Abstract
Background and aims
Patients after transplantation need medical management for the rest of their lives, and self‐management seems to lead to greater adherence to medical standards, improve early physical changes, and increase patient empowerment. The main objective of this article is to systematic review of the consideration to mobile health applications (m‐Health apps) used in transplantation.
Methods
A systematic search was conducted MEDLINE (through PubMed), Web of Science, Scopus, and Science Direct from inception to November 2020. The Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) statement was used in this study. Comprehensive research was carried out using a combination of keywords and MeSH terms associated with m‐Health, empowerment, self‐management, and transplantation. Two independent reviewers screened titles and abstracts, assessed full‐text articles, and extracted data from articles that met inclusion criteria. Eligible studies were original research articles that included posttransplant care and mobile phone‐based applications to support self‐management and self‐care. Also, thesis, book chapters, letters to editors, short briefs, reports, technical reports, book reviews, systematic reviews, or meta‐analysis were excluded.
Results
We divided all the reviewed articles into four categories, self‐management (medication adherence, adherence to medical regimen, and remote monitoring), evaluation, interaction, and interface; 37.5% of the studies were focused on lung transplantation. In 56.25% of the studies, medication adherence was considered because one of the main reasons for the rejection and graft loss is stated medication nonadherence. Also, 62.5% of the studies demonstrated that the use of m‐health improved medication adherence and self‐management in transplantation.
Conclusions
The use of m‐Health apps interventions to self‐management after transplantation has shown promising feasibility and acceptability, and there is modest evidence to support the efficacy of these interventions. We found that m‐Health solutions can help the patient in self‐management in many ways after transplantation.
Keywords: medication adherence, mobile health, posttransplant cares, self‐management
1. INTRODUCTION
Chronic illness is a condition that lasts for 1 year or more and requires constant medical attention. 1 According to the World Health Organization (WHO), chronic diseases such as cardiovascular and respiratory diseases, cancer, and diabetes are the leading causes of death worldwide. 2 On the other hand, transplantation, one of the most effective treatments for end‐stage organ failure, 3 should also be considered a chronic disease because it requires lifelong follow‐up to manage concomitant diseases and prevent transplant loss. 4 In 2018, a total of approximately 146 840 organ transplants were performed worldwide. 5 Patients after transplantation need medical management for the rest of their lives, and they should engage in self‐care behaviors such as medication management, follow‐up medical appointments, and monitoring symptoms regularly. Although biological factors are essential for transplant survival, other factors, such as adherence to medication and medical care, are also influential. 6 For example, one of the main reasons for the rejection and graft loss is stated medication nonadherence. 7
Patients need daily care for daily life, communication with the treatment staff, scheduling an appointment with a doctor and going to the clinic, and adhering to medication. Furthermore, some of the conditions that may cause transplant rejection include infection, neoplasms, recurrence of significant diseases, nephropathy, metabolic syndrome, and surgical complications. Patients may also experience social psychological consequences such as social isolation, job disruption, financial crisis, and emotional burden. 8 Most of the time, transplant recipients encounter many problems that they have to deal with on their own. It is necessary to change the routine of medical practices towards patients centered mode in this particular patient population. Therefore, self‐management is one way to manage or prevent potential health conditions after transplantation and has become increasingly critical for long‐term transplant survivors, which is believed to play a vital role in the improvement of quality of life and health status. 9 Self‐management is the behaviors and activities that a person uses to practically manage the disease and manage the patient's physical or functional effects. 10 Self‐management seems to lead to greater adherence to medical standards and improve early physical changes and increase patients' ability. 11
Advances in information and communication technologies (ICTs) in health care have led to the development of m‐health, and it has revolutionized the provision of health services. 12 , 13 m‐Health to support patients with chronic diseases in self‐management has been widely considered in the last decade. 14 , 15 WHO defines m‐Health as a medical and public health approach supported by mobile devices, patient monitoring devices, personal digital assistants (PDAs), and other wireless devices. 2 Statista website shows that the m‐Health market is growing and projected to exceed US$ 300 billion by 2025. Based on this information, it can be concluded that m‐Health has great potential in medical and public health approaches. 16 Mobile phones have great potential to influence the management of chronic conditions worldwide due to their popularity, availability, portability, and technology capacity. m‐Health applications facilitate communication between patients and treatment staff, reduce chronic diseases costs, adhere to medical regimens, and influence patient outcomes. 14 , 17 , 18
Due to the increasing popularity of m‐Health, some studies have reported the effect of m‐Health interventions on chronic diseases; for example, Fan and Zhao examined the effect of m‐Health on the outcomes of patients with chronic diseases and its limitations. This study showed a positive trend in m‐Health interventions for the management of chronic diseases. 19 Badawy et al examined the evidence for the effectiveness of text messages and m‐Health applications to promote medication adherence in adolescents with chronic health conditions and stated that this approach is effective for promoting drug adherence in adolescents with chronic disease. 20 Badawy et al also found that texting and mobile phone app interventions can correct adherence to preventive behavior in adolescents. 21 Ramsey et al revealed the effectiveness of intervention m‐Health in improving the mental and physical health outcomes of young people undergoing cancer treatment and survivors of youth undergoing cancer treatment and child, adolescent, and young adult survivors of childhood cancer. 22 Furthermore, Badawy et al measured e‐Health interventions for different outcomes of sickle‐cell disease self‐management, and their studies demonstrate the effect of e‐Health on the various components of sickle‐cell disease self‐management. 23 , 24
Although m‐Health interventions can help with patients' self‐management and to improve their care, few systematic studies have focused on the impact of using m‐Health technology on posttransplant self‐management. Therefore, this study was conducted to describe the main features of m‐Health interventions and their effectiveness on posttransplant self‐management outcomes to the following goals:
To investigate the published studies on applying m‐Health in posttransplant care.
