Abstract
Reduction of [SmIII(COT1,4‐SiiPr3)(BH4)(thf)] (COT1,4‐SiiPr3=1,4‐( i Pr3Si)3C8H6) with KC8 resulted in [SmIII/II/III(COT1,4‐SiiPr3)4], the first example of a homoleptic lanthanide quadruple‐decker. As indicated by an analysis of the bond metrics in the solid‐state, the inner Sm ion is present in the divalent oxidation state, while the outer ones are trivalent. This observation could be confirmed by quantum chemical calculations. Mechanistic studies revealed not only insight into possible formation pathways of [SmIII/II/III(COT1,4‐SiiPr3)4] but also resulted in the transformation to other mixed metal sandwich complexes with unique structural properties. These are the 1D‐polymeric chain structured [KSmIII(COT1,4‐SiiPr3)] n and the hexametallic species [(tol)K(COT1,4‐SiiPr3)SmII(COT1,4‐SiiPr3)K]2 which were initially envisioned as possible building blocks as part of different retrosynthetically guided pathways that we developed.
Keywords: coordination chemistry, lanthanides, metallocenes, organometallic chemistry, quadruple-decker complexes
The synthesis of the first well‐defined neutral and homoleptic (same metal, same ligand) all‐carbon‐based quadruple‐decker complexes [SmIII/II/III 3(COT1,4‐SiiPr3)4] (COT1,4‐SiiPr3=1,4‐( i Pr3Si)3C8H6) along with other unique sandwich complexes is shown.

The synthesis and structural characterization of ferrocene [FeCp2] (Cp=C5H5) marked the beginning of a new era in modern organometallic chemistry 70 years ago. [1] Thereafter, sandwich complexes became widely used materials in all kinds of different fields ranging from basic research to industrial applications, fueling the quest for new synthetic approaches towards ever new compounds with striking properties and structural features. [2] In the course of this development, different cyclic, planar and π‐conjugated ligands with ring sizes from 4–9 carbon atoms,[ 1 , 2c , 2d , 3 ] varying peripheral substitution patterns and heteroatom incorporation were successfully employed to stabilize classical sandwich as well as multiple‐decker sandwich complexes. Especially in the case of d‐element and also f‐element sandwich complexes heterocyclic ring systems like boranes or carboranes as well as purely inorganic systems such as Pn5 (Pn=P, As, Sb) and P6 rings have facilitated the synthesis of different multi‐decker compounds. [4] Together with their exclusively carbon ligand‐based analogues, these have been subject to intense research. The interest in the compounds ranges from structural aspects to application approaches such as nano wires. Numerous multiple‐decker complexes have been reported all over the periodic table starting with [M(Cp)] (M=Li, Na, K) [5] s‐block poly‐decker assemblies over classical d‐metal triple‐decker complexes like the archetypal [Ni2(Cp)3][BF4] [6] to p‐block species as the cationic [Sn2(Cp*)3][Ga(C6F5)4] (Cp*=C5Me5) triple‐decker. [7] However, well‐defined neutral and homoleptic (same metal, same ligand) all‐carbon‐based quadruple‐decker complexes are to the best of our knowledge unknown.
In 4f‐element chemistry sandwich complex (e.g. [LnIII(COT)2]−; Scheme 1, I) and multiple‐decker chemistry is considerably underdeveloped and essentially limited to COT (COT=C8H8) derivatives as bridging ligand. [8] Typical examples are the heteroleptic [LnII 2(COT)(Cp*)2] (Ln=Sm, Eu, Yb), [9] the bent and asymmetric [(COT)LnIII(μ:η8,η2‐COT)LnIII(thf)2(COT)] (Ln=La, Ce, Nd, Er) [10] and [LnIII 2(COT′′)3] (Scheme 1, II; COT′′=1,4‐(Me3Si)3C8H6; Ln=La, Ce, Nd, Sm, Tb, Ho, Er, Tm, Lu) [11] triple‐decker compounds, as well as one example of a heteroleptic quadruple‐decker [Cp*2YbII 3(COT′′′)2] (COT′′′=1,3,6‐(Me3Si)3C8H5). [13]
Scheme 1.
Examples for homoleptic lanthanide COT sandwich complexes. Left: Anionic [Ln(COT)2]− sandwich complexes (cation omitted for clarity). [12] Middle: [LnIII 2(COT′′)3] triple‐decker. [11] Right: [SmIII/II/III 3(COT1,4‐SiiPr3)4] (1) quadruple‐decker described in this work.
Aside from these homometallic species, mixed metallic Ln‐K quadruple‐deckers of the type [K2(thf)4LnIII 2(COT)4] (Ln=Gd, Er) [14] have been isolated. Additionally, homoleptic [LnII/III n (COT) m ] (Ln=Sc, Y, La, Ce, Nd, Eu, Tb, Ho, Tm, Yb; up to n=30 for Eu; m=n, n−1, n+1) poly‐decker compounds have been extensively studied as molecular nanowires. However, these species, which are usually prepared by combination of laser vaporization and molecular beam methods as a mixture of compounds are not accessible as well‐defined species via standard synthetic methods. [15] Note, complexes ligated by nitrogen‐based macrocycles like tetrapyrrolates are not considered as sandwich complexes from a classical point of view, as they do not have planar and π‐conjugated aromatic ligands and do not feature Ln−C bonds. [16]
Despite decades of research in this area, to the best of our knowledge homoleptic quadruple‐decker complexes of the f‐elements are unknown. This missing link of textbook complexes may be theoretically constructed from four COT‐ligands and three lanthanide ions. The corresponding neutral compound would necessarily have to be mixed‐valent (vide infra). While examples for mixed‐valent d‐metal carbon‐based sandwich compounds have been known for a long time, [17] analogue 4f species are hitherto unknown.
