Abstract
Aims
Iron deficiency is common in patients with heart failure (HF). In AFFIRM‐AHF, ferric carboxymaltose (FCM) reduced the risk of hospitalisations for HF (HHF) and improved quality of life vs. placebo in iron‐deficient patients with a recent episode of acute HF. The objective of this study was to estimate the cost‐effectiveness of FCM compared with placebo in iron‐deficient patients with left ventricular ejection fraction <50%, stabilised after an episode of acute HF, using data from the AFFIRM‐AHF trial from Italian, UK, US and Swiss payer perspectives.
Methods and results
A lifetime Markov model was built to characterise outcomes in patients according to the AFFIRM‐AHF trial. Health states were defined using the 12‐item Kansas City Cardiomyopathy Questionnaire (KCCQ‐12). Subsequent HHF were incorporated using a negative binomial regression model with cardiovascular and all‐cause mortality incorporated via parametric survival analysis. Direct healthcare costs (2020 GBP/USD/EUR/CHF) and utility values were sourced from published literature and AFFIRM‐AHF. Modelled outcomes indicated that treatment with FCM was dominant (cost saving with additional health gains) in the UK, USA and Switzerland, and highly cost‐effective in Italy [incremental cost‐effectiveness ratio (ICER) EUR 1269 per quality‐adjusted life‐year (QALY)]. Results were driven by reduced costs for HHF events combined with QALY gains of 0.43–0.44, attributable to increased time in higher KCCQ states (representing better functional outcomes). Sensitivity and subgroup analyses demonstrated data robustness, with the ICER remaining dominant or highly cost‐effective under a wide range of scenarios, including increasing treatment costs and various patient subgroups, despite a moderate increase in costs for de novo HF and smaller QALY gains for ischaemic aetiology.
Conclusion
Ferric carboxymaltose is estimated to be a highly cost‐effective treatment across countries (Italy, UK, USA and Switzerland) representing different healthcare systems.
Keywords: Heart failure, Iron deficiency, Ferric carboxymaltose, Cost‐effectiveness
Introduction
Heart failure (HF) is a major global public health burden, affecting an estimated 64 million people worldwide. 1 Patients with HF experience significantly impaired health‐related quality of life (HRQoL), 2 which correlates with increased hospitalisation times and mortality rates. 3 Indeed, HF is one of the biggest causes of hospitalisations in the USA and Europe, 4 and hospitalisations for HF (HHF) are associated with long lengths of stay, a high readmission rate and a large economic burden. 1 , 5 , 6 Despite recent advances in HF treatment, prognosis remains poor 1 and the significant morbidity associated with HF places considerable strain on the healthcare system.
Iron deficiency (ID) is prevalent in up to 50% of patients with chronic HF 7 , 8 and has been suggested to be present in up to 80% of patients hospitalised for an episode of acute HF (AHF). 9 , 10 In patients with HF, ID is associated with reduced exercise capacity, 11 impaired HRQoL, 5 increased risk of hospitalisation and mortality 11 regardless of the presence or absence of anaemia. This high prevalence and the association with adverse outcomes mean that ID represents a substantial unmet medical need in patients with HF and presents a novel therapeutic target. Several recent studies have demonstrated the efficacy and safety of intravenous (IV) ferric carboxymaltose (FCM) for the improvement of the symptomatic and functional health status among adults with HF with reduced ejection fraction and ID. 12 , 13
The results of AFFIRM‐AHF, a randomised, double‐bind, placebo‐controlled trial, demonstrated that treatment with FCM reduced the risk of HHF and improved HRQoL, with no apparent effect on the risk of cardiovascular (CV) death in patients who were iron‐deficient and had a left ventricular ejection fraction (LVEF) <50% and were stabilised after a recent episode of AHF. 12
Given the potential benefits of IV iron in this patient population, the objective of this study was to estimate the cost‐effectiveness of FCM compared with placebo for the treatment of ID in patients with HF from the payer perspective in four archetypes of health systems: UK, USA, Italy and Switzerland.
Methods
Trial design and patient population
The rationale and design of the AFFIRM‐AHF trial have been previously published. 14
Briefly, the AFFIRM‐AHF was a randomised, double‐blind, placebo‐controlled trial. Eligible patients were aged ≥18 years, were hospitalised for AHF, and had a LVEF <50% and concomitant ID, defined as serum ferritin <100 ng/mL, or 100–299 ng/mL with transferrin saturation <20%. During the index hospitalisation, patients received at least 40 mg of IV furosemide (or equivalent). Prior to discharge, patients were randomised to receive either IV FCM or placebo. The total FCM dose required was calculated using baseline haemoglobin and body weight, and the repletion dose was administered at discharge and at week 6 (administered doses were up to 1000 mg FCM or placebo). At weeks 12 and 24, if ID persisted, additional FCM doses of 500 mg (or placebo) were administered.
The protocol was approved by the institutional review board at each participating centre. Written informed consent was obtained from all participants before any study‐related procedures were performed.
Trial data included HHF data reporting first and subsequent events. Hospitalisation for any reason was also recorded, from which HHF events were removed to determine hospitalisation for non‐HF (HnHF) events. Twelve‐item Kansas City Cardiomyopathy Questionnaire (KCCQ‐12) was used to evaluate the HF‐specific health status at baseline and weeks 2, 4, 6, 12, 24, 36 and 52. The KCCQ‐12 is a self‐administered, disease‐specific instrument that quantifies physical function, symptoms (frequency, severity and recent change), social function, self‐efficacy and knowledge, and quality of life. 15
Economic model
Target population and subgroups
The base case population was aligned with the AFFIRM‐AHF trial population. 12 The following pre‐specified subgroups were also analysed: LVEF <25%; LVEF 25–40%; LVEF 40–50%; ischaemic HF aetiology; de novo HF or previous HF at index hospitalisation; anaemia status (yes/no); and patients who were on triple therapy for HF at baseline (one of angiotensin‐converting enzyme inhibitor, angiotensin receptor blocker, or angiotensin receptor–neprilysin inhibitor plus beta‐blocker plus mineralocorticoid receptor antagonist).
