Abstract
Brain arteriovenous malformation (AVM), a presumed congenital lesion, may involve traditional language areas but usually does not lead to language dysfunction unless it ruptures. The objective of this research was to study right‐hemispheric language reorganization patterns in patients with brain AVMs using functional magnetic resonance imaging (fMRI). We prospectively enrolled 30 AVM patients with lesions involving language areas and 32 age‐ and sex‐matched healthy controls. Each subject underwent fMRI during three language tasks: visual synonym judgment, oral word reading, and auditory sentence comprehension. The activation differences between the AVM and control groups were investigated by voxelwise analysis. Lateralization indices (LIs) for the frontal lobe, temporal lobe, and cerebellum were compared between the two groups, respectively. Results suggested that the language functions of AVM patients and controls were all normal. Voxelwise analysis showed no significantly different activations between the two groups in visual synonym judgment and oral word reading tasks. In auditory sentence comprehension task, AVM patients had significantly more activations in the right precentral gyrus (BA 6) and right cerebellar lobule VI (AAL 9042). According to the LI results, the frontal lobe in oral word reading task and the temporal lobe in auditory sentence comprehension task were significantly more right‐lateralized in the AVM group. These findings suggest that for patients with AVMs involving language cortex, different language reorganization patterns may develop for different language functions. The recruitment of brain areas in the right cerebral and cerebellar hemispheres may play a compensatory role in the reorganized language network of AVM patients.
Keywords: arteriovenous malformation, functional magnetic resonance imaging, language, reorganization
The objective of this research was to study right‐hemispheric language reorganization patterns in patients with brain AVMs using functional magnetic resonance imaging (fMRI). Findings suggest that for patients with AVMs involving language cortex, different language reorganization patterns may develop for different language functions. The recruitment of brain areas in the right cerebral and cerebellar hemispheres may play a compensatory role in the reorganized language network of AVM patients.
1. INTRODUCTION
Brain arteriovenous malformation (AVM) is generally presumed to be a congenital lesion, and the characteristic of its pathological change is the direct connections between arteries and veins with no capillaries (Fleetwood & Steinberg, 2002; Solomon & Connolly Jr, 2017). Unlike brain tumors or strokes, language dysfunction is an uncommon manifestation for unruptured brain AVMs involving language areas (Breitenstein et al., 2017; C. J. Chen et al., 2020; Duffau, 2021; Hartwigsen & Saur, 2019; Mohr, Koennecke, & Hartmann, 2020; Stockert et al., 2020). This phenomenon might be explained by language cortex reorganization caused by the AVM nidus, considering its congenital nature (Deng et al., 2015; Rousseau et al., 2019). However, it is still uncertain which brain areas are included in the reorganized language network.
Pertinently, we believe that a brain AVM involving language areas is a special model for studying language reorganization. Brain AVMs are generally regarded as a congenital disease (Fleetwood & Steinberg, 2002; Solomon & Connolly Jr, 2017), but language is mainly learned after birth (Feldman, 2019). Therefore, the formation of brain AVMs and damage to the “supposed” language areas should occur earlier than the establishment of the “real” language areas. Patients with AVMs involving language areas can be regarded as a model of language areas that are congenitally “knocked out,” but these patients still acquired normal language functions in the subsequent language learning process (Deng et al., 2015). This feature is quite different from those of acquired diseases, such as stroke or glioma, which usually occur in adults, and language dysfunction is a common manifestation when the language cortex is involved by these acquired lesions (Cirillo, Caulo, Pieri, Falini, & Castellano, 2019; Gajardo‐Vidal et al., 2021; Ille, Engel, Kelm, Meyer, & Krieg, 2018; Li et al., 2019; Stockert et al., 2020). Therefore, the language reorganization mechanisms of AVM patients might be distinct from those of acquired diseases (Deng et al., 2016).
However, unlike extensive studies of stroke, studies regarding language reorganization in patients with brain AVMs are limited. Previous studies have shown that atypical language dominance is not rare in AVM patients (Deng et al., 2015; Rousseau et al., 2019). For example, Lee, Pouratian, Bookheimer, and Martin (2010) reported 15 right‐handed patients with an AVM nidus involving language areas; 5 of the patients (30%) presented with right dominance. In addition, our preliminary studies found that right‐sided lateralization of blood oxygen level‐dependent (BOLD) signals was observed in 36.5% of AVM patients, and there seemed to be a “mirror phenomenon” in language reorganization patterns; thus, a nidus involving the Broca area mainly led to right‐sided lateralization of the Broca area. Also, a nidus involving the Wernicke area mainly led to right‐sided lateralization of the Wernicke area (Deng et al., 2015, 2020). However, there are still many issues to be addressed regarding language reorganization in patients with brain AVMs. One basic and important issue is the identification of the specific brain areas that participate in the reorganized language network. Therefore, we designed this study to map the reorganized language cortices in patients with brain AVMs using BOLD functional magnetic resonance imaging (fMRI), with a focus on right‐hemisphere reorganization.
Moreover, language is a complex and advanced neural function involving many brain cortices, and fMRI activations while subjects perform different language tasks reflect different aspects of language functions (Hagoort, 2019; Qiu, Tan, Siok, Zhou, & Khong, 2011; Yang & Tan, 2019). For example, Wu, Ho, and Chen (2012) performed a meta‐analysis studying networks for orthographic, phonological, and semantic processing of Chinese characters in healthy Chinese populations. The results showed that there were converging activations among three tasks, including the left middle frontal gyrus, the left superior parietal lobule and the left mid‐fusiform gyrus. Moreover, the left inferior parietal lobule and the right superior temporal gyrus were shown to be specialized for phonological processing, and the left middle temporal gyrus was shown to be specialized for semantic processing. Therefore, we applied a set of Chinese language tasks in fMRI, including visual synonym judgment, oral word reading, and auditory sentence comprehension tasks. Using multitask fMRI, we aimed to study the effect of the AVM nidus on language functions and the patterns of language reorganization more comprehensively.
2. METHODS
2.1. Subjects
This study was approved by the Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University (number: KY2018–103–01), and written informed consent was obtained from all participants. It was registered in the Chinese Trial Registry (clinical trial number: ChiCTR1900020993). Subjects were prospectively enrolled between January 2016 and November 2020.
The inclusion criteria of AVM patients were as follows: (1) right‐handedness confirmed by the Edinburgh handedness inventory; (2) native Mandarin Chinese speakers from mainland China; (3) age: 18–60 years old; (4) education level: high school or above; (5) AVM lesion located in the left hemisphere, involving language areas (mainly including the middle and inferior frontal gyrus, precentral gyrus, superior and middle temporal gyrus, supra‐marginal gyrus and angular gyrus); and (6) no rupture of the AVM or no rupture within 1 month. In this study, patients with an AVM that had ruptured more than 1 month prior to the study were also included for two reasons: first, patients with AVMs involving language areas are relatively rare; second, the bleeding would be absorbed and the clinical conditions would be stabilized in the majority of patients after 1 month. The exclusion criteria were as follows: (1) a treatment history of the AVM nidus, including craniotomy, interventional surgeries, and radiotherapy; (2) other neurological or psychiatric conditions; and (3) inability to cooperate with fMRI tasks.
For comparison, age‐ and sex‐matched healthy controls were recruited with the abovementioned inclusion criteria (1)–(4) and exclusion criteria (2) and (3).
2.2. Language function assessment
Each subject underwent language assessment using the Chinese version of the Western Aphasia Battery (WAB), which was composed of four domains: fluency (range 0–20), comprehension (range 0–200), repetition (range 0–100), and naming (range 0–100). Aphasia quotients (AQs; range 0–100) were applied to estimate the severity of aphasia using the formula (Ren et al., 2019):
A diagnosis of aphasia was considered if the patient's AQ score was less than 93.8 (Wilmskoetter et al., 2019).
2.3. Image acquisition
MRI data of all participants were acquired at the Institute of Biophysics, Chinese Academy of Sciences using a 3‐T Prisma‐fit scanner (Siemens, Erlangen, Germany) and a 20‐element head–neck coil. Each subject underwent a localizing image scan, then completed three language‐task fMRI examinations and T1‐weighted image scans.
For language‐task fMRI assessment, to acquire functional data that covered the whole cerebrum and cerebellum, an interleaved T2‐weighted axial gradient‐echo echo‐planar imaging (EPI) pulse sequence was applied, with repetition time = 2,010 ms, echo time = 30 ms, flip angle = 90°, field of view = 192 × 192 mm2, matrix = 64 × 64, voxel size = 3.0 × 3.0 × 3.5 mm3, number of slices = 33, and slice gap = 0 mm. The total acquisition time was approximately 15 min.
For anatomical data, a T1‐weighted magnetization‐prepared rapid‐acquisition gradient‐echo (MP‐RAGE) sequence was employed, with repetition time = 2,530 ms, echo time = 3.37 ms, flip angle = 7°, field of view = 256 × 256 mm2, matrix = 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm3, and number of slices = 176. The scanning time was 5 min and 53 s.
2.4. fMRI block‐design tasks
We used three language tasks in fMRI examination, including visual synonym judgment, oral word reading and auditory sentence comprehension tasks. A blocked design was used, and each task had six experimental blocks (experimental task) that were alternated with six control blocks (baseline task). Each block lasted for 24 s, and the time required to complete one task was 288 s. Before scanning, each subject was trained to ensure that they could properly perform the task.
