Abstract
Background and Aims
NAFLD is the most common liver disease worldwide. NASH, the progressive form of NAFLD, and advanced fibrosis are associated with poor outcomes. We searched for their noninvasive biomarkers.
Approach and Results
Global RNA sequencing of liver tissue from 98 patients with biopsy‐proven NAFLD was performed. Unsupervised hierarchical clustering well distinguished NASH from nonalcoholic fatty liver (NAFL), and patients with NASH exhibited molecular abnormalities reflecting their pathological features. Transcriptomic analysis identified proteins up‐regulated in NASH and/or advanced fibrosis (stage F3‐F4), including matricellular glycoprotein thrombospondin‐2 (TSP‐2), encoded by the thrombospondin 2 (THBS2) gene. The intrahepatic THBS2 expression level showed the highest areas under the receiver operating characteristic curves (AUROCs) of 0.915 and 0.957 for diagnosing NASH and advanced fibrosis, respectively. THBS2 positively correlated with inflammation and ballooning according to NAFLD activity score, serum aspartate aminotransferase and hyaluronic acid (HA) levels, and NAFLD Fibrosis Score (NFS). THBS2 was associated with extracellular matrix and collagen biosynthesis, platelet activation, caspase‐mediated cleavage of cytoskeletal proteins, and immune cell infiltration. Serum TSP‐2 expression was measured in 213 patients with biopsy‐proven NAFLD, was significantly higher in NASH than in NAFL, and increased parallel to fibrosis stage. The AUROCs for predicting NASH and advanced fibrosis were 0.776 and 0.856, respectively, which were comparable to Fibrosis‐4 index, serum HA level, and NFS in advanced fibrosis diagnosis. Serum TSP‐2 level and platelet count were independent predictors of NASH and advanced fibrosis. Serum TSP‐2 levels could stratify patients with NAFLD according to the risk of hepatic complications, including liver cancer and decompensated cirrhotic events.
Conclusions
TSP‐2 may be a useful biomarker for NASH and advanced fibrosis diagnosis in patients with NAFLD.
Abbreviations
- AST
aspartate aminotransferase
- AUROC
area under the receiver operating characteristic curve
- COL4A4
collagen type IV alpha 4 chain
- ECM
extracellular matrix
- FIB‐4
Fibrosis‐4
- HA
hyaluronic acid
- LAMA2
laminin subunit alpha 2
- LAMC3
laminin subunit gamma 3
- LUM
lumican
- NAFL
nonalcoholic fatty liver
- NAS
NAFLD activity score
- NFS
NAFLD Fibrosis Score
- qPCR
quantitative PCR
- THBS2
thrombospondin 2
- TSP‐2
thrombospondin‐2
The incidence and prevalence of NAFLD has increased over time, and it is now the most common cause of chronic liver disease worldwide, affecting approximately one‐fourth of the global population.( 1 ) NAFLD is thus becoming a major cause of end‐stage liver disease, HCC, and liver transplantation globally.( 1 , 2 ) The spectrum of NAFLD ranges from simple benign steatosis to NASH, which is characterized by hepatic necroinflammation and hepatocyte ballooning, leading to fibrosis progression. Several recent reports have shown that fibrosis severity is strongly associated with adverse hepatic outcomes.( 3 , 4 , 5 ) Therefore, it is critically important to identify patients at high risk of NASH and advanced fibrosis. The gold standard for the diagnosis of NASH and advanced liver fibrosis is liver biopsy. However, there are a number of drawbacks to liver biopsy, including sampling error due to the small size of biopsy specimens, procedure‐related complications, interobserver variability, and high medical expenses.( 1 , 6 ) Hence, there is an urgent medical need to develop alternative noninvasive biomarkers to differentiate NASH from simple steatosis and identify advanced hepatic fibrosis in patients with NAFLD.
Transcriptome analysis of liver tissues from patients with NAFLD has been performed to comprehensively understand molecular abnormalities involved in its disease progression.( 7 , 8 , 9 , 10 , 11 , 12 ) This analysis is also potentially useful to identify serum biomarkers secreted from the liver in patients with NASH and/or advanced fibrosis. However, omics‐based biomarker discovery has not been fully exploited. Therefore, in this study, we conducted whole‐transcriptome analysis of liver tissues from 98 patients with NAFLD and identified a variety of proteins predicted to be secreted from the livers of patients with NASH and/or fibrosis. We focused on the thrombospondin 2 (THBS2) gene and its protein thrombospondin‐2 (TSP‐2) as a candidate biomarker for NASH and advanced fibrosis; we subsequently evaluated the diagnostic ability of TSP‐2 using the serum of 213 patients with biopsy‐proven NAFLD.
Materials and Methods
Study Patients
This study was a retrospective multicenter cohort study; a total of 311 patients with biopsy‐proven NAFLD diagnosed in three Japanese hospitals between 2014 and 2020 were enrolled in this study. These patients satisfied the following criteria: (1) presence of fatty liver by liver imaging test (e.g., hepatorenal contrast by ultrasonography), (2) persistent increases in liver enzyme levels, (3) the absence of chronic liver disease other than NAFLD (e.g., viral hepatitis or autoimmune hepatitis), and (4) alcohol consumption less than 20 g/day. Liver tissue samples for global RNA sequencing were obtained from 98 patients with NAFLD who underwent liver biopsy at Sendai Kousei Hospital from 2016 to 2018 (Transcriptome cohort). These samples were snap‐frozen at the time of liver biopsy. A total of 213 serum samples were obtained from 164 and 49 patients with NAFLD who underwent liver biopsy at either Osaka University Hospital or Kaizuka City Hospital, respectively, from 2014 to 2020 (Serum cohort). In this serum cohort, snap‐frozen liver tissue samples at the time of liver biopsy were obtained from 27 patients with NAFLD who underwent liver biopsy at Osaka University Hospital. All patients provided informed consent, and the study design was consistent with the principles of the Declaration of Helsinki. The protocol of the study using patient serum and tissues was approved by the Institutional Review Board Committees at Osaka University Hospital (Institutional Review Board No. 17097 and 19551).
Liver Histology
For all subjects, diagnoses were established histologically through liver biopsy specimens. The liver samples were embedded in paraffin blocks following standard procedures and stained with hematoxylin and eosin and Masson’s trichrome stains. All liver biopsies were centrally evaluated by an experienced hepatopathologist at one time for this study using the NASH Clinical Research Network histological scoring system.( 13 ) Hepatic fibrosis was scored on a 5‐point scale (0‐4) according to the Kleiner classification.( 13 ) Advanced fibrosis was classified as a score of 3‐4. Hepatic steatosis and lobular inflammation were scored on a 4‐point scale (0‐3), and hepatic ballooning was scored on a 3‐point scale (0‐2).( 13 ) The NAFLD activity score (NAS) was calculated as the sum of the steatosis, lobular inflammation, and ballooning scores, ranging from 0 to 8.( 13 ) We categorized patients with NAFLD into four groups according to Matteoni classification as follows: patients with steatosis alone (type 1), patients with steatosis and inflammation (type 2), patients with steatosis and ballooning hepatocytes (type 3), and patients with steatosis, ballooning hepatocytes, and fibrosis (type 4).( 14 ) We defined type 3 and 4 patients as NASH in this study.
