Abstract
Background
Scrotal color Doppler ultrasonography and transrectal ultrasonography provide crucial information about the clinical status of testes and male accessory glands.
Objective
To analyze the impact of ultrasound in the evaluation of infertile males.
Materials and Methods
A total of 1120 records from infertile men were retrospectively evaluated (from January 2016 up to June 2020). Data on physical examination, semen analysis, sperm culture, scrotal color Doppler ultrasonography and transrectal ultrasonography, as well as sex hormones were analyzed. Among them, 238 reports from oligozoospermic/azoospermic infertile patients (P) fulfilling the inclusion criteria were considered for data analysis. Patients were subdivided into two groups according to follicle‐stimulating hormone (FSH) values (Pa with FSH < 8 U/L and Pb with FSH ≥ 8 U/L). Sixty‐three fertile volunteers (mean ± SD years) were enrolled as controls (C).
Results
A higher prevalence of ultrasound abnormalities was recorded in P compared to C. Pb group had significantly lower bitesticular volume compared to Pa and C. Pa had a higher prevalence of transrectal ultrasonography abnormalities than Pb (69.9% vs. 38.4%), whereas Pb had a higher prevalence of abnormalities at scrotal color Doppler ultrasonography (60.0% vs. 28.3%, both p < 0.01). Bitesticular volume was inversely proportional to the number of altered seminal parameters and able to predict gonadotropin levels. A bitesticular volume <17 cc was associated with a higher risk of azoospermia (odds ratio = 1.799). Intratesticular vascularization was inversely correlated with gonadotropin levels and directly correlated with sperm count. A higher prevalence of prostate and seminal vesicle alterations was detected in patients and in Pa group, when compared with Pb group.
Discussion and Conclusion
Ultrasound abnormalities are correlated with seminal parameters and may guide the clinician in the diagnostic workflow of male infertility, suggesting spermatogenesis impairment or genital tract obstructions.
Keywords: color Doppler, male infertility, oligozoospermia, reproductive hormones, testicular vascularization, ultrasound
1. INTRODUCTION
Couple infertility, defined as the lack of conception after at least 12 months of regular unprotected sexual intercourse aimed at pregnancy, 1 is a common clinical condition. About 10% of couples in the world are infertile with 56% of them seeking medical care. 2 This condition is caused by a male factor in about 50% of the cases. 3 , 4 As for other pathological conditions, the clinical approach to the diagnosis of male infertility requires different levels of intervention. Medical history, physical examination, and semen analysis should be performed as first diagnostic step. 5 Although semen parameters do not lead to a clear‐cut discrimination between fertile and infertile men, semen analysis represents the cornerstone in the evaluation of male infertility. 6
If dyspermia occurs, sperm culture, scrotal Doppler ultrasound, transrectal ultrasound, reproductive hormones, and genetic tests can allow the clinician to complete the diagnostic procedure. Many conditions of infertility, particularly those associated with sperm anomalies, derive from alterations of the genital apparatus that often are not detectable through a physical examination. In these cases, the ultrasonographic examination could be of primary importance to provide the proper diagnosis. Indeed, ultrasonography (US) of the male genital tract, if performed by an expert sonographer, can supply crucial information about the clinical status of testes and male accessory glands, both using the grayscale and color Doppler ultrasonography (CDUS). 7 Ultrasound and CDUS, although different methodologies, are generally performed together in the same examination to provide information about both the structure and the vascularization of the evaluated tract.
In particular, scrotal ultrasonography, associated with color Doppler ultrasonography (sCDUS), provides information about testicular localization, volume, parenchymal texture, intratesticular vascularization, presence of isolated or multiple calcifications, existence and grade of varicocoele, and presence of masses or cysts. Moreover, it can provide evidence on epididymal morphology and diameters and on the status of proximal portion of vas deferens. 8 In the same way, transrectal ultrasonography (TRUS) can reveal important details on prostatic volume, its parenchymal texture, vascularization, presence of masses or cysts, and on the status of ejaculatory ducts, distal vas deferens, and seminal vesicles. 9
In recent years, the ultrasonographic imaging, performed with progressively advanced instruments, allowed clinicians to reach a more accurate diagnosis in many fields of medicine, reducing the operator‐dependent bias implicit in this diagnostic tool and opening new therapeutic perspectives. 10 In addition, recently, the European Academy of Andrology(EAA) has promoted a multicenter study to assess the CDUS characteristics of healthy, fertile men providing, for the first time, normative parameters. 11 Despite this evidence, the US evaluation of the infertile male is still often considered as a second level diagnostic tool.
The aim of this study was to analyze the impact of US abnormalities in the diagnostic workflow of male infertility, namely, in predicting spermatogenesis impairment and/or male genital tract obstruction, and to correlate US abnormalities with seminal and hormonal parameters.
2. MATERIALS AND METHODS
2.1. Patients
A total of 1120 records from infertile men attending the infertility clinic of a tertiary‐care university hospital (Andrology and Reproductive Medicine Unit, University of Padua) between January 2016 and June 2020 were retrospectively evaluated. Data on physical examination, semen analysis, sperm culture, sCDUS and TRUS, sex hormones, and, when present, reports of Y‐ chromosome microdeletions, karyotype, and CFTR gene mutations were considered.
