of the pandemic was spreading out in Europe in spring 2020 with lockdown measures, a remote surveillance system named COVIDOM – based on an alert system triggered by abnormal vital signs – was set up for home monitoring of patients with suspected or proven COVID-19 living in the Ile-de-France region [17]. To complement this alert system, as access to family physicians was limited, we set up in early spring 2020 a prospective weekly medical follow up of these COVID-19 outpatients. We aim to report our experience and to describe the dynamics of the disease in these outpatients, the prevalence and the risk factors for persistent symptoms.
2. Methods
This observational, prospective, single-center study enrolled all consenting symptomatic adult outpatients (> 18 years), with a positive nasopharyngeal RT-PCR for SARS-CoV-2 or positive anti-SARS-CoV-2 antibodies detected by ELISA (IgM or IgG) who self-registered in the COVIDOM Pitié-Salpêtrière (PSL) between March 10 and May 18, 2020. Initially, hospitalized patients, patients from whom consent was not obtained and patients with no follow-up visits were excluded from the analysis. The primary outcome was the probability of complete recovery defined as the absence of symptoms for at least three days at day 30 (D30). Secondary outcomes were the frequency of symptoms at disease onset and their evolution over time, the probability of complete recovery at day 60 (D60), the proportion of COVID-19 relapse defined as the proportion of individuals with reemergence of symptoms after complete recovery, the profile and factors associated with persistent disease defined as the persistence of symptoms at D30.
Mild COVID-19 was defined as having any of the various signs and symptoms of COVID-19, a positive nasopharyngeal RT-PCR for SARS-CoV-2 or positive anti-SARS-CoV-2 antibodies detected by ELISA and not requiring oxygen therapy or hospitalization.
Patients were prospectively followed with weekly phone visits by a physician from clinical onset of COVID-19 (day 0) until D30 (day 0, day 7, day 14, day 30) and D60 for patients with persistent symptoms at D30. Data were collected at each visit using a standardized questionnaire including demographic data, disease onset date, and all symptoms reported. All items were registered in the computerized data system NADISABL SA version 5.
The Cobas® test (Roche), the RealStar® Kit 1.0 assay (ALTONA), and a real-time RT-PCR (National Reference Center) were used to detect SARS-CoV-2 RNA on nasopharyngeal swabs. Only the cycle threshold (Ct) of Cobas® SARS-CoV-2 assay was used as a proxy for quantification for estimation of SARS-CoV-2 viral load. Detection of SARS-CoV-2 IgG antibodies was assessed using commercially available immunoassay (SARS-CoV-2 IgG, Architect, and Abbott). The SARS-CoV-2 RT-PCR and serology results were collected through the database of our hospital virology laboratory.
2.1. Statistical analysis
Descriptive statistics included frequency analysis (percentages) for categorical variables and median and interquartile (IQR) for continuous variables. Chi2/Fisher's exact test was used to compare categorical variables and Mann–Whitney U test for continuous variables. We compared the frequency of symptoms at disease onset according to age (< 60 vs. >60 years) and gender using Fisher's exact test. The probability of recovery and time to recovery were calculated using the Kaplan–Meier method and factors associated with persistent disease were evaluated using COX model accounting for staggered entries in the study. The following variables were assessed: age, sex, profession, SARS-CoV-2 RT-PCR Ct, time from onset until PCR, number of people per household, number and type of initial symptoms. Factors associated with baseline Ct provided for patients assessed with the RT-PCR E-Cobas gene Ct technique were evaluated using a linear regression model. Variables with univariable P-value < 0.20 were retained for the multivariable analysis. Analyses were performed using the software SAS version 9.4 (SAS Institute, Cary, NC).
2.2. Ethics
This study was conducted in accordance with the Helsinki declaration. The research was approved by an institutional review board (CPP Ile-de-France X, Paris, France, No. 47-2020; ClinicalTrials.gov Identifier: NCT04402905). All patients gave their consent.
