Abstract
Smoking is the most important preventable cause of morbidity and mortality worldwide. Recent genome-wide association studies highlighted a human haplotype on chromosome 15 underlying the risk for tobacco dependence and lung cancer. Several polymorphisms in the CHRNA3-CHRNA5-CHRNB4 cluster coding for the nicotinic acetylcholine receptor (nAChR) α3, α5 and β4 subunits were implicated. In mouse models, we define a key role in the control of sensitivity to nicotine for the α5 subunit in dopaminergic (DAergic) neurons of the ventral tegmental area (VTA). We first investigated the reinforcing effects of nicotine in drug-naive α5−/− mice using an acute intravenous nicotine self-administration task and ex vivo and in vivo electrophysiological recordings of nicotine-elicited DA cell activation. We designed lentiviral re-expression vectors to achieve targeted re-expression of wild-type or mutant α5 in the VTA, in general, or in DA neurons exclusively. Our results establish a crucial role for α5*-nAChRs in DAergic neurons. These receptors are key regulators that determine the minimum nicotine dose necessary for DA cell activation and thus nicotine reinforcement. Finally, we demonstrate that a single-nucleotide polymorphism, the non-synonymous α5 variant rs16969968, frequent in many human populations, exhibits a partial loss of function of the protein in vivo. This leads to increased nicotine consumption in the self-administration paradigm. We thus define a critical link between a human predisposition marker, its expression in DA neurons and nicotine intake.
Keywords: dopamine system, human polymorphisms, in vivo electrophysiology, lentiviral vectors, mouse models, nicotinic receptor, nicotine self-administration, smoking
INTRODUCTION
Nicotine addiction is the single most important preventable cause of morbidity and mortality worldwide (World Health Organization, http://www.who.int/tobacco/statistics/tobacco_atlas/en/). Current smoking cessation medications are only moderately successful and novel drug targets need to be defined.1,2 Genome-wide association studies have recently identified a strong link between increased vulnerability to tobacco addiction and risk of lung cancer in humans, and a haplotype on chromosome 15 encompassing the CHRNA3/A5/B4 gene cluster coding for subunits of the nicotinic acetylcholine receptor (nAChR).1–5
These subunits are expressed in discrete regions of the mammalian central nervous system, including the cerebral cortex, cerebellum, thalamus, striatum, hippocampus, substantia nigra, interpeduncular nucleus, ventral tegmental area (VTA) and medial habenula (mHb).6–9 Among these structures, VTA, mHb and interpeduncular nucleus are of particular interest.10 Recent evidence indicates a critical involvement of the α5*-nAChR subunit in neurons connecting the mHb to the interpeduncular nucleus (habenulo-interpeduncular pathway) in two rodent models of nicotine addiction. This pathway is thought to be implicated in signaling the aversive properties of nicotine.8,11 It is well established that nicotine shares with other addictive drugs the ability to activate the dopaminergic (DAergic) neurons of the VTA. Those neurons project to, and release DA in the nucleus accumbens (mesolimbic DA neurons), an effect underlying their rewarding and addictive properties of drugs of abuse.1,12–14 Although the α5 subunit is expressed in 80% of DAergic neurons,7,9 there are no data so far implicating α5 in VTA DA neuron function or reinforcement.
MATERIALS AND METHODS
Full Methods and any associated references are available in the Supplementary Information.
Subjects
Male C57BL/6 J (Charles River, L’Arbresle, France), α5 nAChR knockout15 (α5−/−) mice and their corresponding wild-type (WT) controls were used, weighing 24–28 g.
Drugs
For all experiments, (–)-nicotine bitartrate (Sigma, Milan, Italy) was freshly dissolved in 0.9% saline, pH adjusted to 7.4 ± 0.1 (nicotine concentration, μg kg−1 per infusion free base). Dimethylphenylpiperazinium (DMPP, Sigma) was used at a concentration of 100 μM.
Nicotine self-administration task (intravenous self-administration task)
Nicotine-naive mice were tested in pairs as previously described.16,17
In vivo electrophysiological recordings of VTA DA neurons
Single-unit extracellular recordings and nicotine tartrate injection into the saphenous vein were performed as described.14,18,19
Ex vivo electrophysiological recordings of VTA DA neurons
Slice recordings were performed as detailed in Supplementary Information.
Lentiviral expression vectors
Mice aged 10–12 weeks were injected bilaterally into the VTA as detailed in Supplementary Information. The lentiviral expression vectors are derived from the pHR’s expression vectors first described by Naldini et al.,20 with several subsequent modifications.19,21,22 To create the conditional lentivectors, a previously described sub-cloning strategy was used.14,19,21,22
Data analysis
Behavioral data.
The number of nose pokes (NPs) for both A and P mice in each treatment group was analyzed, first with a Shapiro test, then with two-way analysis of variance to evaluate effects of the drug delivery mode, unit dose and interactions between group and drug dose. Student’s t-tests were used for post hoc comparisons. The whole study was designed as a between-subjects (independent groups) experiment, because each treatment was performed on a single set of animals. Differences between the self-administration profiles of the α5−/−-Lv-α5WT and α5−/−-Lv-α5SNP (single-nucleotide polymorphism) mice were evaluated using the Student’s t-test with repeated Bonferroni corrections.
Electrophysiological data.
