Abstract
New diagnostics and treatment options for the radical cure of Plasmodium vivax malaria are now available. At the 2019 annual meeting of the Vivax Working Group of the Asia Pacific Malaria Elimination Network, participants took part in a roundtable discussion to identify further evidence required to introduce these new tools into policy and practice. Key gaps identified were accuracy and reliability of glucose‐6‐phosphate‐dehydrogenase deficiency tests, health system capacity, and feasibility and cost effectiveness of novel treatment strategies in routine clinical practice. As expected, there were differences in the priorities between country partners and researcher partners. To achieve the 2030 target for the regional elimination of malaria, evidence to address these issues should be generated as a matter of priority. Review of global guidelines alongside locally generated data will help to ensure the timely revision and optimisation of national treatment guidelines that will be vital to meet regional elimination goals.
Keywords: Asia Pacific, evidence gaps, malaria elimination, malaria health policy, Plasmodium vivax malaria, policy implementation, radical cure
1. INTRODUCTION
Plasmodium vivax malaria continues to exert huge public health burden in the Asia‐Pacific region, South America and the Horn of Africa with more than 14.3 million clinical cases in 2017 (Battle et al., 2019). Major gains have been made in reducing this burden that reflects, in part, sustained global attention to malaria and renewed attention to the United Nations' Sustainable Development Goals (SDG). However, these successes in disease control are often focal in nature, vulnerable to reversal and consequently threaten national, regional and international health security. In 2014, governments in the Asia‐Pacific region committed to eliminating malaria by 2030 (Department of Foreign Affairs and Trade, 2014). Until recently, efforts to control and eliminate P. vivax malaria were limited by the availability of effective radical cure options.
Currently, the treatment for P. vivax malaria recommended in most countries in the Asia‐Pacific and the Horn of Africa is a combination of a blood schizonticide such as chloroquine (CQ) or an artemisinin combination therapy (ACT) plus a 14‐day course of primaquine (PQ) to kill the dormant liver stages called hypnozoites (World Health Organization [WHO], 2015b). In these regions, a supervised course of PQ14 (at a dose of 3.5 mg/kg total dose) has variable efficacy—achieving greater than 70% at 6 months in some, but not all locations (John et al., 2012; Llanos‐Cuentas et al., 2019). However, in reality, there are several challenges including the lack of implementation of radical cure policies (Recht et al., 2018) resulting in low prescription rates often due to concerns about haemolysis in patients with glucose‐phosphate‐dehydrogenase deficiency (G6PD) (Ley et al., 2017). But even if policies are implemented, the low adherence to a prolonged course of treatment results in lower effectiveness (Abreha et al., 2017; Douglas et al., 2017; Ley et al., 2017).
While the burden of malaria continues to fall across the Asia‐Pacific, the proportionate burden of P. vivax malaria cases has increased, highlighting the importance of improving the radical cure of malaria to achieve the 2030 target for regional elimination (APMEN, 2019). The recently published Lancet Commission highlights the need and feasibility of malaria elimination but also emphasises the importance of accelerating the introduction of novel strategies (Feachem et al., 2019). In the last 12 months, there have been three major advances in the tools available to tackle P. vivax relapses: (i) a short course high daily dose PQ regimen (Taylor et al., 2019); (ii) a novel quantitative point‐of‐care G6DP test (Alam et al., 2018; Pal et al., 2019); and (iii) single dose Tafenoquine (TQ) (Lacerda et al., 2019; Llanos‐Cuentas et al., 2019). While broad global policy recommendations addressing the use of those tools are expected from the World Health Organization's (WHO) Global Malaria Programme (GMP) within the next 1 or 2 years, the adoption of new tools will need to be tailored to individual country needs and capacities. After 60 years of limited biotechnical innovation for the control of P. vivax malaria, National Malaria Control Programs (NMCPs) are now faced with the challenge of deciding between several new strategies. Options include, but are not limited to, improving adherence to current guidelines, introducing quantitative or qualitative G6PD testing with current treatment, shorter high dose PQ regimens and TQ with quantitative G6PD testing. Introduced strategies may include a mix of these options at different levels of the health system to be rolled out simultaneously or as phased implementation depending on the country context and capacity.
Considering that globally the estimated time between the availability of scientific evidence and its introduction into clinical practice is approximately 17 years (Balas & Boren, 2000; Green et al., 2009; Morris et al., 2011; Trochim, 2010; Westfall et al., 2007), we aimed to bring policymakers together to bridge this gap at the same time that NMCPs are preparing revisions of their National Strategic Plans (NSP) and Global Fund (GF) concept notes. Previous experience with policy changes and their implementation for the treatment of falciparum malaria further highlights the need to institutionalise national malaria policy review and improve communication regarding treatment policy at all levels (Amin et al., 2007; Kamya et al., 2002; Mulligan et al., 2006; Shretta et al., 2000; Williams et al., 2004). Key recommendations suggested are to improve communications from government to health worker level to support implementation, inclusion of pharmaceutical companies in the process to ensure timely registration of novel drugs, active lobbying by the international community to ensure funding for novel tools at country level and to ensure that countries address national level questions in a timely manner (Kshirsagar, 2006; Williams et al., 2004).
NMCPs and national policymakers must consider several factors when revising treatment policies including the available scientific evidence, WHO recommendations, country‐specific considerations regarding malaria epidemiology and G6PD deficiency (including in‐country heterogeneity), local socio‐ecological variations, cost‐effectiveness, logistics and health system capacity. Countries' national planning cycles include National Strategic Plans (NSP), Mid Term Reviews (MTR) and Malaria Programme Reviews (MPRs) which again affect the timely approval of new policy. In addition, many NMCPs rely on external funding from organisations such as the GF that influence decision‐making and timelines at country level. The GF, in turn, relies on WHO prequalification and policy guidance for new tools at the global level. Factors influencing the uptake of these tools include NMCP or Ministry of Health perceptions of ownership of research (countrywide and on a larger scale), the timeliness at which evidence becomes available, the reliability of research data, available funding and cost‐effectiveness (Nutley et al., 2007; Peters, 2018; Smith, 2013). Finally, the incentive to implement the process of policy change can be driven by national goals such as the target for malaria elimination.
In 2019, the Asia Pacific Malaria Elimination Network's (APMEN) Vivax Working Group (VxWG) convened its annual meeting in Kathmandu, Nepal, and was attended by over 140 participants. Roundtable discussions and a workshop were held to identify evidence gaps and potential research questions pertinent to NMCPs' policy change processes in a timely manner. The aim was to identify these questions as NMCPs are preparing revisions of their NSPs and GF concept notes. While countries await the revised WHO global recommendations, which are anticipated by mid‐2021, NMCPs and regional policymakers need to consider the additional information required to initiate country‐level changes once these recommendations are in place.
This article explores the additional evidence required by NMCPs that aim to initiate policy change to implement safe and more effective radical cure for P. vivax malaria. It highlights the common issues faced, as well as country‐specific questions that need to be addressed. The aim of this work was to facilitate an NMCP‐guided translational research agenda for the short to medium term to assist the decision‐making processes for the potential introduction of novel radical cure strategies.
2. METHODS
2.1. The Vivax Working Group of the Asia Pacific Malaria Elimination Network (APMEN)
The VxWG of the APMEN was formed in 2009 at the network's inaugural meeting. P. vivax was identified as a critical challenge for regional malaria elimination. The group now comprises representatives from 21 NMCPs, research partners, the WHO, as well as key funding, global health and industry stakeholders. Together with the Asia Pacific Leaders Malaria Alliance (APLMA), APMEN forms a collaborative network working to overcome the challenges to eliminating malaria in the Asia‐Pacific region (Thriemer et al., 2017, 2018; Vivax Working Group, 2015). Over the last 9 years, the VxWG has fostered dynamic interactions between stakeholders to guide the development and optimisation of tools and products for the diagnosis and treatment of P. vivax . With the availability of novel tools (Alam et al., 2018; Lacerda et al., 2019; Llanos‐Cuentas et al., 2019; Pal et al., 2019; Taylor et al., 2019), the time has now come to shift the focus from product development to the implementation of these tools into policy and practice.
2.2. Participant characteristics
All participants taking part in the main annual meeting were included in the roundtable discussion and were categorised into two groups: country partners (CPs) comprising delegates from NMCPs, and research partners (RPs). RPs included researchers from endemic countries and non‐endemic countries, Civil Society Organisations (CSOs), Product Development Partnerships (PDPs), manufacturers, donors, WHO, APMEN and APLMA. During the consequent workshop, only CPs participated. Three post‐meeting interview CP participants were selected based on their availability in the weeks after the meeting and all three interviewees were present during both the main meeting and the workshop.
2.3. Data collection
Data was collected in roundtable discussions at the annual meeting, in a consequent CP workshop and in follow‐up interviews with select CPs who attended the annual meeting.
2.3.1. Roundtable discussions
During the main meeting, a roundtable discussion was held, focusing on defining the evidence gaps relevant for policy change. The aim of these discussions was to initiate dialogue around what evidence was required to implement national level policy change for adopting novel radical cure options. Conference attendees were allocated to separate tables for CPs and RPs to ensure opinions were captured differentially. CP table allocation was done across subregions to encourage diversity of opinions on each table. In the case of the CPs, there were three tables with 12–14 participants each. RPs were represented in six tables with 10–11 participants each. Each table had a facilitator and a note taker to ensure that table discussions were focused, and discussion outcomes were recorded accurately (Appendix A). Prior to the meeting, facilitators and note takers were briefed on the key objectives of the discussions. At the end of the discussions, the CP tables presented their discussion outcomes to the plenary. Outcomes from the RP discussion were collated separately. Notes and outcome summaries were used for data analysis.