3. Results
Out of 671 patients registered in the COVIDOM-PSL database between March 10 and May 18, 429 patients fulfilling inclusion criteria were assessed. We excluded from the analysis 242 individuals for the following reasons: prior COVID-19-related hospitalization (n = 66), missing consent (n = 30), absence of proven SARS-CoV-2 diagnosis (n = 119), and absence of follow-up visits (n = 27). A total of 1710 visits were performed with a median of four [IQR 3–5] visits per patient; median follow-up duration was 27 [IQR 16–35] days. All 429 patients were followed up to D30 and 175 up to D60 with no loss to follow-up.
Patients were predominantly women (72.5%) with a median age of 41.6 years [IQR 30–51.5], mostly healthcare workers (72.5%). Diagnosis was assessed by RT-PCR in 412/429 (96%) performed within a median time of 3 days [IQR 1–6] from disease onset, or by positive SARS-CoV-2 antibody test in 17/429 (4%) within a median time of 66 days (IQR: 60–75). An RT-PCR Ct value using the E-Cobas gene Ct assay was available for 260 patients (63.1%); median value was 25.8 [IQR 21–32] at diagnosis.
Clinical symptoms presented by the 429 patients over the course of COVID-19 are shown in Table 1 . The main symptoms (> 60%) were cough (69.7%), asthenia (68.8%), anosmia (64.8%), headaches (64.6%), myalgia (62.7%), gastrointestinal symptoms (61.8%), fever (61.5%), and ageusia (60.8%). There were differences in terms of clinical presentation: diarrhea and fever were more frequent in patients > 60 years (24.6% vs. 15.4%, p = 0.02 and 69.2% vs. 48.8%, p < 0.001, respectively), and anosmia was more prevalent in patients < 60 years (42.1% vs. 28.5%, p = 0.007). Fever was more frequent in men (65.2 vs. 51.1%, p = 0.08) while headaches were more frequent in women (56.6 vs. 45.8%, p = 0.04).
Table 1.
Prevalence of symptoms associated with COVID-19 in 429 patients.
| Symptoms | Frequency (%) |
|---|---|
| Cough | 299 (69.7) |
| Asthenia | 295 (68.8) |
| Anosmia | 278 (64.8) |
| Headaches | 277 (64.6) |
| Myalgia | 269 (62.7) |
| Gastrointestinal symptoms | 265 (61.8) |
| Fever | 264 (61.5) |
| Ageusia | 261 (60.8) |
| Rhinorrhea | 190 (44.3) |
| Dyspnea | 139 (32.4) |
| Sore throat | 77 (17.9) |
By multivariable analysis, we found a higher SARS-CoV-2 viral load (lower Ct) in healthcare workers (HCW) compared with non-HCWs (−7.2Ct [95%CI −11.13 to −3.18]; p < 0.001) and in patients presenting with fever at disease onset compared with non-febrile patients (−2.4 Ct [95%CI −4.32 to −0.46]; p = 0.016).
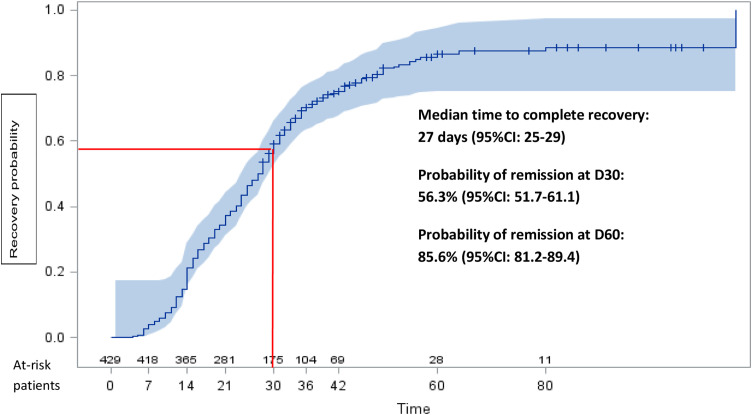
The median time to complete recovery was 27 days (95%CI: 25–29). Three male patients had to be hospitalized at day 13, 14, and 18 for acute respiratory failure (65 years), pulmonary embolism (34 years), and lower limb paresthesia (34 years), respectively.
