Abstract
Overcrowded alkenes are expeditiously prepared by the versatile Barton–Kellogg olefination and have remarkable applications as functional molecules owing to their unique stereochemical features. The induced stereodynamics thereby enable the controlled motion of molecular switches and motors, while the high configurational stability prevents undesired isomeric scrambling. Bistricyclic aromatic enes are prototypical overcrowded alkenes with outstanding stereochemical properties, but their stereocontrolled preparation was thus far only feasible in stereospecific reactions and with chiral auxiliaries. Herein we report that direct catalyst control is achieved by a stereoselective Barton–Kellogg olefination with enantio‐ and diastereocontrol for various bistricyclic aromatic enes. Using Rh2(S‐PTAD)4 as catalyst, several diazo compounds were selectively coupled with a thioketone to give one of the four anti‐folded overcrowded alkene stereoisomers upon reduction. Complete stereodivergence was reached by catalyst control in combination with distinct thiirane reductions to provide all four stereoisomers with e.r. values of up to 99:1. We envision that this strategy will enable the synthesis of topologically unique overcrowded alkenes for functional materials, catalysis, energy‐ and electron transfer, and bioactive compounds.
Keywords: Barton–Kellogg olefination, higher-order stereogenicity, overcrowded alkenes, stereodivergent catalysis, stereoselective catalysis
An unprecedented thiiranation controlled by chiral dirhodium catalysts combined with a highly stereospecific thiirane reduction render the versatile Barton–Kellogg olefination a stereoselective process (see scheme). The fourfold stereogenicity of pertinent overcrowded alkenes was thereby tractable with divergent stereocontrol, thus enabling access to all four stereoisomers from identical substrates.
Introduction
The eminent Feringa motors and bistricyclic aromatic enes are overcrowded alkenes that typically adopt a characteristic anti‐folded geometry upon complementary pyramidalization of the alkene carbons (Figure 1 A).[ 1 , 2 , 3 ] Since the discovery of overcrowded alkenes,[ 4 , 5 ] the stereoisomerism and stereodynamics of bistricyclic aromatic enes and related systems have been studied in great detail.[ 3 , 6 , 7 ] When unsymmetrically disubstituted, the anti‐folded structures exist as four stereoisomers in the form of two enantiomeric pairs of diastereomers (Figure 1 B). Intriguingly, this isomerism results from the different alkene geometries (E or Z) together with their helicity (enantiomeric M or P) within an irreducible stereogenic unit characterized by its fourfold stereogenicity. [8] In their pioneering work, Wynberg and Feringa uncovered that such anti‐folded overcrowded alkenes exhibit particularly high configurational stability and that stereoisomer separation is feasible. [9] Because of their well‐defined topology, the high thermal racemization barriers and the possibility to induce stereospecific isomerization by photochemical stimuli, they have been utilized as molecular switches and catalysts.[ 10 , 11 ] Enantiomerically enriched overcrowded alkenes were classically obtained by HPLC separation[ 12 , 13 , 14 ] of a racemate prepared by a Barton–Kellogg olefination.[ 15 , 16 , 17 ] When comprising stereocenters for instance in the form of a chiral auxiliary, the diastereoselective assembly of overcrowded alkenes provides an indirect stereospecific route to enantioenriched products by a range of synthetic methods, including the McMurry coupling and cascade cyclizations besides the prevalent Barton–Kellogg olefination (Figure 1 C).[ 18 , 19 , 20 , 21 , 22 ] Indirect catalyst control was observed by Tanaka and co‐workers in a rhodium‐catalyzed cascade reaction with selectivity for the configuration of a stereocenter (Figure 1 D). [23] Direct catalyst control over two stereoisomers was recently achieved by the Miller group in a dynamic kinetic resolution of a stereodynamic precursor by a peptide‐catalyzed N‐oxidation (Figure 1 E). [24] Interestingly, the catalyst‐controlled stereoselective synthesis of bistricyclic aromatic enes by the particularly effective Barton–Kellogg olefination has, to the best of our knowledge, not yet been described.
