Abstract
The trachea is a rigid air duct with some mobility, which comprises the upper region of the respiratory tract and delivers inhaled air to alveoli for gas exchange. During development, the tracheal primordium is first established at the ventral anterior foregut by interactions between the epithelium and mesenchyme through various signaling pathways, such as Wnt, Bmp, retinoic acid, Shh, and Fgf, and then segregates from digestive organs. Abnormalities in this crosstalk result in lethal congenital diseases, such as tracheal agenesis. Interestingly, these molecular mechanisms also play roles in tissue regeneration in adulthood, although it remains less understood compared with their roles in embryonic development. In this review, we discuss cellular and molecular mechanisms of trachea development that regulate the morphogenesis of this simple tubular structure and identities of individual differentiated cells. We also discuss how the facultative regeneration capacity of the epithelium is established during development and maintained in adulthood.
Keywords: differentiation, dorsal–ventral patterning, endodermal patterning, epithelial‐mesenchmal interaction, organogenesis, respiratory organ, trachea, trachea‐esophageal separation, tubulogenesis
Key Findings
Organogenesis, respiratory system, epithelial‐mesenchymal interaction, congenital diseases, regeneration.
1. INTRODUCTION
Healthy adult humans can exhale up to 6 L of air per second. 1 The establishment of trachea during development is essential for establishing this high ventilation capacity in adulthood. The trachea comprises the upper region of the respiratory tract in mammals and delivers inhaled air to the alveoli, which are saccular structures for gas exchange (Figure 1A). The developing trachea is shaped as a single tube with rigidity and some mobility and connects the larynx and the lower airway to segregate the respiratory system from digestive organs. Abnormal development of the trachea leads to lethal congenital diseases, such as tracheal agenesis and stenosis. 2 Mesenchymal tissues provide rigidity and some mobility to this organ by giving rise to cartilage and smooth muscles, which are located in ventral and dorsal regions of the trachea, respectively (Figure 1B). The tracheal epithelium acts as the primary physical barrier against various noxious substances. Tracheal integrity is maintained throughout life because of the presence of facultative stem cell populations, such as basal cells (Figure 1D). Primordial tracheal epithelium and mesenchyme are derived from the endodermal foregut 3 , 4 and the surrounding splanchnic mesoderm. They are segregated from the digestive tract through a dynamic cellular reorganization process called “trachea‐esophageal separation,” during which the cellular crosstalk between epithelial and mesenchymal cells is crucial for the spatiotemporal regulation of tissue deformation as well as cellular specification along anterior‐posterior (AP) and dorsoventral (DV) axes. The importance of cellular communication between the epithelium and mesenchyme was demonstrated using chimeric coculture experiments. 5 Fetal distal lung epithelium cocultured with tracheal mesenchyme led to the formation of a cystic structure instead of branching morphogenesis, which is characteristic of distal lung development for the formation of an airway network, indicating that mesenchymal cells can regulate epithelial cell identity. Since then, several studies have demonstrated the significance of the tightly regulated cellular interactions during tracheal development, assisted by advances in molecular biology, such as techniques for gene manipulation in the cell lineage of interest, lineage tracing, and comprehensive transcriptomic analysis at single‐cell resolution. However, several questions remain unanswered. For example, the mechanism by which mesenchymal cells, which acts as a niche for epithelial stem cell populations, 6 differentiate and mature during tracheal development and regeneration 7 , 8 is unclear.
FIGURE 1.
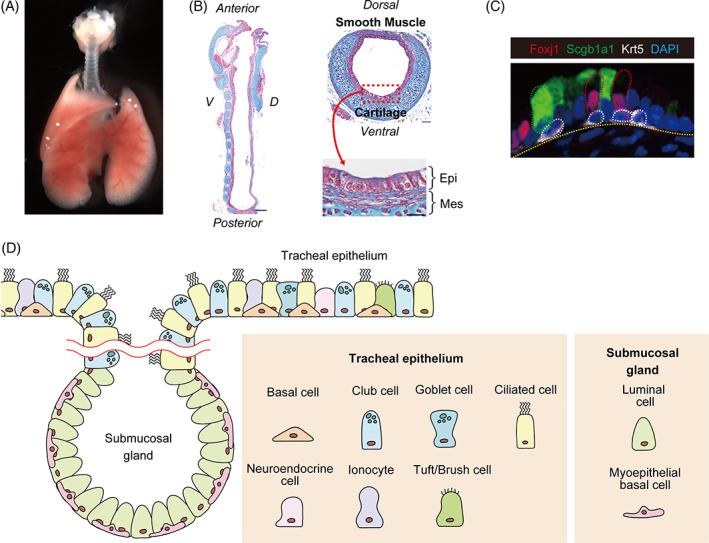
Tissue structure and cellular composition of the trachea. (a) Gross morphology of the trachea at E18.5. (b) Azan staining of the trachea at E18.5 reveals that the pseudostratified tracheal epithelium is surrounded by c‐shaped cartilage rings and smooth muscle cells in ventral and dorsal regions, respectively. (c) Immunostaining reveals several cell types in the tracheal epithelium, such as Krt5+ basal (white), Scgb1a1+ club (green), and Foxj1+ ciliated (red) Cells. (d) The tracheal epithelium contains various specialized cell types. Myoepithelial basal cells and luminal cells reside in submucosal gland structures, which appear in the upper part of the tracheal epithelium after birth
In this review, we will present an overview of molecular mechanisms of tracheal development that regulate its simple but precise tubular morphology and identities of individual differentiated cells, which is mainly coordinated by the crosstalk between epithelial and mesenchymal cells. Studies have revealed these mechanisms are conserved in adult tissue regeneration. In addition, we will discuss future perspectives in the field of trachea development by discussing key outstanding questions for clinical application.
2. FOUR STEPS IN TRACHEAL DEVELOPMENT
Tracheal development consists of four steps, namely regionalization with AP patterning (E7‐E8.5), lineage specification with DV patterning (E8.5‐E10.5), trachea‐esophageal separation (E9.5‐12.5), and tube elongation and expansion (E10.5‐adulthood) (Figure 2). Although there is a partial overlap with these steps, each step progresses through various signaling pathways, such as Wnt, Bmp, retinoic acid (RA), Shh, and Fgf, which are spatially regulated and change dynamically throughout development. The integration of these signaling pathways is needed to specify tracheal progenitors, segregate the respiratory tract from digestive organs, and complete large tube morphogenesis. In this section, we will review each step focusing on the key signaling pathway. Notably, mounting evidence suggests that the regulatory mechanism for trachea development is different from that for lung development. 5 , 9 , 10 Recent studies have identified molecular mechanisms of trachea‐esophageal separation using new technologies, such as comprehensive transcriptomics at single‐cell resolution. 11
FIGURE 2.
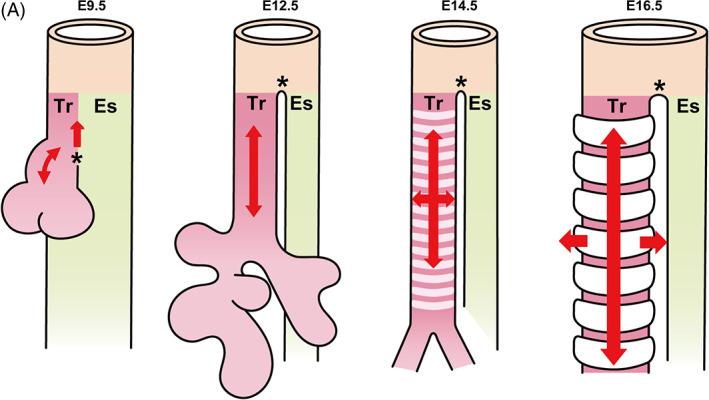
Overview of tracheal development. (a) The tracheal primordium appears at the ventral anterior foregut around E9.5 through trachea‐esophageal separation. The trachea elongates without changing its diameter through splitting and extension until E14.5. From E14.5, the trachea elongates and widens simultaneously to form a long and wide tube, which is led by cartilage growth. Es: Esophagus, Tr: Trachea
2.1. Regionalization with AP patterning (E7.0‐E8.5)
2.1.1. Endodermal patterning
The regional identity of the trachea is established during endodermal patterning, which determines the spatial distribution of endodermal organs, such as the thyroid, liver, and pancreas, along the anterior‐posterior axis within the naïve endodermal sheet by the orchestration of Wnt, RA, Shh, Bmp, and Fgf signaling (Figure 3). 3 , 4
FIGURE 3.