We included in the study records from infertile patients (unable to conceive for at least 12 months) with oligozoospermia (alone or associated with other semen abnormalities) or azoospermia and in which reports of sCDUS and TRUS semen analysis, semen culture, and hormonal assays were available. All exams had to be performed at our center in the same day. Exclusion criteria were as follows: (i) infection at semen culture or signs of acute abacterial prostatitis, (ii) hyperprolactinemia or hypogonadotropic hypogonadism, and (iii) genetic abnormalities. We excluded patients with previously known causes of oligozoospermia/azoospermia, caused by acute infections or genetic or hormonal diseases, thus including oligozoospermic/azoospermic patients in which ultrasound evaluation had been performed in the diagnostic workflow of male infertility to identify the infertility cause.
A total of 238 reports from oligozoospermic/azoospermic infertile patients (P) fulfilled the inclusion criteria and were considered for data analysis. Sixty‐three fertile subjects in which the female partner conceived within 12 months before the start of the study were used as controls (C).
2.2. Semen analysis
Semen samples had been performed according to WHO guidelines. 12 We considered for this study the following seminal parameters: volume, pH, sperm concentration, sperm motility, sperm morphology, and vitality.
Semen culture was considered negative in presence of uniform growth of less than 103 CFU/ml of pathogenic bacteria (including mycoplasmas and chlamydia) in seminal plasma and/or secretions obtained after prostatic massage.
2.3. Sex hormones
For the aim of this study, we retrospectively considered the following hormones: testosterone (T), luteinizing hormone (LH), and follicle‐stimulating hormone (FSH). T had been assayed in duplicate by RIA with the use of commercial kits by Radim (Radim, Pomezia, Italy). LH and FSH had been assayed by immunoradiometric methods on a solid‐phase (coated tube), based on a monoclonal double‐antibody technique. Moreover, free T was calculated according to the formula proposed by Vermeulen et al. 13 FSH was considered within the normal range if <8 U/L. 14 Patients were further subdivided into two groups (Pa and Pb) on the basis of normal or high FSH plasma level (<8 or ≥8, respectively).
2.4. Scrotal color Doppler ultrasonography
This procedure had been performed by the same experienced operator (P.P.), blinded to patient clinical status, using a Toshiba ultrasound machine (Toshiba Aplio XV), equipped with a linear multifrequency (5–12 MHz) probe. Testes echotexture and parenchymal vascular pattern, epididymal and proximal vas deferens patency, and spermatic cord status (including color and pulsed Doppler evaluation of pampiniform plexus) were evaluated as previously described. 15 Testicular volume had been calculated with the ellipsoid formula (Long × LL × AP × 0.52) and volume <12 ml was considered hypotrophic. Testicular parenchyma was defined as normal or inhomogeneous. In order to evaluate testicular vascularization, power Doppler was set at the minimum noise‐free pulse repetition frequency (PRF) level. US images had been recorded and Doppler spots were properly counted on records. Intraparenchymal vascularization was calculated for the first time counting the number of Doppler spots in each testis as seen at power Doppler in longitudinal scan. We considered only vascular spots far, at least, 3 mm or more from the tunica albuginea, thus excluding spots at the extreme periphery of the gonads. Moreover, we excluded transmediastinic artery if present. We considered for this study the number of the identified spots in the two testes (bitesticular vascularization), as reported in Figure 1.
FIGURE 1.
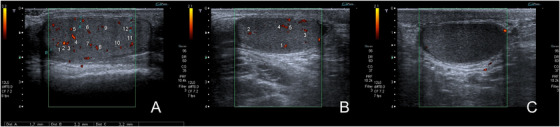
Examples of intraparenchymal vascularization, by counting the number of testicular spots, as seen at power Doppler in longitudinal scan. (A) Normal vascularization (number of spots = 12). (B) Reduced vascularization (number of spots = 5). (C) Absent vascularization (number of spots = 0)
The epididymis was considered abnormal in presence of dilations (at head, body, or tail level), cysts, congestion, and hyperemia.
Finally, we recorded the presence and the degree of varicocoele, classified by Sarteschi's scale, and considered the presence of hydrocele. 16
2.5. Transrectal color Doppler ultrasonography
This procedure had been performed by the same experienced operator (P.P.), blinded to patient clinical status, with the same Toshiba Aplio XV mentioned above, equipped with an “end‐fire” transrectal multifrequency (5–9 MHz) probe. Exam was performed with patient lying on his left side in fetal position. Prostatic gland echotexture and volume, presence of obstructions at ejaculatory ducts/ampullae/distal vas deferens level, signs of acute prostate–vesicular inflammation such as prostate central zone oedema, congestion and prostate calcifications according to previously reported criteria, 9 , 17 seminal vesicle dilation and congestion as showed by enlarged anteroposterior diameter in longitudinal scan, wall thickening, and endoluminal septa were evaluated.
2.6. Statistics
All statistical analyses were performed using SPSS software (Version 27, IBM, Segrate [MI], Italy). All data were first analyzed for normality of distribution using the Kolmogorov–Smirnov test of normality. The results were expressed as mean ± SD when normally distributed, or as median [quartiles] when non‐normally distributed. The differences between continuous variables were analyzed by ANOVA. The differences between discrete variables were analyzed by Chi‐square test or by Fisher test (if the expected count was <5). Values of p < 0.05 were considered statistically significant.
