Abstract
Background and purpose
In its initial stages, Guillain–Barré syndrome (GBS) is difficult to identify, because diagnostic criteria may not always be fulfilled. With this retrospective study, we wanted to identify the most common electrophysiological abnormalities seen on neurophysiological examination of GBS patients and its variants in the early phases.
Methods
We reviewed the clinical records of patients admitted to our Neurology Unit with a confirmed diagnosis of GBS. The study sample was divided in two subgroups according to whether the neurophysiological examination was performed: within 7 days (very early group) or within 7–15 days (early group). H reflex, F waves, and motor and sensory conduction parameters were judged abnormal if they were outside the normal range for at least two nerves. We evaluated neurophysiological findings in Miller–Fisher syndrome (MFS) separately.
Results
The study sample comprised 36 patients. In GBS, the most frequent abnormal neurophysiological parameter was the bilateral absence of the H reflex, followed by F wave abnormalities. Motor conduction parameters were altered in less than 50% of patients, and even less common were sensory nerve action potential reduction and the "sural‐sparing" pattern. In MFS, H reflex was absent bilaterally in 100% of patients, followed by a predominant peripheral sensory involvement, whereas motor conduction parameters were frequently normal.
Conclusions
Bilateral absence of the H reflex is the most sensitive parameter in early diagnosis of GBS and its variants.
Keywords: early diagnosis, electrodiagnostic criteria, Guillain–Barré syndrome, H reflex bilateral absence, Miller–Fisher syndrome
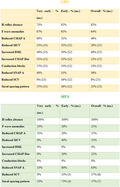
Electrodiagnostic findings in GBS and MFS patients: percentage of patients with abnormal results in at least two nerves. When patients were excluded due to the lack of CMAPs, SNAPs or normal sural nerve SNAP, frequencies are calculated based on the number of patients reported in brackets. MCV: motor conduction velocity. DML: distal motor latency. CMAP Dur: compound motor action potential duration. CMAP A: compound motor action potential amplitude. SNAP A: sensory nerve action potential amplitude. SCV: sensory conduction velocity.
INTRODUCTION
Guillain–Barré syndrome (GBS) and its variants are the most commonly acquired acute polyneuropathies and the leading cause of acute flaccid paralysis worldwide [1]. GBS more often affects males and adults between 50 and 70 years of age (incidence = 0.81–1.89/100,000) [2]. There are several clinical presentations [3] but the more common features are progressive bilateral weakness of the lower limbs often ascending to the arms and bulbar muscles, associated with absent or reduced tendon reflexes. Diagnosis is based on findings from clinical history and neurological examination [4, 5]. Electrophysiological and cerebrospinal fluid (CSF) examinations can corroborate the diagnosis [4]. Abnormal findings in both are necessary to meet Brighton criteria Level 1 (highest level of certainty) or 2 [6]. Early detection and early initiation of treatment can limit disease severity, obviating the need for mechanical ventilation and improving the chances of early recovery [7].
Moreover, electrophysiological studies are key to diagnosis, subtype classification, and prognosis [8]. Serial studies may be necessary for the diagnosis of subtypes, and a second study is recommended in patients lacking demyelinating features or with conduction blocks (CBs) without temporal dispersion [9]. Early neurophysiological examinations can reveal anomalies that are not specific for primary demyelinating neuropathy [10] and may not meet neurophysiological criteria for GBS [11]. With the present study, we wanted to identify the most common electrophysiological abnormalities within 15 days from symptom onset in patients with GBS.
MATERIALS AND METHODS
We retrospectively reviewed the medical charts of patients admitted to our Neurology Unit with a confirmed diagnosis of GBS from January 2016 to January 2020. Data were collected from patients who had undergone a nerve conduction study within 15 days of symptom onset; patients with chronic radiculopathies, mononeuropathies, or polyneuropathies were excluded. The study sample was divided into two subgroups according to the interval between symptom onset and time of neurophysiological examination: within 7 days (very early group) and within 7–15 days (early group). All electrophysiological examinations were performed in the same laboratory according to a standardized protocol with skin temperature > 32°C. Motor nerve conduction (MNC) of the upper limbs was performed using pregelled surface electrodes in a belly tendon montage, stimulating the median and the ulnar nerve at the wrist and the forearm; the motor response was recorded at the abductor pollicis brevis and the abductor digiti minimi, respectively; similarly, MNC of the lower limbs was performed by stimulating the peroneal and the tibial nerve at the ankle and the knee and recording the motor response at the extensor digitorum brevis and the abductor hallucis muscles, respectively.
