Abstract
Objective
To evaluate the nature and burden of residual disease in rheumatoid arthritis (RA) in patients who meet treatment targets. Second, for those who did not meet targets, to evaluate how much is due to patient symptoms.
Methods
Prospective and retrospective studies were searched in Medline, Embase, and Cochrane Library in the English language from January 1, 2008 to April 18, 2018; conference abstracts (from January 2016 to April 2018) and reference lists of relevant studies were also screened.
Results
Of 8,339 records identified, 55 were included in the review; 53 were unique studies, including 10 randomized controlled trials. Of these, 48 reported on patients who achieved low disease activity (LDA) or remission. Studies varied in population, treatment goals, and outcome reporting. The proportions of patients with residual symptoms in these studies varied by the definitions used for LDA or remission and were more often reported in patients with LDA than those in remission. The most commonly reported outcome measures were functional disability (n = 34 studies), tender or swollen joints (n = 18), pain (n = 17), patient global assessment (n = 15), and fatigue (n = 14). However, few studies reported the percentage of patients achieving a specific threshold, which could then be used to easily define the presence of residual symptoms.
Conclusion
Residual symptoms are present in some patients despite their achieving LDA or remission, highlighting an unmet need, especially with respect to improving pain, fatigue, and function. Standardized reporting in future observational studies would facilitate better understanding of this issue in defined RA populations.
INTRODUCTION
Rheumatoid arthritis (RA), the world’s most prevalent chronic autoimmune inflammatory arthritis, is characterized by joint inflammation directly leading to pain, functional decline, and fatigue, all of which negatively impact health‐related quality of life (HRQoL) and reduce a patient’s ability to work (1). A combination of new therapeutics and new treat‐to‐target strategies has made sustained clinical remission a primary goal in treating patients with RA. Reaching this goal requires regular assessment of RA activity composite measures and the ability to escalate treatment regimens (2, 3, 4).
Significance & Innovations.
Based on a very large literature search, we have identified that there is a paucity of data and lack of standardization for reporting residual patient‐centered symptoms and outcomes in those patients attaining a treatment target.
Reliance solely on traditional rheumatoid arthritis disease activity targets as acceptable treatment goals risks underestimating the impact on patients’ pain, function, health, and true burden of illness for a meaningful proportion of patients.
The use of patient‐reported outcomes in addition to a treat‐to‐target approach may provide information that will inform a management decision necessary to address residual symptoms.
Despite successes from this approach, remission may not be achievable, and a low disease activity (LDA) state may be an acceptable alternative goal (2). However, for some patients, particularly in the more established phase of disease, it may not be possible to achieve and sustain even LDA, as evidenced in real‐world data (5, 6). In addition, patients with RA and moderate disease activity may prefer staying with their current treatment over changing treatment and risking the possibility of developing worse symptoms, including possible side effects (7). Whether or not a disease activity treatment target is achieved, many symptoms most troublesome to individuals living with RA are subjective in nature, and their true impact is known only to the patients themselves and not routinely captured in disease activity scores (8). These concerns highlight the importance of understanding and measuring other HRQoL domains that are impacted by RA and that might be ameliorated by using therapeutic approaches employed adjunctively to a goal‐oriented strategy determined solely by composite measures of disease activity.
Recognizing that individuals with RA who meet remission or LDA as defined by composite scores of disease activity may continue to have symptoms (referred to subsequently as “residual symptoms”), we set out in this study to understand the nature of such residual symptoms as reported in the literature and how these symptoms impact patients’ lives.
MATERIALS AND METHODS
A systematic literature review (SLR) was performed, following Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines, in Medline, Embase, and the Cochrane Library. Searches were conducted on April 18, 2018 and were limited to articles published in the English language after January 1, 2008. Conference abstracts (European Alliance of Associations for Rheumatology [EULAR], American College of Rheumatology [ACR], International Society of Pharmacoeconomics and Outcomes Research, and International Society for Pharmacoepidemiology) were also searched from January 2016 to April 2018, as well as reference lists of any relevant systematic reviews and meta‐analyses published in the previous 5 years. Search terms included combinations of free text and Emtree/MeSH subject headings for terms relating to RA, LDA, treatment targets, treatment outcomes, and disease impact. Full details of the search strategy are in Supplementary Table 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24369/abstract.
Study eligibility criteria were created using the population, interventions, and outcomes process (see Supplementary Table 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24369/abstract). Studies of interest were prospective (including randomized controlled trials [RCTs]) and retrospective studies in patients with RA age ≥18 years who were treated with conventional synthetic disease‐modifying antirheumatic drugs (csDMARDs), biologic DMARDs (bDMARDs), or JAK inhibitors according to a treat‐to‐target strategy. We included studies that reported outcomes for patients who did or did not meet treat‐to‐target goals (LDA or remission). Outcomes of interest included any patient symptoms (e.g., pain, fatigue, functioning) or disease impact (e.g., HRQoL, absenteeism, or presenteeism) for studies where patients met treat‐to‐target goals. For patients who did not achieve treat‐to‐target goals, outcomes of interest were disease impact or reasons for not achieving goals. Due to the wealth of literature, studies were limited to the following countries where treat‐to‐target is known to be implemented: Canada, France, Germany, Italy, Japan, the Netherlands, Spain, Sweden, the UK, and the US. Multinational studies that included these countries were included.
Titles and abstracts and full texts were screened by one researcher to determine eligibility according to the predefined inclusion and exclusion criteria. A separate researcher performed a random 10% quality check of the included and excluded studies; any disagreements or uncertainties about relevance were determined by consensus. Data from eligible studies were extracted from full‐text publications where possible. Data extraction was verified against the source by a second researcher.
RESULTS
Of the 8,339 records identified after removal of duplicates, 55 met the inclusion criteria for this review (Figure 1). Three articles reported on the same RCT; hence, 53 unique studies were included.
Figure 1.
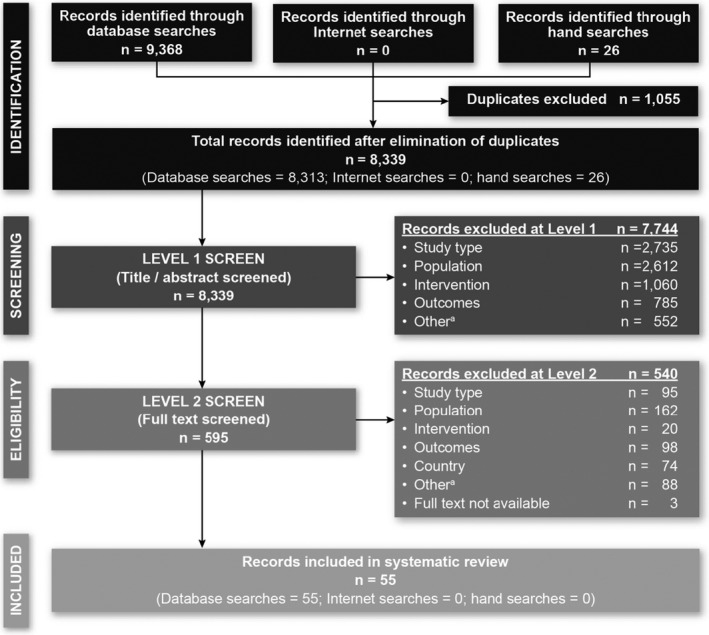
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) diagram. No abstracts were identified from the internet searches because the 2016 and 2017 meetings were indexed in Embase, and the 2018 meetings had not occurred at the time of the search. a = duplicate references, abstracts that were published before 2016, and studies performed in countries not of interest.