The Pearson correlation index or the Spearman correlation index for non‐normally distributed variables was used to describe the correlations between variables and to select the main independent variables to be used later in multivariate analyses. On the basis of correlation analyses, we then performed multilinear regression analyses to compare the most important predictors in determining sperm count. The inclusion and exclusion criteria were defined for p < 0.05 and p > 0.1, respectively.
Because concentration, total sperm count, and motility were strongly heterogeneous, these data were transformed into square root before analysis. Given the presence of multicollinearity, the highly correlated variables (i.e., sperm concentration, non‐motile spermatozoa) were removed. Ordinal regression analyses were performed to assess the impact of the increase in the number of prostatic or testicular sonographic alterations in determining the variables of interest. The Jonckheere–Terpstra Test was performed to calculate the p‐value for trend in the association between number of seminal changes and variables of interest.
Receiver Operating Characteristic (ROC) curves have been calculated by plotting sensitivity values on the y‐axis and 1‐sensitivity values on the x‐axis. The area under the curve (AUC) has been determined and evaluated by Sweets classification:
AUC = 0.5: test is not accurate;
0.5 < AUC ≤ 0.7: test is not enough accurate;
0.7 < AUC ≤ 0.9: test is enough accurate;
0.9 < AUC < 1.0: test is very accurate; and
AUC = 1: test is perfect.
The cutoff values for each considered variable have been calculated with Youden's S‐statistics.
3. RESULTS
The 238 infertile patients included in the study had a mean age of 37 ± 7 years, not significantly different from that of the 63 controls (36 ± 8 years, p = 0.45). Semen analysis of patients (P) showed: 81 oligozoospermic (34%), 50 azoospermic (21%), and 107 subjects with combined alterations (oligoasthenozoospermia, oligoteratozoospermia, and “oligoasthenoteratozoospermia) (45%). Seminal parameters in controls and patients are reported in Table 1.
TABLE 1.
Seminal parameters in controls and patients
| C group (n = 63) | P group (n = 238) | |
|---|---|---|
| Volume (ml) | 3.6 ± 1.6 | 2.7 ± 1.6* |
| pH | 7.5 ± 0.2 | 7.5 ± 0.4 |
| Sperm concentration (106/ml) | 51.9 ± 61.9 | 4.0 ± 8.4* |
| Total sperm count (106) | 184.1 ± 283.6 | 6.9 ± 10.0* |
| Total sperm motility (%) | 58.5 ± 12.6 | 32.2 ± 20.4* |
| Normal morphology (%) | 10.2 ± 5.5 | 6.6 ± 8.0* |
| Vitality | 79.5 ± 6.6 | 67.2 ± 19.3* |
Abbreviations: C, controls; P, Infertile pts.
p < 0.05.
Patients were subdivided into two groups: group Pa with normal FSH plasma levels (<8 U/L) and group Pb with FSH ≥ 8 U/L. In Table 2 are reported mean hormonal values and bitesticular volumes in the three groups. Pb had significantly lower bitesticular volume compared to Pa and C as expected.
TABLE 2.
Hormonal values, bitesticular volume and prevalence of varicocoele in fertile control subjects and in oligo/azoospermic patients divided on the basis of gonadotropin values
| C group (n = 63) | Pa group (n = 113) | Pb group (n = 125) | |
|---|---|---|---|
| Average FSH (U/L) | 3.9 ± 1.5 | 4.6 ± 1.7 | 12.3 ± 6.1* |
| Average LH (U/L) | 3.7 ± 1.4 | 4.2 ± 1.3 | 8.1 ± 3.8* |
| Average free testosterone (nmol/L) | 13.5 ± 4.8 | 13.8 ± 5.6 | 12.7 ± 5.1 |
| Average bitesticular volume (cc) | 33.6 ± 3.7 | 31.5 ± 4.0 | 24.0 ± 4.3* |
Abbreviations: C, controls; Pa, infertile pts with FSH < 8 U/L; Pb, infertile pts with FSH > 8 U/L.
p < 0.05 vs. C and vs. Pa
Considering patients as a whole, a higher prevalence of ultrasound abnormalities with respect to controls was found (Table 3). The total prevalence of US abnormalities was similar in Pa and Pb. In details, Pa had a higher prevalence of TRUS abnormalities (alone or associated to sCDUS alterations) than Pb (69.9% vs. 38.4%), whereas Pb had a higher prevalence of abnormalities at sCDUS (alone or associated to TRUS alterations) (60.0% vs. 28.3%, both p < 0.01).
TABLE 3.