To assess the efficacy of m‐Health in self‐management.
To review the application quality evaluation scale.
2. METHODS
The current systematic review was based on the PRISMA checklist to ensure the inclusion of relevant studies. 25
2.1. Literature search
We searched MEDLINE (through PubMed), Web of Science, Scopus, and Science Direct from inception to November 2020. Comprehensive research was carried out using a combination of keywords and MeSH terms associated with m‐Health, empowerment, self‐management, and transplantation. Table 1 shows a combination of keywords and MeSH terms used in our search.
TABLE 1.
Keywords and search strategy
| Keywords | Transplantation, mobile health, m‐Health, telemedicine, self‐management, self‐care |
| Search strategy |
|
|
Articles were included if met the following criteria: (a) studies conducted on posttransplant care, (b) m‐Health applications interventions, and (c) only articles in the English language. Articles were excluded if they met the following criteria: (a) Self‐management applications without the use of m‐Health, (b) articles related to hematopoietic stem cell transplantation, bone marrow transplant, before solid organ transplant, etc., and (c) thesis, book chapters, letters to editors, short briefs, reports, technical reports, book reviews, review, or meta‐analyses.
2.2. Data extraction
After article retrieval, all titles and abstracts of articles were examined based on the main objectives, and reviewers selected relevant studies. Abasi and Kiani screened all titles and abstracts to find relevant articles. Articles that met our inclusion criteria were selected for full‐text review. Subsequently, full texts of relevant studies were screened thoroughly by two reviewers (Abasi and Kiani). Any conflicts were resolved by discussion with the senior authors (Yazdani and Mahmoudzadeh‐Sagheb). Lastly, after selected final articles, specific categories were considered to classify and analyze relevant articles. The categories were considered for the qualitative analysis of the articles present in Figure 1. Critical articles were summarized and entered into customized extraction forms based on these categories to diminish bias. Two authors (Abasi and Kiani) independently extracted the study characteristics from each article based on the classification. The information extracted by the researchers was reexamined to reach an agreement. The next reviewer (Yazdani and Mahmoudzadeh‐Sagheb) assessed and verified the extracted information. EndNote software was used for resource management. All synthesis and analysis were performed using SPSS v25.
FIGURE 1.
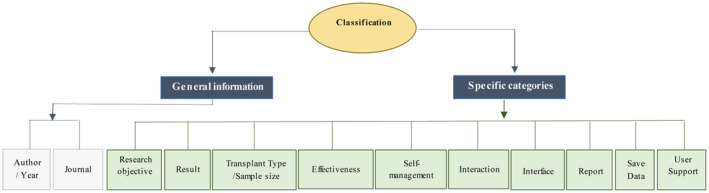
The classification of reviewed articles
3. RESULT
Based on our search strategy, 500 articles were retrieved. In addition, 12 studies were obtained by a simple search in Google Scholar. The procedure of screening articles based on the PRISMA method is displayed in Figure 2. After eliminating duplicate studies, 470 studies were retrieved. After evaluating the title and abstract of the studies, based on our inclusion criteria, 51 articles were selected to evaluate the full text. A total of 16 journal articles met our inclusion criteria. In this study, five categories, description of studies, self‐management, evaluation, interaction, and interface, were evaluated.
FIGURE 2.
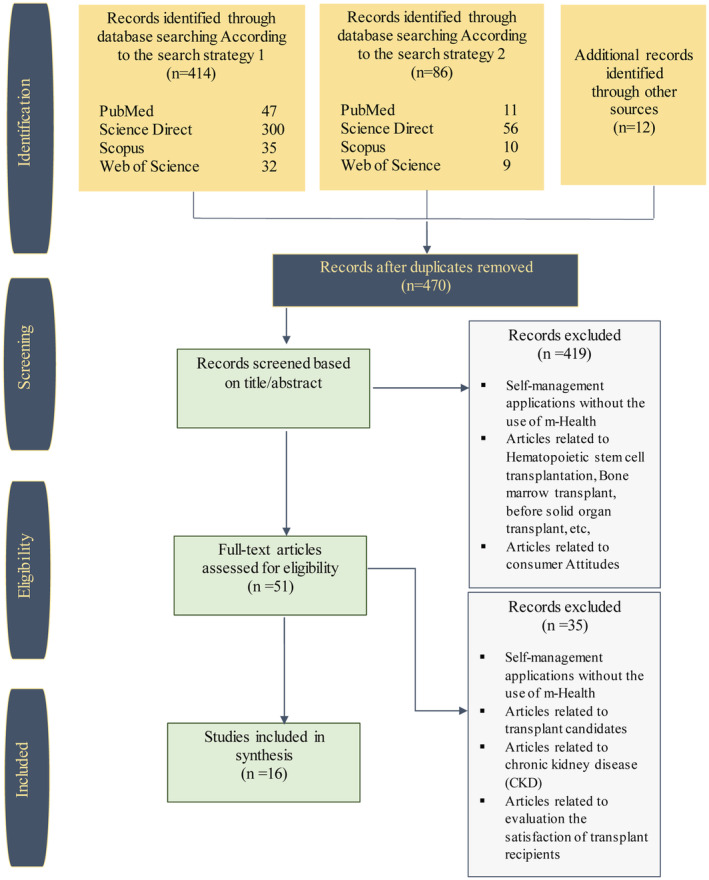
The flow diagram for the identification, screening, and eligibility of studies based on PRISMA
3.1. Description of studies
The distribution of studies by publication year is represented in Figure 3. Reviewed articles were diverse regarding the study design of the research. 50% of studies were randomized controlled trials (RCT), and most of the studies examined more than one outcome; 93.75% of studies reported the sample size. The sample size ranged from 7 to 201 with an average sample size of 80.6000 (SD = ±59.54806); 93.75% of studies reported the participant's age; in 37.5% of studies, participants were over 18 years old; in 18.75% of studies, a mean age of the participants was 57 years, and in 6.25% of studies, and participants' age span was 11 to 18 years. As shown in Figures 4, 68.75% of studies focused on kidney and lung transplant recipients, 12.5% of studies involved heart transplant recipients, and 18.75% of studies involving recipients of multiple transplants.