Herein, we present the unprecedented synthesis and characterization of the mixed‐valent and homoleptic samarium quadruple‐decker [SmIII/II/III 3(COT1,4‐SiiPr3)4] (COT1,4‐SiiPr3=1,4‐(iPr3Si)3C8H6) by partial reduction of [Sm(COT1,4‐SiiPr3)(BH4)(thf)] with KC8.
The synthesis of a neutral COT‐based homoleptic lanthanide quadruple‐decker requires one divalent and two trivalent lanthanide ions to balance the eightfold negative charge of four COT ligands in the anticipated stacked assembly (Scheme 2, left). We developed some retrosynthetic pathways towards a rational approach and defined a few requirements. These are: i) As center metal a lanthanide is needed, which is sufficiently stable in di‐ and trivalent oxidation state to allow the synthesis of the necessary building blocks in both oxidation states. As COT ligands are known to act as reducing agents towards YbIII and COT‐EuIII species are completely unknown, samarium was chosen based on literature data. [18] ii) Solubility and steric protection: the bulky disilylated COT1,4‐SiiPr3 was employed as ligand. As result, [SmIII/II/III 3(COT1,4‐SiiPr3)4] (1, Scheme 2) was defined as target compound.
Scheme 2.
Possible retrosynthetic fragmentation of a Sm(III/II/III) quadruple‐decker to its formal building blocks. The synthons 2′, 3′ and 4′ are depicted structurally simplified and do not correspond to the actual structures of the synthetic equivalents 2, 3 and 4 as determined by single‐crystal X‐ray diffraction.
Next, three retro‐synthetic pathways were defined: A) [SmIII/II/III 3(COT1,4‐SiiPr3)4] (1) can be interpreted as the adduct of the trivalent synthon [SmIII(COT1,4‐SiiPr3)2]− (2′) with a SmII dication (route A Scheme 2). B) [SmIII(COT1,4‐SiiPr3)]+ (3′) may react with the divalent synthon [SmII(COT1,4‐SiiPr3)2]2− (4′) to give 1 (route B Scheme 2). Routes A and B are presumed to be accessible by salt elimination reactions. C) partial in situ reduction of a suitable [SmIII(COT1,4‐SiiPr3)]+ (3′) synthon (route C Scheme 2).
First, we synthesized and characterized the corresponding synthetic equivalents of the proposed synthons. These are [KSmIII(COT1,4‐SiiPr3)] n (2) as reagent for 2′, the elsewhere reported compound [SmIII(COT1,4‐SiiPr3)(BH4)(thf)] (3), [19] as reagent for 3′ and [(tol)K(COT1,4‐SiiPr3)SmII(COT1,4‐SiiPr3)K]2 (4) as reagent for 4′ (Scheme 3).
Scheme 3.
Synthesis of [KSmIII(COT1,4‐SiiPr3)] n 2 and [(tol)K(COT1,4‐SiiPr3)SmII(COT1,4‐SiiPr3)K]2 4.
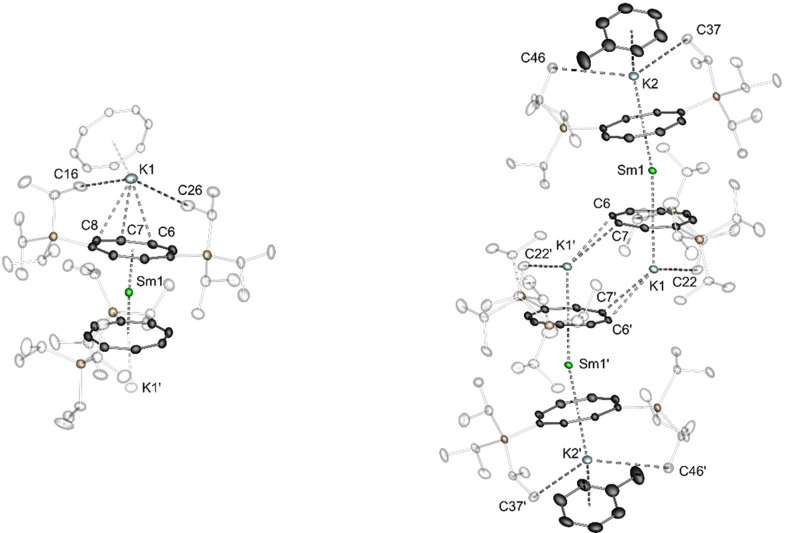
The trivalent building block [KSmIII(COT1,4‐SiiPr3)] n (2), was obtained from SmI3 and [K2(COT1,4‐SiiPr3)] [20] at room temperature in THF (Scheme 3, Figure 1). Compound 2 exhibits a Lewis‐base‐free polymeric structure in the solid state when crystallized from toluene. The repeating [SmIII(COT1,4‐SiiPr3)2]− moieties are interconnected by potassium cations in a μ‐η3:η8 bridging mode (Figure 1 and Figure S19). This is in sharp contrast to K[LnIII(COT)2] compounds, which usually only crystallize, when the potassium atom is coordinated by ethereal solvents, crown ethers or cryptands. In this case the