Follow‐up Evaluation
The incidence of liver cancer or decompensated cirrhotic events (the new emergence of esophageal varix, ascites, and hepatic encephalopathy [HE]) was retrospectively analyzed for all 164 patients with NAFLD enrolled in this study at Kaizuka City Hospital. All patients underwent physical examination and laboratory testing every 3‐6 months and imaging, including ultrasonography, computed tomography, or magnetic resonance imaging, approximately every 6 months. Liver cancer was diagnosed based on histopathological findings by liver biopsy or radiological findings by the evaluation of dynamic contrast‐enhanced computed tomography and magnetic resonance imaging. The start of the observation period was defined as the date of liver biopsy, and the end of the observation period was defined as the date of event diagnosis or last hospital visit. The median observation period was 52 months (interquartile range: 22‐70). These patients were split into two groups by the mean serum TSP‐2 levels (with 60 patients in the TSP‐2–High group and 104 patients in the TSP‐2–Low group), and the incidences of liver cancer or decompensated cirrhotic events were compared. One patient in the TSP‐2–High group was excluded from analysis of decompensated cirrhotic events because of the presence of ascites before the liver biopsy.
RNA‐Sequencing Analysis
Total RNA was isolated from liver tissues as previously described.( 15 ) Library preparation was performed using the TruSeq Stranded mRNA Sample Prep Kit (Illumina, San Diego, CA) on an Apollo Library Prep System (Takara, Shiga, Japan). Sequencing was performed on an Illumina HiSeq 3000 platform in 75‐base single‐end mode. Sequenced reads were mapped to the human reference genome sequences (hg19) using TopHat v2.1.1. The fragments per kilobase of exon per million mapped reads values were calculated using Cuffnorm version 2.2.1. RNA‐sequencing data are available on the National Center for Biotechnology Information Gene Expression Omnibus repository (GSE167523). RNA‐sequencing data from GSE135251 containing hepatic transcriptome data of 206 patients with histologically characterized NAFLD derived from the European NAFLD Registry( 12 , 16 ) were also analyzed for the validation study.
Statistical Analysis
Data are expressed as the mean + SD. Statistical analysis was performed with Mann‐Whitney U tests to assess differences between unpaired groups. One‐way ANOVA followed by the Kruskal‐Wallis test or Tukey’s test was performed for multiple comparisons. Fisher’s exact test was used to analyze categorical data. Correlations were assessed using the Pearson product‐moment correlation coefficient. The Kaplan‐Meier method and log‐rank test were used to analyze differences in event‐free survival. Univariate and multivariate logistic regression analyses were used to analyze factors associated with NASH and advanced liver fibrosis. Odds ratios and 95% CIs were calculated. To assess the diagnostic performance of tissue and serum biomarkers, receiver operating characteristic curve analysis was performed, and the area under the receiver operating characteristic curve (AUROC) was used to evaluate the predictive power. The AUROCs were compared using the DeLong test. A P value < 0.05 was considered to indicate statistical significance, unless otherwise indicated. Prism v.8.4.2 for Mac (GraphPad Prism, research resource identifier [RRID]: SCR_002798; San Diego, CA) and JMP 13 (RRID: SCR_014242; SAS Institute Inc., Cary, NC) were used for the analyses.
All other information regarding materials and methods is provided in the Supporting Data.
Results
Hepatic Transcriptome Profiling of NAFLD Patient Samples Revealed That THBS2 Was Significantly Up‐regulated in NASH
We performed hepatic RNA‐sequencing analysis of 98 patients with biopsy‐proven NAFLD (51 patients with nonalcoholic fatty liver [NAFL] and 47 patients with NASH). Compared to patients with NAFL, patients with NASH were older and showed significantly higher serum aspartate aminotransferase (AST) levels and Fibrosis‐4 (FIB‐4) index values but lower platelet counts (Supporting Table S1). Unsupervised hierarchical clustering of whole‐transcriptome data separated patients with NAFL and NASH, suggesting that their pathological and clinical differences were reflected at the molecular level (Fig. 1A). Pathway analysis indicated strong activation of biological processes related to fibrosis, such as “extracellular structure and matrix organization” and “wound healing,” in addition to processes related to inflammation, such as “leukocyte chemotaxis, migration, differentiation, cell–cell adhesion, and activation,” in NASH patients (Supporting Fig. S1). We also performed CIBERSORT analysis to estimate the immune cell population in the livers of patients with NAFLD from the transcriptome data. Patients with NASH had greater intrahepatic infiltration of immune cells, especially innate immune cells, including M1 macrophages, natural killer cells, mast cells, and dendritic cells, than patients with NAFL (Supporting Fig. S2A,B). In agreement with this active immune environment, the expression of a variety of cytokines and chemokines was also up‐regulated in the livers of patients with NASH (Supporting Fig. S3). These molecular abnormalities highlight the important pathological features, liver inflammation and fibrosis, in the NASH liver.
FIG. 1.
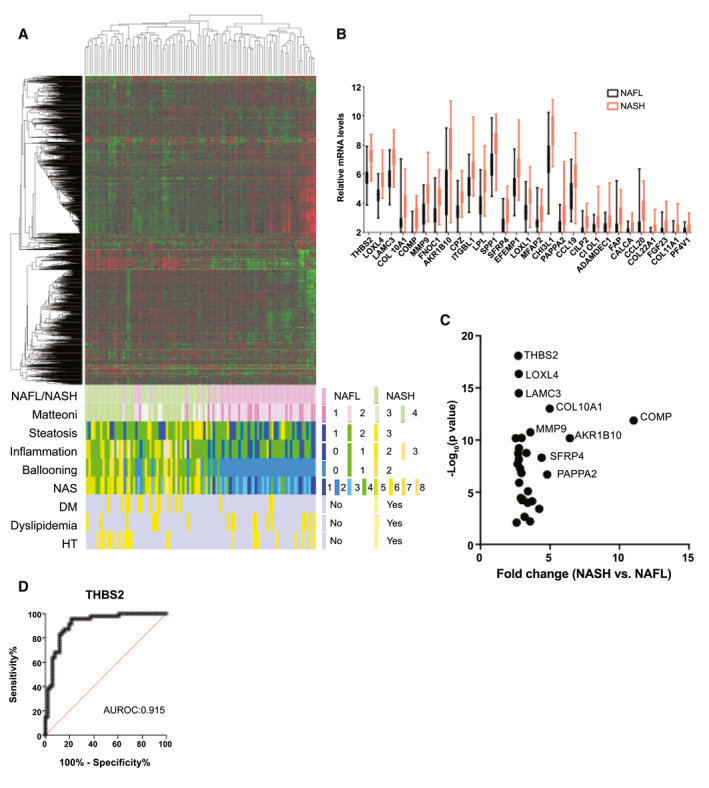
Hepatic transcriptome profiling of NAFLD patient samples revealed that THBS2 was significantly up‐regulated in NASH. (A) Unsupervised clustering based on the RNA‐sequencing data of liver tissues from 98 patients with biopsy‐proven NAFLD and the distribution of clinicopathological features. (B) Relative intrahepatic mRNA levels of 29 significantly up‐regulated genes in patients with NASH (n = 47) compared to patients with NAFL patients (n = 51). (C) mRNA fold change (NASH vs. NAFL) and P value of 29 significantly up‐regulated genes in patients with NASH compared to patients with NAFL. (D) Receiver operating characteristic curve indicating the performance of intrahepatic THBS2 mRNA levels in the diagnosis of NASH among patients with NAFLD. The AUROC is shown.