Distal motor latency (DML), distal compound motor action potential (CMAP) peak‐to‐peak amplitude, distal CMAP negative peak duration, motor conduction velocity (MCV), and F wave abnormalities (absence or increased minimum latency) were measured. The presence of definite partial CBs was evaluated according to American Association of Electrodiagnostic Medicine criteria [12] only in the distal segments: across the elbow and in the forearm segment for the upper limbs and across the knee and in the leg segment for the lower limbs. Sensory nerve action potentials (SNAPs) were obtained using an antidromic technique: at the upper limbs by stimulating the median and the ulnar nerve at the wrist and recording from the third and fifth finger, respectively; at the lower limbs by stimulating the sural and the superficial peroneal nerves of both sides according to Squintani and coworkers' antidromic technique [13]. Peak‐to‐peak amplitude and sensory conduction velocity (SCV) were measured using pregelled surface electrodes applied to the upper limbs and needle recording at the legs. When the sural nerve SNAP was normal, we searched for the “sural‐sparing” pattern (normal sural nerve amplitude with a concomitant reduction in ulnar nerve SNAP).
The H reflex was obtained by stimulating the tibial nerve at the popliteal fossa and recorded from the soleus muscle bilaterally. Neurophysiological parameters were judged abnormal only if they were outside our normal reference values in at least two nerves. Neurophysiological findings were analyzed both including all patients affected by GBS and its variants, and evaluating Miller–Fisher syndrome (MFS) separately.
We also evaluated the presence of albuminocytologic dissociation in CSF (i.e., CSF normal white cell count associated with protein content > 0.45 g/L).
RESULTS
The study population was 36 patients (12 females and 24 males; mean age = 47.9 ± 17.5 years, range = 3–71). All met the diagnostic criteria of GBS [4, 5]; 18 were categorized in the “very early” group and the remaining 18 in the “early” group. Based on clinical features [3], 28 presented GBS (classic subtype in 25, pharyngeal–cervical–brachial weakness in one, and bifacial weakness with paresthesia in two), whereas eight presented the variant MFS (Table 1). None of the patients tested positive to severe acute respiratory syndrome coronavirus 2 infection.
TABLE 1.
Patient distribution by clinical features [3]
| Feature | Very early, n (%) | Early, n (%) | Overall, n (%) |
|---|---|---|---|
| Classic GBS | 12 (66%) | 13 (72%) | 25 (69%) |
| Pharyngeal–cervical–brachial weakness | 1 (6%) | 0 (0%) | 1 (3%) |
| Bifacial weakness with paresthesias | 2 (11%) | 0 (0%) | 2 (6%) |
| MFS | 3 (17%) | 5 (28%) | 8 (22%) |
Abbreviations: GBS, Guillain–Barré syndrome; MFS, Miller–Fisher syndrome.
According to Uncini's electrodiagnostic criteria [8], neurophysiological examination results were equivocal in 19 (53%) patients, acute inflammatory demyelinating polyradiculoneuropathy (AIDP) in 11 (31%), acute motor axonal neuropathy (AMAN) in three (8%), and unexcitable in three (8%; Table 2). By CSF analysis, albuminocytologic dissociation was detected in 55% of the entire population: 44% in the “very early” and 66% in the “early” group. All received intravenous immunoglobulin therapy, and two were monitored in the intensive care unit. According to neurophysiological evaluations, we analyzed GBS and MFS separately; in particular, Table 3 reports neurophysiological parameters of GBS patients, whereas Table 4 displays findings obtained from MFS patients.
TABLE 2.
Patient distribution by electrodiagnostic criteria [8]
| Criterion | Very early, n (%) | Early, n (%) | Overall, n (%) |
|---|---|---|---|
| AIDP | 6 (33%) | 5 (28%) | 11 (31%) |
| AMAN | 3 (17%) | 0 (0%) | 3 (8%) |
| Equivocal | 7 (39%) | 12 (66%) | 19 (53%) |
| Unexcitable | 2 (11%) | 1 (6%) | 3 (8%) |
Abbreviations: AIDP, acute inflammatory demyelinating polyradiculoneuropathy; AMAN, acute motor axonal neuropathy.
TABLE 3.