Model framework
A cohort state‐transition Markov model was developed to estimate outcomes in patients hospitalised for AHF with concomitant ID and treated with either FCM or placebo in the UK, USA, Italy and Switzerland, based on a previously published economic model for patients with HF. 16 In brief, a decision node assigned patients to receive either FCM or placebo per trial specifications. Each treatment arm consisted of a cohort state‐transition Markov model, which reflects disease progression between health states defined by KCCQ clinical summary score (KCCQ‐CSS) quartiles. Consistent with previous approaches, 16 KCCQ quartiles were used to ensure an appropriate balance between granularity and sufficient patient numbers within each subgroup to allow for statistically robust analysis.
Events included HHF, HnHF, and select adverse events (AEs) modelled as transient events captured as event‐specific costs and utility decrements applied to the utility in each health state. Mortality (all‐cause mortality and CV‐specific) represented an absorbing state that could be reached from any of the other health states. Figure 1 shows the model structure.
Figure 1.
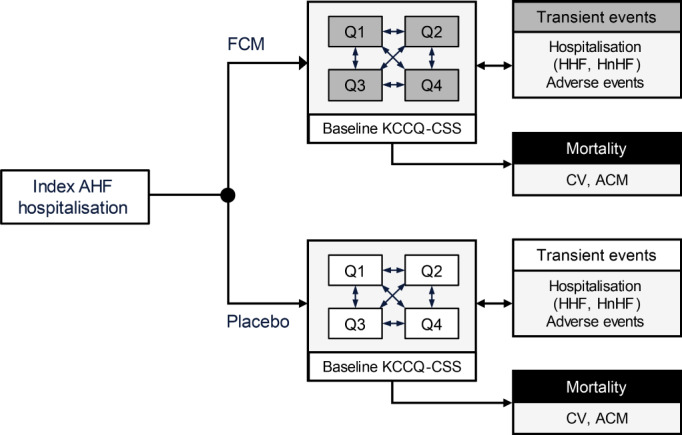
Model structure. After the index hospitalisation for acute heart failure (AHF) event, disease progression was modelled through transitions between discrete health states characterised by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ‐CSS) quartiles (Q1 through Q4). Events such as hospitalisation for heart failure (HHF), hospitalisation for non‐heart failure reasons (HnHF), and adverse events were modelled as transient events that occurred during a KCCQ‐CSS‐defined state, incurring the associated event‐specific costs and utility decrements. All‐cause mortality (ACM) and cardiovascular (CV) mortality represent an absorbing state that could be reached from any of the KCCQ‐CSS health states. FCM, ferric carboxymaltose.
The model base case considers results on a per‐patient basis; events were scaled for presentation as rates per 1000 patients. The model used a lifetime horizon with a cycle length of 4 weeks, reflecting the chronic, progressive course of the disease. Model outcomes were costs, life‐years, quality‐adjusted life‐years (QALYs) and the incremental cost‐effectiveness ratio (ICER; cost per QALY gained). The analysis was performed from the payer perspective. Country‐specific annual discount rates were applied: 3.5% for the UK and 3.0% for the USA, Italy and Switzerland.
Disease progression
The clinical inputs used were derived from the AFFIRM‐AHF trial, using the intention‐to‐treat population dataset. 12 Disease status was captured using transitions between health states represented by KCCQ‐CSS quartiles of baseline scores. Transition probabilities between KCCQ‐CSS health states were derived using multinomial model fits to individual patient‐level data from the AFFIRM‐AHF trial. See online supplementary materials for additional details regarding calculations.
The model captures mortality (CV and all‐cause), hospitalisation (HHF and HnHF) and AEs as events. Probabilities of CV mortality and all‐cause mortality over time were incorporated via parametric multivariable survival analysis, adjusted for time‐updated KCCQ‐CSS (to capture increased risk of mortality with worsening KCCQ‐defined state). Likewise, additional covariates were determined according to clinically pre‐specified subgroup definitions, comprising age, sex, anaemia status, de novo and chronic HF diagnosis, estimated glomerular filtration rate, LVEF, and ischaemic origin of HF. Mortality in the base case analysis was modelled using a Weibull distribution, which was selected following assessment of model fits in accordance with the recommendations for clinical trials published by the National Institute of Health and Care Excellence Decision Support Unit. 17 General mortality life tables (according to age and sex) were obtained from the national statistics offices of the respective countries analysed (UK, USA, Italy, Switzerland) with 2017 data as the most recent year consistently available for all nations. The baseline mortality was adjusted using country‐specific World Health Organization estimates of CV mortality by age bracket to generate non‐CV mortality life tables for use in modelling.
Repeat events (HHF, HnHF) were modelled using generalised estimating equations with a negative binomial distribution. AEs (classified as serious) were selected on the basis of occurrence in at least 1% of the trial population, independently of treatment arm. These were atrial fibrillation, acute kidney injury, pneumonia, and sepsis; AEs of cardiac origin were expected to be captured elsewhere (HHF events and mortality). See online supplementary materials for further details.
Health‐related quality of life
Quality‐of‐life analysis was performed using AFFIRM‐AHF EuroQoL 5 Dimension 5 Level (EQ‐5D‐5L) data to which UK utility values had been applied. 12 Utility estimates for each health state were calculated as the average utility index score across all patients in the corresponding KCCQ‐CSS quartile at baseline. Event‐associated utility decrements (HHF, HnHF, AEs) were estimated using a mixed effects linear regression model to account for repeat measures. The equation was adjusted for the indicated events as well as factors expected to influence EQ‐5D‐5L index score: age, sex, geographical region (Western Europe, Eastern Europe, Latin America, and rest of world) and KCCQ‐CSS health state. Only events occurring in the 4 weeks prior to an EQ‐5D‐5L assessment (constituting one model cycle) were included for consideration. Utility decrements were calculated from trial data or from literature in which too few events were recorded in the target window (online supplementary materials).
Resource use and cost
A health state cost was assigned to each health state, including transient event‐specific costs. All patients were assigned a background resource use associated with HF. Specific costs for events (HHF and AEs) were applied as one‐off costs at the time of the event. Treatment costs associated with FCM were informed by mean reported dose per patient. Most patients received the total dose within two infusions (index hospital admission and week 6 study visit) with an average total dose of 1354 mg. Because doses were typically administered in 1000 mg infusions (adjusted by patient characteristics according to the study protocol), 12 the cost for this infusion was applied in the first cycle. The second cycle comprised the cost for the remaining mean dose (354 mg) with no additional administration or monitoring costs applied. Event costs were derived from published literature. All costs were adjusted to 2020 currency units of the respective countries (UK, USA, Switzerland, Italy; see online supplementary materials for details). The willingness‐to‐pay (WTP) thresholds used for each country were GBP20 000 for the UK, USD100 000 for the USA, CHF50 000 for Switzerland and EUR30 000 for Italy.