Of the 53 included studies, 10 were RCTs and 43 were nonrandomized studies; 42 reported outcomes of interest for patients who achieved treat‐to‐target goals (8 RCTs and 34 nonrandomized studies), and 5 reported on patients who did not achieve goals (5 nonrandomized studies); 6 reported data of interest for both groups of patients (see Supplementary Tables 3 and 4, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24369/abstract). Studies were identified from all 10 specified countries, the greatest number of which were from Japan, the Netherlands, the UK, and the US.
Substantial heterogeneity among the studies made direct comparisons difficult. For example, study designs included RCTs, prospective observational studies, retrospective studies, and patient interviews. Patient characteristics varied in terms of duration and severity of disease and prior treatments, and studies were diverse in their use of tools for assessing outcomes and the manner in which they were reported. Minimum clinically important differences were often not reported, even for those instruments that have them defined. Furthermore, the studies used a variety of treatment goals for remission or LDA; Disease Activity Score in 28 joints (DAS28) was the most commonly used measure, with a DAS28 score of <3.2 used to define LDA, and a DAS28 score of <2.6 to define remission (Figure 2).
Figure 2.
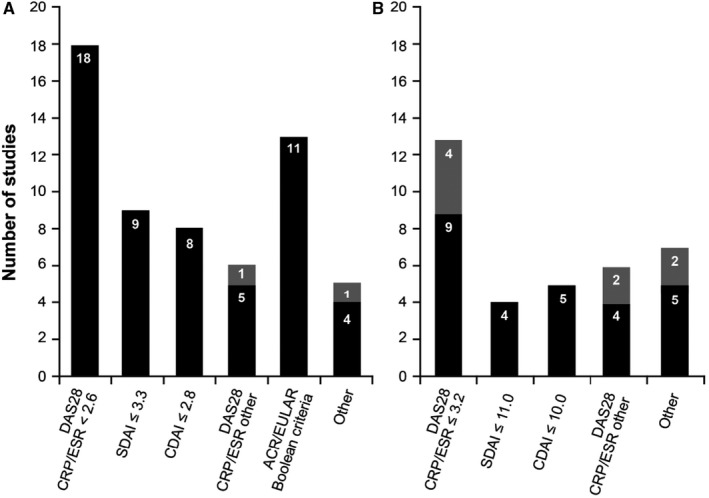
Definitions of remission (A) and low disease activity (B) used in the studies for randomized controlled trials (solid bars) and nonrandomized studies (shaded bars). Some studies compared different treatment goals. ACR = American College of Rheumatology; CDAI = Clinical Disease Activity Index; CRP = C‐reactive protein; DAS28 = Disease Activity Score in 28 joints; ESR = erythrocyte sedimentation rate; EULAR = European Alliance of Associations for Rheumatology; SDAI = Simplified Disease Activity Index.
Residual symptoms
Studies reported on a range of symptoms among patients who achieved treat‐to‐target goals; various tools/measurements were used (see Supplementary Table 3, available at http://onlinelibrary.wiley.com/doi/10.1002/acr.24369/abstract). Most studies reported mean or median values; however, a handful reported the proportion of patients achieving a certain threshold (Tables 1 and 2).
Table 1.
Studies reporting percentage of patients achieving a threshold value for functional disability, pain, and fatigue*
| Author, year (ref.) | Country | Outcome definition and assessment tool | Definition of remission or LDA | Results, no./total no. (%) of patients |
|---|---|---|---|---|
| Functional disability | ||||
| C‐EARLY study (13, 15, 55) | HAQ DI score >0.5 at week 52 | Sustained LDA (DAS28‐ESR score ≤3.2 both at weeks 40 and 52) | CZP standard dose 17/84 (19.3) | |
| CZP reduced frequency 25/126 (19.8) | ||||
| CZP stopped 19/79 (24.1) | ||||
| Perrotta et al, 2018 (18) | Italy | Residual functional impairment (HAQ DI score >0.5) | DAS28‐CRP score <2.6 | 9/47 (19.1) |
| ACR/EULAR remission† | 0/12 (0) | |||
| CDAI score <2.8 | 1/16 (6.25) | |||
| SDAI score <3.3 | 2/19 (10.5) | |||
| DAS28‐CRP score <3.2 | 15/59 (25.4) | |||
| CDAI score <10 | 14/51 (27.4) | |||
| SDAI score <11 | 14/56 (25) | |||
| Sakellariou et al, 2013 (19) | Italy | HAQ DI score >0.5 | DAS28‐ESR score <1.6 | 10/72 (13.9) |
| DAS28‐ESR score <2.6 | 7/56 (12.5) | |||
| DAS28‐ESR score <2.0 | 1/22 (4.5) | |||
| ACR/EULAR remission | 1/23 (4.3) | |||
| SDAI score ≤3.3 | 1/28 (3.6) | |||
| Einarsson et al, 2016 (25) | Sweden | Full physical function (HAQ DI score = 0) after 6 years | DAS28 sustained remission | 43‡ |
| No DAS28 sustained remission§ | 12‡ | |||
| SDAI sustained remission¶ | 60‡ | |||
| Svensson et al, 2016 (26) | Sweden | HAQ DI score ≥1 at 8 years | Remission (DAS score <2.6) at years 1, 2, 5, and 8 | 17.5‡ |
| Navarro‐Millán et al, 2013 (37) | US | M‐HAQ score = 0 | ACR/EULAR remission† | 1,857/2,351 (79) |
| M‐HAQ score >0 and ≤0.5 | 423/2,351 (18) | |||
| M‐HAQ score >0.5 | 71/2,351 (3) | |||
| Pain | ||||
| Perrotta et al, 2018 (18) | Italy | Residual pain defined as VAS score >10 mm (range 0–100 mm) | DAS28‐CRP score <2.6 | 24/47 (51.1) |
| CDAI score <2.8 | 1/16 (6.25) | |||
| SDAI score <3.3 | 2/19 (10.5) | |||
| ACR/EULAR remission† | 0/12 (0) | |||
| DAS28‐CRP score <3.2 | 34/59 (57.6) | |||
| CDAI score <10 | 33/51 (58.9) | |||
| SDAI score <11 | 31/56 (55.3) | |||
| Navarro‐Millán et al, 2013 (37) | US | VAS score = 0 (range 0–10 cm) | ACR/EULAR remission† | 658/2,351 (28) |
| VAS score = 1 (range 0–10 cm) | 1,364/2,351(58) | |||
| VAS score = 2 (range 0–10 cm) | 212/2,351 (9) | |||
| VAS score ≥3 (range 0–10 cm) | 118/2,351 (5) | |||
| Altawil et al, 2016 (42) | Sweden | Significant remaining pain, VAS score >20 (range 0–100 mm) | Good EULAR response | 123/421 (29) |
| Moderate EULAR response | 280/402 (70) | |||
| Fatigue | ||||
| Druce et al, 2016 (47) | UK | Partial remission of fatigue: SF‐36 VT domain >5th percentile from a matched general population (>12.5 on the SF‐36 VT [scale 0–100]) | DAS28 score <2.6 | 255/271 (83) |
| Complete remission of fatigue: SF‐36 VT domain >25th percentile from a matched general population (>50 on the SF‐36 VT [scale 0‐100]) | 101/271 (37.3) | |||
| Nonremission of fatigue | 170/271 (62.7) | |||
| >62.5 on the SF‐36 VT (score ≥ general population median) | 44/271 (16.2) | |||
| Navarro‐Millán et al, 2013 (37) | US | 0 on VAS (range 0–10 cm) | ACR/EULAR remission† | 682/2,351 (29) |
| 1 on VAS (range 0–10 cm) | 1,011/2,351 (43) | |||
| 2 on VAS (range 0–10 cm) | 282/2,351 (12) | |||
| ≥3 on VAS (range 0–10 cm) | 353/2,351 (15) |
ACR = American College of Rheumatology; CDAI = Clinical Disease Activity Index; CZP = certolizumab pegol; DAS28‐CRP = Disease Activity Score in 28 joints using the C‐reactive protein level; DAS28‐ESR = DAS28 using the erythrocyte sedimentation rate; EULAR = European Alliance of Associations for Rheumatology; HAQ = Health Assessment Questionnaire; HAQ DI = Health Assessment Questionnaire disability index; LDA = low disease activity; M‐HAQ = modified Health Assessment Questionnaire; ref. = reference; SDAI = Simplified Disease Activity Index; SF‐36 = Short Form 36 (health survey); SF‐36 VT = SF‐36 vitality scale; VAS = visual analog scale.