Five patients had relapsing disease in a median time of 8 days [IQR 7–9] after an initial complete recovery with new symptoms in three of them. Relapse symptoms were chest pain (n = 3), dyspnea (n = 3), headaches (n = 2), asthenia (n = 2), cough (n = 2), vertigo (n = 2), and gastrointestinal symptoms (n = 2). They all had completely recovered at D30 after the initial episode.
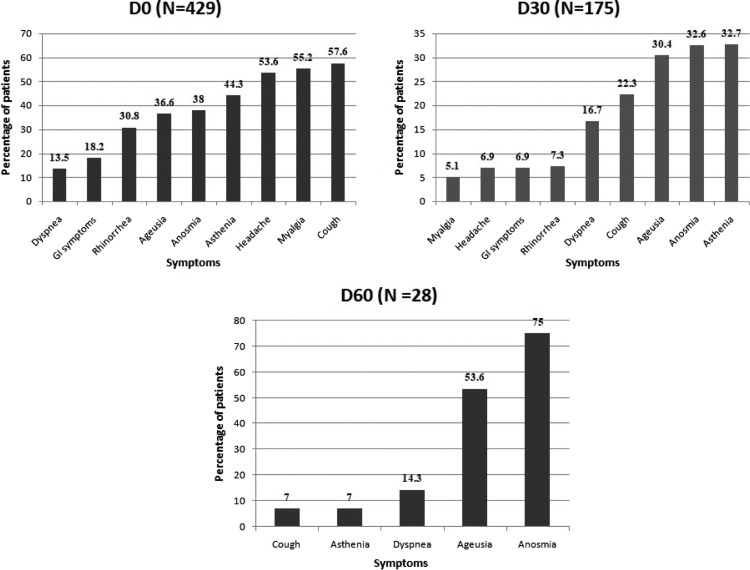
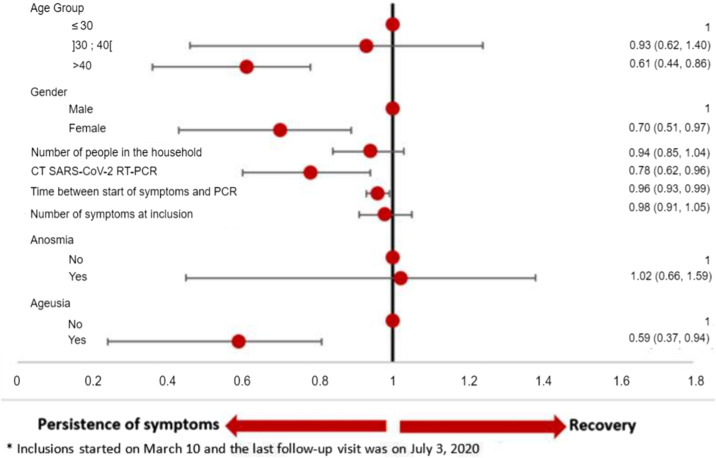
A total of 175 (40.8%) patients reported persistent symptoms at D30 (Fig. 1 ) mainly consisting in asthenia (32.6%), anosmia (32.6%), and ageusia (30.4%). At D60, 28 (6.5%) patients were still symptomatic mainly with anosmia (75%), ageusia (53.6%), and dyspnea (14.3%). None of them required hospitalization after D30. Overall, the probability of complete recovery from COVID-19 was 56.3% (95%CI: 51.7–61.1) at D30, increasing to 85.6% (95%CI: 81.2–89.4) (Fig. 2 ) at D60. By multivariable analysis, persistent symptoms at D30 were associated with the following baseline factors: female sex (HR 0.70), age > 40 (HR 0.61), ageusia (HR 0.59), longer duration of symptoms prior to PCR testing (HR 0.96), and a higher viral load (HR 0.78) (Table 2 and Fig. 3 ).
Fig. 1.
Frequency of symptoms at day 0, day 30, and day 60. GI: gastrointestinal.
Fig. 2.
Probability of complete recovery from mild-to-moderate COVID-19 in 429 patients.
Table 2.