formation of monomeric complexes is observed. [21] One example for a similar polymeric compound is [Li(dme)Tb(COT′′)2] n , which was reported to crystallize as a Li‐bridged polymer from n pentane. [22] In compound 2 the potassium ions are each bound to one neighboring COT1,4‐SiiPr3‐ligand in a η8‐mode (K1‐Ct2=2.4996(6) Å) while the opposing ligand is coordinated in a η3‐allyl‐type‐mode (K1‐C6=3.123(2); C7=2.883(2); C8=3.170(2)) in [KSmIII(COT1,4‐SiiPr3)] n . (2). The bent K‐COT1,4‐SiiPr3 fragment leads to a zigzag‐type polymer chain with a bending angle of 149.78(2)° (Ct1‐K1‐Ct2) at the potassium positions. The open face of the potassium atom is sterically shielded by isopropyl groups of the η8‐coordinated COT1,4‐SiiPr3 ligand in the solid state. As expected, the samarium ions are each μ‐η8:η8 coordinated by two COT1,4‐SiiPr3 ligands with Sm‐Ct (Ct=centroid) distances of 1.9718(4) Å (Ct1) and 2.0128(4) Å (Ct2) and a Ct1‐Sm1‐Ct2 bending angle of 171.32(2)°. The Ct‐Sm‐Ct angle slightly deviates from the perfectly linear 180° arrangement reported for the unsubstituted complex. [21b]
Figure 1.
Left: Cut‐out of the polymeric structure of [KSmIII(COT COT1,4‐SiiPr3)] n 2 in the solid‐state. Right: Molecular structure of [(tol)K(COT COT1,4‐SiiPr3)SmII(COT1,4‐SiiPr3)K]2 4 in the solid‐state. Only one part of the disordered toluene ligands is depicted. Triisopropylsilyl‐groups are transparent and hydrogen atoms are omitted for clarity. For detailed bond lengths and angles see Figures S20 and S21. Sm green, K sky blue, Si tan, C dark grey.
The divalent building block [(tol)K(COT1,4‐SiiPr3)SmII(COT1,4‐SiiPr3)K]2 (4), which was synthesized from [SmIII2(thf)2] and [K2(COT1,4‐SiiPr3)], [20] does not form a polymeric structure in the solid state when crystallized from toluene (Scheme 3). Instead, two K2[SmII(COT1,4‐SiiPr3)2] units formally dimerize, whereas the outer potassium ions are capped by toluene molecules (Figure 1). The resulting structure can be considered as Sm2/K4 hexa‐decker with an interconnecting slipped dipotassium middle deck. This is to the best of our knowledge an unprecedented structural motif in lanthanide coordination chemistry, which is in stark contrast to reported, unsubstituted COT‐based [(K(L) n )2LnII(COT)2] (L=thf (n=3), 18‐c‐6 (n=1); Ln=Eu, Tm, Yb) species. Similar to the above‐described trivalent compounds, these are commonly isolated as monomeric species where the potassium ions are saturated by Lewis‐base‐ligands. [23] The inner potassium ions are each bound to one COT1,4‐SiiPr3 ligand in the expected η8‐coordination mode (K1‐Ct1=2.3000(5) and connected to the neighboring K2[SmII(COT1,4‐SiiPr3)2] fragment via a η2‐bonding mode to a COT1,4‐SiiPr3 ligand (K‐C6′=3.045(2) Å and K‐C7′=3.168(2) Å).
Similar to compound 2, isopropyl groups of the COT1,4‐SiiPr3 ligand sterically shield the open potassium atom faces of the inner complex fragment. The [SmII(COT1,4‐SiiPr3)2]2− fragments exhibit a twofold η8‐coordinated Sm ion with Sm‐Ct distances of 2.1876(3) Å (Ct1) and 2.1541(3) Å (Ct2) and a Ct1‐Sm‐Ct2 bending angle of 168.98(2)°.