FIGURE 3.
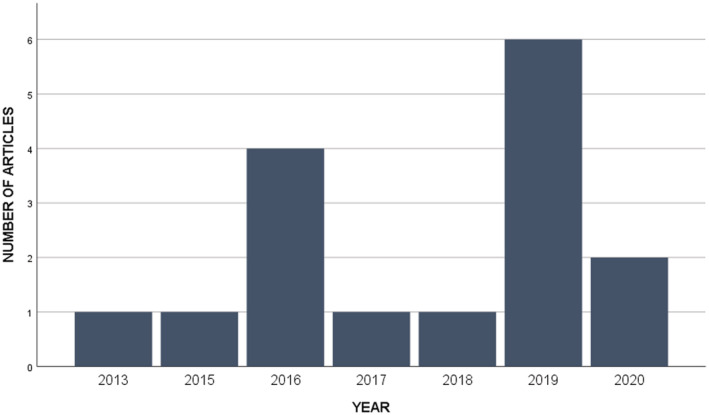
The distribution of papers by publication year
FIGURE 4.
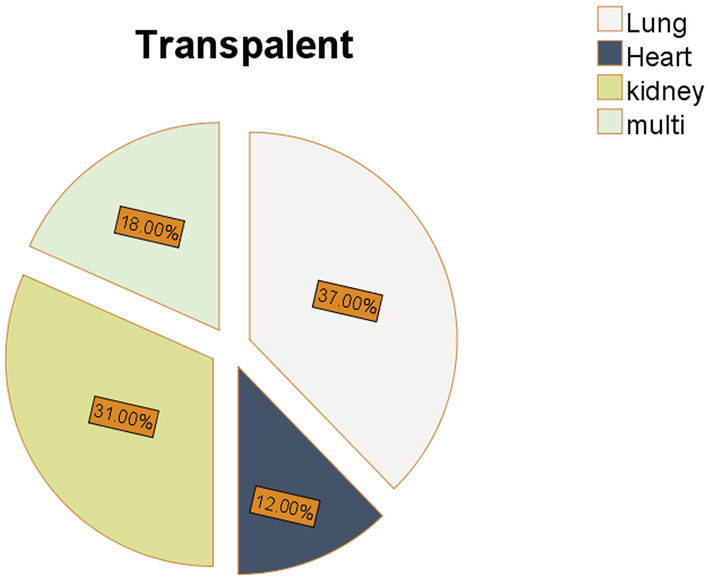
Frequency of studies according to organ transplantation
3.2. Self‐management
The posttransplant care starts immediately after transplantation surgery. Continuous follow‐up care should be provided for transplant patients to improve survival and outcomes. This critical care must continue after the patient is discharged from the hospital. All reviewed articles were categorized into three groups based on their objectives. These three topics include medication adherence, adherence to the medical regimen, and remote monitoring. In the following, the reviewed articles are described based on these three categories.
3.2.1. Medication adherence
Medication adherence means nonobservance and deviation of the drug regimen and adverse effects on the prescribed regimen. 26 Adherence to the medication regimen is especially important for immunosuppressive drugs in posttransplant patients. Due to the proven side effects of missing medication doses, there have long been intense efforts to increase medication adherence in transplant management. Among the included articles, 56.25% of studies were published on medication adherence. 27
McGillicuddy et al 28 , 29 , 30 performed studies to improve medication adherence (using automated reminders from an electronic medication tray) and blood pressure control in patients after kidney transplantation with uncontrolled hypertension.
Teen Pocket PATH is an m‐Health technology designed by Shellmer et al to promote adolescent independence in medication self‐management. 7 Teen Pocket PATH is an m‐Health technology designed to promote adolescent independence in medication self‐management. Their goals were to support adolescents' independence, improve drug management, improve communication between adolescents and their primary caregiver, and remind as soon as possible to avoid missed and late doses. In Levine et al 27 study, mobile application “Transplant Hero” has an interactive alarm and medication reminder for patients, it has also been used to provide educational content. The application uses a “Pebble Smart Technology” smart watch to display reminder notifications. Taber et al 31 in their study mentioned the development, testing, and preliminary validation of an intervention using technology and under the supervision of a pharmacist. The goal was to improve the results and safety of the drug in kidney transplant recipients. The main reasons for poor outcomes after the kidney transplantation of medication safety issues include medication errors, medication nonadherence, and adverse drug events (ADEs). Gomis‐Pastor et al 32 described the implementation of the “mHeart” model, and Gomis et al 33 measured the therapeutical adherence improvement by mHeart. The mHeart platform is a mobile application and website used to facilitate patient communication and to record the timing of medication intake, drug interactions, vital signs, side effects, and symptoms. Cole et al study 34 is a prospective research study for adult kidney transplant recipients, which aimed at improving the results of cardiovascular disease (CVD) risk factors and patient self‐efficacy and improving drug safety. They also described issues of racial segregation in transplant and the details of the intervention.