DA cell firing was analyzed with respect to the average firing rate and the percentage of spikes within a burst.14,18,19,23,24 To quantify nicotine effects, we determined the maximum of fluctuation on a 3-min period before and after injection. To study differences between WT and α5−/− mouse dose-response curves, we used the Kruskall–Wallis non-parametric test. For α5−/−-Lv-α5WT and α5−/−-Lv-α5SNP analyses, we used a Wilcoxon non-paired test with Bonferroni corrections. For all analyses, statistical significance was set at P<0.05.
RESULTS
Nicotine is the principal psychoactive component in tobacco smoke that drives continued addiction.18,25 It exerts its reinforcing effects through its action on neuronal nAChRs, a family of pentameric ligand-gated ion channels.1,17,21,26 Recent genome-wide association studies identified a series of polymorphisms composing a human haplotype. Among them, the rs16969968 SNP leads to a substitution of aspartic acid 398 by asparagine (D398N) in the human α5 subunit.5 It represents the only non-synonymous variant. In heterologous expression systems, the resulting protein exhibits diminished calcium permeability in high-affinity α4α5β2*-nAChRs.5,27 Experimental evidence supports the potential role of α5*-nAChRs in smoking behavior, as mice lacking functional α5*-nAChRs (α5−/−) are less sensitive to nicotine-elicited behaviors.15,28,29 They also exhibit increased self-administration of high nicotine doses in a chronic procedure that assesses the maintenance of drug taking.8 We have used an acute intravenous self-administration task (IVSA), schematically presented in Figure 1a, to address the initiation of drug-taking behavior.17 In this paradigm, both the ‘active’ (A) and the ‘passive’ (P) drug-naive mice are exposed at the same time to the same amount of nicotine that is injected contingently on each NP of the A mouse. Thus, the A mouse is able to associate its NP activity with nicotine delivery, whereas the P mouse is not.
Figure 1.
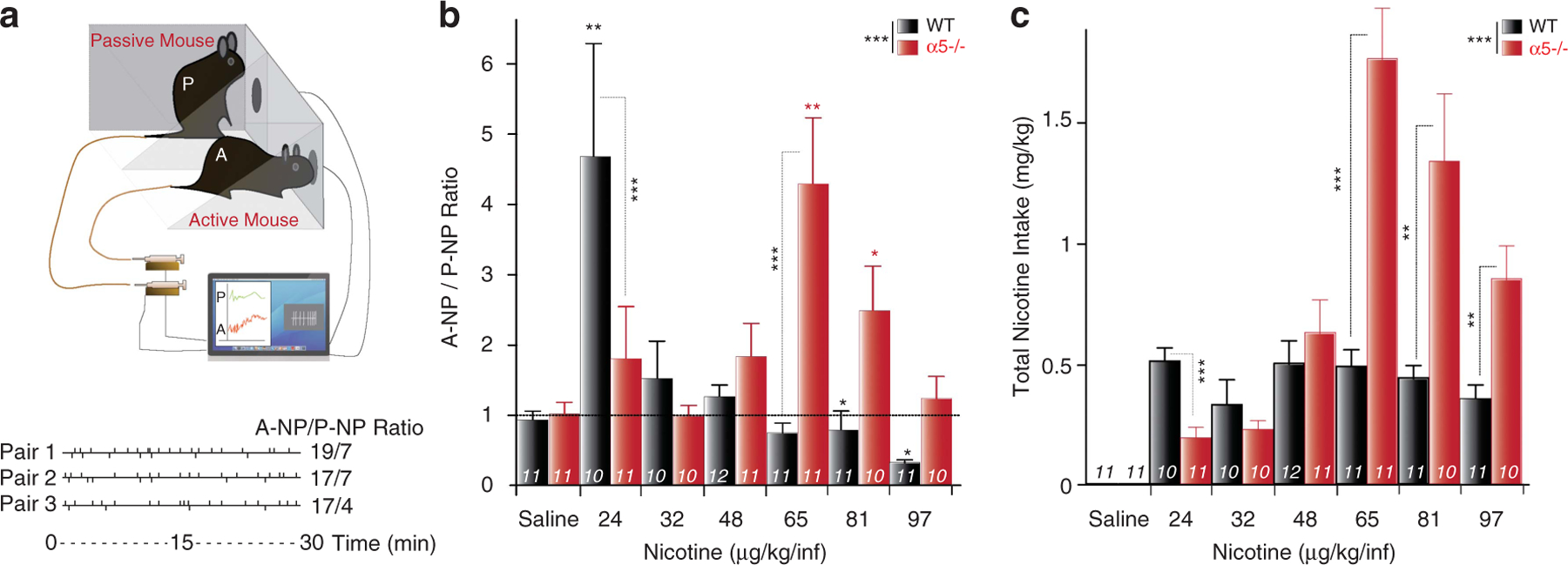
Critical role of the α5 subunit in intravenous self-administration task (IVSA). (a) IVSA set-up. Top: Scheme of the set-up for the intravenous self-administration task (IVSA). Mice are tested in pairs. The active mouse (A) is tested for nicotine reinforcement, the passive mouse (P) is used as a control mouse. Each nose poke (NP) of the A mouse activates a computer-operated syringe pump that delivers a nicotine injection into the tail vein of both the A and the yoked P mouse. Bottom: Event records from three representative paired mice during nicotine IVSA sessions. The vertical deflections above the horizontal line mark the time of each individual NP of the active mouse (A-NP), whereas each deflection below the line represents NP of the passive mouse (P-NP), over the 30-min experimental session. (b, c) α5−/− mice shift to higher doses in IVSA. (b) Mean ± s.e.m. A-NP/P-NP ratio for wild-type (WT; black) and α5−/− (red) mice self-administering different nicotine concentrations (μg kg−1 per infusion). (c) Mean ± s.e.m of total nicotine intake (mg kg−1) by A mice. ***P<0.001 WT vs α5−/−, analysis of variance (ANOVA). *P<0.05, **P<0.01 vs yoked P mice, Student t-test. Number of mice tested is indicated for each group.