Figure 1.
Background and conceptualization.
The Barton–Kellogg olefination essentially involves the coupling of diazo compounds with thioketones—typically in an uncatalyzed reaction—followed by the reduction of the resulting thiirane intermediate.[ 25 , 26 ] Interestingly, a few reported examples indicated that the formation of Rh‐carbenoids with Rh2(OAc)4 facilitates challenging Barton–Kellogg olefinations.[ 27 , 28 , 29 , 30 ] Related to the formation of the thiirane intermediate, an analogous Rh2(OAc)4‐catalyzed epoxidation was reported by Doyle and co‐workers (Figure 1 F). [31] Motivated by our interest in catalyst control over higher‐order stereogenicity and the addressability of specific states of halted molecular motors,[ 32 , 33 ] we thus considered the feasibility of stereocontrol with the chiral dirhodium tetracarboxylate catalysts designed by the Davies group.[ 34 , 35 , 36 ] We specifically envisioned the possibility of an unprecedented stereoselective rhodium‐catalyzed thiiranation[ 37 , 38 , 39 ] combined with a stereospecific reduction for a stereoselective Barton–Kellogg olefination. Our study was furthermore encouraged by the notable prospects for stereodivergent control to provide all four stereoisomers of anti‐folded overcrowded alkenes from the same diazo‐ and thioketone substrates (Figure 1 H). If successful, this method would circumvent tedious separations of enantiomers, resolutions or auxiliary methods to provide a most direct, general and stereoselective assembly of valuable overcrowded alkenes. We now disclose that the stereoselective Barton–Kellogg olefination is viable with rhodium catalysis and that a striking stereodivergence for pertinent overcrowded alkenes with fourfold stereogenicity is tractable.
Results and Discussion
We approached our first aim to achieve catalyst control in a stereoselective Barton–Kellogg olefination by developing an efficient synthesis of monosubstituted diazo substrates 2 either by direct assembly or by diversification using cross‐coupling reactions (see the Supporting Information for details). Thioketone substrate 1 was subsequently coupled with the diazo compound 2 a activated by dirhodium catalysts, where the outcome of the thiirane formation provided first insights into the stereoselectivity of the reactions (Table S1 in the Supporting Information). Different reductants [40] to convert the thiiranes to overcrowded alkenes were tested and the treatment with P(NMe2)3 in toluene was thereby identified as ideal protocol. Whilst full conversion was achieved with P(NMe2)3 in 3 h with excellent stereospecificity, the reduction with PPh3 required long reaction times and led to loss of stereoisomeric enrichment (see the Supporting Information for the optimization of the reduction). The lack of diastereoselectivity in substrate‐controlled reactions was also confirmed with Rh2(OAc)4 (C1) as catalyst, providing a 1:1 mixture of the racemic E‐ and Z‐products (Figure 2, entry 1). In striking contrast, Rh2(R‐DOSP)4 (C2) showed a 1:1.7 d.r. for the Z‐isomer with excellent enantioselectivity (94:6 e.r.) and a 97 % yield at a catalyst loading of 5 mol % (entry 2). Furthermore, deconstructing the processes by the analysis of the thiirane intermediate indicated that an identical level of stereoselectivity is already reached in the coupling step, rendering the thiirane reduction exceptionally stereospecific. Interestingly, Rh2(S‐PTAD)4 (C3) provided an increased d.r. of 2.6:1 for the E‐diastereomer with a 98:2 e.r. for the major overcrowded alkene diastereomer (entry 3, 95:5 e.r. for the minor Z‐diastereomer). In