ABSTRACT
Background
Asymmetric hemispheric loss of dopaminergic neurons is one of the characteristic features of Parkinson's disease (PD). However, it is still debated if right or left asymmetry differently affects cognitive and motor progression.
Objectives
The objective of this study was to investigate, for the first time, the relevance of dopamine transporter (DAT) asymmetry on cognitive and motor manifestations at onset and at 4‐year progression in drug‐naïve PD.
Methods
From the Parkinson's Progression Markers Initiative multicenter cohort, we identified 249 right‐handed patients with PD with baseline asymmetry greater than 20% in putamen DAT binding at single‐photon emission computed tomography. A predominant putamen asymmetry was found on the left in 143 patients (PD‐left), and on the right side in 106 patients (PD‐right); we compared them with 196 healthy controls. Patients were followed longitudinally (2‐year and 4‐year visits), examining their clinical, cognitive, and imaging data.
Results
At baseline, the PD‐left group showed worse performance on the Symbol Digit Modality Test, an attention and processing‐speed test, and lower cerebrospinal fluid β‐amyloid levels than the PD‐right group. These differences were maintained at follow‐up, declining over time in both groups. By contrast, the PD‐right group showed greater motor impairment at baseline, which increased over 4 years. Striatal DAT binding decreased over time in both groups, but the PD‐right group showed a steeper decline, particularly during the first 2‐year follow‐up. Putaminal asymmetry assessed at baseline was maintained over time.
Conclusions
These findings suggest that hemispheric asymmetric dopaminergic denervation influences PD cognitive and motor performance as well as progression. Predominant right hemisphere nigrostriatal dopaminergic loss is associated with greater motor severity, whereas more pronounced left hemisphere denervation affects cognitive manifestations at onset and their progression. © 2021 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society
Keywords: Parkinson's disease, asymmetry, cognitive impairment, DAT‐SPECT, nigrostriatal dopaminergic system
Parkinson's disease (PD) is characterized by hemispheric asymmetric loss of nigrostriatal dopaminergic neurons, which drives motor symptoms lateralization. 1 Hemispheric asymmetry in PD seems to affect not only motor symptoms but also nonmotor features, such as cognition, pain, fatigue, and neuropsychiatric and autonomic manifestations. 2 , 3 , 4 However, previous evidence regarding the patterns and progression of clinical PD phenotypes associated with the hemispheric lateralization of neurodegeneration yielded mixed results, particularly for motor and cognitive functions. 3 , 5 , 6
Methodological heterogeneity of previous studies perhaps contributed to those inconsistent findings, including different designs (mostly cross‐sectional studies, with the longitudinal studies having only short‐term follow‐up), 2 , 7 sample characteristics, and different methods to determine PD lateralization, mainly based on motor symptoms. 8 Indeed, to establish laterality, most studies adopted a subjective method using motor scale scores and patients' reports of their initial side of onset rather than an objective assessment of dopamine denervation, such as the dopamine transporter (DAT) binding single‐photon emission computed tomography (SPECT) imaging. 9 In addition, only one previous work 2 included the clinical spectrum of patients with PD with a stable asymmetry over time, excluding those who turned out to be symmetric overtime, possibly nonidiopathic PD. 2
The Parkinson's Progression Markers Initiative (PPMI)—an observational, international, multicenter study—offers the opportunity to access a large PD cohort with yearly prospective cognitive and motor evaluations and striatal DAT‐SPECT imaging data 10 to improve the understanding of disease mechanisms and evolution.
Hence, in the present study we aimed at exploring for the first time the role of hemispheric asymmetric loss of putaminal DAT‐SPECT uptake—an objective measure of the lateralized motor symptoms severity 11 —on early cognitive and motor manifestations in right‐handed drug‐naïve patients with PD and their progression over 4 years.
We believe our results can expand the current knowledge about the role of hemispheric asymmetry of nigrostriatal degeneration on disease course and possibly provide important clinical implications for treatment plans and intervention strategies.
1. Methods
1.1. Study Design and Participants
In this cohort study, we obtained approval to access the PPMI database and investigate clinical, cognitive, and DAT‐SPECT data. Objectives, methodology, and details of the PPMI study assessments have been published and are available at www.ppmi-info.org/study-design.
From the PPMI data set, as of September 2020, there were 435 newly diagnosed, drug‐naïve patients with PD who had DAT‐SPECT imaging at recruitment using the DAT radiotracer ioflupane ([123I]N‐ω‐fluoropropyl‐2β‐carbomethoxy‐3β‐(4‐iodophenyl)nortropane).
We selected only right‐handed patients with PD with marked putaminal asymmetry (n = 265). The asymmetry index was computed according to the formula described elsewhere9, 12 and we considered only those values above 20%. Of note, we applied a more conservative criteria compared with previous evidence 12 to ensure clear‐cut asymmetry. Furthermore, we excluded patients with PD with motor symptoms on the side ipsilateral to the predominant dopaminergic putaminal deficit (n = 16); this led to a final sample of 249 right‐handed patients with PD: 143 with left putaminal (PD‐left) versus 106 with right putaminal lateralization (PD‐right).
These patients were followed longitudinally, and we examined their data at baseline (or the screening visit 45 days before) and at 2‐year and 4‐year visits. At the 2‐year visit, the sample consisted of 112 patients with PD‐left and 87 patients with PD‐right (n = 199), and at the 4‐year visit there were 92 patients with PD‐left and 67 patients with PD‐right (n = 159). At both follow‐up visits, patients were on treatment; hence, the levodopa equivalent daily dose (LEDD) was calculated at each time point.
Finally, we analyzed the data of healthy controls (HC) in the PPMI cohort and included only those having no cognitive impairment (Montreal Cognitive Assessment [MoCA] score ≥26), normal DAT‐SPECT binding, and right‐handedness, leading to a final sample of 196 HC (for more details, see the flowchart [Fig. 1]). We also evaluated HC cognitive assessment at the 2‐year (n = 166) and 4‐year visits (n = 154) to derive the normative scores at each time point and calculate z scores for the PD sample (as described in the next paragraph).
