Abstract
Drimane‐type sesquiterpenes exhibit various biological activities and are widely present in eukaryotes. Here, we completely elucidated the biosynthetic pathway of the drimane‐type sesquiterpene esters isolated from Aspergillus calidoustus and we discovered that it involves a drimenol cyclase having the same catalytic function previously only reported in plants. Moreover, since many fungal drimenol derivatives possess a γ‐butyrolactone ring, we clarified the functions of the cluster‐associated cytochrome P450 and FAD‐binding oxidoreductase discovering that these two enzymes are solely responsible for the formation of those structures. Furthermore, swapping of the enoyl reductase domain in the identified polyketide synthase led to the production of metabolites containing various polyketide chains with different levels of saturation. These findings have deepened our understanding of how fungi synthesize drimane‐type sesquiterpenes and the corresponding esters.
Keywords: Aspergillus calidoustus, biosynthesis, drimane, natural products, terpenoids
Reported here is the complete elucidation of the biosynthetic pathway of drimane‐type sesquiterpene esters from Aspergillus calidoustus, including the first identified fungal drimenol cyclase. The cluster‐associated acyltransferase is then employed to transfer different lengths of ACP‐activated polyketides, but also very diverse CoA‐esters.
Introduction
Drimane‐type sesquiterpenes are a large group of natural products with unique C15 bicyclic skeletons. They have been identified in various eukaryotes including plants, [1] liverworts, [2] molluscs, [2] sponges, [2] and fungi (primarily Aspergillus [3] and Penicillium [4] species). Many of them possess “drug‐like” chemical properties and display diverse biological activities, including antimicrobial, [5] anti‐inflammatory, [6] cytotoxic, [7] neurotransmission, [8] anti‐diabetic, [1a] and antihyperlipidemic [9] activity. Moreover, the well‐known drimane dialdehydes act as antifeedants against insects and have potential to be used as alternative insecticides. [10]
Because of their interesting structural features and biological activities, fungi‐derived drimanes have attracted increasing attention. Intriguingly, fungi‐derived drimane‐type sesquiterpenes can possess a γ‐butyrolactone ring and are generally esterified (Figure 1). In some cases, esterification helps to increase the activities.[ 3b , 11 ] However, so far the research on this class of compounds has been mainly focused on the isolation, structure elucidation, and bioactivity characterization, with the only exception of astellolides, which are partially characterized at a genetic level in Aspergillus oryzae. [12] The previous studies on the biosynthesis of astellolides suggested that drim‐8‐ene‐11‐ol is the used precursor, produced by the haloacid dehalogenase‐like (HAD‐like) terpene cyclase AstC and two dephosphorylases. Nonetheless, while astellolides harbor a Δ8,9 double bond, other isolated compounds like nanangenines, 22‐hydroxyxylodonin B and purpuride F contain a double bond at a different position (Δ7,8), suggesting a different biosynthesis (Figure 1).
Figure 1.
Structures of representative fungal drimane‐type sesquiterpene esters.
As part of our program to discover novel bioactive molecules from Aspergilli, we performed a chemical investigation on Aspergillus calidoustus that led to the isolation of a series of drimane‐type sesquiterpenes and their esters (1–16; Scheme 1), which were the dominant secondary metabolites. Meanwhile, we employed bioinformatics analysis and a gene deletion campaign to characterize the related biosynthetic gene cluster (BGC). Here, we demonstrated that the isolated drimane‐type molecules originate from drimenol and that the identified terpene cyclase has the same activity observed in plant‐derived cyclases. [13] Furthermore, to better clarify the individual biosynthetic steps, the heterologous expressions of different combinations of the identified genes in Aspergillus fumigatus and Saccharomyces cerevisiae were conducted. Additionally, given the similarity between these compounds and nanangenines (Figure 1), we swapped the enoyl reductase (ER) domain of the identified polyketide synthase (PKS) with the ER domain from Aspergillus nanangensis, resulting in metabolites containing various polyketide chains. Lastly, because of the diversity of the ester moieties in drimane‐type sesquiterpene esters, we determined the substrate specificity of the involved acyl transferase, demonstrating that this enzyme is able to use both ACP‐ and CoA‐activated substrates.
Scheme 1.