comparison, the tetrachlorinated catalyst Rh2(S‐TCPTAD)4 (C4) led to substantially lower stereocontrol (entry 4) and also dirhodium cyclopropane carboxylate catalysts C5 and C6 provided inferior selectivities (see the Supporting Information). Interestingly, Rh2(S‐PTAD)4 (C3) was previously also identified as optimal catalyst for the stereoselective cyclopropanation of styrenes with diaryldiazomethanes that bear donor and acceptor substituents to induce a differentiated alignment of the two aryl groups with respect to the rhodium carbene. [41] Using the catalyst C3, an increase of the temperature from −78 °C to room temperature was found to strongly reduce stereoselectivity (see the Supporting Information). The coupling also showed a strong solvent effect and reactions in CH2Cl2 or THF both resulted in lower d.r. and e.r. values (entries 5 and 6), confirming that toluene is an ideal solvent for the catalyst‐controlled stereoselective thiiranation. More dilute reactions led to a moderate improvement of diastereocontrol (entry 7). Moreover, the excellent stereospecificity and yield of the reduction remained when performed at 60 °C, which allowed to further enhance the mild conditions of the method. Gratifyingly, the catalyst loading was successfully decreased to 2.5 mol % without loss of stereocontrol, providing 3 a in a d.r. of 2.7:1 and an e.r. of 99:1 for the major E‐diastereomer (entry 8, 95:5 for the minor Z‐isomer). When the catalyst loading was further reduced to 1 mol %, the selectivity was marginally compromised to an e.r. of 97:3 (entry 9) and the remainder of the study was therefore carried out with 2.5 mol % dirhodium catalysts.
Figure 2.
Optimization of the catalyst‐controlled stereoselective Barton–Kellogg olefination. [a] Reaction conditions: 1 (50.0 μmol, 1.0 equiv), 2 a (1.5 equiv), Rh2L4, solvent, −78 °C, 3 h, then P(NMe2)3 (50 equiv), toluene, 80 °C or 60 °C. [b] Determined for the crude product by 1H NMR spectroscopy and confirmed by HPLC on a chiral stationary phase. [c] Determined for the isolated product by HPLC on a chiral stationary phase. [d] Yield over both steps for the isolated product. [e] Reduction at 80 °C. [f] Reduction at 60 °C.
Having identified the optimal conditions for the stereoselective catalyst‐controlled Barton–Kellogg olefination (Figure 2, entry 8), the scope and limitations of the method were explored (Figure 3). The reaction scale was successfully increased from 50.0 μmol to 100 μmol without affecting selectivity, providing 4 a in 95 % yield with 2.7:1 d.r. and 98:2 e.r. for the major E‐diastereomer. X‐ray crystallographic analysis of a single crystal confirmed the anti‐folded geometry and established the absolute configuration as (P‐E)‐4 a, thus revealing a catalyst‐controlled selectivity towards P‐helicity. We next varied the dimethoxyphenyl moiety to probe the impact of electronic and steric alterations on the diazo building block. Interestingly, the rather small change in 4 b led to a dramatic loss of stereoselectivity and also the di‐ortho‐methoxy‐substituted 4 c initially resulted in lower enantiocontrol, thus challenging the breadth of the method. Nonetheless, the catalyst Rh2(R‐BTPCP)4 (C5) restored selectivity and provided 4 c in 95 % yield in 4:1 d.r. with 89:11 e.r. for the major E‐isomer. Since the ortho‐ and para‐ methoxy substituents exert a strong electronic influence, this higher stereoselectivity using C5 as complementary catalyst was likely observed due to the rigidity of the 2,6‐substituted diazo substrate 2 c. Moreover, X‐ray crystallographic analysis confirmed that with Rh2(R‐BTPCP)4, the (M‐E)‐4 c‐configured product is formed. In contrast, we were pleased to find that the remainder of the scope studies could be performed with the Rh2(S‐PTAD)4 catalyst (C3) under the previously optimized conditions, thus confirming the generality of the catalyst‐controlled Barton–Kellogg olefination. The dichloroaryl moiety in 4 d led to a notable enantioselectivity of 96:4 (E) and 95:5 (Z) when using catalyst C3. While 4‐fluorophenyl substitution in 4 e resulted in a lower level of stereocontrol, excellent results were achieved with 3,4,5‐trifluorophenyl‐substituted 4 f which was obtained with 99:1 e.r. for both the E‐ and Z‐isomers. Conversely, the phenyl‐substituted overcrowded alkene 4 g was obtained in 90 % yield with lower enantiocontrol. Having evaluated the electronic and steric effects of various aryl substituents, sterically less‐demanding substituents were examined. Remarkably, a chloro substituent was found to already ensure ample differentiation to provide bistricyclic aromatic ene 4 h with 1.8:1 d.r. and 90:10 e.r. for the major E‐diastereomer. Furthermore, in contrast to previous substrates, a pronounced difference of enantioenrichment of the E‐ and Z‐diastereomers was observed. Similar enantioselectivity and an improved diastereoselectivity was established when changing from chlorine to bromine substitution in 4 i where a 2.4:1 d.r. was measured. To our delight, also a strongly electron‐withdrawing functionality was amenable and nitro‐substituted 4 j showed excellent enantioselectivity (99:1 e.r.) for the major P‐E‐diastereomer. Methyl‐substituted overcrowded alkene (P‐E)‐4 k, which has been reported as potential molecular switch, [7] was also obtained with 91:9 e.r. and a d.r. of 1.3:1. Notably, 4 l with an electron‐withdrawing CF3 group was accessible with similar enantioselectivity and an improved diastereomeric ratio. To systematically explore the scope and limitations of the method, also the most challenging disubstituted diazothioxanthene substrates 2 m and 2 n were employed in the stereoselective Barton–Kellogg olefination. While fluoro‐dimethoxyphenyl substituted 4 m was obtained with a valuable level of enantiocontrol (88:12 e.r.), the reaction to brominated 4 n was less selective, indicating the boundaries of enantioselectivity of the method. Consistent with the stereoselectivity for acyclic rhodium diarylcarbenes,[ 41 , 42 , 43 ] electronic effects of the substituents may impact stereocontrol by differentiating the torsion angles between the aryl groups and the rhodium carbene, albeit to a reduced extent within the cyclic structures compared to open analogues. Altogether, the comprehensive scope underscores the capacity of chiral dirhodium tetracarboxylate catalysts for diazo–thioketone couplings through Rh‐carbenoids by the unique stereoselective thiiranation, whereas the breadth of the approach for distinct bistricyclic aromatic ene stereoisomers is highlighted by the remarkable stereospecificity of the thiirane reduction.
Figure 3.
Scope and limitations of the catalyst‐controlled stereoselective Barton–Kellogg olefination. [a] Reaction conditions: 1) 1 (100 μmol, 1.0 equiv), 2 (1.5 equiv), C3 (2.5 mol %), toluene (100 mL), −78 °C, 3–5 h; 2) P(NMe2)3 (50 equiv), toluene (10 mL), 60 °C, 18 h. Yields are for the isolated product after both steps. The d.r. values were measured by 1H NMR spectroscopy for the crude product. The e.r. values were determined for the isolated product by HPLC on a chiral stationary phase. [b] The reaction was carried out with catalyst C5. [c] The reaction was carried out with 1.0 equivalent of 2 f.