Prevalence of different ultrasound abnormalities in fertile control subjects and in oligo/azoospermic patients subdivided on the basis of gonadotropin values
| C group (n = 63) | P group (n = 238) | Pa group (n = 113) | Pb group (n = 125) | |
|---|---|---|---|---|
| Ultrasound abnormalities | 28.5% (18) | 78.1% (186)* | 80.5% (91)* | 76.0% (95)* |
| sCDUS abnormalities | 12.7% (8) | 24.8% (59)° | 10.6% (12) | 37.6% (47) # |
| TRUS abnormalities | 9.5% (6) | 33.2% (79)* | 52.2% (59) § | 16.0% (20) |
| sCDUS + TRUS abnormalities | 6.3% (4) | 20.2% (48)* | 17.7% (20)° | 22.4% (28)* |
Abbreviations: C, controls; Pa, infertile pts with FSH < 8 U/L; Pb, infertile pts with FSH > 8 U/L.
p < 0.01 vs. C.
p < 0.05 vs. C.
p < 0.01 vs. C and Pb.
p < 0.01 vs. C and Pa.
Figure 2 reports the association between sperm count and the number of abnormalities recorded at sCDUS (Figure 2A; r = –0.176, p = 0.01) and TRUS (Figure 2B; r = –0.136, p < 0.05). A higher number of abnormalities at sCDUS or at TRUS was associated with a more severe reduction in total sperm count.
FIGURE 2.
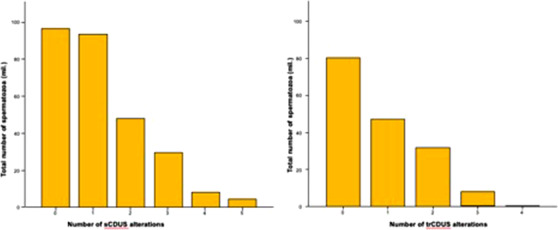
Association between sperm count (A) and number of sCDUS and TRUS alterations (B)
The prevalence of the different sCDUS abnormalities in fertile control subjects and in oligo/azoospermic patients, classified according to gonadotropin values, is shown in Table 4. Testicular hypotrophy and testicular inhomogeneity were more often observed in Pb group compared with Pa and C. Patients had a higher prevalence of sCDUS alterations at epididymis level with respect to controls.
TABLE 4.
Prevalence of different ultrasound abnormalities in fertile control subjects and in oligo/azoospermic patients subdivided on the basis of gonadotropin values
| C group (n = 63) | P group (n = 238) | Pa group (n = 113) | Pb group (n = 125) | |
|---|---|---|---|---|
| Testicular hypotrophy | 6.3% (4) | 49.2% (117)* | 9.7% (11) | 84.8% (106)* , # |
| Testicular inhomogeneity | 1.6% (1) | 35.7% (85)* | 7.1% (8) | 61.6% (77)* , # |
| Epididymis inhomogeneity or cists | 12.7% (8) | 25.2% (60) ° | 27.4% (31)* | 23.2% (29)* |
| Epididymis congestion/hyperemia | 1.6% (1) | 14.7% (35)* | 16.8% (19)* | 12.8% (16)* |
| Varicocoele | 7.9% (5) | 23.5% (56)* | 18.6% (21)* | 28% (35)* |
Abbreviations: C, controls; P, infertile pts; Pa, infertile pts with FSH < 8 U/L; Pb, infertile pts with FSH > 8 U/L.
p < 0.01 vs C.
p < 0.05 vs. C.
p < 0.05 vs. Pa.
Either Pa or Pb had significantly higher rates of varicocoele (18.6% and 28.0%, respectively) than group C (7.9%, both p < 0.05). Despite the prevalence of varicocoele was higher in Pb than Pa, the difference was not statistically different.
Figure 3 shows the relationship between bitesticular volume and the number of altered sperm parameters (r = –0.248, p < 0.001). Bitesticular volume, recorded at sCDUS, was inversely related to the number of abnormal seminal parameters. The sperm count was also inversely related with the bitesticular volume (Figure S1). ROC curve analysis showed that a bitesticular volume lower than 17 cc resulted as a cutoff value, associated with a higher risk of azoospermia (odds ratio = 1.799 [1.255–2.579]).
FIGURE 3.
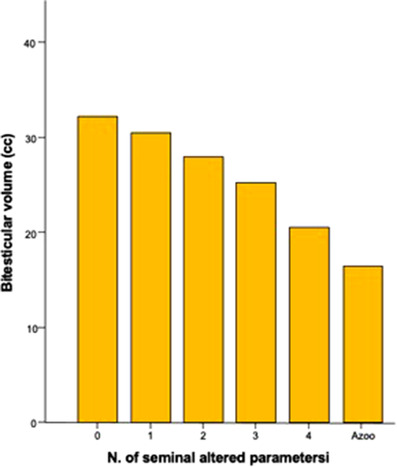
Association between bitesticular volume and increasing number of seminal changes found
Moreover, ROC analysis showed that the bitesticular volume evaluated at sCDUS was able to predict gonadotropin levels as well (Figure 4). The threshold value of bitesticular volume able to predict an increase in FSH levels was 26.1 cc, with an accuracy of 83.3% (95% CI: 78.4–88.2), sensitivity of 73.5% (95% CI: 73.4–81.6), and specificity of 75.8% (95% CI: 69.0–81.5).
FIGURE 4.
