Abstract
Background
Varicocoeles have been considered for a long time potentially correctable causes for male infertility, even though the correlation of this condition with infertility and sperm damage is still debated.
Objective
To present a summary of the evidence evaluation for imaging varicocoeles, to underline the need for a standardized examination technique and for a unique classification, and to focus on pitfalls in image interpretation.
Methods
Based on the evidence of the literature, the current role of ultrasound (US) imaging for varicocoeles has been reported and illustrated, with emphasis on examination technique, classification, and pitfalls.
Results
US is the imaging modality of choice. It is widely used in Europe, while in other countries clinical classification of varicocoeles is considered sufficient to manage the patient. A number of US classifications exist for varicocoeles, in which the examinnation is performed in different ways.
Discussion
An effort toward standardization is mandatory, since lack of standardization contributes to the confusion of the available literature, and has a negative impact on the understanding of the role itself of imaging in patients with varicocoeles.
Conclusion
Use of the Sarteschi/Liguori classification for varicocoeles is recommended, since it is the most complete and widely used US scoring system available today.
Tubular extratesticular structures resembling varicocoeles, either at palpation or at US, should be identified and correctly characterized.
Keywords: classification, infertility, pitfalls, varicocele
1. INTRODUCTION
Varicocoeles are abnormal dilatations of the pampiniform plexus with reflux of venous blood flow. It is present in 15% of the general male population, but it is more often identified in patients seeking medical attention for infertility. 1 , 2 This is why varicocoeles have been considered for a long time as potentially correctable causes for male infertility. However, a recent multicentric international study promoted by the European Academy of Andrology 3 , 4 reported in healthy, fertile men a prevalence of varicocoeles (∼37%) similar to that reported in primary infertile men. 5 , 6 , 7 These data suggest that varicocoele may exert a scanty effect on male fertility, and that its surgical correction should be limited to highly selected populations. Accordingly, current EAU Guidelines on Male Infertility support nowadays very specific indications for varicocoele treatment both in adults and adolescents. 8
Ultrasound (US) is the imaging modality of choice for varicocoeles. 8 The body of published investigations is large, but exceedingly heterogeneous, and the role of imaging itself in the management of these patients is debated. 9 , 10 Outside Europe, US is not routinely used. Most important, both in and outside Europe US is performed in different ways, and several classifications are used. 2
Recently, ESUR‐SPIWG ‐ the Scrotal and Penile Imaging Working group of the European Society of Urogenital Radiology ‐ released two papers to promote standardization of US for varicocoeles. 5 , 6 Recommendations are based on the evidence of the available literature and, when evidence is lacking, on best clinical practice and expert opinion. In these two papers, the most important features to consider when investigating a patient for varicocoeles are discussed, how to perform the US examination, and which classification is best.
1.1. Clinical classification of varicocoeles
Association between infertility, ipsilateral testicular atrophy, and varicocoeles regards clinically palpable, rather than non‐palpable disease. 11 According to the criteria introduced in 1970 by Dubin and Amelar, varicocoeles are detected and scored clinically in three grades. 12 Grade 1 varicocoele is palpable only while standing during Valsalva manoeuvre. Grade 2 is palpable also at rest while standing. Grade 3 is visible through the scrotal skin. Varicocoeles identified only at US (subclinical) are not considered. Some investigators suggest that clinical classification of varicocoeles is sufficient to manage the patient. 8 Clinical scoring, however, is subjective, and depends significantly on the expertise of the sonologist. Also, the progression of subclinical varicocoeles to clinically evident disease is well documented, 13 , 14 and other pathologies can mimic varicocoeles at palpation. 5 Based on these facts, there is a broad consensus among investigators that imaging plays a major role in the diagnosis of varicocoeles. 5 , 6
1.2. US classification of varicocoeles
There is not a universally accepted system to classify varicocoeles. A number of classifications exist in which the exam is performed in different ways and a variety of parameters is evaluated 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 (Table 1). This fact has a negative impact on the understanding of the role of imaging in patients with varicocoeles, and contributes to the confusion of the available literature. An effort toward a standardization is mandatory. Both grey‐scale, color Doppler US and spectral analysis should be performed bilaterally, with the patient standing and supine, with and without Valsalva. Valuable information is obtained combining grey‐scale and Doppler interrogation. Once dilated veins around and/or above the testis are identified, key features to be evaluated are presence and characteristics of venous reflux, and testicular volume. According with ESUR‐SPIWG, 5 , 6 a maximum diameter ≥3 mm is considered diagnostic for a varicocoele (Figure 1). With the patient standing, during Valsalva manoeuvre, reflux > 2s is considered abnormal. Use of the Sarteschi/Liguori classification is recommended. 24 , 25 This is the most complete and widely used classification available today because the examination technique is clearly defined, and most of the parameters evaluated in the different classifications are included. In particular, characteristics of reflux are fully evaluated, as well as the position and site of the dilated veins and testis volume.