3.2.2. Adherence to medical regimen
Three studies used personal assistant Pocket PATH for tracking health. Pocket PATH is one of the few m‐health programs for self‐management, which has been carefully developed, formally tested, and evaluated. Currently, its features are tailored to the posttransplant regimen for lung transplant recipients. These include alerts and reminders about taking immunosuppressive medications. 35 , 36 , 37
Geramita et al 35 used a Pocket PATH for lung transplant recipients in a randomized controlled trial (RCT). They measured the extent to which lung transplant recipients adhere to a medical regimen compared to lung transplant recipients receiving usual care during the first year after transplantation and other factors the potential risk for long‐term non‐compliance.
Rosenberger et al 36 in a randomized controlled trial examined the association between those who had used Pocket PATH for 1 year and long‐term clinical outcomes, mortality, and bronchiolitis obliterans syndrome among 182 recipients.
In a randomized trial by DeVito Dabbs et al, 37 201 transplant recipients participated to compare effectiveness for promoting self‐management behaviors. They placed 99 people in the m‐Health intervention group and 102 in the usual care group. They also examined self‐care agency, re‐hospitalization, and mortality as secondary outcomes.
3.2.3. Remote monitoring
Schenkel et al 38 used Bluetooth‐based tablet technology in their study and, using this technology, transmitted patient vital signs information and respiratory parameters to real‐time transplant coordinators to improve transplant results. The platform also enables the communication between patients and providers and includes a comprehensive tutorial library with custom video content and daily reports. Lerret et al 39 conducted a study based on the theory of individual and family self‐management and focused on the family self‐management of children transplant recipients at home. This pilot study performed a family self‐management intervention (myFAMI) using m‐Health technology to facilitate and support child family management and communication between family members and the healthcare team.
Jiang et al 40 , 41 intervened using Pocket PATH, an example m‐Health. The objectives of their study are the following: (a) To describe the use of mobile technology for self‐monitoring and acceptance rate of lung transplant recipients, and (b) To check the lung transplant recipients' follow‐up of decision support messages to report the recorded vital values and explore appropriate decision‐making predictors after technology support by reporting critical values during the first year after transplantation.
3.3. Evaluation
An important part of software development is quality evaluation. For example, user satisfaction is one of the evaluations that can be measured by examining the usability of the system 42 ; 37/5% of the studies did not have an application quality evaluation scale, 27 , 31 , 33 , 34 , 37 , 38 and 31/25% of the articles evaluated acceptability and usability. 7 , 28 , 29 , 30 , 40 Three studies used the PSSUQ or ASQ questionnaire for evaluation, 7 , 40 , 41 and the other studies used surveys and interviews. 32 , 39
3.4. Interaction
According to Table 2, most research studies had interaction features, and we considered the interactive nature of m‐Health application feedback, alert, and reminder.
TABLE 2.
The characteristics of reviewed articles
| Author/year | Journal/conference name | Research objective | Result | Transplant type /sample size | Effectiveness | Self‐management | Interaction (feedback/reminder/alarm) | Interface | Report | Save data | User support | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Schenkel 38 /2020 | American Journal of Transplantation | Improve outcomes in lung transplantation. | It is helpful in preventing readmission, reducing subsequent hospitalization days, and controlling hospital costs. | Lung transplantation/28 | + | Remote monitoring | Inspirational messages/there are two types of alerts: email notifications for noncritical values but out of range, and phone alerts for critical values. | − | − | + | Face‐to‐face communication between patients and providers. |
| 2 | Gomis‐Pastor 32 /2020 | JMIR Cardio | The main objective of this study was to describe the implementation of the mHeart model and to outline the main facilitators identified when conceiving an m‐Health approach. | Improve therapy management, patient empowerment, and patient‐provider interactions. | Heart transplantation/135 | + | Medication adherence | Alerts, reminders, notifications, messages, and video calls. | − | + | + | The software was interactive with additional human support; a website was also designed for providers. |
| 3 | Geramita 35 /2020 | Transplantation | To evaluate whether Pocket PATH had sustained effects on lung transplant recipients' medical regimen. | Showed better adherence to the medical regimen than lung transplant recipients receiving usual care during the first year post‐transplant. | Lung transplant/105 lung transplant recipients | + | Adhered to medical regimen | Its features include alerts and reminders, and decision support tools guiding patients on when to seek transplant team assistance. | + | − | + | Telephone |
| 4 | Lerret 43 /2019 | Journal of Pediatric Nursing | myFAMI intervention with family members of pediatric transplant recipients and testing the initial effect on post‐discharge. | Facilitate patient/family‐nurse communication and family self‐management behaviors. | Heart, kidney, or liver transplant/include 40 family | + | Remote monitoring | The in‐app notification or prompt serves as a reminder. | − | + | − | Videoconference or a telephone |
| 5 | Levine 27 /2019 | American Journal of Surgery | A mobile app (Transplant Hero) for medication adherence for transplant recipients. | Did not show an increase in medication adherence through the use of mobile health apps. | Adult kidney, pancreas, and/or liver transplant recipient/A total of 108 patients | − | Medication adherence | Interactive alerts and reminders of patients in the use of their medications as well as the preparation of educational content. | − | − | − | Not mentioned |
| 6 | McGillicuddy 28 /2019 | JMIR Research Protocols | Improve medication adherence and sustain blood pressure control among kidney transplantation recipients. | Effectiveness of SMASK to improve medication adherence and blood pressure control in a group of hypertensive kidney transplant recipients. | Kidney transplantation/80 | + | Medication adherence | Automated reminders from an electronic medication tray; proportional text messages and motivational feedback, guided by the self‐determination theory, and auto‐summary report for providers. | + | + | + | |