Under these conditions, a nicotine concentration of 24 μg kg−1 per infusion significantly increased nicotine self-administration in the WT A mice. In contrast, the same behavior required 65 μg kg−1 per infusion nicotine for A α5−/− mice (Figure 1b). When we considered the cumulative amount of nicotine self-administered during the 30-min IVSA session, WT mice adjusted their NP activity rate according to the different nicotine concentrations tested. Although WT mice self-administered fairly constant amounts of the drug, α5−/− mice did not (Figure 1c). We previously showed that systemic nicotine reinforcement in drug-naive mice is under the control of high-affinity nicotinic receptors in the VTA.17,21 The α5 subunit is strongly expressed in this DAergic nucleus underlying nicotine reinforcement.7,9,14,30
In order to address the role of the α5 subunit specifically in the VTA, we used a lentiviral re-expression vector to transduce the WT or polymorphic α5 subunit in the VTA of α5−/− mice (Figures 2a (top), b, c), designated respectively α5−/−-Lv-α5WT and α5−/−-Lv-α5SNP mice. As in WT mice, a nicotine concentration of 24 μg kg−1 per infusion significantly increased nicotine IVSA in α5−/−-Lv-α5WT A mice (Figure 3a, Supplementary Figures S1a and b). We observed a rightward shift of the IVSA dose-response curve between the α5−/−-Lv-α5WT and the α5−/−-Lv-α5SNP mice, as α5−/−-Lv-α5SNP animals started to self-administer nicotine at the 32 μg kg−1 per infusion dose, which is intermediate between the α5−/−-Lv-α5WT (24 μg kg−1 per infusion) and the α5−/− mice (65 μg kg−1 per infusion) (Figure 3a). We calculated the total nicotine intake that remained once again fairly constant in α5−/−-Lv-α5WT mice, but not in α5−/−-Lv-α5SNP mice, with a peak at 65 μg kg−1 per infusion as for knockout mice. As expected, re-expressing enhanced green fluorescent protein (eGFP) in the VTA of α5−/− mice did not result in any modification of behaviour (Supplementary Figure S1c).
Figure 2.

Re-expression of wild-type (WT; α5−/−-Lv-α5WT) and polymorphic (α5−/−-Lv-α5SNP) α5 in the ventral tegmental area (VTA) of α5−/− mice. (a) Scheme of lentiviral vectors, see Supplementary Information for details. (b) Localization of lentivirus reporter gene eGFP expression in VTA. Arrowheads indicate tyrosine hydroxylase (red) and eGFP (green) co-expression by DA cells. (c) Example of a recorded neuron (single plane): tyrosine hydroxylase, eGFP and biocytine identify, respectively, DA cells (red), the neuron re-expressing the α5 subunit (green), and a recorded cell (blue). eGFP, enhanced green fluorescent protein.
Figure 3.
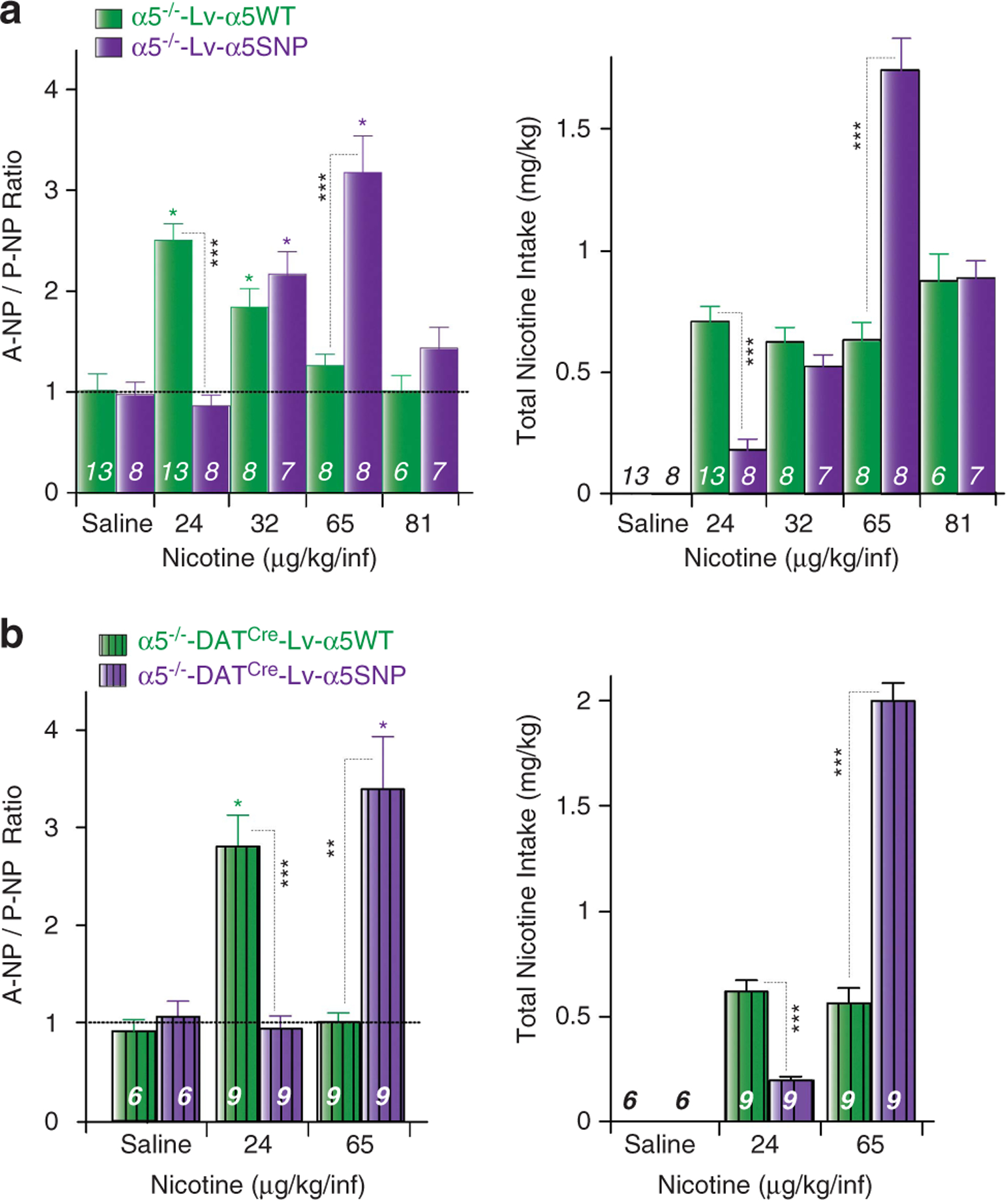