Electrodiagnostic findings in Guillain–Barré syndrome patients: percentage of patients with abnormal results in at least two nerves
| Finding | Very early, % (n) | Early, % (n) | Overall, % (n) |
|---|---|---|---|
| H reflex absence | 73% | 92% | 82% |
| F wave anomalies | 67% | 61% | 64% |
| Reduced CMAP A | 60% | 31% | 46% |
| Reduced MCV | 23% (13) | 33% (12) | 28% (25) |
| Increased DML | 46% (13) | 50% (12) | 48% (25) |
| Increased CMAP Dur | 31% (13) | 33% (12) | 32% (25) |
| Conduction blocks | 15% (13) | 33% (12) | 24% (25) |
| Reduced SNAP A | 40% | 31% | 36% |
| Reduced SCV | 0% (13) | 16% (12) | 8% (25) |
| Sural‐sparing pattern | 25% (11) | 18% (12) | 21% (23) |
When patients were excluded due to the lack of CMAPs, SNAPs, or normal sural nerve SNAPs, frequencies are calculated based on the number of patients reported in parentheses.
Abbreviations: CMAP A, compound motor action potential amplitude; CMAP Dur, compound motor action potential duration; DML, distal motor latency; MCV, motor conduction velocity; SCV, sensory conduction velocity; SNAP A, sensory nerve action potential amplitude.
TABLE 4.
Electrodiagnostic findings in Miller–Fisher syndrome patients: percentage of patients with abnormal results in at least two nerves
| Finding | Very early, % (n) | Early, % (n) | Overall, % (n) |
|---|---|---|---|
| H reflex absence | 100% | 100% | 100% |
| F wave anomalies | 33% | 20% | 25% |
| Reduced CMAP A | 33% | 20% | 25% |
| Reduced MCV | 0% | 40% | 25% |
| Increased DML | 0% | 0% | 0% |
| Increased CMAP Dur | 0% | 20% | 12% |
| Conduction blocks | 0% | 0% | 0% |
| Reduced SNAP A | 33% | 80% | 63% |
| Reduced SCV | 0% | 33% (3) | 17% (6) |
| Sural‐sparing pattern | 33% | 75% (4) | 57% (7) |
When patients were excluded due to the lack of CMAPs, SNAPs, or normal sural nerve SNAPs, frequencies are calculated based on the number of patients reported in parentheses.
Abbreviations: CMAP A, compound motor action potential amplitude; CMAP Dur, compound motor action potential duration; DML, distal motor latency; MCV, motor conduction velocity; SCV, sensory conduction velocity; SNAP A, sensory nerve action potential amplitude.
The most common abnormal neurophysiological parameter in both variants was bilateral absence of the H reflex; it was absent in 73% of the “very early” and 92% of the “early” group (overall frequency = 82%) in GBS patients, whereas it reached 100% in MFS patients.
All motor conduction parameters were more commonly abnormal in GBS patients than in the MFS subtype. In particular, F wave abnormalities were noted in 67% in the “very early” and 61% in the “early” group, respectively, with an overall frequency of 64%. Reduced CMAP amplitude was observed in 46% of patients and was more frequent in the “very early” group (60%). Moreover, it was present in all patients fulfilling the diagnostic criteria for AMAN at further electrodiagnostic examinations. Demyelinating features involving MNC were noted in 25 patients (three had no evocable CMAPs); DML was increased in 46% of the entire population; reduced MCV, increased duration of CMAP, and CBs were even less frequent (see Table 3 for details). All motor conduction parameters anomalies were rare in MFS; in particular, two patients presented F wave anomalies, CMAP amplitude reduction, or MCV reduction. Increased CMAP duration was present in only one patient. None of MFS patients presented motor CBs or increased DML.
As for MCV, SCV was evaluated only in patients with evocable SNAP in at least two different nerves (n = 31, 25 in the GBS group and six in MFS), and sural‐sparing pattern frequency was calculated only in patients with normal sural conduction (n = 30, 23 in the GBS group and seven in MFS). In GBS patients, sensory conduction was normal in more than half of patients in both subgroups and sural‐sparing was found in 21% of all cases with prevalence in the “very early” group (25%). MFS electrophysiological examinations displayed more common sensory conduction anomalies; reduced SNAP amplitude was present in 80% of all “early” patients (one patient had nonevocable SNAPs, and another presented only sural nerve SNAPs) and in 33% of the “very early” subgroup, with an overall frequency of 63%. SCV reduction was present in three patients of the “early” subgroup, whereas sural‐sparing pattern was seen in 57% of patients with normal sural SNAP amplitude (one in the “very early” group and three in the “early” group).