| 7 | Taber 31 /2019 | American Journal of Health‐System Pharmacy | The development, testing, and preliminary validation of a technology‐enabled, pharmacist‐led intervention aimed at improving medication safety and outcomes in kidney transplant recipients. | The study demonstrated improved monitoring, management, and goal attainment for hypertension and diabetes control along with patient acceptability and the feasibility of the m‐Health system. | Kidney transplant/60 | + | Medication adherence | Follow‐up survey/provider‐based feedback/reminders. | − | − | + | Text/telephone/email |
| 8 | Gomis 33 /2019 | The Journal of Heart and Lung Transplantation | Measure the therapeutical adherence (TA) improvement by means of a personalized care program following heart transplant (HTx). | The effectiveness of the pharmaceutical interventions implemented through the mHeart tool was high. | Heart transplant32/ | + | Medication adherence | Not mentioned. | − | − | + | Not mentioned |
| 9 | Cole 34 /2018 | Contemporary Clinical Trials Communications | Demonstrate improved medication safety and CVD risk factor control in adult kidney transplant recipients at least one‐year posttransplant with a functioning graft. | The study will provide important and novel information regarding potential interventional methods to improve CVD risk factor control using innovative technology and pharmacist‐led interventions. | Kidney transplant/ | + | Medication adherence | Not mentioned. | − | + | + | Mentioned |
| 10 | Rosenberger 36 /2017 | American Journal of Transplantation | Examined Pocket PATH during the first year posttransplant. | Pocket PATH exposure had no direct effect on outcomes. | Lung transplant/182 | + | Adhered to medical regimen | Alerts and reminders. | + | − | + | Mentioned |
| 11 | DeVito Dabbs 37 /2016 | American Journal of Transplantation | The study was a randomized controlled trial comparing Pocket PATH and routine care. Outcomes were evaluated over 12 months. | Support for the potential benefits of Pocket PATH, an m‐Health intervention to promote self‐management. | Lung transplantation/201 recipients | + | Adhered to medical regimen | Automatically sends a reminder to the patient feedback. | + | + | + | Not mentioned |
| 12 | Shellmer 7 /2016 | Pediatric Transplantation | The TPP prototype, an m‐Health application to promote medication adherence and strengthen communication on medication management between adolescents and primary caregivers, was developed and tested. | TPP generally easy to use and effective in prompting adolescents to adhere to their medications. | Adolescent solid organ recipients/7 | ++ | Medication adherence | Warning system/reminder. | + | + | − | NOT mentioned |
| 13 | Jiang 41 /2016 | International Journal of Medical Informatics | To examine the degree to which LTR followed decision support messages to report recorded critical values, and to explore predictors of appropriately following technology decision support by reporting critical values. | The majority of LTR responded appropriately to mobile technology‐based decision support for reporting recorded critical values. | Lung transplantation/96 | + | Remote monitoring | Automatic feedback messages. | + | − | + | Messages |
| 14 | Jiang 40 /2016 | Applied Clinical Informatics | To describe lung transplant recipients acceptance and use of mobile technology for health self‐monitoring during the first year posttransplantation. | Correlates were different for short‐ and long‐term use of mobile technology for health self‐monitoring in the first year posttransplantation. | Lung transplantation/96 | ++ | Remote monitoring | Automatic feedback messages/reminding LTR to take action, including reporting the critical values to transplant clinicians. | + | − | + | A user support manual and a toll‐free number were given to LTR to call for help with technical problems. |
| 15 | McGillicuddy 29 /2015 | Progress in Transplantation | Evaluate a mobile health pilot program to improve blood pressure and medication adherence. | Improvements seen in medication adherence and blood pressure control were promising. | Kidney transplant/19 | ++ | Medication adherence | Electronically delivered medication and blood pressure reminder alerts, motivational and reinforcement messages for adherence, and the auditory and visual feedback of blood pressure control and medication adherence. | − | − | − | Not mentioned |
| 16 | McGillicuddy 30 /2013 | JMIR Research Protocols | Assess the feasibility, acceptability, and preliminary outcomes of a prototype mobile health (m‐Health) medication and blood pressure (BP) self‐management system for kidney transplant patients with uncontrolled hypertension. | m‐Health intervention group exhibited significant improvements in medication adherence and significant reductions in clinic‐measured systolic blood pressures across the monthly evaluations. | Renal transplant/20 | ++ | Medication adherence | Feedback/reminder | − | − | + | Text, email, or phone |
3.5. Interface
In most studies, design criteria (use appropriate font sizes, use meaningful colors, use graphs, the contrast between text, and background, etc.) were not considered, but a few studies followed the principles of user‐centered design, and some used design criteria. 7 , 28 , 35 , 36 , 37 , 40 , 41
4. DISCUSSION
In this research, we performed a study to investigate 16 related articles through a standard strategy. An initial objective of this study was to identify the published literature concerning solid organ transplantation and m‐Health.
Most studies have been performed on lung transplants. Lung transplant recipients are more likely to receive transplant complications than recipients of other solid organs, leading to increased mortality and increased use of health resources, so self‐management in this group is important. 37 Pocket PATH is a well‐used and well‐known application in this field. Most studies demonstrated improved medication adherence 7 , 28 , 29 , 30 , 33 and increased self‐care following the use of this technology, and patient empowerment, improved adherence to medical regimen, remote monitoring, and preventing readmission are examples of benefits obtained. 31 , 32 , 35 , 38 , 41 Two studies mentioned that although m‐Health applications are a promising strategy, exposure had no direct effect on outcomes, and further research is required to determine how to best use this technology. 27 , 36
Medication management following solid organ transplants can be complex and burdensome. Patients often have multiple dosing times throughout the day and restrictions on what medications can take together. 7 One of the main reasons for the rejection and graft loss is the stated medication nonadherence. 44 , 45 Rates of medication nonadherence are reported as high as 65%. 27 Due to the importance of this issue, all medication adherence studies were considered.