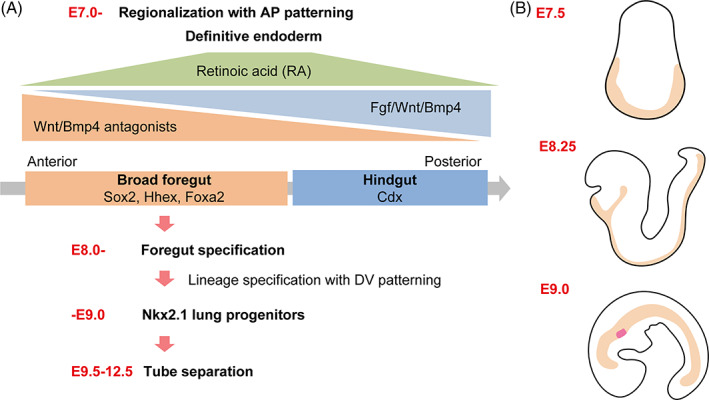
Endodermal patterning. (a) Initial patterning of the anterior–posterior (AP) axis occurs at around E7.0 to 8.0. Hhex and Cdx represent foregut and hindgut regions, respectively, which are determined by the gradient concentration of Fgf/Wnt/Bmp. Further subdivision of the endoderm into the foregut, midgut, and hindgut is initiated by retinoic acid (RA) signaling. By E9.0, Nkx2.1+ respiratory epithelial progenitors, including the trachea and lungs, appear at the ventral part of the foregut endoderm through the gradient expression of Wnt/Bmp ligands along the dorso–ventral (DV) axis. The tracheal primordium segregates from digestive organs through a dynamic cellular reorganization process called “trachea‐esophageal separation” between E9.5‐12.5. (b) Beige regions represent the endoderm during embryogenesis from E7.5 to E9.0. At E9.0, Nkx2.1+ respiratory epithelial progenitors, including the trachea and lungs, are labeled in red at the ventral part of the foregut endoderm
Initial patterning of the AP axis occurs at E7.0 to 8.0, which is characterized by Hhex expression in the future anterior endoderm. The high expression of nodal growth differentiation factor, or NODAL, in the early primitive streak promotes anterior endoderm fate and expression of Hhex, 12 whereas Fgf, Wnt, and Bmp ligands appear to maintain the identity of the hindgut and posterior part of the endoderm and actively repress foregut fate in the posterior part. 13
Reciprocal interactions between the endoderm and adjacent mesoderm further divide the endoderm into the foregut, midgut, and hindgut. By E9.0, respiratory epithelial progenitors, including those of the trachea and lung, appear at the ventral part of the foregut endoderm, which expresses Nkx2.1, a key transcription factor (TF) involved in trachea and lung epithelia development (Figure 4).
FIGURE 4.
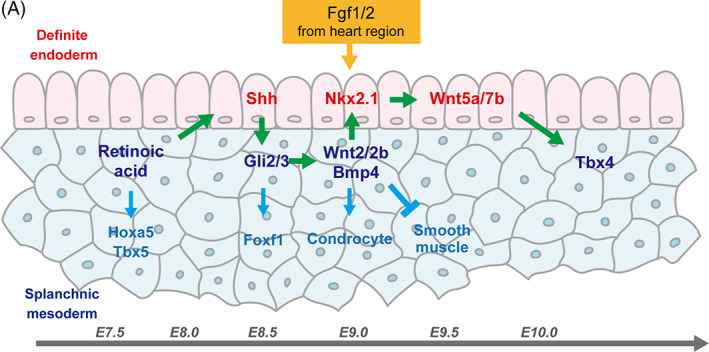
Crosstalk between endoderm and mesoderm during tracheal development. RA expression around E7.5 is an initial cue for the determination of the presumptive trachea in both the epithelium and mesenchyme. RA promotes Shh expression in the epithelium, which in turn initiates Wnt2/2b and Bmp4 expression in the mesenchyme through Gli2/3 expression. As a result, Nkx2.1 is expressed in the presumptive respiratory epithelium by E9.0. Wnt ligands, such as Wnt5a and Wnt7b, secreted from Nkx2.1+ epithelial cells, induce Tbx4 expression in the tracheal mesenchyme around E10.5
Endodermal patterns become more distinct as development proceeds. During this process, RA seems to be the initial cue for the determination of presumptive tracheal regions by inducing key TFs 14 , 15 , 16 in both the epithelium and mesenchyme. RA activity was detected from E7.5 in a transgenic RA‐reporter mouse strain containing lacZ linked to an RA‐response element. 14 RA is the biologically active derivative of vitamin A, which activates key genes through direct binding to nuclear receptors, and is essential for embryogenesis, such as the development of organs, including the hindbrain, spinal cord, forelimb bud, and lung. 16 The ablation of retinaldehyde dehydrogenase 2 (Raldh2), which oxidizes retinal to RA, was reported to lead to failure of trachea and lung development, demonstrating that RA is required for the development of the respiratory system. 17 , 18 , 19 RA plays a critical role in the determination of endodermal identity by inducing Shh expression in endodermal cells 20 ; simultaneously, it initiates the expression of key TFs, such as Tbx4/5, and Hoxa5, in the respiratory mesoderm. 15 Shh appears at E8.5 20 and activates Gli2/3 transcriptional factors, which are downstream of Shh signaling, in the foregut mesoderm. 21 The expression of these genes in the endoderm and mesoderm is essential for tracheal specification because Shh knockout (KO) leads in tracheal and pulmonary anomalies, 22 which are recapitulated in Gli2/3 null mice. 23 Gli2/3 play critical roles in the determination of the DV axis by directly promoting the expression of key factors, such as Wnt2/2b and Bmp4, in the ventral part of the anterior foregut mesoderm 20 (also see Section 2.2 Lineage specification with DV patterning (E8.5‐E10.5)).
In addition to RA and Shh signaling, Fgf signaling plays a critical role in determining the AP patterning. For example, Fgf ligands secreted by the cardiac mesoderm contribute to the induction of different organ markers dose dependently. 24 Using ventral foregut endoderm explants, Serls et al demonstrated that high and moderate levels of Fgf induce Nkx2.1+ lung and thyroid progenitors and albumin‐expressing liver progenitors, respectively, whereas a low dose of Fgf induces Pdx1+ ventral pancreas and duodenum progenitors. 24 This idea is controversial because Fgf treatment is essential for the specification of thyroid progenitors in de novo derivation from pluripotent stem cells but not for that of lung progenitors, suggesting that the requirement for Fgf signaling is different in the context of organ development. 25 In addition, the requirement for Fgf signaling is different within respiratory development (trachea vs. lung). Fgf10‐null mice exhibit normal tracheal specification, but fail to form mainstem bronchi as well as all subsequent pulmonary branching morphogenesis. 9 , 26 This interesting phenotype was recapitulated in Fgf receptor 2 (Fgfr2) knockout mice. 10 These studies demonstrated the requirement of the Fgf10 to Fgfr2 axis for lung development, such as branching morphogenesis, and not for tracheal specification. Furthermore, loss of Bmp signaling disrupts tracheal development but not lung‐bud induction (also see Section 2.2 Lineage specification with DV patterning). Thus, lung and trachea development are regulated by different signaling pathways and machineries.
2.1.2. Mesodermal patterning
The AP patterning is determined through reciprocal interactions between the endoderm and mesoderm; however, specification of the tracheal mesenchyme is not as well characterized as that of the epithelium. Studies on mesodermal development through temporal transcriptional analyses at single‐cell resolution revealed reliable markers for organ‐specific mesodermal cells. 27 , 28 Detailed analyses of transcriptome profiles revealed a dynamic process of organ‐specific mesodermal development.
The tracheal mesenchyme is derived from the lateral plate mesoderm (LPM) through RA, Shh, and Wnt signaling pathways. 4 The T‐box genes Tbx4 and Tbx5 are the most reliable genetic markers and essential TFs for the developing trachea or lung mesenchyme. 29 First, Tbx4 expression occurs in the lung‐bud region around E9.5, followed by the expression of Tbx4 in tracheal mesenchymal cells, independent of the lung buds around E10.5. 30 These Tbx4+ mesenchyme progenitors were reported to contribute to all respiratory mesenchymal lineages through the lineage tracing experiment. 31 Tbx5 expression can be detected in the presumptive tracheal mesenchyme before that of Tbx4. Arora et al reported that loss of Tbx5 leads to loss of tracheal specification in organ culture because Nkx2.1 expression is altered due to decrease expression of Wnt2/2b in the mesenchyme. 32 This observation underscores the importance of Tbx5+ mesenchyme in the initial step of tracheal epithelium specification.
RA signaling is the first cue for mesenchymal patterning. The presumptive respiratory mesenchyme in LPM begins expressing Raldh2/Rdh10 around E8.0, which initiates the expression of the foregut mesoderm‐specific TFs, such as Foxf1, Osr1, Gata4/5/6, and Tbx4/5. 4 RA signaling first pre‐patterns the LPM by inducing key TFs, such as Tbx4/5 and Hoxa5, known as RA signaling targets 16 and epithelial Shh expression. 20 In turn, epithelial Shh stimulates adjacent mesenchymal cells to induce the expression of Wnt2/2b and Bmp4, which are essential ligands for respiratory specification, and Foxf1 in the mesenchyme. 20
Foxf1 is also known as an essential TF in the developing respiratory mesenchyme and a target of RA‐HH signaling. 33 , 34 In humans, mutations in the FOXF1 locus result in alveolar capillary dysplasia with pulmonary vein misalignment. 35 Consistently, the loss one Foxf1 allele in mice results in hypoplastic and malformed tracheal cartilage. 36
Recently, Zorn et al delineated the developmental road map of organ‐specific mesenchymal progenitors, including respiratory progenitors, with detailed analyses using time‐series single‐cell RNA‐sequencing (scRNA‐seq). 27 They reported that the specification of the respiratory mesenchyme occurs around E9.0‐9.5 and identified the key epithelial‐mesenchymal interaction signaling as well as new markers for the developing respiratory mesenchyme. The detailed lineage map and stepwise developmental process needs to be clarified.