Visual synonym judgment task: The experimental task required visual synonym judgment (to determine if a pair of Chinese characters had the same meaning; Figure 1a,b) and the baseline task was a font size judgment task (to decide if two synchronously presented symbols had the same physical size; Figure 1c,d). Each block consisted of 12 trials. In each trial, a pair of Chinese characters (or symbols) was synchronously presented on the screen for 1,000 ms, with one above and another below the fixation cross (Figure 1a–d), followed by the presentation of a fixation cross that lasted for 1,000 ms. Subjects were asked to press a key using their right hand for positive indication (same meaning Chinese characters, or same size symbols) as accurately and quickly as possible.
Oral word reading task: Each block consisted of 12 trials. In the experimental task, a Chinese character (regular or irregular) was presented on the screen for 1,000 ms and the subject was asked to read the character aloud (Figure 1e), followed by a 1,000 ms blank interval. In the baseline task, when a fixation cross (lasting for 24 s) was presented on the screen (Figure 1f), the subject moved his or her tongue up and down continuously, and then followed by a 1,000 ms blank interval.
Auditory sentence comprehension task: In each experimental block, the participant heard news report. In the following control block, the news in the experiment task was played in reverse order and the subjects heard a senseless sound. After the experiment began, the subjects were required to close their eyes, listen carefully, and try to understand the content of the listening materials throughout the experiment. This task was a passive auditory sentence comprehension task, and subjects did not need to provide feedback.
FIGURE 1.
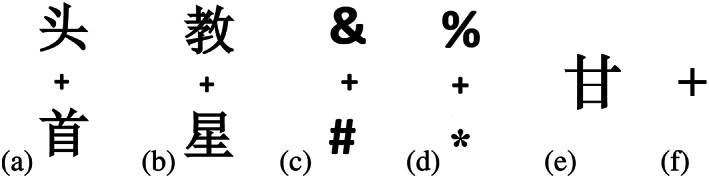
Examples of experimental stimuli. (a) and (b) show experimental stimuli used in the visual synonym judgment task. (a) shows two Chinese characters with the same meaning; both the upper and lower characters mean head. (b) shows two Chinese characters with different meanings; the upper character means teaching and the lower character means stars. (c) and (d) depict baseline stimuli of the visual synonym judgment task. The upper and lower symbols are either of the same font size (c) or not (d). (e) and (f) show stimuli used in the oral word reading task. In the experimental task, one Chinese character is presented, which is pronounced/gan1/ (e); in the baseline task, only a fixation cross is presented on the screen (f)
2.5. fMRI data analysis
-
Lesion mapping
The lesion boundaries of AVMs were manually delineated slice by slice from structural MRI slices on the horizontal plane of three‐dimensional MP‐RAGE images by one neurosurgeon (Dr. Xiaofeng Deng) using MRIcron (http://www.mricro.com; University of South Carolina, Columbia, SC). Then a three‐dimensional lesion mask was reconstructed for each patient. All lesion maps were re‐evaluated by a senior neurosurgeon (Dr. Yan Zhang).
Each lesion mask was registered and then normalized to a standard brain template (MNI152) with SPM12 (http://www.fil.ion.ucl.ac.uk/spm). An overlapping lesion image was created by using the MRIcron toolbox. Lesion volume was calculated according to lesion voxels.
-
fMRI data preprocessing
Data preprocessing was performed with SPM12. A standard preprocessing procedure was conducted (Siok, Jin, Fletcher, & Tan, 2003). EPI functional images were spatially realigned, coregistered with anatomical images, normalized to a standard Montreal Neurological Institute (MNI) reference brain space, and finally smoothed with an isotropic 8‐mm Gaussian kernel.
-
Voxelwise analysis
Two levels of voxelwise analysis were carried out. In the first level, the general linear model (GLM) was employed to analyze each individual subject to provide a measure of the effect of interest at each voxel. In the second level, called group‐level analysis, the effect of interest at each voxel in standard space was combined across subjects using paired t‐test (intragroup analysis) or independent two sample t‐test in a random effect (REX) approach. Then, group inferences were made with a general claim about a hypothesized population from which the sampled subjects were recruited. Multiple comparisons were controlled through voxels >10 with p <.05 familywise error (FWE) correction at the cluster level (p <.001 FWE correction was used in the analysis of the oral work reading task data due to its strong activations).
Activated brain areas were reported with both Brodmann areas (BAs) and Anatomical Automatic Labeling (AAL) atlases (Rondina et al., 2018). Significantly activated brain areas in the bilateral cerebral hemispheres in the AVM and control groups were selected and saved as masks of volumes of interest (VOIs). In addition, brain areas with significant differences (p <.05, FWE correction) in participants in the AVM group compared to those in the control group in voxelwise analysis were defined as regions of interest (ROIs).
-
Further analysis of ROI
In each task with significantly different activations between the two groups, the BOLD signals in experimental blocks and control blocks were separately extracted from the ROIs for each subject. Averaged BOLD signals of the experimental blocks were compared with those of the control blocks in each task, in the AVM group and in the control group, respectively.
-
Correlation of BOLD signal changes between ROIs and VOIs
In each task, the fMRI responses (BOLD signal changes) of experimental blocks were calculated with its control blocks as baseline (after linear trend removal and intensity normalization of the BOLD signal within each scan). To investigate the relationships between the identified brain areas with significant differences between the two groups (ROIs) and the main activated language areas in two groups (VOIs), Pearson correlation was performed to study the correlations between their averaged BOLD signal changes, in the control group and the AVM group, respectively.
-
Lateralization index (LI) and dominant hemisphere assessment
The LI‐toolbox (https://www.medizin.uni‐tuebingen.de/de/das‐klinikum/einrichtungen/kliniken/kinderklinik/kinderheilkunde‐iii/forschung‐iii/software) was used to compute the LI and to estimate the lateralization at a single‐subject level (Wilke & Lidzba, 2007). Bootstrap‐analysis was applied to determine statistical thresholds. In each task, LIs were calculated with three different masks: the frontal lobe, temporal lobe, and cerebellum. All these masks were provided in the toolbox. Voxels within ±5 mm around the midline were excluded to avoid interference caused by activations around the midline and to make the results more reliable.
The LI values ranged from −1 to +1. According to previous reports (Rolinski et al., 2020), if the LI was greater than or equal to +0.2, the subject was considered left‐dominant, a subject with an LI between −0.2 and +0.2 was considered to have no clear hemispheric preference, and a subject with an LI less than or equal to −0.2 was regarded as right‐dominant. Based on these criteria, the dominant hemisphere of the frontal lobe, temporal lobe, and cerebellum were separately evaluated. In this study, we only focused on the dominant hemispheres of the main language areas. The results of other language areas, such as the parietal lobes, insular lobes and basal ganglia, were not analyzed. Finally, LIs and hemisphere dominance were compared between the AVM and control groups.
2.6. Statistical analysis
Statistical analyses were performed with SPSS 22.0 (IBM corporation, New York, NY). Independent sample t‐tests were used to assess the differences between the two groups in age, WAB scores, and LIs. Difference in sex between the two groups was estimated using the chi‐square test. The Mann–Whitney test (rank‐sum test) was used to compare the dominant hemispheres between the two groups. BOLD signal activations in the ROI study were performed with paired t‐tests. Pearson correlation analysis was performed to determine the correlations between lesion size and LIs in three tasks, and among the BOLD signal changes retrieved from the ROIs and VOIs. To analyze whether there was a relationship between the lesion location and patterns of reorganization, AVM patients were categorized into three subgroups according to lesion locations: the frontal subgroup, temporal subgroup and parietal subgroup. One‐way ANOVA was performed to analyze the differences in LIs among the three subgroups. A probability value <.05 was considered statistically significant.
3. RESULTS
3.1. Demographics
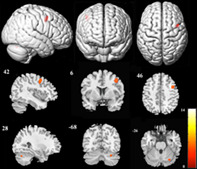
We ultimately enrolled 30 AVM patients (14 males and 16 females) and 32 healthy controls (15 males and 17 females). The demographic and lesion data of the included AVM patients are shown in Table S1. Participants' ages ranged from 18 to 51 years (mean ± SD, 30.23 ± 9.59 years) in the AVM group and from 21 to 57 years (mean ± SD, 29.28 ± 9.93 years) in the control group. There was no significant difference in sex (p = .987) or age (p = .703) between participants in the two groups. The main clinical manifestations of the AVM patients included headache in eight patients (three of them had an AVM rupture), dizziness in five patients, seizures in four patients, transient weakness of limbs in two patients, transient aphasia in one patient and no clinical symptoms in ten patients. The lesion volume ranged from 1.89 to 34.22 cm3 (mean ± SD, 10.46 ± 9.09 cm3). Lesions were located in the left frontal lobe in 13 patients (frontal subgroup), in the left temporal lobe in 9 patients (temporal subgroup) and in the left parietal lobe in 8 patients (parietal group). A lesion overlay map is presented in Figure 2.
FIGURE 2.
Lesion overlay map. The color bar represents the number of patients with a lesion in this area
3.2. Language functions
According to the WAB assessment, the language functions of all participants were normal. No significant difference was observed between the two groups in spontaneous speech, auditory comprehension, repetition, naming, or AQ scores (Table 1).