Transplant survival decreases with time, and long‐term survival rates vary significantly depending on the type of transplant and age group. Biological bases in the reduction of patient and graft survival play strong roles, but other factors such as adherence to medical care and the medication regimen are also effective. These factors are less common among pediatric transplant candidates and adolescents. Nearly 45% of all pediatric transplant candidates and recipients are adolescents. The usage of m‐Health to promote the performance of self‐care behaviors among adolescent transplant recipients offers a new approach to solving this problem. 7 However, few studies have focused only on adolescents. In this research, only two studies of children and adolescents were considered and parents were involved as an active component in the field of m‐Health. 7 , 39
In addition, children and adolescents have many psychosocial consequences in the COVID‐19 epidemic, with lengthy school closures, disruption of daily schedules, and peer interactions around the world. People with chronic health conditions, such as solid organ transplant recipients who have already experienced a psychosocial burden, are more likely to experience more consequences such as increased anxiety about health, academic and social challenges associated with school closures, and increased risk of family stress. Now more than ever, digital approaches to optimize the delivery of health care for children and adolescents have become important. Smartphones are one of the favorite technologies for children and teenagers available to almost all of them. Therefore, m‐Health can be used to reduce psychosocial consequences and improve self‐management skills. 46 , 47 , 48
Telemedicine is defined as “remote delivery of healthcare services over the telecommunication infrastructure.” Concepts of telemedicine with web‐based platforms, mobile applications, video conferencing, chat, remote vital sign monitoring, or a variety of those combinations are suitable for transplantation follow‐up. However, the additional advantage of new telemedicine concepts over standard posttransplant care must be demonstrated to justify the use of additional costs. 49 The development and implementation of these systems entail several different costs such as the cost of equipment, personnel, and communications. 50 Two of the most common methods of economic evaluation are cost‐utility analysis (CUA) and cost‐effectiveness analysis (CEA). CUA is especially used in the evaluation of health technology. The main purpose of the CUA is to estimate the cost ratio of a health‐related intervention and its benefits in terms of the number of years that users live in perfect health. 50 , 51 Today, the introduction of new e‐Health technologies significantly increases the cost‐effectiveness of the healthcare system. 52 Although m‐Health is a cost‐effective one of telemedicine technology with a low‐cost and efficient strategy, the evidence is limited, and most studies have not reported it and lacked a comprehensive analysis. 53 , 54 In addition, a study found the development and successful implementation of a m‐Health application costly and time‐consuming. 32 According to previous studies, the main limitations of the economic evaluation of telemedicine are the lack of randomized controlled trials, the size of small samples, and the lack of quality data and appropriate measures. 50
To develop effective m‐Health applications to support self‐management, end‐user participation is essential, and previous research has shown that end‐user engagement leads to higher levels of user acceptance and satisfaction. User satisfaction when using m‐Health applications as a tool to support self‐management is of particular importance. Because satisfaction reduces the barriers to successful implementation. 7
Lack of usability can be a major barrier to the rapid adoption of mobile services. 55 Most of the studies reviewed in this study did not use standard usability tools to evaluate the program, and most applications reach consumers with little to no empirical evaluation, but Pocket PATH is one of a limited number of m‐Health interventions to have undergone user‐centered development and testing. 35
The unavailability of the studied mobile health applications in the application market is considered a limitation in our study because their installation could be useful in order to more closely examine the capabilities of applications and evaluation.
5. CONCLUSION
We conducted a comprehensive review of m‐Health applications in posttransplant care. We found that m‐Health solutions can help the patient in self‐management in many ways after transplantation. Medication management is important after solid organ transplantation, and according to the evidence, this study shows that m‐Health in this field strengthens medication adherence and may help empower patients. For more exploitation and better results, it is suggested that more attention be paid to understanding the end‐user's expectations and participation in developing an m‐Health application; also more attention should be paid to user design and usability factors.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: Sanaz Abasi, Azita Yazdani.
Data curation: Sanaz Abasi, Shamim Kiani
First Draft Preparation: Sanaz Abasi, Shamim Kiani
Review and Editing: Azita Yazdani, Zahra Mahmoudzadeh‐Sagheb
Supervision: Azita Yazdani, Zahra Mahmoudzadeh‐Sagheb
All authors have read and approved the final version of the manuscript.
Zahra Mahmoudzadeh‐Sagheb had full access to all of the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis.
TRANSPARENCY STATEMENT
Zahra Mahmoudzadeh‐Sagheb and Azita Yazdani affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENT
We express our gratitude to the Shiraz University of Medical Sciences.
Abasi S, Yazdani A, Kiani S, Mahmoudzadeh‐Sagheb Z. Effectiveness of mobile health‐based self‐management application for posttransplant cares: A systematic review. Health Sci Rep. 2021;4:e434. doi: 10.1002/hsr2.434
Sanaz Abasi and Azita Yazdani are the co‐first authors and contributed equally to this article.
Funding information This study was extracted from an MSc thesis supported financially by the Shiraz University of Medical Sciences (Project Number: 22542) with the ethics code of IR.SUMS.REC.1400.049.
DATA AVAILABILITY STATEMENT
All data analyzed for and presented in this article are from the 16 studies we reviewed. The data are accessible via referenced articles.
REFERENCES
- 1. About Chronic Diseases: National Center for Chronic Disease Prevention and Health Promotion. Available from https://www.cdc.gov/chronicdisease/about/index.htm. April 28, 2021.