FIG. 1.
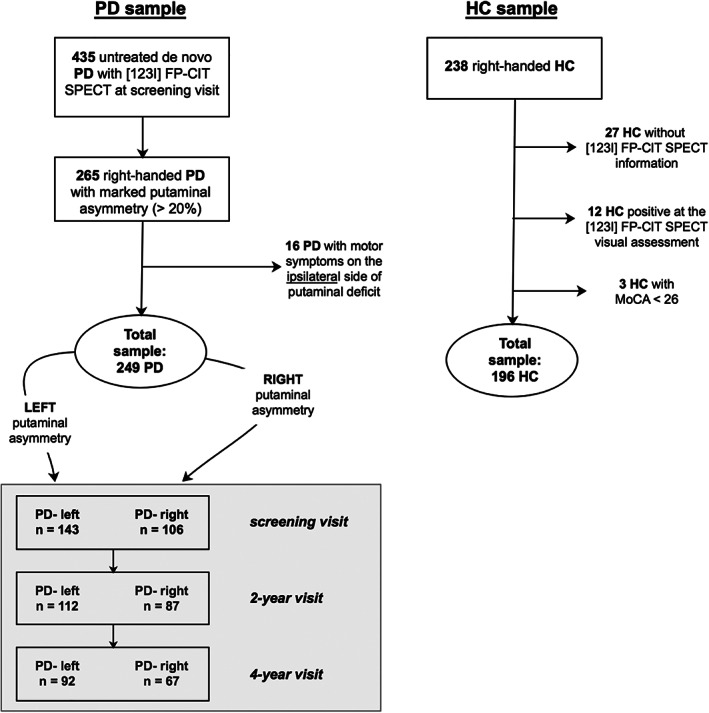
Flowchart of the studied population. [123I] FP‐CIT, [123I]N‐ω‐fluoropropyl‐2β‐carbomethoxy‐3β‐(4‐iodophenyl)nortropane; HC, healthy controls; MoCA, Montreal Cognitive Assessment; PD, Parkinson's Disease; SPECT, single‐photon emission computed tomography.
The PPMI study is registered with ClinicalTrials.gov (NCT01141023), and all participants gave their written informed consent to participate in the program.
1.2. Cognitive and Clinical Assessment
Demographic and clinical variables included age, years of education, sex, disease duration, LEDD, dopamine agonist equivalent daily dose, Movement Disorder Society Unified Parkinson's Disease Rating Scale motor score (MDS‐UPDRS III), and daily functioning by the Activities of Daily Living (ADL) scale. All participants were drug‐naïve for PD medication at the screening visit. To assess each patient's motor asymmetry, lateralized subscores consisting of the sum of side‐specific MDS‐UPDRS III items were calculated (items 3–8, 15, and 17).
Global cognition was assessed using MoCA scores at the time of imaging examination. Additional cognitive tests included the Benton Judgment of Line Orientation 15‐item version (JLO) for visuospatial domain 13 ; the Symbol Digit Modalities Test (SDMT) for attention, visual scanning, and motor speed 14 , 15 ; the Hopkins Verbal Learning Test–Revised with immediate and delayed recall for memory 16 ; the Letter Number Sequencing (LNS) for attention and working memory 17 ; and the semantic fluency test for language abilities. 18 According to the PPMI protocol, motor and cognitive evaluations were performed with patients on levodopa treatment at follow‐up visits.
PD cognitive scores were converted to z scores for each patient after computing internal age‐based norms using HC scores at each time point, allowing us to account for practice effect. Age‐normative data were computed using the following age ranges as previously proposed 19 (age‐based norms are presented in Table e‐1 in Appendix S1, expressed as mean and standard deviation [SD] at each time point): 30 to 45, 46 to 60, 61 to 75 and 76–90 years. We classified patients as clinically impaired in a given neuropsychological test, if the z score was at least −1.5 SD below the appropriate norms. Depression was evaluated with the 15‐item Geriatric Depression Scale. There were no patients with PD with dementia, as all patients were independent in daily living activities as assessed by ADL scores >80/100.
1.3. Cerebrospinal Fluid Sample Measures
A subsample of patients with PD (n = 231 [131 PD‐left, 100 PD‐right]) and HC (n = 178) underwent lumbar puncture at baseline and then patients were followed longitudinally at 2‐year (n = 169 [94 PD‐left, 75 PD‐right]) and 3‐year visits (n = 118 [68 PD‐left, 50 PD‐right]), which was the last available visit. β‐Amyloid (Aβ42), total tau, tau phosphorylated at the threonine (p‐tau181), and the ratio of Aβ42 to total tau were measured in the cerebrospinal fluid (CSF) using the Roche Elecsys fully automated immunoassay developed by Roche Diagnostics (Basel, Switzerland). Indeed, this method seems to reduce CSF measurement variability across laboratories, improving its reliability. The Aβ42 assay has a measuring range of 200 to 1700 pg/mL; the total tau assay, 80 to 1300 pg/mL; and the p‐tau181 assay, 8 to 120 pg/mL. For Aβ42, a cutoff of 1100 pg/mL has been proposed. 20 The α‐synuclein concentration was analyzed using an enzyme‐linked immunosorbent assay available commercially from BioLegend (San Diego, CA). The reference standards used in the assay ranged from 6.1 to 1500 pg/mL. Further methodological details are described comprehensively on the PPMI website (http://www.ppmi‐info.org/studydesign/research‐documents‐and‐sops/).
1.4. DAT‐SPECT
The nigrostriatal pathway integrity was obtained with DAT‐SPECT at the screening visit and annually at each PPMI imaging site following standardized imaging protocols (http://www.ppmi-info.org/data). For this study, we examined the acquisition at the screening (to establish the presence of asymmetry) and at 2 years and 4 years (to assess progression). SPECT raw projection data were reconstructed using iterative reconstruction algorithm at a central SPECT Core laboratory in New Haven, CT. Iterative reconstruction was done without any filtering applied. The methods of SPECT data reconstruction and analysis have been reported previously 21 , 22 (further details are reported in the Methods section in Appendix S1); circular regions of interest were placed on the left and right putamen and left and right caudate nucleus as well as on occipital reference regions.