The Ln‐Ct values are in good agreement with the Eu‐Ct distance of 2.153 Å in [(K(thf)3)2EuII(COT)2]. In contrast, the strong bending of this fragment is rather surprising compared to literature examples: All reported [(K(thf)3)2LnII(COT)2] (Ln=Eu, Tm, Yb) compounds exhibit a [LnII(COT)2]2− moiety with almost no deviation from linearity. [23] Solely [(K(18‐c‐6))2TmII(COT)2] features a bent central complex unit with a Ct‐Tm‐Ct angle of 173.9°. Consequently, the coordination spheres of the potassium cations seem to play a significant role for the electrostatic bonding interactions in the lanthanide sandwich fragment. The outer potassium deck is assembled by a η8‐bound COT1,4‐SiiPr3 ligand (K2‐Ct2=2.2832(5) Å) and a disordered η6‐coordinated toluene moiety (K2‐Ct3A/B=2.9041(5)/2.8874(5) Å) with a bending angle of Ct2‐K‐Ct3A/B of 148.90(2)/155.30(2)°. In agreement with the other potassium‐COT1,4‐SiiPr3 assemblies described before, steric shielding by isopropyl groups stabilizes the outer fragments of the hexa‐decker type sandwich complex. Aside from investigations towards the application of complex 4 as novel reducing agent in organic and inorganic transformation, the unprecedented hexa‐decker motif is of great interest in terms of a possible thulium analogue. Especially, considering recent advances in the synthesis of divalent thulium SMMs. [23c]
Following the strategies outlined in Scheme 2, [KSmIII(COT1,4‐SiiPr3)] n (2) was reacted with [SmIII2(thf)2] (Scheme 2, A and Scheme 4 top) first. Surprisingly, we were not able to isolate the desired product from these attempts. Only intractable product mixtures were obtained even in different solvents and under varying conditions. Also the reaction of [(tol)K(COT1,4‐SiiPr3)SmII(COT1,4‐SiiPr3)K]2 (4) with [SmIII(COT1,4‐SiiPr3)(BH4)(thf)] (3) (Scheme 2, B) in different solvents and under varying conditions did not result in the desired product. Instead, only the trivalent compound [KSmIII(COT1,4‐SiiPr3)] n (2) could be isolated as the sole crystalline product (experimental details for all attempts can be found in the SI, page S6). This indicates that ligand rearrangement pathways might be active in addition to the anticipated salt elimination. The fate of the employed divalent SmII species could not be clarified. Following the unsuccessful salt elimination approach, we aimed to synthesize the mixed valent quadruple‐decker by partial reduction of a trivalent precursor (Scheme 2, C), which might induce ligand rearrangement and formation of the otherwise inaccessible quadruple‐decker. Indeed, 3 rapidly reacts with KC8 at −78 °C as indicated by a quick color change from deep purple to brown. The desired quadruple‐decker 1 can be isolated as a yellow‐brown crystalline solid in 48 % yield (as single crystals based on the proposed side products listed in Scheme 4) after workup. The molecular structure of 1 in the solid state consists of four COT1,4‐SiiPr3 ligands and three Sm ions forming the anticipated homoleptic quadruple‐decker motif (Figure 2). As result of the partial reduction one of these three Sm ions is in the divalent oxidation state. The resulting eightfold positive charge of all Sm atoms is balanced by the eightfold negative charge of four COT1,4‐SiiPr3 ligands. An initial analysis of Sm‐Ct distances in compound 1 suggests, that the inner samarium ion Sm2 is the divalent one, as the Sm‐Ct distances (Sm2‐Ct2=2.2262(4) Å and Sm2‐Ct3=2.2157(4) Å) are longer than the corresponding distances for the outer samarium ions Sm1 and Sm3 (Sm1‐Ct1=1.8803(4) Å, Sm1‐Ct2=2.1336(4) Å and Sm3‐Ct3=2.1594(4) Å, Sm3‐Ct4=1.8942(3) Å). The inner Sm‐Ct distances are in good agreement with the corresponding Sm‐Ct distances in 4 (vide supra), underpinning a III/II/III oxidation state distribution in the sandwich complex.
Scheme 4.
Transformations based on the SmII/III‐ COT1,4‐SiiPr3 system, that are accessible by reduction with KC8. The denotation “NMR” on reaction arrows indicates that the reaction was performed on an NMR scale and no yields were determined.
Figure 2.

Molecular structure of [SmIII/II/III 3(COT1,4‐SiiPr3)4] 1 in the solid‐state. Triisopropylsilyl‐groups are transparent, one disordered molecule of diethyl ether and hydrogen atoms are omitted for clarity. For detailed bond lengths and angles see Figures S19. Sm green, Si tan, C dark grey.
Interestingly, the presumably trivalent outer samarium ions Sm1 and Sm3 exhibit a significantly longer distance to the corresponding inner COT1,4‐SiiPr3 ligands compared to the outer ones. This implies that the bonding situation in compound 1 resembles a dianionic [SmII(COT1,4‐SiiPr3)2]2− fragment that is capped by two [SmIII(COT1,4‐SiiPr3)]+ cations. The COT1,4‐SiiPr3 ligands adapt a staggered conformation with an average torsion angle of 84°. Additionally, the complete sandwich complex is slightly bent with a Sm1‐Sm2‐Sm3 angle of 156.508(13)° whereas the inner Sm(COT1,4‐SiiPr3)2 unit (Ct2‐Sm2‐Ct3=159.59(2)°) is bent stronger than the outer ones (Ct1‐Sm1‐Ct2=172.75(2)° and Ct3‐Sm3‐Ct4=168.49(2)°). The bent metallocene structure of the presumably divalent fragment is even more pronounced than in complex 4 and to the best of our knowledge unprecedented for divalent Ln‐COT complexes. A possible explanation is the presence of eight bulky i Pr3Si groups in the quadruple‐decker, inducing a comparatively strong bending of the central fragment to relief steric pressure from the system.