- 2. Organization WH . MHealth: New Horizons for Health through Mobile Technologies. Geneva: World Health Organization; 2011. [Google Scholar]
- 3. Shimazono Y. The state of the international organ trade: a provisional picture based on integration of available information. Bull World Health Organ. 2007;85:955‐962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iglesias K, De Geest S, Berben L, et al. Validation of the patient assessment of chronic illness care (PACIC) short form scale in heart transplant recipients: the international cross‐sectional bright study. BMC Health Serv Res. 2020;20(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elflein J. Global number of organ transplantations 2018; October 20, 2020. Available from https://www.statista.com/statistics/398645/global-estimation-of-organ-transplantations/
- 6. Kazemi A, Kazemi K, Sami A, Sharifian R. Identifying factors that affect patient survival after Orthotopic liver transplant using machine‐learning techniques. Exp Clin Transplant. 2019;17(6):775‐783. [DOI] [PubMed] [Google Scholar]
- 7. Shellmer DA, Dew MA, Mazariegos G, DeVito Dabbs AJ. Development and field testing of Teen Pocket PATH®, a mobile health application to improve medication adherence in adolescent solid organ recipients. Pediatr Transplant. 2016;20(1):130‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ko D, Muehrer RJ, Bratzke LC. Self‐management in liver transplant recipients: a narrative review. Prog Transplant. 2018;28(2):100‐115. [DOI] [PubMed] [Google Scholar]
- 9. Xing L, Chen Q‐Y, Li J‐N, et al. Self‐management and self‐efficacy status in liver recipients. Hepatobiliary Pancreat Dis Int. 2015;14(3):253‐262. [DOI] [PubMed] [Google Scholar]
- 10. Bratzke LC, Muehrer RJ, Kehl KA, Lee KS, Ward EC, Kwekkeboom KL. Self‐management priority setting and decision‐making in adults with multimorbidity: a narrative review of literature. Int J Nurs Stud. 2015;52(3):744‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ganjali R, Khoshrounejad F, Habibi MRM, et al. Effect and features of information technology‐based interventions on self‐management in adolescent and young adult kidney transplant recipients: a systematic review. Adolesc Health Med Ther. 2019;10:173‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shahmoradi L, Safdari R, Ahmadi H, Zahmatkeshan M. Clinical decision support systems‐based interventions to improve medication outcomes: a systematic literature review on features and effects. Med J Islam Repub Iran. 2021;35(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khammarnia M, Sharifian R, Zand F, Keshtkaran A, Barati O. Designing computerized provider order entry software in Iran: the Nurses' and Physicians' viewpoints. Comput Inform Nurs. 2016;34(9):413‐420. [DOI] [PubMed] [Google Scholar]
- 14. Song T, Deng N, Cui T, et al. Measuring success of Patients' continuous use of Mobile health Services for Self‐management of chronic conditions: model development and validation. J Med Internet Res. 2021;23(7):e26670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zahmatkeshan M, Zakerabasali S, Farjam M, Gholampour Y, Seraji M, Yazdani A. The use of mobile health interventions for gestational diabetes mellitus: a descriptive literature review. J Med Life. 2021;14(2):131‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mikulic M MHealth‐Statistics & Facts. October 23, 2020. Available from https://www.statista.com/topics/2263/mhealth/
- 17. Hamine S, Gerth‐Guyette E, Faulx D, Green BB, Ginsburg AS. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res. 2015;17(2):e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perski O, Blandford A, West R, Michie S. Conceptualising engagement with digital behaviour change interventions: a systematic review using principles from critical interpretive synthesis. Transl Behav Med. 2017;7(2):254‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan K, Zhao Y. Mobile health technology: a novel tool in chronic disease management. Intell Med. 2021. 10.1016/j.imed.2021.06.003 [DOI] [Google Scholar]
- 20. Badawy SM, Barrera L, Sinno MG, Kaviany S, O'Dwyer LC, Kuhns LM. Text messaging and Mobile phone apps as interventions to improve adherence in adolescents with chronic health conditions: a systematic review. JMIR Mhealth Uhealth. 2017;5(5):e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Badawy SM, Kuhns LM. Texting and Mobile phone app interventions for improving adherence to preventive behavior in adolescents: a systematic review. JMIR Mhealth Uhealth. 2017;5(4):e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramsey WA, Heidelberg RE, Gilbert AM, Heneghan MB, Badawy SM, Alberts NM. eHealth and mHealth interventions in pediatric cancer: a systematic review of interventions across the cancer continuum. Psychooncology. 2020;29(1):17‐37. [DOI] [PubMed] [Google Scholar]
- 23. Badawy SM, Thompson AA, Liem RI. Technology access and smartphone app preferences for medication adherence in adolescents and young adults with sickle cell disease. Pediatr Blood Cancer. 2016;63(5):848‐852. [DOI] [PubMed] [Google Scholar]
- 24. Badawy SM, Cronin RM, Hankins J, et al. Patient‐centered eHealth interventions for children, adolescents, and adults with sickle cell disease: systematic review. J Med Internet Res. 2018;20(7):e10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fine RN, Becker Y, De Geest S, et al. Nonadherence consensus conference summary report. Am J Transplant. 2009;9(1):35‐41. [DOI] [PubMed] [Google Scholar]
- 27. Levine D, Torabi J, Choinski K, Rocca JP, Graham JA. Transplant surgery enters a new era: increasing immunosuppressive medication adherence through mobile apps and smart watches. Am J Surg. 2019;218(1):18‐20. [DOI] [PubMed] [Google Scholar]