Proposed biosynthetic pathway for drimane sesquiterpenoids isolated from Aspergillus calidoustus. Gray color represents an as yet unidentified Aspergillus calidoustus endogenous enzyme. Boxed compounds are shunt products isolated from the ΔdrtF mutant strain.
Results and Discussion
Biosynthesis of Drimane Sesquiterpenoids in A. calidoustus
By fermenting A. calidoustus in V8 production medium, we observed the abundant production of different secondary metabolites. Large scale fermentation resulted in the isolation of sixteen compounds (1–16; Scheme 1 and Table S1). The extensive analysis of the HRESIMS and NMR data revealed that these molecules were all structurally related to drimane sesquiterpenoids with compounds 5–16 coupled to polyketide chains with different lengths (C6 or C8, Tables S2–S17). The here identified compounds 1–6, 8–10, 12–14 and 16, have already been reported in Aspergillus ustus, [14] with 5 and 6 also having been identified in Aspergillus flavus. [15] However, we also isolated a few novel derivatives, named calidoustene A (7), calidoustene B (11) and calidoustene C (15, Scheme 1).
As the identified compounds are structurally similar to the astellolides, drimane‐type esters found in A. oryzae, we performed a genome mining analysis using the sequence of the characterized HAD‐like terpene cyclase as probe, [12] and identified an orthologue on chromosome 2 (ASPCAL02 978), confirming previous computational analysis. [11] Investigation of neighboring genes revealed the presence of six open reading frames, coding for potential proteins proposed to be likely involved in sesquiterpene ester biosynthesis (Figure 2 A), specifically: a polyketide synthase (PKS; named DrtA) and the above mentioned HAD‐like terpene cyclase (DrtB) theoretically involved in the biosynthesis of the C6/C8 polyketide chains and drimane backbone, respectively, an alpha/beta hydrolase (DrtE), a FAD‐binding oxidoreductase (DrtC), a cytochrome P450 (DrtD), and a short‐chain dehydrogenase (DrtF) possibly involved in further modifications of the obtained drimane sesquiterpene and the polyketide chain. To establish an efficient gene deletion campaign, for the confirmation of gene functions and cluster boundaries, we firstly deleted the gene coding for the AkuA DNA helicase (ASPCAL00120) in A. calidoustus, thereby suppressing the non‐homologous end‐joining repair mechanism. [16] Chemical analysis confirmed that the obtained A. calidoustus ΔakuA mutant still produced compounds 1–16 (Figure S1); thus, we used the obtained strain as the recipient for further deletions.
Figure 2.
Analysis of drt gene deletion mutants. A) The identified drt gene cluster from Aspergillus calidoustus. B) HPLC analysis (λ=254 nm) of the crude extracts from gene deletion strains. The identified genes were deleted using the ΔakuA mutant as recipient strain. C) A. calidoustus ΔakuA/ΔdrtB mutant supplemented with drimenol.
The deletion of the PKS coding gene drtA showed complete loss of all the sesquiterpene ester compounds, whereas 1–4 were still produced (Figure 2 B). This confirmed that the identified DrtA was indeed responsible for the drimane sesquiterpenoids' biosynthesis. Moreover, we observed the same chemical pattern by deleting the gene coding for the alpha/beta hydrolase DrtE. This implies that DrtE is responsible for the loading of the polyketide moiety on the drimane backbone. Additionally, conserved domain database (CDD) [17] analysis of the deduced DrtA amino acid sequence has shown that this PKS is missing a thioesterase (TE) domain, suggesting that DrtE mainly functions as an acyltransferase but it likely executes an accessory thioesterase activity. [18]
The deletion of drtB, coding for the putative HAD‐like terpene cyclase, as expected, completely abolished the drimane sesquiterpenes' biosynthesis (Figure 2 B), while deletion of drtC resulted in a large accumulation of 13, with all compounds harboring the γ‐butyrolactone ring (5–11 and 14–16) or containing a carboxylic acid at C‐11 (12) disappearing. This implies that DrtC is able to catalyze the formation of carboxylic acids at C‐11 and C‐12. Meanwhile, based on the feature of 13, the remaining two enzymes, DrtD and DrtF, are potential candidates involved in the formation of hydroxy groups at C‐6, C‐9 and C‐12 in 13. Subsequently, deletion of drtD also led to complete absence of the drimane sesquiterpenes, confirming that DrtD plays a role in supplying oxidized drimane precursors in the biosynthesis.