TABLE 1.
WAB results of the AVM and control groups
| Group | Spontaneous speech (0–20) | Auditory comprehension (0–200) | Repetition (0–100) | Naming (0–100) | Aphasia quotient (AQ) (0–100) |
|---|---|---|---|---|---|
| Control | 20.0 ± 0.1 | 197.5 ± 3.5 | 99.4 ± 1.2 | 98.9 ± 1.1 | 99.3 ± 0.5 |
| AVM | 19.9 ± 0.3 | 197.5 ± 3.7 | 99.5 ± 1.5 | 98.7 ± 1.3 | 99.1 ± 0.8 |
| P value | 0.292 | 0.971 | 0.788 | 0.389 | 0.280 |
3.3. Voxelwise analysis results
-
Results of the control group
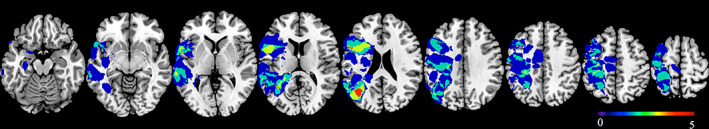
Significant activations of the control group are shown in Table 2 and Figure 3. Visual synonym judgment mainly activated participants' left cerebral and right cerebellar hemispheres, including the left inferior frontal gyrus, left precentral gyrus (premotor cortex), left occipital lobe, left superior and inferior temporal gyrus, left supplementary motor area (SMA), left fusiform gyrus, left caudate, and right cerebellar hemisphere. The oral word reading task mainly activated participants' bilateral cerebral and cerebellar hemispheres, including the left SMA, bilateral precentral gyrus (premotor cortex), bilateral superior temporal gyrus, bilateral cerebellar hemispheres, left occipital lobe, and right putamen. The auditory sentence comprehension task activated participants' left superior and middle temporal gyrus.
-
Results of the AVM group
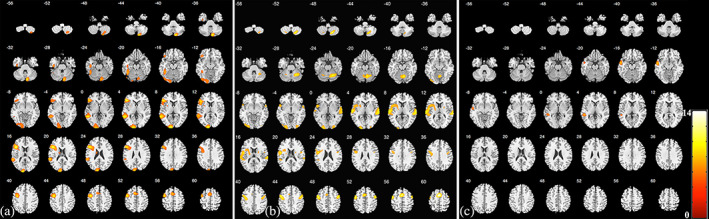
Significant activations of the AVM group are shown in Table 3 and Figure 4. Visual synonym judgment task mainly activated participants' left cerebral and right cerebellar hemispheres, including the left occipital lobe, left precentral (premotor cortex) and inferior frontal gyrus, left SMA left middle temporal gyrus, right cerebellar hemisphere, right insula, and left putamen. The oral word reading task mainly activated participants' bilateral cerebral and cerebellar hemispheres, including the bilateral superior temporal gyrus, bilateral precentral gyrus (premotor cortex), left SMA, bilateral inferior frontal gyrus, left fusiform gyrus, right cingulum, left occipital lobe, and bilateral cerebellar hemispheres. Activations of the auditory sentence comprehension task included the left superior and middle temporal gyrus, left precentral gyrus (premotor cortex), left SMA, right temporal pole, and right cerebellar hemisphere.
-
Activation differences of the AVM group compared to the control group
In the visual synonym judgment and oral word reading tasks, compared to the activations of the control group, the activations of the AVM group showed no significant difference that survived the cluster level p <.05 FWE threshold. However, in the auditory sentence comprehension task, as shown in Table 4 and Figure 5, we identified two significant clusters (ROIs), where stronger activities were found in participants in the AVM group than those in the control group. In one cluster, the peak activation was located in the right premotor cortex (BA 6), which actually included part of the right precentral gyrus (Precentral_R, 75 voxels) and posterior part of the right middle frontal gyrus (Frontal_Mid_R, 59 voxels). The other cluster was located in the right cerebellar lobule VI (AAL 9042, Cerebelum_6_R).
TABLE 2.
Brain areas with significant activation in the control group
| Activation areas | Voxels | BA | AAL | AAL name | Peak MNI coordinates | Peak t | Peak Z | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Task: Visual synonym judgment (threshold at p <.05 FWE correction) | |||||||||
| L inferior frontal gyrus and precentral gyrus | 3,769 | 44 | 2,301 | Frontal_Inf_Oper‐L | −44 | 18 | 18 | 11.12 | 7.01 |
| 45 | 2,311 | Frontal_Inf_Tri_L | −46 | 32 | 4 | 10.7 | 6.87 | ||
| 6 | 2,001 | Precentral_L | −40 | −4 | 46 | 9.52 | 6.46 | ||
| L occipital lobe | 2,321 | 18 | 5,201 | Occpital_Mid_L | −12 | −102 | 8 | 10.5 | 6.81 |
| 18 | 5,021 | Lingual_L | −14 | −92 | −12 | 6.4 | 5.07 | ||
| 19 | 5,301 | Occpital_inf_L | −42 | −84 | −12 | 6.06 | 4.89 | ||
| L fusiform gyrus and superior temporal gyrus | 1,616 | 37 | 8,201 | Temporal_Mid_L | −58 | −48 | 4 | 9.74 | 6.54 |
| 22 | 8,111 | Temporal_Sup_L | −60 | −38 | 20 | 8.58 | 6.1 | ||
| R cerebellar hemisphere | 1,103 | — | 9,012 | Cerebelum_Crus2_R | 12 | −82 | −40 | 9.71 | 6.53 |
| — | 9,062 | Cerebelum_8_R | 26 | −68 | −50 | 6.7 | 5.23 | ||
| L supplementary motor area | 707 | 6 | 2,401 | Supp_Motor_Area_L | −8 | 10 | 52 | 8.39 | 6.02 |
| 6 | 2,401 | Supp_Motor_Area_L | −2 | 2 | 62 | 7.25 | 5.5 | ||
| L fusiform gyrus | 486 | 37 | 5,401 | Fusiform_L | −40 | −50 | −18 | 7.69 | 5.71 |
| L inferior temporal gyrus | 222 | 20 | 8,301 | Temporal_Inf_L | −42 | −18 | −26 | 6.86 | 5.31 |
| L caudate | 36 | 48 | 7,001 | Caudate_L | −12 | −8 | 22 | 5.77 | 4.72 |
| Task: Oral word reading (threshold at p <.001 FWE correction) | |||||||||
| L supplementary motor area | 1,390 | 6 | 2,401 | Supp_Motor_Area_L | −2 | 4 | 64 | 14.79 | Inf |
| L precentral gyrus and superior temporal gyrus | 4,279 | 6 | 2,001 | Precentral_L | −46 | −2 | 50 | 11.57 | 7.15 |
| 41 | 8,111 | Temporal_Sup_L | −62 | −18 | 8 | 10.21 | 6.71 | ||
| 6 | 2,001 | Precentral_L | −50 | −2 | 20 | 9.43 | 6.43 | ||
| R cerebellar hemisphere | 461 | — | 9,062 | Cerebelum_8_R | 22 | −68 | −48 | 11.31 | 7.07 |
| R precentral gyrus | 737 | 6 | 2,002 | Precentral_R | 48 | −2 | 48 | 10.67 | 6.87 |
| 6 | 2,002 | Precentral_R | 48 | 0 | 22 | 7.92 | 5.81 | ||
| R superior temporal gyrus | 1,153 | 41 | 8,112 | Temporal_Sup_R | 68 | −22 | 10 | 10.62 | 6.85 |
| 22 | 8,122 | Temporal_Pole_Sup_R | 60 | 8 | −8 | 7.8 | 5.76 | ||
| R cerebellar hemisphere | 1,225 | — | 9,042 | Cerebelum_6_R | 24 | −58 | −26 | 10.17 | 6.69 |
| — | 9,042 | Cerebelum_6_R | 10 | −66 | −18 | 9.8 | 6.56 | ||
| L occipital lobe | 670 | 18 | 5,201 | Occipital_Mid_L | −20 | −100 | 2 | 8.47 | 6.05 |
| 18 | 5,301 | Occipital_Inf_L | −26 | −92 | −6 | 8.38 | 6.01 | ||
| 19 | 5,301 | Occipital_Inf_L | −40 | −84 | −8 | 7.8 | 5.76 | ||
| L occipital lobe | 225 | 18 | 5,302 | Occipital_Inf_R | 30 | −92 | −2 | 7.92 | 5.81 |
| L cerebellar hemisphere | 44 | — | 9,061 | Cerebelum_8_L | −24 | −66 | −50 | 7.73 | 5.73 |
| L cerebellar hemisphere | 67 | — | 9,041 | Cerebelum_6_L | −24 | −58 | −26 | 7.63 | 5.68 |
| L paracentral lobule | 14 | 4 | 6,401 | paracentral_Lobule_L | −18 | −26 | 64 | 7.32 | 5.54 |
| R putamen | 51 | 49 | 7,012 | Putamen_R a | 22 | −2 | 16 | 7.1 | 5.43 |
| Task: Auditory sentence comprehension (threshold at p <.05 FWE correction) | |||||||||
| L superior temporal gyrus | 376 | 22 | 8,201 | Temporal_Mid_L | −54 | 0 | −14 | 8.01 | 5.85 |
| L middle temporal gyrus | 249 | 21 | 8,201 | Temporal_Mid_L | −52 | −38 | 4 | 7.48 | 5.61 |
Located in white matter and the nearest brain area was reported.