Univariable and multivariable analysis of factors associated with persistence of symptoms (between March 10 and July 3, 2020)a.
| Variables | n | Complete resolution at day 30 |
Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|---|---|---|
| No. (n = 175) | Yes (n = 254) | HR (95%CI) | P-value | HR (95%CI) | P-value | ||
| Age | 429 | 44.9 (34.2–52.7) | 37.2 (29.4–50.2) | 0.99 (0.98–0.99) | 0.0058 | ||
| ≤ 30 | 429 | 30 (28.0) | 77 (72.0) | 1 | 0.0007 | 1 | 0.0065 |
| [30; 40] | 33 (33.3) | 66 (66.7) | 0.82 (0.61–1.11) | 0.93 (0.62–1.40) | |||
| >40 | 112 (50.2) | 111 (49.8) | 0.62 (0.48–0.80) | 0.61 (0.44–0.86) | |||
| Gender | 429 | ||||||
| Male | 38 (32.2) | 80 (67.8) | 1 | 0.0036 | 1 | 0.0332 | |
| Female | 137 (44.0) | 174 (56.0) | 0.71 (0.56–0.89) | 0.70 (0.51–0.97) | |||
| Place of work | 428 | ||||||
| Healthcare facility | 150 (39.6) | 229 (60.4) | 1 | 0.941 | |||
| Non-healthcare facility | 25 (51.0) | 24 (49.0) | 1.01 (0.73–1.42) | ||||
| Number of people in the household | 424 | 3 (2–4) | 2 (2–4) | 0.92 (0.85–0.99) | 0.02 | 0.94 (0.85–1.04) | 0.2532 |
| RT-PCR SARS-CoV-2 Ct, per additional 10 unit | 260 | 24.8 (20.6–32.5) | 25.9 (21.1–31.6) | 0.87 (0.71–1.06) | 0.1657 | 0.78 (0.62–0.96) | 0.0201 |
| Time from symptom onset to RT-PCR test | 411 | 4 (1–7) | 3 (1–5) | 0.97 (0.95–0.99) | 0.0112 | 0.96 (0.93–0.99) | 0.0161 |
| Number of symptoms at inclusion | 429 | 4 (3–6) | 4 (3–6) | 0.95 (0.90–1.00) | 0.0607 | 0.98 (0.91–1.05) | 0.5046 |
| Diarrhea | 429 | ||||||
| No | 140 (39.9) | 211 (60.1) | 1 | 0.6502 | |||
| Yes | 35 (44.9) | 43 (55.1) | 0.94 (0.71–1.24) | ||||
| Myalgia | 429 | ||||||
| No | 77 (40.1) | 115 (59.9) | 1 | 0.8001 | |||
| Yes | 98 (41.4) | 139 (58.6) | 1.03 (0.83–1.28) | ||||
| Asthenia | 429 | ||||||
| No | 95 (39.8) | 144 (60.2) | 1 | 0.2749 | |||
| Yes | 80 (42.1) | 110 (57.9) | 0.89 (0.72–1.10) | ||||
| Fever | 429 | ||||||
| No | 82 (42.5) | 111 (57.5) | 1 | 0.2768 | |||
| Yes | 93 (39.4) | 143 (60.6) | 1.13 (0.91–1.40) | ||||
| Cough | 429 | ||||||
| No | 69 (37.9) | 113 (62.1) | 1 | 0.5786 | |||
| Yes | 106 (42.9) | 141 (57.1) | 0.94 (0.76–1.17) | ||||
| Rhinorrhea | 429 | ||||||
| No | 125 (42.1) | 172 (57.9) | 1 | 0.9524 | |||
| Yes | 50 (37.9) | 82 (62.1) | 0.99 (0.79–1.25) | ||||
| Headaches | 429 | ||||||
| No | 89 (44.7) | 110 (55.3) | 1 | 0.7725 | |||
| Yes | 86 (37.4) | 144 (62.6) | 1.03 (0.83–1.28) | ||||
| Anosmia | 429 | ||||||
| No | 103 (38.7) | 163 (61.3) | 1 | 0.1658 | 1 | 0.9294 | |
| Yes | 72 (44.2) | 91 (55.8) | 0.86 (0.69–1.07) | 1.02 (0.66–1.59) | |||
| Ageusia | 429 | ||||||
| No | 104 (38.2) | 168 (61.8) | 1 | 0.09 | 1 | 0.0248 | |
| Yes | 71 (45.2) | 86 (54.8) | 0.82 (0.66–1.03) | 0.59 (0.37–0.94) | |||
| Dyspnea | 429 | ||||||
| No | 149 (40.2) | 222 (59.8) | 1 | 0.4923 | |||
| Yes | 26 (44.8) | 32 (55.2) | 0.89 (0.65–1.23) | ||||
HR: hazard ratio; Cox proportional hazard model was used to assess factors associated with symptom persistence.