To support the structure‐implied hypothesis of a mixed valent quadruple‐decker complex with a III/II/III oxidation state distribution, quantum chemical calculations [24] at DFT level (PBE0 [25] /def2‐SV(P) [26] ) were performed for 1. With the Fermi‐smearing approach [27] applied to the calculations of the experimental structure, a high‐spin state with altogether 16 unpaired electrons was found. For this state, the valence s/p/d/f contributions from the Sm atoms to total and spin density were calculated with the natural population analysis [28] and compared to that of the typical SmII and SmIII compounds SmIIX2 and SmIIIX3, (X=Cl, Br, I). The results are shown in Table 1. Concerning the total density, the most striking differences of SmIIX2 compared to SmIIIX3 are found in the contributions of the f‐ and the d‐orbitals. For SmIII, f/d valence orbital contributions amount to ca. 5.26/0.80 electrons, for SmII to 6.01/0.26 electrons. The f/d contributions of Sm1, 5.40/0.87 and Sm3, 5.41/0.84 electrons, are very close to that of SmIIIX3, while that of Sm2, 5.99/0.33 electrons, are very close to that of SmIIX2. s and p contributions are small and similar for all situations and compounds. For the spin density, the f‐orbital contributions again show the corresponding agreements within 0.1 electrons, and the other contributions are below 0.1 electrons. Thus, the assignment of oxidation states +3 for the outer atoms Sm1 and Sm3 and +2 for the inner Sm2 is very well supported by the calculations. Also, interpreting 1 as dianionic [SmII(COT1,4‐SiiPr3)2]2− capped by two [SmIII(COT1,4‐SiiPr3)]+ cations (vide supra) is in line with the calculated partial charges for the three parts, amounting to −1.22 and twice +0.61. We finally note that the magnetic coupling between the Sm atoms is small. The difference of the high‐spin state to the broken‐symmetry state (with a surplus of six beta electrons for Sm2) amounts to less than 0.5 kJ mol−1; moreover, the s/p/d/f contributions of the Sm atoms for this state are virtually identical to that for the high‐spin state, see Table S5.
Table 1.
Valence s/p/d/f populations (sα+sβ etc.) of Sm1, Sm2, Sm3 in 1 and mean values of for Sm in SmIIIX3 and SmIIX2 (X=Cl, Br, I) obtained with the natural population analysis of the total density, as well as corresponding numbers of unpaired electrons (sα‐sβ etc.) obtained from the spin density with the same method. The numbers for X=Cl, Br, I show high similarity and are given in the Supporting Information (Table S5).
|
Valence population |
SmIIIX3 |
Sm1 in 1 |
Sm3 in 1 |
SmIIX2 |
Sm2 in 1 |
|---|---|---|---|---|---|
|
sα+sβ |
0.198 |
0.074 |
0.073 |
0.099 |
0.054 |
|
pα+pβ |
0.038 |
0.000 |
0.000 |
0.047 |
0.007 |
|
dα+dβ |
0.800 |
0.870 |
0.836 |
0.262 |
0.334 |
|
fα+fβ |
5.259 |
5.395 |
5.414 |
6.010 |
5.978 |
|
sα‐sβ |
0.013 |
0.015 |
0.014 |
0.013 |
0.011 |
|
pα‐pβ |
0.017 |
0.057 |
0.053 |
0.037 |
0.048 |
|
dα‐dβ |
0.068 |
0.090 |
0.086 |
0.040 |
0.052 |
|
fα‐fβ |
5.188 |
5.332 |
5.356 |
5.989 |
5.957 |
To gain some insight into the formation mechanism of the quadruple‐decker 1 NMR experiments were performed. Especially the origin of the fourth COT1,4‐SiiPr3 ligand is in question at this point. The stoichiometry of the reaction can either be balanced by the formation of [Sm(BH4)3(thf)3] or elemental Sm0, both of which explain the presence of four ligands per three Sm ions. The existence of [Sm(BH4)3(thf)3] in the reaction mixture can easily be precluded, as no signs of this species could be found in the bulk reaction mixture or in NMR experiments. Additionally, it could be shown that [Sm(BH4)3(thf)3] is not stable under the here applied reducing conditions. NMR‐scale reactions of [Sm(BH4)3(thf)3] with KC8 in [D8]THF result in silent 1H‐ and 11B‐NMR spectra, accompanied by the formation of a dark grey solid, presumably elemental samarium. [29] Likewise, the simultaneous formation of Sm0 and [K2(COT1,4‐SiiPr3)] by reduction of the starting material 3 seems feasible. To further proof the presence of [K2(COT1,4‐SiiPr3)], NMR experiments with varying KC8 ratios were performed (Scheme 4). Reaction of 3 and KC8 in a 4:1 ratio in d8‐THF yields a 2:1 mixture of complexes 2 and 3 (Figure S12). Compound 2 could be produced by salt elimination between 3 and in situ formed [K2(COT1,4‐SiiPr3)]. Residual starting material 3 can be explained by insufficient amounts of KC8 for full conversion. If 3 and KC8 are conversely reacted in a 1:4 ratio in d8‐THF, clean and quantitative conversion to the divalent compound 4 was observed. In concordance with the 4:1 reaction this can again be explained by formation of [K2(COT1,4‐SiiPr3)] which quickly reacts to 2. The trivalent species 2 is subsequently reduced by excess KC8 to give compound 4 (Scheme 4). A similar reactivity was recently described for the reduction of trivalent [K(thf)3Tm(COT)2]. [23c] Likewise, isolated 2 can be reduced by KC8 to give 4 in an NMR scale reaction. Finally, the 1:1 reaction of 3 and KC8 yields the quadruple‐decker 1, as already described above. Consequently, 1, 2, and 4 can be formed by reducing 3 with KC8 depending on the exact stoichiometric ratio of the precursors. This rather surprising diversity of possible transformations in this simple system is depicted in Scheme 4. Moreover, it is feasible that the formation of the quadruple‐decker 1 is initiated by complete reduction of parts of the employed starting material 3 to give Sm0 and [K2(COT1,4‐SiiPr3)], which reacts with excess starting material to form higher sandwich aggregates. Nevertheless, the exact mechanism of the reaction cannot be fully deduced by these findings. Most notably the isolated hexa‐decker 4, which was found to be the end point of the reaction with an excess of reducing agent, does not react with 3 in bulk or NMR reactions as anticipated (Scheme 1, route B). Similarly, isolated 2 cannot be fused by divalent samarium sources (Scheme 1, route A). As a result, additional transient species must exist in the reaction cascade, yielding the title compound 1.