2.2. Lineage specification with DV patterning (E8.5‐E10.5)
DV‐axis patterning is the first step in trachea‐esophageal separation and proceeds through cellular interactions between the mesenchyme and epithelium through Wnt and Bmp signaling gradient along the DV axis (Figure 5). An important event during this process involves the specification of the Nkx2.1+ trachea and lung progenitors in the endoderm. Nkx2.1 expresses in the ventral region of the foregut, whereas Sox2 expresses in the dorsal region. The complementary distribution of Nkx2.1 and Sox2 determines the DV pattern of the foregut tube. 37 , 38 , 39
FIGURE 5.
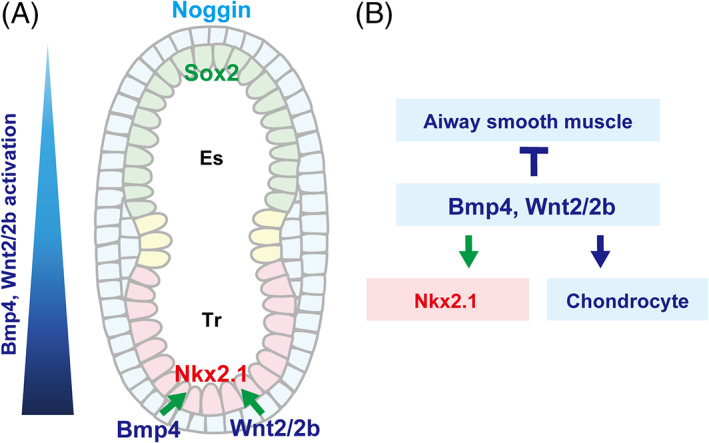
Lineage specification with dorsal–ventral patterning (E8.5–E10.5). (a) Dominant Bmp4 and Wnt2/2b expression in the ventral mesenchyme contributes to the establishment of the Wnt/Bmp gradient along the dorso–ventral axis, which is reinforced by the dominant expression of Noggin, a Bmp4 antagonist, in the dorsal side. (b) Bmp4 and Wnt2/2b expression promotes the specification of Nkx2.1+ respiratory epithelial cells and chondrocytes and inhibits the specification of airway smooth muscle cells. Es: Esophageal region, Tr: Tracheal region
Nkx2.1, an essential TF for trachea and lung specification, 40 is initially expressed by E9.0, 24 specifically in the ventral area of the anterior foregut endoderm. In Nkx2.1 null mice, the trachea fails to appear, resulting in tracheal agenesis, 38 although bilateral bronchial tubes arise from the esophagus and connect to profoundly hypoplastic lungs. Recent analysis of Nkx2.1 null mice using single‐cell or bulk RNA‐seq and ChiP‐seq revealed that Nkx2.1 plays critical roles in the determination of tracheal mesenchyme identity through direct regulation of Shh and Wnt7b expression in the epithelium. 11 Nkx2.1 null mice exhibit ectopic airway smooth muscle cells (A‐SMCs) in the ventral region at the expense of chondrocytes, which suggests that Nkx2.1 KO leads to a dorsalized phenotype. 38 Thus, Nkx2.1 expression is necessary for trachea and lung development and for determining DV patterning.
In contrast to Nkx2.1 expression in the ventral foregut endoderm, Sox2 is dominantly expressed in the dorsal part and determines dorsal identity in the endoderm and mesoderm. 37 , 39 Consistently, Sox2 conditional KO in the epithelium alters the dorsal identity in both the epithelium and mesenchyme and converts the esophagus to trachea. 39 In addition, Sox2 directly contributes to esophageal specification in part by repressing Wnt activity. 41
2.2.1. Signaling pathway responsible for DV patterning
Wnt and Bmp signaling pathways play critical roles in the DV patterning of the foregut. As described, Nkx2.1 is an essential TF for trachea and lung development and DV patterning. Nkx2.1 expression in the endoderm is induced by Wnt2/2b ligands derived from the ventral foregut mesenchyme. 42 Wnt2/2b null mice exhibit lung agenesis due to the loss of Nkx2.1 expression, which is recapitulated by beta‐catenin (Ctnnb1) conditional KO mutants in the endodermal epithelium. By contrast, Ctnnb1 overexpression triggers the ectopic Nkx2.1 expression in esophageal region. 42 , 43
Although Nkx2.1 is a key player in respiratory development, Nkx2.1 KO mice are able to form lung buds and induce Tbx4 expression in the mesenchyme. 11 , 30 , 38 These results suggest the presence of Nkx2.1‐independent mechanisms for respiratory specification. One such mechanism is the canonical Wnt activity in the epithelium. The Wnt2/2b KO and endodermal Ctnnb1 conditional KO express a phenotype in which lung buds do not form and mesenchymal Tbx4 is not induced. We reported that epithelial Wnt ligand expression is independent of Nkx2.1. In addition, Wnt2/2b‐dependent canonical Wnt activity in the epithelium was required for the induction of Tbx4 expression in the mesenchyme. Therefore, bidirectional Wnt activation between the mesoderm and endoderm in the ventral anterior foregut has an important role in respiratory specification independent of Nkx2.1. 30 Epithelial Wnt ligands are also required for tracheal‐mesenchymal development, such as cartilage formation, at later stages 30 , 44 , 45 (See the details in Section 4. Mesenchymal development). The exclusive expression of Dickkopf homolog 1 (DKK1), a Wnt‐antagonist, in the dorsal area also contributes to DV patterning. 46
Bmp signaling is also indispensable for the determination of presumptive tracheal regions and trachea‐esophageal separation by defining the DV patterning. Bmp4 appears in the ventral foregut mesoderm at E8.5. 47 Both Bmp4 conditional KO within the splanchnic mesoderm and Bmpr1a/b conditional KO within the endodermal epithelium result in tracheal agenesis due to the reduced proliferation of epithelial and mesenchymal cells. Interestingly, ectopic primary bronchi arise from the esophagus despite an obvious defect in tracheal development. 47 , 48 These results also support the idea that tracheal development is regulated by a mechanism distinct from that of lung development. The phenotype of Bmpr1a/b conditional KO mice was rescued by Sox2 inactivation, suggesting that Bmp4 to Bmpr1a/b promotes tracheal formation through the repression of Sox2 in the ventral foregut region. 48 The dominant expression of Noggin, a Bmp4 antagonist, in the dorsal side is involved in DV patterning by forming Bmp gradient along the DV axis. 49
2.3. Trachea‐esophageal separation: E9.5 to E12.5
Trachea‐esophageal separation (Figure 6) is a dynamic tissue deformation process, which segregates the respiratory system from the digestive tract by reorganizing epithelial and mesenchymal cells. Several trachea‐esophageal separation models, such as outgrowth, watershed, and septation, have been proposed, 50 but a study suggested that the splitting and extension model is highly likely 51 (Figure 6C).
FIGURE 6.