1.5. Statistical Analysis
Preliminary analyses were conducted to assess the normal distribution using the Kolmogorov–Smirnov test. Demographics and clinical characteristics were compared across groups using analysis of variance for continuous variables, whereas the chi‐square test was used for categorical variables. Neuropsychological performance was compared between groups (HC vs. all PD and PD‐left vs. PD‐right) using analyses of covariance including age and education as covariates plus motor severity when comparing SDMT performance as this cognitive task has a motor component.
Pearson correlations were performed to explore the association between the DAT‐SPECT specific binding ratio (SBR) of the four striatal regions and neuropsychological measures. To investigate the progression of striatal asymmetry as well as clinical and cognitive performance, follow‐up data were analyzed by means of linear mixed effects models. DAT‐SPECT, CSF, and clinical and cognitive measures were set as dependent variables in each model. The time points (screening, 2‐year and 4‐year visit) and the putaminal asymmetry categorization (PD‐left vs. PD‐right) were entered as predictors and included as fixed effects of the model together with their interactions. The individual variability was included in the model by entering patients as a random factor (random intercept model). LEDD or MDS‐UPDRS III was entered as covariates in the model when cognitive performances were tested to exclude their possible influence. Statistical analyses were performed using R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). Specifically, for mixed models, the lme4 package was used, degrees of freedom of such comparisons were computed using the Satterthwaite approximation method, and conditional R 2 was calculated as a measure of goodness of fit of the tested models. The statistical significance threshold was set at P < 0.050 using the false discovery rate (FDR) correction for multiple comparisons when appropriate.
2. Results
As shown in Table 1, we found no significant differences between HC and the whole PD group in terms of age, education, and sex. A reduced caudate and putaminal DAT‐SPECT uptake was observed in patients with PD compared with HC (P < 0.001) as well as lower levels in CSF measurements of α‐synuclein, total tau, and p‐tau181 biomarkers. Patients with PD showed poorer cognitive performance in all cognitive tests except for the LNS and Benton JLO.
TABLE 1.
Demographic, DAT‐SPECT, clinical, and cognitive characteristics of HC and PD groups according to the hemispheric asymmetry of nigrostriatal degeneration
| HC, n = 196 | PD‐left, n = 143 | PD‐right, n = 106 | P value, HC vs. all PD | P value, PD‐left vs. right | |
|---|---|---|---|---|---|
| Demographic and clinical characteristics | |||||
| Age, y | 59.83 (11.25) | 60.30 (9.80) | 59.29 (9.68) | 0.752 | 0.420 |
| Sex, female/male | 69/127 | 50/93 | 45/61 | 0.229 | |
| Education, y | 16.02 (2.96) | 15.64 (3.00) | 15.3 (3.01) | 0.130 | 0.376 |
| Disease duration from symptoms, mo | 5.67 (6.51) | 5.69 (6.72) | 0.986 | ||
| DAT‐SPECT | |||||
| Mean caudate, SBR | 2.99 (0.61) | 2.02 (0.56) | 2.02 (0.53) | <0.001 | 1 |
| Caudate left | 3.00 (0.64) | 1.84 (0.56) | 2.24 (0.56) | <0.001 | <0.001 |
| Caudate right | 2.97 (0.62) | 2.21 (0.60) | 1.80 (0.53) | <0.001 | <0.001 |
| Mean putamen, SBR | 2.16 (0.55) | 0.85 (0.29) | 0.85 (0.29) | <0.001 | 0.954 |
| Putamen left | 2.15 (0.56) | 0.63 (0.24) | 1.06 (0.38) | <0.001 | <0.001 |
| Putamen right | 2.16 (0.57) | 1.06 (0.37) | 0.64 (0.22) | <0.001 | <0.001 |
| CSF markers, pg/mL | |||||
| Aβ42 | 1024.17 (497.45) | 883.84 (359.80) | 1002.21 (517.14) | 0.056 | 0.042 |
| α‐synuclein | 1702.59 (764.83) | 1527.37 (666.30) | 1577.79 (701.31) | 0.033 | 0.578 |
| Total tau | 189.91 (80.45) | 163.53 (53.64) | 177.13 (64.75) | 0.003 | 0.082 |
| p‐tau181 | 16.84 (8.54) | 13.95 (4.90) | 14.92 (5.93) | <0.001 | 0.172 |
| Aβ42:total tau ratio | 5.59 (1.65) | 5.49 (1.46) | 5.68 (1.68) | 0.891 | 0.366 |
| Motor characteristics | |||||
| MDS‐UPDRS III | 19.02 (8.62) | 22.3 (8.51) | 0.003 | ||
| MDS‐UPDRS III –asymmetry subscores | |||||
| Left side | 2.76 (3.48) | 12.17 (4.3) | <0.001 | ||
| Right side | 10.23 (3.95) | 3.39 (3.38) | <0.001 | ||
| Cognitive characteristics | |||||
| MoCA | 28.07 (1.14) | 27.10 (2.29) | 26.86 (2.52) | <0.001 | 0.422 |
| Benton JLO a | 26.17 (4.03) | 25.65 (4.08) | 25.47 (4.30) | 0.286 | 0.939 |
| HVLT‐R Immediate Recall a | 26.08 (4.57) | 24.88 (5.12) | 24.25 (4.41) | 0.004 | 0.391 |
| HVLT‐R Delayed Recall a | 9.28 (2.36) | 8.58 (2.48) | 8.15 (2.36) | <0.001 | 0.154 |
| HVLT‐R Recognition a | 11.48 (0.83) | 11.24 (1.34) | 11.16 (1.17) | 0.026 | 0.965 |
| LNS a | 10.85 (2.64) | 10.44 (2.81) | 10.75 (2.46) | 0.460 | 0.458 |
| SDMT a | 46.99 (10.64) | 41.18 (8.76) | 43.37 (10.71) | <0.001 | 0.017 |
| Semantic fluency a | 52.09 (11.31) | 48.52 (12.24) | 48.53 (11.33) | 0.004 | 0.909 |
Note. Values represent mean (standard deviation). Significant results are reported in bold.