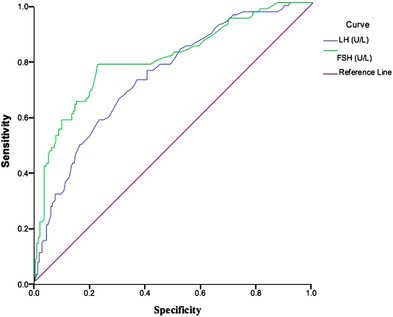
ROC curve for FSH and LH values that identify subjects with testicular hypotrophy
Finally, ROC analysis also indicated a tight association between testicular vascularization and both sperm count (Figure 5A) and FSH (Figure 5B). The AUC of ROC analysis for bitesticular vascularization was 0.619 (CI: 0.553–0.684) and 0.808 (CI: 0.757–0.859), respectively, pointing out a high accuracy of bitesticular vascularization in predicting sperm count (p < 0.001) and FSH levels (p < 0.0001). In particular, the presence of a number lower than 29 bitesticular vascular spots resulted as a cutoff value for reduced sperm count (<39 millions) and elevated FSH levels (≥8 mUI/ml) with a sensitivity of 74.2% (95% CI: 62.5–83.1) and 76.5% (95% CI: 66.7–84.3), specificity of 76.5% (95% CI: 69.1–86.2) and 74.2% (95% CI: 67.4–80.1), and accuracy of 61.9% (95% CI = 55.3–68.4) and 80.8% (95% CI = 75.7–85.9), respectively.
FIGURE 5.
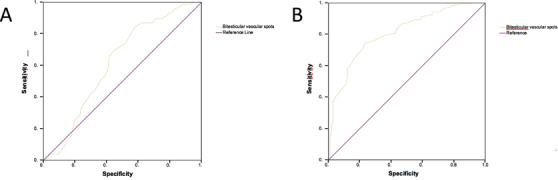
(A) ROC curve for bitesticular vascular spots that identify subjects with oligo/azoospermia. (B) ROC curve for bitesticular vascular spots values that identify subjects with FSH increased
Table 5 reports the association between the degree of bitesticular vascularization, observed in infertile patients versus sperm count, sperm concentration, gonadotropin levels, and bitesticular volume. In patients with reduced bitesticular vascularization (<29 vascular spots), sperm count and concentration were reduced (p < 0.01). Moreover, reduction of intratesticular vascularization was inversely associated with gonadotropin levels, whereas no difference was observed for T; however, in patients with reduced vascularization a trend toward T reduction was present.
TABLE 5.
Association between intratesticular vascularization and sperm count, hormones, and bitesticular volume
| Sperm count (mil.) | Sperm concentration (mil./ml) | LH (U/L) | FSH (U/L) | Free testosterone (nmol/L) | Bitesticular volume (cc) | |
|---|---|---|---|---|---|---|
| Normal vascularization (242) | 69.2 ± 11.3* | 22.4 ± 3.0* | 5.3 ± 2.7 # | 6.8 ± 5.2 # | 14.77 ± 2.4 | 29.8 ± 5.0 # |
| Reduced vascularization (59) | 9.1 ± 2.6 | 2.9 ± 0.7 | 7.2 ± 2.3 | 12.8 ± 4.0 | 13.22 ± 2.0 | 22.0 ± 5.0 |
p < 0.01 vs. reduced vascularization.
p < 0.05 vs. reduced vascularization.
The prevalence of the different TRUS abnormalities in fertile control subjects and in patients, subdivided on the basis of gonadotropin values, is reported in Table 6. A higher prevalence of prostate and seminal vesicle alterations was detected in patients when compared to controls. In particular, Pa, when compared with Pb, had a significantly higher prevalence of prostate inhomogeneity, hyperemia, seminal vesicle ectasia or inhomogeneity, and ectasia of the deferens ducts.
TABLE 6.
Prevalence of the different TRUS abnormalities in fertile control subjects and in oligo/azoospermic patients subdivided on the basis of gonadotropin values. Prostate hyperemia has been diagnosed in presence of areas of moderate increase in vascularity, as previously reported 9
| C group (n = 63) | P group (n = 238) | Pa group (n = 113) | Pb group (n = 125) | |
|---|---|---|---|---|
| Prostate hyperplasia | 6.3% (4) | 13.9% (33) | 17.7% (20)* | 10.4% (13) |
| Prostate inhomogeneity | 9.5% (6) | 34.4% (82)* | 41.6% (47)* | 28.0% (35)* , # |
| Prostate hyperemia | 1.6% (1) | 16.0% (38)* | 21.2% (24)* | 11.2% (14)* , # |
| Seminal vesicle ectasia or inhomogeneity | 3.2% (2) | 18.1% (43)* | 23.9% (27)* | 12.8% (16)* , # |
| Ectasia of deferens ducts | 0% (0) | 4.6% (11) | 8.0% (9)* | 1.6% (2) # |
Abbreviations: C, controls; P, infertile pts; Pa, infertile pts with FSH < 8 U/L; Pb, infertile pts with FSH > 8 U/L.
p < 0.05 vs. C.
p < 0.05 vs. Pa.
Collaterally, we performed the ROC analysis for gonadotropins and total sperm count observed in our population, demonstrating that LH is associated with alteration of the sperm count for values ≥5.65 U/L, whereas the threshold value for FSH is 5.35 (Figure S2).