Our findings highlight that the structural diversity of 4f‐element metallocenes is still far from being exhausted. During our attempts to rationally synthesize a homoleptic samarium quadruple‐decker, two building blocks with unique structural properties could be isolated. These are likely induced by the peripheral substitution pattern of the employed COT1,4‐SiiPr3 ligands. Especially the eye‐catching molecular solid‐state structure of compound 2 with its mixed‐metallic hexa‐decker type sandwich assembly demonstrates that ligand substitution can greatly influence the structure of lanthanide‐COT metallocenes. Moreover, we could show that the reduction of the half‐sandwich complex 3 with KC8 yields the archetypal homoleptic quadruple‐decker 1. This metallocene is the first homoleptic quadruple‐decker of the f‐elements and due to the inherent need of charge balance the only classical mixed valent sandwich assembly of the lanthanides reported to date. Additionally, NMR experiments to probe the influence of the stoichiometric ratio (3/KC8) on the formation of 1 unveiled a surprising variety of reductive transformations that are accessible starting from compound 3. Therefore, we hope that our findings will, aside from their importance in basic research and structural chemistry, stimulate further investigations in this area based on uncommon reaction pathways in this highly flexible and versatile system.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Acknowledgements
Xiaofei Sun, Christina Zovko and Helga Berberich are acknowledged for support of NMR‐experiments. Deutsche Forschungsgemeinschaft (DFG) is acknowledged for financial support within the Reinhart Koselleck‐Projekt 440644676, RO 2008/19‐1. Open Access funding enabled and organized by Projekt DEAL.
L. Münzfeld, A. Hauser, P. Hädinger, F. Weigend, P. W. Roesky, Angew. Chem. Int. Ed. 2021, 60, 24493.
Dedicated to Professor Glen B. Deacon on the occasion of his 85th birthday
Contributor Information
Prof. Dr. Florian Weigend, Email: florian.weigend@chemie.uni-marburg.de.
Prof. Dr. Peter W. Roesky, Email: roesky@kit.edu.
References
- 1.
- 1a. Kealy T. J., Pauson P. L., Nature 1951, 168, 1039; [Google Scholar]
- 1b. Fischer E. O., Pfab W., Z. Naturforsch. B 1952, 7, 377; [Google Scholar]
- 1c. Wilkinson G., Rosenblum M., Whiting M. C., Woodward R. B., J. Am. Chem. Soc. 1952, 74, 2125. [Google Scholar]
- 2.
- 2a. Štěpnička P., Ferrocenes: Ligands, Materials and Biomolecules, Wiley, Chichester, 2008; [Google Scholar]
- 2b. Elschenbroich C., Organometallchemie, Vol. 6, Vieweg+Teubner Verlag, Wiesbaden, 2008; [Google Scholar]
- 2c. Fischer E. O., Hafner W., Z. Naturforsch. B 1955, 10, 665; [Google Scholar]
- 2d. Streitwieser A., Mueller-Westerhoff U., J. Am. Chem. Soc. 1968, 90, 7364; [Google Scholar]
- 2e. Guo F.-S., Day B. M., Chen Y.-C., Tong M.-L., Mansikkamäki A., Layfield R. A., Science 2018, 362, 1400. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a. Riley P. E., Davis R. E., J. Organomet. Chem. 1976, 113, 157; [Google Scholar]
- 3b. Engebretson G., Rundle R. E., J. Am. Chem. Soc. 1963, 85, 481; [Google Scholar]
- 3c. Kawasaki K., Sugiyama R., Tsuji T., Iwasa T., Tsunoyama H., Mizuhata Y., Tokitoh N., Nakajima A., Chem. Commun. 2017, 53, 6557; [DOI] [PubMed] [Google Scholar]
- 3d. Xémard M., Zimmer S., Cordier M., Goudy V., Ricard L., Clavaguéra C., Nocton G., J. Am. Chem. Soc. 2018, 140, 14433; [DOI] [PubMed] [Google Scholar]
- 3e. Münzfeld L., Schoo C., Bestgen S., Moreno-Pineda E., Köppe R., Ruben M., Roesky P. W., Nat. Commun. 2019, 10, 3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.