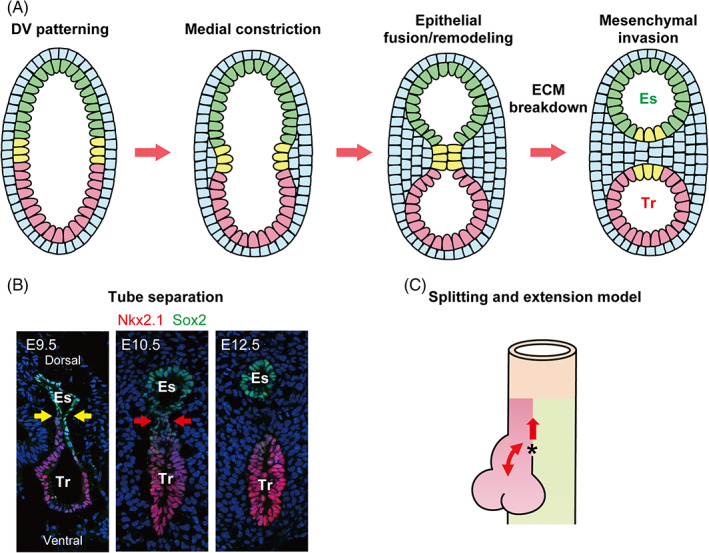
Trachea‐esophageal separation. (a, b) Trachea‐esophageal separation involves five steps; dorso–ventral (DV) patterning, medial constriction, epithelial fusion, or remodeling, ECM breakdown, and mesenchymal invasion. Sox2 (green) and Nkx2.1 (red) reveal complementary expression patterns along the DV axis. Foxf1+ mesenchyme promotes medial constriction at the Sox2 and Nkx2.1 boundary, which is indicated by yellow arrows in the E9.5 image. The fusion and remodeling of the polarized epithelium are required for the separation of the trachea and esophagus, which is indicated by red arrows in the E10.5 image. Mesenchymal invasion completes trachea‐esophageal separation of the foregut into a distinct trachea and esophagus, as shown in the E12.5 image. (c) The “Splitting and extension” model explains the growth of the trachea during trachea‐esophageal separation. The saddle‐shaped structure indicated by the asterisk moves rostrally to divide the foregut into the trachea and esophagus, which grow equally and simultaneously after separation. Es: Esophagus, Tr: Trachea
The recent study suggests that trachea‐esophageal separation can be subdivided into five steps, namely DV patterning, medial constriction, epithelial fusion and remodeling, extracellular matrix (ECM) breakdown, and mesenchymal invasion 51 (Figure 6A,B). First, the presumptive tracheal epithelium and mesenchyme acquire their identity at the ventral anterior foregut as a part of DV patterning. 48 Then, the Foxf1+ splanchnic mesenchyme promotes medial constriction of the foregut at the boundary between the presumptive Nkx2.1+ tracheal and Sox2+ esophageal epithelium. 51 Two studies have reported the significance of a newly‐defined epithelial population appearing at the boundary of the DV axis called midline epithelial cells, which coexpress both Nkx2.1 and Sox2. 51 , 52 During epithelial remodeling, this population forms a transient septum and contributes to the separation, giving rise to both tracheal and esophageal epithelia. Kim et al demonstrated that Isl1 is an essential TF for this subset of progenitor population during trachea‐esophageal separation, 52 whereas the comprehensive gene regulatory network remains largely unknown. Detailed analyses, such as temporal transcriptome analyses and gene manipulation, are needed to better understand the molecular mechanisms regulating trachea‐esophageal separation. Molecular analysis of mutant mice, such as Barx1 KO, that lack trachea‐esophageal separation despite the normal establishment of DV patterning might allow better understanding of the molecular mechanism of this process. 53
2.4. Tube elongation and expansion: E10.5‐adulthood
After trachea‐esophageal separation, the trachea becomes longer and wider to acquire sufficient lumen for efficient gas exchange. We have described this process in detail using mice as a mammalian model and found that the length of the trachea continuously extended overtime, whereas the expansion of its diameter was not detectable via micro‐CT measurements until E14.5. 54 During this period, cellular specification and patterning of A‐SMCs and chondrocytes occur in the mesenchyme (see Section 4. Mesenchymal development). Tracheal smooth muscle progenitors migrate toward the subepithelial region, coordinated by Wnt5a‐Ror2‐mediated synchronized mesenchymal cellular polarity. This is necessary for normal smooth muscle morphogenesis and tube elongation along the anterior‐posterior axis. From E14.5, the trachea elongates and widens simultaneously to shape a long and wide tube. 54 The expansion of tube diameter is led by cartilage growth. During this period, tracheal epithelial cells start the process of specification and maturation through cellular interactions between the epithelium and mesenchyme as well as among epithelial cells (see Section 3. epithelial development).
3. EPITHELIAL DEVELOPMENT
The single lumen of the trachea has a pseudostratified columnar epithelium, which contains several distinct cell populations, including basal, club, goblet, neuroendocrine, and ciliated cells 55 , 56 (Figure 1D). In addition, a recent study using comprehensive scRNA‐seq analysis identified several minor populations, such as ionocytes, hillock cells, and tuft/brush cells. 57 There are some notable differences in airway structure and composition between mice and humans; basal cells reside only in the trachea and main bronchi in mice, whereas these cells are present in the more distal part of the airway, including terminal bronchioles, in humans. 58 This distal expansion of the basal cells is accompanied by the expansion of cartilage ring distribution to the intrapulmonary airways in humans. In contrast, the cartilage rings are present only in the extrapulmonary airways in mice.
The tracheal epithelium functions as the primary physical barrier against inhaled insults, such as chemical particles, viruses, and bacteria. Once the epithelium is injured, tissue structure and cellular composition are repaired by the facultative regeneration capacity of the tracheal epithelium. In this section, we will summarize developmental processes of epithelial cell populations in the developing trachea, especially that of facultative stem cell populations, such as basal, neuroendocrine, club, and myoepithelial basal cells in the submucosal gland.
From E10.5 to E16.5, the proximal‐distal axis in the epithelium of the branching airway system is defined by the complementary expression pattern of Sox2 vs. Sox9. 55 , 56 In mice, Sox2 is expressed in the proximal region, whereas Sox9 is expressed in the most distal region, which forms alveoli. In contrast, in humans, SOX2 can be detected in the distal region as well, though its expression is lower in the distal area than in the proximal area. 59 Meanwhile, SOX9 expression still marks the distal region in humans. Thus, the production of Sox2 and Sox9 commonly defines the proximal‐distal axis in both species. Because the trachea consists of the upper region of the respiratory tract, Sox2 plays critical roles in the specification of tracheal epithelial cells during development. Sox2 conditional KO in the ventral epithelial domain of the early anterior foregut using Nkx2.5‐Cre mice line impairs epithelial differentiation and results in a smaller basal cell population. 60 By contrast, Sox2 overexpression in alveolar type 2 cells and distal epithelial cells biases their identity into the proximal epithelium by inducing the expression of proximal epithelium‐specific markers, such as Scgb1a1 and Foxj1. 61
In addition to Sox2, p63 is an essential TF for the normal development of the tracheal epithelium, especially for basal cells. p63 expression can be detected in the tracheal epithelium throughout development. 37 p63+ progenitors at early stages of trachea development are multipotent 37 , 62 and can give rise to both airway and alveolar descendants until E10.5. 62 Although p63+ progenitors give rise to both basal and non‐basal cells between E10.5 to E13.5, lineage constriction occurs around E13.5 to 14.5 and most p63+ cells contribute to the basal cell pool. 62
Tracheal basal cells are tissue stem cells that maintain epithelium and are the major cell source during epithelial regeneration. 63 , 64 The TF p63 is indispensable for maintaining basal cell identity; p63 KO results in the complete disappearance of the basal cell population. 65 , 66 Although p63+ progenitors are ancestors of mature basal cells, the molecular mechanism for the specification of basal cells is unclear. Two studies, using time‐series scRNA‐seq in humans 67 and mice, 8 revealed that dual Smad activation through Tgf and Bmp signaling is necessary for the specification of basal cells. In contrast to Smad activation during specification, dual Smad inhibition promotes the maturation of basal progenitors and induces the expression of mature markers, such as Krt5 and NGFR. 8 , 67 , 68 Using this two‐step processes, Miller et al induced the formation of human mature basal cells from the multipotent distal‐tip progenitors in vitro. 67 During basal cell specification, Tgf ligands are secreted from the mesenchyme during development in a spatiotemporal fashion, 8 which suggests the significance of the crosstalk between epithelial and mesenchymal cells.
In addition to Tgf/Bmp signaling, Fgf activation triggered by Fgf10 ligand from the mesenchyme controls the specification and maintenance of the basal cell population. 6 , 45 Fgf receptor 2 (Fgfr2), which binds Fgf10, conditional KO in the epithelium reduced basal cells. 45 Hou et al demonstrated that the Wnt‐Fgf crosstalk is required for the specification of basal cells. 45 Beta‐catenin 1 (Ctnnb1) conditional KO in the mesenchyme of the developing trachea using the Dermo1‐Cre mice line led to a decrease in the basal cell population and Fgf10 expression in the mesenchyme. This phenotype is recapitulated by Wntless (Wls) conditional KO in the epithelium. Wls encodes a G‐protein receptor that is essential for the intracellular transportation of Wnt ligands for secretion. These results suggest that Wnt/Fgf crosstalk between the epithelium and mesenchyme contributes to basal cell specification. In addition, Volckaert et al demonstrated that the Fgf10 to Fgfr2 axis is essential for maturation after specification and maintenance in adulthood. 6 , 69
The main function of club cells under normal condition includes secreting mucus with anti‐pathogenic proteins to enhance the clearance of inhaled insults. Club cells can also act as a facultative stem cell population during airway regeneration, especially in lower airways where basal cells do not reside. Club cells are derived from early lung progenitors and can be detected at E14.5 to E16.5, 70 of which lineage specification is largely dependent on Notch signaling. 71 , 72 Using time‐series scRNA‐seq during tracheal development, we identified Scgb3a2 + intermediate progenitors, which can give rise to non‐basal cell populations, such as club cells and ciliated cells, in a Notch‐dependent manner. 8 Scgb3a2 is known as a club cell marker and the readout of Notch signaling pathway. 73 Consistent with this, RBPj, a core component of Notch signaling, conditional KO in the tracheal epithelium eliminates all Scgb3a2 + intermediate progenitors, 8 suggesting that Notch pathway is critical for the binary cell fate choice between basal vs. non‐basal progenitors in the epithelium of the developing trachea. These results imply that the crosstalk within epithelial progenitors is also important in the establishment of mature epithelial populations, because Notch pathway is activated by the direct interaction between a ligand‐expressing cell and receptor‐expressing cell to achieve alternative cell fate decisions, known as Notch‐mediated lateral inhibition. Notch‐mediated lateral inhibition also regulates the number of lung neuroendocrine population, 71 , 72 which is a rare population with multiple functions, including facultative regeneration. 74 , 75 , 76 The specification of neuroendocrine cells occurs earliest among tracheal epithelial cell populations around E12.5. 77 Tsao et al and Morimoto et al demonstrated that Notch inhibition results in the expansion of neuroendocrine cells during development by generating endodermal epithelium‐specific deletions of Notch‐related components. 71 , 72 Interestingly, during airway regeneration in adulthood, Notch activation in neuroendocrine cells reprogrammed their identity into other cell fates, such as club cells and ciliated cells, through the down‐regulation of neuroendocrine markers. 76 These studies suggest the consistent role of Notch signaling in development and regeneration of neuroendocrine cells.