DISCUSSION
Neurophysiological examination is key to establishing a diagnosis of GBS, but indications for appropriate timing after symptom onset are less clear‐cut. MNC findings may be normal in the early phases and even beyond in atypical variants such as MFS [14, 15, 16]. Depending on the diagnostic criteria utilized [8, 17, 18], neurophysiology performed during the first 7 days after symptom onset has low sensitivity in most cases [11, 19, 20]. Our data confirm that electromyography is usually insufficient to make a definite diagnosis in the first weeks; our results were equivocal in 53% of this cohort.
As described elsewhere [10, 11, 19, 21, 22], we noted that an absent H reflex was more frequent than other neurophysiological parameters, and more frequent than CSF hyperproteinorrachia as well. Although a usual finding in S1 radiculopathy [23], the absence of bilateral H reflex could be a meaningful electrophysiological finding in the diagnosis of GBS in its early stages. An absent H reflex may be a very sensitive parameter, but it is not included among diagnostic criteria [8, 17, 18] and it does not necessarily infer demyelination or axonal damage [11, 16]. The high sensitivity we noted is probably due to the H reflex exploring sensory and motor fibers in both their distal and proximal segments, as demonstrated by its high sensitivity in the diagnosis of atypical GBS variants and MFS, which affects motor and sensory neurons predominantly across proximal segments [23, 24, 25] Although it is the most common finding in early GBS and its variants, an increased H/M ratio has been reported in atypical AMAN presentation [26, 27, 28], characterized by hyperreflexia and reflex spread, and it could be seen in up to 26% of cases [28].
In GBS patients, F wave abnormalities were the second most common alteration, demonstrating an early involvement of the proximal tract of the peripheral nerve system. Furthermore, the frequency of F wave abnormal findings was comparable to those reported previously [10, 11, 20, 21, 29, 30, 31]. In our series, F wave anomalies seem to be slightly more frequent in the “very early” group; this finding could be explained by AMAN patients being included in this group. Conversely, in MFS the F waves were abnormal in a low percentage, and the finding was in line with literature data [16, 25, 32].
Although CBs and slowed conduction velocity are typical electrophysiological hallmarks of peripheral demyelination damage [33], they were not as frequently observed as late responses and reflex anomalies; increased CMAP duration, increased DML, and reduced MCV were present in less than 50% of our GBS patients. This supports the hypothesis that damage to the more proximal segment of the nerves (e.g., the nerve roots) occurs before the peripheral nerves, probably because of their less efficient blood–nerve interface [34, 35]. In this regard, Griffin and collaborators found in autopsy samples inflammatory demyelination or Wallerian‐like degeneration in the intrathecal spinal nerve roots and the ventral rami of spinal nerves [36]. Such prominent proximal involvement has been reported in magnetic resonance imaging and ultrasonography studies showing contrast enhancement of the cauda equina or root enlargement, respectively [37, 38].
In our series, CBs were evaluated only on the distal segments, so their detection was less frequent. We believe that performing more proximal motor stimulation (at Erb's point or needle electric root stimulation) could reveal more CBs, as reported elsewhere [21, 39, 40]. Distal CMAP amplitude reduction could be due to distal demyelination, distal conduction failure, or axonal degeneration, and it was more frequent in the “very early” group probably because AMAN and two of three unexcitable variants were included in the group. Regarding distal segment motor conduction in GBS patients, our finding was similar to previous studies [10, 11, 20, 29, 30, 31]. SNAP amplitude reduction was noted in less than half of GBS patients, and SCV reduction was occasional, as reported previously [10, 11, 29, 30, 31]. Whereas motor conduction abnormalities were rarer in MFS than GBS, distal sensory anomalies were more common in MFS. In line with other reports [16, 25, 32], our results confirmed that SNAP amplitude reduction is the most common abnormal distal conduction parameter, even more frequent than motor conduction anomalies. Reduced SNAP amplitude in MFS is mainly determined by a nodal–paranodal dysfunction of the axolemma, as demonstrated by the presence of reversible sensory CBs [14, 15, 32, 41], whereas in chronic inflammatory demyelinating polyneuropathy (CIDP) and some cases of acute inflammatory demyelinating polyradiculoneuropathy, reduced SNAP amplitude is frequently associated with increased SNAP duration and other signs of demyelination [42, 43].