2.3.2. Workshop
Following the initial two‐day meeting, a workshop restricted to CPs was held to explore the additional evidence required by each country to develop a road map for policy change and implementation. CPs were divided into nine discussion tables (with 2–3 countries per table) and each table was allocated a facilitator and note taker. Workshop discussions were based on the assumption that a WHO recommendation was available for high dose short course PQ and TQ. Directly after the end of the roundtable discussions of the main meeting and in a consultative process with the facilitators, the authors distilled nine research questions relevant for policy change. The questions were written on flipcharts and participants were asked to rank them by priority.
2.3.3. Follow‐up interviews
Follow‐up Skype or email semi‐structured interviews were held with three CPs—Ethiopia, Indonesia and Cambodia—in November and December 2019. The objectives of these additional interviews were to verify the priority research questions ranked during the workshop, investigate if any further evidence gaps had been identified after the workshop and understand if further discussion with other NMCP members may have altered priority questions.
2.4. Data analysis
Our approach to analysis was qualitative and semi‐quantitative, as follows:
2.4.1. Group discussion analysis
Notes from discussions of both CPs and RPs were analysed to identify research topics that both groups perceived as important for policy change. To further understand the relative importance of each research topic to the two groups, all notes were combined, and a count made of the frequency with which each of the emergent research topics were discussed—we refer to these as research topic frequency scores.
In addition, all notes for CPs and RPs were combined and a word cloud generated to conceptualise emerging major themes—this consisted of a visual representation of text data with words weighted by their frequency of use in a document. The word cloud was created using the online software tool http://www.wordclouds.com by merging all group work notes, removing country names, correcting spelling, and checking frequently used words for variances in how they were expressed (for instance PQ‐14 and 14‐day PQ were both expressed as PQ14). Once the word cloud was generated, the frequent word list was double‐checked for duplicated words with incorrect spelling or similar meanings.
2.4.2. Prioritisation of research topics
During the workshop, facilitators developed a list of research topics that emerged from the roundtable discussions of both CPs and RPs. A total of nine topics areas were developed. During the workshop, CPs were asked to prioritise research questions they perceived as important for policy change by ranking them from a score of 1 (the highest priority) to 5 (the lowest). Questions which were not ranked by participants were given a score of six. The average country priority ranking for each research question was then calculated to identify the most important research questions.
2.4.3. Semi‐structured interviews
One interview was conducted via Skype and notes were taken, and the two other interviews were done via email. All transcripts were manually coded for confirmed priority areas, novel priority areas, potential other opinions and future actions. Passages of text were read, and codes assigned based on themes, and examined for frequency, trends, similarities, and differences. When necessary, direct speech in the results section was corrected for grammatical errors to improve readability; words added by investigators to improve clarity were marked by square brackets.
2.4.4. Comparison of country partner and research partner priorities
We compared RP and CP priority topics by assessing research priority frequency scores from group discussions. As there were twice the number of RPs than CPs during discussion groups, we incorporated CP ranking of research topics into the CP frequency scores by multiplying the original frequency score by average rank scored. We adapted lessons learned expressed in Williams et al. (2004) to develop a continuum of policy change along which we plotted research topic frequency scores. Four key phases were identified up to policy change: (i) current implementation; (ii) clinical studies to guide global policy; (iii) national adaptation of new tools; and (iv) national policy change to new tools. Research topics related to current practice were included under ‘current implementation’. Efficacy trials were included under ‘clinical studies’ and ‘national adaptation of new tools’ included feasibility and effectiveness studies—or any studies that would allow CPs to assess the use of new tools within their respective health systems. ‘National policy change’ included elements such as translation of evidence and coordination between RPs and CPs. The placement of circles for research topics along the continuum of change was done by two authors and agreed through follow‐up discussions. Topics were represented by circles, the size of which was related to the frequency score with larger circles representing higher scores.
3. RESULTS
3.1. Participants
A total of 43 CPs and 97 RPs attended the meeting (Appendix B and Appendix C). CP representation within the Asia‐Pacific region included participants from Afghanistan (n = 2), Bangladesh (n = 1), Bhutan (n = 3), Cambodia (n = 2), China (n = 1), India (n = 2), Indonesia (n = 2), Lao (n = 3), Malaysia (n = 2), Nepal (n = 10), Pakistan (n = 1), the Philippines (n = 1), Papua New Guinea (PNG) (n = 1), Republic of Korea (n = 2), Solomon Islands (n = 2), Sri Lanka (n = 2), Thailand (n = 2), Vanuatu (n = 1) and Vietnam (n = 2). An additional observer from Ethiopia (n = 1) also attended. Among the 97 RPs there were 39 (39.8%) researchers from endemic countries, 24 (24.7%) researchers from non‐endemic countries, 7 (7.2%) representatives from CSOs, 10 (10.3%) delegates from PDPs, 3 (3.1%) manufacturers, 1 (1.0%) international WHO representative and 2 (2.1%) officials from the WHO office in Nepal, 3 (3.1%) APMEN representatives, 3 (3.1%) APLMA officials and 3 (3.1%) donor representatives. Most researchers from both endemic and non‐endemic countries had a biomedical research focus including clinical and lab science or modelling and statistics skills (92.1%; 58/63) and 5 (7.9%) participants worked in the field of social science or had a health economics focus.
3.2. Key research questions identified by country partners during main meeting
The most frequent topics identified as being key to inform policy change and implementation were cost‐effectiveness and cost–benefit, understanding policy change processes, operational feasibility and health system capacity, G6PD test accuracy and reliability, treatment efficacy and G6PD prevalence. In addition, safety, drug acceptability, treatment effectiveness and adherence and P. vivax prevalence were also ranked as important (Appendix D and Appendix E).
3.3. Key research questions identified by research partners during main meeting
Research areas, questions and topics identified by the six RP tables during the meeting encompassed a wide variety of issues. Overarching topics included the safety and appropriate testing for G6PD, effectiveness and adherence of radical cure options, operational feasibility and health care system capacity (Table 1 and Figure 1). RPs also highlighted other challenges, beyond research, that require better understanding or strengthening such as variations in government regulation of the public and private health sector in different APMEN countries that affects policy regulation, revision and implementation. Understanding P. vivax epidemiology and associated mortality to facilitate advocacy as well as the need to improve communication with patients to highlight the benefits of radical cure were also mentioned.
TABLE 1.
Evidence gaps and research questions identified by research partners
| Overarching topics | Identified evidence gaps and research questions |
|---|---|
| Safety and appropriate testing for G6PD |
• How good is the reliability and field applicability of G6PD tests? • Is a single time point to test for G6PD activity sufficient (i.e., one life‐long diagnosis)? • How to conduct effective training G6PD testing including QC? • What is the more comprehensive picture on G6PD deficiency levels across different populations? • Country‐specific data on the adverse effects of PQ and TQ is required as well as better definitions to describe, predict and manage haemolytic events |
| Effectiveness and adherence of radical cure options |
• Lack of comparative effectiveness data between PQ7 and PQ14 and TQ • Limited evidence to support country claims that PQ14 is working • In which areas is low dose PQ with increased adherence (supervision) sufficient? • Pragmatic qualitative studies on adherence |
| Feasibility and health care system capacity |
• Need for large‐scale implementation and feasibility studies particularly in large countries such as Indonesia and India with many ethnic groups • Cost‐effectiveness on subnational level • Pharmacovigilance strengthening • Feasibility of implementation of different radical cure options on primary, secondary, and tertiary health care levels • Operational challenges for roll out of easy‐to‐use, safe and reliable G6PD testing |
FIGURE 1.
Word cloud identifying key topics from research partner discussions
3.4. Prioritisation of questions by country partners during the workshop
To clarify which of these topics had the highest priority for CPs, nine key questions were distilled after the initial discussions at the main meeting. These included: (i) What is the effectiveness of different treatment options (14 day low dose PQ (PQ14), 7 day high dose PQ (PQ7), TQ) and adherence between different regimens? (ii) What are the overall vivax dynamics in my country? (iii) How effective is current practice? (iv) What is the prevalence of G6PD deficiency in my country? (v) How well do new diagnostics work in the field (usability, robustness, etc.)? (vi) How can we ensure safe delivery of the different options? (vii) What is the cost effectiveness of the different regimens? (viii) Feasibility of new interventions at different levels of health system (including supply chain capacity and quality assurance)? (ix) What is the best way to improve patients' adherence?
Of these nine research questions, CPs ranked effectiveness of different radical cure options as the highest priority followed by questions around how well G6PD diagnostics work in the field. Evaluating methods to improve adherence was ranked as the third priority. Cost‐effectiveness of different options and feasibility as well as more data on G6PD prevalence within countries were ranked equally following the initial three priorities (Table 2). There were significant differences in rankings by country (Appendix F).
TABLE 2.