Upon deletion of drtF, HPLC and LC–HRMS analyses showed that 5–11 disappeared, and 12–16 with fully unsaturated acyl chains could be detected (Figure 2 B). This suggests that the short‐chain dehydrogenase DrtF can catalyze the single or multiple oxidations occurring on the PKS chain. Interestingly, this strain yielded higher titers of 17 and 18, the former was previously identified in A. ustus, [19] and permitted the isolation of two novel compounds, calidoustene D (19) and calidoustene E (20, Figure 2 B and Scheme 1).
Lastly, we confirmed the BGC boundaries. We deleted genes ASPCAL02975 (coding for putative alpha/beta hydrolase) and ASPCAL02983 (coding for ankyrin repeats), and observed the same production pattern as for the wild type and the ΔakuA strain (Figure S1). We also attempted to delete the putative gene ASPCAL02976. However, the deletion of this locus probably failed due to its highly similarity to a second locus present in the genome (ASPCAL01670).
The gene deletion campaign was very useful for assigning gene functions to all open reading frames composing the drt BGC. However, we could not fully elucidate the structure of the HAD‐like terpene cyclase product. During astellolide biosynthesis, the terpene cyclase is responsible for the formation of drimanyl pyrophosphate, which is then dephosphorylated leading to the synthesis of drim‐8‐ene‐11‐ol (Figure 1). [12] Since the hydroxy group at C‐9 can potentially cause the migration of the double bond to Δ7,8, drim‐8‐ene‐11‐ol was also predicted to be the nanangenines' precursor (Figure 1). [11] However, we observed that not all of the intermediates isolated from A. calidoustus harbor a hydroxy group at C‐9, such as 3, 4 and 18. Also, previously isolated compounds, such as 22‐hydroxyxylodonin B and purpuride F, are also missing the hydroxy group at C‐9 (Figure 1). Based on these observations, we postulated that drimenol is the likely upstream precursor and the product of the terpene cyclase DrtB. Next, A. calidoustus ΔdrtB mutant strain was treated with drimenol, resulting in the re‐detection of drimane‐type sesquiterpenes and their esters (Figure 2 C), which validates our hypothesis.
To verify the activity of DrtB, we performed heterologous expression of the isolated cDNA in Escherichia coli. Expression of the native open reading frame resulted in no accumulation of the recombinant protein. Subsequently, we deleted the highly hydrophobic C‐terminal part of the enzyme, which led to an observable production of drimenol in vivo (Figure 3 A and Figure S2). Furthermore, the incubation of the purified DrtB with farnesyl pyrophosphate (FPP) led to the in vitro synthesis of drimenol (Figure S3), thus confirming that DrtB is a drimenol cyclase.
Figure 3.
Heterologous expression in E. coli and S. cerevisiae. Extracted ion chromatograms (EIC) showing A) the production of drimenol by DrtB in vivo, B) the production of 17, 18 and 21–23 from S. cerevisiae transformants: i) control with empty plasmid, ii) expression of drtB, iii) drtD, iv) co‐expression of drtB and drtD, and v) co‐expression of drtB, drtD and drtC, and C) the metabolites produced after feeding drimenol to i) S. cerevisiae expressing drtD and ii) control. D) The structures of compounds 21–26 (marked with “) were deduced based on their HRESIMS spectra.
To better characterize the role played by DrtC and DrtD in modifying drimenol, we heterologously expressed three genes, namely drtB, drtD and drtC, in S. cerevisiae using yeast expression plasmids. The individual expression of drtB or drtD produced no detectable drimenol or drimenol derivatives (Figure 3 B, ii and iii), suggesting that the drimenol produced by DrtB might be further metabolized in S. cerevisiae. The bicistronic expression of drtB and drtD however led to the production of 17 and 18 (Figure 3 B, iv). Unexpectedly, this strain also produced 21, which possesses the same molecular weight as 18. Co‐expression of drtB, D and C produced 22 and 23, in which C‐11 is oxidized into carboxylic acid and condensed to a γ‐butyrolactone ring (Figure 3 B, v). Therefore, the results confirmed that DrtD catalyzes the hydroxylation at C‐6 and C‐9 position, and it is also able to oxidize the hydroxy group at C‐11 to an aldehyde. Additionally, DrtD seems to further oxidize the hydroxy group at C‐6 to form a ketone.