Sensitivity analyses
The robustness of the model to assumptions made about survival distributions, model parameters, utility values and costs was assessed using deterministic sensitivity analysis (DSA), while probabilistic sensitivity analysis (PSA) was used to assess overall uncertainty in the modelled outcomes.
Results
Baseline characteristics of the modelled patient cohort are presented in Table 1 , the utility values and country‐specific costs applied in the model are shown in Table 2 and online supplementary materials, respectively. 18
Table 1.
Baseline patient characteristics
| Ferric carboxymaltose (n = 558) | Placebo (n = 550) | |
|---|---|---|
| Age, years | 71.20 (10.80) | 70.89 (11.14) |
| Sex | ||
| Female | 244 (43.7) | 250 (45.5) |
| Male | 314 (56.3) | 300 (54.5) |
| Race | ||
| American Indian or Alaska Native | 1 (0.2) | 0 |
| Asian | 26 (4.7) | 22 (4.0) |
| Black or African American | 3 (0.5) | 4 (0.7) |
| Other | 0 | 1 (0.2) |
| White | 528 (94.6) | 523 (95.1) |
| Hypertension | 468 (83.9) | 471 (85.6) |
| Dyslipidaemia | 300 (53.8) | 292 (53.1) |
| Atrial fibrillation | 314 (56.3) | 305 (55.5) |
| Smoking | 217 (38.9) | 202 (36.7) |
| Myocardial infarction | 229 (41.0) | 213 (38.7) |
| Diabetes mellitus | 227 (40.7) | 243 (44.2) |
| Coronary revascularization | 195 (34.9) | 206 (37.5) |
| Angina pectoris | 91 (16.3) | 78 (14.2) |
| Stroke | 53 (9.5) | 66 (12.0) |
| Baseline diastolic blood pressure, mmHg | 72.59 (10.31) | 71.87 (9.91) |
| Baseline systolic blood pressure, mmHg | 119.76 (15.24) | 119.69 (15.64) |
| Baseline heart rate, bpm | 74.49 (13.18) | 74.23 (12.76) |
| Baseline BMI, kg/m2 | 28.13 (5.64) | 28.03 (5.71) |
| Baseline eGFR (CKD‐EPI), mL/min/1.73 m2 | 55.38 (21.36) | 55.75 (23.07) |
| Baseline NYHA class | 1 (0.2) | 3 (0.5) |
| I | 14 (2.5) | 8 (1.5) |
| II | 255 (45.7) | 240 (43.6) |
| III | 272 (48.7) | 277 (50.4) |
| IV | 16 (2.9) | 22 (4.0) |
| Baseline LVEF, % | 32.64 (9.59) | 32.74 (9.94) |
| <25% | 104 (18.6) | 122 (22.2) |
| ≥25% and <40% | 288 (51.6) | 243 (44.2) |
| ≥40% and <50% | 166 (29.7) | 184 (33.5) |
| Heart failure aetiology | ||
| Ischaemic | 265 (47.5) | 257 (46.7) |
| Non‐ischaemic | 282 (50.5) | 275 (50.0) |
| Unknown | 11 (2.0) | 18 (3.3) |
| Baseline haemoglobin category | ||
| Anaemic a | 292 (52.3) | 312 (56.7) |
| Non‐anaemic | 265 (47.5) | 238 (43.3) |
| Newly diagnosed heart failure | 153 (27.4) | 165 (30.0) |
| Patients with ARNI, yes/no | 35 (6.3) | 36 (6.5) |
| Patients with ACEi or ARB or ARNI, yes/no | 420 (75.3) | 414 (75.3) |
| Patients with beta‐blockers | 453 (81.2) | 461 (83.8) |
| Patients with aldosterone antagonists | 376 (67.4) | 352 (64.0) |
| Patients with triple therapy | 247 (44.3) | 238 (43.3) |
| Baseline haemoglobin, g/dL | 12.26 (1.62) | 12.14 (1.60) |
| Baseline serum phosphorus, mg/dL | 4.28 (12.58) | 3.83 (0.98) |
| Baseline serum ferritin, ng/mL | 83.85 (62.15) | 88.47 (68.64) |
| Baseline TSAT, % | 15.15 (8.31) | 14.23 (7.47) |
| NT‐proBNP pg/mL | 4743 (2781–8128) | 4684 (2785–8695) |
For continuous variables, numbers represent mean (standard deviation); for categorical variables, numbers represent n (%).
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; BMI, body mass index; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; NYHA, New York Heart Association; TSAT, transferrin saturation.
Defined as <12 g/dL in non‐pregnant females and <13 g/dL in males.
Table 2.
Utility inputs
| Health state | Mean | SE | Reference |
|---|---|---|---|
| KCCQ‐CSS Q1: 0–<25 | 0.54 | 0.02 | AFFIRM‐AHF |
| KCCQ‐CSS Q2: 25–<39 | 0.65 | 0.01 | AFFIRM‐AHF |
| KCCQ‐CSS Q3: 39–<54 | 0.70 | 0.01 | AFFIRM‐AHF |
| KCCQ‐CSS Q4: 54–<100 | 0.83 | 0.01 | AFFIRM‐AHF |
| Hospitalisation | |||
| Hospitalisation for HF | −0.07 | 0.02 | AFFIRM‐AHF |
| Hospitalisation for non‐HF | −0.02 | 0.02 | AFFIRM‐AHF |
| Adverse events | |||
| Atrial fibrillation | −0.02 | 0.11 | AFFIRM‐AHF |
| Pneumonia | −0.10 | 0.08 | AFFIRM‐AHF |
| Acute kidney injury | −0.04 | 0.09 | AFFIRM‐AHF |
| Sepsis | −0.30 | 0.10 a | Galante et al. 18 |
HF, heart failure; KCCQ‐CSS, Kansas City Cardiomyopathy Questionnaire clinical summary score; SE, standard error.
Assumed to be 10% of the mean value.