Next, we searched for the differentially expressed genes between NAFL and NASH and identified 137 significantly up‐regulated genes in patients with NASH (Supporting Table S2). To identify genes whose hepatic expression changes were reflected peripherally, we focused on the predicted secreted proteins according to the Protein Atlas database.( 17 ) Among the 137 genes, 29 genes were classified as secreted proteins and thus were considered potential candidate blood biomarkers for discriminating NASH from NAFL in patients with NAFLD (Fig. 1B). Among these genes, THBS2 was most significantly up‐regulated (Fig. 1C), and intrahepatic THBS2 mRNA levels were able to differentiate NASH from NAFL at an AUROC of 0.915 (Fig. 1D). This finding was validated by individual quantitative PCR (qPCR) analysis (Supporting Fig. S4A,B) or when using NAS score equal or above 5 for the diagnosis of NASH (Supporting Fig. S5A,B). This was further validated by the transcriptome data set of another recently published histologically characterized European NAFLD cohort (Supporting Fig. S6A,B).( 12 )
Hepatic Transcriptomic Profiling of NAFLD Patient Samples Identified THBS2 as Strongly Associated With Liver Fibrosis
Because hepatic fibrosis is the most important predictor of lethal outcomes in patients with NAFLD, we also comprehensively searched for secreted proteins up‐regulated in the fibrotic livers of patients with NAFLD. To this end, we classified patients with NAFLD into three groups according to the pathological fibrosis stage (F0, F1‐F2, and F3‐F4) (Supporting Table S3) and compared their transcriptome profiles. The levels of several clinically available markers of liver fibrosis, including serum hyaluronic acid (HA) level, platelet count, NAFLD Fibrosis Score (NFS), and FIB‐4 index, were associated with fibrosis stage, suggesting the validity of the histological diagnosis (Supporting Table S3). Principal component analysis of whole‐transcriptome data separated these three groups (Supporting Fig. S7). Then, we searched for genes that showed stepwise up‐regulation parallel to fibrosis stage. We identified 87 significantly up‐regulated genes in the F1‐F2 group compared with the F0 group and 265 genes in the F3‐F4 group compared with the F1‐F2 group (Supporting Fig. S8). Of the 17 genes commonly identified in both comparisons, 8 genes (including THBS2) were classified as predicted secreted proteins by the Protein Atlas database( 17 ) (Fig. 2A‐H). We evaluated the ability to diagnose advanced liver fibrosis (F3‐F4) according to the hepatic expression levels of eight genes and clinical parameters. All eight genes were individually capable of discriminating between the early and advanced stages of fibrosis (F0‐F2 vs. F3‐F4) at high AUROCs (Table 1). The AUROC of intrahepatic THBS2 mRNA levels (0.957) was significantly higher than the AUROCs of other well‐known clinical factors associated with fibrosis, including FIB‐4 index (0.836, P < 0.05) and HA (0.824, P < 0.05), and was comparable to the NFS (0.881, P = 0.08) (Table 1). The finding was validated by individual qPCR analysis of this cohort (Supporting Fig. S9A,B). In the transcriptome data set of the European NAFLD cohort, THBS2 expression levels also showed stepwise up‐regulation parallel to fibrosis stage (Supporting Fig. S10A), and the AUROC of intrahepatic THBS2 mRNA levels for advanced fibrosis (0.853) was significantly higher than that of the FIB‐4 index (0.768, P < 0.05) (Supporting Fig. S10B).( 12 )
FIG. 2.
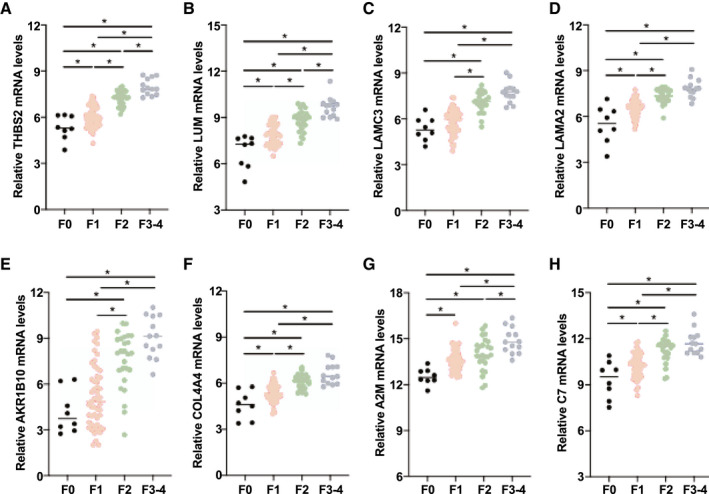
Hepatic transcriptomic profiling of NAFLD patient samples identified THBS2 as strongly associated with liver fibrosis. (A‐H) Relative intrahepatic mRNA levels of eight genes based on fibrosis stage among 98 patients with biopsy‐proven NAFLD (*P < 0.05): THBS2 (A), LUM (B), LAMC3 (C), LAMA2 (D), AKR1B10 (E), COL4A4 (F), A2M (G), and C7 (H).
TABLE 1.
AUROC for the Diagnosis of Advanced Fibrosis (F3‐F4) in 98 Patients With Biopsy‐Proven NAFLD
| Gene Name | AUROC | 95% CI |
|---|---|---|
| THBS2 | 0.957 | 0.916‐0.998 |
| LUM | 0.943 | 0.896‐0.990 |
| LAMC3 | 0.910 | 0.843‐0.976 |
| LAMA2 | 0.896 | 0.815‐0.977 |
| AKR1B10 | 0.878 | 0.799‐0.957 |
| COL4A4 | 0.861 | 0.770‐0.951 |
| A2M | 0.856 | 0.767‐0.946 |
| C7 | 0.844 | 0.753‐0.935 |
| Clinical Variable | AUROC | 95% CI |
| NFS | 0.881 | 0.810‐0.951 |
| FIB‐4 index | 0.836 | 0.728‐0.944 |
| HA | 0.824 | 0.720‐0.929 |
Hepatic THBS2 Levels Were Associated With NAFLD Activity and Fibrosis
Because hepatic THBS2 levels can identify both patients with NASH and patients with advanced fibrosis, we sought the clinicopathological factors associated with hepatic THBS2 levels. Consistent with the stepwise up‐regulation of hepatic THBS2 levels along with fibrosis stage (Fig. 2A), THBS2 expression was positively correlated with serum HA levels and NFS (Fig. 3A,B and Supporting Table S4), suggesting that the hepatic THBS2 level reflects liver fibrosis. It was also positively correlated with serum AST levels (Fig. 3C and Supporting Table S4). The hepatic THBS2 level increased along with NAS (Fig. 3D) and was positively associated with inflammation and ballooning but not with steatosis (Fig. 3E). These findings suggest that hepatic THBS2 levels may also reflect liver injury, histologically exhibited as inflammation and hepatocyte ballooning.