To further confirm this hypothesis, drimenol was fed to S. cerevisiae expressing drtD alone, with a control strain containing the empty plasmid (Figure 3 C). With the feeding experiments, the production of 17, 18 and 21 was confirmed, and a small amount of 18 was also produced by the control. Additionally, the production of 24 further confirmed that DrtD could oxidize the alcohol at C‐11 to an aldehyde, while the presence of 25 and 26 confirmed that this P450 also catalyzes the hydroxylation at C‐12. Taken together, the heterologous expression in yeast demonstrated that the P450 DrtD is responsible for the hydroxylations at C‐6, C‐9 and C‐12, as well as the oxidation of hydroxy groups at C‐6 and C‐11 to a ketone and an aldehyde, respectively; then, the C‐11 aldehyde can be further oxidized into a carboxylic acid by DrtC. Moreover, these results show that DrtB, DrtD and DrtC are solely responsible for the formation of the different drimane structures observed during drimane sesquiterpene biosynthesis in A. calidoustus, and that the different degree of oxidation at C‐11 and C‐12 determines the divergent γ‐butyrolactone conformations observed in 15, 16 and 20.
Enoyl Reductase Domain Swapping of the Polyketide Synthase DrtA
Among all the drimane‐type sesquiterpene esters isolated from fungi, the structures of nanangenines (Figure 1), with the acyl chains fully saturated, suggest a divergent evolution of the involved PKSs. It is known that the α–β double bond formed by a PKS dehydratase (DH) domain is reduced by an enoyl reductase (ER) domain to generate a single bond in the nascent polyketide. [18] Therefore, we assume that fungal PKSs involved in drimane‐type sesquiterpene ester biosynthesis would present variations in their ER domains. Genome mining on the available fungal genomes identified that the drt BGC is conserved in twelve different Aspergillus species (Figure 4 A). Phylogenetic analysis based on the deduced amino acid sequences of the identified PKS–ER domains revealed the presence of two distinct clades: clade I includes A. calidoustus and A. ustus, both producing sesquiterpene esters with different levels of desaturation in the polyketide chain, and clade II, which includes A. nanangensis. To validate the phylogenetic analysis, we aimed to swap the ER domain present in DrtA with the PKS–ER potentially involved in nanangenine biosynthesis. The ER region to be swapped was determined based on the amino acid sequence alignment of the closely related PKS–ER domains (Figure S4), and the modified PKS was named DrtA* (Figure 4 B).
Figure 4.
Modification of the polyketide synthase DrtA by domain swapping of the enoyl reductase (ER). A) Phylogenetic analysis based on PKS–ER domains. The analysis was performed by comparison with orthologue genes identified from other Aspergilli. Putative clusters from the analyzed species are also reported, including scaffold numbers and chromosomal coordinates. B) The ER domain in the DrtA sequence was swapped with the ER domain from the A. nanangensis PKS (FE257_006541, labeled in fuchsia), leading to the synthetic construct DrtA*. KS, ketosynthase; AT, acyltransferase; DH, dehydratase; KR, ketoreductase; P, acyl carrier protein. C) HPLC analysis (UV λ=254 nm) of the crude extracts from ectopic integration of the drtA* in A. calidoustus wild‐type strain. The mutant strain (wt/drtA*) and the control strain (wild type) were grown under non‐inducing (Tet−) and inducing (Tet+) conditions. D) Structure of compound 27 isolated from wt/drtA* inducing strain.