TABLE 1.
Ultrasonographic classifications of varicocoeles
| Study, year | Grades | Position | ||||
|---|---|---|---|---|---|---|
| Sarteschi et al. (1993) | Grade 1: Inguinal reflux only during Valsalva in not enlarged vessels | Grade 2: Supra‐testicular varicosities with reflux only during Valsalva | Grade 3: Peri‐testicular reflux only during Valsalva in enlarged vessels. Visible but not dilated vessels when supine. Enlarged when standing | Grade 4: Enlarged vessels in supine and standing position, with increasing caliber during Valsalva. Reflux at rest, increasing during Valsalva. Possible testicular hypothrophy | Grade 5: Enlarged vessels in supine and standing position, with caliber not increasing with Valsalva. Reflux at rest, not increasing during Valsalva. Testicular hypothrophy. Intratesticular varices may be present | Standing & Supine |
| Hirsh et al. (1980) | Grade 1: No spontaneous reflux, inducible with Valsalva | Grade 2: Intermittent spontaneous reflux | Grade 3: Continuous spontaneous reflux | Standing | ||
| Dhabuwala et al (1989) | Grade 1: Reflux < 2s | Grade 2: Reflux > 2s | Grade 3: Spontaneous reflux increasing with Valsalva | Supine | ||
| Hoekstra & Witt (1995) | Grade 1: Dilated veins < 2.5 mm without flow reversal after Valsalva | Grade 2: Dilated veins 2.5‐3.5 mm and flow reversal after Valsalva | Grade 3: Dilated veins > 3.5 mm and flow reversal after Valsalva | Standing | ||
| Cornud et al. (1999) | Grade 1: Brief reflux < 1s | Grade 2: Intermediate reflux < 2s decreasing during and stopping prior to the end of Valsalva | Grade 3: Permanent reflux > 2s and with a plateau aspect throughout the abdominal strain | Not specified | ||
| Oyen (2002) | Grade 1: Slight reflux (< 2s) during Valsalva | Grade 2: Reflux (> 2s) during Valsalva, not continuous | Grade 3: Reflux at rest or continuous during the entire Valsalva maneuver | Supine | ||
| Pauroso (2011) | Grade 1: No varicosities seen. Reflux in the vessels of the inguinal canal that is observed only during Valsalva | Grade 2: Small varicosities with reflux seen only during Valsalva | Grade 3: Enlarged vessels whose caliber increases during Valsalva | Grade 4: Vessel enlargement with basal reflux that does not increase during Valsalva | Supine | |
| Iosa & Lazzarini (2013) | Grade 1: Reflux > 1s only during Valsalva | Grade 2: Spontaneous, discontinuous reflux not increasing by Valsalva | Grade 3: Spontaneous, discontinuous reflux increased by Valsalva | Grade 4A: Spontaneous, continuous reflux not increased by Valsalva | Grade 4B: Spontaneous, continuous reflux increased by Valsalva | Standing & Supine |
| Patil et al. (2016) | Grade 0: Reflux time < 1s | Grade 1: Reflux time 1s‐2.5s | Grade 2: Reflux time 2.5s‐4s | Grade 3: Reflux time > 4s | Standing | |
| Chiou (1997) |
Maximum vein diameter (mm) 0: < 2.5 mm 1:2.5‐2.9 mm 2:3.0‐3.9 mm 3:≥4 mm |
Plexus/sum of diameter of veins 0: No plexus identified 1: Plexus with sum diameter > 3 mm 2: Plexus with sum diameter 3–5.9 mm 3: Plexus with sum diameter ≥6 mm |
Change of flow velocity on Valsalva maneuver 0: < 2 cm/s or duration n < 1s 1: 2–4.9 cm/s 2: 5–9.9 cm/s 3: ≥10 cm/s |
Total score 0–9 ≥4: presence of varicocoele |
Supine | |
FIGURE 1.