(See our review papers for more detailed information on the roles of Notch signaling 78 and neuroendocrine cells 79 in the mammalian respiratory system)
In addition to basal and club cells, the tracheal epithelium has a stem cell population called myoepithelial basal cells, which are activated upon severe tissue damage. The myoepithelial basal cells are maintained in submucosal glands (SMGs) that appear at the upper part of the tracheal epithelium after birth 80 and exhibits a saccular structure (Figure 1D). 81 , 82 , 83 SMGs are composed of four structures, namely ciliated ducts, collecting ducts, mucus tubules, and serous acini. 84 Myoepithelial basal cells appear in the early phase of elongation of SMG in a Wnt‐dependent manner 80 , 85 and reside in all structures except the ciliated duct. 86 Lynch et al also found that Wnt signals induce the regeneration capacity of myoepithelial basal cells in response to airway injury in adulthood, 86 suggesting that the role of Wnt signaling in myoepithelial basal cells is conserved through the lifetime.
As discussed in this section, the interactions between epithelial and mesenchymal cells as well as among epithelial cells coordinate the specification, maintenance, and reactivation of the facultative stem cell populations at appropriate time and space in the tracheal epithelium (Figure 7). This knowledge is utilized to induce human airway epithelial cells from human iPSCs and progenitors in vitro. 67 , 68 Because most studies were done using nonhuman models, clinical applications of these studies in human regeneration therapy are limited. Basic studies using human samples are desired for better understanding of mechanisms underlying human trachea development, such as a study that identified rare subsets, ionocytes, responsible for cystic fibrosis, a life‐threatening airway disease. 57
FIGURE 7.
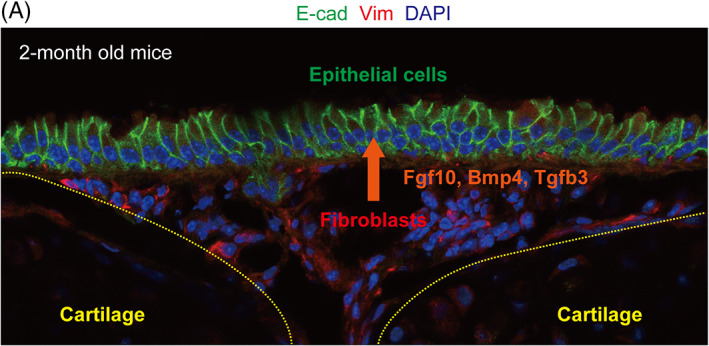
Fibroblasts as a “regeneration hub.” Vimentin(Vim)+ subepithelial fibroblasts, located between tracheal cartilages, control airway basal stem cell behavior by secreting various growth factors, such as Fgf10, Bmp4, and Tgfb3
4. MESENCHYMAL DEVELOPMENT
Tracheal mesenchymal cells are derived from the LPM. Although Tbx4+ mesenchymal cells are known to give rise to all mesenchymal populations during development, 31 the detailed lineage map remains to be elucidated. Mesenchyme surrounding the tracheal epithelial sheet is composed of various subsets of cells, such as A‐SMCs, chondrocytes, fibroblasts, endothelial cells, bronchial vessel cells, and vascular SMCs. 28 Tracheal A‐SMCs and chondrocytes reside in dorsal and ventral regions, respectively (Figure 1B), play critical roles in tracheal tube morphogenesis, 54 and provide rigidity and some mobility to this organ. Fibroblasts adjacent to the basement membrane maintain stem cell populations and regulate the regeneration process by providing several growth factors to the epithelium for proliferation, 7 , 8 , 69 although the characterization of this population including cell markers remains unclear. In this section, we will summarize the developmental processes of trachea mesenchymal cell population focusing on the specification of trahceal A‐SMCs and chondrocytes along the DV axis through Shh, Bmp, and Wnt pathways.
4.1. Airway smooth muscle cells
Airway smooth muscle cells (A‐SMCs) appear at nearly E11.0 around bifurcating bronchi and extend in a cranial to rostral fashion to cover the dorsal side of the trachea. 4 , 87 , 88 The specification of A‐SMCs and chondrocytes is controlled by Wnt and Bmp signaling, the gradient distribution pattern of which along the DV axis influences their complementary distribution. 87
In general, Shh and Bmp signaling pathways play opposing roles in the differentiation and patterning of SMCs; Bmp inhibits the differentiation of SMCs, whereas Shh promotes it. 89 The dominant expression of Noggin, a Bmp antagonist, in the dorsal side is believed to contribute to the dorsal specification of A‐SMCs. Wnt ligands, including Wnt7b and Wn5b, which are secreted from the epithelium of the developing trachea, cooperate with Bmp4 to inhibit the proliferation and differentiation of tracheal A‐SMCs. Snowball et al found that Wntless (Wls) conditional KO in the epithelium resulted in the expansion of A‐SMCs at the expense of chondrocyte formation due to the failure of DV patterning. In Wls conditional KO mice, the expression of Msx1/2 was significantly decreased. 44 Msx1/2 are targets of Bmp signaling and inhibit the differentiation of A‐SMCs. 90 In contrast to the inhibitory effect of Wnt signaling on tracheal A‐SMCs, Wnt7b activates the proliferation and differentiation of A‐SMC precursors in the lower airway. 91 Wnt7b KO and beta‐catenin conditional KO in A‐SMCs using SM22a Cre :Ctnnb1 f/f result in decreased proliferation of A‐SMC precursors. Moreover, loss of Wnt2 in the mesenchyme alters A‐SMC specification in the branching region, with decreased expression of Wnt7b in the epithelium and Fgf10 in the mesenchyme. 92 Given that these factors are necessary for A‐SMC development, 91 , 93 Wnt2 is placed high in the hierarchy of signaling molecules responsible for lower A‐SMC development, excluding those of the trachea. Notably, these studies underscore different functions of Wnt signaling in the development of tracheal vs. lower A‐SMCs. Wnt inhibits A‐SMC differentiation in the developing trachea and promotes it in the lower airway, suggesting that characteristics of trachea vs. lower airway mesenchyme are controlled by different machineries.
4.2. Chondrocytes
The specification of tracheal chondrocytes starts around E10.5, which is characterized by Sox9 expression. 94 Sox9 plays a central role in the specification of chondrocytes, 87 , 95 because Sox9 KO results in the loss of chondrocytes. During chondrocyte development, epithelial cells play leading roles along the DV axis. Sox2, which characterizes the dorsal epithelium, conditional KO in the anterior foregut endoderm results in the increase of A‐SMCs at the expense of chondrocytes due to the loss of the DV axis. 60 Wnt and Bmp signaling was reported to promote chondrocyte specification. 96 , 97 Consistently, germline deletions of Wnt‐related components, such as R‐spondin 2 (Rspo2) and lipoprotein receptor‐related protein 6 (Lrp6), leads to severe malformations of the tracheal cartilage due to decreased intracellular β‐catenin activation, 98 demonstrating that Wnt ligands secreted from the epithelium to the mesenchyme, such as Wnt5a and Wnt7b, are necessary for cartilage formation. 30 , 44 , 45 Bmp4 may promote the differentiation of chondrocyte progenitors through the positive regulation of Sox9 expression. 97 Around E13.5, Sox9+ chondrocyte progenitors start to periodically condensate along the anterior‐posterior axis to form the cartilage rings. 99 Despite its significance in tracheal development, the detailed mechanism regulating the patterning of chondrocyte progenitors remains to be elucidated, although Shh signaling is known to play important roles in chondrocyte patterning and proliferation. 99 , 100 Shh expression in the endodermal epithelium induces Sox9 expression and contributes to the formation of tracheal cartilage; Shh KO mice exhibit anomalous cartilage formation, which can be rescued by Bmp4 or Noggin treatment. 101
4.3. Fibroblasts
Mounting evidence suggests that fibroblasts are composed of stem cell niches in various organs, such as the intestine, skin, and respiratory system. 102 Tracheal fibroblasts, which reside between tracheal cartilages, act as a stem cell niche by providing several growth factors to the basal cell population 7 , 8 , 45 , 69 (Figure 7). As mentioned in Section 3, epithelial‐mesenchymal interactions promote basal cell specification; mesenchymal Fgf10 stimulates Fgfr2 of epithelial progenitors in response to Wnt ligands from the epithelium. 45 Fgf10 to Fgfbr2 signaling is also required for maintaining basal cell identity in adulthood. 69 Fgfr2 conditional KO in the tracheal epithelium results in the loss of mature basal cells, whereas Fgf10 overexpression expands the basal cell population. During maintenance, Wnt7a secreted by basal cells activates Fgf10 expression in fibroblasts, indicating that the Wnt‐Fgf crosstalk between the epithelium and mesenchyme is indispensable for basal cell homeostasis in adulthood and specification during development.