The modelled outcomes indicated that treatment with FCM was associated with discounted QALY gains of 0.43, 0.43, 0.44 and 0.44, in the UK, Italy, Switzerland and USA, respectively (Table 3 ). FCM was dominant (cost saving with additional health gain) in the UK, USA, and Switzerland, and highly cost‐effective in Italy with an ICER of EUR1269/QALY gained. In the Italian setting, the small incremental cost is attributable to the relatively higher background HF maintenance cost in comparison to that in the other countries. In any case, the ICER estimated in Italy is far below the accepted threshold of EUR30 000/QALY. The QALY gains (improved life expectancy) were attributable to a combination of increased time spent in better KCCQ states (representing better scores on symptom, physical and social functional scales) and a reduction in HHF events, for patients who received FCM. Over a lifetime horizon, treatment with FCM was associated with an estimated 199 fewer HHF events per 1000 patients. The reduction in HHF events is associated with cost savings of USD5422, GBP561, EUR1389 and CHF2709 per average patient in the USA, UK, Italy and Switzerland, respectively. The cost‐effectiveness results were largely driven by cost offsets associated with reduced HHF events and QALY improvements.
Table 3.
Base‐case results
| Country | Ferric carboxymaltose | Placebo | Incremental |
|---|---|---|---|
| United Kingdom | |||
| Total costs | GBP10 700 | GBP10 839 | –GBP139 |
| Treatment (intervention) | GBP242 | GBP0 | GBP242 |
| Background medical management | GBP3694 | GBP3268 | GBP426 |
| Hospitalisation for HF | GBP3177 | GBP3738 | –GBP561 |
| Hospitalisation for non‐HF | GBP787 | GBP774 | GBP13 |
| CV mortality | GBP1831 | GBP2006 | –GBP175 |
| Adverse events | GBP969 | GBP1053 | –GBP84 |
| Total LYs | 4.21 | 3.72 | 0.49 |
| Total QALYs | 2.98 | 2.55 | 0.43 |
| ICER | – | – | Dominant |
| United States of America | |||
| Total costs | USD67 475 | USD70 896 | –USD3421 |
| Treatment (intervention) | USD2112 | USD0 | USD2112 |
| Background medical management | USD8507 | USD7508 | USD998 |
| Hospitalisation for HF | USD31 141 | USD36 564 | –USD5422 |
| Hospitalisation for non‐HF | USD9996 | USD9809 | USD187 |
| CV mortality | USD15 007 | USD16 401 | –USD1394 |
| Adverse events | USD1036 | USD900 | USD136 |
| Total LYs | 4.27 | 3.77 | 0.50 |
| Total QALYs | 3.02 | 2.58 | 0.44 |
| ICER | – | – | Dominant |
| Italy | |||
| Total costs | EUR26 489 | EUR25 939 | EUR550 |
| Treatment (intervention) | EUR270 | EUR0 | EUR270 |
| Background medical management | EUR14 226 | EUR12 572 | EUR1654 |
| Hospitalisation for HF | EUR7915 | EUR9305 | –EUR1389 |
| Hospitalisation for non‐HF | EUR1775 | EUR1744 | EUR31 |
| CV mortality | EUR1522 | EUR1666 | –EUR144 |
| Adverse events | EUR781 | EUR653 | EUR128 |
| Total LYs | 4.25 | 3.76 | 0.49 |
| Total QALYs | 3.01 | 2.57 | 0.43 |
| ICER | – | – | EUR1269/QALY |
| Switzerland | |||
| Total costs | CHF45 028 | CHF47 733 | –CHF2705 |
| Treatment (intervention) | CHF379 | CHF0 | CHF379 |
| Background medical management | CHF5687 | CHF5020 | CHF667 |
| Hospitalisation for HF | CHF15 563 | CHF18 272 | –CHF2709 |
| Hospitalisation for non‐HF | CHF544 | CHF5734 | CHF110 |
| CV mortality | CHF15 226 | CHF16 638 | –CHF1412 |
| Adverse events | CHF2330 | CHF2070 | CHF260 |
| Total LYs | 4.28 | 3.78 | 0.50 |
| Total QALYs | 3.03 | 2.59 | 0.44 |
| ICER | – | – | Dominant |
CV, cardiovascular; HF, heart failure; ICER, incremental cost‐effectiveness ratio; LY, life‐year; QALY, quality‐adjusted life‐year.
Subgroup analysis
Ferric carboxymaltose remained either dominant or cost‐effective vs. current WTP thresholds in all subgroups analysed (Figure 2 ). In all four countries, use of FCM vs. placebo in patients with de novo HF was associated with the greatest cost increase per QALY vs. the overall trial population (range: CHF‐1, 205 in Switzerland to EUR1, 361 in Italy); nevertheless, FCM remained dominant vs. placebo in the USA and Switzerland and cost‐effective in the UK and Italy, with ICERs remaining below the WTP threshold in each country. In patients with a history of HF, the cost of FCM vs. placebo decreased compared with the overall trial population, with FCM resulting in greater cost savings in the UK, USA and Switzerland and improved cost‐effectiveness in Italy. The greatest cost savings vs. the overall population were consistently seen in the LVEF <25% subgroup, where cost differences ranged from USD –5117 in the USA to EUR –108 in Italy. Patients in the de novo HF group had modestly greater life expectancy (0.54–0.57 in this group vs. approximately 0.43 overall); the increase in costs for these patients was therefore attributable to this modest survival benefit as they accrued greater HF maintenance costs. In contrast, for patients with LVEF <25%, the cost savings were driven by fewer HHF events, while the mortality difference was minor. Given that patients who receive triple therapy for HF are receiving a very high standard of care at baseline, it is of particular relevance that, for these patients, FCM is a dominant option in the USA, UK and Switzerland, and also exhibits a very low ICER in Italy.
Figure 2.