Identification of varicocoele at gray‐scale US. Serpiginous varicosities are seen (arrowheads) larger than 3 mm above the testis (T) with low‐level internal echoes
The Sarteschi/Liguori classification divides varicocoeles in five grades, depending on presence of varicosities, either in supine or standing position, and depending on the relationships of the dilated veins with the testis, testicular size, and characteristics of reflux. Grade 1 varicocoele is characterized by inguinal reflux in non‐enlarging vessels while standing during Valsalva manoeuvre (Figure 2). Grade 2 is characterized by varicosities with reflux only while standing during Valsalva that reach the superior pole of the testis (Figure 3). Grade 3 is characterized by varicosities also around the testis with reflux in standing position and during Valsalva maneuvre (Figure 4). Grade 4 is diagnosed if there are varicosities in supine and standing position which enlarge during Valsalva (Figure 5). Reflux is already present at rest and increases during Valsalva. Testicular hypotrophy may be present. Grade 5 is characterized by enlarged veins in supine and standing position. Reflux is already present at rest, and does not increase during Valsalva. Testicular hypotrophy is common.
FIGURE 2.

Grade 1 varicocoele according to the Sarteschi/Liguori scoring system. Images obtained at rest (A) and during Valsalva (B) showing inguinal reflux in non‐enlarging veins in standing position during Valsalva's manoeuver
FIGURE 3.

Grade 2 varicocoele according to the Sarteschi/Liguori scoring system. Images obtained at rest (A) and during Valsalva (B) showing reflux in supratesticular veins in standing position during Valsalva's manoeuver (T = testis)
FIGURE 4.

Grade 3 varicocoele according to the Sarteschi/Liguori scoring system. Images obtained at rest (A) and during Valsalva (B) showing reflux in the peritesticular veins in standing position during Valsalva's manoeuver (T = testis)
FIGURE 5.

Grade 4 varicocoele according to the Sarteschi/Liguori scoring system. Images obtained at rest (A) and during Valsalva (B) showing reflux at rest in the peritesticular veins which increases during Valsalva's manoeuver (T = testis)
Interestingly, the EAA US consortium defined “severe” varicocoele a venous vessel dilation (> 3 mm) characterized by a continuous venous reflux at rest, increasing or not during a Valsalva maneuvre, consistent with grade 4 and 5 varicocoeles according to Sarteschi/Liguori classification. 4
1.3. How to perform US examination for varicocoeles
Gray‐scale, color Doppler, and spectral analysis have to be done. All parameters should be assessed bilaterally. The patient should be evaluated in both the supine and upright position, in general, upright position is more informative. This approach helps comparison among different studies and improves standardization, even though in clinical practice it might be unnecessary in some cases. Grey‐scale US is performed first. With the patient lying supine, enlarged veins are evaluated and testes volume are measured. The patient is then placed in standing position. The largest varicosity is identified and measured during the Valsalva maneuvre. However, measurement of the largest vein at rest is suggested by the EAA US consortium, to avoid the possible size variability due to Valsalva maneuvre. 4 Colour Doppler and spectral analysis are then performed at the inguinal canal, in the supratesticular area, and in the veins around the testis.