Average ranking of country research priorities, with 1 being highest priority and 5 lowest (no priority = score of 6)
| Question | Average rank |
|---|---|
| What is the effectiveness of different treatment options (PQ14, PQ7, TQ) and adherence between different regimens? | 2.6 |
| How well do new diagnostics work in the field (usability, robustness, etc.)? | 3.9 |
| What is the best way to improve patients' adherence? | 4.1 |
| What is the cost effectiveness of different regimen options? | 4.4 |
| Feasibility of new interventions at different levels of health system (including supply chain capacity and quality assurance)? | 4.4 |
| What is the prevalence of G6PD deficiency in my country? | 4.4 |
| How can we ensure safe delivery of the different options? | 4.9 |
| How effective is the current practice? | 5.3 |
| What are the overall vivax dynamics in my country? | 5.6 |
3.5. Validation of country partner priority research topics
3.5.1. Confirmation of priority ranking
All three CP participants interviewed confirmed that the priorities developed during the APMEN VxWG workshop remain priorities even after discussion with other NMCP members.
The efficacy and effectiveness of PQ7 remained the top‐ranking question for Cambodia:
We don't know about the efficacy of PQ treatment plans, but we think that 7‐day dose might be appropriate, and an increased dose might be effective, based on what other APMEN countries are doing also. (Cambodian NMCP representative)
The Indonesian NMCP representative confirmed that feasibility studies are their priority whereas the Ethiopian representative highlighted that operational feasibility of novel tools and cost effectiveness of G6PD testing at community level were the most pressing questions to address for their country as well as assessing gaps in current practice.
We need to assess whether new policy related to vivax radical cure could be implemented in a routine situation. (Indonesian NMCP representative)
It is good first to have conclusive evidence that shows the current practice has adherence issues. If not, the decision might be to continue with PQ14 to be on the safe side. (Ethiopian NMCP representative)
3.5.2. Other opinions within the NMCPs
The Cambodian and Indonesian respondents reported that other NMCP colleagues and their respective Ministries of Health are also in agreement that these are each of their countries' key priorities. The Indonesian NMCP discussed these priorities with their national expert committee on October 24, 2019 after the 2019 APMEN meeting and before the interview, suggesting that there is a reasonable consensus within this expert community. However, the Indonesian respondent mentioned that there might be other opinions with diverging priorities at a higher Ministerial level. The Ethiopian respondent highlighted the need to reach full consensus among decision‐makers and that more discussions were needed within the country.
3.5.3. Future action
In line with the identified priorities, Cambodia planned pilot studies to inform potential policy change.
Cambodia will only implement evidence‐based policy. We plan to investigate PQ7 and we would also like to explore how to implement TQ starting in the military. (Cambodian NMCP representative)
Similarly, feasibility studies were planned in Indonesia and in Ethiopia to generate data to support decision‐making.
TQ is not a priority at this point, we have huge amount of P. vivax . So, to minimise any unintended consequence as a result of this drug, we have to wait for some time [and gather more evidence]. (Ethiopian NMCP representative)
For Indonesia and Ethiopia, respondents mentioned that this did not yet influence national planning whereas in Cambodia these plans were closely linked to the currently developed NSP and these activities will be included in an upcoming application to the GF.
3.6. Research priorities on the continuum of change
By combining CP topics and ranking into frequency scores, the highest priority topics raised by CPs became the use and reliability of point‐of‐care (PoC) G6PD tests on a par with cost‐effectiveness of new tools, G6PD prevalence, and feasibility and health system capacity, closely followed by patient adherence and effectiveness of novel treatment options (Figure 2). CPs emphasised costing, budget impact and cost–benefit analysis as well as cost‐effectiveness. Lower ranked priorities were P. vivax prevalence, current treatment effectiveness, understanding change processes, drug acceptability and safety. RPs prioritised patient adherence and effectiveness, safety, and feasibility and health system capacity. Additional priorities that were ranked lower were drug acceptability and cost‐effectiveness. The lowest ranked topic by RPs was P. vivax prevalence.
FIGURE 2.
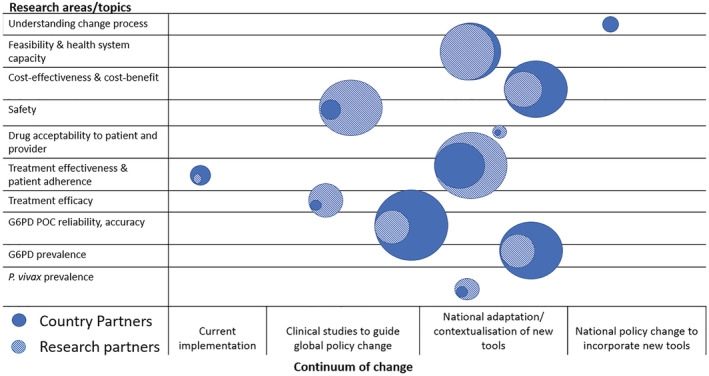
Research areas/topic priorities for country and research partners plotted along the continuum of change. Notes: Topics identified in the main meeting and the workshop are represented by circles. Circle sizes represent the frequency of topic discussed, with larger circles representing higher frequency
The greatest overlap between CPs and RPs was the need to understand the operational feasibility of implementing new tools in country health systems which was similarly ranked between CPs and RPs. Treatment effectiveness and patient adherence was the next greatest overlap followed by cost effectiveness and cost–benefit of different treatments. The greatest divergence between partners was regarding G6PD PoC accuracy and reliability, safety, and cost‐effectiveness. CPs highlighted the need to understand policy processes including how to better increase uptake of evidence into policy. RPs tended to highlight the need to map P. vivax prevalence or mortality to highlight to policymakers the need for vivax to be prioritised. Finally, research topics of interest to both CPs and RPs clustered around the national adaptation or contextualisation of new tools with CPs highlighting the need to understand policy processes or how to translate results from studies into guidance (Figure 2).
4. DISCUSSION
P. vivax malaria continues to be a major global public health threat. In the Asia‐Pacific region and the Horn of Africa, the highest caseloads are often in remote communities where access to healthcare is poor. With the recent approval of and evidence for the efficacy of new tools for better radical cure (Lacerda et al., 2019; Llanos‐Cuentas et al., 2019; Taylor et al., 2019; White, 2019), discussions were facilitated to identify key research questions faced by CPs seeking to adapt new tools into their respective settings. The overall results of our analysis highlight a call by CPs for additional national‐level evidence around PoC G6PD accuracy and reliability, operational feasibility of radical cure options within health systems and cost‐effectiveness analysis of new tools. This work highlights key questions CPs will face when aiming to change national policy and proposes areas which researchers and donors can focus their work and resources on. It indicates the need for an increased understanding of treatment effectiveness under real‐life conditions as compared to efficacy which is measured in carefully controlled settings (Thriemer et al., 2017). This is in line with questions faced by CPs around a national level understanding of how to incorporate these new tools into clinical practice. Previous work on malaria treatment policy change highlights the importance of undertaking effectiveness studies as well as efficacy studies to drive policy change (Durrheim & William, 2005). Countries need to contextualise research findings and global policy recommendations to suit their countries' socio‐economic needs and specific epidemiological contexts (Davis & Walker, 2019). Those studies are crucial to support policy change and ‘bridge the "know–do" gap’ as highlighted previously (WHO, 2005). Health policy change is complex requiring different types of evidence along the continuum of change (Lewin et al., 2012; Peters & Bennett, 2012). Malaria programs have the benefit of receiving global guidance from the WHO that collates and assesses global clinical trial and safety data for new treatments. However, WHO guidance is global by necessity thus early country adopters of new tools often need to determine whether and how these tools will work in their local contexts.
The need for greater understanding of PoC G6PD accuracy and reliability prior to policy change was prioritised by CPs and RPs indicating the need to pilot new G6PD diagnostic tools especially before implementing TQ or high dose PQ regimens. Both CPs and RPs expressed concerns regarding drug tolerability and safety. However, in this context, CPs seem to express this as ‘wanting to better understand the reliability of G6PD tests’ to ensure that these tools will correctly identify G6PD deficient (or intermediate) patients when used in tandem with higher dose regimens. Historically, the availability of G6PD PoC tests has been very limited. NMCPs' exposure to quantitative tests, which are now in the process of more widespread commercialisation and availability, has been limited but concerted efforts have been undertaken by partners to raise awareness of these products and how they can be used to support malaria control and elimination goals (Ley et al., 2015). The difference in the level of priority given to PoC G6PD by RPs and CPs likely reflects an asymmetry in information between the two groups possibly indicating the need for better knowledge transfer through synthesis and accessible presentation of G6PD literature as well as further studies to answer CPs' questions. Discussions also highlighted the need to have a broader understanding about safety of radical cure away from a focus on adequate testing only to a more holistic approach, including adequate detection of early signs of haemolysis and risk mitigation strategies.
While both CPs and RPs expressed the need for feasibility studies, there was little discussion as to what exactly should be included in these studies. RPs highlighted the need to understand how pharmacovigilance systems could be strengthened down to the lowest levels where new tools could be used. This could indicate that feasibility for CPs and RPs means understanding how new tools will be deployed within the health system. Previous qualitative research to understand the processes and timelines for the introduction of ACTs and mandatory confirmation of malaria prior to treatment (Ajayi et al., 2008; Swana et al., 2016) indicates that exploratory studies provide useful and effective pre‐implementation data. Other health system elements highlighted from previous research on malaria treatment policy change pathways include increasing the uptake of integrated community case management (iCCM) (WHO, 2018) and strengthening the health workforce (WHO, 2015a) to manage the use of novel tools. This begs the question as to how and at what cost can countries provide adequate supportive supervision to their health workforce to ensure effective and safe radical cure, particularly when scaled up.