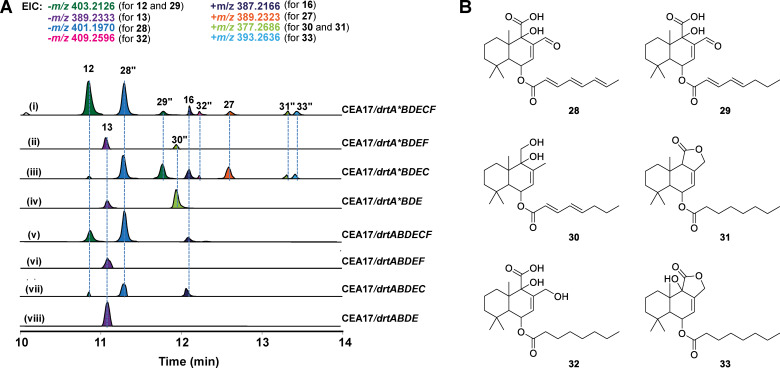
Because of the lack of available selection markers, any attempt to introduce the drtA* into the A. calidoustus ΔdrtA mutant failed. Therefore, we firstly expressed the synthetic drtA* gene in the A. calidoustus wild‐type strain under the control of a tetracycline‐inducible promoter (tet ON). [20] Although the overall production of drimane sesquiterpenes in the wt/drtA* mutant was lower than that of the wild type, the expression of the modified DrtA* led to the identification of a novel metabolite, calidoustene F (27, Figure 4 C,D). This compound was successfully isolated from a large‐scale cultivation, and, as expected, it was a drimane‐type sesquiterpene ester having the PKS chain with a reduced terminal double bond (Figure 4 D). However, with this experiment we did not identify any molecules harboring a fully saturated polyketide. To further examine the function of the ER‐swapped drtA* gene we performed heterologous expression (Figure 5 A). We first expressed each PKS gene, drtA or drtA*, in A. fumigatus to yield mutant strains CEA17/drtA and CEA17/drtA*. Afterwards, different combinations of additional drt genes were polycistronically expressed in CEA17/drtA and CEA17/drtA*, respectively. The mutant strains missing the drtC gene mainly produced 13 (Figure 5 A, ii, iv, vi and viii), as observed in the A. calidoustus ΔdrtC strain (Figure 2 B). However, those strains lacking DrtC but expressing the ER‐swapped PKS, namely drtA*BDEF (Figure 5 A, ii) and drtA*BDE (Figure 5 A, iv), also produced metabolite 30, with a partially saturated polyketide tail. The addition of drtC to the polycistron led to the production of 12, 16 and 28 (Figure 5 A, i, iii, v and vii), intermediates with highly unsaturated polyketide chains. Nonetheless, in mutants containing the swapped ER‐domain (Figure 5 A, i and iii), we identified compounds 27 and 29, with partially reduced acyl chains, and as well 31–33 containing fully saturated acyl chains (Figure 5 B). These results confirmed that, upon ER domain swap, the obtained PKS could produce fully saturated acyl chains. However, we also observed partially saturated and fully unsaturated acyl chains, indicating that the specificity of the ER domain is influenced by other structural domains. It is known that the reductive steps during polyketide elongation are optional, suggesting that the ER reductive step is always skipped by DrtA. [18] Nevertheless, the ER‐domain in the drtA* mutants is able to optionally skip the reducing steps during polyketide chain synthesis (Figure S5). Moreover, the heterologous expression in A. fumigatus revealed another interesting aspect of the biosynthesis: since we did not detect compounds 1–4, we assume that hydroxylations at C‐2 and C‐3 are catalyzed by endogenous enzymes in A. calidoustus not associated to the drt BGC (Scheme 1).
Figure 5.
Heterologous expression in A. fumigatus. Genes of drtA and drtA* were expressed in A. fumigatus in combination with the other drt genes. A) LC–HRMS EIC of the metabolites from A. fumigatus transformants. B) The structures of compounds 28–33 (marked with “) were deduced based on their HRESIMS spectra.