FIGURE 3.
Activation results of the control group. The results of the visual synonym judgment task (a), oral word reading task (b), and auditory sentence comprehension task (c). The left side of the brain faces left, and the right side faces right. The color bar indicates t‐statistics. (p <.05, FWE‐corrected for multiple comparisons at the cluster level for the visual synonym judgment and auditory sentence comprehension tasks; p <.001, FWE‐corrected for the oral word reading task)
TABLE 3.
Brain areas with significant activation in the AVM group
| Activation areas | Voxels | BA | AAL | AAL name | Peak MNI coordinates | Peak t | Peak Z | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Task: Visual synonym judgment (threshold at p <.05 FWE correction) | |||||||||
| L occipital lobe | 2,738 | 18 | 5,201 | Occipital_Mid_L | −20 | −94 | 2 | 12.46 | 7.27 |
| 18 | 5,301 | Occipital_Inf_L | −24 | −86 | −10 | 10.68 | 6.75 | ||
| L precentral gyrus and inferior frontal gyrus | 4,768 | 6 | 2,001 | Precentral_L | −44 | 2 | 44 | 11.75 | 7.08 |
| 44 | 2,311 | Frontal_Inf_Tri_L | −42 | 18 | 31 | 10.25 | 6.61 | ||
| 45 | 2,311 | Frontal_Inf_Tri_L | −48 | 26 | 15 | 9.56 | 6.38 | ||
| L supplementary motor area | 1,027 | 6 | 2,401 | Supp_Motor_Area_L | −6 | 12 | 56 | 10.51 | 6.7 |
| R cerebellar hemisphere | 2,523 | — | 9,062 | Cerebelum_8_R | 28 | −66 | −48 | 8.7 | 6.05 |
| — | 9,012 | Cerebelum_Crus2_R | 26 | −76 | −44 | 8.53 | 5.99 | ||
| — | 9,012 | Cerebelum_Crus2_R | 14 | −78 | −32 | 8.26 | 5.88 | ||
| L middle temporal gyrus | 346 | 21 | 8,201 | Temporal_Mid_L | −58 | −46 | 0 | 7.33 | 5.47 |
| R insula | 219 | 13 | 3,002 | Insula_R | 36 | 30 | −2 | 6.92 | 5.27 |
| L putamen | 70 | — | 7,011 | Putamen_L | −18 | −2 | 12 | 6.25 | 4.94 |
| Task: Oral word reading (threshold at p <.001 FWE correction) | |||||||||
| R superior temporal gyrus | 1,186 | 22 | 8,112 | Temporal_Sup_R | 62 | −16 | 2 | 12.93 | 7.39 |
| 22 | 8,122 | Temporal_Pole_Sup_R | 56 | 8 | −8 | 8.42 | 5.94 | ||
| — | 8,112 | Temporal_Sup_R a | 36 | −36 | 8 | 7.84 | 5.7 | ||
| L precentral gyrus | 286 | 6 | 2,001 | Precentral_L | −50 | 0 | 54 | 11.2 | 6.91 |
| R precentral gyrus | 359 | 6 | 2,002 | Precentral_R | 52 | 0 | 52 | 10.26 | 6.62 |
| R cerebellar hemisphere | 1,372 | — | 9,062 | Cerebelum_8_R | 8 | −72 | −38 | 10.04 | 6.54 |
| — | 9,062 | Cerebelum_8_R | 16 | −68 | −48 | 9.81 | 6.46 | ||
| — | 9,130 | Vermis_6 | 6 | −64 | −16 | 9.76 | 6.45 | ||
| L supplementary motor area | 531 | 6 | 2,401 | Supp_Motor_Area_L | −4 | 8 | 60 | 9.81 | 6.46 |
| L superior temporal gyrus | 52 | 22 | 8,201 | Temporal_Mid_L | −52 | −38 | 10 | 8.35 | 5.91 |
| L inferior frontal gyrus | 177 | 45 | 3,001 | Insula_L | −26 | 28 | 8 | 8.32 | 5.9 |
| L fusiform gyrus | 138 | 37 | 5,401 | Fusiform_L | −44 | −54 | −14 | 8.31 | 5.9 |
| R cingulum | 108 | — | 4,012 | Cingulum_Mid_R a | 10 | 4 | 28 | 8.3 | 5.89 |
| L occipital lobe | 564 | 19 | 5,301 | Occipital_Inf_L | −38 | −84 | −12 | 8.12 | 5.82 |
| 18 | 5,301 | Occipital_Inf_L | −20 | −92 | −6 | 7.75 | 5.66 | ||
| 18 | 5,201 | Occipital_Mid_L | −24 | −88 | 0 | 7.68 | 5.63 | ||
| R inferior frontal gyrus | 48 | 45 | 2,312 | Frontal‐Inf‐tri‐R a | 28 | 30 | 10 | 7.91 | 5.73 |
| L superior temporal gyrus | 157 | 41 | 8,111 | Temporal_Sup_L | −66 | −22 | 6 | 7.86 | 5.71 |
| 41 | 8,111 | Temporal_Sup_L | −60 | −10 | 2 | 7.71 | 5.64 | ||
| R cerebellar hemisphere | 30 | — | 9,042 | Cerebelum_6_R | 30 | −56 | −32 | 7.35 | 5.48 |
| Task: Auditory sentence comprehension (threshold at p <.05 FWE correction) | |||||||||
| L superior and middle temporal gyrus | 199 | 22 | 8,201 | Temporal_Mid_L | −62 | −44 | 8 | 6.8 | 5.22 |
| 21 | 8,201 | Temporal_Mid_L | −54 | −32 | 2 | 5.66 | 4.61 | ||
| L precentral gyrus | 53 | 6 | 2,001 | Precentral_L | −52 | 4 | 50 | 6.55 | 5.09 |
| R cerebellar hemisphere | 66 | — | 9,042 | Cerebelum_6_R | 30 | −66 | −26 | 6.24 | 4.93 |
| R temporal pole | 17 | 38 | 8,122 | Temporal_Pol_R | 54 | 14 | −14 | 5.85 | 4.71 |
| L supplementary motor area | 20 | 6 | 2,401 | Supp_Motor_Area_L | −6 | 6 | 64 | 5.81 | 4.69 |
Located in white matter and the nearest brain area was reported.
FIGURE 4.
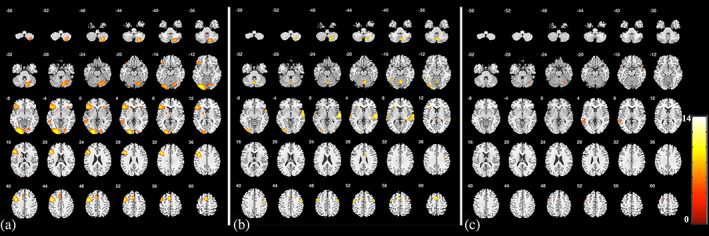
Activation results of the AVM group. The results of the visual synonym judgment task (a), oral word reading task (b), and auditory sentence comprehension task (c). The color bar indicates t‐statistics. (p <.05, FWE‐corrected for the visual synonym judgment and auditory sentence comprehension tasks; p <.001, FWE‐corrected for the oral word reading task)
TABLE 4.
Significant clusters of the AVM group compared to the control group in auditory sentence comprehension task (threshold at p <.05, FWE correction)
| Activation areas | Voxels | BA | AAL | AAL name | MNI coordinates | Peak t | Peak Z | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| R precentral gyrus | 139 | 6 | 2,002 | Precentral_R | 42 | 6 | 46 | 5.73 | 5.1 |
| R cerebellar hemisphere | 22 | — | 9,042 | Cerebelum_6_R | 28 | −68 | −26 | 5.23 | 4.73 |
FIGURE 5.
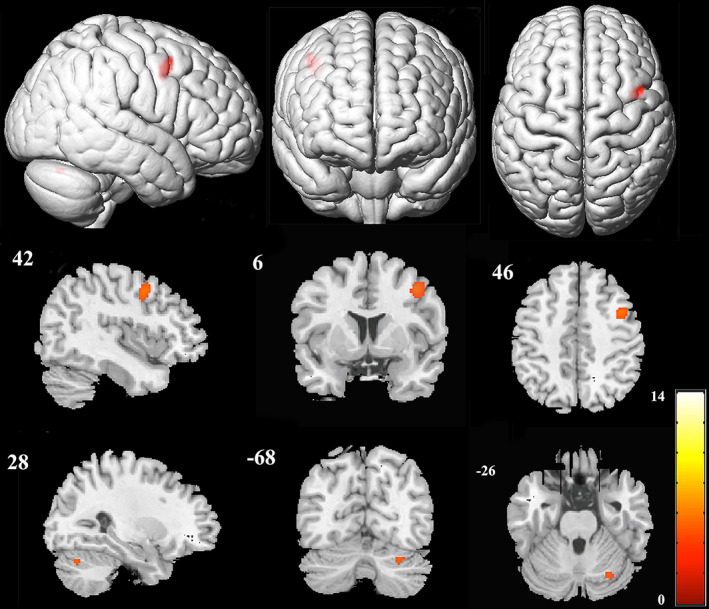
Significant activations (AVM >control) in the auditory sentence comprehension task. (p <.05, FWE‐corrected)
3.4. ROI study
According to the group‐level analysis results, two ROIs were identified in the auditory sentence comprehension task. In the ROI of the right precentral gyrus (BA 6), the mean difference in the experimental task compared to the control task was 0.004 ± 0.103 (p = .827) in the AVM group and −0.055 ± 0.152 (p = .051) in the control group. Meanwhile, in the ROI of the right cerebellar lobule VI (AAL 9042), the difference was 0.091 ± 0.126 (p <.001) in the AVM group and −0.000 ± 0.075 (p = .980) in the control group.