Inclusions started on March 10, 2020 and the last follow-up visit was on July 3, 2020.
Fig. 3.
Multivariable analysis of factors associated with persistence of symptoms (between March 10 and July 3, 2020)*. *: inclusions started on March 10 and the last follow-up was on July 3, 2020.
4. Discussion
This prospective study of 429 non-hospitalized patients shows that mild COVID-19 is a long infectious disease process as only 56% of individuals had cleared their COVID-19 symptoms at 1 month. Furthermore, 14% of patients had persistent symptoms after 2 months.
Initial COVID-19 clinical presentation in our population of young individuals with mild COVID-19 was consistent with other reports (most frequently cough, asthenia, headaches, fever, gastrointestinal symptoms, and anosmia/ageusia) [12], [18], [19]. Interestingly, we found, as reported in other studies, gender and age differences in initial clinical presentations with fever more frequently observed in men [18], [20], headaches more frequent in women [18], anosmia more prevalent in young individuals while older patients more frequently had fever and diarrhea [18].
SARS-CoV-2 viral load has been shown to be a prognostic factor in critically ill patients [21], [22] and a predictor of transmissibility [23], [24]. One study correlated olfactory and taste dysfunction to a lower Ct value at diagnosis [25]. Higher viral load in HCWs may be explained by a faster access to RT-PCR earlier in the course of COVID-19. Similarly, for febrile patients, fever could have been a warning sign motivating early testing or/and a consequence of an inflammatory response to a higher viral load.
Persistence of symptoms beyond what is commonly expected in a flu-like syndrome, seems to be a common feature of COVID-19 [11], [12], [13], [14], [15], [16], [26]. Whereas in early 2020, the World Health Organization (WHO) reported a median time to recovery of 2 weeks in mild cases and 3–6 weeks in critically ill patients [10], several studies reported persistence of symptoms after several weeks in up to 80% of cases [11], [12], [26], [27], [28]. However, most of these persistent symptoms were reported in hospitalized patients and/or severe cases [11], [12], [27], [28]. In our younger outpatient population of 429 individuals with a mild illness, the median time to recovery was 4 weeks, longer than what was initially reported by the WHO [10] or by Lechien [18] with a mean disease duration of around 2 weeks.
Moreover, Petersen et al. from the Faroe Islands [26] reported at least one symptom after 2 months in 53% of 180 non-hospitalized COVID-19 patients. Logue et al. reported at least one symptom after 6 months in 32.7% of 150 COVID-19 outpatients [15] and Boscolo–Rizzo et al. reported at least one symptom after 12 months in 53% of 304 mild-to-moderate COVID-19 patients [14]. In contrast, in our cohort of 429 mild COVID-19 outpatients, the probability of persistent symptoms at 1 month was 46% and 14% after 2 months. Altogether these data suggest that SARS-CoV-2 infection is more complex than a standard respiratory viral infection.
Persistence of symptoms is not an uncommon finding in acute viral infections such as CMV infection or primary EBV infection [29], [30], [31]. Within the coronaviruses family, SARS-CoV-1 has also been associated with persistent symptoms, such as reported in 40% of individuals one year after initial infection with 25% meeting chronic asthenia syndrome criteria [32].