A typical feature of sensory nerve conduction abnormalities in demyelinating and axonal forms of GBS, as well as MFS and CIDP, is reduced SNAP amplitude higher in the median, ulnar, or radial nerve compared to the sural nerve: the so‐called sural‐sparing pattern [16, 44]. Median, ulnar, and radial sensory nerve conduction studies test the most distal part of the sensory axons, whereas the intermediate nerve segment is examined in sural nerve conduction studies.
The sural‐sparing pattern happens because the distal nerve terminals are preferentially affected by demyelination or nodal immune attack [45, 46, 47] compared to the intermediate nerve segments, probably because the blood–nerve barrier in distal nerve terminals and nerve roots is anatomically less efficient [48]. In our patients, the sural‐sparing pattern was observed more frequently in MFS than GBS. According to the literature, its frequency is reported to vary between 17.8% [21] and 73% [22].
There are multiple reasons for such a wide range. First, definitions of sural‐sparing differ [11, 19, 29, 44, 49]. Second, the variability may also depend on stimulation technique; some authors [10, 21, 29] used antidromic stimulation for both upper limb and sural nerve and reported a lower percentage of sural‐sparing compared to others. This is probably because the SNAP amplitude obtained with the orthodromic technique is generally lower than that obtained with the antidromic method. Third, site of stimulation (proximal/distal stimulation at forearm/wrist for radial nerve) may also influence the amount of sural‐sparing [49]. Finally, another factor is the timing of the neurophysiological examination; a sural‐sparing pattern was reported in 81.8% of patients with an electrodiagnosis performed within 4 days after symptom onset and in 60% of those with a nerve conduction study done beyond 10 days after symptom onset [31].
CONCLUSIONS
The diagnosis of GBS within 15 days of symptom onset can be challenging, especially in clinical atypical subtypes. Although neurophysiological findings do not usually fulfill diagnostic criteria, certain findings could be suggestive of acute inflammatory polyneuropathy. Bilateral absence of the H reflex is the most sensitive parameter, especially in MFS, although it is not suggestive of demyelination by itself. Our findings show that proximal segments are most frequently affected in the early stages of the disease. A thorough nerve neurophysiological evaluation, including H reflex and F wave abnormalities, is key to early diagnosis of GBS and its variants.
CONFLICT OF INTEREST
None of the authors has potential conflicts of interest to be disclosed.
AUTHOR CONTRIBUTIONS
Andrea Rasera: Conceptualization (equal), data curation (lead), formal analysis (lead), investigation (lead), writing–original draft (lead). Silvia Romito: Data curation (equal), investigation (equal), writing–review & editing (equal). Alessia Segatti: Data curation (equal), investigation (equal). Elisa Concon: Data curation (equal), investigation (equal). Luca Alessandrini: Data curation (equal), investigation (equal). Federica Basaldella: Data curation (equal), investigation (equal). Andrea Badari: Data curation (equal), investigation (equal). Bruno Bonetti: Resources (equal), supervision (equal). Giovanna Squintani: Conceptualization (lead), data curation (equal), formal analysis (equal), investigation (equal), supervision (lead), writing–original draft (equal), writing–review & editing (lead).
Rasera A, Romito S, Segatti A, et al. Very early and early neurophysiological abnormalities in Guillain–Barré syndrome: A 4‐year retrospective study. Eur J Neurol. 2021;28:3768–3773. 10.1111/ene.15011
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors, Open Access Funding provided by Universita degli Studi di Verona within the CRUI‐CARE Agreement.
WOA Institution: Universita degli Studi di Verona.
Blended DEAL: CARE
DATA AVAILABILITY STATEMENT
Data are available on request from the authors.