3.5. Correlation of BOLD signal changes among ROIs and VOIs
Based on the activations of brain regions in the control and AVM groups, a total of 13 cerebral VOIs were identified, including 7 in the left frontal lobe, 2 in the right frontal lobe, 1 in the bilateral SMA, 2 in the left temporal lobe, and 1 in the right temporal lobe (Table S2).
Correlations of BOLD signal changes among 2 ROIs and 13 VOIs are shown in Table S2. We evaluated the correlation changes in participants in the AVM group compared to those in the control group. For the ROI of the right precentral gyrus (BA 6), in AVM patients, the main changes in the correlations with left frontal and temporal VOIs were positive correlations that changed to no correlation or negative correlations; regions showing such changes included the left precentral gyrus, inferior frontal gyrus and superior and middle temporal gyri. In contrast, the right frontal VOIs (right precentral gyrus, right middle frontal gyrus, and bilateral SMAs) showed positive correlations with the right precentral gyrus in AVM patients, especially in the visual synonym judgment task. For the ROI of right cerebellar lobule VI, correlations with part of the pars opercularis and left temporal lobe disappeared, while new positive correlations with the left precentral gyrus, left inferior frontal gyrus, and right temporal pole were observed.
3.6. LI and dominant hemisphere results
The LI results of three brain areas, namely the frontal and temporal lobes and cerebellum, are shown in Table 5. Different language activation patterns were observed among the three tasks. In the visual synonym judgment task, typical left dominance of the frontal and temporal lobes (LI > +0.20) and right dominance of the cerebellum (LI < −0.20) were observed in both control and AVM groups. The LIs of the three regions showed no significant difference between the two groups. However, in the oral word reading task, the LIs of both groups showed no clear hemisphere preference (−0.20 < LI < +0.20) in any of the three regions. And the LI of the frontal lobe was significantly more right‐lateralized in the AVM group than in the control group (−0.01 ± 0.32 vs. 0.19 ± 0.26, p = .009). No significant difference in the LIs of the temporal lobe and cerebellum was observed between the two groups. Moreover, in the auditory sentence comprehension task, the LIs of both groups showed left dominance of temporal lobe (LI > +0.20), and no clear hemisphere preference (−0.20 < LI < +0.20) in either the frontal lobe or the cerebellum. The LI of the temporal lobe was significantly more right‐lateralized in the AVM group than in the control group (0.24 ± 0.39 vs. 0.50 ± 0.23, p = .003). No significant difference was observed in the LIs of the frontal lobe and cerebellum between the two groups.
TABLE 5.
Differences of the LIs between two groups in three tasks
| Group | Visual synonym judgment | Oral word reading | Auditory sentence comprehension | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LIFL | LITL | LICB | LIFL | LITL | LICB | LIFL | LITL | LICB | |
| Control | 0.56 ± 0.14 | 0.54 ± 0.22 | −0.28 ± 0.41 | 0.19 ± 0.26 | 0.04 ± 0.35 | −0.10 ± 0.31 | 0.18 ± 0.37 | 0.50 ± 0.23 | −0.13 ± 0.41 |
| AVM | 0.61 ± 0.20 | 0.57 ± 0.20 | −0.46 ± 0.32 | −0.01 ± 0.32 | −0.06 ± 0.30 | −0.11 ± 0.33 | 0.15 ± 0.38 | 0.24 ± 0.39 | −0.18 ± 0.36 |
| p value | .278 | .609 | .062 | .009 | .225 | .939 | .785 | .003 | .616 |
Abbreviations: LICB, LI of cerebellum; LIFL, LI of frontal lobe; LITL, LI of temporal lobe.
The dominant hemispheres of three brain areas (the frontal lobe, temporal lobe and cerebellum) are shown in Table 6. Consistent with the results of the LIs, in the visual synonym judgment task, the majority of participants showed left dominance of both the frontal and temporal lobes and right dominance of the cerebellum. According to the Mann–Whitney test, no significant difference between participants in the two groups was observed in the three brain regions. In the oral word reading task, the results of both groups showed bilateral activations (no clear hemisphere preference) in three regions. However, the frontal lobe was significantly more right‐dominant in participants in the AVM group than in participants in the control group (p = .003). In the auditory sentence comprehension task, the frontal lobe and cerebellum showed no clear hemisphere preference, but the temporal lobe was left‐dominant in the majority of participants. In addition, the temporal lobe was significantly more right‐dominant in participants in the AVM group than in participants in the control group (p = .020).
TABLE 6.
Differences of dominant hemisphere between two groups in three brain lobes (Mann–Whitney Test)
| Dominant hemisphere of control group | Dominant hemisphere of AVM group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tasks | Brain lobes | Left (%) | NHP (%) | Right (%) | Left (%) | NHP (%) | Right (%) | Z | p value |
| Visual synonym judgment | Frontal lobe | 31 (96.9) | 1 (3.1) | 0 (0) | 29 (96.7) | 0 (0) | 1 (3.3) | −0.069 | .945 |
| Temporal lobe | 28 (87.5) | 4 (12.5) | 0 (0) | 29 (96.7) | 0 (0) | 1 (3.3) | −1.254 | .210 | |
| Cerebellum | 6 (18.8) | 7 (21.9) | 19 (59.4) | 2 (6.7) | 4 (13.3) | 24 (80.0) | −1.805 | .071 | |
| Oral word reading | Frontal lobe | 16 (50.0) | 13 (40.6) | 3 (9.4) | 8 (26.7) | 12 (40.0) | 10 (33.3) | −2.972 | .003 |
| Temporal lobe | 12 (37.5) | 9 (28.1) | 11 (34.4) | 5 (16.7) | 16 (53.3) | 9 (30.0) | −0.788 | .431 | |
| Cerebellum | 6 (18.8) | 15 (46.9) | 11 (34.4) | 7 (23.3) | 11 (36.7) | 12 (40.0) | −0.114 | .910 | |
| Auditory sentence comprehension | Frontal lobe | 18 (56.3) | 7 (21.9) | 7 (21.9) | 15 (50.0) | 8 (26.7) | 7 (23.3) | −0.411 | .681 |
| Temporal lobe | 29 (90.6) | 2 (6.3) | 1 (3.1) | 20 (66.7) | 5 (16.7) | 5 (16.7) | −2.331 | .020 | |
| Cerebellum | 9 (28.1) | 7 (21.9) | 16 (50.0) | 7 (23.3) | 10 (33.3) | 13 (43.3) | −0.175 | .861 | |
Abbreviation: NHP, no hemispheric preference.
Correlation analysis showed that there was no significant correlation between lesion size and LIs in the three tasks, and detailed correlation coefficients and p values are shown in Table S3. Similarly, ANOVA results suggested no significant difference in LIs among the three subgroups categorized by lesion location (Table S3).
4. DISCUSSION
The current study investigated language reorganization patterns in AVM patients using task‐based fMRI technique, aiming to explain how these patients are exempted from language dysfunction in cases where the traditional language areas are involved by the congenital lesions. The results suggested that the AVM nidus involving language areas might have distinct effects on different language functions, leading to different reorganization patterns. Possible brain areas involved in language reorganization include perilesion areas in the left hemisphere, the right frontal lobe, the right temporal lobe and the right cerebellar hemisphere. However, the scopes in which these areas participate in language and the ways these areas function in the reorganized language network might be different.
4.1. Language reorganization patterns of AVM patients
The lateralization differences between two groups varied among tasks, suggesting that AVM patients exhibited different language reorganization patterns for different language functions.
In the visual synonym judgment task, there was no significant difference between the two groups, in either the LI or the activated brain areas, demonstrating no significant reorganization in the right hemisphere. Considering that the traditional language areas were involved by the AVM nidus, we therefore speculate that perilesion reorganization should occur in the left hemisphere, but the location of the reorganized areas might be scattered, leading to nonsignificant differences.
In the oral word reading task, the LI results suggested that the dominant hemisphere of the frontal lobe (the presumed main brain area responsible for oral production) was significantly more right‐lateralized in participants in the AVM group than in participants in the control group. Similarly, in the auditory sentence comprehension task, the dominant hemisphere of the temporal lobe (the presumed main brain area responsible for language comprehension) was significantly more right‐lateralized in participants in the AVM group than in participants in the control group. However, no significantly different brain areas were identified in the voxelwise analysis in these two lobes. The possible reason for this outcome might be that the activation areas of the right hemisphere were inconsistent, which led to negative results in the group‐level analysis.