FIG. 3.
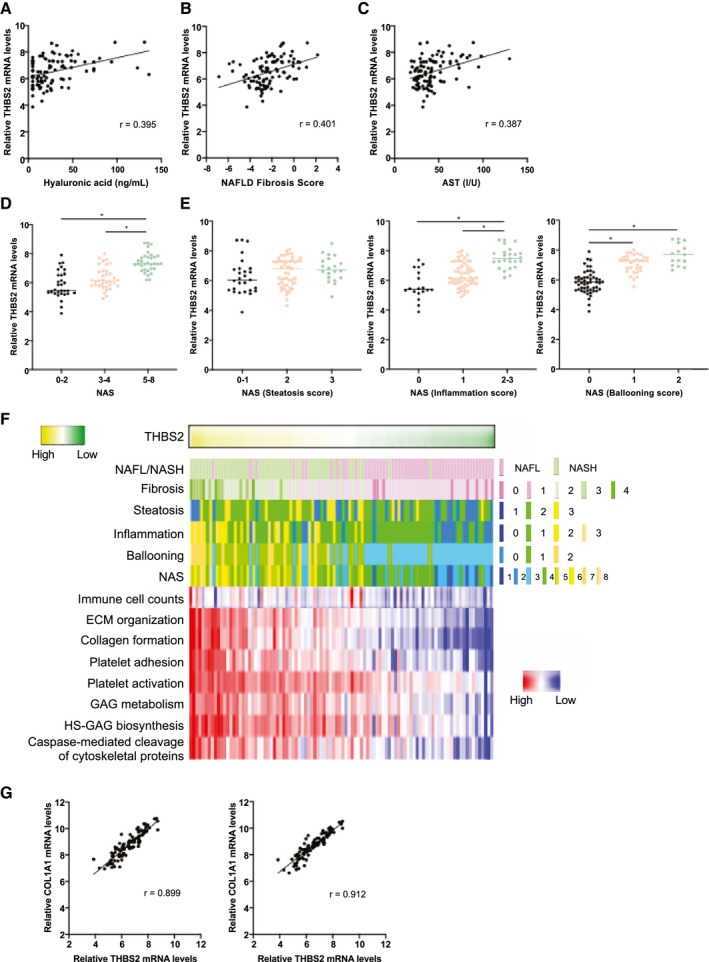
Hepatic THBS2 levels were associated with NAFLD activity and fibrosis. (A‐C) Correlations between the intrahepatic THBS2 mRNA levels and serum HA levels (A), NFS (B), and serum AST levels (C) of 98 patients with biopsy‐proven NAFLD. (D) Relative intrahepatic THBS2 mRNA levels based on the NAS. (*P < 0.01). (E) Relative intrahepatic THBS2 mRNA levels based on the steatosis (left), inflammation (middle,) and ballooning (right) scores of the NAS (*P < 0.01). (F) Correlation of intrahepatic THBS2 mRNA levels with clinicopathological features, intrahepatic immune cell infiltration, and molecular pathways. (G) Correlation between intrahepatic THBS2 and COL1A1 (left) or COL1A2 (right) mRNA levels. Abbreviation: HS‐GAG, heparan sulfate glycosaminoglycan.
We then investigated the pathways and molecules associated with intrahepatic THBS2 levels. To this end, we performed single‐sample gene‐set enrichment analysis of our transcriptomic data and identified 55 REACTOME pathways strongly correlated with intrahepatic THBS2 levels (r > 0.75) (Supporting Table S5). These pathways included multiple pathways involved in the development and progression of NASH, such as extracellular matrix (ECM)/collagen biosynthesis,( 18 ) platelet activation,( 19 ) glycosaminoglycan biosynthesis,( 20 ) and caspase‐mediated cleavage of cytoskeletal proteins( 21 ) (Fig. 3F). CIBERSORT( 22 ) analysis identified a positive correlation between intrahepatic THBS2 levels and immune cell infiltration (r = 0.42) (Fig. 3F). Weighted correlation network analysis( 23 ) identified a gene network in which THBS2 was connected to 98 genes, including 11 collagen genes (Supporting Fig. S11 and Supporting Table S6). The expression levels of type I collagens, major ECM components of the fibrotic liver, were strongly correlated with THBS2 mRNA levels in the livers of patients with NAFLD (Fig. 3G and Supporting Table S7). Correlation between expression levels of type I collagens and THBS2 was validated by individual qPCR analysis in this cohort (Supporting Fig. S12A,B) and the transcriptome data set of the European NAFLD cohort (Supporting Fig. S13A,B).( 12 ) Taken together, these associations at the molecular and pathway levels may further support our claim that hepatic THBS2 is an excellent marker to identify NASH and advanced fibrosis in patients with NAFLD.
Serum TSP‐2 Level Discriminated NASH From NAFL Among Patients With NAFLD
Next, we investigated the potential of TSP‐2, a secreted protein encoded by THBS2, as a noninvasive serum biomarker to distinguish patients with NASH from patients with NAFLD using another cohort of 213 patients with biopsy‐proven NAFLD (121 patients with NAFL and 92 patients with NASH). Patients with NASH were significantly older and showed significantly higher serum AST levels, alanine aminotransferase levels, and FIB‐4 index values and lower platelet counts (Supporting Table S8). First, we preliminarily examined the correlation between intrahepatic THBS2 mRNA levels by qPCR and serum TSP‐2 levels by ELISA in 27 patients with NAFLD. A significant association was observed between the mRNA levels and the serum levels (r = 0.75), suggesting that hepatic expression changes of THBS2 were reflected peripherally (Supporting Fig. S14). We then measured the serum levels of TSP‐2 for all 213 patients. Serum TSP‐2 levels were significantly higher in patients with NASH than in patients with NAFL (Fig. 4A). Serum TSP‐2 levels differentiated NASH from NAFL with an AUROC of 0.776 (Fig. 4B). These findings were validated when using NAS score equal or above 5 for the diagnosis of NASH (Supporting Fig. S15A,B). Multivariate logistic regression analysis revealed that serum TSP‐2 level, platelet count, and age were independent predictors of NASH in patients with NAFLD (Table 2). Similar to the intrahepatic THBS2 mRNA level, the serum TSP‐2 level also increased along with the NAS and was significantly higher in patients with positive inflammation or ballooning scores than in those with negative scores (Fig. 4C,D).
FIG. 4.