Determining the Substrate Specificity of the Acyltransferase DrtE
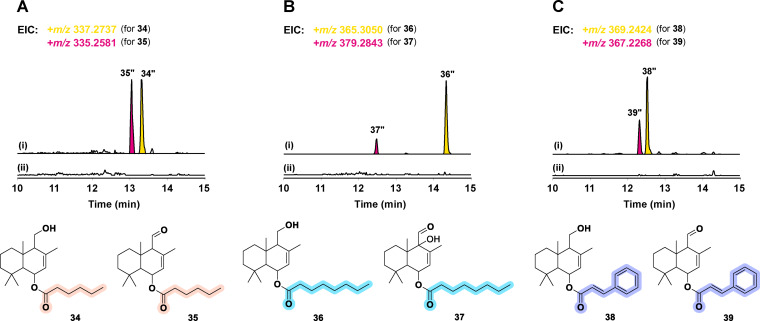
Motivated by the diversity of the ester moieties in fungi‐derived drimane‐type sesquiterpene esters, we determined the substrate specificity of the involved acyl transferase DrtE by feeding different potential substrates together with drimenol to S. cerevisiae expressing drtD, drtE and two different CoA ligases. The 4‐coumaroyl‐CoA ligase from Nicotiana tabacum (4CL) [21] and the long‐chain‐fatty‐acid‐CoA ligase from E. coli (FadD) [22] were used to esterify selected substrates, namely hexanoic, octanoic, and cinnamic acid. Interestingly, DrtE was able to use all tested CoA‐activated substrates, not only the different lengths of fatty acyl‐CoA (C6 and C8), but also the cinnamoyl‐CoA (Figure 6).
Figure 6.
Substrate specificity of DrtE. A) S. cerevisiae expressing drtD, drtE and the coumaroyl‐CoA ligase 4CL fed with hexanoic acid i) with and ii) without drimenol. B) S. cerevisiae expressing drtD, drtE and the fatty‐acid CoA‐ligase fadD fed with octanoic acid i) with and ii) without drimenol. C) S. cerevisiae expressing drtD, drtE and 4CL fed with cinnamic acid i) with and ii) without drimenol. The structures of 34–39 were inferred based on HRESIMS spectra.
Conclusion
Here, we elucidated the complete biosynthetic pathway of the fungal natural products drimane‐type sesquiterpenes and their esters (1–16) isolated from A. calidoustus, established through gene inactivation, heterologous expression and feeding experiments. Firstly, the backbone of these compounds, drimenol, is produced by the terpene cyclase DrtB. Next, the P450 DrtD catalyzes the hydroxylation at C‐6, C‐9 and C‐12, and it is also responsible for the oxidation of hydroxy groups at C‐6 and C‐11 to ketone and aldehyde, respectively. Then, the biosynthesis can go in two directions, either the hydroxylated drimenol is further hydroxylated at C‐2 and C‐3 by an enzyme(s) not associated with the drt BGC, or the FAD‐binding oxidoreductase DrtC further oxidizes C‐11 or C‐12 obtaining a carboxylic acid, which is then condensed with the γ‐OH to form the butyrolactone ring. The polyketide synthase DrtA synthesizes different lengths (C6 and C8) of PKS chains, which are then oxidized to varying degrees by the short‐chain dehydrogenase DrtF. Finally, these PKS chains are transferred onto drimane sesquiterpenes by the acyltransferase DrtE, forming the sesquiterpene esters. We further demonstrated that upon swapping of the ER domain, we could obtain partially and fully saturated polyketide chains, confirming the evolutionary divergence observed among the BGCs identified in closely related Aspergillus species. Furthermore, the acyltransferase DrtE was shown to utilize both ACP‐ and CoA‐activated substrates, which could be an efficient tool for further applications, such as the substrate‐driven derivatization of drimane‐type sesquiterpene esters.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
We thank Daniela Hildebrandt for the excellent technical support and Heike Heinecke for conducting NMR measurements. This work was mainly supported by a grant of the European Social Fund ESF “Europe for Thuringia” projects SphinX (2017FGR0073) and by the Leibniz Research Cluster (LRC) in the framework of the BMBF Strategic Process Biotechnology 2020+ (031A360A). Open Access funding enabled and organized by Projekt DEAL.
Y. Huang, S. Hoefgen, V. Valiante, Angew. Chem. Int. Ed. 2021, 60, 23763.
References
- 1.