Intravenous self-administration task (IVSA) is restored by α5WT, but not α5SNP re-expression in DA neurons. (a) (Left) Mean ± s.e.m A-NP/P-NP ratio for α5−/−-Lv-α5WT (green) and α5−/−-Lv-α5SNP (purple) mice acutely self-administering nicotine (μg kg−1 per infusion). (Right) Mean ± s.e.m of total nicotine intake (mg kg−1) by A mice. (b) (Left) Mean ± s.e.m A-NP/P-NP ratio for α5−/−-DATCre-Lv-α5WT (green) and α5−/−-Lv-α5−/−-DATCre-Lv-α5SNP (purple) mice acutely self-administering nicotine (μg kg−1 per infusion). (Right) Mean ± s.e.m of total nicotine intake (mg kg−1) by A mice. ***P<0.001, **P<0.01, *P<0.05, Student’s t-test. n, number of recorded of mice tested.
Thereafter, to address and ascertain the specific role of α5*-nAChRs in VTA DA cells, a DA cell-specific expression system was generated similar to Tolu et al.14,22 α5−/−-DATCre mice were generated by crossing α5−/− with DAT-Cre expressing transgenic mice. These mice were injected with a Cre recombinase-dependent conditional lentiviral expression vector to drive α5WT or α5SNP exclusively in VTA DA neurons (Figure 2a, bottom). Selective re-expression of WT α5 subunit in VTA DA neurons was sufficient to induce nicotine reinforcement for a low nicotine dose. We also observed a rightward shift of the IVSA dose-response curve between α5−/−-DATCre-Lv-α5WT and α5−/−-DATCre-Lv-α5SNP mice (Figure 3b). The first line self-administered nicotine at the 24 μg kg−1 per infusion dose, but not the second. Conversely, the α5−/−-DATCre-Lv-α5SNP mice self-administered nicotine at the 65 μg kg−1 per infusion dose, whereas α5−/−-DATCre-Lv-α5WT did not, similar to the results obtained with the generalized re-expression in the VTA (see above). When total nicotine intake was examined, mice expressing WT α5 in DA cells self-administered constant amount of nicotine, whereas mice expressing the α5 SNP did not. The amounts self-administered at the 24 and 65 μg kg−1 per infusion doses were identical between mice with either generalized or DA-specific expression.
These clear-cut behavioral data made us aware that the α5 would have a major role specifically in DA neurons, and that the human polymorphism alters this function. Therefore, DA cell responses to nicotine were assessed by in vivo electrophysiological recordings. We first analyzed the spontaneous activity of VTA DA cells in WT and mutant mice, and found no differences in the spontaneous firing rate or percentage of spike within bursts (Supplementary Figures S2a and b). Systemic administration of nicotine resulted in a rapid and pronounced increase in firing rate in both WT and α5−/− mice (Figure 4a). In both cases, the nicotine-elicited mean firing rate increased with nicotine concentration (Figure 4b). However, while 15 μg kg−1 nicotine was sufficient to trigger an increase of WT DA neuron firing frequency, 120 μg kg−1 nicotine was necessary to activate α5−/− DA cells (Figure 4c). This result correlates with the shift observed in the nicotine IVSA task. Similar results were obtained when analyzing nicotine-elicited modification of bursting activities (Supplementary Figures S2c and d). Thus, α5*-nAChRs are key in defining the minimal nicotine dose initiating a DA response and thus reinforcement.
Figure 4.
