Abstract
Background
With the advent of low‐cost generic direct‐acting antivirals (DAA), hepatitis C (HCV) elimination is now achievable even in low‐/middle‐income settings. We assessed the feasibility and effectiveness of a simplified clinical pathway using point‐of‐care diagnostic testing and non‐specialist‐led care in a decentralized, community‐based setting.
Methods
This feasibility study was conducted at two sites in Yangon, Myanmar: one for people who inject drugs (PWID), and the other for people with liver disease. Participants underwent on‐site rapid anti‐HCV testing and HCV RNA testing using GeneXpert(R). General practitioners determined whether participants started DAA therapy immediately or required specialist evaluation. Primary outcome measures were progression through the HCV care cascade, including uptake of RNA testing and treatment, and treatment outcomes.
Findings
All 633 participants underwent anti‐HCV testing; 606 (96%) were anti‐HCV positive and had HCV RNA testing. Of 606 tested, 535 (88%) were RNA positive and had pre‐treatment assessments; 30 (6%) completed specialist evaluation. Of 535 RNA positive participants, 489 (91%) were eligible to initiate DAAs, 477 (98%) completed DAA therapy and 421 achieved SVR12 (92%; 421/456). Outcomes were similar by site: PWID site: 91% [146/161], and liver disease site: 93% [275/295]). Compensated cirrhotic patients were treated in the community; they achieved an SVR12 of 83% (19/23). Median time from RNA test to DAA initiation was 3 days (IQR 2‐5).
Conclusions
Delivering a simplified, non‐specialist‐led HCV treatment pathway in a decentralized community setting was feasible in Yangon, Myanmar; retention in care and treatment success rates were very high. This care model could be integral in scaling up HCV services in Myanmar and other low‐ and middle‐income settings.
Keywords: cirrhosis, general practitioners, hepatitis C, Myanmar, non‐specialist, people who inject drugs, point‐of‐care testing, retention in care, South East Asia
Abbreviations
- ALT
Alanine aminotransferase
- APRI
aspartate aminotransferase to platelet ratio index
- AST
Aspartate aminotransferase
- BI
Burnet Institute
- CT2
Community‐based Testing and Treatment Study
- DAAs
Direct‐acting antivirals
- eGFR
estimated glomerular filtration rate
- FIND
Foundation for Innovative New Diagnostics
- GPs
general practitioners
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- ITT
intention‐to‐treat
- LMICs
low‐ and middle‐income countries
- mITT
modified intention‐to‐treat
- MLF
Myanmar Liver Foundation
- PoC
point‐of‐care
- PWID
people who inject drugs
- RDT
rapid diagnostic test
- SVR12
sustained virological response at 12 weeks post‐treatment completion
- VL
viral load
- WHO
World Health Organization
Lay summary.
We conducted a feasibility study of a decentralized, community‐based, general practitioner‐led ‘one‐stop‐shop’ model of care for hepatitis C in Myanmar. We found very high retention in care across the care cascade and high cure rates, including for people who inject drugs and for people with compensated cirrhosis who were treated by a trained general practitioner in the community. New innovative models of care must be developed to ensure access for all, especially in low‐ and middle‐income settings that have the bulk of the disease burden; this care model could be integral in scaling up hepatitis C services in Myanmar and other low‐ and middle‐income settings.
1. INTRODUCTION
An estimated 71 million people live with hepatitis C (HCV) infection, 75% in low‐ and middle‐income countries (LMICs). 1 The World Health Organization (WHO) set the goal of eliminating HCV as a public health threat by 2030, 2 but few countries are on track. 3 For LMICs, limited specialized workforces and the cost of diagnostics and treatment are major barriers to elimination. 4 To achieve elimination targets, considerable investment is required 5 , 6 and treatment access must be scaled up via decentralized, simplified clinical pathways utilizing non‐specialist physicians. 7
Direct‐acting antivirals (DAAs)—well‐tolerated, short‐duration (typically 8‐12 weeks), all‐oral regimens, effective for all virus genotypes—have revolutionized the global response to HCV. 8 Clinical trial data of most DAA regimens demonstrate sustained virological response at 12 weeks post‐treatment (SVR12) rates of over 95%, with many close to 100%. 9 Their simplicity and low toxicity profile 9 allow prescribing by general practitioners (GPs—aka primary care physicians), facilitating treatment of most HCV patients in the community, with complex cases referred for specialist review. 8 In many settings, the diagnostic pathway requires multiple blood draws and appointments, with substantial loss to follow‐up at each step. 10 Rapid point‐of‐care (PoC) tests now enable diagnosis of active infection within one encounter, without centralized laboratory facilities.
These advances allow implementation of a ‘one‐stop‐shop’ model of testing and treatment in decentralized health facilities, with task shifting from specialists to GPs, supported by WHO guidelines. 8 Treatment outcomes of non‐specialist‐led HCV clinical pathways are non‐inferior to those in specialist‐led care. 11 , 12 Moreover, models of care in Egypt, Pakistan, Iran and Australia providing on‐site rapid anti‐HCV testing, phlebotomy (including on‐site PoC RNA testing or sample referral) and treatment prescription have demonstrated high retention 13 , 14 , 15 , 16 and treatment uptake. 13 , 14 , 15 Evidence from a Cambodian GP‐led programme suggest that iterative simplification of HCV clinical pathways does not reduce safety or effectiveness of care, 17 but does reduce cost per cure. 18 ‘One‐stop‐shop’ models can deliver acceptable, convenient and accessible service in the community at reduced cost. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20
Myanmar is a South‐East Asian LMIC with 53 million people, 21 an estimated 1.4 million (2.7%) of whom are anti‐HCV positive. 22 Before 2000, when blood donation screening was improved, 23 most HCV transmission probably occurred through formal and informal healthcare settings, 8 and likely persists in informal settings. Among people who inject drugs (PWID), estimated anti‐HCV prevalence is 56%, but 70‐85% in areas of Kachin State 24 ; transmission of HCV via shared injecting equipment continues.
In 2016, Myanmar launched its National Hepatitis Control Program and first National Action Plan (2017‐2020), outlining targets for viral hepatitis for 2030. 23 For HCV, targets included diagnosing 50% and treating 50% of people with HCV infection by 2030. 23 The Myanmar National Simplified Treatment Guidelines (2017, 2019) for HCV infection, 25 , 26 based on the WHO HCV care and treatment guidelines, 27 support a simplified testing and treatment algorithm. Individuals must undertake the minimum set of pre‐treatment assessments, but no genotyping is required. The pan‐genotypic DAA regimens currently recommended are sofosbuvir/velpatasvir and sofosbuvir/daclatasvir. 26 Importantly, the guidelines allow for both specialist physicians (e.g. hepatologists, infectious disease physicians) and GPs to prescribe DAAs; patients with physical signs of liver decompensation should be referred to specialists. 25 , 26 Therefore, the guidelines allow simplified GP‐led clinical pathways to be implemented in community settings in Myanmar.