A better understanding of the risk factors for persistent symptoms is key to better advise patients with COVID-19 particularly in a context of mild illness where a rapid resumption to normal condition is expected. We identified four independent factors associated with symptom persistence: age over 40 years, female gender, ageusia, and high SARS-CoV-2 viral load.
Age is a marker of severity in COVID-19 [33], [34], potentially related to a lesser immune response leading to longer persistence of the virus. While the mechanisms remain unknown, gender is a known risk factor for developing severe COVID-19 [33], [34] and the role of estrogen as an immune response modulator has been suggested [35]. Experiencing anosmia and ageusia has been reported to last potentially as long as several months as they may be due to SARS-CoV-2 injury to nerves [36], [37]. Importantly, our study is the first to report the role of a higher baseline SARS-CoV-2 viral load in nasal cavities and of a long time between symptom onset and RT-PCR test in symptom persistence. This contrasts with a recent study by Peghin M et al. that did not find any correlation between Ct values and post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients [16]. SARS-CoV-2 high viral load and its persistence is a marker associated with severity and mortality in COVID-19 [21], [22], [23], [24]. This is in accordance with many other viral diseases where the viral replication intensity is correlated with the severity of virus-induced damages either directly to the infected cells or tissues and/or indirectly through the inflammatory response [38], [39], and delay in recovery is expected. This reinforces the urgent need for effective strategies on SARS-CoV-2 replication such as antiviral agents or monoclonal antibodies. Other studies, most of them including hospitalized patients, identified risk factors associated with persistent symptoms: older age [12], [14], [26], severe COVID-19 requiring hospitalization [12], body mass index [14], number of symptoms on admission [13] or in the acute phase [14], and presence of dyspnea [12].
Our study has several limitations. In the initial context of the first wave of COVID-19 during which this study was set up, the main focus was the symptoms of patients diagnosed with COVID-19, highly anxious about their immediate prognosis; therefore we did not thoroughly collect their medical history including comorbidities (even though they were young) and tobacco use. Nevertheless, comorbidities and tobacco use do not seem to be associated with persistent symptoms [12], [13], [18], [26]. Weekly visits during the lockdown period were performed by phone calls which may have led to overestimate subjective perceptions from patients without any physical, biological, or imaging examination. Knowing that lockdowns and social distancing can influence some common complaints (such as asthenia) and in the absence of a control group, it is difficult to determine the role of the viral illness itself in the clinical picture. We tried to control this bias by using a standardized questionnaire administered by medical doctors. For the above-mentioned reasons, we did not perform any psychological evaluation; we were thus not able to assess the influence of psychological disorders on symptom persistence. Moreover, our study mainly included HCWs, women, and people who registered in the COVIDOM system which could influence the generalizability of results to other populations since HCWs and patients registered in the COVIDOM system may be a unique population with high awareness of their medical condition. Finally, we presumed that our patients were infected by either the initial strain of SARS-CoV-2 or by the strain carrying the D614G mutation in the spike protein since they were the two dominant strains circulating globally at the time of the study [40]. It would be important, in the near future, to assess whether the emergence of new variants will impact the whole clinical course of COVID-19.
5. Conclusion
COVID-19 even in its mild clinical presentation is an acute infection with symptoms persisting over one month in up to 44% of outpatients. However, most of them had recovered at two months. Older people, women, individuals with a high nasal viral load, and patients with initial ageusia were more likely to suffer from persistent symptoms. A better understanding of the pathogenesis of persistent symptoms is highly needed. Decreasing virus load might help to shorten disease duration.
Ethical approval
All procedures performed in studies involving human partic-pants were in accordance with the 1964 Helsinki declaration and its later amendments.
Contributions of authors
AF, BS, RT, M-AV, CK contributed to the conception and design of the study.
AF, BS, CB, YD, LS, RT, M-AV, SS, RP, AB, GM, NG, OI, OP, VP, NK, AC, CK collected clinical and biological data.
BA performed the virological analysis.
AN, LA performed the statistical analyses.
AF, BS, RT, SS, RP, AB, VP, EC, BA, CK wrote the article.