Cost‐effectiveness studies on new tools and treatment regimens were also raised by CPs and RPs. Some countries require Cost‐Effectiveness Analyses (CEA) to allow the incorporation of new tools into insurance schemes (Escribano Ferrer et al., 2017; Hansen et al., 2017). In the APMEN region, China, Malaysia, Philippines and Thailand have a requirement for Health Technology Assessments (HTA) of new tools (Sivalal, 2009) akin to the NICE process in the United Kingdom (NICE, 2019), but this is not a requirement in most regional countries. The HTA requirement is likely correlated to the proportion of the malaria programs supported by domestic versus external funding. Some countries are more reliant on GF for procurement of tools which need to be WHO approved or prequalified prior to GF procurement (Pigott et al., 2012). Others have substantial domestic budgets that may require different sets of evidence or information for policy change (Pigott et al., 2012). While limited, some CPs discussed the need for cost–benefit analysis and budget impact studies in addition to cost‐effectiveness. This likely reflects their cognizance of having to advocate for additional resources within their respective ministries of health, providing cost data in different ways to do so.
Understanding policy change processes and uptake of evidence was also raised. CPs requested more and better translation of evidence into policy especially by neutral or non‐technical parties who could translate evidence into simple language. In addition, they specified the need for stronger coordination with researchers and better understanding of regulatory and policy change pathways (see Figure 2). The gap between scientific recommendations based on research findings and countries' motivations to change policy is often difficult to bridge (Smith, 2013). CPs' requests align with findings from Smith's review of knowledge transfer in which an increase in the use of research into policy and practice explored the following strategies: ‘ensuring research is accessible’, ‘developing ongoing, collaborative relationships’, ‘improving structural communication channels’ and ensuring that there are sufficiently high incentives among researchers and research users to engage in knowledge exchange (Smith, 2013, p. 21).
CPs were also keenly aware of their implementation needs and unsurprisingly were concerned about the success of current treatment practice which will need to continue beyond feasibility studies while countries await global WHO guidance. In addition, G6PD deficient patients will continue to receive either the current standard treatment or 8‐week PQ. Understanding current adherence rates and reasons for non‐adherence will be essential for designing more effective behaviour‐centred strategies to improve the effectiveness of current regimens (Aunger & Curtis, 2016). RPs agreed with CPs regarding the need to assess current treatment guidelines but more from the perspective of proving that those treatments have low effectiveness (Abreha et al., 2017; Douglas et al., 2017; Maneeboonyang et al., 2011; Takeuchi et al., 2010) and thus strengthen the advocacy and business case for a policy change.
There are several limitations to this work. First, although APMEN serves as a unique forum for exchange between the research community and NMCPs, inevitable time restrictions prevent in‐depth discussions and more detailed analysis. CPs often have greater experience and capacity in policy implementation than in identifying and commissioning research to suit country needs.
Second, topics raised by RPs are shaped by their expertise and knowledge background, which is biased towards clinical and basic science with a lack of expertise in the fields of policy and health system research as well as limited participation by scientists with behavioural and social science backgrounds. This might also explain why participants did not raise topics around particular strategies to provide treatment to mobile populations and other hard‐to‐reach populations.
Third, the selection of research questions used for the ranking was done ad hoc at the end of the main meeting without considering the more detailed notes from each of the tables due to time limitations.
Fourth, there is a risk of potential power imbalance between RPs and CPs as RPs tended to be either native English speakers or have a very good working knowledge of English and were more confident in expressing their views. Whereas CPs may be less confident in expressing their views in English despite being highly knowledgeable in their country context. This results in CP voices often being heard less. To address this issue, roundtable discussions during the main meeting were separated for CPs and RPs and a follow‐up workshop was held exclusively for CPs.
Fifth, follow‐up interviews to verify some of the discussion outcomes were only held with three NMCPs. Interview participants were selected based on their availability within the timeframe of writing this article. Further in‐depth investigation is warranted to fully understand the evidence gaps.
Sixth, average priority scores across the region only have limited significance and given the heterogeneity between countries, localised research agendas need to be developed. In addition, individual CPs might not accurately represent country program priorities, but might be biased based on the specific area of work within the respective programs.
Seventh, the methods used to obtain frequency scores combined the average ranking of countries with roundtable scores to consider fewer CP than RP participants which could have skewed priorities towards those that were ranked.
Finally, we attempted to position research questions along a continuum of policy change while acknowledging that change is an iterative, non‐linear process that is influenced by many factors beyond evidence that require better understanding. As well as defining these evidence gaps, there is a need to understand the political economy of policy change processes. Providing data on ongoing research for CPs to draw from for timely decision‐making, packaging evidence in a manner that it translates clearly from RPs to CPs and continuing to strengthen the communication interface between RPs and CPs is also ideal. Work addressing these needs has already started and will inform policy change in the short and long term.
5. CONCLUSION
The safe and effective roll out of radical cure has repeatedly been identified by CPs as one of the major challenges of moving towards malaria elimination in vivax endemic countries (Thriemer et al., 2017). With the availability of novel tools and treatment strategies, the Asia‐Pacific target of elimination within the next 10 years appears more feasible. However, given the relatively short timeframe to achieve this 2030 target, the evidence gaps identified in our meetings need to be addressed urgently so that results become available at the same time as WHO provides a policy recommendation and revised global guidance on P. vivax treatment—anticipated in mid‐2021. This would enable NMCPs to examine global recommendations alongside nationally generated data relevant to their respective contexts.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
KT and RP conceived the overall meeting. KT, CAL, SN, EGG and VR planned and organised the workshop. VR collated data gathered by notetakers and facilitators at the meeting and the workshop. VR, MT, LD and MH contributed in‐depth data collection from Indonesia, Cambodia and Ethiopia. VR, KT, CAL and KP analysed the data. VR wrote the first draft of the article. KT, CAL, RP and KP have reviewed and edited the manuscript. All authors have read and approved the final manuscript.
FUNDING INFORMATION
VR is funded by an Australian Government Research Training Program stipend from Charles Darwin University and an Australian Centre of Research Excellence in Malaria travel grant (APP 1134989). KT is funded by a CSL Centenary Fellowship, and a Bill and Melinda Gates Foundation grant (OPP1164105).
ACKNOWLEDGEMENTS
We would especially like to acknowledge and thank facilitators of the roundtable discussions and workshop: Spike Novak, Nick Luter, Robert Commons, Jutta Marfurt, Angela Devine, Sophie Weston, Leanne Robinson, Rosalind Howes, Samuel McEwen, Chris Mercado, Melanie Larson, Kylie Mannion, Vajra Allan, Yucheng Tsai, Rittika Datta, Josselyn Neukom, Kemi Tesfazghi, Evelyn Wong, Daniel Pfeffer, Annisa Rahmalia, Inessa Ba and Charlotte Gryseels. We would also like to thank all participants of the 2019 VxWG annual meeting (Appendix B and Appendix C) and the Nepalese NMCP for hosting this event.
Biographies
Varunika Ruwanpura is a social sciences researcher and journalist. She is a PhD student at the Menzies School of Health Research in Darwin, Australia. Her research focusses on malaria policy analysis in select endemic countries.
Spike Nowak serves as a regional advisor to the PATH/MMV VivAccess project. His work focuses on market landscaping, demand forecasting, market entry, coordination, and stakeholder engagement for G6PD deficiency testing, high sensitivity RDTs, and malaria radical cure drugs.
Emily Gerth‐Guyette is a public health researcher with 10 years of experience implementing health program and technology innovations at the community level. She has a graduate degree from the University of Washington and since joining PATH in 2014 has focused primarily on diagnostic technology development and introduction.
Dr Minerva Theodora received her medical degree from North Sumatera University, Medan, and qualified as an epidemiologist of public health at Indonesia University, Depok. She works as an epidemiologist and health worker for the National Malaria Control Programme in the Ministry of Health of Indonesia.
Dr Lek Dysoley is the deputy director for research at the National Center for Parasitology, Entomology and Malaria Control (CNM), Cambodia. He leads the Cambodia Malaria Program management team in collaboration with WHO, and guides current nationwide malaria elimination intervention aiming to achieve eradication by 2030.
Mebrahtom Haile is a National Malaria Program Manager and Senior Public Health specialist at the Federal Ministry of Health, Ethiopia. He also leads the National Malaria Program advisor committee in Ethiopia and works closely with research institutes, and international and local organisations to generate evidence for policy decision‐making and mobilisation of resources for the program.
Professor Koen Peeters Grietens heads the Medical Anthropology Unit at the Institute of Tropical Medicine in Antwerp and is a senior lecturer at Nagasaki University. He holds a PhD in Social and Cultural Anthropology and has conducted extensive research on sociocultural factors related to infectious disease transmission dynamics, community perceptions on health and illness, and their impact on the effectiveness of prevention, control and elimination strategies.
Professor Ric Price is an infectious diseases physician and clinical researcher, a Fellow of the Royal Australian College of Physicians, the Royal College of Physicians (UK) and the Royal College of Pathologists (UK). He is a Welcome Trust Senior Research Fellow, with dual affiliation as Professor at the Menzies School of Health Research and at the Centre of Tropical Medicine and Global Health, University of Oxford. He is also the chair of the Vivax Working Group of the Asia Pacific Malaria Elimination Network.