The CT2 Study (Hepatitis C: Community‐based Testing and Treatment) was part of the Foundation for Innovative New Diagnostics (FIND)‐led Hepatitis C Elimination through Access to Diagnostics (HEAD‐Start) project supported by Unitaid. The CT2 Study implemented a ‘one‐stop‐shop’ model of care, utilizing PoC diagnostic testing conducted by laboratory technicians and a simplified GP‐led clinical pathway. This manuscript describes the care cascade outcomes of the CT2 Study, which demonstrate the feasibility and effectiveness of this simplified clinical pathway at two sites.
2. METHODS
Our methods are described briefly below, and in detail elsewhere. 28
The study was conducted at two study sites in Yangon, Myanmar: Burnet Institute (BI) Thingangyun Key Population Service Centre for PWID and the Myanmar Liver Foundation (MLF) Than Sitt Charity Clinic for patients with liver‐related concerns. The Myanmar Department of Medical Research Ethics Review Committee (#2019‐144) and the Alfred Hospital Human Research Ethics Committee (#244/17) approved the study. The trial was registered at ClinicalTrials.gov (NCT03939013) on 6 May 2019. The investigators are responsible for study design, data collection, data analysis, interpretation of data and manuscript preparation.
2.1. Study procedures
Interested participants were screened using the pre‐enrolment criteria, 28 and eligible participants signed informed consent forms. GPs took short medical histories and referred them to on‐site laboratory technicians (Figure 1).
FIGURE 1.
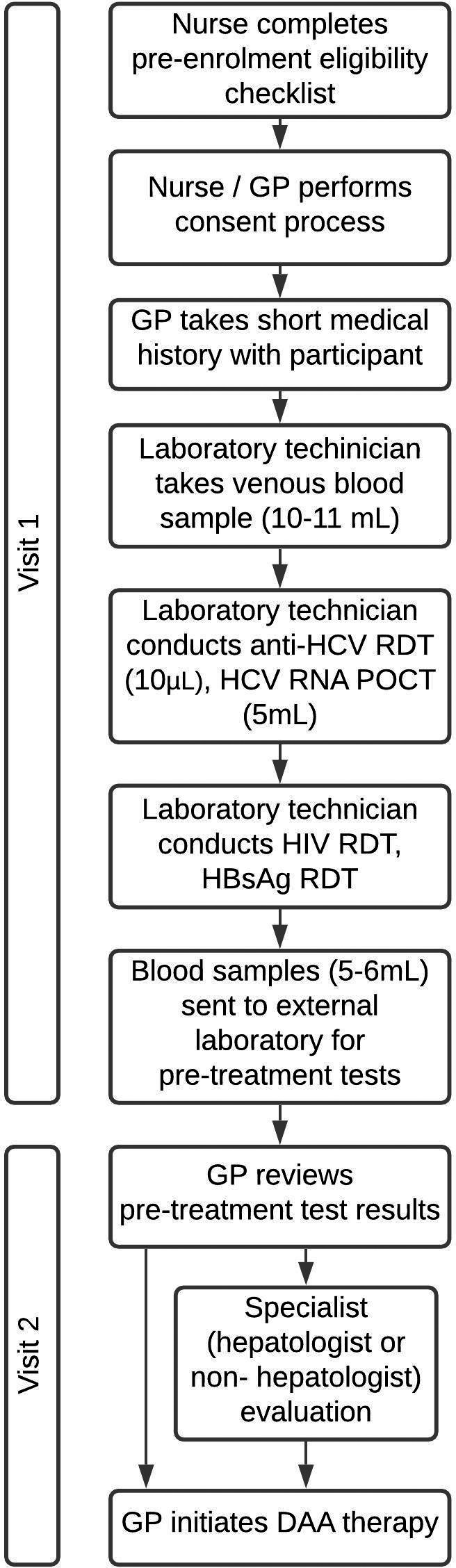
Study procedures diagram. Study procedure steps from pre‐enrolment screening to initiating DAA therapy
Technicians took 10‐11 mL of blood, then conducted the WHO prequalified PoC rapid diagnostic test (RDT) for anti‐HCV (SD BIOLINE). Following a reactive RDT result, HCV on‐site RNA testing involved the WHO prequalified Xpert hepatitis C VL assay on the GeneXpert® System (Cepheid). All HCV‐RNA‐positive participants then had HIV and HBV RDTs, liver function tests, renal function tests and a full blood examination. RDTs were conducted on‐site and all other blood tests at a private laboratory nearby, with samples collected from study sites daily and results emailed within 24 hours. The aspartate aminotransferase to platelet ratio index (APRI) score was calculated to assess cirrhosis and inform length of treatment (12 or 24 weeks). The APRI score is calculated as:
Participants were asked to return for review of pre‐treatment assessments; GPs performed a clinical assessment for physical signs of decompensated cirrhosis, and determined whether the participant required hepatologist or other specialist evaluation. Participants with (i) ALT or AST >200 U/L, (ii) bilirubin above 1.14 mg/dL, (iii) albumin <3.5 g/dL without other obvious cause, or with past or current, (iv) jaundice, (v) ascites, (vi) hepatic encephalopathy or (vii) haematemesis and melena met the criteria for hepatologist review. Participants reviewed by a specialist then returned to the GP for DAA initiation, if deemed appropriate. Participants were ineligible for DAA therapy if they presented with (i) HIV co‐infection, (ii) hepatitis B co‐infection, (iii) active tuberculosis, (iv) eGFR <30 mL/min/1.73 m2, (v) or pregnancy or (vi) were taking medications with serious interactions with sofosbuvir/daclatasvir.
For eligible participants, GPs prescribed and dispensed generic oral sofosbuvir 400 mg and daclatasvir 60 mg on‐site. The APRI cut‐off for cirrhosis was 2.0 as per the 2017 Myanmar National Guidelines, 25 but was changed to 1.5 on 6 September 2019 in line with the second edition. 26 Participants with an APRI score below the national cut‐off received a 12‐week course, and those with APRI score above cut‐off received a 24‐week course. Participants returned to the clinic every four weeks for short on‐treatment study‐related monitoring visits, which included medication dispensing and questions about alcohol use, injecting drug use, medication adherence and side effects.
Participants’ blood was tested using the hepatitis C VL assay on the GeneXpert® System (Cepheid) 12 weeks after completing treatment to assess sustained virological response (SVR12), defined as no HCV RNA detected. Participants with HCV detectable, VL <10 IU/mL were asked to return for a second HCV RNA test at SVR24; if the result was ‘not detected’, this was classified as SVR12 achieved.