1.4. Testicular volume
In a large series of healthy, fertile men a recent multicenter study reports a mean testicular volume of 20.4 ± 4.0 mL (measured with the Prader orchidometer). The 5th percentile of the testicular volume distribution is 15.0 and 14.0 mL for the right and the left testis, respectively. 4
In varicocoeles, venous reflux is related with testicular hypotrophy, and repair can result in an increase of the testicular volume. 26 , 27 , 28
In testis, volume is obtained more accurately from measurement of the three diameters at US rather than using an orchidometer, or with physical examination. Measurement of the testicular height (H), width (W), and length, (L) should be as accurate as possible. Testis compression should be avoided, since it influences significantly the measurements of the diameters. Estimation of the volume varies significantly using different mathematical formulas. The ellipsoid formula is widely used, also implemented in the US equipment for automated volume calculation from the three diameters. Testicular ellipsoid volume is obtained by multiplying the product of the three diameters by 0.52 (V = HxWxLx0.52). According to this formula, the 5th percentile of the testicular volume distribution is 12.0 and 11.0 mL for the right and the left testis, respectively. 4 Hence, testicular hypotrophy can be defined for volumes below these values. An empirical formula introduced by Lambert et al., has been shown more accurate than the ellipsoid formula. 29 , 30 , 31 According to this formula, testicular volume is obtained by multiplying the three diameters by 0.71 (V = H×W×L×0.71). Lambert's formula is preferred by the ESUR‐SPIWG guidelines. 5 , 6 In a clinical setting, however, volumes calculated with the ellipsoid formula and measured using the Prader orchidometers fit better, while volume derived from Lambert's formula is larger. Hence, ellipsoid formula is preferred by the EAA. 4 It must be underlined that volume calculated with the Lambert's formula is 27% larger than with the ellipsoid formula. Therefore, reporting the method used to calculate the volume is of paramount importance when imaging varicocoeles. It is possible to move from the volume obtained with the ellipsoid formula to Lambert's formula and the other way around multiplying by 1.36 and 0.73, respectively.
1.5. Presence, duration, and characteristics of reflux
The mainstay of the US examination for varicocoeles is Doppler evaluation of the duration of reflux. The therapeutic strategies for varicocoele correction are based on the assumption that the negative effect on spermatogenesis could reverse, if reflux is eliminated. 32
Venous reflux is identified by combining color Doppler interrogation and spectral analysis.
Color Doppler interrogation of the spermatic vessels is panoramic. It is necessary to identify the varicosities and their relationship with testis. Moreover, it provides real‐time information on flow direction, and on how it changes in different positions and during the Valsalva maneuver. However, color Doppler assessment is subjective. Findings must be substantiated with spectral Doppler analysis which provides a measure of the duration and of the characteristics of reflux (Figure 6). The threshold fixed by the ESUR‐SPIWG guidelines for the diagnosis of varicocoeles is > 2s, measured in standing position during Valsalva. 5 , 6
FIGURE 6.

Waveform changes of varicocoeles in standing position during Valsalva manoeuver (arrowhead). (A) Inversion of reflux direction. (B) Increase of flow showing a plateau
1.6. Reflux peak velocity
Evaluation of reflux peak velocity is considered by several investigators a potentially useful Doppler parameter to predict the need for varicocoele repair. 33 This is an active research field that might provide, in the future, important clinical information, but at present cannot be recommended for routine clinical use. Unfortunately, it is difficult to compare the results of the different available studies, since they differ in many critical points. Peak velocity is measured with the patient supine or while standing, either breathing normally, or during Valsalva. Measurements are performed in a variety of positions. Most important, in several investigations angle correction is not performed. The ESUR‐SPIWG does not recommend evaluation of reflux peak velocity in routine clinical practice because angle correction is essential in all Doppler velocity measurements, which also depend critically on the sampling site, patient position, and Valsalva. 5 , 6 Further studies obtained with a standardized examination technique are needed to substantiate the role of this parameter in the management of patients with varicocoeles.