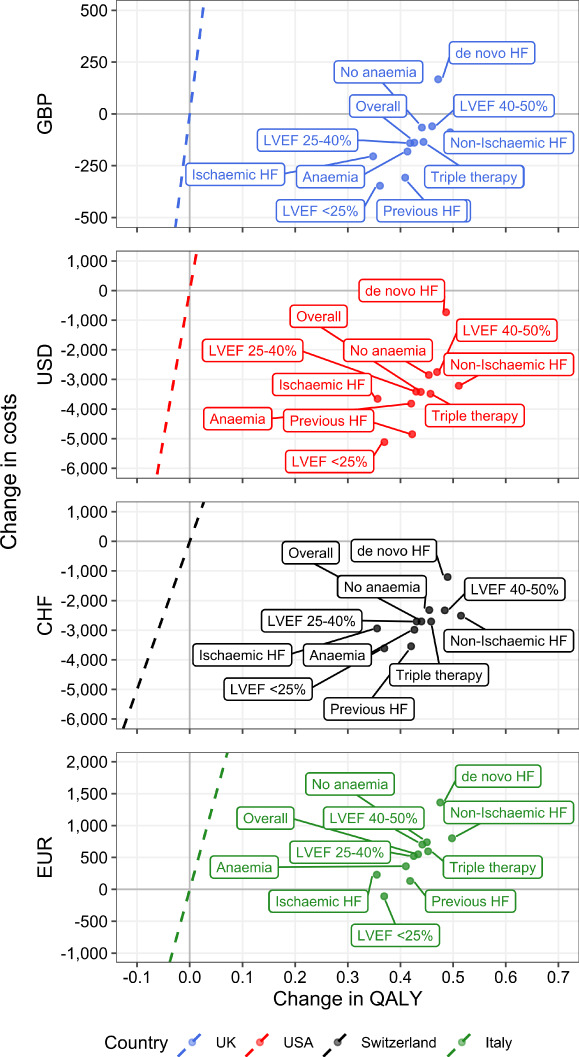
Subgroup analysis. Points are shown on the cost‐effectiveness plane for the mean value analysis of indicated patient subgroups. Dashed lines correspond to the willingness‐to‐pay threshold for each country. Points to the right of the dashed line would be considered cost‐effective at the given willingness‐to‐pay threshold, while points to the right of the y‐axis [positive change in quality‐adjusted life‐year (QALY)] and below the x‐axis (decreased costs) are considered dominant. CHF, Swiss Francs; EUR, Euros; GBP, Great Britain Pounds Sterling; HF, heart failure; LVEF, left ventricular ejection fraction; USD, United States Dollars.
In terms of utility, the subgroup with HF of non‐ischaemic origin was associated with the greatest increase in QALYs gained (0.50–0.52 QALYs) and the group of ischaemic origin the lowest (0.35–0.36 QALYs). As in the overall analysis, this result was driven by time spent in the KCCQ‐defined health states, with more time in the topmost quartiles for non‐ischaemic patients.
In all subgroups, although the magnitude of change in QALYs differed, there was a consistent net increase. The other component of the ICER, change in costs, demonstrated either increased cost‐savings (LVEF <25%) or in the most extreme case, a moderate increase in costs (de novo HF), but the result remained highly cost‐effective.
Sensitivity analysis
The robustness of model results was assessed through deterministic and probabilistic sensitivity analyses. Results of the PSA are presented in Figure 3 . For each country, the bulk of the simulations falls to the right of the WTP threshold (Figure 3A ), indicating these results would be considered cost‐effective at the indicated threshold. To assess the cost‐effectiveness across different WTP thresholds, cost‐effectiveness acceptability curves were also generated (Figure 3B ). A high proportion of simulations would be considered cost‐effective across a range of thresholds in the different countries for the parameters sampled. In the base case threshold WTP definitions, 86.9%, 82.9%, 90.1% and 89.4% of simulations would be considered cost‐effective in the UK, USA, Switzerland and Italy, respectively.
Figure 3.
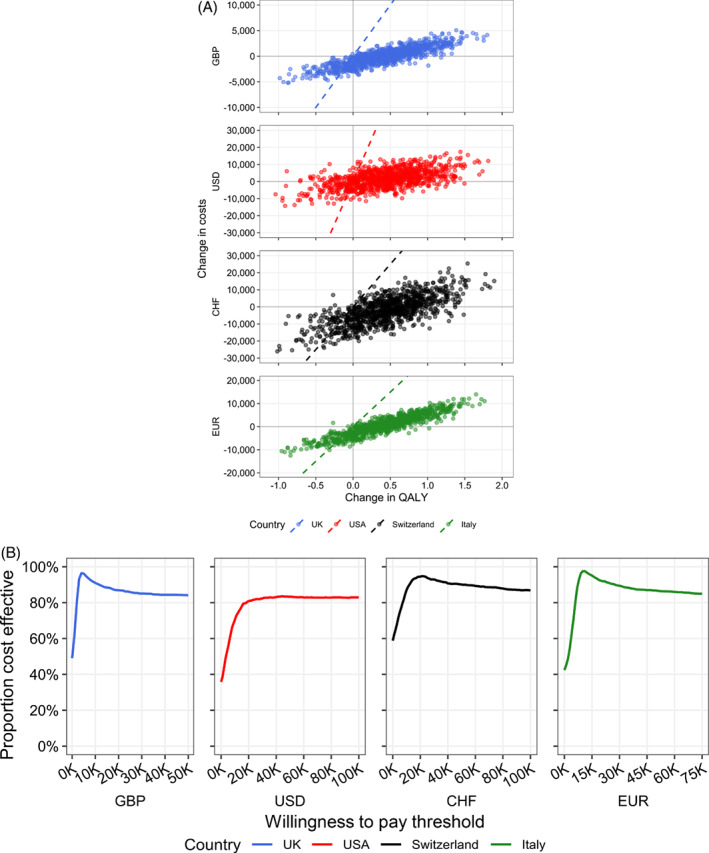
Probabilistic sensitivity results. Probabilistic sensitivity results are shown for incremental costs and benefits on the cost‐effectiveness plane (A). Individual points represent separate simulations based on random sampling of model parameters and the dashed line indicates the willingness‐to‐pay threshold for each country. Points to the right of the line are considered cost‐effective. In the cost‐effectiveness acceptability curves (B), the percentage of simulations is shown as a function of varying the willingness‐to‐pay threshold. CHF, Swiss Francs; EUR, Euros; GBP, Great Britain Pounds Sterling; QALY, quality‐adjusted life‐year; USD, United States Dollars.