All authors read and approved the final article.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
We thank our collaborators of the COVIDOM-19 PSL research group (F. Adda, E. Bourzam, P. Bouvet, G. Brucker, P. Charles, C. Dehais, S. Epelbaum, C. Ewenczyk, A. Hartmann, H. Gobillot, E. Hainque, N. Hamani, P. Lavagna, F. Laylavoix, N. Le Forestier, L. Lenclume, S. Lhuiller, C. Lubetzki, E. Maillart, C. Masgnaux, E. Mayer, M. Menel, V. Meric, N. Qatib, and M. Trogneux) for their help with this study.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss P., Murdoch D.R. Clinical course and mortality risk of severe COVID-19. Lancet Lond Engl. 2020;395:1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. 2020:7. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sachdeva M., Gianotti R., Shah M., Bradanini L., Tosi D., Veraldi S., et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75–81. doi: 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nouchi A., Chastang J., Miyara M., Lejeune J., Soares A., Ibanez G., et al. Prevalence of hyposmia and hypogeusia in 390 COVID-19 hospitalized patients and outpatients: a cross-sectional study. Eur J Clin Microbiol Infect Dis. 2020:1–7. doi: 10.1007/s10096-020-04056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whittaker A., Anson M., Harky A. Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand. 2020 doi: 10.1111/ane.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 10.Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) n.d. ; 2020. https://www.who.int/publications-detail-redirect/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19).(accessed May 30, 2021).
- 11.Carfì A., Bernabei R., Landi F., Gemelli Against COVID-19 Post-Acute Care Study Group Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosn J., Piroth L., Epaulard O., Turnier P.L., Mentré F., Bachelet D., et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boscolo-Rizzo P., Guida F., Polesel J., Marcuzzo A.V., Capriotti V., D’Alessandro A., et al. Sequelae in adults at 12 months after mild-to-moderate coronavirus disease 2019 (COVID-19) Int Forum Allergy Rhinol. 2021 doi: 10.1002/alr.22832. [alr.22832] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logue J.K., Franko N.M., McCulloch D.J., McDonald D., Magedson A., Wolf C.R., et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4:e210830. doi: 10.1001/jamanetworkopen.2021.0830. [10.1001/jamanetworkopen.2021.0830] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peghin M., Palese A., Venturini M., De Martino M., Gerussi V., Graziano E., et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.05.033. [S1198743X21002810s] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yordanov Y., Dechartres A., Lescure X., Apra C., Villie P., Marchand-Arvier J., et al. Covidom, a telesurveillance solution for home monitoring patients with COVID-19. J Med Internet Res. 2020:22. doi: 10.2196/20748. [10.2196/20748] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q., et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020 doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vahey G.M., Marshall K.E., McDonald E., Martin S.W., Tate J.E., Midgley C.M., et al. Symptom profiles and progression in hospitalized and nonhospitalized patients with coronavirus disease, Colorado, USA, 2020. Emerg Infect Dis. 2021;27:385–395. doi: 10.3201/eid2702.203729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien J., Du K.Y., Peng C. Incidence, clinical features, and outcomes of COVID-19 in Canada: impact of sex and age. J Ovarian Res. 2020;13:137. doi: 10.1186/s13048-020-00734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller E.H., Zucker J., Castor D., Annavajhala M.K., Sepulveda J.L., Green D.A., et al. Pretest symptom duration and cycle threshold values for severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction predict coronavirus disease 2019 mortality. Open Forum Infect Dis. 2021:8. doi: 10.1093/ofid/ofab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choudhuri J., Carter J., Nelson R., Skalina K., Osterbur-Badhey M., Johnston A., et al. SARS-CoV-2 PCR cycle threshold at hospital admission associated with patient mortality. PLoS ONE. 2020:15. doi: 10.1371/journal.pone.0244777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcus J.E., Frankel D.N., Pawlak M.T., Casey T.M., Cybulski R.J., Enriquez E., et al. Risk factors associated with COVID-19 transmission among US air force trainees in a congregant setting. JAMA Netw Open. 2021:4. doi: 10.1001/jamanetworkopen.