2.2. Data collection
Our data collection procedures have been described previously. 28 In brief, we collected clinical case report forms and participants completed behavioural and acceptability questionnaires. GPs completed case report forms using an electronic database (OpenMRS) at every clinical visit. Participants completed questionnaires in Burmese (the primary language spoken in Yangon) through REDCap, with optional assistance from the study nurse.
2.3. Outcomes
Feasibility was determined by progression through the care cascade measured by the primary outcomes outlined in the protocol 28 and two secondary outcome measures comparing the care cascade by site and clinical characteristics and treatment outcomes by cirrhosis status. The primary outcome measures were (i) proportion of anti‐HCV‐positive participants receiving an RNA test, (ii) proportion of RNA‐positive participants initiated on DAAs, (iii) proportion of participants initiated on treatment completing therapy per protocol (defined as collecting last 28‐day bottle of medication and not reporting more than 7 days missed doses) and (iv) proportion of participants completing treatment achieving SVR12. The secondary outcome measures presented here were (i) proportion of participants requiring specialist review before DAA initiation, (ii) primary outcomes by site, (iii) days from RNA test to treatment initiation and (iv) liver and other clinical characteristics, and treatment outcomes, of the participant group initiated on DAA therapy, by cirrhosis status.
We will undertake (and publish separately) an in‐depth feasibility and scalability assessment of the model through document review and analysis of key informant interviews, covering factors contributing to successful implementation and key requirements for scalability.
2.4. Statistical analysis
Descriptive statistics of progression through the care cascade are presented. Differences in baseline characteristics and the proportion progressing through the care cascade steps (primary outcome measures) between sites, and in clinical characteristics and outcomes by cirrhosis status, were compared using Chi‐squared tests or Fisher's exact tests. Achievement of SVR12 was assessed in a per‐protocol population of those who completed treatment per protocol, in an intention‐to‐treat (ITT) population, and in a modified intention‐to‐treat (mITT) population, in which only those tested for treatment outcome were included in SVR12 analysis (includes those who did not complete treatment per protocol). SVR12 among the ITT population, restricted to those who completed DAA treatment per protocol, was also assessed. Participants who returned for their test one week early or within 12 weeks after scheduled SVR12 test were included in analysis. The median and interquartile range for number of days from RDT to DAA treatment initiation and RNA test to DAA initiation were calculated and compared by specialist review and site, respectively. Differences between sites were determined using Wilcoxon rank sum tests. All data management and analyses were conducted in StataSE v15.0 (College Station, TX: StataCorp LLC).
3. RESULTS
3.1. Participant characteristics
Between 30 January and 30 September 2019, 634 patients were enrolled; one subsequently withdrew consent and data. Data for 633 participants are shown in Table 1, disaggregated by clinic site. Most participants were male, employed, resided in Yangon and reported previous anti‐HCV testing. Participant characteristics differed by site; participants at MLF site were older, more likely to live outside Yangon, were unemployed and reported unknown source of HCV infection. Almost all participants at the BI site were male and reported injecting drugs recently. Most were prescribed methadone and one‐fifth reported a history of incarceration. Very few participants reported hazardous alcohol consumption in the past 12 months (2%, 11/599).
TABLE 1.
Baseline demographic and behavioural characteristics
|
Total N = 633 n (%) |
MLF Than Sitt Charity Clinic n = 380 n (%) |
Burnet Institute Thingangyun Clinic n = 253 n (%) |
Pearson's chi‐square test e /Wilcoxon rank sum test P‐value |
|
|---|---|---|---|---|
| Sex, male | 405 (64) | 166 (44) | 239 (94) | P < .001 |
| Median age, years (IQR) | 42 (31‐53) | 50.5 (39‐59) | 32 (27‐40) | P < .001 |
| Residence location | ||||
| Yangon | 466 (74) | 223 (59) | 243 (96) | P < .001 |
| Outside of Yangon | 167 (26) | 157 (41) | 10 (4) | |
| Employment status a | ||||
| Employed | 352 (56) | 197 (52) | 155 (61) | P = .005 |
| Unemployed | 231 (36) | 159 (42) | 72 (28) | |
| Retired/student | 45 (7) | 24 (6) | 21 (8) | |
| Ever‐injected drugs b | 265 (42) | 12 (3) | 253 (100) | P < .001 |
| Injected drugs in the past 6 months |
236/264 (89) |
1/12 (8) |
235/253 (93) |
P < .001 |
| Currently prescribed methadone at screening | 161 (25) | 0 (0) | 161 (64) | P < .001 |
| Ever incarcerated c | 65 (10) | 7 (2) | 58 (24) | P < .001 |
| Self‐reported mode of hepatitis C acquisition d : | ||||
| Injecting drug use | 218 (36) | 6 (2) | 212 (88) | P < .001 |
| Tattoo/scarification | 24 (4) | 11 (3) | 13 (5) | |
| Healthcare or dental care related | 106 (18) | 102 (28) | 4 (2) | |
| Family history | 78 (13) | 75 (21) | 3 (1) | |
| Unprotected sex | 22 (4) | 15 (4) | 7 (3) | |
| Unknown | 157 (26) | 156 (43) | 1 (0.4) | |
| Previously tested for anti‐HCV antibodies (self‐report) | ||||
| Never tested | 91 (14) | 12 (3) | 79 (31) | P < .001 |
| Tested previously | 542 (86) | 368 (97) | 174 (69) | |
‘Prefer not to answer’ responses included in denominator:
5 responded ‘prefer not to answer’.
1 responded ‘prefer not to answer’.
13 responded ‘prefer not to answer’.
28 had no mode of acquisition selected and were excluded from the denominator.
Fisher's exact test used for variables where there was a cell n < 5.
3.2. Care cascade progression
The cascade of care for the 633 study participants is presented in Figure 2. Progression through the care cascade, including primary and secondary outcome measures, is presented by site in Table 2. Of the participants screened for anti‐HCV, 96% were antibody positive; of these, 100% had RNA testing and 88% were positive (Figure 2); anti‐HCV and HCV RNA positivity were similar by site (Table 2).
FIGURE 2.