The DSA results are presented in the online supplementary materials. Considering the time horizon, restricting the model to a within‐trial (1 year) horizon resulted in FCM dominance for all countries. Over this short interval, small QALY increases were observed combined with cost savings due to reduced HHF events that overcame smaller cost increases due to HnHF and treatment costs. In the present analysis, no additional monitoring or administration costs for FCM were applied, because, per trial protocol, patients in both arms would have received the same level of care, including study visits where infusions would occur. Increasing treatment costs in the DSA by 150% to partially account for a scenario where the treatment may incur additional costs, the result remained dominant for the UK, USA and Switzerland, with an ICER of EUR1499 for Italy, well below the considered WTP threshold. Similar patterns of dominance and ICERs were observed for variations in utilities and distributions for mortality modelling.
Discussion
In this base case analysis, management of ID with IV FCM vs. placebo (in addition to standard HF therapy) in patients stabilised after hospitalisation for an AHF episode was found to be cost‐saving from a UK, US and Swiss health system perspective, and cost‐effective from an Italian health system perspective based on the current WTP thresholds. The difference in a small incremental cost for gain in QALYs, as for Italy, is in part due to the higher estimated background HF maintenance cost for Italy. The cost was derived from the work of Rognoni and Gerzeli 19 who, in performing an economic analysis of FCM in the Italian setting, reported costs for a model based on New York Heart Association (NYHA) classes. If these costs were lower, then, like the other countries, the intervention tends towards dominance. We accept that there may be alternative estimates for this maintenance cost, but we note that changing this cost (per the deterministic sensitivity analysis) does not change the conclusion and that the ICER in the Italian setting only changes from dominance after year 1 (within trial analysis with a time horizon of 1 year yields a result dominant for FCM).
In general, cost‐effectiveness was mainly driven by cost offsets associated with reductions in HHF, in addition to QALY gains derived from greater time spent with a better HF‐specific health status with FCM vs. placebo. In previous published cost‐effectiveness analyses in HF, results were principally driven by reductions in CV and all‐cause mortality. 16 , 20
In our analyses from the UK, Italy, Switzerland and US health service perspectives, patients treated with FCM gained 0.43–0.44 QALYs compared with placebo. This gain in QALYs should be viewed in the context of the AFFIRM‐AHF population, who were elderly with an impaired LVEF, a high prevalence of comorbidities, and recent admission to hospital for a potentially life‐threatening event. 12 Indeed, the average lifespan of patients discharged following HHF has been previously estimated at approximately 5.5 years, with an impaired LVEF associated with an even shorter life span. 21 Thus, a gain of 0.43–0.44 QALYs represents a clinically meaningful improvement in our patients. Similar QALY gains in patients with HF and a reduced LVEF were observed with dapagliflozin vs. placebo in the DAPA‐HF trial (UK: 0.48) 16 and with sacubitril/valsartan vs. enalapril in the PARADIGM‐HF trial (UK: 0.52; Italy: ∼0.28; Switzerland: 0.42; and USA: 0.78). 20 , 22 , 23 This was despite the DAPA‐HF and PARADIGM‐HF trial populations being younger (mean age ∼66 and 64 vs. 71 years, respectively) and having less severely elevated N‐terminal pro B‐type natriuretic peptide (NT‐proBNP, median across treatment groups: 1428–1446 and 1594–1631 vs. 4684–4743 pg/mL, respectively) compared with AFFIRM‐AHF. 12 , 16 , 24 Additionally, CV mortality rates were generally lower in DAPA‐HF compared with AFFIRM‐AHF (11.5% over a median of 18 months vs. 14.2% over 12 months in the placebo group), 12 , 16 indicating a baseline population with lower CV risk. It is worth noting that reductions in CV and all‐cause mortality were major contributors to the QALY gains observed in DAPA‐HF and PARADIGM‐HF, 16 , 24 whereas in AFFIRM‐AHF, the major contributor was a reduction in HHF. Thus, the QALY gains in AFFIRM‐AHF largely reflect clinically relevant improvements in a patient's life while they are alive.
An increase in time spent with a higher KCCQ‐CSS (indicating better HF‐specific health status) with FCM vs. placebo also contributed to the QALY gains observed. Use of KCCQ‐CSS vs. NYHA class is a relatively novel approach to economic evaluation in HF. While NYHA class is a physician‐reported outcome with four discrete categories, the KCCQ‐CSS is patient‐reported and quantitatively measures symptom frequency, symptom burden and physical limitation, providing a more precise reflection of clinical severity and limitations in patients with HF. 25 The estimated health utilities associated with the different KCCQ‐CSS quartiles in AFFIRM‐AHF were similar to those derived from DAPA‐HF data, offering additional, external validity. 16
The reduction in HHF events with FCM vs. placebo in AFFIRM‐AHF translated into lower hospitalisation costs in all four countries, offsetting FCM treatment costs and providing additional cost savings in the UK, USA and Switzerland. Importantly, cost savings came not from reducing CV mortality, the effect on which was minimal with FCM vs. placebo in AFFIRM‐AHF, but from preventing HHF. Given the high prevalence of ID in HF, 7 , 8 the impact of FCM on HHF and related cost‐savings could be substantial globally, provided patients are appropriately screened and treated for ID. Reducing hospitalisation rates is also an important health‐economic and resourcing advantage in the COVID‐19 era. 26
Subgroup analyses revealed that treatment with FCM was highly cost‐effective vs. placebo in all four countries, irrespective of characteristics such as anaemia status, LVEF, HF aetiology and HF history, and in the context of a high baseline standard of care, such as for patients receiving triple therapy.
Nevertheless, characteristics reflective of a more severe disease state, such as a LVEF <25%, anaemia, and a history of HF, were associated with greater cost savings. Similar results were demonstrated in DAPA‐HF, where better cost‐effectiveness results were observed in patients with longer HF duration, lower LVEF and higher NT‐proBNP. 16 This is likely due to the higher frequency of events expected in patients with a higher baseline CV risk and thus a greater opportunity to prevent events from occurring through treatment.
This economic analysis was based on data for patients with a recent AHF episode, representing a very high‐risk subgroup of patients with HF; however, the findings are applicable to all patients with HF and align with those from prior economic analyses in patients with chronic HF and ID: in the FAIR‐HF trial, FCM was cost‐effective vs. placebo from UK, 27 Spanish 5 and Swedish 28 health service perspectives. Similarly, a pooled analysis of patient‐level data from FAIR‐HF, FER‐CARS‐01, CONFIRM‐HF and EFFICACY‐HF trials found that FCM was dominant vs. placebo in an Italian healthcare setting 19 and cost‐saving over 5 years in a French healthcare setting. 29 These data support our findings and suggest that FCM is cost‐effective in a broad spectrum of patients with HF and ID.