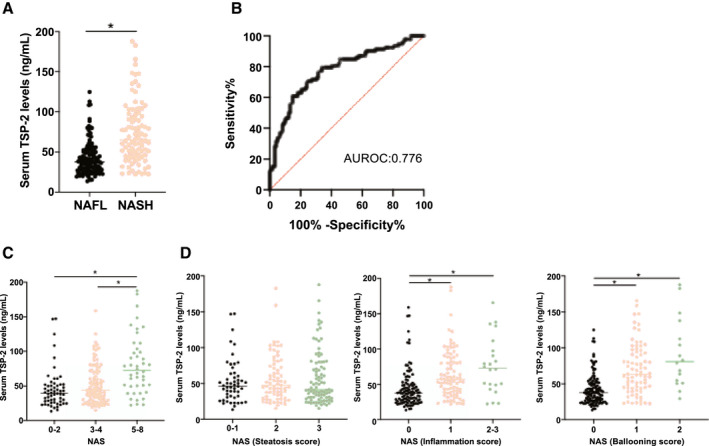
Serum TSP‐2 levels discriminated NASH from NAFL among patients with NAFLD patients. (A) Relative serum TSP‐2 levels in 121 patients with NAFL and 92 patients with NASH (*P < 0.01). (B) Receiver operating characteristic curve of the performance of serum TSP‐2 levels in the diagnosis of NASH among 213 patients with biopsy‐proven NAFLD. The AUROC is shown. (C) Relative serum TSP‐2 levels based on the NAS (*P < 0.01). (D) Relative serum TSP‐2 levels based on the steatosis (left), inflammation (middle), and ballooning (right) scores of the NAS (*P < 0.01).
TABLE 2.
Logistic Regression Analysis for the Prediction of NASH in 213 Patients With Biopsy‐Proven NAFLD
| Factor | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | ||
| Age (years) | Per year | 1.057 | 1.031‐1.083 | <0.05 | 1.042 | 1.008‐1.078 | <0.05 |
| Sex (M/F) | 0.8 | 0.454‐1.409 | N.S. | ||||
| Body mass index (kg/m2) | per 1 kg/m2 | 1.018 | 0.961‐1.077 | N.S. | |||
| AST (U/L) | per 10 U/L | 1.3 | 1.162‐1.454 | <0.05 | N.S. | ||
| ALT (U/L) | per 10 U/L | 1.071 | 1.009‐1.136 | <0.05 | N.S. | ||
| GGT (U/L) | per 10 U/L | 1.021 | 0.983‐1.060 | N.S. | |||
| ALP (U/L) | per 40 U/L | 1.027 | 0.925‐1.141 | N.S. | |||
| Triglyceride (mg/dL) | per 10 mg/dL | 1.019 | 0.985‐1.054 | N.S. | |||
| LDL‐C (mg/dL) | per 10 mg/dL | 0.951 | 0.873‐1.036 | N.S. | |||
| Fasting blood sugar (mg/dL) | per 10 mg/dL | 1.057 | 0.989‐1.129 | N.S. | |||
| HbA1c (%) | per 1 % | 1.219 | 0.970‐1.531 | N.S. | |||
| AFP (ng/mL) | per 1 ng/mL | 1.318 | 1.147‐1.514 | <0.05 | N.S. | ||
| Albumin (g/dL) | per 1 g/dL | 0.259 | 0.103‐0.653 | <0.05 | N.S. | ||
| Platelet count (×104/μL) | per 1X 104/μL | 0.88 | 0.837‐0.926 | <0.05 | 0.928 | 0.875‐0.984 | <0.05 |
| TSP‐2 (ng/mL) | per 1 ng/mL | 1.039 | 1.026‐1.052 | <0.05 | 1.03 | 1.016‐1.045 | <0.05 |
Abbreviations: AFP, alpha‐fetoprotein; F, female; GGT, γ‐glutamyl transpeptidase; LDL‐C, low density lipoprotein cholesterol; M, male; N.S., not significant.
Serum TSP‐2 Levels Identified Patients With Advanced Fibrosis Among Patients With NAFLD
We also investigated the association between serum TSP‐2 levels and fibrosis in patients with NAFLD. The clinical and serological characteristics of patients with NAFLD stratified by fibrosis stage are provided in Supporting Table S9. A stepwise increase in serum HA levels, FIB‐4 index, and NFS according to fibrosis stage were observed, suggesting their usefulness as fibrosis markers (Supporting Table S9). Serum TSP‐2 levels also showed a stepwise elevation along with the fibrosis stage (Fig. 5A), and the AUROC of TSP‐2 levels for diagnosis of F3‐F4 stage advanced liver fibrosis was 0.856, which was comparable to that of other fibrosis markers, including FIB‐4 index (AUROC: 0.784, P = 0.05), HA (AUROC: 0.783, P = 0.06), and NFS (AUROC: 0.808, P = 0.21) (Fig. 5B). Similar to the hepatic THBS2 mRNA level, the serum TSP‐2 level positively correlated with the serum AST level, HA level, and NFS (Fig. 5C‐E and Supporting Table S10). Multivariate logistic regression analysis revealed that serum TSP‐2 level and platelet count were independent predictors of advanced liver fibrosis in patients with NAFLD (Table 3). Taken together, these findings suggest that the serum TSP‐2 level may be a good noninvasive biomarker to identify NASH and advanced fibrosis in patients with NAFLD.
FIG. 5.
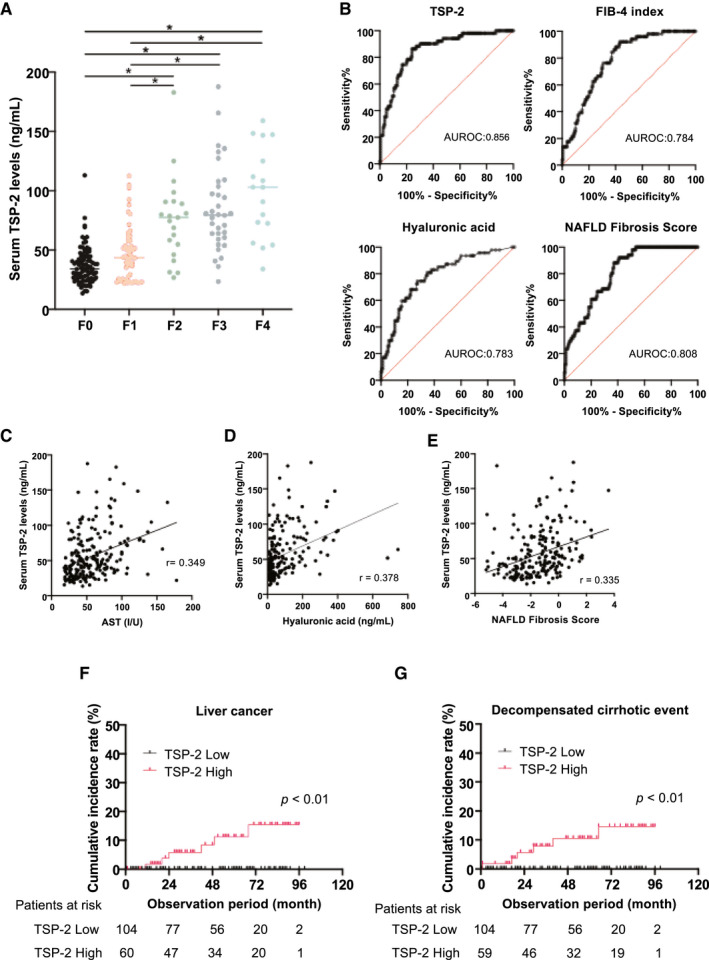
Serum TSP‐2 levels identified advanced fibrosis patients among patients with NAFLD. (A) Relative serum TSP‐2 levels based on fibrosis staging in 213 patients with biopsy‐proven NAFLD (*P < 0.01). (B) Receiver operating characteristic curve of the performance of serum TSP‐2 levels, FIB‐4 index, HA, and NFS in the diagnosis of advanced liver fibrosis (F3‐F4) among patients with NAFLD. The AUROC is shown. (C‐E) Correlation of serum TSP‐2 levels with serum AST levels (C), serum HA levels (D), and NFS (E). (F,G) Kaplan‐Meier curves of the cumulative incidence rate of liver cancer (F) in 164 patients with NAFLD and decompensated cirrhotic events, including esophageal varices, ascites, and hepatic encephalopathy (G) in 163 patients with NAFLD stratified by serum TSP‐2 levels. Number of patients at risk is shown at each observation period.