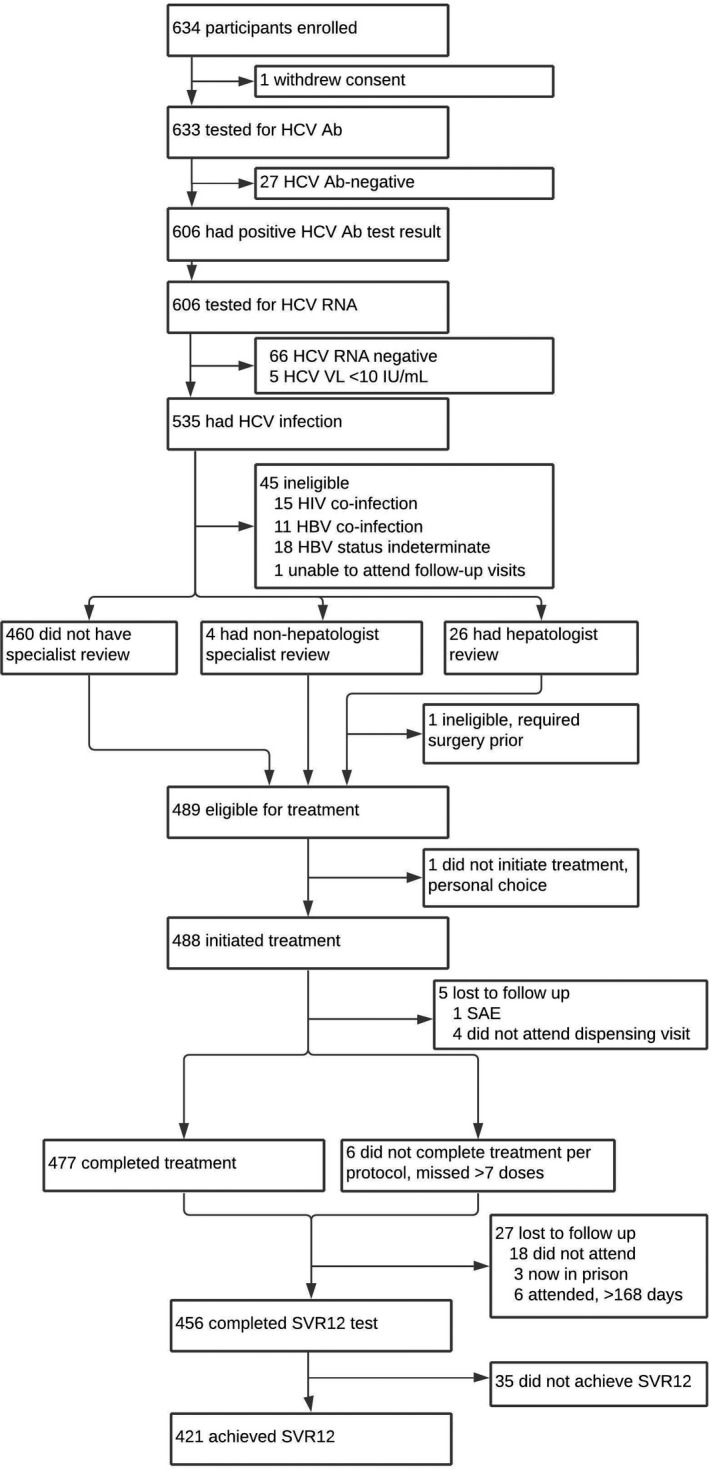
Clinical pathway progression. Participant progression through clinical pathway, including ineligible participants and those lost to follow‐up
TABLE 2.
Care cascade outcomes, by clinic and total
|
Total N = 633 n (%) |
MLF Than Sitt Charity Clinic n = 380 n (%) |
Burnet Institute Thingangyun Clinic n = 253 n (%) |
Pearson's Chi‐square/Fisher's exact test (P‐value) | |
|---|---|---|---|---|
| Positive for anti‐hepatitis C antibodies | 606 (96) | 368 (97) | 238 (94) | P = .091 |
| Received GeneXpert(R) RNA test a | 606 (100) | 368 (100) | 238 (100) | NA |
| Positive for hepatitis C RNA | 535 (88) | 331 (90) | 204 (86) | P = .114 |
| Number of RNA‐positive participants: | 535 | 331 | 204 | |
| Patients who underwent specialist review b | 30 (6) | 13 (4) | 17 (8) | P = .027 |
| Patients eligible for DAA treatment | 489 (91) | 312 (94) | 177 (87) | P = .003 |
| Patients initiated on DAA treatment a | 488 (91) | 312 (94) | 176 (86) | P = .002 |
| Patients who initiated DAA treatment: | 488 | 312 | 176 | |
| Patients who completed treatment per protocol a | 477 (98) | 308 (99) | 169 (96) | P = .054 |
| Patients who achieve SVR12 a (ITT analysis) | 421 (86) | 275 (88) | 146 (83) | P = .110 |
| Patients who complete treatment per protocol: | 477 | 308 | 169 | |
| Patients who achieve SVR12 (per protocol analysis) a | 419 (88) | 275 (89) | 144 (85) | P = .192 |
| Patients who underwent SVR12 testing: | 456 | 295 | 161 | |
| Patients who achieve SVR12 a (mITT analysis) | 421 (92) | 275 (93) | 146 (91) | P = .331 |
Per protocol analysis = analysis of SVR12 achievement, among those who completed treatment.
ITT analysis = analysis of SVR12 achievement among all those initiated on DAA treatment.
mITT analysis = analysis of SVR12 achievement, only among those tested for SVR12.
Primary outcome measures.
Secondary outcome measures.
Among the RNA‐positive participants, 86% (459/535) were eligible to initiate DAA therapy by the GP directly and 6% (30/535) required specialist evaluation first (Figure 2). A higher proportion underwent specialist evaluation at the BI site (Table 2). The most common reason for specialist review was elevated liver function test results (Table 3). In all, 26 participants were reviewed by a hepatologist, and four by other specialists; all but one were eligible for treatment.
TABLE 3.
Reasons for specialist evaluation
| N = 30 | |
|---|---|
| Elevated liver function test (ALT, AST, bilirubin) | 22 |
| Low platelet count | 1 |
| Review of abnormal abdomen ultrasound | 1 |
| Referral for chest X‐ray to exclude tuberculosis/pneumonia | 2 |
| Review for suspected gall stones | 1 |
| Advice re steroid interaction with DAAs | 1 |
| Review of concurrent cardiac issue | 1 |
| Referral to urosurgeon | 1 |
In total, 489/535 participants with current HCV were eligible for treatment; 488 started treatment and were managed by GPs in community‐based settings. Proportions initiating DAA treatment differed by site due to the proportion eligible (Table 2). Forty‐six RNA‐positive participants were ineligible to commence DAA therapy, mostly due to HIV or HBV co‐infection (Figure 2). Those ineligible for DAA therapy in the study were referred to other treatment programmes.
Of 488 DAA initiators, 483 (99%) received all monthly treatment refills and 477 (98%) completed treatment per protocol (Figure 2). Per protocol treatment completion did not differ significantly by site (Table 2), and only five participants discontinued treatment (Figure 2). Forty‐seven participants reported missing at least one dose, but 39 (83%) missed fewer than seven doses. Most participants (91%) reported no side effects at monitoring visits.