The above results showed that the same lesions had different effects on different language functions, leading to different language reorganization patterns. Three language reorganization patterns were observed in this study. In the oral word reading and auditory sentence comprehension tasks, right cerebral hemisphere reorganization existed in patients' frontal lobe and temporal lobe, respectively. In visual synonym judgment processing, there was no obvious right‐hemisphere reorganization and perilesion reorganization in the left hemisphere might have played a role. Although some authors questioned the role of the right hemisphere in aphasia patients (Teki et al., 2013; Winhuisen et al., 2005), our findings in this congenital disease model supported that the right hemisphere might contribute to the maintenance of intact language functions.
Notably, we did not find a significant correlation between lesion sizes and LIs or between lesion locations and LIs. This result is not consistent with results of our previous studies, as we previously found that language reorganization patterns had anatomic specificity in AVM patients (Deng et al., 2015, 2020). We believe the possible reason for this outcome in the current study might be the limited number of patients included.
4.2. Two brain areas (AVM > control) identified in the auditory sentence comprehension task
Compared to the control group, the AVM group showed no significant differences in brain activation during the visual synonym judgment and oral word reading tasks. However, in the auditory sentence comprehension task, two significantly different brain areas were identified: the right premotor cortex (BA 6) and the right cerebellar lobule VI (AAL 9042). Considering that no language dysfunction exists in these patients whose traditional language areas are involved by AVM nidus, we speculate that these two brain areas might play a role in compensation for the impairment of language function caused by the lesions. However, the mechanisms of these two brain areas might be different and are discussed separately.
-
Role of the right cerebellar lobule VI
The role of the cerebellum in language has been recognized for decades. Although its exact function is still controversial, the cerebellum is currently believed to be engaged in verbal fluency, grammar, syntactics, comprehension, and so on (King, Hernandez‐Castillo, Poldrack, Ivry, & Diedrichsen, 2019; Pleger & Timmann, 2018; Starowicz‐Filip et al., 2017). Clinical studies have found that patients with cerebellar damage could present with various types of language disorders, including phonological, lexicosemantic, and syntactic dysfunctions (Ackermann, 2013; De Smet, Paquier, Verhoeven, & Marien, 2013; Marien et al., 2014). Moreover, there is a consensus that the brain regions of the cerebellum responsible for language are located in the lateral posterior cerebellar hemisphere and include lobule VI, Crus I, and Crus II (Booth, Wood, Lu, Houk, & Bitan, 2007; D'Mello & Stoodley, 2015). It has been reported that the right cerebellar lobule VI mainly contributes to verbal fluency (King et al., 2019; J. L. Chen, Penhune, & Zatorre, 2008). In addition, the cerebellar hemispheres have laterality, and the right hemisphere is dominant in the majority of the right‐handed healthy population (Ashida, Cerminara, Edwards, Apps, & Brooks, 2019; Gao et al., 2017; Gilligan & Rafal, 2019; Lesage, Hansen, & Miall, 2017; McAvoy et al., 2016).
Our results were basically consistent with previous literature reports. As shown in Table 2, there were significant activations in participants' right cerebellar lobule VI during the oral word reading task in the control group, suggesting the role of the right cerebellar lobule VI in oral production. In addition to lobules VI and Crus II, lobule VIII was involved in language processing, evident by the signals recorded when participants were performing visual synonym judgment and oral word reading tasks. Meanwhile, we found that the laterality of cerebellum varied with tasks, and bilateral activation was common in oral word reading task (Lidzba, Wilke, Staudt, Krageloh‐Mann, & Grodd, 2008).
Moreover, in the auditory sentence comprehension task, the right cerebellar lobule VI exhibited no significant difference between the experimental and the control tasks in the control group, while significantly positive activations were observed in the AVM group. In addition, positive correlations between the right cerebellar lobule VI and other language areas, including the left precentral gyrus, left inferior frontal gyrus and right temporal pole, were found in the AVM group but not in the control group. Based on these findings, we speculate that the right cerebellar lobule VI might not make a significant contribution to auditory comprehension in the healthy population. However, when the language areas of the left cerebral hemisphere are impaired by the AVM nidus, the right cerebellar lobule VI might participate in the reorganized language network and contribute to the upward regulation of language functions to prevent language function deterioration.
-
Role of the right premotor cortex
The left premotor cortex (BA 6) also contributes to language functions, and it participates in the process of gestural communication, speech and other language functions (local syntactic dependencies, etc.) (Friederici, 2006; Gajardo‐Vidal et al., 2021; Häberling, Corballis, & Corballis, 2016; Mugler et al., 2018). In addition, the middle part of the premotor cortex (mid‐PMC) is reported to be involved in general auditory processing, which might be relevant to movement anticipation (J. L. Chen et al., 2008).
Although the right premotor cortex was also identified to be more significantly activated in participants in the AVM group, the mechanism of this cortex might be different from that of the right cerebellar lobule VI. In the ROI of right premotor cortex, the main reason for the significant difference between the two groups was the negative activation (task‐dependent decrease) in the control group, rather than positive activation in the AVM group. In other words, the premotor cortex and posterior part of the right middle frontal gyrus were negatively activated in auditory sentence comprehension processing in the healthy population; however, when language areas of the left cerebral hemisphere were impaired by the AVM nidus, this negative activation disappeared. Intriguingly, the correlation analysis results showed that the correlations between right premotor cortex and some left frontotemporal language areas changed from positive correlations to no correlation or negative correlations in AVM patients.
We believe these phenomena might be explained by interhemispheric inhibition theory. It has been speculated that there is interhemispheric inhibition between hemispheres mediated by the corpus callosum, which connects the homotopic regions of the left and right hemispheres (McAvoy et al., 2016; Tzourio‐Mazoyer, Perrone‐Bertolotti, Jobard, Mazoyer, & Baciu, 2017). In typical healthy brains, interhemispheric inhibition mainly exerts from the left cerebral hemisphere to the right cerebral hemisphere, permitting the left hemisphere to maintain language dominance. However, in pathological conditions, interhemispheric transcallosal inhibitory connections might be decreased, leading to disinhibition of the right hemisphere (Chu, Meltzer, & Bitan, 2018; Hartwigsen & Saur, 2019).
Pertinently, interhemispheric inhibition might also be exerted from the right hemisphere to the left hemisphere. Clinical studies found that inhibitory low‐frequency repetitive transcranial magnetic stimulation (LFrTMS) over the right hemisphere (especially the right inferior frontal gyrus and superior temporal gyrus) could improve language function in poststroke aphasic patients with left‐hemispheric lesions, and speculated the mechanism for this improvement was the reduction in interhemispheric inhibition from the right to the left hemisphere by suppressive LFrTMS (Ren et al., 2019).
According to our findings and previous reports, we hypothesize that damage to the language areas in the left hemisphere due to the AVM nidus and the activation of the right premotor cortex may be reciprocal causations. A possible mechanism for this occurrence is that in normal controls, the right premotor cortex is inhibited by the left language areas, showing negative activation in this area. However, when the traditional language areas in the left hemisphere are impaired by the AVM nidus, interhemispheric inhibitory effects decrease; thus, the negative activation in the right hemisphere disappears. This effect in turn decreases the inhibitory effect from the right hemisphere to the left hemisphere and then enhances the function of language areas of the left hemisphere, which accordingly maintains normal language functions.
Therefore, we speculate that these two brain areas may share different mechanisms in language regulation. The right cerebellar lobule VI in AVM patients might upregulate the function of language areas in the left hemisphere, while the right precentral gyrus and posterior part of the right middle frontal gyrus might downregulate the inhibition of language areas in the left hemisphere. These inferences are speculated based on the results of our study, and their validity needs to be confirmed. Furthermore, this hypothesis may be verified by longitudinal studies researching short‐term and long‐term postsurgery fMRI changes in AVM patients. In addition, studies using other imaging methods might be helpful. For example, using diffusion tensor imaging (DTI) to demonstrate the anatomical connections in AVM patients, especially changes in white matter connecting two hemispheres, might be useful to investigate the underlying reorganization mechanism.
4.3. Limitations of the study
First, the number of participants was limited. Second, lesion locations were not concentrated, which included the frontal, temporal and parietal lobes. Some findings might be concealed in group‐level analysis. Third, we included three patients with AVM rupture histories, but fMRI activations could potentially have been affected by prior bleeding. To minimize the impact of previous bleeding on the results, for patients with a history of AVM rupture, we only enrolled those with mild symptoms after bleeding, with bleeding more than 1 month prior to the enrollment and without neurological deficits when enrolled. Fourth, it is controversial whether BOLD signals are reliable in AVM patients, considering the hemodynamic changes caused by the AVM nidus in surrounding brain areas. For example, Lehéricy et al. (2002) reported that the dominant hemisphere indicated by the LIs changed from pre‐embolization right‐side dominance to postembolization symmetric or left‐side dominance in two patients. The authors therefore speculated that the pre‐embolization abnormal LIs were at least partly due to flow abnormalities that impaired the detection of the BOLD signal intensity. However, much clinical, imaging and electrophysiological evidence supports the reliability of BOLD signals. For example, as mentioned in our previous paper, patients with unruptured AVMs involving important traditional language areas usually do not present with language dysfunction; one possible explanation for this phenomenon is right‐side hemisphere reorganization (Deng et al., 2015; Deng et al., 2020).In addition, the results of the Wada test and fMRI in some studies also demonstrated the reliability of using LI of BOLD signals to predict the dominant hemisphere in AVM patients (Lee et al., 2010). Therefore, although controversy exists, we believe right‐lateralization of BOLD signals suggests right‐hemisphere reorganization to a large extent. Even so, it is uncertain that the subtle patterns of activation are not affected by the lesions, or that the perfusion and cerebrovascular reactivity of the right hemisphere are absolutely normal. We believe these are the inherent limitations of this congenital vascular model.