TABLE 3.
Logistic Regression Analysis for the Prediction of Advanced Fibrosis (F3‐F4) in 213 Patients With Biopsy‐Proven NAFLD
| Factor | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | ||
| Age (years) | Per year | 1.037 | 1.010‐1.065 | <0.05 | N.S. | ||
| Sex (M/F) | 1.157 | 0.605‐2.210 | N.S. | ||||
| Body mass index (kg/m2) | per 1 kg/m2 | 1.077 | 1.011‐1.147 | <0.05 | N.S. | ||
| AST (U/L) | per 10 U/L | 1.079 | 0.976‐1.194 | N.S. | |||
| ALT (U/L) | per 10 U/L | 0.947 | 0.878‐1.021 | N.S. | |||
| GGT(U/L) | per 10 U/L | 1.009 | 0.967‐1.052 | N.S. | |||
| ALP (U/L) | per 40 U/L | 1.01 | 0.894‐1.140 | N.S. | |||
| Triglyceride (mg/dL) | per 10 mg/dL | 0.998 | 0.994‐1.002 | N.S. | |||
| LDL‐C (mg/dL) | per 10 mg/dL | 0.866 | 0.779‐0.962 | <0.05 | N.S. | ||
| Fasting blood sugar (mg/dL) per 10 mg/dL | 1.06 | 0.991‐1.134 | N.S. | ||||
| HbA1c (%) | per 1 % | 1.282 | 1.015‐1.619 | <0.05 | N.S. | ||
| AFP (ng/mL) | per 1 ng/mL | 1.257 | 1.105‐1.430 | <0.05 | N.S. | ||
| Albumin (g/dL) | per 1 g/dL | 0.198 | 0.072‐0.545 | <0.05 | N.S. | ||
| HA (ng/mL) | per 10 ng/mL | 1.094 | 1.053‐1.136 | <0.05 | N.S. | ||
| Platelet count (×104/μL) | per 1X 104/μL | 0.816 | 0.757‐0.879 | <0.05 | 0.865 | 0.795‐0.940 | <0.05 |
| TSP‐2 (ng/mL) | per 1 ng/mL | 1.043 | 1.030‐1.057 | <0.05 | 1.037 | 1.020‐1.055 | <0.05 |
Abbreviations: F, female; GGT, γ‐glutamyl transpeptidase; LDL‐C, low density lipoprotein cholesterol; M, male; N.S., not significant.
Liver cancer and decompensated cirrhotic events such as esophageal varix, ascites, and HE are important lethal outcomes in patients with NAFLD. We therefore retrospectively analyzed the incidence of liver cancer and decompensated cirrhotic events (the new emergence of esophageal varix, ascites, and HE) for 164 patients with NAFLD who were followed up under a standardized schedule (the median observation period was 52 months). The patients were sorted into two groups according to the mean serum TSP‐2 level. Liver cancer was observed in 6 patients among 60 patients with high serum TSP‐2 levels but none of the 104 patients with low TSP‐2 levels during the observation period. The cumulative incidence rates of liver cancer at 2 years and 4 years were 5.78% and 8.40%, respectively, among patients with high serum TSP‐2 levels (Fig. 5F). Decompensated events were observed in 6 patients of those with high serum TSP‐2 levels (esophageal varix, 4 patients; ascites, 2 patients) but none of those with low levels. The cumulative incidence rates of decompensated events at 2 years and 4 years were 5.64% and 10.54%, respectively, among patients with high serum TSP‐2 levels (Fig. 5G). These cumulative incidence rates were significantly higher in patients with NAFLD with high serum TSP‐2 levels than in those with low levels (Fig. 5F,G), suggesting the usefulness of TSP‐2 in identifying patients with NAFLD with a high risk of these complications.
Discussion
In this study, we performed global RNA sequencing of liver tissues from nearly 100 patients with NAFLD and achieved comprehensive profiling of molecular abnormalities involved in NASH development. Interestingly, unsupervised clustering of transcriptome data successfully discriminated NASH from NAFL, strongly suggesting the molecular differences between these two disease entities. Pathway analysis also identified two major pathological characteristics, hepatic inflammation and fibrosis, in the livers of patients with NASH, suggesting the potential usefulness of omics approaches to identify patients with NASH patients. We provided 137 significantly up‐regulated genes possibly involved in molecular pathogenesis of NASH. We also evaluated those genes in the recently published transcriptome data set of the NAFLD cohort derived from the European NAFLD Registry and found that 95 genes were also significantly up‐regulated in their patients with NASH (Supporting Table S2). This considerable overlap strengthens the validity of our transcriptome analysis.
Through transcriptome analysis, we identified that THBS2 was most significantly up‐regulated in the livers of patients with NASH and advanced fibrosis. Although a positive association of THBS2 with NASH and advanced fibrosis was also observed in several other omics studies of liver tissues from patients with NAFLD,( 7 , 9 , 12 ) the diagnostic potential of THBS2 for NASH and advanced fibrosis has not been fully evaluated. In the current study, we validated the strong positive association of hepatic THBS2 levels with NAFLD disease progression. Moreover, we present data from patients derived from Japan and several European countries, making THBS2 a much more plausible hepatic biomarker with greater generalizability. Subsequently, we also showed the usefulness of serum TSP‐2 as a noninvasive biomarker for identifying NASH and advanced fibrosis. Recently, Kimura et al.( 24 ) have also shown the utility of serum TSP‐2 levels as predictor for disease severity in patients with NAFLD. The authors showed a correlation of serum TSP‐2 levels with hepatocyte ballooning and fibrosis stage among patients with NAFLD, which is consistent with our findings.