Of 488 who commenced treatment, 421 (86%) achieved SVR12 using ITT analysis, including 12 patients with detectable but unquantifiable HCV VL (<10 IU/L) at SVR12 who had confirmed clearance at SVR24. Similarly, high rates of SVR12 were achieved in ITT analysis across both sites (Table 2). mITT analysis of SVR12, restricted to treatment outcomes among those tested, showed high rates of SVR12 at both sites (Table 2).
3.3. Clinical characteristics
Clinical characteristics of the 488 treatment initiators are presented in Table 4, disaggregated by cirrhosis status, defined as APRI score below or above cut‐off of 2.0 (or 1.5 after 6 September 2019).
TABLE 4.
Liver and renal function characteristics and treatment outcomes among DAA initiators by cirrhosis status and total
|
Total N = 488 |
Non‐cirrhotic (APRI <cut‐off) n = 463 |
Cirrhotic (APRI >cut‐off) n = 25 |
Chi‐square or Fisher's exact test | |
|---|---|---|---|---|
| Liver function | ||||
| AST | ||||
| Normal | 304 (62) | 304 (66) | 0 (0) | P < .001 |
| Above normal cut‐off | 184 (38) | 159 (34) | 25 (100) | |
| ALT | ||||
| Normal | 352 (72) | 344 (74) | 8 (32) | P < .001 |
| Above normal cut‐off | 136 (28) | 119 (26) | 17 (68) | |
| Bilirubin | ||||
| Normal | 481 (99) | 459 (99) | 22 (88) | P = .004 |
| Above normal cut‐off | 7 (1) | 4 (1) | 3 (12) | |
| Albumin | ||||
| Normal or high | 478 (98) | 456 (98) | 22 (88) | P = .011 |
| Low | 10 (2) | 7 (2) | 3 (12) | |
| Other clinical parameters | ||||
| eGFR | ||||
| Normal | 337 (69) | 320 (69) | 17 (68) | P = .907 |
| Below normal | 151 (31) | 143 (31) | 8 (32) | |
| Creatinine | ||||
| Normal or low | 464 (95) | 441 (95) | 23 (92) | P = .352 |
| Above normal | 24 (5) | 22 (5) | 2 (8) | |
| Platelet count | ||||
| Low (<150 × 109/L) | 45 (9) | 24 (5) | 21 (84) | P < .001 |
| Normal (150 × 109/L–400 × 109/L) | 432 (89) | 428 (92) | 4 (16) | |
| Above normal (>400 × 109/L) | 11 (2) | 11 (2) | 0 (0) | |
| Treatment decisions and outcomes | ||||
| DAA course length | ||||
| 12‐week course | 461 (94) | 461 (99.6) | 0 (0) | P < .001 |
| 24‐week course | 27 (6) | 2 (0.4) | 25 (100) | |
| SVR12 (ITT, among those who started DAAs) | ||||
| Achieved | 421 (86) | 402 (87) | 19 (76) | P = .126 |
| Not achieved | 67 (14) | 61 (13) | 6 (24) | |
| SVR12 (per protocol analysis) | n = 477 | n = 456 | n = 21 | |
| Achieved | 419 (88) | 400 (88) | 19 (91) | P = .705 |
| Not achieved | 58 (12) | 56 (12) | 2 (10) | |
| SVR12 (mITT, among those tested) | n = 456 | n = 433 | n = 23 | |
| Achieved | 421 (92) | 402 (93) | 19 (83) | P = .090 |
| Not achieved | 35 (8) | 31 (7) | 4 (17) | |
Normal ranges for sex, as per laboratory standards: AST normal cut‐off: <59 U/L for male, <36 U/L for female; ALT normal cut‐off: <72 U/L for male, <52 U/L for female; Bilirubin normal cut‐off: >1.14 mg/dL; eGFR normal cut‐off: >90 mL/min/1.73 m2; Albumin cut‐off: <3.5 g/dL; Creatinine normal cut‐off: 1.2 mg/dL.
Per protocol analysis = analysis of SVR12 achievement, among those who completed treatment.
ITT analysis = analysis of SVR12 achievement among all those initiated on DAA treatment.
mITT analysis = analysis of SVR12 achievement, only among those tested for SVR12.
Most participants with cirrhosis were from the MLF Clinic (n = 19, 76%). Compared to non‐cirrhotic participants, those with cirrhosis were more likely to have AST and ALT levels outside the normal range (100% vs 34%, 68% vs 26%, respectively), and to have lower platelet counts consistent with portal hypertension (84% vs 8%). Treatment outcomes in non‐cirrhotic and cirrhotic participants were 87% vs 76% (P = .126, ITT) and 93% vs 83% (P = .090, mITT) when restricted to those tested, with a (non‐significantly) lower proportion of cirrhotic participants achieving SVR12. Treatment outcomes were similar when restricted to those who completed treatment per protocol (non‐cirrhotic: 88% vs cirrhotic: 91%, P = .705).
Of those who did not achieve SVR12 (n = 35), five reported missing doses: two missed fewer than seven doses and three seven or more doses. Of those who did not achieve SVR12, 12 (34%) participants reported injecting drug use in the past 6 months at SVR12, therefore were possibly re‐infected with hepatitis C. Dried blood spot samples were taken at screening and at SVR12 for further testing to distinguish reinfection from treatment failure. (Testing for reinfection was delayed due to COVID‐19 restrictions, so these findings will be reported in a subsequent paper.)
3.4. Time taken to progress through cascade of care
Most participants (n = 444, 91%) required two visits before initiating treatment and receiving their first month of DAA medication: one visit for HCV testing (RDT and RNA testing) and one visit for review of pre‐treatment investigations and treatment initiation. RDT and RNA tests were conducted on the same day for 587 participants (97%).
Median time from RDT to DAA initiation was 3 days (IQR 2‐5). There was a statistically significant difference between median time for those not requiring specialist review (3 days, IQR 2‐5) and those requiring review (20 days, IQR 15‐36, P < .001). Time from final RNA test date to DAA initiation date was a median of 3 days (IQR 2‐5) across both sites. Median time from final RNA test date to DAA initiation date differed by site: 2 days (IQR 2‐4) for MLF Clinic, and 4 days (IQR 3‐7) for BI Clinic.
4. DISCUSSION
Implementing a simplified one‐stop‐shop clinical pathway, using PoC diagnostic testing and GP‐initiated DAA therapy, in a decentralized, community‐based setting, was feasible in Myanmar. It was also feasible for GPs to manage compensated cirrhotic patients in the community setting and to facilitate timely access to specialist review, including hepatologists and other relevant specialists, through a ‘hub‐and‐spoke’ approach where required.