Limitations
The cost‐effectiveness framework employed herein was designed to predict whether the acquisition cost of FCM would be justified given its expected health gains and cost offsets. The data suggest that, from the payers' perspective, this is likely to be the case; however, our analysis quantifies value from one stakeholder's view (the payer). For example, it considers the impact of HHF reductions on costs, but does not evaluate whether there is sufficient capacity within the system to cope with demand. Furthermore, our analysis does not consider disease incidence and prevalence over time which, for a chronic, progressive condition such as HF that is associated with a high rate of hospitalisations and readmissions, 1 , 4 , 5 is an important limitation, particularly given the continually expanding and increasingly ageing nature of the HF population. 1 , 6 Consequently, our analysis likely underestimates the holistic value of FCM in terms of planning service delivery, staffing, resource requirements and policy decisions; further studies investigating FCM from these perspectives would be beneficial. The AFFIRM‐AHF study was a multinational trial in which health utility was converted into a single summary index using the UK tariff. Ideally, we would have utilised country‐specific utility values to address regional variability; however, our objective was to provide an estimate of the cost‐effectiveness of FCM from an international perspective acknowledging that the cost profile across countries is considerable. The recent cost‐effectiveness evaluation of dapagliflozin in HF that used country‐specific tariffs reported incremental utility (QALY) gains that differed by 0.02 across countries, 16 suggesting regional variation in utility is modest. Nevertheless, the results of our analysis should be interpreted with this limitation in mind.
A further limitation is the need to extrapolate 1 year of follow‐up data to a lifetime horizon, limiting the certainty of future predictions. This includes the extrapolation of numerical differences in survival observed across trial arms. However, it is noteworthy that the results remain robust even when restricting the time horizon to 1 year.
In addition, although AFFIRM‐AHF was a multinational trial with sites across Europe, South America and Singapore, variations in race were limited (95% of patients were white) and the representation from each country was modest. 12 Thus, the trial population does not necessarily represent the typical HF populations of the four countries.
Conclusion
In conclusion, our results indicate the health‐economic value of IV FCM for the treatment of ID in patients stabilised after an AHF episode, with cost‐saving observed in the UK, USA and Switzerland, and cost‐effectiveness and QALY gains in all four countries, including Italy. Therefore, FCM improves the lives of patients with ID and HF while allowing health services to reclaim treatment costs or, in certain cases, gain financially. Given the prevalence of ID in HF, the potential for global cost savings from reductions in HHF with FCM is substantial.
Funding
This work was supported by Vifor Pharma Ltd., who provided support for model development, data analysis and medical writing for this study.
Conflict of interest: ARdR, F.D. and D.O. are full‐time employees of Vifor Pharma Ltd. P.M. and J.A.D. are employees of Health Economics and Outcomes Research Ltd. Health Economics and Outcomes Research Ltd. received fees from Vifor Pharma Ltd in relation to this study. A.J.S.C. reports personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, Gore, Impulse Dynamics, and Respicardia, outside the submitted work. PP reports participation in clinical trials for and grants and personal fees from Vifor Pharma during the conduct of the study; participation in clinical trials for and personal fees from Amgen, Bayer, Novartis, AbbottVascular, Boehringer Ingelheim, Pfizer, Servier, Astra Zeneca, Cibiem, BMS, and Impulse Dynamics, outside the submitted work; participation in clinical trials for Cardiac Dimensions, outside the submitted work; and personal fees from Berlin Chemie, outside the submitted work. E.A.J. reports personal fees from Vifor Pharma, during the conduct of the study; grants and personal fees from Vifor Pharma, personal fees from Bayer, personal fees from Novartis, personal fees from Abbott, personal fees from Boehringer Ingelheim, from Pfizer, from Servier, from AstraZeneca, from Berlin Chemie, from Cardiac Dimensions, from Fresenius, from Gedeon Richter, outside the submitted work. G.R. has nothing to disclose.
Supporting information
Table S1. Deterministic sensitivity results.
Table S2. Parameterisations for the adjusted mortality equations.
Table S3. KCCQ‐CSS transition probabilities.
Table S4. Regression models developed for hospitalisation for heart failure and non‐heart failure.
Table S5. Adverse event rates.
Table S6. Estimation of utility values.
Table S7. Cost inputs for the model.
Acknowledgements
The authors thank Laurence Richards, Tinevimbo Shiri and Kerrie Ford of Health Economics and Outcomes Research Ltd. for additional analytical and medical writing support, and Helen Sims of AXON Communications for providing medical writing support and editorial support, which was funded by Vifor Pharma Ltd. in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
References
- 1. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail 2020;22:1342–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Juenger J, Schellberg D, Kraemer S, Haunstetter A, Zugck C, Herzog W, Haass M. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart 2002;87:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moradi M, Daneshi F, Behzadmehr R, Rafiemanesh H, Bouya S, Raeisi M. Quality of life of chronic heart failure patients: a systematic review and meta‐analysis. Heart Fail Rev 2020;25:993–1006. [DOI] [PubMed] [Google Scholar]
- 4. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–1133. [DOI] [PubMed] [Google Scholar]
- 5. Comín‐Colet J, Rubio‐Rodríguez D, Rubio‐Terrés C, Enjuanes‐Grau C, Gutzwiller FS, Anker SD, Ponikowski P. A cost‐effectiveness analysis of ferric carboxymaltose in patients with iron deficiency and chronic heart failure in Spain. Rev Esp Cardiol 2015;68:846–851. [DOI] [PubMed] [Google Scholar]
- 6. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW. Heart disease and stroke statistics – 2020 update: a report from the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 7. Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation 2018;138:80–98. [DOI] [PubMed] [Google Scholar]