- 4a. Scherer O. J., Schwalb J., Swarowsky H., Wolmershäuser G., Kaim W., Gross R., Chem. Ber. 1988, 121, 443; [Google Scholar]
- 4b. Scherer O. J., Brück T., Wolmershäuser G., Chem. Ber. 1989, 122, 2049; [Google Scholar]
- 4c. Scherer O. J., Blath C., Wolmershäuser G., J. Organomet. Chem. 1990, 387, C21; [Google Scholar]
- 4d. Scherer O. J., Sitzmann H., Wolmershäuser G., Angew. Chem. Int. Ed. Engl. 1985, 24, 351; [Google Scholar]; Angew. Chem. 1985, 97, 358; [Google Scholar]
- 4e. Grimes R. N., J. Organomet. Chem. 1999, 581, 1; [Google Scholar]
- 4f. Breunig H. J., Burford N., Rösler R., Angew. Chem. Int. Ed. 2000, 39, 4148; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2000, 112, 4320; [Google Scholar]
- 4g. Ghosh S., Beatty A. M., Fehlner T. P., J. Am. Chem. Soc. 2001, 123, 9188; [DOI] [PubMed] [Google Scholar]
- 4h. Yao H., Grimes R. N., Organometallics 2003, 22, 4539; [Google Scholar]
- 4i.R. N. Grimes, Carboranes, Vol. 3, London, Academic Press, 2016;
- 4j. Beck V., O'Hare D., J. Organomet. Chem. 2004, 689, 3920; [Google Scholar]
- 4k. Herberich G. E., Englert U., Ganter B., Lamertz C., Organometallics 1996, 15, 5236; [Google Scholar]
- 4l. Herberich G. E., Ganter B., Organometallics 1997, 16, 522; [Google Scholar]
- 4m. Kuwabara T., Guo J.-D., Nagase S., Sasamori T., Tokitoh N., Saito M., J. Am. Chem. Soc. 2014, 136, 13059; [DOI] [PubMed] [Google Scholar]
- 4n. Mills D. P., Evans P., Chem. Eur. J. 2021, 27, 6645.33453062 [Google Scholar]
- 5. Dinnebier R. E., Behrens U., Olbrich F., Organometallics 1997, 16, 3855. [Google Scholar]
- 6. Salzer A., Werner H., Angew. Chem. Int. Ed. Engl. 1972, 11, 930; [Google Scholar]; Angew. Chem. 1972, 84, 949. [Google Scholar]
- 7. Cowley A. H., Macdonald C. L. B., Silverman J. S., Gorden J. D., Voigt A., Chem. Commun. 2001, 175. [Google Scholar]
- 8. Edelmann F. T., New J. Chem. 2011, 35, 517. [Google Scholar]
- 9.
- 9a. Evans W. J., Clark R. D., Ansari M. A., Ziller J. W., J. Am. Chem. Soc. 1998, 120, 9555; [Google Scholar]
- 9b. Evans W. J., Johnston M. A., Greci M. A., Ziller J. W., Organometallics 1999, 18, 1460; [Google Scholar]
- 9c. Evans W. J., Johnston M. A., Clark R. D., Ziller J. W., J. Chem. Soc. Dalton Trans. 2000, 1609. [Google Scholar]
- 10. DeKock C. W., Ely S. R., Hopkins T. E., Brault M. A., Inorg. Chem. 1978, 17, 625. [Google Scholar]
- 11.
- 11a. Poremba P., Edelmann F. T., J. Organomet. Chem. 1998, 553, 393; [Google Scholar]
- 11b. Lorenz V., Blaurock S., Hrib C. G., Edelmann F. T., Organometallics 2010, 29, 4787; [Google Scholar]
- 11c. Le Roy J. J., Jeletic M., Gorelsky S. I., Korobkov I., Ungur L., Chibotaru L. F., Murugesu M., J. Am. Chem. Soc. 2013, 135, 3502; [DOI] [PubMed] [Google Scholar]
- 11d. Lorenz V., Liebing P., Bathelier A., Engelhardt F., Maron L., Hilfert L., Busse S., Edelmann F. T., Chem. Commun. 2018, 54, 10280; [DOI] [PubMed] [Google Scholar]
- 11e. Edelmann A., Lorenz V., Hrib C. G., Hilfert L., Blaurock S., Edelmann F. T., Organometallics 2013, 32, 1435. [Google Scholar]
- 12. Mares F., Hodgson K., Streitwieser A., J. Organomet. Chem. 1970, 24, C68. [Google Scholar]
- 13. Edelmann A., Blaurock S., Lorenz V., Hilfert L., Edelmann F. T., Angew. Chem. Int. Ed. 2007, 46, 6732; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 6855. [Google Scholar]
- 14.
- 14a. Xia J., Jin Z., Chen W., J. Chem. Soc. Chem. Commun. 1991, 1214; [Google Scholar]
- 14b. Le Roy J. J., Ungur L., Korobkov I., Chibotaru L. F., Murugesu M., J. Am. Chem. Soc. 2014, 136, 8003. [DOI] [PubMed] [Google Scholar]
- 15.