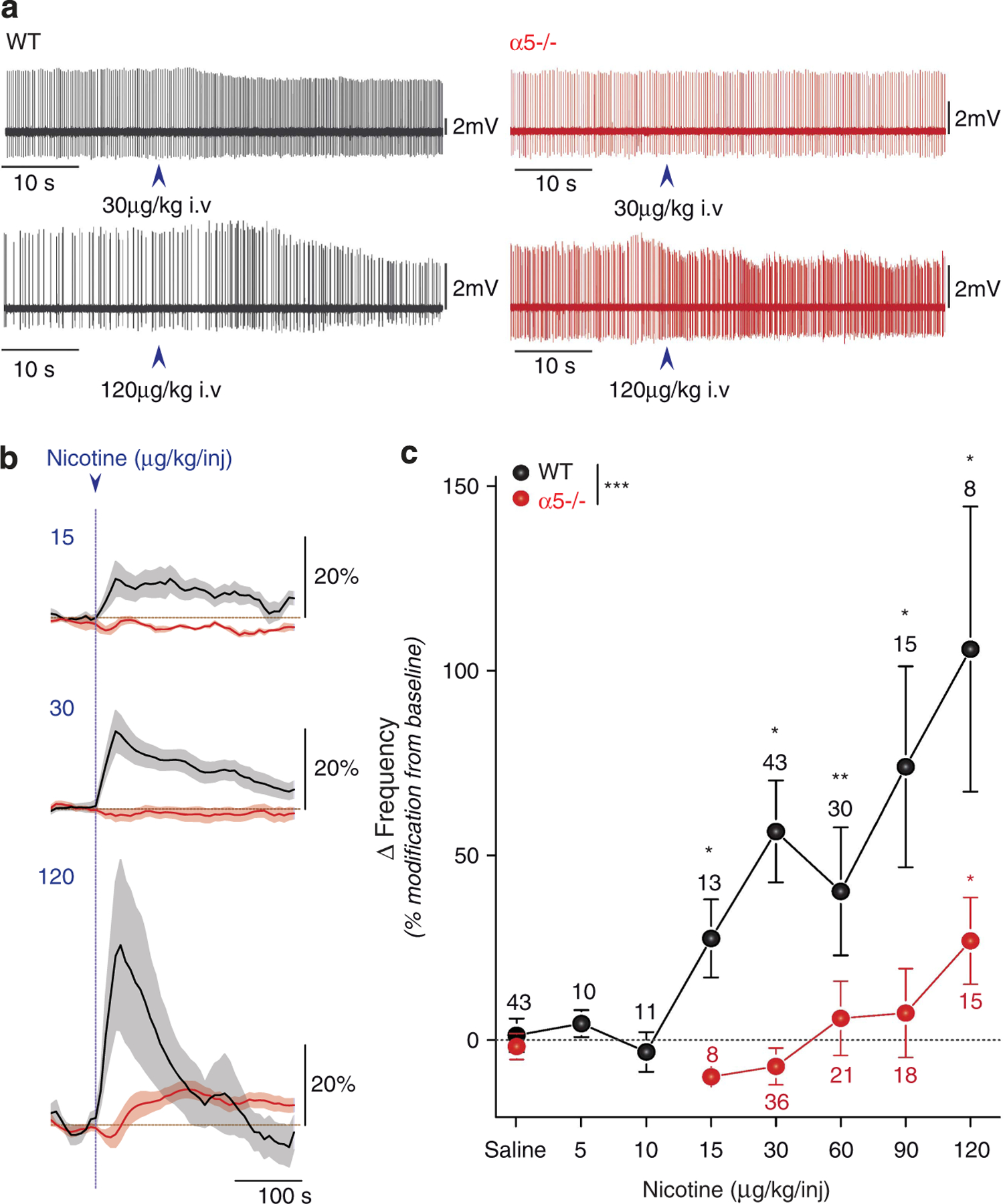
Nicotine-elicited increase in ventral tegmental ares (VTA) DA cell firing is shifted to higher doses in α5−/− mice. (a) Typical electrophysiological recording depicting the changes in firing pattern elicited by 30 μg kg−1 or 120 μg kg−1 intravenous (i.v.) nicotine injection (arrow) in wild-type (WT) and α5−/− mice. (b) Mean ± s.e.m DA cell firing frequency increase after injection of the indicated nicotine concentration, in WT and α5−/− mice. (c) Rightward shift in the dose-response curve of nicotine-elicited DA cell activation in α5−/− mice. Mean ± s.e.m of increased variation from baseline in firing frequency for WT and α5−/− mice injected with the indicated nicotine concentrations. WT: black; α5−/−: red. ***P<0.001, Kruskall–Wallis test; *P<0.05, **P<0.01 Wilcoxon test. n, number of recorded neurons.
We then analyzed the mice with targeted re-expression of WT and SNP α5 subunit. First, to exclude any potential effect of the SNP in the translation, trafficking, surface expression or affinity to nicotine of the ‘mutant’ subunit, we carried out slice electro-physiology on vectorized mice. As expected, α5−/−-Lv-α5WT mice displayed a WT profile in response to a saturating dose of DMPP (100 μM) (Figure 5a). At a saturating dose of DMPP (100 μM), no differences in amplitude of current were observed between WT, α5−/−-Lv-α5WT and α5−/−-Lv-α5SNP (Figure 5a) mice. This serves as a control to confirm comparable amounts of α5 protein expression between each lentivector used.
Figure 5.