1.7. Testicular and extratesticular abnormalities
In patients investigated for varicocoeles, a variety of atrophic parenchymal changes can be seen. Small, relatively hypoechoic testes with inhomogeneous echotexture or striated appearance can be identified by US.
Testicular hypotrophy can be secondary to high‐grade varicocoeles or, more often, an incidental finding due to prior cryptorchidism, infarction, infection/inflammation, or traumas. 34 , 35 Karyotype abnormalities should also be specifically considered, particularly Kleinfelter syndrome, 36 showing hypergonadotropic hypogonadism. Hypogonadotropic hypogonadism should be checked too. It is important to identify testicular hypotrophy in infertile patients with varicocoeles since improvement of semen quality after repair is unlikely.
Intratesticular varicocoele can occur, either isolated or associated with extratesticular varicocoeles 37 (Figure 7). US reveals dilated intratesticular veins with reflux during Valsalva manoeuvre. Small, nonpalpable testicular lesions can be discovered, whose nature cannot be assessed based on imaging and laboratory findings. Benign neoplasms and non‐neoplastic lesions are prevailing for nodules < 5 mm, making orchidectomy an inappropriately aggressive treatment. If tumour markers are negative, US surveillance is appropriate for the majority of testicular incidentalomas in infertile men. 38
FIGURE 7.

Intratesticular varicocoele associated with extratesticular varicocoele. Images obtained at rest (A) and during Valsalva's manoeuver (B). At rest (A) US reveals dilated intratesticular (arrowheads) and peritesticular (asterisks) veins with reflux during Valsalva manoeuver (B). (T = testis)
Extratesticular masses are often identified. Most of them are simple epididymal cysts, easily characterized by US. 37 Solid and mixed nodules include a variety of neoplastic and non‐neoplastic lesions, the majority of which are benign. Differential diagnosis, however, is difficult. 39
1.8. Reporting
Since in the various medical centers classification of varicocoeles may change, when comparing different US studies inconsistency of reporting is an issue. The correct evaluation of patients requires detailed description in the report of US and Doppler features. A standard report is welcome in which all the relevant features of the varicocoele are described. Regardless of the classification used, the following should be enclosed in the medical report: volume, echogenicity and echotexture of the testes; presence of varicosities and relationships to the testes; size of the largest vein measured while standing at rest (EAA standard operating procedures) and during the Valsalva maneuver (ESUR‐SPIWG operating procedures), irrespective of the location; characteristics of reflux before and during Valsalva, depending on the patient's position; incidental findings. 6
1.9. Pitfalls
Tubular extratesticular structures resembling varicocoeles, either at palpation or at US, are often other pathologies. Spermatoceles, clusters of cyst, tubular ectasia, and other tubular structures such and post‐vasectomy changes are easily characterized at gray‐scale US. 37 Cavernous haemangiomas may mimic a varicocoele on gray‐scale US. They show increased through‐transmission, heterogeneous echotexture, and enlarged vascular spaces that enhance at CEUS, but usually display no flows at Doppler interrogation, since velocities are too slow. Phleboliths may be seen as echogenic foci with distal acoustic shadowing. 39 , 40 Lymphangiomas may resemble haemangiomas at gray‐scale US, or present with cystic‐like appearance. The dilated lymphatics do not enhance at CEUS. 40
Arteriovenous malformations show large arteries with high velocity flows. This feature allows differentiation from varicocoeles, in which only venous flows are recorded 41 (Figure 8).
FIGURE 8.

Scotal arteriovenous malformation mimicking varicocoele. (A) Colour Doppler US shows dilated vessels above the testis, resembling supratesticular varicocoele. (B) Spectral Doppler interrogation reveals high velocity arterial flows. (T = testis)
Another mimic for varicocoele could be Zinner syndrome. 42 The dilated vas deferens and epididymis can simulate venous dilatation, and during the Valsalva maneuver a Doppler signal resembling reflux can be artefactually recorded, due to spermatozoa movement.