4. DISCUSSION
Couple infertility remains a largely unresolved problem with an estimated 35% of idiopathic infertility. 18
Male factor has long been poorly studied and the normal semen parameters for male fertility have often been modified by the scientific community, 12 making it difficult to compare studies carried out at different times and in different settings. Among the many uncertainties existing in the field of diagnosis and treatment of male infertility, the role of ultrasound is still debated and generally it is considered as a supplementary tool.
In this manuscript, we have demonstrated the pivotal role of ultrasound in the diagnostics of male infertility. At first, bitesticular volume is strongly predictive of spermatogenic efficiency. Literature showed very few studies correlating testis volume evaluated by US with hormonal and seminal parameters. In 2008, Sakamoto et al. correlated US testis volume of 408 infertile men (816 testes) with seminal parameters and gonadotropins, showing a positive strong relationship between sperm density, total sperm count, and total motile sperm count, whereas a negative correlation was found between testis volume/semen parameters and gonadotropins. 19 Unlike this study, authors used Lambert formula (L × D × W × 0.71) to assess testis volume and infertile patients with various degrees of seminiferous tubular damage (i.e., cryptorchid patients) were excluded from the study. Nonetheless, their conclusions were similar to those presented here. More recently, Condorelli et al. demonstrated in a small group of 78 patients, using for the first time the 2010 WHO reference values for semen analysis and modern ultrasound technology, the association between low testicular volume (evaluated by ellipsoid formula) and both conventional and non‐conventional semen parameters. 20 Similar data were reported by other authors. 11 , 21 In the present retrospective study, we selected 238 oligozoospermic or azoospermic infertile patients and 63 proven fertile subjects. At the best of our knowledge, this study analyzes, for the first time in a big population of selected infertile patients, the correlation between US‐defined bitesticular volume and hormonal and seminal findings. As observed by Condorelli et al., we confirmed the direct correlation between bitesticular volume and sperm count and demonstrated for the first time that 17 cc represents a cutoff value associated with a higher risk of azoospermia. Unlike Condorelli et al., who did not report significant differences in FSH and LH levels among patients with normal and reduced testicular volume, we observed that a threshold value of 26.1 cc was able to predict a significant increase in gonadotropin levels. Despite this discrepancy between the studies might be explained by substantial differences in the studied population, further investigations are required to clarify this aspect.
There are very few studies in literature on parenchymal vascularization of testis in relation to fertility. Our group previously demonstrated that the testicular vascularization, evaluated by sCDUS, is able to predict tubular function, improving sperm recovery by TESE. 15 Recently Nowroozi et al. replicated our study in a larger cohort of patients, reaching the same conclusions. 22 However, in both studies, the analysis of intraparenchymal vascularization had been performed by roughly grouping testes into three categories according to testicular perfusion: grade 1 (no echo‐detectable vessel), grade 2 (one to three detectable vessels), grade 3 (three or more detectable vessels). In the present study, we made the effort to count the number of vascular parenchymal spots, thus analyzing vascularization as a continuous variable. Vascularization, quantified in this way, enabled us to predict the spermatogenic function. Indeed, the presence of <29.5 bitesticular vascular spots represents a cutoff value for the diagnosis of non‐obstructive oligozoospermia.
We moreover observed a higher prevalence of varicocoele in patients than in controls. A higher, although not significant, prevalence of varicocoele has been observed in Pb group. This is consistent with previous literature data confirming both the higher prevalence of varicocoele in infertile patients and the inhomogeneous increase of FSH values in patients with varicocoele, so that patients may have varicocoele and normal FSH values. 24 Furthermore, we found that TRUS abnormalities of prostate, seminal vesicles or deferent ducts are more frequent in oligozoospermic patients with normal FSH levels. The presence of obstructive/inflammatory signs at epididymis, prostate, seminal vesicles or deferent ducts might represent an ultrasound fingerprint of obstructive oligozoospermia, that may benefit from the appropriate treatments. We previously demonstrated that oligozoospermic patients benefit from prednisone treatment if an ultrasound pattern of prostate–vesicular inflammation is observed at TRUS. 10
In the present study, we also confirmed the strict relationship between FSH and tubular function. Although FSH < 8 U/L is generally accepted as reflecting a normal spermatogenesis, thus representing the cutoff for gonadotrophin treatment, 23 here we reported that even FSH levels higher than 5.35 U/L may reflect a mild alteration of spermatogenesis. Hence, we reported, for the first time, the existence of a possible “gray‐zone” of FSH values, between 5.35 and 8 UI/L in which FSH levels in the upper limits of the normal range might reflect an initial decrease in spermatogenic function.