Specific re-expression of α5*-nAChRs in ventral tegmental area (VTA) cells. (a) (Left) DMPP-evoked currents in DA cells. (Right) DA cell mean ± s.e.m of current amplitude in response to dimethylphenylpiperazinium (DMPP; 100 μM) in wild-type (WT), α5−/−-Lv-α5WT, α5−/−-Lv-α5SNP, α5−/−-Lv-α5GFP, α5−/−-DATCre-Lv-α5WT, α5−/−-DATCre-Lv-α5SNP and α5−/− mice. (b) (Top) Electrophysiological recording depicting the changes in firing pattern elicited by 15 and 30 μg kg−1 intravenous (i.v.) nicotine injection (arrow) in α5−/−-DATCre-Lv-α5WT and α5−/−-DATCre-Lv-α5SNP mice. (Middle) Mean ± s.e.m DA cell firing frequency increase after injection of the indicated nicotine concentration in α5−/−-Lv-α5SNP, α5−/−-DATCre-Lv-α5WT and (bottom) α5−/−-DATCre-Lv-α5WT and α5−/−-DATCre-Lv-α5SNP mice. (c) Mean ± s.e.m of maximum DA cell firing rate elicited by the indicated nicotine concentration in WT, α5−/−-Lv-5WT, α5−/−-Lv-α5SNP, α5−/−-Lv-α5SNP, α5−/−-DATCre-Lv-α5WT, α5−/−-DATCre-Lv-α5SNP and α5−/− mice. WT: black; α5−/−-Lv-α5WT: green; α5−/−-Lv-α5SNP: purple; α5−/−-Lv-α5GFP: blue; α5−/−-DATCre-Lv-α5WT: striped-green; α5−/−-DATCre-Lv-α5SNP: striped-purple; α5−/−: red. *P<0.05, Wilcoxon test, ***P<0.001, Wilcoxon test with Bonferroni corrections. n, number of recorded neurons.
We then analyzed the mice with generalized VTA expression. First of all, re-expressing eGFP in the VTA of α5−/− mice did not result in any modification of the nicotine-evoked response (Supplementary Figure S3a). Similar to WT mice, α5−/−-Lv-α5WT mice exhibited an increase in firing (Figure 5b) and bursting (Supplementary Figure S3b) in response to a 15 μg kg−1 nicotine dose. Confirming this phenotype, α5−/−-Lv-α5SNP DA cells required twice the nicotine dose to exhibit activation in response to an injection compared with cells from α5−/−-Lv-α5WT mice (Figure 5b and Supplementary Figure S3b for bursting analysis). Thus, the D398N SNP, a risk factor for nicotine dependence and lung cancer, induces a partial loss of function in the DA system. We then confirmed that DA neuron-specific expression of WT and mutant α5 is sufficient to confer the same response profiles. In vivo recordings of DA cells showed that twice the dose of nicotine is required to elicit DA cell activation for α5−/−-DATCre-Lv-α5SNP as compared with α5−/−-DATCre-Lv-α5WT mice, similar to the results obtained using ubiquitous expression in the VTA (Figure 5c and Supplementary Figure S3c for burst analysis).
These observations support our general conclusion that α5*-nAChRs specifically in VTA DA cells drive sensitivity to nicotine reward. In addition, α5 SNP expression leads to a partial loss of function, thereby increasing the dose of nicotine that animals perceive as rewarding. Our data establish that α5*-nAChRs located in the VTA are crucial determinant of the minimal dose for nicotine reinforcement, and hence nicotine intake.
DISCUSSION
We have comprehensively analyzed the role of the α5 nAChR subunit in the DA system. Using ex vivo and in vivo models for electrophysiological recordings and nicotine self-administration behavior, we demonstrate that the α5 subunit has a critical role in defining the sensitivity of the VTA DA system to nicotine. This has a direct and immediate consequence for nicotine reinforcement. Furthermore, we demonstrate a direct link between the α5SNP, which induces a partial loss of function and increased nicotine intake.
An unexpected role for the α5 subunit
We have defined novel major, largely unexpected roles for α5 nAChR subunit. Although it does not contribute to the nicotine binding site,26,31,32 as it can function only as an accessory subunit, its deletion leads to a dramatic shift in several nicotine-elicited functions in the brain. This loss of function can be demonstrated by changes in slice and in vivo electrophysiological responses to nicotine or nicotinic agonist exposure. A dramatic shift to high nicotine doses in an IVSA paradigm is also observed. These outcomes are entirely dependent on the presence of α5 in VTA DA cells. Normal responses are restored by targeted, generalized lentiviral re-expression in all VTA cells, but also in VTA DA cells, specifically. These data are in accordance with the finding that in the VTA, α5 mRNA is significantly more prevalent in DA cells than in GABA cells.7 They also clearly demonstrate that, surprisingly, α4β2 nAChRs that do not comprise α5 have a minor role in the nicotine-evoked response of VTA DA cells, and that the functional contribution of α5 is critical. This is a novel, specific role for a nicotinic receptor subunit. Previous analyses of nAChR genes, for example β2, α4 or α6, indicated an all-or-none phenotype associated with a receptor subunit, that is, the function was entirely lost.14,17,19,33 Alpha5 is different in the sense that, while not essential for receptor function, it can powerfully ‘modulate’ the nicotine sensitivity of the DAergic system.