- 8. Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013;34:816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohen‐Solal A, Damy T, Terbah M, Kerebel S, Baguet JP, Hanon O, Zannad F, Laperche T, Leclercq C, Concas V, Duvillié L, Darné B, Anker SD, Mebazaa A. High prevalence of iron deficiency in patients with acute decompensated heart failure. Eur J Heart Fail 2014;16:984–991. [DOI] [PubMed] [Google Scholar]
- 10. Wexler D, Silverberg D, Sheps D, Blum M, Keren G, Iaina A, Schwartz D. Prevalence of anemia in patients admitted to hospital with a primary diagnosis of congestive heart failure. Int J Cardiol 2004;96:79–87. [DOI] [PubMed] [Google Scholar]
- 11. Martens P, Nijst P, Verbrugge FH, Smeets K, Dupont M, Mullens W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid‐range and preserved ejection fraction. Acta Cardiol 2018;73:115–123. [DOI] [PubMed] [Google Scholar]
- 12. Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Göhring UM, Keren A, Khintibidze I, Kragten H, Martinez FA, Metra M, Milicic D, Nicolau JC, Ohlsson M, Parkhomenko A, Pascual‐Figal DA, Ruschitzka F, Sim D, Skouri H, van der Meer P, Lewis BS, Comin‐Colet J, von Haehling S, Cohen‐Solal A, Danchin N, Doehner W, Dargie HJ, Motro M, Butler J, Friede T, Jensen KH, Pocock S, Jankowska EA; AFFIRM‐AHF Investigators . Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double‐blind, randomised, controlled trial. Lancet 2020;396:1895–1904. [DOI] [PubMed] [Google Scholar]
- 13. van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Böhm M, Doletsky A, Voors AA, Macdougall IC, Anker SD, Roubert B, Zakin L, Cohen‐Solal A; EFFECT‐HF Investigators . Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation 2017;136:1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ponikowski P, Kirwan BA, Anker SD, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Haboubi T, Keren A, Khintibidze I, Kragten H, Martinez FA, McDonagh T, Metra M, Milicic D, Nicolau JC, Ohlsson M, Parhomenko A, Pascual‐Figal DA, Ruschitzka F, Sim D, Skouri H, van der Meer P, Jankowska EA. Rationale and design of the AFFIRM‐AHF trial: a randomised, double‐blind, placebo‐controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalisations and mortality in iron‐deficient patients admitted for acute heart failure. Eur J Heart Fail 2019;21:1651–1658. [DOI] [PubMed] [Google Scholar]
- 15. Spertus JA, Jones PG. Development and validation of a short version of the Kansas City cardiomyopathy questionnaire. Circ Cardiovasc Qual Outcomes 2015;8:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McEwan P, Darlington O, McMurray JJV, Jhund PS, Docherty KF, Böhm M, Petrie MC, Bergenheim K, Qin L. Cost‐effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health‐economic analysis of DAPA‐HF. Eur J Heart Fail 2020;22:2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bagust A, Beale S. Survival analysis and extrapolation modeling of time‐to‐event clinical trial data for economic evaluation: an alternative approach. Med Decis Making 2014;34:343–351. [DOI] [PubMed] [Google Scholar]
- 18. Galante J, Augustovski F, Colantonio L, Bardach A, Caporale J, Marti SG, Kind P. Estimation and comparison of EQ‐5D health states' utility weights for pneumoccocal and human papillomavirus diseases in Argentina, Chile, and the United Kingdom. Value Health 2011;14(5 Suppl 1):S60–S64. [DOI] [PubMed] [Google Scholar]
- 19. Rognoni C, Gerzeli S. Ferric carboxymaltose for patients with heart failure and iron deficiency in Italy: cost‐effectiveness and budget impact. J Comp Eff Res 2019;8:1099–1110. [DOI] [PubMed] [Google Scholar]
- 20. McMurray JJ, Trueman D, Hancock E, Cowie MR, Briggs A, Taylor M, Mumby‐Croft J, Woodcock F, Lacey M, Haroun R, Deschaseaux C. Cost‐effectiveness of sacubitril/valsartan in the treatment of heart failure with reduced ejection fraction. Heart 2018;104:1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alter DA, Ko DT, Tu JV, Stukel TA, Lee DS, Laupacis A, Chong A, Austin PC. The average lifespan of patients discharged from hospital with heart failure. J Gen Intern Med 2012;27:1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ademi Z, Pfeil AM, Hancock E, Trueman D, Haroun RH, Deschaseaux C, Schwenkglenks M. Cost‐effectiveness of sacubitril/valsartan in chronic heart‐failure patients with reduced ejection fraction. Swiss Med Wkly 2017;147:w14533. [DOI] [PubMed] [Google Scholar]
- 23. D'Angiolella LS, Cortesi PA, Pitotti C, Ritrovato D, Mantovani LG, Senni M. Sacubitril/valsartan in heart failure with reduced ejection fraction: cost and effectiveness in the Italian context. Eur J Heart Fail 2017;19:1551–1553. [DOI] [PubMed] [Google Scholar]
- 24. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 25. Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in clinical trials and clinical care: JACC state‐of‐the‐art review. J Am Coll Cardiol 2020;76:2379–2390. [DOI] [PubMed] [Google Scholar]
- 26. McCabe R, Schmit N, Christen P, D'Aeth JC, Løchen A, Rizmie D, Nayagam S, Miraldo M, Aylin P, Bottle A, Perez‐Guzman PN, Ghani AC, Ferguson NM, White PJ, Hauck K. Adapting hospital capacity to meet changing demands during the COVID‐19 pandemic. BMC Med 2020;18:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gutzwiller FS, Schwenkglenks M, Blank PR, Braunhofer PG, Mori C, Szucs TD, Ponikowski P, Anker SD. Health economic assessment of ferric carboxymaltose in patients with iron deficiency and chronic heart failure based on the FAIR‐HF trial: an analysis for the UK. Eur J Heart Fail 2012;14:782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hofmarcher T, Borg S. Cost‐effectiveness analysis of ferric carboxymaltose in iron‐deficient patients with chronic heart failure in Sweden. J Med Econ 2015;18:492–501. [DOI] [PubMed] [Google Scholar]
- 29. Bourguignon S, Faller M, Champs F‐O, Moutier H, Levesque K, Caranhac G, Cohen‐Solal A. Budget impact of intravenous ferric carboxymaltose in patients with chronic heart failure and iron deficiency in France. ESC Heart Fail 2019;6:559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Deterministic sensitivity results.
Table S2. Parameterisations for the adjusted mortality equations.
Table S3. KCCQ‐CSS transition probabilities.
Table S4. Regression models developed for hospitalisation for heart failure and non‐heart failure.
Table S5. Adverse event rates.
Table S6. Estimation of utility values.
Table S7. Cost inputs for the model.