Studies, including ours, have shown that tracheal subepithelial fibroblasts regulate the proliferative status of the mature basal cell population in adults by secreting Tgf‐β/Bmp ligands to the epithelium. 7 , 8 Under homeostasis, these ligands maintain basal cells in a quiescent state to prevent the exhaustion of the stem cell pool by reducing the number of cell divisions. In response to airway injury, such as exposure to SO2, which is an experimental model for acute epithelial injury, the expression of these ligands decreases in fibroblasts, and then, basal cells become proliferative for airway regeneration and compensate for cell loss. These results demonstrated that fibroblasts act as a “regeneration hub” by controlling the proliferation mode of stem cell populations during airway regeneration. Moreover, we found that this inhibitory effect of the Tgf‐β pathway on epithelial proliferation is conserved in the specification of basal cells during development. 8 The highly proliferative and multipotent epithelial progenitors in fetal airways commit to each cell lineage through cell‐cycle slowdown as development proceeds. In this process, Tgf ligands slow down the cell cycle in mesenchymal cells by suppressing the expression of Id genes, which are key TFs for promoting the proliferation of respiratory progenitors. 8
4.4. Tracheal vasculature
The tracheobronchial vasculature is known to appear during the canalicular stage (16‐26 weeks in humans and E16.5‐17.5 in mice) and become the systemic circulation for nutrient supply. 103 In humans, systemic blood vessels supply the trachea down to the carina and mainstem bronchi, whereas in mice, the lung does not have a normal systemic circulation to the intraparenchymal airways. 104 However, details of the process involved in the development of tracheal vasculature remain unknown. Proximal vessels are established through angiogenesis, whereas distal vessels are formed through vasculogenesis. 105 Cardiopulmonary mesoderm progenitors (CPPs) were shown to contribute to the formation of the proximal vasculature, including the trachea, by giving rise to airway or vascular SMCs, proximal vascular endothelia, and pericyte‐like cells. 106 CPPs arise from lsl1+ cardiac progenitors before lung development and are regulated by Shh signaling from the foregut endoderm. However, detailed analyses are warranted to clarify the development of tracheal vasculature.
5. FUTURE PERSPECTIVES
Studies in the last two decades accompanied by emergent technologies have significantly deepened our understanding of the mechanisms underlying tracheal development. The trachea develops through stepwise cellular processes to acquire a sizable lumen with rigidity and some mobility. Trachea‐esophageal separation is a representative event that requires cellular reorganization, such as epithelial fusion or remodeling and mesenchymal invasion. 51 Although the comprehensive gene regulatory network for trachea‐esophageal separation remains elusive, rapid progress in technology, such as time‐series transcriptional analyses with spatial information 107 and next‐generation lineage tracing methods, 108 will enable better understating of the molecular mechanisms regulating trachea development and development of efficient treatments for life‐threatening congenital diseases, including tracheal agenesis and stenosis.
Mutually directed cellular interactions between epithelial and mesenchymal progenitor cells ensure the specification and maturation of their identities during development. However, the developmental road map of mesenchymal lineage has been less clear compared to that of epithelial lineage. A study using time‐series scRNA‐seq identified key TFs involved in mesenchymal development, 27 which can be useful to recapitulate tracheal development in vitro using human iPSCs. In terms of regenerative medicine, making a transplantable artificial trachea composed of mature epithelial and mesenchymal cells would be the final goal. Therefore, knowledge on human trachea development is needed because model animals, such as mice and chick, have been used for research.
As discussed in Section 4, subepithelial fibroblasts located between the tracheal cartilage are involved in the maintenance and reactivation of the epithelial stem cell population. Despite their significance, the origin and regulation of fibroblasts remain unknown. This information is required to understand the tissue regeneration with stem cells and its regulation by epithelial‐mesenchymal interactions. Decoding stem cell regulation will be useful for future regenerative medicine.
AUTHOR CONTRIBUTIONS
Hirofumi Kiyokawa: Conceptualization; funding acquisition; project administration; writing‐original draft. Mitsuru Morimoto: Conceptualization; funding acquisition; project administration; supervision; writing‐original draft; writing‐review & editing.
ACKNOWLEDGMENTS
The authors apologize for omitting any references due to word limitation. These studies are supported by funding from Grants‐in‐Aid for Scientific Research (B) (20H03693) (M. M.), Young Scientists (19K17691) (H. K.) of the Ministry of Education, and Culture, Sports, Science and Technology, Japan and from the Special Postdoctoral Researcher (SPDR) Program of RIKEN (H. K.). We also thank Yuka Kiyokawa and Suzuka Takahashi, who drew and refined the illustrations in the figures. We also thank Keishi Kishimoto for reviewing the manuscript and give us constructive comments.
Kiyokawa H, Morimoto M. Molecular crosstalk in tracheal development and its recurrence in adult tissue regeneration. Developmental Dynamics. 2021;250(11):1552–1567. 10.1002/dvdy.345
Funding information Grants‐in‐Aid for Scientific Researchof the Ministry of Education, and Culture, Sports, Science and Technology, Japan, Grant/Award Numbers: 20H03693, 19K17691; RIKEN
Contributor Information
Hirofumi Kiyokawa, Email: hirofumi.kiyokawa@riken.jp.
Mitsuru Morimoto, Email: mitsuru.morimoto@riken.jp.
REFERENCES
- 1. Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med. 2008;177(3):253‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arooj Sher Z. J Liu K. congenital tracheal defects: embryonic development and animal models. AIMS Genetics. 2016;3(1):60‐73. 10.3934/genet.2016.1.60. [DOI] [Google Scholar]
- 3. Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221‐251. 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han L, Nasr T, Zorn AM. Mesodermal lineages in the developing respiratory system. Dev Biol. 2016;9(25):221–251. [PMC free article] [PubMed] [Google Scholar]
- 5. Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn. 1998;212(4):482‐494. https://doi.org10.1002/(SICI)1097–0177(199808)212:4<482::AID-AJA2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 6. Volckaert T, Campbell A, Dill E, Li C, Minoo P, De Langhe S. Localized Fgf10 expression is not required for lung branching morphogenesis but prevents differentiation of epithelial progenitors. Develop. 2013;140(18):3731‐3742. 10.1242/dev.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tadokoro T, Gao X, Hong CC, Hotten D, Hogan BLM. BMP signaling and cellular dynamics during regeneration of airway epithelium from basal progenitors. Develop. 2016;143(5):764‐773. 10.1242/dev.126656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kiyokawa H, Yamaoka A, Matsuoka C, Tokuhara T, Abe T, Morimoto M. Airway tissue stem cells reutilize the embryonic proliferation regulator, Tgfß‐Id2 axis, for tissue regeneration. bioRxiv . 2020: 10.1101/2020.11.23.394908. [DOI] [PubMed]
- 9. Min H, Danilenko DM, Scully SA, et al. Fgf‐10 is required for both limb and lung development and exhibits striking functional similarity to drosophila branchless. Genes Dev. 1998;12(20):3156‐3161. 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arman E, Haffner‐Krausz R, Gorivodsky M, Lonai P. Fgfr2 is required for limb outgrowth and lung‐branching morphogenesis. Proc Natl Acad Sci U S A. 1999;96(21):11895‐11899. 10.1073/pnas.96.21.11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuwahara A, Lewis AE, Coombes C, Leung FS, Percharde M, Bush JO. Delineating the early transcriptional specification of the mammalian trachea and esophagus. elife. 2020;9:1‐23. 10.7554/eLife.55526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas PQ, Brown A, Beddington RSP. Hex: a homeobox gene revealing peri‐implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors. Development. 1998;125(1):85‐94. [DOI] [PubMed] [Google Scholar]
- 13. Kraus MRC, Grapin‐Botton A. Patterning and shaping the endoderm in vivo and in culture. Curr Opin Genet Dev. 2012;22(4):347‐353. 10.1016/j.gde.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 14. Mic FA, Haselbeck RJ, Cuenca AE, Duester G. Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development. 2002;129(9):2271‐2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen F, Desai TJ, Qian J, Niederreither K, Lü J, Cardoso WV. Inhibition of Tgfβ signaling by endogenous retinoic acid is essential for primary lung bud induction. Development. 2007;134(16):2969‐2979. 10.1242/dev.006221. [DOI] [PubMed] [Google Scholar]
- 16. Ghyselinck NB, Duester G. Retinoic acid signaling pathways. Development. 2019;146(13):1‐7. 10.1242/dev.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molotkov A, Molotkova N, Duester G. Retinoic acid generated by raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev Dyn. 2005. 232(4):950–957. 10.1002/dvdy.20256. [DOI] [PubMed] [Google Scholar]