HC, n = 185; PD‐right, n = 102; and PD‐left, n = 137.
Abbreviations: DAT, dopamine transporter; SPECT, single‐photon emission computed tomography; HC, healthy controls; PD, Parkinson's disease; SBR, specific binding ratio; CSF, cerebrospinal fluid; Aβ42, β‐amyloid; p‐tau181, tau phosphorylated at the threonine; MDS‐UPDRS III, Movement Disorder Society Unified Parkinson's Disease Rating Scale motor score; MoCA, Montreal Cognitive Assessment; JLO, Judgment of Line Orientation; HVLT‐R, The Hopkins Verbal Learning Test–Revised; LNS, Letter Number Sequencing; SDMT, Symbol Digit Modalities Test.
Patients with PD‐right versus PD‐left did not differ in demographic variables. There was no difference in mean caudate and putamen DAT‐SPECT SBR, which ensures that disease severity was uniform between PD groups; in patients with PD‐left, the SBR was more reduced in the left than in the right caudate (P < 0.001) as opposed to patients with PD‐right, where the DAT deficit was more prominent in the right than in the left caudate (P < 0.001). We found differences in CSF with the PD‐left group showing lower Aβ42 levels (P = 0.042), and in MDS‐UPDRS III scores with the PD‐right group presenting with more severe motor symptoms (P = 0.003). The lateralized motor subscores confirmed the asymmetrical motor symptoms of the two subgroups (P < 0.001). Of note, the MDS‐UPDRS motor score negatively correlated with the right putamen and caudate DAT‐SPECT uptake (r = −0.33 and r = −0.25, P < 0.001, respectively; FDR corrected).
We also observed worse SDMT performance in the PD‐left group versus the PD‐right group (F 1,234 = 5.75, P < 0.017), confirmed by z score analysis (Table e‐2 in Appendix S1). SDMT positively correlated with the left putamen and caudate (r = 0.16, P = 0.019 and r = 0.26, P < 0.001, respectively; FDR corrected) and the right caudate (r = 0.19, P = 0.008; FDR corrected).
Longitudinal mixed model results (see Table e‐3 in Appendix S1) revealed that motor severity and LEDD significantly increased during the 4‐year period, showing an effect of time (F 2,398 = 13.45, P < 0.001 and F 2,442 = 314.54, P < 0.001, respectively; Fig. 2A,B). In addition, we found an asymmetry effect for the motor severity (F 1,240 = 5.31, P = 0.022), wherein patients with PD‐right maintained significantly greater motor deficits over time when compared with the PD‐left group. However, no time × asymmetry interaction was observed. CSF Aβ42 levels showed a significant reduction over time (3 years), with time (F 2,286 = 5.26, P = 0.006) and asymmetry effects (F 1,233 = 4.46, P = 0.036), with patients with PD‐left maintaining significantly lower CSF Aβ42 levels over time than patients with PD‐right (Fig. 2C).
FIG. 2.
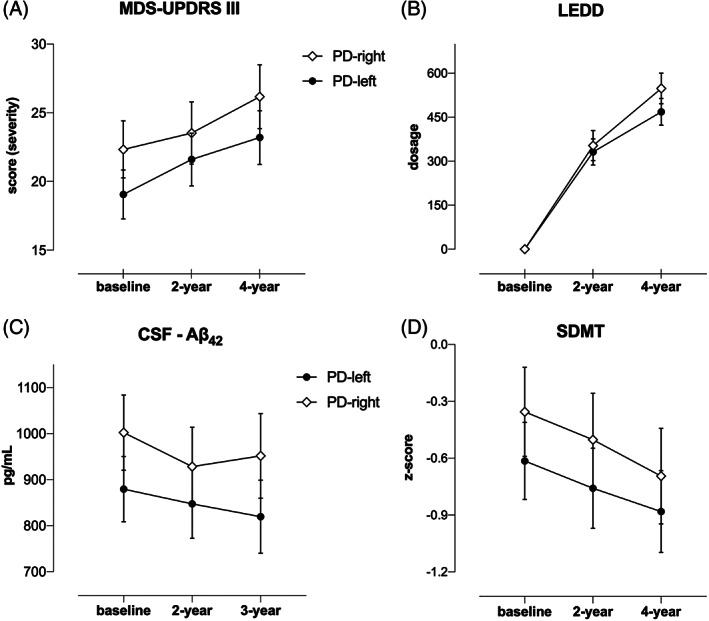
Clinical and cognitive progression in PD with predominant dopaminergic putaminal deficit on the left (PD‐left) versus the right side (PD‐right), namely in the (A) MDS‐UPDRS III, (B) LEDD, (C) CSF β‐amyloid, and (D) SDMT. Aβ42, β‐amyloid; CSF, cerebrospinal fluid; LEDD, levodopa equivalent daily dose; MDS‐UPDRS III, Movement Disorder Society Unified Parkinson's Disease Rating Scale motor score; PD, Parkinson's disease; SDMT, Symbol Digit Modalities Test.
SDMT declined linearly in 4 years, showing main effects of time and asymmetry (F 2,397 = 4.84, P < 0.046 and F1,246 = 2.93, P < 0.032, respectively), with the PD‐left group maintaining poorer SDMT performance than the PD‐right group over 4 years (Fig. 2D). Regarding all the other cognitive measures, we found no time or asymmetry effects (data not shown).