Dr Caroline Lynch is regional advisor for Medicines for Malaria Venture, based in Thailand. Currently, Caroline works on VivAccess—a joint PATH/MMV initiative. She holds a PhD in malaria epidemiology and MSc in Applied Parasitology and Medical Entomology and has extensive experience in research and implementation of malaria control programs.
Dr Kamala Thriemer is a Senior Research Fellow at the Menzies School of Health Research in Darwin, Australia. She has extensive field experience in Africa and Asia including work on malaria, typhoid and cholera. Her current research program focuses on optimising treatment options for. She is co‐currently a lecturer at Charles Darwin University and coordinates the Vivax Working Group of the Asia Pacific Malaria Elimination Network (APMEN).
APPENDIX A. ROUNDTABLE ALLOCATION
| Table | Facilitators | Note taker | NMCPs |
|---|---|---|---|
| CP table 1 | Josselyn Neukom, APLMA | Rob Commons, Menzies School of Health Research | Afghanistan, Bhutan, Cambodia, India, Lao (PDR), Malaysia, Pakistan, PNG, Solomon Islands, Sri Lanka, Vanuatu, Ethiopia, China |
| CP table 2 | Evelyn Wong, CHAI | Rittika Datta, APLMA | Afghanistan, Bhutan, China Indonesia, Lao (PDR), Nepal, Pakistan, Republic of Korea, Solomon Islands, Thailand, Vietnam, Ethiopia, PNG, Vanuatu |
| CP table 3 | Spike Novak, PATH | Kemi Tesfezghi, PSI | Bangladesh, Cambodia, India, Indonesia, Malaysia, Nepal, Philippines, Republic of Korea, Sri Lanka, Thailand, Vietnam, Ethiopia |
| RP table 1 | Nick Luter, PATH | Jutta Marfurt, Menzies School of Health Research |
Researchers, endemic countries n = 6 Researchers, non‐endemic countries n = 3 PDP n = 1 WHO n = 1 Manufacturers n = 2 |
| RP table 2 | Angela Devine, Menzies School of Health Research | Sophie Weston, Menzies School of Health Research |
Researchers, endemic countries n = 6 Researchers, non‐endemic countries n = 2 CSO n = 1 PDP n = 1 NMCP n = 1 Donor n = 1 |
| RP table 3 | Ros Howes, FIND | Varunika Ruwanpura, Menzies School of Health Research |
Researchers, endemic countries n = 6 Researchers, non‐endemic countries n = 2 CSO n = 1 PDP n = 1 Donor n = 2 |
| RP table 4 | Sam McEvan, Burnet Institute | Chris Mercado, APMEN |
Researchers, endemic countries n = 6 Researchers, non‐endemic countries n = 2 CSO n = 1 PDP n = 1 WHO n = 1 Manufacturers n = 1 NMCP n = 1 |
| RP table 5 | Melanie Larson, PATH | Kylie Mannion, APMEN |
Researchers, endemic countries n = 4 Researchers, non‐endemic countries n = 4 CSO n = 1 Manufacturers n = 1 APMEN = 1 |
| RP table 6 | Vajra Allen, PATH | Yucheng Tsai, CHAI |
Researchers, endemic countries n = 5 Researchers, non‐endemic countries n = 3 WHO n = 1 Manufacturers n = 1 APLMA n = 1 |
APPENDIX B. APMEN VxWG 2019 CP PARTICIPANTS
| Country | Participant name |
|---|---|
| Afghanistan | Hamida Hamid |
| Afghanistan | Sami Nahzat |
| Bangladesh | MM Aktaruzzaman |
| Bhutan | Kinley Penjor |
| Bhutan | Rixin Jamtsho |
| Bhutan | Tobgyel Tobgyel |
| Cambodia | Chea Nguon |
| Cambodia | Lek Dysoley |
| China | Gao Qi |
| Ethiopia | Mebrahtom Haile Zeweli |
| India | Suman Lata Wattal |
| India | Sunil V Gittee |
| Indonesia | Minerva Theodora |
| Indonesia | Nurul Amin |
| Lao | Keobouphaphone Chindavongsa |
| Lao | Viengxay Vanisaveth |
| Lao | Vonethalom Thongpaseuth |
| Malaysia | Jenarun Jelip |
| Malaysia | Rose Nani Binti Mudin |
| Nepal | Anub Bastola |
| Nepal | Bibek Lal |
| Nepal | Ghanashyam Pokharel |
| Nepal | Kumar P Pokharel |
| Nepal | Leela Bikram Thapa |
| Nepal | Prakash P Sah |
| Nepal | Uttam Koirala |
| Nepal | Uttam Pyakurel |
| Nepal | Yuva Raj Pokharel |
| Pakistan | Abdul Majeed |
| Papua New Guinea | Leo Sora Makita |
| Philippines | Raffy Deray |
| Republic of Korea | Byoung Hak Jeon |
| Republic of Korea | Sang‐Eun Lee |
| Solomon Islands | Albino Bobogare |
| Solomon Islands | Leonard Boaz |
| Sri Lanka | K. D. N. P. Ranaweera |
| Sri Lanka | Megala Ravichandran |
| Thailand | Darin Areechokchai |
| Thailand | Prayuth Sudathip |
| Vanuatu | Esau Naket |
| Vietnam | Ngo Duc Thang |
| Vietnam | Nguyen Van Dung |
Abbreviations: CPs, country partners; CSOs, Civil Society Organisations; PDPs, Product Development Partnerships; RPs, research partners.
Note: All CPs are leaders or members of NMCPs or the respective disease control divisions in their countries.
APPENDIX C. APMEN VxWG 2019 RP PARTICIPANTS
| ACCESSBIO | Young S Hong |
|---|---|
| ACCESSBIO | BD Han |
| ACCESSBIO | Jacob Yang |
| APLMA, Singapore | Amita Chebbi |
| APLMA, Singapore | Josselyn Naukom |
| APLMA, Singapore | Rittika Datta |
| APLMA, Singapore | Vaibhav Gupta |
| APMEN, Singapore | Chris Mercado |
| APMEN, Singapore | Kylie Mannion |
| Australian National University, Australia | Kinley Wangdi |
| Burnet Institute, Australia | Jack Richards |
| Burnet Institute, Australia | Leanne Robinson |
| Burnet Institute, Australia | Rebecca Dabbs |
| Burnet Institute, Australia | Samuel McEwen |
| Clinton Health Access Initiative | Evelyn Wong |
| Clinton Health Access Initiative | Inessa Ba |
| Clinton Health Access Initiative | Yucheng Tsai |
| Clinton Health Access Initiative, Nepal | Kiran Awasti |
| Department of Foreign Affairs and Trade, Australia | Alyson Auliff |
| Eijkman Institute for Molecular Biology, Indonesia | Ari Satyagraha |
| Eijkman‐Oxford Clinical Research Unit, Indonesia | Kevin Baird |
| Ethiopian Public Health Institute, Ethiopia | Adugna Woyessa |
| Ethiopian Public Health Institute, Ethiopia | Ashenafi Assefa |
| FIND, Switzerland | Christian Ghergu |
| FIND, Switzerland | Rosalind Howes |
| GSK, UK | Andy Walker |
| icddr,b, Bangladesh | Wasif Khan |
| Institute of Cholera and Enteric Diseases, India | Arunansu Talukdar |
| Institute of Cholera and Enteric Diseases, India | Santasabuj Das |
| Institute of Tropical Medicine, Belgium | Charlotte Gryseels |
| Kasturba Medical College, India | Kavitha Saravu |
| London School of Hygiene & Tropical Medicine, UK | Henry Suhendra |
| Mahidol Vivax Research Center, Thailand | Wang Nguitragool |
| Mahidol Vivax Research Center, Thailand | Wanlapa Roobsoong |
| Malaria Atlas Project (MAP), UK | Katherine Battle |
| Malaria Medicines Venture (MMV), Switzerland | Melanie Larson |
| Malaria Medicines Venture (MMV), Switzerland | Caroline Lynch |
| Malaria Medicines Venture (MMV), Switzerland | Eric‐Marie Dupuy |
| Malaria Medicines Venture (MMV), Switzerland | George Jagoe |
| Menzies School of Health Research, Australia | Angela Devine |
| Menzies School of Health Research, Australia | Daniel Pfeffer |
| Menzies School of Health Research, Australia | Jutta Marfurt |
| Menzies School of Health Research, Australia | Kamala Thriemer |
| Menzies School of Health Research, Australia | Matthew Grigg |
| Menzies School of Health Research, Australia | Ric Price |
| Menzies School of Health Research, Australia | Robert Commons |
| Menzies School of Health Research, Australia | Sophie Weston |
| Menzies School of Health Research, Australia | Varunika Ruwanpura |
| Mahidol‐Oxford Research unit (MORU), Thailand | Cindy Chu |
| Nangarhar University, Afghanistan | Awab Ghulam Rahim |
| National Institute of Malariology, Parasitology and Entomology (NIMPE), Vietnam | Bui Quang Phuc |
| Oxford University Clinical Research Unit (OUCRU), Vietnam | Nguyen Chau |
| Oxford University, UK | Bipin Adhikari |
| Papua New Guinea Institute of Medical Research, PNG | Livingstone Tavul |
| Pasteur Institute, France | Narimane Nekkab |
| PATH, US | Nick Luter |
| PATH, US | Sampa Pal |
| PATH, US | Spike Nowak |
| PATH, US | Vajra Allan |
| Population Services International (PSI), Lao | Kemi Tesfazghi |
| Population Services International (PSI), Myanmar | Phone Si Hein |
| Research Institute for Tropical Medicine, Philippines | Effie Espino |
| Research Institute for Tropical Medicine, Philippines | Veronica L. Tallo |
| Retired Malaria Specialist, Nepal | Garib Das Thakur |
| Retired Malaria Specialist, Nepal | Padam B Chand |
| Save the Children, Nepal | Suman Thapa |
| SD Biosensor, Korea | Eun Kang Ye |
| Sukra Raj Tropical & Infectious Disease Hospital, Nepal | Basudev Pandey |
| Swedish Committee for Afghanistan, Afghanistan | Mohammad Anwar |
| Tribhuvan University, Nepal | Binad Dhungal |
| Tribhuvan University, Nepal | Jeevan B Sherchand |
| Tribhuvan University, Nepal | Keshab Parajuli |
| Tribhuvan University, Nepal | Komal Raj Rijal |
| Tribhuvan University, Nepal | Megha Raj Banjara |
| Tribhuvan University, Nepal | Naba Raj Adhikari |
| Tribhuvan University, Nepal | Prakash Ghimire |
| Tribhuvan University, Nepal | Upendra Thapa Shrestha |
| US President's Malaria Initiative/USAID, Myanmar | Nu Nu Khin |
| UCSF Global Health Group, US | Adam Bennett |
| Unitaid, Switzerland | Katerina Gallizzo |
| University of Gadjah Mada, Indonesia | Supargiyono Supargiyono |