- 15a. Hayes R. G., Thomas J. L., J. Am. Chem. Soc. 1969, 91, 6876; [Google Scholar]
- 15b. Kurikawa T., Negishi Y., Hayakawa F., Nagao S., Miyajima K., Nakajima A., Kaya K., J. Am. Chem. Soc. 1998, 120, 11766; [Google Scholar]
- 15c. Hosoya N., Takegami R., Suzumura J.-i., Yada K., Koyasu K., Miyajima K., Mitsui M., Knickelbein M. B., Yabushita S., Nakajima A., J. Phys. Chem. A 2005, 109, 9; [DOI] [PubMed] [Google Scholar]
- 15d. Miyajima K., Knickelbein M. B., Nakajima A., J. Phys. Chem. A 2008, 112, 366; [DOI] [PubMed] [Google Scholar]
- 15e. Lee J. S., Lei Y., Kumari S., Yang D.-S., J. Chem. Phys. 2009, 131, 104304; [Google Scholar]
- 15f. Hosoya N., Takegami R., Suzumura J.-i., Yada K., Miyajima K., Mitsui M., Knickelbein M. B., Yabushita S., Nakajima A., J. Phys. Chem. A 2014, 118, 8298; [DOI] [PubMed] [Google Scholar]
- 15g. Huttmann F., Schleheck N., Atodiresei N., Michely T., J. Am. Chem. Soc. 2017, 139, 9895. [DOI] [PubMed] [Google Scholar]
- 16.
- 16a. Jiang J., Ng D. K. P., Acc. Chem. Res. 2009, 42, 79; [DOI] [PubMed] [Google Scholar]
- 16b. Fukuda T., Biyajima T., Kobayashi N., J. Am. Chem. Soc. 2010, 132, 6278; [DOI] [PubMed] [Google Scholar]
- 16c. Deacon G. B., Guo Z., Junk P. C., Wang J., Angew. Chem. Int. Ed. 2017, 56, 8486; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 8606. [Google Scholar]
- 17. Wang X., Sabat M., Grimes R. N., J. Am. Chem. Soc. 1995, 117, 12227. [Google Scholar]
- 18.
- 18a. Cotton S., Lanthanide and Actinide Chemistry, Wiley, Chichester, 2006; [Google Scholar]
- 18b. Edelmann A., Hrib C. G., Blaurock S., Edelmann F. T., J. Organomet. Chem. 2010, 695, 2732. [Google Scholar]
- 19.
- 19a.L. Münzfeld, X. Sun, S. Schlittenhardt, A. Hauser, C. Schoo, S. Gillhuber, F. Weigend, M. Ruben, P. W. Roesky, unpublished results; [DOI] [PMC free article] [PubMed]
- 19b. Cendrowski-Guillaume S. M., Le Gland G., Nierlich M., Ephritikhine M., Organometallics 2000, 19, 5654. [Google Scholar]
- 20. Summerscales O. T., Cloke F. G. N., Hitchcock P. B., Green J. C., Hazari N., Science 2006, 311, 829. [DOI] [PubMed] [Google Scholar]
- 21.
- 21a. Hodgson K. O., Raymond K. N., Inorg. Chem. 1972, 11, 3030; [Google Scholar]
- 21b. Meihaus K. R., Long J. R., J. Am. Chem. Soc. 2013, 135, 17952; [DOI] [PubMed] [Google Scholar]
- 21c. Ungur L., Le Roy J. J., Korobkov I., Murugesu M., Chibotaru L. F., Angew. Chem. Int. Ed. 2014, 53, 4413; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 4502; [Google Scholar]
- 21d. Palumbo C. T., Fieser M. E., Ziller J. W., Evans W. J., Organometallics 2017, 36, 3721. [Google Scholar]
- 22. Lorenz V., Edelmann A., Blaurock S., Freise F., Edelmann F. T., Organometallics 2007, 26, 6681. [Google Scholar]
- 23.
- 23a. Kinsley S. A., Streitwieser A., Zalkin A., Organometallics 1985, 4, 52; [Google Scholar]
- 23b. Evans W. J., Shreeve J. L., Ziller J. W., Polyhedron 1995, 14, 2945; [Google Scholar]
- 23c. Moutet J., Schleinitz J., La Droitte L., Tricoire M., Pointillart F., Gendron F., Simler T., Clavaguéra C., Le Guennic B., Cador O., Nocton G., Angew. Chem. Int. Ed. 2021, 60, 6042; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 6107. [Google Scholar]
- 24.TURBOMOLE Version 7.5.1, TURBOMOLE GmbH 2021. TURBOMOLE is a development of University of Karlsruhe and Forschungszentrum Karlsruhe 1989–2007, TURBOMOLE GmbH since 2007.
- 25. Perdew J. P., Ernzerhof M., Burke K., J. Chem. Phys. 1996, 105, 9982. [Google Scholar]
- 26.
- 26a. Weigend F., Ahlrichs R., Phys. Chem. Chem. Phys. 2005, 7, 3297; [DOI] [PubMed] [Google Scholar]
- 26b. Gulde R., Pollak P., Weigend F., J. Chem. Theory Comput. 2012, 8, 4062. [DOI] [PubMed] [Google Scholar]
- 27. Rabuck A. D., Scuseria G. E., J. Chem. Phys. 1999, 110, 695. [Google Scholar]
- 28. Reed A. E., Weinstock R. B., Weinhold F., J. Chem. Phys. 1985, 83, 735. [Google Scholar]
- 29. Schöttle C., Rudel S., Popescu R., Gerthsen D., Kraus F., Feldmann C., ACS Omega 2017, 2, 9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deposition Numbers 2104261 (for 1), 2104262 (for 2), and 2104263 (for 4) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information