- 28. McGillicuddy J, Chandler J, Sox L, et al. “Smartphone medication adherence saves kidneys” for kidney transplantation recipients: protocol for a randomized controlled trial. JMIR Res Protoc. 2019;8(6):e13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGillicuddy JW, Taber DJ, Mueller M, et al. Sustainability of improvements in medication adherence through a mobile health intervention. Prog Transplant. 2015;25(3):217‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGillicuddy JW, Gregoski MJ, Weiland AK, et al. Mobile health medication adherence and blood pressure control in renal transplant recipients: a proof‐of‐concept randomized controlled trial. JMIR Res Protoc. 2013;2(2):e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taber DJ, Pilch NA, McGillicuddy JW, Mardis C, Treiber F, Fleming JN. Using informatics and mobile health to improve medication safety monitoring in kidney transplant recipients. Am J Health Syst Pharm. 2019;76(15):1143‐1149. [DOI] [PubMed] [Google Scholar]
- 32. Gomis‐Pastor M, Mirabet S, Roig E, et al. Interdisciplinary Mobile health model to improve clinical care after heart transplantation: implementation strategy study. JMIR Cardio. 2020;4(1):e19065‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gomis M, Mirabet S, Mangues M, et al. Impact of Mobile health in heart transplant management: the mHeart study. J Heart Lung Transplant. 2019;38(4, Supplement):S273‐S274. [Google Scholar]
- 34. Cole AJ, Johnson RW 2nd, Egede LE, Baliga PK, Taber DJ. Improving medication safety and cardiovascular risk factor control to mitigate disparities in African‐American kidney transplant recipients: design and methods. Contemp Clin Trials Commun. 2018;9:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Geramita EM, DeVito Dabbs AJ, DiMartini AF, et al. Impact of a Mobile health intervention on long‐term nonadherence after lung transplantation: follow‐up after a randomized controlled trial. Transplantation. 2020;104(3):640‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosenberger EM, DeVito Dabbs AJ, DiMartini AF, Landsittel DP, Pilewski JM, Dew MA. Long‐term follow‐up of a randomized controlled trial evaluating a Mobile health intervention for self‐Management in Lung Transplant Recipients. Am J Transplant. 2017;17(5):1286‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. DeVito Dabbs A, Song MK, Myers BA, et al. A randomized controlled trial of a mobile health intervention to promote self‐management after lung transplantation. Am J Transplant. 2016;16(7):2172‐2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schenkel FA, Barr ML, McCloskey CC, et al. Use of a bluetooth tablet‐based technology to improve outcomes in lung transplantation: a pilot study. Am J Transplant. 2020;20(12):3649‐3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lerret SM, White‐Traut R, Medoff‐Cooper B, et al. Pilot study protocol of a mHealth self‐management intervention for family members of pediatric transplant recipients. Res Nurs Health. 2020;43(2):145‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiang Y, Sereika SM, Dabbs AD, Handler SM, Schlenk EA. Acceptance and use of mobile technology for health self‐monitoring in lung transplant recipients during the first year post‐transplantation. Appl Clin Inform. 2016;7(2):430‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang Y, Sereika SM, Dabbs AD, Handler SM, Schlenk EA. Using mobile health technology to deliver decision support for self‐monitoring after lung transplantation. Int J Med Inform. 2016;94:164‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alwashmi MF, Hawboldt J, Davis E, Fetters MD. The iterative convergent design for mobile health usability testing: mixed methods approach. JMIR Mhealth Uhealth. 2019;7(4):e11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lerret SM. Using MHealth to improve outcomes for children following solid organ. Transplantation. 2019;46:134‐135. [DOI] [PubMed] [Google Scholar]
- 44. Lurie S, Shemesh E, Sheiner PA, et al. Non‐adherence in pediatric liver transplant recipients‐an assessment of risk factors and natural history. Pediatr Transplant. 2000;4(3):200‐206. [DOI] [PubMed] [Google Scholar]
- 45. Simons LE, Blount RL. Identifying barriers to medication adherence in adolescent transplant recipients. J Pediatr Psychol. 2007;32(7):831‐844. [DOI] [PubMed] [Google Scholar]
- 46. Badawy SM, Radovic A. Digital approaches to remote pediatric health care delivery during the COVID‐19 pandemic: existing evidence and a call for further research. JMIR Pediatr Parent. 2020;3(1):e20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Serlachius A, Badawy SM, Thabrew H. Psychosocial challenges and opportunities for youth with chronic health conditions during the COVID‐19 pandemic. JMIR Pediatr Parent. 2020;3(2):e23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Badawy SM, Thompson AA, Kuhns LM. Medication adherence and technology‐based interventions for adolescents with chronic health conditions: a few key considerations. JMIR Mhealth Uhealth. 2017;5(12):e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duettmann W, Naik MG, Zukunft B, et al. eHealth in transplantation. Transplant Int. 2021;34(1):16‐26. [DOI] [PubMed] [Google Scholar]
- 50. de la Torre‐Díez I, López‐Coronado M, Vaca C, Aguado JS, de Castro C. Cost‐utility and cost‐effectiveness studies of telemedicine, electronic, and mobile health systems in the literature: a systematic review. Telemed J E Health. 2015;21(2):81‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Devlin N, Parkin D. Does NICE have a cost‐effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ. 2004;13(5):437‐452. [DOI] [PubMed] [Google Scholar]
- 52. Dávalos ME, French MT, Burdick AE, Simmons SC. Economic evaluation of telemedicine: review of the literature and research guidelines for benefit‐cost analysis. Telemed J E Health. 2009;15(10):933‐948. [DOI] [PubMed] [Google Scholar]
- 53. Badawy SM, Kuhns LM. Economic evaluation of text‐messaging and smartphone‐based interventions to improve medication adherence in adolescents with chronic health conditions: a systematic review. JMIR Mhealth Uhealth. 2016;4(4):e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Iribarren SJ, Cato K, Falzon L, Stone PW. What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PloS One. 2017;12(2):e0170581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Islam MN, Karim M, Inan T, Islam AKMN. Investigating usability of mobile health applications in Bangladesh. BMC Med Inform Decis Mak. 2020;20:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed for and presented in this article are from the 16 studies we reviewed. The data are accessible via referenced articles.