Swollen joint count of 28 Joints, tender joint count of 28 Joints, CRP level, and patient global assessment of disease activity VAS ≤1.
Values are the percentage.
DAS28 score of <2.6 on at least 2 consecutive occasions and for at least 6 months.
SDAI score of <3.3 on at least 2 consecutive occasions and for at least 6 months.
Table 2.
Summary of thresholds used in studies*
| Outcome threshold | No. of studies reporting percentages | |
|---|---|---|
| Functional disability | HAQ score ≤0.5 | 2 |
| HAQ score >0.5 | 1 | |
| HAQ score = 0 | 1 | |
| HAQ score ≥1 | 1 | |
| M‐HAQ score = 0; >0 and ≤0.5; >0.5 | 1 | |
| Pain | Residual pain: VAS score >10 mm | 1 |
| Significant remaining pain: VAS score >20 mm | 1 | |
| VAS scores = 0, 1, 2, ≥3 | 1 | |
| Fatigue | Partial remission/complete of fatigue: SF‐36 vitality domain >5th/25th percentile from a matched general population | 1 |
| VAS score = 0, 1, 2, ≥3 cm | 1 | |
| Tender or swollen joints | Any tender or swollen joints | 1 |
| TJC >1 | 2 | |
| SJC >1 | 2 | |
| SJC28 = 0; 1; ≥2 | 1 | |
| TJC28 = 0; 1; ≥2 | 1 | |
| TJC28 or SJC28 in feet >0 | 1 | |
| Physician or patient global assessment of disease activity | PtGA VAS score >1 cm | 1 |
| PhGA VAS score >1 cm | 1 | |
| Patient assessment of general health, VAS score >1 cm | 1 | |
| Other symptoms | PASS, yes/no (yes indicates that symptoms are at an acceptable level to the patient) | 1 |
| Presence of morning stiffness | 1 |
HAQ = Health Assessment Questionnaire; M‐HAQ = modified HAQ; PASS = Patient Acceptable Symptom State; PhGA = physician global assessment of disease activity; PtGA = patient global assessment of disease activity; SF‐36 = Short Form 36 (health survey); SJC = swollen joint count; SJC28 = SJC in 28 joints; TJC = tender joint count; TJC28 = TJC in 28 joints; VAS = visual analog scale.
The most commonly reported outcomes were functional disability, pain, fatigue, tender or swollen joints, and physician global assessment of disease activity (PhGA) or patient global assessment of disease activity (PtGA) (Figure 3). Other symptoms such as anxiety, depression, sleep disturbances, and morning stiffness were reported less often.
Figure 3.
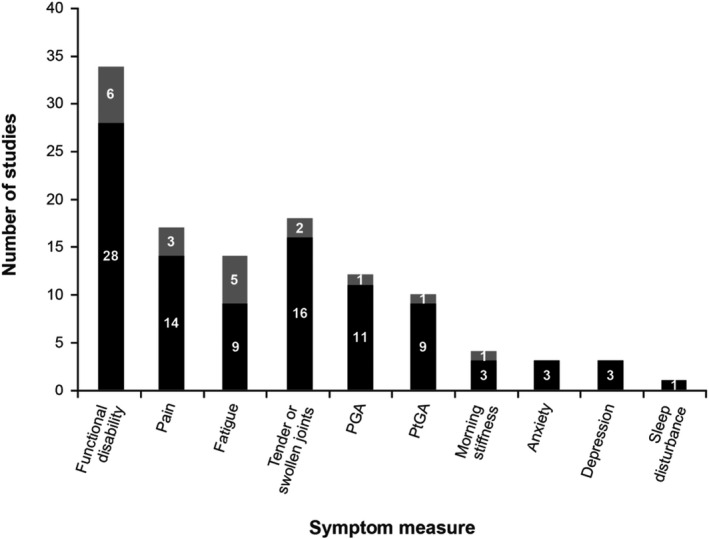
Outcome measures used in the studies to inform on symptoms for randomized controlled trials (solid bars) and nonrandomized studies (shaded bars). PGA = physician global assessment of disease activity; PtGA= patient global assessment of disease activity.
Functional disability
Functional disability was the most commonly reported symptom, reported in 34 studies (including 6 RCTs) (results in Supplementary Table 5, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24369/abstract). The tool most often used was the Health Assessment Questionnaire (HAQ) or its derivatives: the HAQ disability index (HAQ DI; 27 studies), modified HAQ (M‐HAQ; 3 studies), and multidimensional HAQ (MD‐HAQ; 1 study). Other tools included the Patient‐Reported Outcomes Measurement Information System (PROMIS‐29) physical function domain, Michigan Hand Outcome Questionnaire, Funktionsfragebogen Hannover patient questionnaire, McMaster Toronto Arthritis Patient Preference Questionnaire, Signals of Functional Impairment, and Steinbrocker Functional Classification.
Of the 6 RCTs that used the HAQ DI, all defined LDA using the DAS28 (C‐reactive protein [CRP] or erythrocyte sedimentation rate [ESR]) (9, 10, 11, 12, 13, 14). A HAQ DI score of <0.5 was often used by studies to describe normative physical function. Mean ± SD scores for the HAQ DI ranged from 0.23 ± 0.33 for patients who experienced early remission (DAS score <1.6) after 4 months of methotrexate in the IMPROVED study (9) to 0.81 in patients with LDA at ≥2 visits in the DRESS study (10). The C‐EARLY study reported that, although the mean HAQ DI score was 0.3, ~20% of patients had residual effects on physical function (15). Additionally, 21 nonrandomized studies reported results from the HAQ (5, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35), 8 of which included patients in LDA or remission with a mean HAQ score of >0.5 (16, 17, 21, 24, 25, 27, 28, 32). Mean ± SD scores ranged from 0.10 ± 0.02 in patients with a swollen joint count (SJC) of ≤1, tender joint count (TJC) of ≤1, and PtGA score of ≤1 (20) to 1.4 ± 0.6 in patients with a DAS28 score of <3.2 (28).