Finally, we clearly showed the power of sCDUS and TRUS and their central role in the clinical workflow of male infertility. sCDUS and TRUS are today performed with more and more advanced instruments. Moreover, procedures have been recently standardized in a multicenter study supported by the EAA, 11 thus reducing the operator‐dependent bias. More recently, the prognostic value of sCDUS in the diagnostic evaluation of male infertility has been confirmed. Pozza et al. reported that the “testicular ultrasound score” (based on bitesticular volume and testicular asymmetry, parenchymal echotexture, echogenicity, and presence of microlithiasis, solid lesions, and varicocoele) predicts both spermatogenesis impairment and hypogonadism. 24 Despite these advancements and evidences, current guidelines still consider ultrasound as a supplementary diagnostic tool in the evaluation of the infertile male. According to the guidelines of American Society of Reproductive Medicine (ASRM) and the American Urology Association (AUA), “scrotal ultrasound is indicated in those patients in whom a physical examination of the scrotum is difficult or inadequate or in whom a testicular mass is suspected.” 25 Authors do not recommend, as a consequence, scrotal US as a routine evaluation in the diagnostic workflow in oligozoospermic infertile patients. In contrast, the European Academy of Andrology in its guidelines recommend scrotal US as a part of routine investigation, at least in men with oligoasthenoteratozoospermia. 26 Furthermore, the European Association of Urology (EAU) recommend only scrotal ultrasound in patients with infertility, in view of a higher risk of testis cancer. 27 Here, we reported the high diagnostic power of scrotal ultrasound in all oligozoospermic patients. In details, our data show that the US parameters—bitesticular volume and intratesticular vascularization—may predict sperm count and FSH plasma levels, and consequently spermatogenic function, in a population of infertile men (not only with oligoasthenoteratozoospermia). As a consequence, our evidence supports the idea that it is time for a revision of current guidelines, including sCDUS as a routine assessment in the evaluation of oligozoospermic infertile patients. Furthermore, we also found that TRUS abnormalities are frequent in patients with seminal alterations and namely with normal FSH levels. Similar results were previously reported by other authors. 28 , 29 , 30 Once again, ASRM, AUA, and EAU suggest TRUS for a small group of infertile patients and namely those with azoospermia or when a partial or complete distal obstruction is suspected. 25 , 31 On the other side, EAA suggests TRUSin patients with oligoasthenoteratozoospermia, low semen volume and/or significant semen parameters fluctuations, acidic pH, and low fructose. 26
In the present study, we confirmed the diagnostic role of TRUS in the routine investigation of the male partner. Therefore, TRUS represents a pivotal exam in every infertile man with altered sperm parameters. Data from this study clearly demonstrated that infertile patients with oligozoospermia and normal FSH plasma levels frequently have US abnormalities at sexual accessory glands. About 70% of patients in this population in fact showed US alterations at prostate or at distal seminal tract level. Therefore, when quantitative and/or qualitative sperm defects exist, TRUS can give useful diagnostic information and predict the therapeutic outcome.
The present study shows some limitations. Ultrasound procedures suffer from a well‐known problem related to the low reproducibility comparing different operators. However, this is a monocentric study, so that all patients have been evaluated by the same sonographer, thus reducing the variability of results. Furthermore, the detection of Doppler spots represents a new and still non‐standardized parameter that can be influenced by many factors, such as patient's characteristics, device, and operator, thus requiring more and more extensive studies for its validation.
Considering the ultrarapid development of US medical technology, increasingly up‐to‐date US devices are continuously available, which allow the vascular pattern of specific areas of interest to be assessed with ever greater precision, with or without the contrast‐enhanced US approach. Further perspective studies are therefore needed in future to investigate testicular vascularization with these new US applications and devices.
In conclusion, the present study demonstrated the great impact of US abnormalities in the diagnostic workflow of male infertility. US abnormalities are correlated with seminal and hormonal parameters and may guide the clinician in predicting spermatogenesis impairment and/or male genital tract obstruction, thus suggesting the infertility cause and guiding toward the most appropriate treatment.
Supporting information
SUPPORTING INFORMATION
SUPPORTING INFORMATION
Garolla A, Grande G, Palego P, et al., Central role of ultrasound in the evaluation of testicular function and genital tract obstruction in infertile males. Andrology. 2021;9:1490–1498. 10.1111/andr.13060
Giuseppe Grande and Pierfrancesco Palego are co‐first authors.