| University of Indonesia, Indonesia | Erni Nelwan |
| University of Indonesia, Indonesia | Inge Sutanto |
| University of Melbourne, Australia | Julie Simpson |
| University of Sumatera Utara, Indonesia | Ayodhia Pitaloka |
| University of Sumatera Utara, Indonesia | Inke Nadia Diniyanti Lubis |
| University of Western Australia, Australia | Brioni Moore |
| University of Western Australia, Australia | Laurens Manning |
| Vector Borne Disease Research and Training Centre, Nepal | Yadu Chandra Ghimire |
| Walter and Eliza Hall Institute (WEHI), Australia | Robert James |
| Walter and Eliza Hall Institute (WEHI), Australia | Harin Karunajeewa |
| WHO, Nepal | Lungten Wangchuk |
| WHO, Nepal | Jos Vandealor |
| WHO, Switzerland | Jane Cunningham |
| WorldWide Antimalarial Resistance Network, UK | Philippe Guerin |
| YPKMP Timika, Indonesia | Annisa Rahmalia |
| YPKMP Timika, Indonesia | Rini Poespoprodjo |
APPENDIX D. INITIAL EVIDENCE GAPS IDENTIFIED BY COUNTRY PARTNERS DURING THE MEETING'S ROUNDTABLE DISCUSSION
| Discussion table number | Country partners | Identified research areas |
|---|---|---|
| 1 | Afghanistan, Bhutan, Cambodia, India, Lao (PDR), Malaysia, Pakistan, Papua New Guinea (PNG), Solomon Islands, Sri Lanka, Vanuatu, Ethiopia, Democratic People's Republic of Korea (DPRK), China | Operational research and policy implementation research |
| Better knowledge translation and advocacy from research organisations for available evidence | ||
| Better understanding of local data and context including G6PD prevalence in each country/Countries had different views regarding whether G6PD data should be population wide, on specific populations, or vivax specific. | ||
| Further national studies relating to the global studies for radical cure options | ||
| Comparison of efficacy, cost effectiveness, and safety of PQ7 versus PQ14 versus TQ | ||
| 2 | Afghanistan, Bhutan, China, Indonesia, Lao (PDR), Nepal, Pakistan, DPRK, Solomon Islands, Thailand, Vietnam, Republic of Korea (ROK), Ethiopia, PNG, Vanuatu | Better coordination between NMCPs and research institutes in countries |
| Additional data and research on G6PD prevalence | ||
| Efficacy and accuracy of qualitative and quantitative G6PD tests | ||
| Research on addressing asymptomatic P. vivax malaria cases | ||
| Research on the success of existing malaria control programmes | ||
| 3 | Bangladesh, Cambodia, India, Indonesia, Malaysia, Nepal, Philippines, Republic of Korea (ROK), Sri Lanka, Thailand, Vietnam, India | Technical evidence on efficacy of new diagnostic tests |
| WHO recommendation on new drugs and the process for drug registration | ||
| Operational feasibility based on level of health system, supply chain factors, human resource capacity and quality assurance procedures | ||
| Cost benefit analysis for implementing radical cure and an assessment of the impact on national health budgets | ||
| Drug acceptability: for patients and provider acceptability, this may require pilot studies. | ||
| Development of a regional evidence base to influence national policy change |
APPENDIX E. WORD CLOUD GENERATED FROM INITIAL DISCUSSION AMONG COUNTRY PARTNERS DURING ROUNDTABLE DISCUSSION
APPENDIX F. COUNTRY‐SPECIFIC RANKINGS OF RESEARCH PRIORITIES
F.1. Afghanistan
| Research question | Priority ranking |
|---|---|
| What is the prevalence of G6PD deficiency in my country? | 1 |
| What is the cost effectiveness of different regimen options? | 2 |
| What is the effectiveness of different options (PQ14, PQ7, TQ) and adherence between different regimens? | 3 |
| Feasibility of new interventions at different levels of health system (including supply chain capacity and quality assurance)? | 4 |
| What is the best way to improve patients' adherence? | 5 |
F.2. Bangladesh
| Research question | Priority ranking |
|---|---|
| What is the prevalence of G6PD deficiency in my country? | 1 |
| How effective is the current practice? | 2 |
| What is the effectiveness of different options (PQ14, PQ7, TQ) and adherence between different regimens? | 3 |
| What is the best way to improve patients' adherence? | 4 |
| What is the cost effectiveness of different regimen options? | 5 |
F.3. Bhutan
| Research question | Priority ranking |
|---|---|
| What is the prevalence of G6PD deficiency in my country? | 1 |
| What is the effectiveness of different options (PQ14, PQ7, TQ) and adherence between different regimens? | 2 |
| What is the cost effectiveness of different regimen options? | 3 |
| What are the overall vivax dynamics in my country? | 4 |
| How can we ensure safe delivery of the different options? | 5 |
F.4. Cambodia
| Research question | Priority ranking |
|---|---|
| What is the effectiveness of different options (PQ14, PQ7, TQ) and adherence between different regimens? | 1 |
| What is the cost effectiveness of different regimen options? | 2 |
| How can we ensure safe delivery of the different options? | 3 |
| How well do new diagnostics work in the field (usability, robustness, etc.)? | 4 |
| Feasibility of new interventions at different levels of health system (including supply chain capacity and quality assurance)? | 5 |
F.5. Ethiopia
| Research question | Priority ranking |
|---|---|
| What is the best way to improve patients' adherence? | 1 |
| How can we ensure safe delivery of the different options? | 2 |
| What is the prevalence of G6PD deficiency in my country? | 3 |
| What is the cost effectiveness of different regimen options? | 4 |
| How well do new diagnostics work in the field (usability, robustness, etc.)? | 5 |
F.6. India
| Research question | Priority ranking |
|---|---|
| What is the effectiveness of different options (PQ14, PQ7, TQ) and adherence between different regimens? | 1 |
| How well do new diagnostics work in the field (usability, robustness, etc.)? | 2 |
| How can we ensure safe delivery of the different options? | 3 |
| What is the best way to improve patients' adherence? | 4 |
| What is the cost effectiveness of different regimen options? | 5 |
F.7. Indonesia
| Research question | Priority ranking |
|---|---|
| Feasibility of new interventions at different levels of health system (including supply chain capacity and quality assurance)? | 1 |
| How well do new diagnostics work in the field (usability, robustness, etc.)? | 2 |
| How can we ensure safe delivery of the different options? | 3 |
| What is the cost effectiveness of different regimen options? | 4 |
| What is the effectiveness of different options (PQ14, PQ7, TQ) and adherence between different regimens? | 5 |
F.8. Lao (PDR)
| Research question | Priority ranking |
|---|---|
| What is the effectiveness of different options (PQ14, PQ7, TQ) and adherence between different regimens? | 1 |
| How well do new diagnostics work in the field (usability, robustness, etc.)? | 2 |
| How can we ensure safe delivery of the different options? | 3 |
| What is the best way to improve patients' adherence? | 4 |
| Feasibility of new interventions at different levels of health system (including supply chain capacity and quality assurance)? | 5 |
F.9. Malaysia
| Research question | Priority ranking |
|---|---|
| How effective is the current practice? | 1 |
| What is the cost effectiveness of different regimen options? | 2 |
| How well do new diagnostics work in the field (usability, robustness, etc.)? | 3 |
| Feasibility of new interventions at different levels of health system (including supply chain capacity and quality assurance)? | 4 |
| Not answered | 5 |
F.10. Nepal
| Research question | Priority ranking |
|---|---|
| What is the best way to improve patients' adherence? | 1 |
| How well do new diagnostics work in the field (usability, robustness, etc.)? | 2 |
| How effective is the current practice? | 3 |
| What is the effectiveness of different options (PQ14, PQ7, TQ) and adherence between different regimens? | 4 |
| Feasibility of new interventions at different levels of health system (including supply chain capacity and quality assurance)? | 5 |
F.11. Pakistan
| Research question | Priority ranking |
|---|---|
| What are the overall vivax dynamics in my country? | 1 |
| What is the prevalence of G6PD deficiency in my country? | 2 |
| What is the effectiveness of different options (PQ14, PQ7, TQ) and adherence between different regimens? | 3 |
| How well do new diagnostics work in the field (usability, robustness, etc.)? | 4 |
| What is the best way to improve patients' adherence? | 5 |
F.12. Papua New Guinea (PNG)
| Research question | Priority ranking |
|---|---|
| What is the prevalence of G6PD deficiency in my country? | 1 |
| What is the best way to improve patients' adherence? | 2 |
| Feasibility of new interventions at different levels of health system (including supply chain capacity and quality assurance)? | 3 |
| What is the cost effectiveness of different regimen options? | 4 |