Regarding NAFLD disease pathogenesis, it is clear that the processes of liver cell damage, inflammation, and fibrogenesis are tightly linked. Therefore, “biomarkers for fibrosis” often reflect a pathway that is involved in this complex setting and thus cross‐sectionally associated with NASH. Indeed, THBS2 is strongly associated with fibrogenesis (type I collagen synthesis) but also showed an association with hepatocyte ballooning and liver inflammation. Consequently, serum TSP‐2 levels serve as a biomarker for both NASH and advanced fibrosis.
Regarding the context for the use of this biomarker, based on the AUROC values of serum TSP‐2 levels for diagnosing advanced fibrosis (AUROC = 0.856; Fig. 5B) and NASH (AUROC = 0.776; Fig. 4B) in patients with NAFLD, it may be more useful as a diagnostic tool for fibrosis. When we focused on patients with advanced fibrosis (F3‐F4), serum TSP‐2 levels were still positively associated with AST levels and the presence of hepatocyte ballooning (Supporting Fig. S16A,B), suggesting that serum TSP‐2 levels might be used to detect patients with advanced fibrosis who have a greater risk for further disease progression. Meanwhile, new simple scoring system using independent predictors (serum TSP‐2 level, platelets, and age) might provide even better diagnostic ability for NASH and advanced fibrosis, and would be worth further evaluation in a future external cohort.
TSP‐2, a member of the thrombospondin family, is a matricellular glycoprotein that mediates cell‐to‐cell and cell‐to‐matrix interactions.( 25 ) Thrombospondins have a variety of tissue‐specific roles in wound healing, angiogenesis, and connective tissue organization.( 25 ) The roles of TSP‐2 have been studied primarily using Thbs2 knockout mice, which displayed normal physical appearance but showed increased fragility and laxity of skin and connective tissues with microscopically abnormal collagen fibrils.( 17 , 25 ) Moreover, a lack of well‐defined collagen fibrils was also observed in ECM derived in vitro from dermal fibroblasts isolated from Thbs2 knockout mice.( 26 ) These data strongly suggest the important role of TSP‐2 in proper collagen fibrillogenesis.( 26 ) In patients with NASH, lipotoxicity‐based chronic liver stimuli induce liver inflammation and turn resident HSCs into activated hepatic myofibroblasts. These myofibroblasts secrete ECM proteins such as type I collagen, leading to fibrosis progression.( 27 ) In this study, we identified a strong correlation between hepatic THBS2 mRNA levels and type I collagen expression, suggesting the possible role of TSP‐2 in collagen synthesis in the fibrotic liver. Recently, Chen et al.( 28 ) showed that TSP‐2 protein was produced primarily in HSCs in the murine fibrotic liver and may activate HSCs in association with activation of the Notch signaling pathway. Taken together, these data illuminate the important role of TSP‐2 in liver fibrogenesis.
Among eight candidate blood biomarkers for advanced liver fibrosis identified in this study, half of them (collagen type IV alpha 4 chain [COL4A4], lumican [LUM], laminin subunit gamma 3 [LAMC3], and laminin subunit alpha 2 [LAMA2]) were ECM proteins (Fig. 2). COL4A4 encodes one of the six subunits of type IV collagen, a fragment of which is already used in the clinic as a reliable serum marker for liver fibrosis.( 29 ) Lumican, encoded by LUM, is an ECM protein, and its plasma levels have been reported to correlate with hepatic collagen production.( 30 ) LAMC3 and LAMA2 encode the gamma 3 chain and the alpha 2 chain of laminins, respectively.( 31 ) The utility of serum laminin as a marker of liver fibrosis in NAFLD has been studied.( 32 ) In addition to those ECM proteins, we found AKR1B10, an aldo/keto reductase, which is involved in lipid and xenobiotic metabolism. A strong association of its hepatic and serum levels with disease activity and fibrosis stage in patients with NAFLD has been shown by several groups.( 12 , 33 ) Meanwhile, the AUROC values of these candidate biomarkers appear to be markedly high. This is possibly due to the high‐throughput screening methodology and/or our transcriptomic cohort bias, which should be noted here as a potential limitation of the study.
The prevalence of NASH and advanced fibrosis in our cohort is approximately 48% and 13% for the transcriptome cohort and 43% and 24% for the serum cohort, respectively. These values may be higher compared with the population‐based or outpatient‐based prevalence because of the bias to only include patients who underwent liver biopsy in this study. Because our discovery cohort is a highly selected cohort, with an overrepresentation of more‐advanced/severe disease cases, it is important to evaluate the utility of our biomarker for validation cohorts with lower prevalence, such as a screening setting at an outpatient clinic or a health check‐up.
In conclusion, through global transcriptome analysis of liver tissues from patients with biopsy‐proven NAFLD, we identified molecular abnormalities important for disease progression. We also discovered and validated THBS2 and its encoding secreted protein TSP‐2 as a diagnostic marker for NASH and advanced fibrosis in a large cohort of patients with histologically characterized NAFLD.
Author Contributions
K.K. was responsible for the data curation, formal analysis, validation, visualization, and original draft of the manuscript. T.K. was responsible for the study concept, formal analysis, funding acquisition, validation, visualization, and original draft of the manuscript. H.M. and S.S. were responsible for the data curation and formal analysis. O.G. and S.C. were responsible for the data curation, formal analysis, validation, and manuscript review and editing. D.M. was responsible for the formal analysis and original draft of the manuscript. N.K., Y.Y., and Y.Ko. were responsible for the resources and data curation. Y.T., R.Y., H.H., R.S., and T.Tat. were responsible for the methodology. Y.Ka. and E.M. were responsible for the methodology and formal analysis. A.K.D. was responsible for the study supervision (validation analysis) and manuscript review and editing. Q.M.A. was responsible for the study supervision (validation analysis), data curation, and manuscript review and editing. T.Tak. was responsible for the study concept, formal analysis, funding acquisition, supervision, and manuscript review and editing.
Supporting information
Supplementary Material
Acknowledgment
The authors thank M. Imai for the technical and experimental help.
Supported by the Japan Agency for Medical Research and Development (JP20fk0210074 to T.K.); a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (20H03661 to (T.K.); the Elucidating Pathways of Steatohepatitis consortium funded by the Horizon 2020 Framework Program of the European Union (634413 to Q.M.A., A.K.D., S.C., and O.G.); the Liver Investigation: Testing Marker Utility in Steatohepatitis consortium funded by the Innovative Medicines Initiative Program of the European Union (777377); and the Newcastle National Institute for Health Research Biomedical Research Centre.