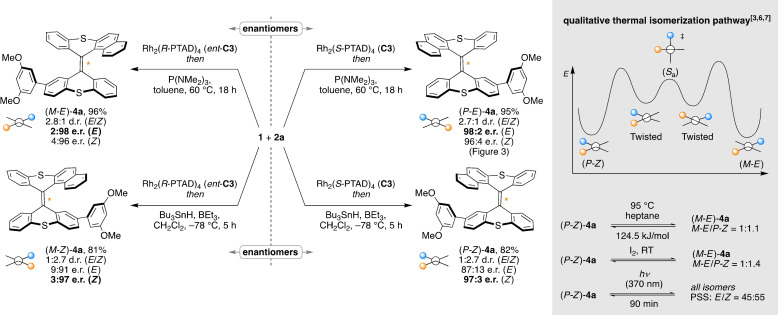
To additionally explore the stereochemical versatility of the developed stereoselective Barton–Kellogg olefination, we also aimed at stereodivergent control over all four stereoisomers of the overcrowded alkenes (Figure 4). The opposite enantiomeric pathway was expediently confirmed by using the Rh2(R‐PTAD)4 catalyst (ent‐C3), providing (M‐E)‐4 a with the expected inverted enantioselectivity of 2:98. For the challenging redirection of diastereoselectivity, we investigated an olefin formation by means of a radical thiirane reduction. However, while the desulfurization of thiiranes to alkenes with Bu3SnH and BEt3 as initiator is known as particularly mild methodology, [44] it has to the best of our knowledge not been described for the synthesis of overcrowded alkenes. Nonetheless, an intriguing reversal of diastereoselectivity and an excellent stereospecificity were found when employing the Bu3SnH/BEt3 system. Under optimized conditions, the diazo–thioketone coupling of 1 and 2 a with Rh2(S‐PTAD)4 (C3) followed by a radical thiirane reduction in CH2Cl2 at −78 °C thereby provided the Z‐configured overcrowded alkene (P‐Z)‐4 a in 1:2.7 d.r. with an e.r. of 97:3. To also obtain the fourth stereoisomer, the enantioselectivity was inverted by using the Rh2(R‐PTAD)4 catalyst (ent‐C3) in combination with the radical Bu3SnH/BEt3 thiirane reduction, giving (M‐Z)‐4 a in 81 % yield with 3:97 e.r. for the major Z‐diastereomer and 9:91 e.r. for the minor E‐diastereomer.
Figure 4.
Stereodivergent catalytic Barton–Kellogg olefination and isomerization studies. Yields are for the isolated product after both steps.
At ambient temperature, none of the alkenes prepared in our studies showed a noticeable tendency for stereoisomerization, thus underscoring the extraordinary configurational stability of the investigated overcrowded alkenes. To determine the isomerization barriers, a sample of nearly enantiopure (P‐Z)‐4 a (>99:1 e.r.; 95:5 d.r.) in heptane was heated to 95 °C, resulting in a stereospecific isomerization to (M‐E)‐4 a.[ 6 , 7 ] The process followed first‐order kinetics with ΔG ≠ 95 °C=124.5 kJ mol−1, reaching an equilibrium state with a (P‐Z)‐4 a/(M‐E)‐4 a ratio of 1.1:1. Similar P‐Z/M‐E isomerization was observed at room temperature under ambient light in the presence of iodine within 9 h. In contrast, the irradiation of a sample of (P‐Z)‐4 a with a UV‐A LED (370 nm, 43 W) resulted in complete isomerization between all four stereoisomers within 90 min, leading to a photostationary state (PSS) of E/Z=45:55. With the distinct stereoisomerization processes established, we conclusively anticipate the integration of the defined overcrowded alkene stereoisomers as switchable moieties or tractable functional units for the design of molecular machinery. Having functional elements with defined starting positions controlled by stereoselective catalysis, interactions during assembly become adjustable, the analytics comprehensible and the particularly well‐defined topology is expected to facilitate the construction of advanced functional molecules and materials.
Conclusion
The catalyst‐controlled stereoselective Barton–Kellogg olefination for the high‐yielding synthesis of overcrowded alkenes with fourfold stereogenicity was developed. The reaction provides access to distinct anti‐folded bistricyclic aromatic enes and features a combination of a highly efficient thiiranation using the Davies’ Rh2(S‐PTAD)4 catalyst and an exceptionally stereospecific thiirane reduction. The scope of the method also comprises the possibility for divergent stereocontrol, rendering all four stereoisomers accessible from the same substrates. Based on the generality of the Barton–Kellogg olefination and the viability of forming the central C=C double bond of the overcrowded alkene in a direct catalyst‐controlled stereoselective coupling, we envision that the rational design and synthesis of various catalyst structures, bioactive compounds and functional molecular scaffolds are feasible with the method presented herein.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Acknowledgements
Financial support from the Swiss National Science Foundation (155902 and 175746), the University of Basel, and the NCCR Molecular Systems Engineering is acknowledged (182895). This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No. 101002471). We thank Dr. Alessandro Prescimone for X‐ray crystallography and Andreas Ostertag for support in the scale‐up synthesis of substrates. Open access funding provided by University of Basel.