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarkar B., Sinha R.N., Sarkar K. Initial viral load of a COVID-19-infected case indicated by its cycle threshold value of polymerase chain reaction could be used as a predictor of its transmissibility – an experience from Gujarat, India. Indian J Community Med. 2020;45:278–282. doi: 10.4103/ijcm.IJCM_593_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain A., Pandey A.K., Kaur J., Kumar L., Singh M., Das S., et al. Is there a correlation between viral load and olfactory & taste dysfunction in COVID-19 patients? Am J Otolaryngol. 2021;42:102911. doi: 10.1016/j.amjoto.2021.102911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen M.S., Kristiansen M.F., Hanusson K.D., Danielsen M.E., á Steig B., Gaini S., et al. Long COVID in the Faroe Islands – a longitudinal study among non-hospitalized patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet Lond Engl. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Leon S., Wegman-Ostrosky T., Perelman C., Sepulveda R., Rebolledo P., Cuapio A., et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Res Sq. 2021 doi: 10.21203/rs.3.rs-266574/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balfour H.H., Holman C.J., Hokanson K.M., Lelonek M.M., Giesbrecht J.E., White D.R., et al. A prospective clinical study of Epstein-Barr virus and host interactions during acute infectious mononucleosis. J Infect Dis. 2005;192:1505–1512. doi: 10.1086/491740. [DOI] [PubMed] [Google Scholar]
- 30.Horwitz C.A., Henle W., Henle G., Snover D., Rudnick H., Balfour H.H., et al. Clinical and laboratory evaluation of cytomegalovirus-induced mononucleosis in previously healthy individuals. Report of 82 cases. Medicine (Baltimore) 1986;65:124–134. doi: 10.1097/00005792-198603000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Wreghitt T.G., Teare E.L., Sule O., Devi R., Rice P. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003;37:1603–1606. doi: 10.1086/379711. [DOI] [PubMed] [Google Scholar]
- 32.Lam M.H.-B., Wing Y.-K., Yu M.W.-M., Leung C.-M., Ma R.C.W., Kong A.P.S., et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169:2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 33.Barek MdA, Aziz MdA, Islam M.S. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID-19 cases: a meta-analysis with 55 studies and 10014 cases. Heliyon. 2020:6. doi: 10.1016/j.heliyon.2020.e05684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vahidy F.S., Pan A.P., Ahnstedt H., Munshi Y., Choi H.A., Tiruneh Y., et al. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: cross-sectional analysis from a diverse US metropolitan area. PLOS ONE. 2021;16:e0245556. doi: 10.1371/journal.pone.0245556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipsa A., Prabhu J.S. Gender disparity in COVID-19: role of sex steroid hormones. Asian Pac J Trop Med. 2021;14:5–9. doi: 10.4103/1995-7645.304293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertlich M., Stihl C., Lüsebrink E., Hellmuth J.C., Scherer C., Freytag S., et al. The course of subjective and objective chemosensory dysfunction in hospitalized patients with COVID-19: a 6-month follow-up. Eur Arch Otorhinolaryngol. 2021:1–7. doi: 10.1007/s00405-021-06796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cousyn L., Sellem B., Palich R., Bendetowicz D., Agher R., Delorme C., et al. Olfactory and gustatory dysfunctions in COVID-19 outpatients: a prospective cohort study. Infect Dis Now. 2021 doi: 10.1016/j.idnow.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muema D.M., Akilimali N.A., Ndumnego O.C., Rasehlo S.S., Durgiah R., Ojwach D.B.A., et al. Association between the cytokine storm, immune cell dynamics, and viral replicative capacity in hyperacute HIV infection. BMC Med. 2020;18:81. doi: 10.1186/s12916-020-01529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selhorst P., Combrinck C., Ndabambi N., Ismail S.D., Abrahams M.-R., Lacerda M., et al. Replication capacity of viruses from acute infection drives HIV-1 disease progression. J Virol. 2017;91 doi: 10.1128/JVI.01806-16. [e01806-16, e01806-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO | SARS-CoV-2 Variants. WHO n.d.; 2020 http://www.who.int/csr/don/31-december-2020-sars-cov2-variants/en/.(accessed May 30, 2021).