REFERENCES
- 1. Zegers‐Hochschild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393‐406. [DOI] [PubMed] [Google Scholar]
- 2. Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment‐seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22(6):1506‐1512. [DOI] [PubMed] [Google Scholar]
- 3. Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. H T, C K, RD O. Concepts in diagnosis and therapy for male reproductive impairment. Lancet Diabetes Endocrinol. 2017;5(7):554‐564. [DOI] [PubMed] [Google Scholar]
- 5. Ferlin A, Foresta C. Infertility: practical clinical issues for routine investigation of the male partner. J Clin Med. 2020;9(6):1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Milardi D, Grande G, Sacchini D, et al. Male fertility and reduction in semen parameters: a single tertiary‐care center experience. Int J Endocrinol. 2012;2012:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Updat. 2015;21(1):56‐83. [DOI] [PubMed] [Google Scholar]
- 8. Armstrong JM, Keihani S, Hotaling JM. Use of ultrasound in male infertility: appropriate selection of men for scrotal ultrasound. Curr Urol Rep. 2018;19(8):58. [DOI] [PubMed] [Google Scholar]
- 9. La Vignera S, Calogero AE, Condorelli RA, et al. Ultrasonographic evaluation of patients with male accessory gland infection. Andrologia. 2012;44:26‐31. [DOI] [PubMed] [Google Scholar]
- 10. Milardi D, Luca G, Grande G, et al. Prednisone treatment in infertile patients with oligozoospermia and accessory gland inflammatory alterations. Andrology. 2017;5(2):268‐273. [DOI] [PubMed] [Google Scholar]
- 11. Lotti F, Frizza F, Balercia G, et al. The European Academy of Andrology (EAA) ultrasound study on healthy, fertile men: clinical, seminal and biochemical characteristics. Andrology. 2020;8(5):1005‐1020. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization (WHO) . WHO laboratory manual for the examination and processing of human semen. Geneva, Switzerland: WHO; 2016. http://www.who.int/reproductivehealth/publications/infertility/9789241547789/en/. Accessed September 12, 2017. [Google Scholar]
- 13. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666‐3672. [DOI] [PubMed] [Google Scholar]
- 14. Casamonti E, Vinci S, Serra E, et al. Short‐term FSH treatment and sperm maturation: a prospective study in idiopathic infertile men. Andrology. 2017;5(3):414‐422. [DOI] [PubMed] [Google Scholar]
- 15. Foresta C, Garolla A, Bettella A, Ferlin A, Rossato M, Candiani F. Doppler ultrasound of the testis in azoospermic subjects as a parameter of testicular function. Hum Reprod. 1998;13(11):3090‐3093. [DOI] [PubMed] [Google Scholar]
- 16. Sarteschi LM. Lo studio del varicocele con eco‐color doppler. G Ital Ultrason. 1993;4:43‐49. [Google Scholar]
- 17. Dellabella M, Milanese G, Muzzonigro G. Correlation between ultrasound alterations of the preprostatic sphincter and symptoms in patients with chronic prostatitis‐chronic pelvic pain syndrome. J Urol. 2006;176(1):112‐118. [DOI] [PubMed] [Google Scholar]
- 18. Eurostat . Fertility statistics ‐ statistics explained. https://ec.europa.eu/eurostat/statistics‐explained/index.php/Fertility_statistics. Accessed August 5, 2020.
- 19. Sakamoto H, Yajima T, Nagata M, Okumura T, Suzuki K, Ogawa Y. Relationship between testicular size by ultrasonography and testicular function: measurement of testicular length, width, and depth in patients with infertility. Int J Urol. 2008;15(6):529‐533. [DOI] [PubMed] [Google Scholar]
- 20. Condorelli R, Calogero AE, La Vignera S. Relationship between testicular volume and conventional or nonconventional sperm parameters. Int J Endocrinol. 2013;2013:145792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lotti F, Corona G, Castellini G, et al. Semen quality impairment is associated with sexual dysfunction according to its severity. Hum Reprod. 2016;31(12):2668‐2680. [DOI] [PubMed] [Google Scholar]
- 22. Nowroozi MR, Ayati M, Amini E, et al. Assessment of testicular perfusion prior to sperm extraction predicts success rate and decreases the number of required biopsies in patients with non‐obstructive azoospermia. Int Urol Nephrol. 2015;47(1):53‐58. [DOI] [PubMed] [Google Scholar]
- 23. Isidori AM, Pozza C, Gianfrilli D, Isidori A. Medical treatment to improve sperm quality. Reprod Biomed Online. 2006;12(6):704‐714. http://www.ncbi.nlm.nih.gov/pubmed/16792845. Accessed August 18, 2017. [DOI] [PubMed] [Google Scholar]
- 24. Pozza C, Kanakis G, Carlomagno F, et al. Testicular ultrasound score: a new proposal for a scoring system to predict testicular function. Andrology. 2020;8(5):1051‐1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Male Infertility Best Practice Policy Committee of the American Urological Association; Practice Committee of the American Society for Reproductive Medicine . Report on optimal evaluation of the infertile male. Fertil Steril. 2004;82(Suppl 1):S123‐S130. 10.1016/j.fertnstert.2004.05.058. [DOI] [PubMed] [Google Scholar]
- 26. Colpi GM, Francavilla S, Haidl G, et al. European Academy of Andrology guideline Management of oligo‐astheno‐teratozoospermia. Andrology. 2018;6(4):513‐524. [DOI] [PubMed] [Google Scholar]
- 27. Jungwirth A, Giwercman A, Tournaye H, et al. European Association of Urology guidelines on Male Infertility: the 2012 update. Eur Urol. 2012;62(2):324‐332. [DOI] [PubMed] [Google Scholar]
- 28. Lotti F, Corona G, Cocci A, et al. The prevalence of midline prostatic cysts and the relationship between cyst size and semen parameters among infertile and fertile men. Hum Reprod. 2018;33(11):2023‐2034. [DOI] [PubMed] [Google Scholar]
- 29. Lotti F, Corona G, Colpi GM, et al. Seminal vesicles ultrasound features in a cohort of infertility patients. Hum Reprod. 2012;27(4):974‐982. [DOI] [PubMed] [Google Scholar]
- 30. Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update. 2015;21(1):56‐83. [DOI] [PubMed] [Google Scholar]
- 31. Jungwirth A, Diemer T, Dohle GR, Kopa Z, Krausz C, Tournaye H. EAU guidelines on male infertility. Eur Urol Eur Urol Eur Urol. 2004;466162(512):555‐8159. http://uroweb.org/wp‐content/uploads/EAU‐Guidelines‐Male‐Infertility‐2016‐pocket.pdf. Accessed June 18, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
SUPPORTING INFORMATION