Intratesticular varicocoeles can resemble lesions when investigated in the supine position at rest, but reveal their vascular nature when the patient is investigated in standing position during Valsalva manoeuver (Figure 9). Venous reflux is identified, a feature that allows differentiation with other vascular intratesticular lesions, such haemangiomas and arteriovenous malformations, which show arterial flows and arterialized‐venous spectral waveform. 5
FIGURE 9.
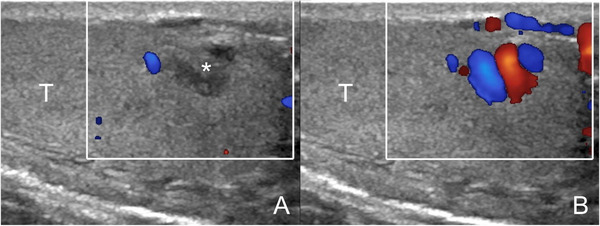
Intratesticular varicocoele. Images obtained at rest (A) and during Valsalva's manoeuver (B). At rest (A) a hypoechoic lesion is seen (asterisk) resembling a tumor. During Valsalva (B) enlarged intratesticular veins with reflux are revealed (T = testis)
2. CONCLUSIONS
Although they are often asymptomatic and detected incidentally, varicocoeles are considered potentially correctable causes for male infertility. Diagnosis is obtained by US, but standardization is necessary, since there is no consensus on the diagnostic criteria, classification, and examination technique. The Sarteschi/Liguori classification is the most complete and widely used scoring system available today. Cysts, spermatoceles, tubular ectasia, post‐vasectomy changes, and other conditions which can mimic clinically varicocoeles are differentiated with multiparametric US.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interest
AUTHOR CONTRIBUTIONS
Guarantors of integrity of entire study, MB, FL; study concepts/study design, all authors; manuscript drafting and revision for important intellectual content, all authors; approval of final version of submitted manuscript, all authors; literature research, MB, FL; manuscript editing, all authors.
Bertolotto M, Cantisani V, Drudi FM, Lotti F. Varicocoele. Classification and pitfalls. Andrology. 2021;9:1322–1330. 10.1111/andr.13053
REFERENCES
- 1. Clavijo RI, Carrasquillo R, Ramasamy R. Varicoceles: prevalence and pathogenesis in adult men. Fertil Steril. 2017;108:364‐369. [DOI] [PubMed] [Google Scholar]
- 2. Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update. 2015;21:56‐83. [DOI] [PubMed] [Google Scholar]
- 3. Lotti F, Frizza F, Balercia G, et al. The European Academy of Andrology (EAA) ultrasound study on healthy, fertile men: clinical, seminal and biochemical characteristics. Andrology. 2020;8:1005‐1020. [DOI] [PubMed] [Google Scholar]
- 4. Lotti F, Frizza F, Balercia G, et al. The European Academy of Andrology (EAA) ultrasound study on healthy, fertile men: scrotal ultrasound reference ranges and associations with clinical, seminal, and biochemical characteristics. Andrology. 2021;9:559‐576. [DOI] [PubMed] [Google Scholar]
- 5. Bertolotto M, Freeman S, Richenberg J, et al. Ultrasound evaluation of varicoceles: systematic literature review and rationale of the ESUR‐SPIWG Guidelines and Recommendations. J Ultrasound. 2020;23:487‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Freeman S, Bertolotto M, Richenberg J, et al. Ultrasound evaluation of varicoceles: guidelines and recommendations of the European Society of Urogenital Radiology Scrotal and Penile Imaging Working Group (ESUR‐SPIWG) for detection, classification, and grading. Eur Radiol. 2020;30:11‐25. [DOI] [PubMed] [Google Scholar]
- 7. Zini A, Boman JM. Varicocele: red flag or red herring. Semin Reprod Med. 2009;27:171‐178. [DOI] [PubMed] [Google Scholar]
- 8. Salonia A, Bettochi C, Carvalho J, et al. 2021.