- 18. Desai TJ, Chen F, Lü J, et al. Distinct roles for retinoic acid receptors alpha and beta in early lung morphogenesis. Dev Biol. 2006;291(1):12‐24. 10.1016/j.ydbio.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 19. Wang Z, Dollé P, Cardoso WV, Niederreither K. Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev Biol. 2006;297(2):433‐445. 10.1016/j.ydbio.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 20. Rankin SA, Han L, McCracken KW, et al. A retinoic acid‐hedgehog Cascade coordinates mesoderm‐inducing signals and endoderm competence during lung specification. Cell Rep. 2016;16(1):66‐78. 10.1016/j.celrep.2016.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hui CC, Slusarski D, Platt KA, Holmgren R, Joyner AL. Expression of three mouse homologs of the drosophila segment polarity gene cubitus interruptus, Gli, Gli‐2, and Gli‐3, in ectoderm‐ and mesoderm‐derived tissues suggests multiple roles during postimplantation development. Dev Biol. 1994;162(2):402‐413. 10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]
- 22. Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20(1):58‐61. 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- 23. Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet. 1998;20(1):54‐57. 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- 24. Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132(1):35‐47. 10.1242/dev.01570. [DOI] [PubMed] [Google Scholar]
- 25. Serra M, Alysandratos KD, Hawkins F, et al. Pluripotent stem cell differentiation reveals distinct developmental pathways regulating lung‐ versus thyroid‐lineage specification. Development. 2017;144(21):3879‐3893. 10.1242/dev.150193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sekine K, Ohuchi H, Fujiwara M, et al. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21(1):138‐141. 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- 27. Han L, Chaturvedi P, Kishimoto K, et al. Single cell transcriptomics identifies a signaling network coordinating endoderm and mesoderm diversification during foregut organogenesis. Nat Commun. 2020;11(1):1‐16. 10.1038/s41467-020-17968-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Travaglini KJ, Nabhan AN, Penland L, et al. A molecular cell atlas of the human lung from single‐cell RNA sequencing. Nature. 2020;587(7835):619‐625. 10.1038/s41586-020-2922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chapman DL, Garvey N, Hancock S, et al. Expression of the T‐box family genes, Tbx1‐Tbx5, during early mouse development. Dev Dyn. 1996;206(4):379‐390. https://doi.org10.1002/(SICI)1097–0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 30. Kishimoto K, Furukawa KT, Luz‐Madrigal A, et al. Bidirectional Wnt signaling between endoderm and mesoderm confers tracheal identity in mouse and human cells. Nat Commun. 2020;11(1):1‐12. 10.1038/s41467-020-17969-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kumar ME, Bogard PE, Espinoza FH, Menke DB, Kingsley DM, Krasnow MA. Defining a mesenchymal progenitor niche at single‐cell resolution. Science. 2014;346(6211):1258810‐1–9. 10.1126/science.1258810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arora R, Metzger RJ, Papaioannou VE. Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet. 2012;8(8):1‐14. 10.1371/journal.pgen.1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kugler MC, Joyner AL, Loomis CA, Munger JS. Sonic hedgehog signaling in the lung: from development to disease. Am J Respir Cell Mol Biol. 2015;52(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ustiyan V, Bolte C, Zhang Y, et al. FOXF1 transcription factor promotes lung morphogenesis by inducing cellular proliferation in fetal lung mesenchyme. Dev Biol. 2018;443(1):50‐63. 10.1016/j.ydbio.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stankiewicz P, Sen P, Bhatt SS, et al. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet. 2009;84(6):780‐791. 10.1016/j.ajhg.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mahlapuu M, Ormestad M, Enerbäck S, Carlsson P. The forkhead transcription factor Foxf1 is required for differentiation of extra‐embryonic and lateral plate mesoderm. Development. 2001;128(2):155‐166. [DOI] [PubMed] [Google Scholar]
- 37. Que J, Okubo T, Goldenring JR, et al. Multiple dose‐dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134(13):2521‐2531. 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(−/−) mouse embryos. Dev Biol. 1999;209(1):60‐71. 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- 39. Teramoto M, Sugawara R, Minegishi K, et al. The absence of SOX2 in the anterior foregut alters the esophagus into trachea and bronchi in both epithelial and mesenchymal components. Biology Open. 2020;9(2):bio048728. 10.1242/bio.048728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kimura S, Hara Y, Pineau T, et al. The T/ebp null mouse: thyroid‐specific enhancer‐binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10(1):60‐69. 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 41. Trisno SL, Philo KED, McCracken KW, et al. Esophageal Organoids from human pluripotent stem cells delineate Sox2 functions during esophageal specification. Cell Stem Cell. 2018;23(4):501‐515.e7. 10.1016/j.stem.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goss AM, Tian Y, Tsukiyama T, et al. Wnt2/2b and β‐catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17(2):290‐298. 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harris‐Johnson KS, Domyan ET, Vezina CM, Sun X. β‐Catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci U S A. 2009;106(38):16287‐16292. 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Snowball J, Ambalavanan M, Whitsett J, Sinner D. Endodermal Wnt signaling is required for tracheal cartilage formation. Dev Biol. 2015;405(1):56‐70. 10.1016/j.ydbio.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hou Z, Wu Q, Sun X, et al. Wnt/Fgf crosstalk is required for the specification of basal cells in the mouse trachea. Development. 2019;146(3):1‐6. 10.1242/dev.171496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen F, Cao Y, Qian J, Shao F, Niederreither K, Cardoso WV. A retinoic acid‐dependent network in the foregut controls formation of the mouse lung primordium. J Clin Investig. 2010;120(6):2040‐2048. 10.1172/JCI40253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li Y, Gordon J, Manley NR, Litingtung Y, Chiang C. Bmp4 is required for tracheal formation: a novel mouse model for tracheal agenesis. Dev Biol. 2008;322(1):145‐155. 10.1016/j.ydbio.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Domyan ET, Ferretti E, Throckmorton K, Mishina Y, Nicolis SK, Sun X. Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development. 2011;138(5):971‐981. 10.1242/dev.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation. 2006;74(7):422‐437. 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 50. Billmyre KK, Hutson M, Klingensmith J. One shall become two: separation of the esophagus and trachea from the common foregut tube. Dev Dyn. 2015;244(3):277‐288. 10.1002/dvdy.24219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nasr T, Mancini P, Rankin SA, et al. Endosome‐mediated epithelial remodeling downstream of hedgehog‐Gli is required for Tracheoesophageal separation. Dev Cell. 2019;51(6):665‐674.e6. 10.1016/j.devcel.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim E, Jiang M, Huang H, et al. Isl1 regulation of Nkx2.1 in the early foregut epithelium is required for trachea‐esophageal separation and lung lobation. Dev Cell. 2019;51(6):675‐683.e4. 10.1016/j.devcel.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Woo J, Miletich I, Kim BM, Sharpe PT, Shivdasani RA. Barx1‐mediated inhibition of Wnt signaling in the mouse thoracic foregut controls Tracheo‐esophageal septation and epithelial differentiation. PLoS One. 2011;6(7):2‐9. 10.1371/journal.pone.0022493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kishimoto K, Tamura M, Nishita M, et al. Synchronized mesenchymal cell polarization and differentiation shape the formation of the murine trachea and esophagus. Nat Commun. 2018;9(1):2816. 10.1038/s41467-018-05189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Morrisey EE, Hogan BLM. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18(1):8‐23. 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141(3):502‐513. 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Montoro DT, Haber AL, Biton M, et al. A revised airway epithelial hierarchy includes CFTR‐expressing ionocytes. Nature. 2018;560(7718):319‐324. 10.1038/s41586-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3(9–10):545‐556. 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nikolić MZ, Caritg O, Jeng Q, et al. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long‐term self‐renewing organoids. elife. 2017;6:1‐33. 10.7554/eLife.26575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Que J, Luo X, Schwartz RJ, Hogan BLM. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136(11):1899‐1907. 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tompkins DH, Besnard V, Lange AW, et al. Sox2 activates cell proliferation and differentiation in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45(1):101‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang Y, Riccio P, Schotsaert M, et al. Spatial‐temporal lineage restrictions of embryonic p63+ progenitors establish distinct stem cell pools in adult airways. Dev Cell. 2018;44(6):752‐761.e4. 10.1016/j.devcel.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rock JR, Onaitis MW, Rawlins EL, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106(31):12771‐12775. 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BLM. Notch‐dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8(6):639‐648. 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang A, Schweitzer R, Sun D, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398(6729):714‐718. 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 66. Daniely Y, Liao G, Dixon D, et al. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004;287(1 56–1):171‐181. 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]