5. CONCLUSION
The results of our study suggested that an AVM nidus involving language areas might have distinct effects on different language functions, leading to different language reorganization patterns. Three language reorganization patterns were observed in this study. In the oral word reading and auditory sentence comprehension tasks, right‐hemisphere reorganization existed in patients' frontal and temporal lobes, respectively. In visual synonym judgment processing, AVM patients demonstrated no right‐hemisphere reorganization and peri‐lesion reorganization in the left hemisphere might have played a role. In addition, two brain areas were found to be involved in the regulation of language functions, the right precentral gyrus and posterior middle frontal gyrus (BA 6) and the right cerebellar lobule VI (AAL 9042); these two regions might have different roles in patients' reorganized language network. The right cerebellar lobule VI might upregulate the function of language areas in the left hemisphere, while the right premotor cortex might downregulate the inhibition of language areas in the left hemisphere. In brief, there appear to be some patterns of right‐hemisphere compensation/reorganization in the population with this form of congenital brain lesion.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Table S1 The demographic and lesion data of AVM patients.
Table S2: Pearson correlation coefficients between 2 ROIs and 13 VOIs in the AVM group and control group.
Table S3: Results of Pearson correlation between lesion size and LI in three tasks, and ANOVA test results of LIs among the three subgroups.
ACKNOWLEDGMENT
The authors acknowledge all individuals who participated in this study.
Deng, X. , Wang, B. , Zong, F. , Yin, H. , Yu, S. , Zhang, D. , Wang, S. , Cao, Y. , Zhao, J. , & Zhang, Y. (2021). Right‐hemispheric language reorganization in patients with brain arteriovenous malformations: A functional magnetic resonance imaging study. Human Brain Mapping, 42(18), 6014–6027. 10.1002/hbm.25666
Funding information National Natural Science Foundation of China, Grant/Award Numbers: 81701088, 81870833, 31730039; Beijing talents project, Grant/Award Number: 2017000021469G211; Chinese Academy of Sciences grants, Grant/Award Numbers: XDB32010300, ZDBS‐LY‐SM028; Ministry of Science and Technology of the People's Republic of China, Grant/Award Numbers: 2020AAA0105601, 2019YFA0707103
Contributor Information
Bo Wang, Email: bwang@bcslab.ibp.ac.cn.
Yan Zhang, Email: yanzhang135@163.com.
DATA AVAILABILITY STATEMENT
Data availability statement: Some or all data used in the study are available from the corresponding author by request.
REFERENCES
- Ackermann, H. (2013). The contribution of the cerebellum to speech and language. Brain and Language, 127(3), 315–316. 10.1016/j.bandl.2013.10.006 [DOI] [PubMed] [Google Scholar]
- Ashida, R. , Cerminara, N. L. , Edwards, R. J. , Apps, R. , & Brooks, J. C. W. (2019). Sensorimotor, language, and working memory representation within the human cerebellum. Human Brain Mapping, 40(16), 4732–4747. 10.1002/hbm.24733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth, J. R. , Wood, L. , Lu, D. , Houk, J. C. , & Bitan, T. (2007). The role of the basal ganglia and cerebellum in language processing. Brain Research, 1133(1), 136–144. 10.1016/j.brainres.2006.11.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenstein, C. , Grewe, T. , Flöel, A. , Ziegler, W. , Springer, L. , Martus, P. , … Baumgaertner, A. (2017). Intensive speech and language therapy in patients with chronic aphasia after stroke: A randomised, open‐label, blinded‐endpoint, controlled trial in a health‐care setting. Lancet, 389(10078), 1528–1538. 10.1016/s0140-6736(17)30067-3 [DOI] [PubMed] [Google Scholar]
- Chen, C. J. , Ding, D. , Derdeyn, C. P. , Lanzino, G. , Friedlander, R. M. , Southerland, A. M. , … Sheehan, J. P. (2020). Brain arteriovenous malformations: A review of natural history, pathobiology, and interventions. Neurology, 95(20), 917–927. 10.1212/wnl.0000000000010968 [DOI] [PubMed] [Google Scholar]
- Chen, J. L. , Penhune, V. B. , & Zatorre, R. J. (2008). Listening to musical rhythms recruits motor regions of the brain. Cerebral Cortex, 18(12), 2844–2854. 10.1093/cercor/bhn042 [DOI] [PubMed] [Google Scholar]
- Chu, R. , Meltzer, J. A. , & Bitan, T. (2018). Interhemispheric interactions during sentence comprehension in patients with aphasia. Cortex, 109, 74–91. 10.1016/j.cortex.2018.08.022 [DOI] [PubMed] [Google Scholar]
- Cirillo, S. , Caulo, M. , Pieri, V. , Falini, A. , & Castellano, A. (2019). Role of functional imaging techniques to assess motor and language cortical plasticity in glioma patients: A systematic review. Neural Plasticity, 2019, 4056436. 10.1155/2019/4056436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello, A. M. , & Stoodley, C. J. (2015). Cerebro‐cerebellar circuits in autism spectrum disorder. Frontiers in Neuroscience, 9, 408. 10.3389/fnins.2015.00408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet, H. J. , Paquier, P. , Verhoeven, J. , & Marien, P. (2013). The cerebellum: Its role in language and related cognitive and affective functions. Brain and Language, 127(3), 334–342. 10.1016/j.bandl.2012.11.001 [DOI] [PubMed] [Google Scholar]
- Deng, X. , Wei, X. , Zhang, Y. , Wang, B. , Zhang, D. , Yu, S. , … Zhao, J. (2020). Impact of AVM location on language cortex right‐hemisphere reorganization: A voxel‐based lesion‐symptom mapping study. Clinical Neurology and Neurosurgery, 189, 105628. 10.1016/j.clineuro.2019.105628 [DOI] [PubMed] [Google Scholar]
- Deng, X. , Xu, L. , Zhang, Y. , Wang, B. , Wang, S. , Zhao, Y. , … Zhao, J. (2016). Difference of language cortex reorganization between cerebral arteriovenous malformations, cavernous malformations, and gliomas: A functional MRI study. Neurosurgical Review, 39(2), 241–249; discussion 249. 10.1007/s10143-015-0682-7 [DOI] [PubMed] [Google Scholar]
- Deng, X. , Zhang, Y. , Xu, L. , Wang, B. , Wang, S. , Wu, J. , … Zhao, J. (2015). Comparison of language cortex reorganization patterns between cerebral arteriovenous malformations and gliomas: A functional MRI study. Journal of Neurosurgery, 122(5), 996–1003. 10.3171/2014.12.JNS14629 [DOI] [PubMed] [Google Scholar]
- Duffau, H. (2021). New philosophy, clinical pearls, and methods for intraoperative cognition mapping and monitoring "à la carte" in brain tumor patients. Neurosurgery, 88, 919–930. 10.1093/neuros/nyaa363 [DOI] [PubMed] [Google Scholar]
- Feldman, H. M. (2019). How young children learn language and speech. Pediatrics in Review, 40(8), 398–411. 10.1542/pir.2017-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleetwood, I. G. , & Steinberg, G. K. (2002). Arteriovenous malformations. Lancet, 359(9309), 863–873. 10.1016/s0140-6736(02)07946-1 [DOI] [PubMed] [Google Scholar]
- Friederici, A. D. (2006). Broca's area and the ventral premotor cortex in language: Functional differentiation and specificity. Cortex, 42(4), 472–475. 10.1016/s0010-9452(08)70380-0 [DOI] [PubMed] [Google Scholar]
- Gajardo‐Vidal, A. , Lorca‐Puls, D. L. , Team, P. , Warner, H. , Pshdary, B. , Crinion, J. T. , … Price, C. J. (2021). Damage to Broca's area does not contribute to long‐term speech production outcome after stroke. Brain, 144, 817–832. 10.1093/brain/awaa460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Q. , Tao, Z. , Cheng, L. , Leng, J. , Wang, J. , Yu, C. , & Chen, H. (2017). Language lateralization during the Chinese semantic task relates to the contralateral cerebra‐cerebellar interactions at rest. Scientific Reports, 7(1), 14056. 10.1038/s41598-017-14600-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan, T. M. , & Rafal, R. D. (2019). An opponent process cerebellar asymmetry for regulating word association priming. Cerebellum, 18(1), 47–55. 10.1007/s12311-018-0949-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häberling, I. S. , Corballis, P. M. , & Corballis, M. C. (2016). Language, gesture, and handedness: Evidence for independent lateralized networks. Cortex, 82, 72–85. 10.1016/j.cortex.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Hagoort, P. (2019). The neurobiology of language beyond single‐word processing. Science, 366(6461), 55–58. 10.1126/science.aax0289 [DOI] [PubMed] [Google Scholar]
- Hartwigsen, G. , & Saur, D. (2019). Neuroimaging of stroke recovery from aphasia—Insights into plasticity of the human language network. NeuroImage, 190, 14–31. 10.1016/j.neuroimage.2017.11.056 [DOI] [PubMed] [Google Scholar]