Potential conflict of interest: Dr. Anstee consults, is on the speakers’ bureau, has active research collaborations, and received grants from Allergan/Tobira. He consults, has active research collaborations, and received grants from AstraZeneca, Novartis, and Pfizer. He consults, is on the speakers’ bureau, and has active research collaborations with Bristol‐Myers Squibb and Genfit. He consults and is on the speakers’ bureau for Abbott and Gilead. He consults and has active research collaborations with Eli Lilly, HistoIndex, Intercept, and Novo Nordisk. He has active research collaborations and received grants from AbbVie, GlaxoSmithKline, and Glympse Bio. He consults for 89Bio, Acuitas, Altimmune, Axcella, Blade, BNN Cardio, Celgene, Cirius, CymaBay, EcoR1, E3Bio, Galmed, Genentech, Grunthal, Indalo, Imperial Innovations, Inventiva, IQVIA, Janssen, Madrigal, Medimmune, Metacrine, NewGene, NGMBio, North Sea, Poxel, ProSciento, Raptor, Servier, Terns, and Viking. He has active research collaborations with Antaros, Boehringer Ingelheim, Echosens, Ellegaard Gottingen Minipigs AS, Exalenz, iXscient, Nordic Bioscience, One Way Liver Genomics, Perspectum, Resoundant, Sanofi‐Aventis, SomaLogic, and Takeda. He is on the speakers’ bureau for Clinical Care Options, Falk, Fishawack, Integritas, Kenes, and MedScape. He received grants from Vertex. He received royalties from Elsevier.
References
Author names in bold designate shared co‐first authorship.
- 1. Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology 2020;158:1851‐1864. [DOI] [PubMed] [Google Scholar]
- 2. Castera L, Friedrich‐Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1264‐1281.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389‐397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy‐proven NAFLD. J Hepatol 2017;67:1265‐1273. [DOI] [PubMed] [Google Scholar]
- 5. Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Gastroenterology 2020;158:1611‐1625.e12. [DOI] [PubMed] [Google Scholar]
- 6. Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005;128:1898‐1906. [DOI] [PubMed] [Google Scholar]
- 7. Moylan CA, Pang H, Dellinger A, Suzuki A, Garrett ME, Guy CD, et al. Hepatic gene expression profiles differentiate presymptomatic patients with mild versus severe nonalcoholic fatty liver disease. Hepatology 2014;59:471‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teufel A, Itzel T, Erhart W, Brosch M, Wang XY, Kim YO, et al. Comparison of gene expression patterns between mouse models of nonalcoholic fatty liver disease and liver tissues from patients. Gastroenterology 2016;151:513‐525.e0. [DOI] [PubMed] [Google Scholar]
- 9. Suppli MP, Rigbolt KTG, Veidal SS, Heebøll S, Eriksen PL, Demant M, et al. Hepatic transcriptome signatures in patients with varying degrees of nonalcoholic fatty liver disease compared with healthy normal‐weight individuals. Am J Physiol Gastrointest Liver Physiol 2019;316:G462‐G472. [DOI] [PubMed] [Google Scholar]
- 10. Hoang SA, Oseini A, Feaver RE, Cole BK, Asgharpour A, Vincent R, et al. Gene expression predicts histological severity and reveals distinct molecular profiles of nonalcoholic fatty liver disease. Sci Rep 2019;9:12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haas JT, Vonghia L, Mogilenko DA, Verrijken AN, Molendi‐Coste O, Fleury S, et al. Transcriptional network analysis implicates altered hepatic immune function in NASH development and resolution. Nat Metab 2019;1:604‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Govaere O, Cockell S, Tiniakos D, Queen R, Younes R, Vacca M, et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis. Sci Transl Med 2020;12:eaba4448. [DOI] [PubMed] [Google Scholar]
- 13. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 14. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413‐1419. [DOI] [PubMed] [Google Scholar]
- 15. Kodama T, Yi J, Newberg JY, Tien JC, Wu H, Finegold MJ, et al. Molecular profiling of nonalcoholic fatty liver disease‐associated hepatocellular carcinoma using SB transposon mutagenesis. Proc Natl Acad Sci USA 2018;115:E10417‐E10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hardy T, Wonders K, Younes R, Aithal GP, Aller R, Allison M, et al. The European NAFLD registry: a real‐world longitudinal cohort study of nonalcoholic fatty liver disease. Contemp Clin Trials 2020;98:106175. [DOI] [PubMed] [Google Scholar]
- 17. Thul PJ, Akesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, et al. A subcellular map of the human proteome. Science 2017;356:eaal3321. [DOI] [PubMed] [Google Scholar]
- 18. Schwabe RF, Tabas I, Pajvani UB. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology 2020;158:1913‐1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diggs LP, Greten TF. The effects of platelet accumulation in fatty liver disease. Nat Rev Gastroenterol Hepatol 2019;16:393‐394. [DOI] [PubMed] [Google Scholar]
- 20. Mardinoglu A, Agren R, Kampf C, Asplund A, Uhlen M, Nielsen J. Genome‐scale metabolic modelling of hepatocytes reveals serine deficiency in patients with non‐alcoholic fatty liver disease. Nat Commun 2014;5:3083. [DOI] [PubMed] [Google Scholar]
- 21. Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin‐18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology 2009;50:1072‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol Biol 2018;1711:243‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 2008;9:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kimura T, Tanaka N, Fujimori N, Yamazaki T, Katsuyama T, Iwashita Y, et al. Serum thrombospondin 2 is a novel predictor for the severity in the patients with NAFLD. Liver Int 2021;41:505‐514. [DOI] [PubMed] [Google Scholar]
- 25. Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol 2011;3:a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kyriakides TR, Zhu Y‐H, Smith LT, Bain SD, Yang Z, Lin MT, et al. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol 1998;140:419‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol 2021;18:151‐166. [DOI] [PubMed] [Google Scholar]
- 28. Chen W, Wu X, Yan X, Xu A, Yang A, You H. Multitranscriptome analyses reveal prioritized genes specifically associated with liver fibrosis progression independent of etiology. Am J Physiol Gastrointest Liver Physiol 2019;316:G744‐G754. [DOI] [PubMed] [Google Scholar]
- 29. Yoneda M, Mawatari H, Fujita K, Yonemitsu K, Kato S, Takahashi H, et al. Type IV collagen 7s domain is an independent clinical marker of the severity of fibrosis in patients with nonalcoholic steatohepatitis before the cirrhotic stage. J Gastroenterol 2007;42:375‐381. [DOI] [PubMed] [Google Scholar]
- 30. Decaris ML, Li KW, Emson CL, Gatmaitan M, Liu S, Wang Y, et al. Identifying nonalcoholic fatty liver disease patients with active fibrosis by measuring extracellular matrix remodeling rates in tissue and blood. Hepatology 2017;65:78‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yao Y. Laminin: loss‐of‐function studies. Cell Mol Life Sci 2017;74:109‐ 1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mak KM, Mei R. Basement membrane type IV collagen and laminin: an overview of their biology and value as fibrosis biomarkers of liver disease. Anat Rec (Hoboken) 2017;300:1371‐1390. [DOI] [PubMed] [Google Scholar]
- 33. Gerhard GS, Legendre C, Still CD, Chu X, Petrick A, DiStefano JK. Transcriptomic profiling of obesity‐related nonalcoholic steatohepatitis reveals a core set of fibrosis‐specific genes. J Endocr Soc 2018;2:710‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