T. A. Schmidt, C. Sparr, Angew. Chem. Int. Ed. 2021, 60, 23911.
Contributor Information
Tanno A. Schmidt, http://sparr.chemie.unibas.ch
Prof. Dr. Christof Sparr, Email: christof.sparr@unibas.ch.
References
- 1. Koumura N., Zijlstra R., van Delden R., Harada N., Feringa B. L., Nature 1999, 401, 152–155. [DOI] [PubMed] [Google Scholar]
- 2. Kassem S., van Leeuwen T., Lubbe A. S., Wilson M. R., Feringa B. L., Leigh D., Chem. Soc. Rev. 2017, 46, 2592–2621. [DOI] [PubMed] [Google Scholar]
- 3. Biedermann P. U., Agranat I., Top. Curr. Chem. 2014, 350, 177–277. [DOI] [PubMed] [Google Scholar]
- 4. Bell F., Waring D. H., J. Chem. Soc. 1949, 2689–2693. [Google Scholar]
- 5. Harnik E., Herbstein F. H., Schmidt G. M. J., Nature 1951, 168, 158–160. [Google Scholar]
- 6. Agranat I., Tapuhi Y., J. Am. Chem. Soc. 1978, 100, 5604–5609. [Google Scholar]
- 7. Feringa B. L., Jager W. F., de Lange B., J. Chem. Soc. Chem. Commun. 1993, 288–290. [Google Scholar]
- 8.The stereogenicity of (irreducible) stereogenic units allows the prediction of the number of stereoisomers according to an extended Le Bel–Van ’t Hoff rule, as: s1 * s2 ,… (s: stereogenicity, n: number of stereogenic units with the specific stereogenicity). See compound 2 e in Ref. [32] for an example with six- and twofold stereogenicity.
- 9. Feringa B., Wynberg H., J. Am. Chem. Soc. 1977, 99, 602–603. [Google Scholar]
- 10. Feringa B. L., van Delden R. A., Koumura N., Geertsema E. M., Chem. Rev. 2000, 100, 1789–1816. [DOI] [PubMed] [Google Scholar]
- 11. Chen C.-T., Tsai C.-C., Tsou P.-K., Huang G.-T., Yu C.-H., Chem. Sci. 2017, 8, 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feringa B. L., Jager W. F., de Lange B., Tetrahedron Lett. 1992, 33, 2887–2890. [Google Scholar]
- 13. Jager W. F., de Lange B., Schoevaars A. M., van Bolhuis F., Feringa B. L., Tetrahedron: Asymmetry 1993, 4, 1481–1497. [Google Scholar]
- 14.Evident enantioenrichment (ee<2 %) was observed upon circular polarized light irradiation: Huck N. P. M., Jager W. F., de Lange B., Feringa B. L., Science 1996, 273, 1686–1168. [Google Scholar]
- 15. Staudinger H., Siegwart J., Helv. Chim. Acta 1920, 3, 833–840. [Google Scholar]
- 16. Barton D. H. R., Willis B. J., J. Chem. Soc. Perkin Trans. 1 1972, 305–310. [Google Scholar]
- 17. Buter J., Wassenaar S., Kellogg R. M., J. Org. Chem. 1972, 37, 4045–4060. [Google Scholar]
- 18. Geertsema E. M., Meetsma A., Feringa B. L., Angew. Chem. Int. Ed. 1999, 38, 2738–2741; [PubMed] [Google Scholar]; Angew. Chem. 1999, 111, 2902–2905. [Google Scholar]
- 19. Chen C.-T., Chou Y. C., J. Am. Chem. Soc. 2000, 122, 7662–7672. [Google Scholar]
- 20. Tietze L. F., Düfert A., Lotz F., Sölter L., Oum K., Lenzer T., Beck T., Herbst-Irmer R., J. Am. Chem. Soc. 2009, 131, 17879–17884. [DOI] [PubMed] [Google Scholar]
- 21. Chen W.-C., Lee Y.-W., Chen C.-T., Org. Lett. 2010, 12, 1472–1475. [DOI] [PubMed] [Google Scholar]