REFERENCES
- 1. Willison HJ, Jacobs BC, van Doorn PA. Guillain‐Barré syndrome. The Lancet. 2016;388(10045):717‐727. 10.1016/S0140-6736(16)00339-1 [DOI] [PubMed] [Google Scholar]
- 2. Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain‐Barré syndrome: a systematic review and meta‐analysis. Neuroepidemiology. 2011;36(2):123‐133. 10.1159/000324710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wakerley BR, Uncini A, Yuki N, GBS Classification Group . Guillain‐Barré and Miller Fisher syndromes–new diagnostic classification. Nat Rev Neurol. 2014;10(9):537‐544. 10.1038/nrneurol.2014.138 [DOI] [PubMed] [Google Scholar]
- 4. Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain‐Barré syndrome. Ann Neurol. 1990;27(Suppl):S21‐S24. 10.1002/ana.410270707 [DOI] [PubMed] [Google Scholar]
- 5. Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain‐Barré syndrome and validation of Brighton criteria. Brain. 2014;137(Pt 1):33‐43. 10.1093/brain/awt285 [DOI] [PubMed] [Google Scholar]
- 6. Sejvar JJ, Kohl KS, Gidudu J, et al. Guillain‐Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29:599‐612. 10.1016/j.vaccine.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 7. Hughes RAC, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain‐Barré syndrome. Cochrane Database Syst Rev. 2014;19(9):CD002063. 10.1002/14651858.CD002063.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uncini A, Ippoliti L, Shahrizaila N, Sekiguchi Y, Kuwabara S. Optimizing the electrodiagnostic accuracy in Guillain‐Barré syndrome subtypes: criteria sets and sparse linear discriminant analysis. Clin Neurophysiol. 2017;128(7):1176‐1183. 10.1016/j.clinph.2017.03.048 [DOI] [PubMed] [Google Scholar]
- 9. Uncini A, Kuwabara S. The electrodiagnosis of Guillain‐Barré syndrome subtypes: where do we stand? Clin Neurophysiol. 2018;129(12):2586‐2593. 10.1016/j.clinph.2018.09.025 [DOI] [PubMed] [Google Scholar]
- 10. Gordon PH, Wilbourn AJ. Early electrodiagnostic findings in Guillain‐Barré syndrome. Arch Neurol. 2001;58(6):913‐917. 10.1001/archneur.58.6.913 [DOI] [PubMed] [Google Scholar]
- 11. Vucic S, Cairns KD, Black KR, Tick Chong PS, Cros D. Neurophysiologic findings in early acute inflammatory demyelinating polyradiculoneuropathy. Clin Neurophysiol. 2004;115(10):2329‐2335. 10.1016/j.clinph.2004.05.009 [DOI] [PubMed] [Google Scholar]
- 12. American Association of Electrodiagnostic Medicine, Olney RK . Guidelines in electrodiagnostic medicine. Consensus criteria for the diagnosis of partial conduction block. Muscle Nerve Suppl. 1999;8:S225‐S229. [PubMed] [Google Scholar]
- 13. Squintani G, Zoppini G, Donato F, et al. Antidromic sensory nerve conduction study of the digital branches of the medial plantar nerve: a novel method to detect early diabetic sensory axonal polyneuropathy. Muscle Nerve. 2014;50(2):193‐199. 10.1002/mus.24135 [DOI] [PubMed] [Google Scholar]
- 14. Guiloff RJ. Peripheral nerve conduction in Miller Fisher syndrome. J Neurol Neurosurg Psychiatry. 1977;40(8):801‐807. 10.1136/jnnp.40.8.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Umapathi T, Tan EY, Kokubun N, Verma K, Yuki N. Non‐demyelinating, reversible conduction failure in Fisher syndrome and related disorders. J Neurol Neurosurg Psychiatry. 2012;83(9):941‐948. 10.1136/jnnp-2012-303079 [DOI] [PubMed] [Google Scholar]
- 16. Kuwabara S, Sekiguchi Y, Misawa S. Electrophysiology in Fisher syndrome. Clin Neurophysiol. 2017;128(1):215‐219. 10.1016/j.clinph.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 17. Hadden RD, Cornblath DR, Hughes RAC, et al. Electrophysiological classification of Guillain‐Barré syndrome: clinical associations and outcome. Ann Neurol. 1998;44:780‐788. 10.1002/ana.410440512 [DOI] [PubMed] [Google Scholar]