- 9. Khera M, Lipshultz LI. Evolving approach to the varicocele. Urol Clin North Am. 2008;35:183‐189. [DOI] [PubMed] [Google Scholar]
- 10. Practice Committee of the American Society for Reproductive M, Society for Male R, Urology . Report on varicocele and infertility: a committee opinion. Fertil Steril. 2014;102:1556‐1560. [DOI] [PubMed] [Google Scholar]
- 11. Mihmanli I, Kurugoglu S, Cantasdemir M, Zulfikar Z, Halit Yilmaz M, Numan F. Color Doppler ultrasound in subclinical varicocele: an attempt to determine new criteria. Eur J Ultrasound. 2000;12:43‐48. [DOI] [PubMed] [Google Scholar]
- 12. Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 1970;21:606‐609. [DOI] [PubMed] [Google Scholar]
- 13. Cervellione RM, Corroppolo M, Bianchi A. Subclinical varicocele in the pediatric age group. J Urol. 2008;179:717‐719. discussion 719. [DOI] [PubMed] [Google Scholar]
- 14. Zampieri N, Dall'Agnola A. Subclinical varicocele and sports: a longitudinal study. Urology. 2011;77:1199‐1202. [DOI] [PubMed] [Google Scholar]
- 15. Chiou RK, Anderson JC, Wobig RK, et al. Color Doppler ultrasound criteria to diagnose varicoceles: correlation of a new scoring system with physical examination. Urology. 1997;50:953‐956. [DOI] [PubMed] [Google Scholar]
- 16. Cornud F, Belin X, Amar E, Delafontaine D, Helenon O, Moreau JF. Varicocele: strategies in diagnosis and treatment. Eur Radiol. 1999;9:536‐545. [DOI] [PubMed] [Google Scholar]
- 17. Dhabuwala CB, Kumar AB, Kerkar PD, Bhutawala A, Pierce J. Patterns of Doppler recordings and its relationship to varicocele in infertile men. Int J Androl. 1989;12:430‐438. [DOI] [PubMed] [Google Scholar]
- 18. Hirsh AV, Cameron KM, Tyler JP, Simpson J, Pryor JP. The Doppler assessment of varicoceles and internal spermatic vein reflux in infertile men. Br J Urol. 1980;52:50‐56. [DOI] [PubMed] [Google Scholar]
- 19. Hoekstra T, Witt MA. The correlation of internal spermatic vein palpability with ultrasonographic diameter and reversal of venous flow. J Urol. 1995;153:82‐84. [DOI] [PubMed] [Google Scholar]
- 20. Iosa G, Lazzarini D. Hemodynamic classification of varicoceles in men: our experience. J Ultrasound. 2013;16:57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oyen RH. Scrotal ultrasound. Eur Radiol. 2002;12:19‐34. [DOI] [PubMed] [Google Scholar]
- 22. Patil V, Shetty SM, Das SK. Redefining the criteria for grading varicoceles based on reflux times: a clinicoradiological correlation. Ultrasound Q. 2016;32:82‐85. [DOI] [PubMed] [Google Scholar]
- 23. Pauroso S, Di Leo N, Fulle I, Di Segni M, Alessi S, Maggini E. Varicocele: ultrasonographic assessment in daily clinical practice. J Ultrasound. 2011;14:199‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sarteschi LM, Paoli R, Bianchini M, Menchini Fabris GF. Lo studio del varicocele con eco‐color‐Doppler. G Ital Ultrasonologia. 1993;4:43‐49. [Google Scholar]
- 25. Liguori G, Trombetta C, Garaffa G, et al. Color Doppler ultrasound investigation of varicocele. World J Urol. 2004;22:378‐381. [DOI] [PubMed] [Google Scholar]
- 26. Sakamoto H, Saito K, Ogawa Y, Yoshida H. Effects of varicocele repair in adults on ultrasonographically determined testicular volume and on semen profile. Urology. 2008;71:485‐489. [DOI] [PubMed] [Google Scholar]