| What are the overall vivax dynamics in my country? | 5 |
F.13. Solomon Islands
| Research question | Priority ranking |
|---|---|
| What is the effectiveness of different options (PQ14, PQ7, TQ) and adherence between different regimens? | 1 |
| Feasibility of new interventions at different levels of health system (including supply chain capacity and quality assurance)? | 2 |
| How well do new diagnostics work in the field (usability, robustness, etc.)? | 3 |
| What is the best way to improve patients' adherence? | 4 |
| How effective is the current practice? | 5 |
F.14. Republic of Korea
| Research question | Priority ranking |
|---|---|
| What is the effectiveness of different options (PQ14, PQ7, TQ) and adherence between different regimens? | 1 |
| Not answered | 2 |
| Not answered | 3 |
| Not answered | 4 |
| Not answered | 5 |
F.15. Sri Lanka
| Research question | Priority ranking |
|---|---|
| What is the effectiveness of different options (PQ14, PQ7, TQ) and adherence between different regimens? | 1 |
| Feasibility of new interventions at different levels of health system (including supply chain capacity and quality assurance)? | 2 |
| How well do new diagnostics work in the field (usability, robustness, etc.)? | 3 |
| How can we ensure safe delivery of the different options? | 4 |
| What is the cost effectiveness of different regimen options? | 5 |
F.16. Thailand
| Research question | Priority ranking |
|---|---|
| What is the effectiveness of different options (PQ14, PQ7, TQ) and adherence between different regimens? | 1 |
| What is the best way to improve patients' adherence? | 2 |
| Feasibility of new interventions at different levels of health system (including supply chain capacity and quality assurance)? | 3 |
| How can we ensure safe delivery of the different options? | 4 |
| How well do new diagnostics work in the field (usability, robustness, etc.)? | 5 |
F.17. Vanuatu
| Research question | Priority ranking |
|---|---|
| What is the effectiveness of different options (PQ14, PQ7, TQ) and adherence between different regimens? | 1 |
| How well do new diagnostics work in the field (usability, robustness, etc.)? | 2 |
| What is the best way to improve patients' adherence? | 3 |
| How can we ensure safe delivery of the different options? | 4 |
| What is the cost effectiveness of different regimen options? | 5 |
F.18. Vietnam
| Research question | Priority ranking |
|---|---|
| What is the effectiveness of different options (PQ14, PQ7, TQ) and adherence between different regimens? | 1 |
| What is the cost effectiveness of different regimen options? | 2 |
| Feasibility of new interventions at different levels of health system (including supply chain capacity and quality assurance)? | 3 |
| How well do new diagnostics work in the field (usability, robustness, etc.)? | 4 |
| What is the prevalence of G6PD deficiency in my country? | 5 |
Ruwanpura VSH, Nowak S, Gerth‐Guyette E, et al. Further evidence needed to change policy for the safe and effective radical cure of vivax malaria: Insights from the 2019 annual APMEN Vivax Working Group meeting. Asia & the Pacific Policy Studies. 2021;8:208–242. 10.1002/app5.314
Caroline Anita Lynch and Kamala Thriemer shared last authorship.
Funding information CSL Centenary Fellowship; Bill and Melinda Gates Foundation, Grant/Award Number: OPP1164105; Australian Centre of Research Excellence in Malaria, Grant/Award Number: APP 1134989; Australian Government Research Training Program
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abreha, T. , Hwang, J. , Thriemer, K. , Tadesse, Y. , Girma, S. , Melaku, Z. , … Price, R. N. (2017). Comparison of artemether‐lumefantrine and chloroquine with and without primaquine for the treatment of Plasmodium vivax infection in Ethiopia: A randomized controlled trial. PLoS Medicine, 14(5), e1002299. 10.1371/journal.pmed.1002299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajayi, I. O. , Browne, E. N. , Garshong, B. , Bateganya, F. , Yusuf, B. , Agyei‐Baffour, P. , … Pagnoni, F. (2008). Feasibility and acceptability of artemisinin‐based combination therapy for the home management of malaria in four African sites. Malaria Journal, 7(1), 6–6. 10.1186/1475-2875-7-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam, M. S. , Kibria, M. G. , Jahan, N. , Thriemer, K. , Hossain, M. S. , Douglas, N. M. , … Ley, B. (2018). Field evaluation of quantitative point of care diagnostics to measure glucose‐6‐phosphate dehydrogenase activity. PloS One, 13(11), e0206331. 10.1371/journal.pone.0206331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin, A. A. , Zurovac, D. , Kangwana, B. B. , Greenfield, J. , Otieno, D. N. , Akhwale, W. S. , & Snow, R. W. (2007). The challenges of changing national malaria drug policy to artemisinin‐based combinations in Kenya. Malaria Journal, 6(1), 72–72. 10.1186/1475-2875-6-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- APMEN . (2019). Asia Pacific Malaria Elimination Network. Retrieved from https://www.apmen.org/about/
- Aunger, R. , & Curtis, V. (2016). Behaviour centred design: Towards an applied science of behaviour change. Health Psychology Review, 10(4), 425–446. 10.1080/17437199.2016.1219673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balas, E. , & Boren, S. (2000). Managing clinical knowledge for health care improvement. Yearbook of Medical Informatics, 9(1), 65–70. 10.1055/s-0038-1637943 [DOI] [PubMed] [Google Scholar]
- Battle, K. E. , Lucas, T. C. D. , Nguyen, M. , Howes, R. E. , Nandi, A. K. , Twohig, K. A. , … Gething, P. W. (2019). Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000–17: A spatial and temporal modelling study. The Lancet, 394(10195), 332–343. 10.1016/S0140-6736(19)31096-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, A. , & Walker, D. (2019). On the path to UHC: Global evidence must go local to be useful. International Journal of Health Policy and Management, 8(3), 181–183. 10.15171/ijhpm.2018.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Foreign Affairs and Trade . (2014). Chairman's Statement of the 9th East Asia Summit, Nay Pyi Taw, Myanmar, November 13. Retrieved from https://dfat.gov.au/international-relations/regional-architecture/eas/Pages/chairmans-statement-of-the-9th-east-asia-summit.aspx
- Douglas, N. M. , Poespoprodjo, J. R. , Patriani, D. , Malloy, M. J. , Kenangalem, E. , Sugiarto, P. , … Price, R. N. (2017). Unsupervised primaquine for the treatment of Plasmodium vivax malaria relapses in southern Papua: A hospital‐based cohort study. PLoS Medicine, 14(8), e1002379. 10.1371/journal.pmed.1002379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrheim, D. , & William, H. (2005). Assuring effective malaria treatment in Africa: Drug efficacy is necessary but not sufficient. Journal of Epidemiology and Community Health, 59(3), 178–179. 10.1136/jech.2004.020826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano Ferrer, B. , Hansen, K. S. , Gyapong, M. , Bruce, J. , Narh Bana, S. A. , Narh, C. T. , … Webster, J. (2017). Cost‐effectiveness analysis of the national implementation of integrated community case management and community‐based health planning and services in Ghana for the treatment of malaria, diarrhoea and pneumonia. Malaria Journal, 16(1), 277–277. 10.1186/s12936-017-1906-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feachem, R. G. A. , Chen, I. , Akbari, O. , Bertozzi‐Villa, A. , Bhatt, S. , Binka, F. , … Mpanju‐Shumbusho, W. (2019). Malaria eradication within a generation: Ambitious, achievable, and necessary. The Lancet, 394(10203), 1056–1112. 10.1016/S0140-6736(19)31139-0 [DOI] [PubMed] [Google Scholar]
- Green, L. W. , Ottoson, J. M. , García, C. , & Hiatt, R. A. (2009). Diffusion theory and knowledge dissemination, utilization, and integration in public health. Annual Review of Public Health, 30(1), 151–174. 10.1146/annurev.publhealth.031308.100049 [DOI] [PubMed] [Google Scholar]
- Hansen, K. S. , Ndyomugyenyi, R. , Magnussen, P. , Lal, S. , & Clarke, S. E. (2017). Cost‐effectiveness analysis of malaria rapid diagnostic tests for appropriate treatment of malaria at the community level in Uganda. Health Policy and Planning, 32(5), 676–689. 10.1093/heapol/czw171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, G. K. , Douglas, N. M. , von Seidlein, L. , Nosten, F. , Baird, J. , White, N. J. , & Price, R. N. (2012). Primaquine radical cure of Plasmodium vivax: A critical review of the literature. Malaria Journal, 11(1), 280–280. 10.1186/1475-2875-11-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamya, M. R. , Bakyaita, N. N. , Talisuna, A. O. , Were, W. M. , & Staedke, S. G. (2002). Increasing antimalarial drug resistance in Uganda and revision of the national drug policy. Tropical Medicine & International Health, 7(12), 1031–1041. 10.1046/j.1365-3156.2002.00974.x [DOI] [PubMed] [Google Scholar]