Retention in care was high throughout our care cascade. Similar simplified, decentralized models of care have been implemented elsewhere, 13 , 14 , 17 , 29 , 30 , 31 , 32 but few are ‘one‐stop‐shops’ with non‐specialist‐led care in the community. 20 Retention in care was slightly higher in our study than in a similar care model implemented in Egypt, where linkage and treatment uptake in the early cascade was over 90%. 13 The Egyptian model involved specialist‐led care, not GP‐led care, and was implemented over one to three days, with same‐day testing and treating implemented by the outreach clinical team, and did not report SVR12 uptake and rate. Retention in care was equally high for PWID in our study. Many studies of HCV models of care focus on lowering barriers to care and reducing attrition among PWID, but most have been conducted in middle‐ and high‐income settings. 19 Barriers to care for PWID include the multi‐step process of accessing treatment and previous experiences of discrimination in healthcare. 33 High retention in care among PWID in our study, most of whom were current injectors, points to the benefit of convenient care; the study site was near a methadone dispensary and the study site provided needle and syringe exchange. This care model appeared convenient for participants at both sites, because they only attended one community‐based location for care, and the simplified pathway reduced visit frequency to two in most instances and the time from screening test to starting treatment to a median of 3 days. Time to start treatment was generally shorter than in other studies, where time varied from zero days (same‐day test and treat) 13 to 100, 14 depending on the clinical pathway and model of care implemented. We will explore the acceptability of this model of care in forthcoming qualitative work.
Most participants were managed by GPs, without referral for specialist evaluation, including participants currently injecting drugs and participants with cirrhosis. The mITT SVR12 rates among those tested were high at 92%, and importantly, cure rates were no worse among PWID nor those with cirrhosis. The observed cure rates were similar to those reported in early clinical trials using sofosbuvir and daclatasvir (sof/dac) regimens: the ALLY‐3 Phase III clinical trial of a sof/dac regimen for genotype 3 patients reported 90% SVR12 achievement among HCV/HIV co‐infected treatment‐naïve patients. 34 Our treatment outcomes were also similar to those of an observational study in Myanmar of sof/dac (+/− ribavirin), treating mostly genotypes 3 and 6. 35 In our study, adherence to DAA therapy was very high; only 9.6% of participants reported missing any doses, and the majority of them missing fewer than seven doses. Adequate adherence to DAA therapy has often been cited among GPs and specialist physicians as a concern for treating people who are actively injecting drugs for their chronic HCV infection. 36 However, results from our study, and from pooled analyses of adherence and SVR rates, 37 , 38 including among people who actively inject drugs, demonstrate that adherence was not of particular concern among this group who consistently report high adherence and SVR rates.
Importantly, we found similarly good treatment outcomes in both cirrhotic (83%) and non‐cirrhotic (93%) groups, when restricted to those who were assessed for SVR12 ( P = .090, mITT). This is consistent with results from a study of outcomes among genotype 3 cirrhotic patients treated with sof/dac (+/− ribavirin) reporting mITT analysis where 80% of those with cirrhosis achieving SVR12. 39 Our findings suggest that treating cirrhotic patients in the community is safe and effective, with few participants not achieving SVR12 and no serious adverse events related to DAA therapy. This is consistent with literature from simplified model of care utilizing sof/dac implemented in Cambodia where those with cirrhosis were safely managed by GPs and 94.9% achieved a cure. 17 Therefore, this care model and clinical pathway will not result in more referrals to specialists to manage treatment failures, further supporting the effectiveness of task‐shifting of care and treatment from tertiary‐based specialists to community‐based non‐specialists, using a ‘hub‐and‐spoke’ referral model. These results support the treatment of all patients without cirrhosis, including PWID, using simplified clinical pathways in community‐based settings, and demonstrate the potential for treatment as prevention for PWID, with 91% achieving SVR12.
4.1. Challenges and limitations
Several limitations to the generalizability of these study findings need to be considered before wider implementation, including characteristics of the participant group and the project setting.
Testing and treatment were provided at no cost to the participants, which may have encouraged retention in care in a setting where no‐cost programmes are rare. Given this was a research study, this model of care likely afforded more staff time for clinical encounters and appointment reminders than if implemented in routine primary care. It is important to note that the national treatment guidelines do not require on‐treatment monitoring visits. Removal of these visits and one‐time dispensing for patients without cirrhosis should be considered, particularly for patients who cannot attend frequently due to employment or caring commitments, or clinic location.
More participants (88%) were RNA positive than is commonly reported (75%) among people with HCV. 40 It is unclear whether this was due to population‐specific variation in spontaneous clearance rates because a population‐level RNA prevalence study has not yet been conducted in Myanmar. Alternatively, the study may have included persons with recent HCV infection. It is also possible that some participants did not reveal their RNA‐positive status to enrol, albeit the high cost of RNA testing in Myanmar makes it unaffordable to most people. This highlights the importance of identifying options for further simplifying diagnosis prior to treatment initiation, by either removing the anti‐HCV test and only performing RNA testing, or removing RNA tests and using result at five minutes for anti‐HCV test as an indicator of RNA status. 41 Trained laboratory technicians are integral to implementation of this model of care and to any simplified diagnostic pathway. However, there is potential for task‐shifting to nurses or community health workers for conducting RNA tests, particularly with the fingerstick assay, or for other workers to perform on‐site phlebotomy and refer blood samples to external laboratories.
This study was conducted in Yangon, a major city in Myanmar, with access to high‐quality private laboratories to perform pre‐treatment assessments, usually within 24 hours. An absence of similar laboratory support may reduce the model's applicability in more remote locations. As PoC devices and/or test kits for pre‐treatment work‐up tests become available, it may be possible to perform these on‐site if access to laboratories is difficult; and such an approach was piloted successfully in Egypt. 13 However, on‐site laboratories have their own challenges, including requirements for continuous and stable electricity supply and sufficient air conditioning/refrigeration. How longer turnaround time from anti‐HCV test to receipt of pre‐treatment assessments would affect retention in care is unknown. While pre‐treatment assessment results were available within 24 hours, not all participants returned the next day for their review, and retention in care from anti‐HCV test to DAA initiation appointment was 100%. Implementation of this model of care in other settings would require scoping of blood sample referral and transport options. Provision of on‐site same‐day phlebotomy may be more important for a convenient care model than on‐site viral load testing; on‐site same‐day phlebotomy is a key component of the ‘one‐stop‐shop’.
Future work should evaluate feasibility of implementation in other settings, such as remote locations, private GP clinics, and integrated HCV testing and treatment at existing methadone treatment sites/decentralized HIV treatment sites; together with options for implementation of simplified or modified pathways for diagnostic testing and pre‐treatment assessments.
5. CONCLUSION
This study demonstrates the feasibility and effectiveness of a simplified HCV treatment pathway implemented in a decentralized, community‐based setting, utilizing PoC diagnostics and non‐specialist led care. Such models are promoted by WHO and other global organizations, recognizing the importance of integrated and fully decentralized simplified care models and task‐shifting to non‐specialists as good practice principles. Our findings support Myanmar's national hepatitis C guidelines and the development of formal recommendations from WHO on simplified service delivery to support the expansion of HCV testing and treatment in other LMICs.