Relation to previous work
Our work complements and considerably extends previous studies that begun to dissect the frequent human haplotype on chromosome 15. Fowler et al.,8,34 observed that α5−/− mice exhibit an increase in IVSA for high nicotine doses, but no modifications at low doses, and found no strong evidence for a role in reward. They proposed that α5*-nAChRs in the mHb trigger an inhibitory motivational signal limiting nicotine intake of high nicotine doses. Together with our previous work,35 those results potentially identify the habenular receptors as α3α5ß4*-nAChRs, although careful immunoprecipitation studies only found low amounts of α5 in this nucleus.36 Here, we analyzed a different receptor combination in VTA. We demonstrate, using different approaches, that α5−/− mice exhibit a decreased sensitivity of the DAergic system to acute nicotine injection. Therefore, α5*-nAChRs expressed in VTA DA neurons are crucial for the control of the minimum nicotine dose necessary for DAergic activation, and thus nicotine reinforcement in nicotine-naive mice. This implies that the α5 subunit has convergent roles in two different brain structures. It defines high-affinity responses to nicotine in both the mHb and the VTA DAergic system. The human SNP ‘linked’ to nicotine intake thus has a dual role. First, its presence in the DA system increases the minimum nicotine concentration necessary for the activation of DA neurons that underlies the reinforcing effects associated with smoking. At the same time, the partial loss of function in mHb neurons reduces the aversive properties of high nicotine doses and promotes continued use once dependence is established.
Deletion of the α5 subunit resulted in a ‘loss of control’ of nicotine consumption at high doses, a behavior restored by selective re-expression of α5 in VTA DA cells. Our recordings of VTA DA cell activity evoked by nicotine do not reveal a population responding with an inverted U-shaped curve, that is, an excitation by low doses and an inhibition by high doses of the drug. Specific populations that drive or specifically code for high doses of nicotine have thus not been identified. This is, however, not the only possible mechanism. Indeed, DA neuron firing is not the sole determinant of reinforcing or aversive effects of a drug. One quintessential feature is the actual amount of DA that is released. For nicotine, this is not alone determined by DA neuron firing but also by presynaptic nicotinic receptors in DA terminals,19 and in particular α5* nAChRs in DA terminals of the dorsal striatum.37 Finally, the effects of DA are also determined by its action on the postsynaptic cell, the medium spiny neurons of the striatum or other cells in different target regions. Low or high release of DA would differentially recruit D1- and D2-type postsynaptic receptors, thus eliciting different behaviors.38
Dissecting the role of a human non-synonymous variant
Beirut et al.5 provided the initial analysis of the functional consequences potentially associated with the α5SNP on α4α5β2*-nAChRs. The incorporation of α5SNP into HEK293T cells transfected with α4β2 cDNA reduces the maximal response to a nicotinic agonist without altered α4α5β2*-nAChRs surface expression.5 Also, Kuryatov et al.27 reported that the α5SNP lowers Ca2+ permeability and increases acute desensitization in (α4β2)2α5 nAChRs expressed in Xenopus oocytes. We have previously elucidated the roles of α4 and β2 nAChRs, the main partners of α5 within the VTA, in nicotine reinforcement.14,19,21 Numerous studies in slices from the DAergic system, such as Tsuneki et al.,31 describe how nicotinic receptors, particularly α4ß2*-nAChRs and thus α4α5ß2 nAChRs, mobilize extracellular and intracellular calcium in DA cells in response to nicotine exposure. Kitai and collaborators39 have long demonstrated a crucial role for calcium and sodium in the slow oscillation potential and firing excitability of DA neurons. In accordance with all these evidences, our expression of the SNP in the VTA results in a partial loss of α4β2α5* nicotine-evoked function, and yields intermediate behavioral and electrophysiological phenotypes compared with those of the α5−/− mice.
Extending our findings to humans, it is known that smokers manipulate their dose of nicotine on a puff-by-puff basis to reach an optimal blood concentration that produces the desired reinforcing effect and satisfactory experience.25 Our results reveal that, despite a similar response at saturating doses in slices, we could observe a partial loss of function generated by expression of polymorphic α5*-nAChRs in α5−/− mice rather than a complete inactivation. Overall, it suggests that humans expressing the D398N risk allele may smoke more because the optimal nicotine concentration required to activate the DAergic system is higher.
Implications for drug design
When analyzing nicotine dependence, experimental research has been extensively dedicated to ß2*-nAChRs and its main partners, α6 and α4, and the homopentameric α7*-nAChRs in the DAergic system.9,14,17,19,30,33,40 Here the α5 subunit emerges as a key determinant for sensitivity to nicotine in DA neurons. This means that the crucial pentamer responsible for nicotine effects in the cell bodies of the VTA does contain the α5 subunit in addition to previously described partners. Our work shows for the first time that a nicotinic subunit is clearly associated with a shift in the sensibility to nicotine-evoked activity. This underlies the mechanism for the critical impact of SNPs on this functional modulatory subunit. This finding should pave the way for ‘personalized’ smoking cessation medication targeting the polymorphic α5 subunit, for example, with a positive allosteric modulator. This strategy could restore the partial loss of function of α5 in the reward system in heterozygous or homozygous carriers.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Stefania Tolu for helpful comments on the manuscript. This work was supported by the Institut Pasteur, Centre National de la Recherche Scientifique CNRS UMR 3571, UMR 7102 and ATIP programme, the Agence Nationale pour la Recherche (ANR Neuroscience, Neurologie et Psychiatrie 2009, and ANR BLANC 2012), la Fondation pour la Recherche Médicale (FRM, équipe 2013 PF), fondation pour la the Neuropole de Recherche Francilien (NeRF) of Ile de France, the Bettencourt Schueller Foundation, National Cancer Institute INCa BIO-SILC programme, Ecole des Neurosciences de Paris (ENP), FP7 ERANET Neuron NICO-GENE network, LabEx GENMED funded by ANR, and NIH grants DA029157 and U19CA148127. This work was supported by the Department of Biomedical Sciences, Division of Neuroscience and Clinical Pharmacology, University of Cagliari, Italy. The laboratories of Philippe Faure, Uwe Maskos and Bertrand Lambolez are part of the École des Neurosciences de Paris Ile-de-France RTRA network. PF and UM are members of the Laboratory of Excellence, LabEx Bio-Psy.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Changeux J-P. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci 2010; 11: 389–401. [DOI] [PubMed] [Google Scholar]