- 22. Liu H., El-Salfiti M., Lautens M., Angew. Chem. Int. Ed. 2012, 51, 9846–9850; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 9984–9988. [Google Scholar]
- 23. Hojo D., Noguchi K., Tanaka K., Angew. Chem. Int. Ed. 2009, 48, 8129–8132; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 8273–8276. [DOI] [PubMed] [Google Scholar]
- 24. Stone E. A., Cutrona K. J., Miller S. J., J. Am. Chem. Soc. 2020, 142, 12690–12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burns J. M., Clark T., Williams C. M., J. Org. Chem. 2021, 86, 7515–7528. [DOI] [PubMed] [Google Scholar]
- 26. Querol M., Stoekli-Evans H., Belser P., Org. Lett. 2002, 4, 1067–1070. [DOI] [PubMed] [Google Scholar]
- 27. Takano S., Tomita S. i., Takahashi M., Ogasawara K., Synthesis 1987, 1116–1117. [Google Scholar]
- 28. Kim G., Chu-Moyer M. Y., Danishefsky S. J., Schulte G. K., J. Am. Chem. Soc. 1993, 115, 30–39. [Google Scholar]
- 29. Mlostón G., Heimgartner H., Helv. Chim. Acta 2014, 79, 1785–1792. [Google Scholar]
- 30. Yang M., Li J., Li A., Nat. Commun. 2015, 6, 6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Doyle M. P., Hu W., Timmons D. J., Org. Lett. 2001, 3, 933–935. [DOI] [PubMed] [Google Scholar]
- 32. Wu X., Witzig R. M., Beaud R., Fischer C., Häussinger D., Sparr C., Nat. Catal. 2021, 4, 457–462. [Google Scholar]
- 33. Schmidt T. A., Sparr C., Acc. Chem. Res. 2021, 54, 2764–2774. [DOI] [PubMed] [Google Scholar]
- 34. Davies H. M. L., Bruzinski P. R., Lake D. H., Kong N., Fall M. J., J. Am. Chem. Soc. 1996, 118, 6897–6907. [Google Scholar]
- 35. Hansen J., Davies H. M. L., Coord. Chem. Rev. 2008, 252, 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reddy R. P., Lee G. H., Davies H. M. L., Org. Lett. 2006, 8, 3437–3440. [DOI] [PubMed] [Google Scholar]
- 37.For examples of diastereoselective thiiranation (episulfidation), see Refs. [38, 39].
- 38. Zhou C., Fu C., Ma S., Angew. Chem. Int. Ed. 2007, 46, 4379–4381; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 4457–4459. [Google Scholar]
- 39. Cano I., Gómez-Bengoa E., Landa A., Maestro M., Mielgo A., Olaizola I., Oiarbide M., Palomo C., Angew. Chem. Int. Ed. 2012, 51, 10856–10860; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 11014–11018. [Google Scholar]
- 40. Larock R. C., Zeni G., Compr. Org. Transform. 2018, 34. [Google Scholar]
- 41. Lee M., Musaev D. G., Davies H. M. L., ACS Catal. 2020, 10, 6240–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bergstrom B. D., Nickerson L. A., Shaw J. T., Souza L. W., Angew. Chem. Int. Ed. 2021, 60, 6864–6878; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 6940–6954. [Google Scholar]
- 43. Zhu D., Chen L., Fan H., Yao Q., Zhu S., Chem. Soc. Rev. 2020, 49, 908–950. [DOI] [PubMed] [Google Scholar]
- 44. Uenishi J. i., Kubo Y., Tetrahedron Lett. 1994, 35, 6697–6700. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information