ETHICS COMMITTEE APPROVAL
The Myanmar Department of Medical Research Ethics Review Committee (#2019‐144) and the Alfred Hospital Human Research Ethics Committee (#244/17) approved the study. The trial was registered at ClinicalTrials.gov (NCT03939013) on 6 May 2019.
DATA SHARING
The anonymized dataset will be held by the chief investigator.
CONFLICT OF INTEREST
MH has received investigator‐initiated grant funding from Gilead Sciences and Abbvie for unrelated work. AP has received investigator‐initiated grant funding from Gilead Sciences, MSD and Abbvie and speaker fees from Gilead Sciences for unrelated work. JH has received investigator‐initiated grant funding and speaker fees from Gilead Sciences for unrelated work. WLY has received Gilead Sciences Fellowship for related work. KPK has received non‐financial support from Mylan, Hetero and Royal Ruby. YYS and WA have received non‐financial support from Mylan. WN has received non‐financial support from Mylan and Cipla. All others declare no potential competing interests.
AUTHORS’ CONTRIBUTION
BD, HH, KPK, WN, SS, JH, AP and MH conceived, planned and designed the study, and prepared the protocol. WLY, NN and BD coordinated the implementation. SS, NN, JM, PE, BD, WLY, HH, AB and WA contributed to the study monitoring. KSA, KPK, WA and WN contributed to the intervention development and implementation of the study. YYS was site investigator for MLF and KTM was site investigator for BI; YYS, WN and KTM contributed to data collection. BD, AB, JH, AP, SS, JM, PE, JH and MH prepared the manuscript. BD and AB did the analysis. BD wrote the first draft of the paper. All authors revised the paper critically for intellectual content and approved the final version.
PATIENT CONSENT STATEMENT
All patients were taken through the informed consent process in Burmese, were provided with a written information sheet and signed an approved written informed consent form.
ACKNOWLEDGEMENTS
The authors acknowledge the contributions of study participants, the clinical team, the specialist hepatologists from Yangon public hospitals, our database developers, and the support of the Myanmar National Hepatitis Control Program for the successful implementation of the study.
Draper BL, Htay H, Pedrana A, et al. Outcomes of the CT2 study: A ‘one‐stop‐shop’ for community‐based hepatitis C testing and treatment in Yangon, Myanmar. Liver Int. 2021;41:2578–2589. 10.1111/liv.14983
Funding information
The CT2 Study was supported by Unitaid, as part of the FIND‐led HEAD‐Start Projects. Unitaid had no role in the writing of the manuscript or decision to submit for publication. All authors had full access to the full data, if requested, and accept responsibility for the decision to submit the manuscript for publication.
Handling Editor: John Dillon
REFERENCES
- 1. World Health Organization (WHO) . Global hepatitis report. 2017. https://www.who.int/hepatitis/publications/global‐hepatitis‐report2017/en/
- 2. World Health Organization (WHO) . Global Health sector strategy on viral hepatitis, 2016–2021. 2016. https://www.who.int/hepatitis/strategy2016‐2021/ghss‐hep/en/
- 3. Center for Disease Analysis Foundation . Polaris observatory—HCV elimination maps. https://cdafound.org/dashboard/polaris/maps.html
- 4. World Health Organization (WHO) . Progress report on access to hepatitis C treatment: focus on overcoming barriers in low‐ and middle‐income countries. 2018. https://apps.who.int/iris/handle/10665/260445
- 5. Tordrup D, Hutin Y, Stenberg K, et al. Additional resource needs for viral hepatitis elimination through universal health coverage: projections in 67 low‐income and middle‐income countries, 2016–30. Lancet Glob Heal. 2019;7(9):e1180‐e1188. 10.1016/S2214-109X(19)30272-4 [DOI] [PubMed] [Google Scholar]
- 6. Schröeder SE, Pedrana A, Scott N, et al. Innovative strategies for the elimination of viral hepatitis at a national level: a country case series. Liver Int. 2019;39(10):1818‐1836. 10.1111/liv.14222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pedrana A, Howell J, Scott N, et al. Global hepatitis C elimination: an investment framework. Lancet Gastroenterol Hepatol. 2020;5(10):927‐939. 10.1016/S2468-1253(20)30010-8 [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization (WHO) . Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. 2018. https://www.who.int/hepatitis/publications/hepatitis‐c‐guidelines‐2018/en/ [PubMed]
- 9. Asselah T, Marcellin P, Schinazi RF. Treatment of hepatitis C virus infection with direct‐acting antiviral agents: 100% cure? Liver Int. 2018;38:7‐13. 10.1111/liv.13673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grebely J, Applegate TL, Cunningham P, Feld JJ. Hepatitis C point‐of‐care diagnostics: in search of a single visit diagnosis. Expert Rev Mol Diagn. 2017;17(12):1109‐1115. 10.1080/14737159.2017.1400385 [DOI] [PubMed] [Google Scholar]
- 11. Castro R, Perazzo H, de Araujo LAMM, Gutierres IG, Grinsztejn B, Veloso VG. Effectiveness of implementing a decentralized delivery of hepatitis C virus treatment with direct‐acting antivirals: A systematic review with meta‐analysis. PLoS One. 2020;15(2):e0229143. 10.1371/journal.pone.0229143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oru E, Trickey A, Shirali R, Kanters S, Easterbrook P. Decentralisation, integration, and task‐shifting in hepatitis C virus infection testing and treatment: a global systematic review and meta‐analysis. Lancet Glob Heal. 2021;9(4):E431‐E445. 10.1016/S2214-109X(20)30505-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shiha G, Soliman R, Serwah A, Mikhail NN, Asselah T, Easterbrook P. A same day “test and treat” model for chronic HCV and HBV infection: results from two community‐based pilot studies in Egypt. J Viral Hepat. 2020;27(6):593‐601. 10.1111/jvh.13268 [DOI] [PubMed] [Google Scholar]
- 14. Khalid GG, Kyaw KWY, Bousquet C, et al. From risk to care: the hepatitis C screening and diagnostic cascade in a primary health care clinic in Karachi, Pakistan—a cohort study. Int Health. 2018;12(1):19‐27. 10.1093/inthealth/ihy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alavi M, Poustchi H, Merat S, et al. An intervention to improve HCV testing, linkage to care, and treatment among people who use drugs in Tehran, Iran: The ENHANCE study. Int J Drug Policy. 2019;72:99‐105. 10.1016/j.drugpo.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 16. Williams B, Howell J, Doyle J, et al. Point‐of‐care hepatitis C testing from needle and syringe programs: an Australian feasibility study. Int J Drug Policy. 2019;72:91‐98. 10.1016/j.drugpo.2019.05.012 [DOI] [PubMed] [Google Scholar]