For DAT‐SPECT imaging (see Table e‐3 in Appendix S1), we found that the mean putamen uptake declined over time (F 2,359 = 226.85, P < 0.001), with a discrete pattern as confirmed by the time × asymmetry interaction (F 2,359 = 5.09, P = 0.007). Specifically, the PD‐right group showed faster putaminal DAT SBR reduction in the first 2 years followed by a slower decline. Indeed, a quadratic trend could best describe the putaminal degeneration in the patients with PD‐right (t 354 = 3.73, P < 0.001), but not in patients with PD‐left (t 354 = 0.64, P = 0.552; Fig. 3A). Similarly, for the mean caudate, DAT‐SPECT SBR significantly decreased over time (F 2,359 = 249.51, P < 0.001), with a discrete pattern based on the asymmetry predictor as confirmed by the time × asymmetry interaction (F 2,358 = 5.09, P = 0.044). Mean caudate DAT SBR declined more rapidly in PD‐right group than in PD‐left group in the first 2‐year follow‐up and was slower afterward. A quadratic trend described the progressive reduction of caudate DAT binding in the PD‐right group (t 355 = 3.48, P < 0.001), but not in PD‐left group (t 355 = 1.11, P = 0.268; Fig. 3B).
FIG. 3.
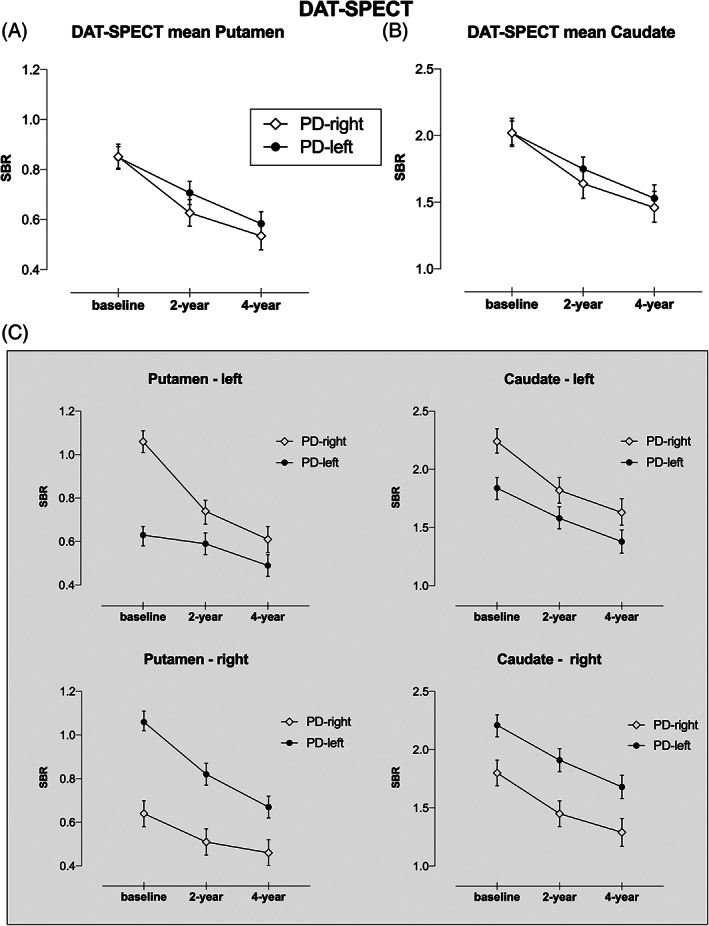
DAT‐SPECT imaging progression in PD with predominant dopaminergic putaminal deficit on the left (PD‐left) versus the right side (PD‐right), namely in the (A) mean putamen, (B) mean caudate, and (C) left/right side of the putamen and caudate nuclei. DAT, dopamine transporter; PD, Parkinson's disease; SPECT, single‐photon emission computed tomography; SBR, specific binding ratio.
Specifically, when analyzing the four striatal regions (Fig. 3C; Table e‐4 in Appendix S1), we found a time × asymmetry interaction except for the caudate right, with DAT‐SPECT SBR reduction in patients with PD‐right showing a decline (quadratic trend) in both left striatal nuclei, whereas in the patients with PD‐left, this decline was observed only in the right putamen. Finally, it is worth noting that an asymmetry effect was present in both left and right putaminal DAT availability (F 1,241 = 50.37, P < 0.001 and F 1,242 = 80.36, P < 0.001), suggesting that the putaminal asymmetry assessed at baseline was maintained during the follow‐up.
3. Discussion
This is the first study to investigate prospectively the role of hemispheric asymmetry of dopaminergic cell loss as a potential biomarker of cognitive and clinical manifestations in a large multicenter cohort of right‐handed patients with PD followed from their initial drug‐naïve disease stage for 4 years.
Right versus left predominant lateralization was assessed objectively through DAT‐SPECT imaging, including only those patients with PD with significant and stable asymmetry (asymmetry index above 20%). We found a significantly greater proportion of right‐handed patients with PD with a marked asymmetric loss of nigrostriatal dopaminergic neurons on the left than on the right side (57% vs. 43%; P < 0.001) and noteworthy, the lateralized DAT difference was maintained during the follow‐up period. This appears consistent with previous clinical evidence reporting higher proportions of right‐handed patients with PD presenting with motor deficits on their right bodyside, which has been explained with greater vulnerability of the dominant hemisphere to PD‐related dysfunction. 9 , 23 This is also aligned with neuropathology 24 and clinical 25 evidence showing persisting asymmetry in dopaminergic degeneration at advanced stages despite less lateralized motor symptoms.
The causes of predominant left‐sided striatal dopaminergic cell loss in right‐handed patients with PD are still unknown, and several hypotheses have been raised 4 —such as the inborn asymmetry of striatal terminals and substantia nigra neurons, increased awareness of symptoms on the dominant bodyside, greater vulnerability associated to the hemispheric dominance or handedness—however, it can be surmised that an interplay between PD pathogenetic mechanisms and hemispheric dominance may increase vulnerability of the left hemispheric nigrostriatal pathway. Therefore, our right‐handed HC group had symmetric nigrostriatal dopaminergic function (data not shown), 26 corroborating the hypothesis that physiological asymmetry in PD cannot be explained solely by hemispheric dominance; yet, mirroring the left‐lateralized pattern also observed in other neurodegenerative conditions, such as fronto‐temporal dementia, Huntington's disease, and Alzheimer's disease (AD). 27 , 28 Furthermore, our results replicate previous evidence 26 where the caudate DAT deficit was more severe in the affected hemisphere following the putaminal lateralization.