- 27. Zampieri N, Cervellione RM. Varicocele in adolescents: a 6‐year longitudinal and followup observational study. J Urol. 2008;180:1653‐1656. discussion 1656. [DOI] [PubMed] [Google Scholar]
- 28. Zhou T, Zhang W, Chen Q, et al. Effect of varicocelectomy on testis volume and semen parameters in adolescents: a meta‐analysis. Asian J Androl. 2015;17:1012‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lambert B. The frequency of mumps and of mumps orchitis and the consequences for sexuality and fertility. Acta Genet Stat Med. 1951;2:1‐166. [PubMed] [Google Scholar]
- 30. Mbaeri TU, Orakwe JC, Nwofor AME, Oranusi CK, Mbonu OO. Ultrasound measurements of testicular volume: comparing the three common formulas with the true testicular volume determined by water displacement. African Journal of Urology. 2013;19:69‐73. [Google Scholar]
- 31. Sakamoto H, Saito K, Oohta M, Inoue K, Ogawa Y, Yoshida H. Testicular volume measurement: comparison of ultrasonography, orchidometry, and water displacement. Urology. 2007;69:152‐157. [DOI] [PubMed] [Google Scholar]
- 32. Liguori G, Ollandini G, Pomara G, et al. Role of renospermatic basal reflow and age on semen quality improvement after sclerotization of varicocele. Urology. 2010;75:1074‐1078. [DOI] [PubMed] [Google Scholar]
- 33. Glassberg KI. My indications for treatment of the adolescent varicocele (and why?). Transl Androl Urol. 2014;3:402‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loberant N, Bhatt S, McLennan GT, Dogra VS. Striated appearance of the testes. Ultrasound Q. 2010;26:37‐44. [DOI] [PubMed] [Google Scholar]
- 35. Mittal PK, Little B, Harri PA, et al. Role of imaging in the evaluation of male infertility. Radiographics. 2017;37:837‐854. [DOI] [PubMed] [Google Scholar]
- 36. Rocher L, Moya L, Correas JM, et al. Testis ultrasound in Klinefelter syndrome infertile men: making the diagnosis and avoiding inappropriate management. Abdom Radiol (NY). 2016;41:1596‐1603. [DOI] [PubMed] [Google Scholar]
- 37. Valentino M, Bertolotto M, Ruggirello M, Pavlica P, Barozzi L, Rossi C. Cystic lesions and scrotal fluid collections in adults: ultrasound findings. J Ultrasound. 2011;14:208‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rocher L, Ramchandani P, Belfield J, et al. Incidentally detected non‐palpable testicular tumours in adults at scrotal ultrasound: impact of radiological findings on management Radiologic review and recommendations of the ESUR scrotal imaging subcommittee. Eur Radiol. 2016;26:2268‐2278. [DOI] [PubMed] [Google Scholar]
- 39. Secil M, Bertolotto M, Rocher L, et al. Imaging features of paratesticular masses. J Ultrasound Med. 2017;36:1487‐1509. [DOI] [PubMed] [Google Scholar]
- 40. Conzi R, Damasio MB, Bertolotto M, et al. Sonography of scrotal wall lesions and correlation with other modalities. J Ultrasound Med. 2017;36:2149‐2163. [DOI] [PubMed] [Google Scholar]
- 41. Yilmaz C, Arslan M, Arslan M. Intrascrotal arteriovenous malformation simulating varicocele. AJR Am J Roentgenol. 2009;192:W351. [DOI] [PubMed] [Google Scholar]
- 42. Pavan N, Bucci S, Mazzon G, Bertolotto M, Trombetta C, Liguori G. It's not always varicocele: a strange case of Zinner syndrome. Can Urol Assoc J. 2015;9:E535‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]