- 17. Zhang M, O’Keefe D, Iwamoto M, et al. High sustained viral response rate in patients with hepatitis C using generic sofosbuvir and daclatasvir in Phnom Penh, Cambodia. J Viral Hepat. 2020;27(9):886‐895. 10.1111/jvh.13311 [DOI] [PubMed] [Google Scholar]
- 18. Walker JG, Mafirakureva N, Iwamoto M, et al. Cost and cost‐effectiveness of a simplified treatment model with direct‐acting antivirals for chronic hepatitis C in Cambodia. Liver Int. 2020;40(10):2356‐2366. 10.1111/liv.14550 [DOI] [PubMed] [Google Scholar]
- 19. Lazarus JV, Pericàs JM, Picchio C, et al. We know DAAs work, so now what? Simplifying models of care to enhance the hepatitis C cascade. J Intern Med. 2019;286:503‐525. 10.1111/joim.12972 [DOI] [PubMed] [Google Scholar]
- 20. Radley A, Robinson E, Aspinall EJ, Angus K, Tan L, Dillon JF. A systematic review and meta‐analysis of community and primary‐care‐based hepatitis C testing and treatment services that employ direct acting antiviral drug treatments. BMC Health Serv Res. 2019;19(1):765. 10.1186/s12913-019-4635-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Bank . Myanmar country profile. 2019. https://databank.worldbank.org/
- 22. Lwin AA, Aye KS, Moh Htun M, et al. Sero‐prevalence of Hepatitis B and C Viral Infections in Myanmar: National and Regional Survey in 2015. Myanmar Health Sci Res J. 2017;29:167–175. [Google Scholar]
- 23. National Hepatitis Control Program, Myanmar Ministry of Health and Sports . Myanmar National Action Plan for Viral Hepatitis Response 2017–2020. 2017. http://mohs.gov.mm/su/NrcAXR
- 24. National AIDS Program, Myanmar Ministry of Health and Sports (2019) . Myanmar Integrated Biological and Behavioural Survey and Population Size Estimates among People Who Inject Drugs (PWID) 2017–2018. 2019. http://www.aidsdatahub.org/resource/myanmar‐ibbs‐population‐size‐estimates‐pwid‐2017‐2018
- 25. Ministry of Health and Sports Myanmar (2017). Myanmar National Simplified Treatment Guidelines for Hepatitis C Infection ‐ first edition, 2017. 2017. https://mohs.gov.mm/Main/content/publication/hepatitis‐simplified‐treatment‐guidelines‐for‐hepatitis‐c‐infection
- 26. Ministry of Health and Sports Myanmar . Myanmar national simplified treatment guidelines for hepatitis C infection—second edition. 2019. https://mohs.gov.mm/page/3212
- 27. World Health Organization (WHO) . Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection. 2016. https://www.who.int/hepatitis/publications/hepatitis‐c‐guidelines‐2016/en/ [PubMed]
- 28. Draper BL, Pedrana A, Howell J, et al. Decentralized, community‐based hepatitis C point‐of‐care testing and direct‐acting antiviral treatment for people who inject drugs and the general population in Myanmar: protocol for a feasibility study. JMIR Res Protoc. 2020;9(7):e16863. 10.2196/16863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dhiman RK, Grover GS, Premkumar M, et al. Decentralized care with generic direct‐acting antivirals in the management of chronic hepatitis C in a public health care setting. J Hepatol. 2019;71(6):1076‐1085. 10.1016/j.jhep.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 30. Wade AJ, Doyle JS, Gane E, et al. Outcomes of treatment for hepatitis C in primary care, compared to hospital‐based care: a randomized, controlled trial in people who inject drugs. Clin Infect Dis. 2020;70(9):1900‐1906. 10.1093/cid/ciz546 [DOI] [PubMed] [Google Scholar]
- 31. Gupta N, Mbituyumuremyi A, Kabahizi J, et al. Treatment of chronic hepatitis C virus infection in Rwanda with ledipasvir–sofosbuvir (SHARED): a single‐arm trial. Lancet Gastroenterol Hepatol. 2019;4(2):119‐126. 10.1016/S2468-1253(18)30382-0 [DOI] [PubMed] [Google Scholar]
- 32. Radley A, de Bruin M, Inglis SK, et al. Clinical effectiveness of pharmacist‐led versus conventionally delivered antiviral treatment for hepatitis C virus in patients receiving opioid substitution therapy: a pragmatic, cluster‐randomised trial. Lancet Gastroenterol Hepatol. 2020;5(9):809‐818. 10.1016/S2468-1253(20)30120-5 [DOI] [PubMed] [Google Scholar]
- 33. Madden A, Hopwood M, Neale J, Treloar C. Beyond interferon side effects: what residual barriers exist to DAA hepatitis C treatment for people who inject drugs? PLoS One. 2018;13(11):e0207226. 10.1371/journal.pone.0207226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV‐1. N Engl J Med. 2015;373(8):714‐725. 10.1056/NEJMoa1503153 [DOI] [PubMed] [Google Scholar]
- 35. Hlaing NKT, Nangia G, Tun KT, et al. High sustained virologic response in genotypes 3 and 6 with generic NS5A inhibitor and sofosbuvir regimens in chronic HCV in Myanmar. J Viral Hepat. 2019;26(10):1186‐1199. 10.1111/jvh.13133 [DOI] [PubMed] [Google Scholar]
- 36. Grebely J, Bruggmann P, Treloar C, Byrne J, Rhodes T, Dore GJ. Expanding access to prevention, care and treatment for hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26(10):893‐898. 10.1016/j.drugpo.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 37. Foster GR, Dore GJ, Wang S, et al. Glecaprevir/pibrentasvir in patients with chronic HCV and recent drug use: an integrated analysis of 7 phase III studies. Drug Alcohol Depend. 2019;194:487‐494. 10.1016/j.drugalcdep.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 38. Brown A, Welzel TM, Conway B, et al. Adherence to pan‐genotypic glecaprevir/pibrentasvir and efficacy in HCV‐infected patients: a pooled analysis of clinical trials. Liver Int. 2020;40(4):778‐786. 10.1111/liv.14266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hézode C, Lebray P, De Ledinghen V, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, for hepatitis C virus genotype 3 in a French early access programme. Liver Int. 2017;37(9):1314‐1324. 10.1111/liv.13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3(2):47‐52. 10.7150/ijms.3.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smookler D, Vanderhoff A, Biondi MJ, et al. Reducing read time of point‐of‐care test does not affect detection of hepatitis C virus and reduces need for reflex RNA. Clin Gastroenterol Hepatol. 2020;19(7):1451‐1458.e4. 10.1016/j.cgh.2020.07.058 [DOI] [PubMed] [Google Scholar]