At the initial assessment, the PD‐left group showed worse focal performance only in SDMT, a cognitive task requiring processing speed and attention executive abilities. In line with previous publications, 22 we found an association between striatal dopaminergic functions and SDMT that positively correlated with bilateral caudate and left putamen DAT SBR, confirming the involvement of dopaminergic activity in attention executive functioning. In addition, during the 4‐year follow‐up, SDMT showed a linear worsening in both groups, with patients with PD‐left maintaining a worse performance than patients with PD‐right, although still in a normal range as above the clinical cutoff. It is worth noting that hemispheric asymmetry of nigrostriatal deficit did not influence the other assessed cognitive measures, including memory and visuospatial and language abilities.
Hence, our findings suggest that SDMT is a sensitive tool in detecting subtle attention executive alterations driven by asymmetric dopaminergic loss of the dominant hemisphere in drug‐naïve patients with early PD as well as in capturing focal subclinical cognitive changes in presence of various factors, such as dopamine replacement therapy, aging, additional neurotransmitter dysfunctions, and pathologies. 29 Indeed, we and others have previously reported that worse performance on the SDMT can possibly be amyloid‐related in early PD30, 31 and here, the patients with PD‐left showed lower concentrations of CSF Aβ42, a marker of cortical amyloid pathology. In addition, a poor performance in this task may also be observed in normal aging, with attention and processing‐speed abilities being proposed as crucial age‐related markers of cognitive dysfunction. 32 Hence, this evidence underlines that SDMT is a clinically useful test to capture the multifactorial nature of PD cognitive dysfunctions.
Interestingly, patients with PD‐left did not show an early AD‐like cognitive pattern but, rather, attention‐processing speed alterations, which correlate with subcortical nuclei—suggesting that Aβ42 plays an unspecific “non‐AD‐like” role and could only amplify cognitive alterations. These data support the view that asymmetric dopaminergic loss, amyloidosis, and aging exhibit independent and interactive contributions to cognitive progression. 7 , 29
In this regard, CSF Aβ42 and asymmetric DAT tracer uptake have been previously identified as useful predictors of cognitive impairment among various clinical markers in early PD. 7 Overall, our findings confirm this evidence and stress the importance of considering the left hemispheric predominance of dopaminergic cell loss as an additional factor affecting PD cognitive functioning over time. Of note, in the overall drug‐naïve PD population, we observed the presence of early subclinical cognitive alterations (average z scores were above −1.5 SD), supporting previous evidence of neuropsychological deficits in newly diagnosed drug‐naïve PD compared with HC. 33
CSF results at baseline highlighted significantly lower levels in α‐synuclein, total tau, and p‐tau181 in patients with PD than in demographically matched HC, converging with previous investigations, 34 with the exception of CSF Aβ42 that differed only in the PD subgroups. Of note, CSF Aβ42 concentrations declined linearly, and this difference was maintained over time in the patients with PD‐left.
Another intriguing observation is that unmedicated patients with PD‐right had greater motor severity documented by higher MDS‐UPDRS motor scores at baseline, which negatively correlated with right putaminal and caudate DAT binding despite similar age and disease duration to patients with PD‐left.
Over time, motor severity linearly increased in both groups, with patients with PD‐right maintaining a more severe motor score at 4 years. In addition, our longitudinal data revealed that patients with PD‐right had steeper declines in mean putamen and caudate DAT binding and more pronounced in the first 2‐year period, which was best described by the quadratic trend. Although the exact reason for this difference in motor severity is unclear, 4 a possible explanation can be that those patients with PD with initial and predominant cell loss in the right nigrostriatal system have longer disease‐related latencies between neurodegeneration onset and subjective awareness of motor deficits in their nondominant bodyside. Therefore, neurodegeneration could have progressed more in these patients before coming to medical attention. Alternatively, it could be that there is greater neural plasticity in patients with motor deficits in their dominant bodyside, as the recruitment of compensatory mechanisms (eg, striatal or extrastriatal) allowed them to better cope with PD‐related motor symptoms 35 ; among striatal compensatory mechanisms, the DAT downregulation seems to be particularly relevant to explain motor symptom severity according to disease onset. 36 Finally, a different response to dopamine replacement therapy according to the hemispheric asymmetries may also be considered.
In this regard, it has been reported that patients with right hemisphere deficits had greater motor and cognitive improvements after dopaminergic treatment. 6 , 37 Unlike prior investigations, 6 , 37 we did not find any significant interaction between dopaminergic hemispheric asymmetry and PD‐replacement therapy despite our results showing a mild tendency for patients with PD‐right to have a higher levodopa intake over time (P = 0.151), which would be aligned with a previous study. 6 The reasons for this inconsistent result may be complicated by the different staging of the disease across studies as well as the assessment of PD lateralization based on motor onset symptoms 6 , 37 rather than DAT imaging.
Our research has limitations as it only includes 4‐year follow‐up after initial diagnosis and in the absence of dementia pathological confirmation, our results cannot establish the exact underlying mechanism of early cognitive decline in PD. Further longitudinal studies with longer observation periods and postmortem data are needed to clarify if early hemispheric asymmetry in dopamine denervation plays a role in development of dementia.
In the PPMI study, handedness was assessed by clinical history rather than using standardized instruments, and it was not possible to evaluate the left‐handed PD sample because of its small sample size. Moreover, there was a substantial drop‐out rate, about the 36% in 4 years, and we hypothesize that patients not showing up at follow‐up were those with more rapid severity.