- 18. Rajabally Y, Durand MC, Mitchell J, Orlikowski D, Nicolas G. Electrophysiological diagnosis of Guillain‐Barré syndrome subtype: could a single study suffice? J Neurol Neurosurg Psychiatry. 2015;86:115‐119. 10.1136/jnnp-2014-307815 [DOI] [PubMed] [Google Scholar]
- 19. Chanson JB, Echaniz‐Laguna A. Early electrodiagnostic abnormalities in acute inflammatory demyelinating polyneuropathy: a retrospective study of 58 patients. Clin Neurophysiol. 2014;125(9):1900‐1905. 10.1016/j.clinph.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 20. Berciano J, Orizaola P, Gallardo E, et al. Very early Guillain‐Barré syndrome: A clinical‐electrophysiological and ultrasonographic study. Clin Neurophysiol Pract. 2019;30(5):1‐9. 10.1016/j.cnp.2019.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baraba R, Sruk A, Sragalj L, Butković‐Soldo S, Bielen I. Electrophysiological findings in early Guillain‐Barré syndrome. Acta Clin Croat. 2011;50(2):201‐207. [PubMed] [Google Scholar]
- 22. Wali A, Kanwar D, Khan SA, Khan S. Early electrophysiological findings in acute inflammatory demyelinating polyradiculoneuropathy variant of Guillain‐Barre syndrome in the Pakistani population ‐ a comparison with global data. J Peripher Nerv Syst. 2017;22(4):451‐454. 10.1111/jns.12241 [DOI] [PubMed] [Google Scholar]
- 23. Burke D. Clinical uses of H reflexes of upper and lower limb muscles. Clin Neurophysiol Pract. 2016;7(1):9‐17. 10.1016/j.cnp.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito M, Kuwabara S, Odaka M, et al. Bickerstaff’s brainstem encephalitis and Fisher syndrome form a continuous spectrum: clinical analysis of 581 cases. J Neurol. 2008;255:674‐682. [DOI] [PubMed] [Google Scholar]
- 25. Sekiguchi Y, Misawa S, Shibuya K, et al. Patterns of sensory nerve conduction abnormalities in Fisher syndrome: more predominant involvement of group Ia afferents than skin afferents. Clin Neurophysiol. 2013;124:1465‐1469. [DOI] [PubMed] [Google Scholar]
- 26. Kuwabara S, Ogawara K, Koga M, Mori M, Hattori T, Yuki N. Hyperreflexia in Guillain‐Barré syndrome: relation with acute motor axonal neuropathy and anti‐GM1 antibody. J Neurol Neurosurg Psychiatry. 1999;67(2):180‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Versace V, Campostrini S, Rastelli E, et al. Understanding hyper‐reflexia in acute motor axonal neuropathy (AMAN). Neurophysiol Clin. 2020;50(3):139‐144. [DOI] [PubMed] [Google Scholar]
- 28. Uncini A, Notturno F, Kuwabara S. Hyper‐reflexia in Guillain‐Barré syndrome: systematic review. J Neurol Neurosurg Psychiatry. 2020;91(3):278‐284. [DOI] [PubMed] [Google Scholar]
- 29. Albertí MA, Alentorn A, Martínez‐Yelamos S, et al. Very early electrodiagnostic findings in Guillain‐Barré syndrome. J Peripher Nerv Syst. 2011;16(2):136‐142. 10.1111/j.1529-8027.2011.00338.x [DOI] [PubMed] [Google Scholar]
- 30. Luigetti M, Servidei S, Modoni A, Rossini PM, Sabatelli M, Lo Monaco M. Admission neurophysiological abnormalities in Guillain‐Barré syndrome: a single‐center experience. Clin Neurol Neurosurg. 2015;135:6‐10. 10.1016/j.clineuro.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 31. Jin J, Hu F, Qin X, et al. Very early neurophysiological study in Guillain‐Barre Syndrome. Eur Neurol. 2018;80(1‐2):100‐105. 10.1159/000494261 [DOI] [PubMed] [Google Scholar]
- 32. Alberti MA, Povedano M, Montero J, Casasnovas C. Early electrophysiological findings in Fisher‐Bickerstaff syndrome. Neurologia. 2020;35(1):40‐45. [DOI] [PubMed] [Google Scholar]
- 33. Sumner AJ. The physiological basis for symptoms in Guillain‐Barré syndrome. Ann Neurol. 1981;9(Suppl):28‐30. 10.1002/ana.410090706 [DOI] [PubMed] [Google Scholar]
- 34. Olsson Y. Topographical differences in the vascular permeability of the peripheral nervous system. Acta Neuropathol. 1968;10(1):26‐33. 10.1007/BF00690507 [DOI] [PubMed] [Google Scholar]