The M‐HAQ was used in 3 studies; mean scores ranged from 0.04 for patients in Clinical Disease Activity Index (CDAI) remission (CDAI score ≤2.8) at year 5 (36) to 0.45 for patients with DAS28‐ESR scores of >2.6 and ≤3.2 (37). M‐HAQ scores were lower for patients in remission than for those with LDA and varied by target used (Table 1). In the one study that used the MD‐HAQ, patients in remission (DAS28‐CRP score <2.6) had a mean ± SD score of 0.32 ± 0.32 (38).
The level of functional disability depends on the target criteria used. Two studies reported a higher proportion of patients with residual functional impairment (HAQ score >0.5) when a target DAS28 score of <2.6 was used compared with more stringent targets such as ACR‐/EULAR‐defined remission (18, 19) (Table 1). Additionally, an analysis of 753 patients from a postmarketing registry (16) also found that residual HAQ scores in patients treated with infliximab or golimumab were higher when the definition of remission used was a DAS28 score of <2.6 than when the definition was a CDAI score of ≤2.8 or an SDAI score of ≤3.3, with mean ± SD scores of 0.76 ± 0.67, 0.57 ± 0.56, and 0.57 ± 0.57, respectively. Additionally, patients with LDA had higher residual HAQ scores than those in remission: 0.86 ± 0.67 for DAS28 score of ≤3.2, 0.94 ± 0.70 for CDAI score of ≤10.0, and 0.89 ± 0.69 for SDAI score of ≤11.0 (16).
Whether remission is sustained also impacts the presence of functional disability. An observational study of patients receiving anti–tumor necrosis factor (anti‐TNF) therapy over 6 years (25) observed that patients in sustained remission (defined as DAS28 score <2.6 on at least 2 consecutive occasions and for ≥6 months) had lower HAQ scores (better physical functioning) than those who had only occasional remission. Full physical function (HAQ score = 0) was achieved by 43% of patients with DAS28‐defined sustained remission, 60% with SDAI‐defined sustained remission, and only 12% with no DAS28‐defined sustained remission. Furthermore, an analysis of the Better Anti‐Rheumatic PharmacOTherapy (BARFOT) trial (26) reported that 17.5% of patients in remission (DAS score <2.6) at years 1, 2, 5, and 8 had a HAQ score of ≥1 at 8 years, indicating that some patients still experienced significant disability despite sustained remission.
Pain
Pain was reported in 17 studies, including 3 RCTs. Thirteen reported pain using a visual analog scale (VAS), 3 used the 36‐item Short Form 36 (SF‐36) health survey bodily pain domain, and 1 each used the PROMIS‐29 pain interference domain and an 11‐point scale (results in Supplementary Table 6, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24369/abstract).
Ten studies (9, 14, 22, 23, 24, 26, 35, 39, 40, 41) reported mean or median VAS scores. For patients in remission, the average VAS score ranged from 3 mm (range 0–12; scale 0–100 mm) for patients in Boolean remission (40) to 3 cm (interquartile range 2–4, scale 0–10 cm) for patients with a DAS in 44 joints score of ≤2.4 and SJC score of ≤1 plus ultrasound remission (22). For patients with LDA, the mean ± SD VAS score ranged from 12.8 ± 15.5 for patients with sustained LDA (14) to 28.3 (scale 0–100 mm) for patients with moderate or good EULAR response who perceived their overall health not to have improved (41). Only 4 studies reported an average pain VAS score of <1 cm (10 mm), and these were all for patients in remission (23, 35, 40, 41).
Three studies (18, 37, 42) reported patients achieving a certain VAS value (Table 1). First, a prospective study of patients taking biologics defined residual pain as a VAS score of >10 mm (18). Residual pain was reported by 51.1% of patients with a DAS28‐CRP score of <2.6 compared with 6.25% with a CDAI score of <2.8, 10.5% with an SDAI score of <3.3, and 0% meeting ACR/EULAR remission criteria. The proportion of patients experiencing residual pain was higher in patients who achieved only LDA. Second, a cross‐sectional analysis of the Corrona registry (37) also found that most patients in ACR/EULAR remission reported low pain scores on the VAS (scale 0–10 cm). However, 9% had a VAS score of 2, and 5% reported pain with a score of ≥3. Finally, a case–control study of patients with early RA treated with methotrexate for 3 months defined remaining pain as a VAS score of >20 (scale 0–100 mm) (42). The study reported that 29% and 70% of patients with a good or moderate EULAR response, respectively, experienced remaining pain. In the good‐response group, remaining pain was significantly associated with high baseline HAQ score and low ESR.
Of the 3 studies (12, 41, 43) that used the SF‐36 bodily pain domain, mean scores ranged from 16.3, for patients with moderate or good EULAR response and who considered their health to have improved after treat‐to‐target strategy aimed at achieving fast remission (41), to 72.1 ± 19.3 for patients with stable LDA (defined as a DAS28 score <3.2 for >6 months prior) (12).
Fatigue
Fatigue was reported in 14 studies (including 5 RCTs); 5 used a VAS, 4 used the Functional Assessment of Chronic Illness–Fatigue (FACIT‐F) subscale; 2 used the Bristol RA Fatigue Multidimensional Questionnaire (BRAF‐MDQ); and 1 study each used the SF‐36 vitality domain, PROMIS‐29 fatigue T score, and an 11‐point scale (results in Supplementary Table 7, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24369/abstract).
Of the 5 studies using a VAS (17, 35, 37, 39, 41), 3 reported the average score, which was >1 cm (10 mm) in all 3 studies. Notably, a study of patients at the Leiden Early Arthritis Clinic (35) showed that patients who achieved early sustained DMARD‐free remission experienced less fatigue than those who achieved remission late or had intermediate remission, with median VAS scores of 3, 13, and 21 (scale 0–100 mm), respectively. Only 1 study reported the proportion of patients achieving certain VAS values (37) (Table 1). In this study, only 29% of patients who met the ACR/EULAR remission criteria reported a fatigue score of 0 (scale 0–10 cm), while 15% reported a score of ≥3.
Four RCTs used the FACIT‐F (scale 0–52) to assess fatigue, with a higher score indicating less fatigue. Two studies reported mean ± SD FACIT‐F scores, which ranged from 42.0 ± 8.7 for patients with sustained LDA (DAS28 score ≤3.2 at week 36 and average DAS28 score ≤3.2 in weeks 12–36) in the PRESERVE study (14) to 44.4 ± 7.3 for responders (DAS28‐ESR score ≤3.2 at week 39 and DAS28‐ESR score <2.6 at week 52) in the PRIZE study (44). The REFLEX study (45) reported that 20.6% of responders (ACR 20% improvement criteria) did not achieve a minimum clinically important difference of ≥3.56. The final study (46) reported only the change from baseline in ACR and EULAR responders.