- 2.De Biasi M, Dani JA. Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci 2011; 34: 105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci 2004; 24: 10035–10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JC, Cruchaga C, Saccone NL, Bertelsen S, Liu P, Budde JP et al. Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet 2009; 18: 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 2008; 165: 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I et al. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol 2009; 78: 703–711. [DOI] [PubMed] [Google Scholar]
- 7.Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 2001; 21: 1452–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature 2011; 471: 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee S, Santos N, Holgate J, Haass-Koffler CL, Hopf FW, Kharazia V et al. The α5 subunit regulates the expression and function of α4*-containing neuronal nicotinic acetylcholine receptors in the ventral-tegmental area. PLoS ONE 2013; 8: e68300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuesta LM, Fowler CD, Kenny PJ. Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochem Pharmacol 2011; 82: 984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci 2009; 29: 3014–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Ciano P, Everitt BJ. Contribution of the ventral tegmental area to cocaine-seeking maintained by a drug-paired conditioned stimulus in rats. Eur J Neurosci 2004; 19: 1661–1667. [DOI] [PubMed] [Google Scholar]
- 13.Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 1996; 382: 255–257. [DOI] [PubMed] [Google Scholar]
- 14.Tolu S, Eddine R, Marti F, David V, Graupner M, Pons S et al. Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol Psychiatry 2012; 18: 382–393. [DOI] [PubMed] [Google Scholar]
- 15.Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol 2003; 63: 1059–1066. [DOI] [PubMed] [Google Scholar]
- 16.Martellotta MC, Kuzmin A, Zvartau E, Cossu G, Gessa GL, Fratta W. Isradipine inhibits nicotine intravenous self-administration in drug-naive mice. Pharmacol Biochem Behav 1995; 52: 271–274. [DOI] [PubMed] [Google Scholar]
- 17.Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci 2008; 28: 12318–12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marti F, Arib O, Morel C, Dufresne V, Maskos U, Corringer P-J et al. Smoke extracts and nicotine, but not tobacco extracts, potentiate firing and burst activity of ventral tegmental area dopaminergic neurons in mice.. Neuropsychopharmacology 2011; 36: 2244–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S et al. Distinct contributions of nicotinic acetylcholine receptor subunit alpha4 and subunit alpha6 to the reinforcing effects of nicotine. Proc Natl Acad Sci USA 2011; 108: 7577–7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996; 272: 263–267. [DOI] [PubMed] [Google Scholar]
- 21.Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux J-P et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 2005; 436: 103–107. [DOI] [PubMed] [Google Scholar]
- 22.Tolu S, Avale ME, Nakatani H, Pons S, Parnaudeau S, Tronche F et al. A versatile system for the neuronal subtype specific expression of lentiviral vectors. FASEB J 2010; 24: 723–730. [DOI] [PubMed] [Google Scholar]
- 23.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 1984; 4: 2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grace AA, Bunney BS.. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci 1984; 4: 2866–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol 2008; 49: 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albuquerque EX, Pereira EFR, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 2009; 89: 73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuryatov A, Berrettini W, Lindstrom J.. Acetylcholine receptor (AChR)Alpha5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (alpha4beta2)2alpha5 AChR function. Mol Pharmacol 2010; 79: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther 2008; 325: 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA et al. The role of alpha5 nicotinic acetylcholine receptors in the pharmacological and behavioral effects of nicotine in mice. J Pharmacol Exp Ther 2010; 334: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux J-P et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron 2006; 50: 911–921. [DOI] [PubMed] [Google Scholar]
- 31.Tsuneki H, Klink R, Léna C, Korn H, Changeux JP. Calcium mobilization elicited by two types of nicotinic acetylcholine receptors in mouse substantia nigra pars compacta. Eur J Neurosci 2000; 12: 2475–2485. [DOI] [PubMed] [Google Scholar]
- 32.Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature 1996; 380: 347–351. [DOI] [PubMed] [Google Scholar]
- 33.Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 1998; 391: 173–177. [DOI] [PubMed] [Google Scholar]
- 34.Fowler CD, Tuesta L, Kenny PJ. Role of α5* nicotinic acetylcholine receptors in the effects of acute and chronic nicotine treatment on brain reward function in mice. Psychopharmacology 2013; 229: 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S et al. Aversion to Nicotine Is Regulated by the Balanced Activity of beta4 and alpha5 Nicotinic Receptor Subunits in the Medial Habenula. Neuron 2011; 70: 522–535. [DOI] [PubMed] [Google Scholar]
- 36.Scholze P, Koth G, Orr-Urtreger A, Huck S. Subunit composition of α5-containing nicotinic receptors in the rodent habenula. J Neurochem 2012; 121: 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Exley R, McIntosh JM, Marks MJ, Maskos U, Cragg SJ. Striatal α5 nicotinic receptor subunit regulates dopamine transmission in dorsal striatum. J Neurosci 2012; 32: 2352–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci 2012; 15: 816–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitai S, Shepard P, Callaway J, Scroggs R. Afferent modulation of dopamine neuron firing patterns. Curr Opin Neurobiol 1999; 9: 1–8. [DOI] [PubMed] [Google Scholar]
- 40.Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 2000; 27: 349–357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