- 35. Berciano J, Sedano MJ, Pelayo‐Negro AL, et al. Proximal nerve lesions in early Guillain‐Barré syndrome: implications for pathogenesis and disease classification. J Neurol. 2017;264(2):221‐236. 10.1007/s00415-016-8204-2 [DOI] [PubMed] [Google Scholar]
- 36. Griffin JW, Li CY, Ho TW, et al. Guillain‐Barré syndrome in northern China. The spectrum of neuropathological changes in clinically defined cases. Brain. 1995;118(Pt 3):577‐595. 10.1093/brain/118.3.577 [DOI] [PubMed] [Google Scholar]
- 37. Gorson KC, Ropper AH, Muriello MA, Blair R. Prospective evaluation of MRI lumbosacral nerve root enhancement in acute Guillain‐Barré syndrome. Neurology. 1996;47(3):813‐817. 10.1212/wnl.47.3.813 [DOI] [PubMed] [Google Scholar]
- 38. Grimm A, Décard BF, Schramm A, et al. Ultrasound and electrophysiologic findings in patients with Guillain‐Barré syndrome at disease onset and over a period of six months. Clinical Trial Clin Neurophysiol. 2016;127(2):1657‐1663. 10.1016/j.clinph.2015.06.032 [DOI] [PubMed] [Google Scholar]
- 39. Ye Y, Zhu D, Liu L, Wang K, Huang K, Hou C. Electrophysiological measurement at Erb's point during the early stage of Guillain‐Barré syndrome. J Clin Neurosci. 2014;21(5):786‐789. 10.1016/j.jocn.2013.07.022 [DOI] [PubMed] [Google Scholar]
- 40. Kurt Incesu T, Secil Y, Tokucoglu F, et al. Diagnostic value of lumbar root stimulation at the early stage of Guillain‐Barré syndrome. Clin Neurophysiol. 2013;124(1):197‐203. 10.1016/j.clinph.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 41. Rajabally YA, Hassan‐Smith G, Notturno F, et al. Motor and sensory conduction failure in overlap of Guillain‐Barré and Miller Fisher syndrome: two simultaneous cases. J Neurol Sci. 2011;303(1‐2):35‐38. 10.1016/j.jns.2011.01.019 [DOI] [PubMed] [Google Scholar]
- 42. Rajabally YA, Samarasekera S. Electrophysiological sensory demyelination in typical chronic inflammatory demyelinating polyneuropathy. Eur J Neurol. 2010;17(7):939‐944. [DOI] [PubMed] [Google Scholar]
- 43. Squintani G, Basaldella F, Donato F, Silipo S, Moretto G. Increase of distal sensory action potential duration as a sensitive electrophysiological parameter in atypical case of acute inflammatory demyelinating polyneuropathy. Neurol Sci. 2016;37(2):305‐307. [DOI] [PubMed] [Google Scholar]
- 44. Umapathi T, Li Z, Verma K, Yuki N. Sural‐sparing is seen in axonal as well as demyelinating forms of Guillain‐Barré syndrome. Clin Neurophysiol. 2015;126(12):2376‐2380. 10.1016/j.clinph.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 45. Bromberg MB, Albers JW. Patterns of sensory nerve conduction abnormalities in demyelinating and axonal peripheral nerve disorders. Muscle Nerve. 1993;16(3):262‐266. 10.1002/mus.880160304 [DOI] [PubMed] [Google Scholar]
- 46. Tamura N, Kuwabara S, Misawa S, Mori M, Nakata M, Hattori T. Superficial radial sensory nerve potentials in immune‐mediated and diabetic neuropathies. Clin Neurophysiol. 2005;116(10):2330‐2333. 10.1016/j.clinph.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 47. Kuwabara S, Isose S, Mori M, et al. Different electrophysiological profiles and treatment response in ‘typical’ and ‘atypical’ chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 2015;86(10):1054‐1059. 10.1136/jnnp-2014-308452 [DOI] [PubMed] [Google Scholar]
- 48. Olsson Y. Microenvironment of the peripheral nervous system under normal and pathological conditions. Review Crit Rev Neurobiol. 1990;5(3):265‐311. [PubMed] [Google Scholar]
- 49. Hiew FL, Rajabally YA. Sural sparing in Guillain‐Barré syndrome subtypes: a reappraisal with historical and recent definitions. Clin Neurophysiol. 2016;127(2):1683‐1688. 10.1016/j.clinph.2015.09.131 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.