The one study using the SF‐36 vitality domain (scale 0–100) found that few patients with a DAS28 score of <2.6 achieved complete fatigue remission (47). Partial fatigue remission was achieved by 83% of patients, while complete fatigue remission was achieved by only 37.3% of patients (Table 1). Those with nonremission of fatigue had a higher proportion of steroid use, stroke, and depression and lower scores for pain and function than the remission group.
Other symptoms
Very few studies reported on symptoms of anxiety, depression, sleep disturbance, or morning stiffness. PtGA and PhGA scores, TJCs, and SJCs were generally low when reported (see Supplementary Tables 8–10, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24369/abstract). These outcomes are often required to be low according to criteria that define LDA and remission. However, many remission criteria still allow for a TJC, SJC, PhGA, or PtGA score of 1.
For example, an observational study in patients with early RA who were treated with DMARDs (23) defined residual disease activity as ≥1 swollen or tender metatarsophalangeal joints. Residual disease activity was present in 10 of 38 patients (26.3%) in Boolean‐based remission (TJC, SJC, CRP, and PtGA ≤1), 12 of 42 patients (28.6%) in Boolean clinical practice remission (TJC, SJC, and PtGA ≤1), 22 of 61 patients (36.1%) with an SDAI score of ≤3.3, and 25 of 63 patients (39.7%) with a CDAI score of ≤2.8. Similarly, an analysis of patients with early RA who were treated with anti‐TNF agents (21) reported on those with an SJC and TJC of ≥1. Fifty‐one percent of patients who met a DAS28‐ESR score of <2.6, and 34.4% who were in ACR/EULAR remission had an SJC in 28 joints of >0. Additionally, 25.2% of patients in remission defined as a DAS28‐ESR score of <2.6, and 21.5% who were in ACR/EULAR remission had a TJC in 28 joints of >0.
Disease impact
The disease impact of illness may include a number of factors such as quality of life, activity impairment, and health care resource utilization. In this SLR, we found that HRQoL and work or activity impairment were 2 aspects of disease impact that were reported in patients who achieved treat‐to‐target goals in 19 studies (8 RCTs and 11 nonrandomized studies) (see Supplementary Table 3, available at http://onlinelibrary.wiley.com/doi/10.1002/acr.24369/abstract). However, the nature of how residual symptoms affected disease impact was generally not reported, and the considerable heterogeneity between the studies makes comparisons difficult.
Patients not achieving treatment goals
Only 5 studies (all nonrandomized, 3 of which were abstracts) reported reasons why patients did not achieve treatment goals (see Supplementary Table 4, available at http://onlinelibrary.wiley.com/doi/10.1002/acr.24369/abstract). No studies were identified that reported how patients’ symptoms contributed to disease burden.
One abstract reported (48) that patients who did not achieve their goals were significantly older, had higher rates of disease activity, had a greater number of TJCs, and had higher PtGA and HAQ DI scores than those who did achieve their goals. Similarly, an analysis of the BARFOT cohort (26) reported that female sex, current smoker, disease activity at baseline, and nonremission status at 6 months were predictive of persistent disease.
Three of the studies linked not achieving treatment goals to the treatment regimens that were used. One abstract (49) reported that, of patients with high disease activity (DAS28‐ESR score >5.1), only 64 patients (9%) were receiving bDMARDs, a much lower proportion than the 18–20% of patients with less active RA. This was reported to be due to failed response to bDMARDs, unwillingness, or contraindications. Yamazaki and Takanashi (48) found that 21 of 60 patients (35%) not achieving LDA or remission were thought to have been insufficiently treated, and 18 of 60 (30%) were insufficiently treated for their complications. Furthermore, 6 of 9 patients from focus groups and interviews (50) stated that they “did not think they had found a treatment regime that controlled their RA properly and expressed disappointment about past drug combination which did not lead to symptom relief.” Three participants reported that they were wary of trying more intensive treatments due to potential side effects.
DISCUSSION
The management of RA has changed greatly over the last 2 decades. This evolution reflects a number of factors. First, earlier intervention in the course of disease was facilitated by the formulation of the ACR/EULAR 2010 classification criteria for RA (51). Second, researchers came to understand that best outcomes are achieved when systemic and local inflammatory disease activity is optimally suppressed over time. This was facilitated by the treat‐to‐target approach (2), which is recommended for use and widely adopted in clinical practice in the geographic regions from which the studies included in this SLR were undertaken. Third, the choices for effective pharmacotherapy have expanded, with introduction of a variety of classes of targeted biologic therapies with differing mechanisms of action (i.e., TNF inhibitor and other bDMARDs) and, more recently, introduction of small molecule, orally available, JAK inhibitors. Because of these advances, the outlook for a contemporary patient presenting with RA is genuinely better than was the case a generation ago. The presence and magnitude of residual symptoms may depend on the stringency of the remission or LDA measure attained as well as on the durability of this level of response. But even when remission criteria are met, it is not straightforward to interpret the cause underlying any symptomatic deficit. In the case of functional disability, it may be the consequence of aging given that HAQ scores increase with age in long‐standing disease; and furthermore, there may be an irreversible component of the HAQ due to past structural damage (52). Everyday clinical experience illustrates that unmet need remains (1). It also raises the question of the underlying pathophysiologic or other causes of residual symptomatology and how this knowledge can be best harnessed to inform a management decision. In this SLR, we sought to better understand the evidence regarding ongoing symptomatology and its relationship to unresolved disease activity and to gain insight into the nature of remaining symptoms experienced by patients despite their attaining the recommended therapeutic target of remission or LDA. These questions are of importance because we have long known that many of the symptoms associated with active RA represent generic features of inflammation, such as pain, fatigue, and functional deficit. But it is also clear that such symptoms have a multifactorial etiology and may be the result of both inflammatory and noninflammatory processes. Furthermore, with respect to inflammatory causes, it might be the case that symptoms in any given individual respond differentially to distinct classes of targeted therapies.
This SLR is notable for revealing a relative paucity of data relevant to these questions. This in part reflects the challenges of extrapolating cohort‐level data to the needs of an individual. It is also a reflection of the tendency to limit data capture to a small set of end points in clinical trials. But perhaps most revealing, in the case of observational studies, is the lack of standardization with respect to the outcome measures and scales employed, therefore complicating the ability to make comparisons. Nevertheless, with respect to the range of symptoms and/or outcomes reported in this SLR, the best symptomatic outcomes show relationships to the attainment and maintenance of the more robust measures of remission. These observations support the treat‐to‐target principle as being an effective approach to the overall goal of patient well‐being in RA. However, it also has to be acknowledged that only a minority of patients both attain and maintain a robust remission over the longer term (53, 54), and the question arises as to whether we can employ patient‐reported outcome measures to identify aspects of life that matter most to the individual and then make subsequent use of this information to inform a management choice that will achieve an overall goal of well‐being. The current treat‐to‐target recommendations have limited utility in a subgroup of patients for whom the desirable target cannot be attained despite a change in pharmacotherapy up to every 3 months. For such patients, it may be more appropriate to focus on personalized treatment goals such as a meaningful reduction in pain or fatigue or patient‐defined functional goals for activities that matter to them. This may require a careful choice of pharmacologic intervention as well as nonpharmacologic approaches that incorporate patient‐centered approaches. The practice of protocol‐driven medicine in a treat‐to‐target approach has the advantage of optimizing outcomes at a cohort level and for the minority who attain and maintain the target, particularly remission. This has the potential disadvantage of treating the disease activity while ignoring the patient who has the disease, thus making care less personalized.