- 1a. Belhadj S., Keskes H., Apel C., Roussi F., Litaudon M., Hentati O., Allouche N., Chem.-Biol. Interact. 2020, 330, 109167; [DOI] [PubMed] [Google Scholar]
- 1b. He D., Slebodnick C., Rakotondraibe L. H., Bioorg. Med. Chem. Lett. 2017, 27, 1754–1759; [DOI] [PubMed] [Google Scholar]
- 1c. Hu Y., Tao L., Tan H., Zhang M., Shimizu K., Zhang F., Zhang C., Inflammation 2017, 40, 1204–1213; [DOI] [PubMed] [Google Scholar]
- 1d. Karmahapatra S., Kientz C., Shetty S., Yalowich J. C., Rakotondraibe L. H., J. Nat. Prod. 2018, 81, 625–629. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. Jansen B. J. M., de Groot A., Nat. Prod. Rep. 2004, 21, 449–477; [DOI] [PubMed] [Google Scholar]
- 2b. Jansen B. J. M., de Groot A., Nat. Prod. Rep. 1991, 8, 309–318. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a. Rahbæk L., Christophersen C., Frisvad J., Bengaard H. S., Larsen S., Rassing B. R., J. Nat. Prod. 1997, 60, 811–813; [Google Scholar]
- 3b. Yurchenko A. N., Trinh P. T. H., Smetanina O. F., Rasin A. B., Popov R. S., Dyshlovoy S. A., von Amsberg G., Menchinskaya E. S., Thanh Van T. T., Afiyatullov S. S., Mar. Drugs 2019, 17, 579; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3c. Li H., Zhang R., Cao F., Wang J., Hu Z., Zhang Y., J. Nat. Prod. 2020, 83, 2200–2206; [DOI] [PubMed] [Google Scholar]
- 3d. Ren R., Chen C. J., Hu S. S., Ge H. M., Zhu W. Y., Tan R. X., Jiao R. H., Chem. Biodiversity 2015, 12, 371–379. [DOI] [PubMed] [Google Scholar]
- 4.
- 4a. Ma M., Ge H., Yi W., Wu B., Zhang Z., Tetrahedron Lett. 2020, 61, 151504; [Google Scholar]
- 4b. Liu H., Li X. M., Liu Y., Zhang P., Wang J. N., Wang B. G., J. Nat. Prod. 2016, 79, 806–811; [DOI] [PubMed] [Google Scholar]
- 4c. Liao L., Lee J. H., You M., Choi T. J., Park W., Lee S. K., Oh D. C., Oh K. B., Shin J., J. Nat. Prod. 2014, 77, 406–410. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. Intaraudom C., Punyain W., Bunbamrung N., Dramae A., Boonruangprapa T., Pittayakhajonwut P., Fitoterapia 2019, 138, 104353; [DOI] [PubMed] [Google Scholar]
- 5b. Flores-Bocanegra L., Augustinović M., Raja H. A., Kurina S. J., Maldonado A. C., Burdette J. E., Falkinham J. O., Pearce C. J., Oberlies N. H., Tetrahedron Lett. 2021, 68, 152896; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5c. Ma X., Li L., Zhu T., Ba M., Li G., Gu Q., Guo Y., Li D., J. Nat. Prod. 2013, 76, 2298–2306; [DOI] [PubMed] [Google Scholar]
- 5d. Zhao J., Feng J., Tan Z., Liu J., Zhao J., Chen R., Xie K., Zhang D., Li Y., Yu L., J. Nat. Prod. 2017, 80, 1819–1826. [DOI] [PubMed] [Google Scholar]
- 6.