- 67. Miller AJ, Yu Q, Czerwinski M, et al. In vitro and in vivo development of the human airway at single‐cell resolution. Dev Cell. 2020;53(1):117‐128.e6. 10.1016/j.devcel.2020.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hawkins FJ, Suzuki S, Beermann ML, et al. Derivation of airway basal stem cells from human pluripotent stem cells. Cell Stem Cell. 2020;28(1):79‐95. 10.1016/j.stem.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Volckaert T, Yuan T, Chao CM, et al. Fgf10‐hippo epithelial‐Mesenchymal crosstalk maintains and recruits lung basal stem cells. Dev Cell. 2017;43(1):48‐59.e5. 10.1016/j.devcel.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rawlins EL, Okubo T, Xue Y, et al. The role of Scgb1a1+ Clara cells in the long‐term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4(6):525‐534. 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136(13):2297‐2307. 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Morimoto M, Liu Z, Cheng HT, Winters N, Bader D, Kopan R. Canonical notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J Cell Sci. 2010;123(2):213‐224. 10.1242/jcs.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Guha A, Vasconcelos M, Cai Y, et al. Neuroepithelial body microenvironment is a niche for a distinct subset of Clara‐like precursors in the developing airways. Proc Natl Acad Sci U S A. 2012;109(31):12592‐12597. 10.1073/pnas.1204710109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci U S A. 2009;106(23):9286‐9291. 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Song H, Yao E, Lin C, Gacayan R, Chen MH, Chuang PT. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc Natl Acad Sci U S A. 2012;109(43):17531‐17536. 10.1073/pnas.1207238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ouadah Y, Rojas ER, Riordan DP, Capostagno S, Kuo CS, Krasnow MA. Rare pulmonary neuroendocrine cells are stem cells regulated by Rb, p53, and notch. Cell. 2019;179(2):403‐416 e23. 10.1016/j.cell.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li Y, Linnoila RI. Multidirectional differentiation of achaete‐scute homologue‐1‐defined progenitors in lung development and injury repair. Am J Respir Cell Mol Biol. 2012;47(6):768‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kiyokawa H, Morimoto M. Notch signaling in the mammalian respiratory system, specifically the trachea and lungs, in development, homeostasis, regeneration, and disease. Develop Growth Differ. 2020;62(1):67‐79. 10.1111/dgd.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Noguchi M, Furukawa KT, Morimoto M. Pulmonary neuroendocrine cells: physiology, tissue homeostasis and disease. Dis Model Mech. 2020;13(12):1‐17. 10.1242/dmm.046920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Anderson PJ, Lynch TJ, Engelhardt JF. Multipotent myoepithelial progenitor cells are born early during airway submucosal gland development. Am J Respir Cell Mol Biol. 2017;56(6):716‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hegab AE, Ha VL, Gilbert JL, et al. Novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential. Stem Cells. 2011;29(8):1283‐1293. 10.1002/stem.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xie W, Fisher JT, Lynch TJ, et al. CGRP induction in cystic fibrosis airways alters the submucosal gland progenitor cell niche in mice. J Clin Invest. 2011;121(8):3144‐3158. 10.1172/JCI41857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lynch TJ, Anderson PJ, Xie W, et al. Wnt signaling regulates airway epithelial stem cells in adult murine submucosal glands. Stem Cells. 2016;34(11):2758‐2771. 10.1002/stem.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Liu X, Driskell RR, Engelhardt JF. Airway glandular development and stem cells. Curr Top Dev Biol. 2004;64:33‐56. https://doi.org10.1016/S0070-2153(04)64003-8. [DOI] [PubMed] [Google Scholar]
- 85. Driskell RR, Goodheart M, Neff T, et al. Wnt3a regulates Lef‐1 expression during airway submucosal gland morphogenesis. Dev Biol. 2007;305(1):90‐102. 10.1016/j.ydbio.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lynch TJ, Anderson PJ, Rotti PG, et al. Submucosal gland myoepithelial cells are reserve stem cells that can regenerate mouse tracheal epithelium. Cell Stem Cell. 2018;22(5):653‐667.e5. 10.1016/j.stem.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hines EA, Jones MKN, Verheyden JM, Harvey JF, Sun X. Establishment of smooth muscle and cartilage juxtaposition in the developing mouse upper airways. Proc Natl Acad Sci U S A. 2013;110(48):19444‐19449. 10.1073/pnas.1313223110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tollet J, Everett AW, Sparrow MP. Spatial and temporal distribution of nerves, ganglia, and smooth muscle during the early pseudoglandular stage of fetal mouse lung development. Dev Dyn. 2001;221(1):48‐60. 10.1002/dvdy.1124. [DOI] [PubMed] [Google Scholar]
- 89. Huycke TR, Miller BM, Gill HK, et al. Genetic and mechanical regulation of intestinal smooth muscle development. Cell. 2019;179(1):90‐105 e21. 10.1016/j.cell.2019.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ki H, Nakamura S, Nishida W, Sobue K. Bone morphogenetic protein‐induced Msx1 and Msx2 inhibit Myocardin‐dependent smooth muscle gene transcription. Mol Cell Biol. 2006;26(24):9456‐9470. 10.1128/mcb.00759-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cohen ED, Ihida‐Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Investig. 2009;119(9):2538‐2549. 10.1172/JCI38079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Goss AM, Tian Y, Cheng L, et al. Wnt2 signaling is necessary and sufficient to activate the airway smooth muscle program in the lung by regulating myocardin/Mrtf‐B and Fgf10 expression. Dev Biol. 2011;356(2):541‐552. 10.1016/j.ydbio.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mailleux AA, Kelly R, Veltmaat JM, et al. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development. 2005;132(9):2157‐2166. 10.1242/dev.01795. [DOI] [PubMed] [Google Scholar]
- 94. Nasr T, Holderbaum AM, Chaturvedi P, et al. Disruption of a hedgehog‐foxf1‐rspo2 signaling axis leads to tracheomalacia and a loss of sox9+ tracheal chondrocytes. Dis Model Mech. 2021;14(2):1‐13. 10.1242/dmm.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Turcatel G, Rubin N, Menke DB, Martin G, Shi W, Warburton D. Lung mesenchymal expression of Sox9 plays a critical role in tracheal development. BMC Biol. 2013;11:1‐15. 10.1186/1741-7007-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Akiyama H, Lyons JP, Mori‐Akiyama Y, et al. Interactions between Sox9 and β‐catenin control chondrocyte differentiation. Genes Dev. 2004;18(9):1072‐1087. 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hatakeyama Y, Tuan RS, Shum L. Distinct functions of BMP4 and GDF5 in the regulation of chondrogenesis. J Cell Biochem. 2004;91(6):1204‐1217. 10.1002/jcb.20019. [DOI] [PubMed] [Google Scholar]
- 98. Bell SM, Schreiner CM, Wert SE, Mucenski ML, Scott WJ, Whitsett JA. R‐spondin 2 is required for normal laryngeal‐tracheal, lung and limb morphogenesis. Development. 2008;135(6):1049‐1058. 10.1242/dev.013359. [DOI] [PubMed] [Google Scholar]
- 99. Sala FG, Del Moral PM, Tiozzo C, et al. FGF10 controls the patterning of the tracheal cartilage rings via Shh. Development. 2011;138(2):273‐282. 10.1242/dev.051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tiozzo C, De Langhe S, Carraro G, et al. Fibroblast growth factor 10 plays a causative role in the tracheal cartilage defects in a mouse model of Apert syndrome. Pediatr Res. 2009;66(4):386‐390. 10.1203/PDR.0b013e3181b45580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Park J, Zhang JJR, Moro A, Kushida M, Wegner M, Kim PCW. Regulation of Sox9 by sonic hedgehog (Shh) is essential for patterning and formation of tracheal cartilage. Dev Dyn. 2010;239(2):514‐526. 10.1002/dvdy.22192. [DOI] [PubMed] [Google Scholar]
- 102. Chacon‐Martinez CA, Koester J, Wickstrom SA. Signaling in the stem cell niche: regulating cell fate, function and plasticity. Development. 2018;145(15):1‐11. 10.1242/dev.165399. [DOI] [PubMed] [Google Scholar]
- 103. McDonald DM, Yao LC, Baluk P. Dynamics of airway blood vessels and lymphatics: lessons from development and inflammation. Proc Am Thorac Soc. 2011;8(6):504‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mitzner W, Lee W, Georgakopoulos D, Wagner E. Angiogenesis in the mouse lung. Am J Pathol. 2000;157(1):93‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. McCulley D, Wienhold M, Sun X. The pulmonary mesenchyme directs lung development. Curr Opin Genet Dev. 2015;32:98‐105. 10.1016/j.gde.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Peng T, Tian Y, Boogerd CJ, et al. Coordination of heart and lung co‐development by a multipotent cardiopulmonary progenitor. Nature. 2013;500(7464):589‐592. 10.1038/nature12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Asp M, Bergenstrahle J, Lundeberg J. Spatially resolved Transcriptomes‐next generation tools for tissue exploration. BioEssays. 2020;42(10):e1900221. 10.1002/bies.201900221. [DOI] [PubMed] [Google Scholar]
- 108. Vanhorn S, Morris SA. Next‐generation lineage tracing and fate mapping to interrogate development. Dev Cell. 2020;56(1):7‐21. 10.1016/j.devcel.2020.10.021. [DOI] [PubMed] [Google Scholar]