The main limitation of this review arises from the multiple sources of heterogeneity among the studies. The range of treatment goals makes comparisons difficult, as the differing requirements lead to varying levels of residual symptoms that can be present. Additionally, outcomes from different countries may not be comparable due to geographic differences in responses to symptoms such as pain.
In conclusion, despite evidence to support adoption of a treat‐to‐target paradigm in the routine management of RA, residual symptoms still occur in patients achieving LDA or remission. This SLR confirms that there is an unmet need, especially with respect to improving pain, fatigue, and function where possible, even when a target of LDA or remission has been met. Standardized reporting in future observational studies and use of measures that inform on the interference of these symptoms in the daily lives of patients would facilitate better understanding of this issue in defined RA populations. From a pragmatic perspective, these findings suggest that setting personalized goals for the individual in addition to the practice of treat‐to‐target may inform individualized management as part of holistic care.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Taylor had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Pope, Curtis, Kannowski, Mitchell, Bell, Workman, Paik, Cardoso, Taylor.
Acquisition of data
Kannowski, Mitchell, Bell, Taylor.
Analysis and interpretation of data
Michaud, Pope, van de Laar, Curtis, Kannowski, Mitchell, Bell, Workman, Paik, Cardoso, Taylor.
ROLE OF THE STUDY SPONSOR
This study was conducted by RTI Health Solutions under the direction of Eli Lilly and Company. The study, analysis of data, and medical writing support were performed by RTI Health Solutions. Eli Lilly and Company provided funding for the research, and employees of Eli Lilly and Company contributed to the study protocol design, data interpretation, and manuscript development and review. Publication of this article was contingent upon approval by Eli Lilly and Company.
Supporting information
Supplementary Material
Supported by Eli Lilly and Company. Dr. Taylor’s work was supported by the NIHR Oxford Biomedical Research Centre.
Dr. Pope has received consulting fees from Janssen, Amgen, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Emerald Health Pharmaceuticals, Novartis, Roche, Samsung, Sandoz, UCB (less than $10,000 each), AbbVie, Eli Lilly and Company, Gilead, Pfizer, and Sanofi (more than $10,000 each). Dr. van de Laar has received consulting fees from AbbVie, Biogen, Sanofi, Janssen, Eli Lilly and Company, and Pfizer (less than $10,000 each) and research support from Merck, Pfizer, AbbVie, Janssen, and Eli Lilly and Company. Dr. Curtis has received consulting fees from AbbVie, Bristol Myers Squibb, Janssen, Regeneron, Roche, UCB (less than $10,000 each), Amgen, Corrona, Eli Lilly and Company, Myriad, and Pfizer (more than $10,000 each) and research support from those companies. Drs. Kannowski, Paik, and Cardoso and Ms Workman own stock or stock options in Eli Lilly and Company. Dr. Taylor has received consulting fees from AbbVie, Biogen, Galapagos, Gilead, GlaxoSmithKline, Janssen, Pfizer, Roche, Sanofi, Nordic Pharma, Fresenius, UCB (less than $10,000 each), and Eli Lilly and Company (more than $10,000) and research support from Celgene, Galapagos, Janssen, and Eli Lilly and Company. No other disclosures relevant to this article were reported.
References
- 1. Taylor PC, Moore A, Vasilescu R, Alvir J, Tarallo M. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol Int 2016;36:685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016;75:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 4. Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 5. Combe B, Logeart I, Belkacemi MC, Dadoun S, Schaeverbeke T, Daures JP, et al. Comparison of the long‐term outcome for patients with rheumatoid arthritis with persistent moderate disease activity or disease remission during the first year after diagnosis: data from the ESPOIR cohort. Ann Rheum Dis 2015;74:724–9. [DOI] [PubMed] [Google Scholar]
- 6. Kavanaugh A, Keystone E, Greenberg JD, Reed GW, Griffith JM, Friedman AW, et al. Benefit of biologics initiation in moderate versus severe rheumatoid arthritis: evidence from a United States registry. Rheumatology (Oxford) 2017;56:1095–101. [DOI] [PubMed] [Google Scholar]
- 7. Wolfe F, Michaud K. Resistance of rheumatoid arthritis patients to changing therapy: discordance between disease activity and patients’ treatment choices. Arthritis Rheum 2007;56:2135–42. [DOI] [PubMed] [Google Scholar]
- 8. Strand V, Wright GC, Bergman MJ, Tambiah J, Taylor PC. Patient expectations and perceptions of goal‐setting strategies for disease management in rheumatoid arthritis. J Rheumatol 2015;42:2046–54. [DOI] [PubMed] [Google Scholar]
- 9. Heimans L, de Boer KV, Koudijs KK, Visser K, Goekoop‐Ruiterman YP, Harbers JB, et al. Health‐related quality of life and functional ability in patients with early arthritis during remission steered treatment: results of the IMPROVED study. Arthritis Res Ther 2013;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Herwaarden N, van Maas AD, Minten MJ, van Den Hoogen FH, Kievit W, van Vollenhoven RF, et al. Disease activity guided dose reduction and withdrawal of adalimumab or etanercept compared with usual care in rheumatoid arthritis: open label, randomised controlled, non‐inferiority trial. BMJ 2015;350:h1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bouman CA, van Herwaarden N, van Den Hoogen FH, Fransen J, van Vollenhoven RF, Bijlsma JW, et al. Long‐term outcomes after disease activity‐guided dose reduction of TNF inhibition in rheumatoid arthritis: 3‐year data of the DRESS study: a randomised controlled pragmatic non‐inferiority strategy trial. Ann Rheum Dis 2017;76:1716–22. [DOI] [PubMed] [Google Scholar]
- 12. Ghiti Moghadam M, ten Klooster PM, Vonkeman HE, Kneepkens EL, Klaasen R, Stolk JN, et al. Impact of stopping tumor necrosis factor inhibitors on rheumatoid arthritis patients’ burden of disease. Arthritis Care Res (Hoboken) 2018;70:516–24. [DOI] [PubMed] [Google Scholar]
- 13. Weinblatt ME, Bingham CO III, Burmester GR, Bykerk VP, Furst DE, Mariette X, et al. A phase III study evaluating continuation, tapering, and withdrawal of certolizumab pegol after one year of therapy in patients with early rheumatoid arthritis. Arthritis Rheumatol 2017;69:1937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Strand V, Jones TV, Li W, Koenig AS, Kotak S. The impact of rheumatoid arthritis on work and predictors of overall work impairment from three therapeutic scenarios. Int J Clin Rheumatol 2015;10:317–28. [Google Scholar]