- 6a. Chen C., Sun W., Liu X., Wei M., Liang Y., Wang J., Zhu H., Zhang Y., Bioorg. Chem. 2019, 91, 103166; [DOI] [PubMed] [Google Scholar]
- 6b. Felix S., Sandjo L. P., Opatz T., Erkel G., Bioorg. Med. Chem. 2014, 22, 2912–2918. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a. Fang W., Lin X., Zhou X., Wan J., Lu X., Yang B., Ai W., Lin J., Zhang T., Tu Z., MedChemComm 2014, 5, 701–705; [Google Scholar]
- 7b. Kwon J., Lee H., Seo Y. H., Yun J., Lee J., Kwon H. C., Guo Y., Kang J. S., Kim J. J., Lee D., J. Nat. Prod. 2018, 81, 1444–1450. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Xu K., Zhou Q., Li X. Q., Luo T., Yuan X. L., Zhang Z. F., Zhang P., Bioorg. Chem. 2020, 104, 104252; [DOI] [PubMed] [Google Scholar]
- 8b. Wang H. L., Li R., Li J., He J., Cao Z. Y., Kurtán T., Mándi A., Zheng G. L., Zhang W., Org. Lett. 2020, 22, 2995–2998. [DOI] [PubMed] [Google Scholar]
- 9. Li Y., Wu C., Liu D., Proksch P., Guo P., Lin W., J. Nat. Prod. 2014, 77, 138–147. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Kubo I., Ganjian I., Experientia 1981, 37, 1063–1064; [DOI] [PubMed] [Google Scholar]
- 10b. Escalera J., von Hehn C. A., Bessac B. F., Sivula M., Jordt S. E., J. Biol. Chem. 2008, 283, 24136–24144; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10c. Inocente E. A., Shaya M., Acosta N., Rakotondraibe L. H., Piermarini P. M., PLoS Neglected Trop. Dis. 2018, 12, e0006265; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10d. Montenegro I., Madrid A., Cuellar M., Seeger M., Alfaro J. F., Besoain X., Martínez J. P., Ramirez I., Olguín Y., Valenzuela M., Molecules 2018, 23, 2053; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10e. Montenegro I., Pino L., Werner E., Madrid A., Espinoza L., Moreno L., Villena J., Cuellar M., Molecules 2013, 18, 4192–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lacey H. J., Gilchrist C. L., Crombie A., Kalaitzis J. A., Vuong D., Rutledge P. J., Turner P., Pitt J. I., Lacey E., Chooi Y. H., Beilstein J. Org. Chem. 2019, 15, 2631–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shinohara Y., Takahashi S., Osada H., Koyama Y., Sci. Rep. 2016, 6, 32865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.
- 13a. Henquet M. G. L., Prota N., van der Hooft J. J., Varbanova-Herde M., Hulzink R. J., de Vos M., Prins M., de Both M. T., Franssen M. C., Bouwmeester H., Plant J. 2017, 90, 1052–1063; [DOI] [PubMed] [Google Scholar]
- 13b. Kwon M., Cochrane S. A., Vederas J. C., Ro D. K., FEBS Lett. 2014, 588, 4597–4603. [DOI] [PubMed] [Google Scholar]
- 14.
- 14a. Lu Z., Wang Y., Miao C., Liu P., Hong K., Zhu W., J. Nat. Prod. 2009, 72, 1761–1767; [DOI] [PubMed] [Google Scholar]
- 14b. Neuhaus G. F., Loesgen S., J. Nat. Prod. 2021, 84, 37–45; [DOI] [PubMed] [Google Scholar]
- 14c. Zhou H., Zhu T., Cai S., Gu Q., Li D., Chem. Pharm. Bull. 2011, 59, 762–766; [DOI] [PubMed] [Google Scholar]
- 14d. Liu H., Edrada-Ebel R., Ebel R., Wang Y., Schulz B., Draeger S., Müller W. E., Wray V., Lin W., Proksch P., J. Nat. Prod. 2009, 72, 1585–1588. [DOI] [PubMed] [Google Scholar]
- 15. Liu Y. F., Yue Y. F., Feng L. X., Zhu H. J., Cao F., Mar. Drugs 2019, 17, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ninomiya Y., Suzuki K., Ishii C., Inoue H., Proc. Natl. Acad. Sci. USA 2004, 101, 12248–12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marchler-Bauer A., Derbyshire M. K., Gonzales N. R., Lu S., Chitsaz F., Geer L. Y., Geer R. C., He J., Gwadz M., Hurwitz D. I., Nucleic Acids Res. 2015, 43, D222–D226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hertweck C., Angew. Chem. Int. Ed. 2009, 48, 4688–4716; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 4782–4811. [Google Scholar]
- 19. Hayes M. A., Wrigley S. K., Chetland I., Reynolds E. E., Ainsworth A. M., Renno D. V., Latif M. A., Cheng X. M., Hupe D. J., Charlton P., J. Antibiot. 1996, 49, 505–512. [DOI] [PubMed] [Google Scholar]
- 20. Meyer V., Wanka F., van Gent J., Arentshorst M., van den Hondel C. A., Ram A. F., Appl. Mech. Mater. Appl. Environ. Microbiol. 2011, 77, 2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Z., Nair S. K., Structure 2015, 23, 2032–2042. [DOI] [PubMed] [Google Scholar]
- 22. Campbell J. W., Morgan-Kiss R. M., J. E. Cronan, Jr. , Mol. Microbiol. 2003, 47, 793–805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information