The last CSF measurements available in the PPMI database (at the 3‐year visit) were not concomitant with all the other DAT imaging and clinical and cognitive measures assessed at the 4‐year follow‐up. Although our CSF longitudinal analysis showed a linear decline, previous evidence in early PD reported that these biomarkers remaining stable over 6 and 12 months. 38 Lastly, the neuropsychological battery was limited in terms of the number and type of tests per cognitive domain. Regarding SDMT, we cannot exclude that its execution was influenced by different bradykinesia severity, but we controlled our analyses for motor severity as well as PD medication intake. To disentangle this issue, the oral version of SDMT can be employed in future work.
The strengths of this study include the large sample of de novo drug‐naïve patients with PD, excluding possible interaction effects between DAT‐SPECT imaging and medications at baseline. Differently from previous investigations, we also assessed asymmetry based on an objective measure as putaminal DAT binding rather than motor assessment, 39 and for consistency we excluded patients with more severe motor symptoms on the ipsilateral side of nigrostriatal degeneration. 12 , 39 , 40
Furthermore, this is the first longitudinal and multicenter study following a representative sample of patients with PD and HC over a 4‐year follow‐up, whereas previous studies examined shorter periods. 2 , 7 Indeed, a major strength was the inclusion of an age‐matched and education‐matched HC group with a longitudinal assessment, allowing us to compute internal normative data, which are based on a more comparable sample, and evaluated with the same test battery at each time point. 19 In this regard, we believe the age‐based norms that we provided (see Table e‐1 in Appendix S1) can be useful for future studies, particularly in the PPMI context.
In conclusion, our findings lend support to a specific influence of hemispheric asymmetry in dopaminergic cell loss on cognitive and motor outcomes in PD. Further research is needed to understand the long‐term evolution of dopaminergic hemispheric asymmetry and the interplay with functional decline and loss of independence with advancing disease.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
E.F.: 1C, 2A, 2B, 3A
A.A.: 1A, 2C, 3B
P.B.: 3B
L.W.: 2C, 3B
R.B.: 1B, 2A, 3B
Financial Disclosures
E.F., P.B., L.W., and R.B. have nothing to report. A.A. has received compensation for consultancy and speaker‐related activities from UCB, Boehringer Ingelheim, GE Healthcare, Britannia, AbbVie, Angelini Pharmaceuticals, Kyowa Kirin, Zambon, Bial, Neuroderm, Theravance Biopharma, Jazz Pharmaceuticals, Roche and Medscape; He receives research support from Chiesi Pharmaceuticals, Bial, Lundbeck, Roche, Horizon 2020 Grants 825785 and 101016902, Ministry of Education University and Research Grant ARS01_01081, and the Cariparo Foundation. He serves as a consultant for Boehringer–Ingelheim for legal cases on pathological gambling.
Supporting information
Appendix S1. Supporting Information.
Acknowledgments
Data used in the preparation of this article were obtained from the Parkinson's Progressive Markers Initiative (PPMI) database (www.ppmi-info.org/data); for up‐to‐date information on the study, visit www.ppmi-info.org. PPMI is sponsored by the Michael J. Fox Foundation for Parkinson's Research and its funding partners including AbbVie, Allergan, Amathus therapeutics, Avid Radiopharmaceuticals, Bial Biotech, Biogen, BioLegend, Bristol Myers Squibb, Calico, Celgene, Denali, 4D pharma plc, GE Healthcare, Genentech, GlaxoSmithKline plc, Golub Capital, Handl Therapeutics, insitro Inc, Janssen Neuroscience, Lilly, Lundbeck, Merck, Meso Scale Discovery, Neurocrine Biosciences, Pfizer, Piramal, Prevail Therapeutics, Roche, Sanofi Genzyme, Servier, Takeda, Teva, UCB, Verily, and Voyager Therapeutics.
Relevant conflict of interest/financial disclosure: Nothing to report.
Funding agencies: No funding.
References
- 1. Hughes AJ, Daniel SE, Ben‐Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 2002;125:861–870. [DOI] [PubMed] [Google Scholar]
- 2. Cubo E, Martín PM, Martin‐Gonzalez JA, Rodríguez‐Blázquez C, Kulisevsky J, ELEP Group Members . Motor laterality asymmetry and nonmotor symptoms in Parkinson's disease. Mov Disord 2010;25:70–75. [DOI] [PubMed] [Google Scholar]
- 3. Riederer P, Jellinger KA, Kolber P, Hipp G, Sian‐Hülsmann J, Krüger R. Lateralisation in Parkinson disease. Cell Tissue Res 2018;373:297–312. [DOI] [PubMed] [Google Scholar]
- 4. Djaldetti R, Ziv I, Melamed E. The mystery of motor asymmetry in Parkinson's disease. Lancet Neurol 2006;5:796–802. [DOI] [PubMed] [Google Scholar]
- 5. Verreyt N, Nys GMS, Santens P, Vingerhoets G. Cognitive differences between patients with left‐sided and right‐sided Parkinson's disease. A review. Neuropsychol Rev 2011;21:405–424. [DOI] [PubMed] [Google Scholar]
- 6. Hanna‐Pladdy B, Pahwa R, Lyons KE. Paradoxical effect of dopamine medication on cognition in Parkinson's disease: relationship to side of motor onset. J Int Neuropsychol Soc 2015;21:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schrag A, Siddiqui UF, Anastasiou Z, Weintraub D, Schott JM. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson's disease: a cohort study. Lancet Neurol 2017;16:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooper CA, Mikos AE, Wood MF, et al. Does laterality of motor impairment tell us something about cognition in Parkinson disease? Parkinsonism Relat Disord 2009;15:315–317. [DOI] [PubMed] [Google Scholar]
- 9. Scherfler C, Seppi K, Mair KJ, et al. Left hemispheric predominance of nigrostriatal dysfunction in Parkinson's disease. Brain 2012;135:3348–3354. [DOI] [PubMed] [Google Scholar]