- 15. Bingham C III, Emery P, Weinblatt M, Burmester GR, Furst DE, Mariette X, et al. Maintenance of improvements in patients’ physical function, workplace and household productivity, and reduction in caregiver burden with 2 years of certolizumab pegol treatment in DMARD‐naive, early RA patients with severe progressive disease [abstract]. Arthritis Rheumatol 2016;68 Suppl 10. URL: https://acrabstracts.org/abstract/maintenance‐of‐improvements‐in‐patients‐physical‐function‐workplace‐and‐household‐productivity‐and‐reduction‐in‐caregiver‐burden‐with‐2‐years‐of‐certolizumab‐pegol‐treatment‐in‐dmard‐naive‐early/. [Google Scholar]
- 16. Bell M, Stewart J, Haraoui B, Keystone E, Baer P, Boulos P, et al. The HAQ reversible and irreversible components measuring function in rheumatoid arthritis [abstract]. Arthritis Rheumatol 2016;68 Suppl 10. URL: https://acrabstracts.org/abstract/the‐haq‐reversible‐and‐irreversible‐components‐measuring‐function‐in‐rheumatoid‐arthritis/. [Google Scholar]
- 17. Barnabe C, Sun Y, Boire G, Hitchon CA, Haraoui B, Thorne JC, et al. Heterogeneous disease trajectories explain variable radiographic, function and quality of life outcomes in the Canadian Early Arthritis Cohort (CATCH). PLoS One 2015;10:e0135327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perrotta FM, De Socio A, Scriffignano S, Lubrano E. Residual disease activity in rheumatoid arthritis patients treated with subcutaneous biologic drugs that achieved remission or low disease activity: a longitudinal observational study. Clinical Rheumatol 2018;37:1449–55. [DOI] [PubMed] [Google Scholar]
- 19. Sakellariou G, Scirè CA, Verstappen SM, Montecucco C, Caporali R. In patients with early rheumatoid arthritis, the new ACR/EULAR definition of remission identifies patients with persistent absence of functional disability and suppression of ultrasonographic synovitis. Ann Rheum Dis 2013;72:245–9. [DOI] [PubMed] [Google Scholar]
- 20. Shidara K, Nakajima A, Inoue E, Hoshi D, Sugimoto N, Seto Y, et al. Continual maintenance of remission defined by the ACR/EULAR criteria in daily practice leads to better functional outcomes in patients with rheumatoid arthritis. J Rheumatol 2017;44:147–53. [DOI] [PubMed] [Google Scholar]
- 21. De Punder YM, Fransen J, Kievit W, Houtman PM, Visser H, van de Laar MA, et al. The prevalence of clinical remission in RA patients treated with anti‐TNF: results from the Dutch Rheumatoid Arthritis Monitoring (DREAM) Registry. Rheumatology (Oxford) 2012;51:1610–7. [DOI] [PubMed] [Google Scholar]
- 22. Van der Ven M, Martijn Kuijper T, Gerards AH, Tchetverikov I, Weel AE, Zeben J, et al. No clear association between ultrasound remission and health status in rheumatoid arthritis patients in clinical remission. Rheumatology (Oxford) 2017;56:1276–81. [DOI] [PubMed] [Google Scholar]
- 23. Van Tuyl LH, Britsemmer K, Wells GA, Smolen JS, Zhang B, Funovits J, et al. Remission in early rheumatoid arthritis defined by 28 joint counts: limited consequences of residual disease activity in the forefeet on outcome. Ann Rheum Dis 2012;71:33–7. [DOI] [PubMed] [Google Scholar]
- 24. Martínez Pérez R, Rubio E, Fernández Alba MD, Hernández B, Menor R, Povedano J. Utility of the ultrasound for the evaluation of activity in patients with rheumatoid arthritis in remission or low clinical activity in treatment with biological in optimization phase [abstract]. Ann Rheum Dis 2016;75:1233. [Google Scholar]
- 25. Einarsson JT, Geborek P, Saxne T, Kristensen LE, Kapetanovic MC. Sustained remission improves physical function in patients with established rheumatoid arthritis, and should be a treatment goal: a prospective observational cohort study from southern Sweden. J Rheumatol 2016;43:1017–23. [DOI] [PubMed] [Google Scholar]
- 26. Svensson B, Andersson M, Forslind K, Ajeganova S, Hafström I, Bala V, et al. Persistently active disease is common in patients with rheumatoid arthritis, particularly in women: a long‐term inception cohort study. Scand J Rheumatol 2016;45:448–55. [DOI] [PubMed] [Google Scholar]
- 27. Barami T, Booth A, Clemes S, Brooke‐Wavell K, Summers G. Physical activity levels in female rheumatoid arthritis patients on long term anti‐tumour necrosis factor therapy compared to patients with active rheumatoid disease and healthy controls [abstract]. Rheumatology (Oxford) 2017;56:ii128–ii9. [Google Scholar]
- 28. Cruz A, Shimizu T, Tanaka M, Mamoto K, Burghardt AJ, Heilmeier U, et al. Radiological changes measured by MRI and high‐resolution peripheral quantitative computed tomography (HR‐pQCT) show correlation with Michigan Hand Outcome Questionnaire (MHQ) in rheumatoid arthritis patients [abstract]. Arthritis Rheumatol 2017;69 Suppl 10. URL: https://acrabstracts.org/abstract/radiological‐changes‐measured‐by‐mri‐and‐high‐resolution‐peripheral‐quantitative‐computed‐tomography‐hr‐pqct‐show‐correlation‐with‐michigan‐hand‐outcome‐questionnaire‐mhq‐in‐rheumatoid‐arthritis‐p/. [Google Scholar]
- 29. Ruyssen‐Witrand A, Guernec G, Nigon D, Tobon G, Jamard B, Rat AC, et al. Aiming for SDAI remission versus low disease activity at 1 year after inclusion in ESPOIR cohort is associated with better 3‐year structural outcomes. Ann Rheum Dis 2015;74:1676–83. [DOI] [PubMed] [Google Scholar]
- 30. Kikuchi J, Kondo T, Shibata A, Sakai R, Okada Y, Chino K, et al. Efficacy and tolerability of six‐week extended dosing interval with tocilizumab therapy in a prospective cohort as remission maintenance in patients with rheumatoid arthritis. Mod Rheumatol 2017;28:444–51. [DOI] [PubMed] [Google Scholar]
- 31. Takeuchi T, Matsubara T, Ohta S, Mukai M, Amano K, Tohma S, et al. Biologic‐free remission of established rheumatoid arthritis after discontinuation of abatacept: a prospective, multicentre, observational study in Japan. Rheumatology (Oxford) 2014;54:683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yasuda S, Ohmura K, Kanazawa H, Kurita T, Kon Y, Ishii T, et al. Maintenance treatment using abatacept with dose reduction after achievement of low disease activity in patients with rheumatoid arthritis (MATADOR): a prospective, multicenter, single arm pilot clinical trial. Mod Rheumatol 2017;27:930–7. [DOI] [PubMed] [Google Scholar]
- 33. Rezaei H, Saevarsdottir S, Forslind K, Albertsson K, Wallin H, Bratt J, et al. In early rheumatoid arthritis, patients with a good initial response to methotrexate have excellent 2‐year clinical outcomes, but radiological progression is not fully prevented: data from the methotrexate responders population in the SWEFOT trial. Ann Rheum Dis 2012;71:186–91. [DOI] [PubMed] [Google Scholar]