- Ille, S. , Engel, L. , Kelm, A. , Meyer, B. , & Krieg, S. M. (2018). Language‐eloquent white matter pathway tractography and the course of language function in glioma patients. Frontiers in Oncology, 8, 572. 10.3389/fonc.2018.00572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, M. , Hernandez‐Castillo, C. R. , Poldrack, R. A. , Ivry, R. B. , & Diedrichsen, J. (2019). Functional boundaries in the human cerebellum revealed by a multi‐domain task battery. Nature Neuroscience, 22(8), 1371–1378. 10.1038/s41593-019-0436-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D. J. , Pouratian, N. , Bookheimer, S. Y. , & Martin, N. A. (2010). Factors predicting language lateralization in patients with perisylvian vascular malformations. Clinical article. Journal of Neurosurgery, 113(4), 723–730. 10.3171/2010.2.JNS091595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy, S. , Biondi, A. , Sourour, N. , Vlaicu, M. , du Montcel, S. T. , Cohen, L. , … Marsault, C. (2002). Arteriovenous brain malformations: Is functional MR imaging reliable for studying language reorganization in patients? Initial observations. Radiology, 223(3), 672–682. 10.1148/radiol.2233010792 [DOI] [PubMed] [Google Scholar]
- Lesage, E. , Hansen, P. C. , & Miall, R. C. (2017). Right lateral cerebellum represents linguistic predictability. The Journal of Neuroscience, 37(26), 6231–6241. 10.1523/JNEUROSCI.3203-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Dong, J. W. , Del Ferraro, G. , Petrovich Brennan, N. , Peck, K. K. , Tabar, V. , … Holodny, A. I. (2019). Functional translocation of Broca's area in a low‐grade left frontal glioma: Graph theory reveals the novel, adaptive network connectivity. Frontiers in Neurology, 10, 702. 10.3389/fneur.2019.00702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidzba, K. , Wilke, M. , Staudt, M. , Krageloh‐Mann, I. , & Grodd, W. (2008). Reorganization of the cerebro‐cerebellar network of language production in patients with congenital left‐hemispheric brain lesions. Brain and Language, 106(3), 204–210. 10.1016/j.bandl.2007.11.003 [DOI] [PubMed] [Google Scholar]
- Marien, P. , Ackermann, H. , Adamaszek, M. , Barwood, C. H. , Beaton, A. , Desmond, J. , … Ziegler, W. (2014). Consensus paper: Language and the cerebellum: An ongoing enigma. Cerebellum, 13(3), 386–410. 10.1007/s12311-013-0540-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy, M. , Mitra, A. , Coalson, R. S. , d'Avossa, G. , Keidel, J. L. , Petersen, S. E. , & Raichle, M. E. (2016). Unmasking language lateralization in human brain intrinsic activity. Cerebral Cortex, 26(4), 1733–1746. 10.1093/cercor/bhv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr, J. P. , Koennecke, H. C. , & Hartmann, A. (2020). Management of brain arteriovenous malformations: Still a long and winding road ahead. Neurology, 95(20), 899–900. 10.1212/wnl.0000000000010964 [DOI] [PubMed] [Google Scholar]
- Mugler, E. M. , Tate, M. C. , Livescu, K. , Templer, J. W. , Goldrick, M. A. , & Slutzky, M. W. (2018). Differential representation of articulatory gestures and phonemes in precentral and inferior frontal gyri. The Journal of Neuroscience, 38(46), 9803–9813. 10.1523/JNEUROSCI.1206-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger, B. , & Timmann, D. (2018). The role of the human cerebellum in linguistic prediction, word generation and verbal working memory: Evidence from brain imaging, non‐invasive cerebellar stimulation and lesion studies. Neuropsychologia, 115, 204–210. 10.1016/j.neuropsychologia.2018.03.012 [DOI] [PubMed] [Google Scholar]
- Qiu, D. , Tan, L. H. , Siok, W. T. , Zhou, K. , & Khong, P. L. (2011). Lateralization of the arcuate fasciculus and its differential correlation with reading ability between young learners and experienced readers: A diffusion tensor tractography study in a Chinese cohort. Human Brain Mapping, 32(12), 2054–2063. 10.1002/hbm.21168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, C. , Zhang, G. , Xu, X. , Hao, J. , Fang, H. , Chen, P. , … Gao, F. (2019). The effect of rTMS over the different targets on language recovery in stroke patients with global aphasia: A randomized sham‐controlled study. BioMed Research International, 2019, 4589056. 10.1155/2019/4589056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolinski, R. , You, X. , Gonzalez‐Castillo, J. , Norato, G. , Reynolds, R. C. , Inati, S. K. , & Theodore, W. H. (2020). Language lateralization from task‐based and resting state functional MRI in patients with epilepsy. Human Brain Mapping, 41(11), 3133–3146. 10.1002/hbm.25003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondina, J. M. , Ferreira, L. K. , de Souza Duran, F. L. , Kubo, R. , Ono, C. R. , Leite, C. C. , … Busatto, G. F. (2018). Selecting the most relevant brain regions to discriminate Alzheimer's disease patients from healthy controls using multiple kernel learning: A comparison across functional and structural imaging modalities and atlases. NeuroImage: Clinical, 17, 628–641. 10.1016/j.nicl.2017.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau, P. N. , La Piana, R. , Chai, X. J. , Chen, J. K. , Klein, D. , & Tampieri, D. (2019). Brain functional organization and structure in patients with arteriovenous malformations. Neuroradiology, 61(9), 1047–1054. 10.1007/s00234-019-02245-6 [DOI] [PubMed] [Google Scholar]
- Siok, W. T. , Jin, Z. , Fletcher, P. , & Tan, L. H. (2003). Distinct brain regions associated with syllable and phoneme. Human Brain Mapping, 18(3), 201–207. 10.1002/hbm.10094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon, R. A. , & Connolly, E. S., Jr. (2017). Arteriovenous malformations of the brain. The New England Journal of Medicine, 376(19), 1859–1866. 10.1056/NEJMra1607407 [DOI] [PubMed] [Google Scholar]
- Starowicz‐Filip, A. , Chrobak, A. A. , Moskala, M. , Krzyzewski, R. M. , Kwinta, B. , Kwiatkowski, S. , … Przewoznik, D. (2017). The role of the cerebellum in the regulation of language functions. Psychiatria Polska, 51(4), 661–671. 10.12740/PP/68547 [DOI] [PubMed] [Google Scholar]
- Stockert, A. , Wawrzyniak, M. , Klingbeil, J. , Wrede, K. , Kummerer, D. , Hartwigsen, G. , … Saur, D. (2020). Dynamics of language reorganization after left temporo‐parietal and frontal stroke. Brain, 143(3), 844–861. 10.1093/brain/awaa023 [DOI] [PubMed] [Google Scholar]
- Teki, S. , Barnes, G. R. , Penny, W. D. , Iverson, P. , Woodhead, Z. V. , Griffiths, T. D. , & Leff, A. P. (2013). The right hemisphere supports but does not replace left hemisphere auditory function in patients with persisting aphasia. Brain, 136(Pt 6), 1901–1912. 10.1093/brain/awt087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Perrone‐Bertolotti, M. , Jobard, G. , Mazoyer, B. , & Baciu, M. (2017). Multi‐factorial modulation of hemispheric specialization and plasticity for language in healthy and pathological conditions: A review. Cortex, 86, 314–339. 10.1016/j.cortex.2016.05.013 [DOI] [PubMed] [Google Scholar]
- Wilke, M. , & Lidzba, K. (2007). LI‐tool: A new toolbox to assess lateralization in functional MR‐data. Journal of Neuroscience Methods, 163(1), 128–136. 10.1016/j.jneumeth.2007.01.026 [DOI] [PubMed] [Google Scholar]
- Wilmskoetter, J. , Marebwa, B. , Basilakos, A. , Fridriksson, J. , Rorden, C. , Stark, B. C. , … Bonilha, L. (2019). Long‐range fibre damage in small vessel brain disease affects aphasia severity. Brain, 142(10), 3190–3201. 10.1093/brain/awz251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhuisen, L. , Thiel, A. , Schumacher, B. , Kessler, J. , Rudolf, J. , Haupt, W. F. , & Heiss, W. D. (2005). Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: A combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke, 36(8), 1759–1763. 10.1161/01.STR.0000174487.81126.ef [DOI] [PubMed] [Google Scholar]
- Wu, C. Y. , Ho, M. H. , & Chen, S. H. (2012). A meta‐analysis of fMRI studies on Chinese orthographic, phonological, and semantic processing. NeuroImage, 63(1), 381–391. 10.1016/j.neuroimage.2012.06.047 [DOI] [PubMed] [Google Scholar]
- Yang, J. , & Tan, L. H. (2019). Whole‐brain functional networks for phonological and orthographic processing in Chinese good and poor readers. Frontiers in Psychology, 10, 2945. 10.3389/fpsyg.2019.02945 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 The demographic and lesion data of AVM patients.
Table S2: Pearson correlation coefficients between 2 ROIs and 13 VOIs in the AVM group and control group.
Table S3: Results of Pearson correlation between lesion size and LI in three tasks, and ANOVA test results of LIs among the three subgroups.
Data Availability Statement
Data availability statement: Some or all data used in the study are available from the corresponding author by request.