- 10. Marek K, Chowdhury S, Siderowf A, et al. The Parkinson's progression markers initiative (PPMI)—establishing a PD biomarker cohort. Ann Clin Transl Neurol 2018;5:1460–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seibyl JP, Marchek KL, Quinlan D, et al. Decreased single‐photon emission computed tomographic {123I} β‐CIT striatal uptake correlates with symptom severity in Parkinson's disease. Ann Neurol 1995;4:589–598. [DOI] [PubMed] [Google Scholar]
- 12. Kaasinen V. Ipsilateral deficits of dopaminergic neurotransmission in Parkinson's disease. Ann Clin Transl Neurol 2016;3:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benton AL, Varney NR, Hamsher KD. Visuospatial judgment: a clinical test. Arch Neurol 1978;35:364–367. [DOI] [PubMed] [Google Scholar]
- 14. Sheridan LK, Fitzgerald HE, Adams KM, et al. Normative symbol digit modalities test performance in a community‐based sample. Arch Clin Neuropsychol 2006;21:23–28. [DOI] [PubMed] [Google Scholar]
- 15. Smith A. Symbol Digit Modalities Test: Manual. Los Angeles, CA: Western Psychological Services; 1982. [Google Scholar]
- 16. Brandt J, Benedict RH. Hopkins Verbal Learning Test‐Revised. Odessa, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 17. Wechsler D. Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV). San Antonio, TX: NCS Pearson; 2008. [Google Scholar]
- 18. Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment 1999;6:147–178. [DOI] [PubMed] [Google Scholar]
- 19. Wyman‐Chick KA, Martin PK, Weintraub D, et al. Selection of normative group affects rates of mild cognitive impairment in Parkinson's disease. Mov Disord 2018;33:839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer's disease concord with amyloid‐beta PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement 2018;14(11):1470–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marek K, Jennings D, Lasch S, et al. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 2011;95:629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siepel FJ, Bronnick KS, Booij J, et al. Cognitive executive impairment and dopaminergic deficits in de novo Parkinson's disease. Mov Disord 2014;29:1802–1808. [DOI] [PubMed] [Google Scholar]
- 23. Iranzo A, Stefani A, Niñerola‐Baizan A, et al. Left‐hemispheric predominance of nigrostriatal deficit in isolated REM sleep behavior disorder. Neurology 2020;94:e1605–e1613. [DOI] [PubMed] [Google Scholar]
- 24. Kempster PA, Gibb WR, Stern GM, Lees AJ. Asymmetry of substantia nigra neuronal loss in Parkinson's disease and its relevance to the mechanism of levodopa related motor fluctuations. J Neurol Neurosurg Psychiatry 1989;52:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller‐Patterson C, Buesa R, McLaughlin N, Jones R, Akbar U, Friedman JH. Motor asymmetry over time in Parkinson's disease. J Neurol Sci 2018;393:14–17. [DOI] [PubMed] [Google Scholar]
- 26. Garrido A, Iranzo A, Stefani A, et al. Lack of asymmetry of nigrostriatal dopaminergic function in healthy subjects. Mov Disord 2020;35:1072–1076. [DOI] [PubMed] [Google Scholar]
- 27. Claassen DO, McDonell KE, Donahue M, et al. Cortical asymmetry in Parkinson's disease: early susceptibility of the left hemisphere. Brain Behav 2016;6:e00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Minkova L, Habich A, Peter J, Kaller CP, Eickhoff SB, Klöppel S. Gray matter asymmetries in aging and neurodegeneration: a review and meta‐analysis. Hum Brain Mapp 2017;38:5890–5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Biundo R, Weis L, Antonini A. Cognitive decline in Parkinson's disease: the complex picture. NPJ Parkinsons Dis 2016;2:16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fiorenzato E, Biundo R, Cecchin D, et al. Brain amyloid contribution to cognitive dysfunction in early‐stage Parkinson's disease: the PPMI dataset. J Alzheimers Dis 2018;66:229–237. [DOI] [PubMed] [Google Scholar]
- 31. McMillan CT, Wolk DA. Presence of cerebral amyloid modulates phenotype and pattern of neurodegeneration in early Parkinson's disease. J Neurol Neurosurg Psychiatry 2016;87:1112–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salthouse TA. The processing‐speed theory of adult age differences in cognition. Psychol Rev 1996;103:403–428. [DOI] [PubMed] [Google Scholar]
- 33. Weintraub D, Simuni T, Caspell‐Garcia C, et al. Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson's disease. Mov Disord 2015;30:919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kang J‐H, Irwin DJ, Chen‐Plotkin AS, et al. Association of cerebrospinal fluid β‐amyloid 1‐42, T‐tau, P‐tau181, and α‐synuclein levels with clinical features of drug‐naive patients with early Parkinson disease. JAMA Neurol 2013;70:1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ham JH, Lee JJ, Kim JS, Lee PH, Sohn YH. Is dominant‐side onset associated with a better motor compensation in Parkinson's disease? Mov Disord 2015;30:1921–1925. [DOI] [PubMed] [Google Scholar]
- 36. Palermo G, Giannoni S, Frosini D, et al. Dopamine transporter, age, and motor complications in Parkinson's disease: a clinical and single‐photon emission computed tomography study. Mov Disord 2020;35:1028–1036. [DOI] [PubMed] [Google Scholar]
- 37. Tomer R, Aharon‐Peretz J, Tsitrinbaum Z. Dopamine asymmetry interacts with medication to affect cognition in Parkinson's disease. Neuropsychologia 2007;45:357–367. [DOI] [PubMed] [Google Scholar]
- 38. Mollenhauer B, Caspell‐Garcia CJ, Coffey CS, et al. Longitudinal CSF biomarkers in patients with early Parkinson disease and healthy controls. Neurology 2017;89:1959–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang P, Tan Y‐Y, Liu D‐Q, et al. Motor‐symptom laterality affects acquisition in Parkinson's disease: a cognitive and functional magnetic resonance imaging study. Mov Disord 2017;32:1047–1055. [DOI] [PubMed] [Google Scholar]
- 40. Aguirregomozcorta M, Stamelou M, Antonini A, et al. Patients with rest‐tremor and scans with ipsilateral dopaminergic deficit. J Neurol 2013;260:1132–1135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