- 34. Saleem B, Brown AK, Quinn M, Karim Z, Hensor EM, Conaghan P, et al. Can flare be predicted in DMARD treated RA patients in remission, and is it important? A cohort study. Ann Rheum Dis 2012;71:1316–21. [DOI] [PubMed] [Google Scholar]
- 35. Ajeganova S, van Steenbergen HW, van Nies JA, Burgers LE, Huizinga TW, van Der Helm‐Van Mil AH. Disease‐modifying antirheumatic drug‐free sustained remission in rheumatoid arthritis: an increasingly achievable outcome with subsidence of disease symptoms. Ann Rheum Dis 2016;75:867–73. [DOI] [PubMed] [Google Scholar]
- 36. Alemao E, Joo S, Kawabata H, Al MJ, Allison PD, Rutten‐Van Mölken MP, et al. Effects of achieving target measures in rheumatoid arthritis on functional status, quality of life, and resource utilization: analysis of clinical practice data. Arthritis Care Res (Hoboken) 2016;68:308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Navarro‐Millán I, Chen L, Greenberg JD, Pappas DA, Curtis JR. Predictors and persistence of new‐onset clinical remission in rheumatoid arthritis patients. Semin Arthritis Rheum 2013;43:137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lemmey AB, Wilkinson TJ, Clayton RJ, Sheikh F, Whale J, Jones HS, et al. Tight control of disease activity fails to improve body composition or physical function in rheumatoid arthritis patients. Rheumatology (Oxford) 2016;55:1736–45. [DOI] [PubMed] [Google Scholar]
- 39. Curtis JR, Shan Y, Harrold L, Zhang J, Greenberg JD, Reed GW. Patient perspectives on achieving treat‐to‐target goals: a critical examination of patient‐reported outcomes. Arthritis Care Res (Hoboken) 2013;65:1707–12. [DOI] [PubMed] [Google Scholar]
- 40. Fusama M, Miura Y, Yukioka K, Kuroiwa T, Yukioka C, Inoue M, et al. Psychological state is related to the remission of the Boolean‐based definition of patient global assessment in patients with rheumatoid arthritis. Mod Rheumatol 2015;25:679–82. [DOI] [PubMed] [Google Scholar]
- 41. Steunebrink LM, Oude Voshaar MA, Taal E, Vonkeman HE, Zijlstra TR, van de Laar MA. Determinants of perceived health nonimprovement in early rheumatoid arthritis patients with favorable treatment outcomes. Arthritis Care Res (Hoboken) 2018;70:510–5. [DOI] [PubMed] [Google Scholar]
- 42. Altawil R, Saevarsdottir S, Wedrén S, Alfredsson L, Klareskog L, Lampa J. Remaining pain in early rheumatoid arthritis patients treated with methotrexate. Arthritis Care Res (Hoboken) 2016;68:1061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McWilliams DF, Walsh DA. Factors predicting pain and early discontinuation of tumour necrosis factor‐α‐inhibitors in people with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. BMC Musculoskelet Disord 2016;17:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wiland P, Dudler J, Veale D, Tahir H, Pedersen R, Bukowski J, et al. The effect of reduced or withdrawn etanercept‐methotrexate therapy on patient‐reported outcomes in patients with early rheumatoid arthritis. J Rheumatol 2016;43:1268–77. [DOI] [PubMed] [Google Scholar]
- 45. Keystone E, Burmester GR, Furie R, Loveless JE, Emery P, Kremer J, et al. Improvement in patient‐reported outcomes in a rituximab trial in patients with severe rheumatoid arthritis refractory to anti–tumor necrosis factor therapy. Arthritis Care Res (Hoboken) 2008;59:785–93. [DOI] [PubMed] [Google Scholar]
- 46. Mease PJ, Revicki DA, Szechinski J, Greenwald M, Kivitz A, Barile‐Fabris L, et al. Improved health‐related quality of life for patients with active rheumatoid arthritis receiving rituximab: results of the Dose‐Ranging Assessment: International Clinical Evaluation of Rituximab in Rheumatoid Arthritis (DANCER) trial. J Rheumatol 2008;35:20–30. [PubMed] [Google Scholar]
- 47. Druce KL, Bhattacharya Y, Jones GT, Macfarlane GJ, Basu N. Most patients who reach disease remission following anti‐TNF therapy continue to report fatigue: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford) 2016;55:1786–90. [DOI] [PubMed] [Google Scholar]
- 48. Yamazaki H, Takanashi T. Analysis of rheumatoid arthritis patients that did not achieve the treatment goal by the treat‐to‐target strategy in daily practice [abstract]. Arthritis Rheumatol 2017;69 Suppl 10. URL: https://acrabstracts.org/abstract/analysis‐of‐rheumatoid‐arthritis‐patients‐that‐did‐not‐achieve‐the‐treatment‐goal‐by‐the‐treat‐to‐target‐strategy‐in‐daily‐practice/. [Google Scholar]
- 49. Gullick NJ, Ibrahim F, Mian A, Vincent A, Panayi G, Tom B, et al. Intensive treatment for rheumatoid arthritis reduces disease activity over time [abstract]. Arthritis Rheumatol 2016;68 Suppl 10. URL: https://acrabstracts.org/abstract/intensive‐treatment‐for‐rheumatoid‐arthritis‐reduces‐disease‐activity‐over‐time/. [Google Scholar]
- 50. Prothero L, Georgopoulou S, Galloway J, Williams R, Bosworth A, Lempp H. Patients’ and carers’ views and expectations about intensive management for moderate rheumatoid arthritis: a qualitative study. Psychol Health Med 2016;21:918–25. [DOI] [PubMed] [Google Scholar]
- 51. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 52. Sokka T, Kautiainen H, Hannonen P, Pincus T. Changes in Health Assessment Questionnaire disability scores over five years in patients with rheumatoid arthritis compared with the general population. Arthritis Rheum 2006;54:3113–8. [DOI] [PubMed] [Google Scholar]
- 53. Shahouri SH, Michaud K, Mikuls TR, Caplan L, Shaver TS, Anderson JD, et al. Remission of rheumatoid arthritis in clinical practice: application of the American College of Rheumatology/European League Against Rheumatism 2011 remission criteria. Arthritis Rheum 2011;63:3204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Prince FH, Bykerk VP, Shadick NA, Lu B, Cui J, Frits M, et al. Sustained rheumatoid arthritis remission is uncommon in clinical practice. Arthritis Res Ther 2012;14:R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bykerk VP, Emery P, Weinblatt M, Burmester GR, Furst DE, Mariette X, et al. Clinical responses and improvements in patient‐reported outcomes are associated with increased productivity in the workplace and at home in rheumatoid arthritis patients treated with certolizumab pegol [abstract]. Arthritis Rheumatol 2016;68 Suppl 10. URL: https://acrabstracts.org/abstract/cinical‐responses‐and‐improvements‐in‐patient‐reported‐outcomes‐are‐associated‐with‐increased‐productivity‐in‐the‐workplace‐and‐at‐home‐in‐rheumatoid‐arthritis‐patients‐treated‐with‐certolizumab‐pegol/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