- Kshirsagar, N. (2006). Malaria: Antimalarial resistance and policy ramifications and challenges. Journal of Postgraduate Medicine, 52(4), 291–293. Retrieved from https://www.jpgmonline.com/text.asp?2006/52/4/291/28156 [PubMed] [Google Scholar]
- Lacerda, M. V. G. , Llanos‐Cuentas, A. , Krudsood, S. , Lon, C. , Saunders, D. L. , Mohammed, R. , … Koh, G. C. K. W. (2019). Single‐dose tafenoquine to prevent relapse of Plasmodium vivax malaria. The New England Journal of Medicine, 380(3), 215–228. 10.1056/nejmoa1710775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin, S. , Bosch‐Capblanch, X. , Oliver, S. , Akl, E. A. , Vist, G. E. , Lavis, J. N. , … Haines, A. (2012). Guidance for evidence‐informed policies about health systems: Assessing how much confidence to place in the research evidence. PLoS Medicine, 9(3), e1001187. 10.1371/journal.pmed.1001187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley, B. , Luter, N. , Espino, F. E. , Devine, A. , Kalnoky, M. , Lubell, Y. , … Domingo, G. J. (2015). The challenges of introducing routine G6PD testing into radical cure: A workshop report. Malaria Journal, 14(1), 377–377. 10.1186/s12936-015-0896-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley, B. , Thriemer, K. , Jaswal, J. , Poirot, E. , Alam, M. S. , Phru, C. S. , … Price, R. N. (2017). Barriers to routine G6PD testing prior to treatment with primaquine. Malaria Journal, 16(1), 329–329. 10.1186/s12936-017-1981-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos‐Cuentas, A. , Lacerda, M. V. G. , Hien, T. T. , Vélez, I. D. , Namaik‐larp, C. , Chu, C. S. , … Green, J. A. (2019). Tafenoquine versus primaquine to prevent relapse of Plasmodium vivax malaria. The New England Journal of Medicine, 380(3), 229–241. 10.1056/nejmoa1802537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneeboonyang, W. , Lawpoolsri, S. , Puangsa‐Art, S. , Yimsamran, S. , Thanyavanich, N. , Wuthisen, P. , … Singhasivanon, P. (2011). Directly observed therapy with primaquine to reduce the recurrence rate of plasmodium vivax infection along the Thai‐Myanmar border. Southeast Asian Journal of Tropical Medicine and Public Health, 42(1), 9–18. Retrieved from https://pubmed.ncbi.nlm.nih.gov/21323159/ [PubMed] [Google Scholar]
- Morris, Z. S. , Wooding, S. , & Grant, J. (2011). The answer is 17 years, what is the question: Understanding time lags in translational research. Journal of the Royal Society of Medicine, 104(12), 510–520. 10.1258/jrsm.2011.110180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan, J. , Mandike, R. , Palmer, N. , Williams, H. , Abdulla, S. , Bloland, P. , & Mills, A. (2006). The costs of changing national policy: Lessons from malaria treatment policy guidelines in Tanzania. Tropical Medicine & International Health, 11(4), 452–461. 10.1111/j.1365-3156.2006.01590.x [DOI] [PubMed] [Google Scholar]
- NICE . (2019). National Institute for Health and Care Excellence. Retrieved from https://www.nice.org.uk/
- Nutley, S. , Walter, I. , & Davies, H. (2007). Using evidence: How research can inform public services. Policy Press. [Google Scholar]
- Pal, S. , Bansil, P. , Bancone, G. , Hrutkay, S. , Kahn, M. , Gornsawun, G. , … Domingo, G. J. (2019). Evaluation of a novel quantitative test for glucose‐6‐phosphate dehydrogenase deficiency: Bringing quantitative testing for glucose‐6‐phosphate dehydrogenase deficiency closer to the patient. The American Journal of Tropical Medicine and Hygiene, 100(1), 213–221. 10.4269/ajtmh.18-0612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, D. H. (2018). Health policy and systems research: The future of the field. Health Research Policy and Systems, 16(1), 84–84. 10.1186/s12961-018-0359-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, D. H. , & Bennett, S. (2012). Better guidance is welcome, but without blinders. PLoS Medicine, 9(3), e1001188. 10.1371/journal.pmed.1001188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott, D. M. , Atun, R. , Moyes, C. L. , Hay, S. I. , & Gething, P. W. (2012). Funding for malaria control 2006–2010: A comprehensive global assessment. Malaria Journal, 11(1), 246–246. 10.1186/1475-2875-11-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recht, J. , Ashley, E. A. , & White, N. J. (2018). Use of primaquine and glucose‐6‐phosphate dehydrogenase deficiency testing: Divergent policies and practices in malaria endemic countries. PLoS Neglected Tropical Diseases, 12(4), e0006230. 10.1371/journal.pntd.0006230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shretta, R. , Omumbo, J. , Rapuoda, B. , & Snow, R. W. (2000). Using evidence to change antimalarial drug policy in Kenya. Tropical Medicine & International Health, 5(11), 755–764. 10.1046/j.1365-3156.2000.00643.x [DOI] [PubMed] [Google Scholar]
- Sivalal, S. (2009). Health technology assessment in the Asia Pacific region. International Journal of Technology Assessment in Health Care, 25(S1), 196–201. 10.1017/S0266462309090631 [DOI] [PubMed] [Google Scholar]
- Smith, K. (2013). Beyond evidence‐based policy in public health: The interplay of ideas. Palgrave Macmillan. [Google Scholar]
- Swana, E. K. , Makan, G. Y. , Mukeng, C. K. , Mupumba, H. I. , Kalaba, G. M. , Luboya, O. N. , & Bangs, M. J. (2016). Feasibility and implementation of community‐based malaria case management with integrated vector control in the Democratic Republic of Congo. Malaria Journal, 15(1), 413–413. 10.1186/s12936-016-1475-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, R. , Lawpoolsri, S. , Imwong, M. , Kobayashi, J. , Kaewkungwal, J. , Pukrittayakamee, S. , … Singhasivanon, P. (2010). Directly‐observed therapy (DOT) for the radical 14‐day primaquine treatment of Plasmodium vivax malaria on the Thai‐Myanmar border. Malaria Journal, 9(1), 308–308. 10.1186/1475-2875-9-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, W. R. J. , Thriemer, K. , von Seidlein, L. , Yuentrakul, P. , Assawariyathipat, T. , Assefa, A. , … Price, R. N. (2019). Short‐course primaquine for the radical cure of Plasmodium vivax malaria: A multicentre, randomised, placebo‐controlled non‐inferiority trial. The Lancet, 394(10202), 929–938. 10.1016/S0140-6736(19)31285-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thriemer, K. , Bobogare, A. , Ley, B. , Gudo, C. S. , Alam, M. S. , Anstey, N. M. , … Price, R. N. (2018). Quantifying primaquine effectiveness and improving adherence: A round table discussion of the APMEN Vivax Working Group. Malaria Journal, 17(1), 241–241. 10.1186/s12936-018-2380-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thriemer, K. , Ley, B. , Bobogare, A. , Dysoley, L. , Alam, M. S. , Pasaribu, A. P. , … Price, R. N. (2017). Challenges for achieving safe and effective radical cure of Plasmodium vivax: A round table discussion of the APMEN Vivax Working Group. Malaria Journal, 16(1), 141–141. 10.1186/s12936-017-1784-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trochim, W. (2010). Translation won't happen without dissemination and implementation: Some measurement and evaluation issues. In 3rd Annual Conference on the Science of Dissemination and Implementation (pp. 581–629). Bethesda, MD.
- Vivax Working Group . (2015). Targeting vivax malaria in the Asia Pacific: The Asia Pacific Malaria Elimination Network Vivax Working Group. Malaria Journal, 14(1), 484–484. 10.1186/s12936-015-0958-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall, J. M. , Mold, J. , & Fagnan, L. (2007). Practice‐based research—“Blue Highways” on the NIH roadmap. JAMA: The Journal of the American Medical Association, 297(4), 403–406. 10.1001/jama.297.4.403 [DOI] [PubMed] [Google Scholar]
- White, N. (2019). Tafenoquine—A radical improvement? The New England Journal of Medicine, 380(3), 285–286. 10.1056/NEJMe1816383 [DOI] [PubMed] [Google Scholar]
- WHO . (2005). Bridging the “know–do” gap meeting on knowledge translation in global health. Retrieved from https://www.measureevaluation.org/resources/training/capacity-building-resources/high-impact-research-training-curricula/bridging-the-know-do-gap.pdf
- WHO . (2015a). Global technical strategy for malaria 2016–2030. Retrieved from https://www.who.int/malaria/publications/atoz/9789241564991/en/
- WHO . (2015b). Guidelines for the treatment of malaria (Third ed.). World Health Organization. Retrieved from https://www.who.int/publications/i/item/9789241549127 [Google Scholar]
- WHO . (2018). World Malaria Report 2018. World Health Organization. Retrieved from https://www.who.int/malaria/publications/world-malaria-report-2018/en/ [Google Scholar]
- Williams, H. A. , Durrheim, D. , & Shretta, R. (2004). The process of changing national malaria treatment policy: Lessons from country‐level studies. Health Policy and Planning, 19(6), 356–370. 10.1093/heapol/czh051 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.


