Abstract
3′ to 5′ RNA degradation is primarily catalyzed by the RNA exosome subunits Dis3 and Rrp6 in the nucleus of Saccharomyces cerevisiae. These enzymes form a complex with the nine-subunit non-catalytic core (Exo9) to carry out their functions in vivo. Protein cofactors Rrp47, Mpp6, and the Mtr4 RNA helicase also assist the complex by modulating its activities and/or recruiting it to specific RNAs for processing or degradation. Here we present our preferred strategy for reconstituting RNA exosomes from S. cerevisiae using purified, recombinantly expressed components.
Keywords: RNA exosome, RNA decay, Exoribonuclease, Budding Yeast
1. Introduction
The S. cerevisiae RNA exosome is an essential complex with functions in both nuclear and cytoplasmic RNA processing and decay [1, 2]. The nuclear complex contains as many as 13 primary components (Exo13), including Rrp6 and Dis3 nucleases, the nine-subunit non-catalytic core (Exo9), and Mpp6 and Rrp47 cofactors, and is involved in diverse biological processes [3, 4]. Additional cofactors such as the RNA helicase Mtr4, a component of the TRAMP polyadenylation complex [5–7], associate with the nuclear RNA exosome and assist it in RNA decay.
Detailed explorations of the structure and functions of individual components and complexes have been greatly aided by the development of in vitro reconstitution strategies, which were pioneered in our laboratory and subsequently applied in Elena Conti’s [8–11]. Biochemical work using reconstituted complexes showed that RNA was channeled through a central pore in Exo9 to engage the catalytic subunits [9, 10, 12], that Mpp6 and Rrp47 stimulated Rrp6 decay activity in the exosome [13], and that Mpp6 and Rrp47 cooperate to recruit the Mtr4 helicase for degradation of structured RNAs [13–16]. Structures of nuclear exosome complexes lacking Mpp6 and Rrp47 have revealed that the catalytic subunits are positioned on opposite ends of the central pore of Exo9 and RNA is threaded into or through the channel towards their respective active sites [11, 12]. Recent structural work has revealed that Mpp6 binds a conserved surface on Rrp40 [13, 15–17] and that the Rrp47/Rrp6 heterodimer interface sits atop the complex forming a ‘lid’ structure [18] that can be displaced by either structured RNA [18] or Mtr4 [16, 17]. For a more detailed description of biochemical and structural achievements using reconstituted exosomes from S. cerevisiae, see introduction chapter: Strategies for generating RNA exosome complexes from recombinant expression hosts.
While many aspects of our current strategy for reconstituting S. cerevisiae exosomes remains similar to previous reports [8, 19], several modifications have enabled higher yields and purity of the Exo9 components and Dis3 (Fig.1). Additionally, we describe purification of the Rrp6/Rrp47 heterodimer, Mpp6, and Mtr4. Finally, we report our optimized reconstitution protocols for Exo9, Exo13, and helicase-dependent RNA decay using Exo13 and Mtr4 (Fig. 1).
Figure 1. Reconstitution scheme for the S. cerevisiae nuclear exosome.
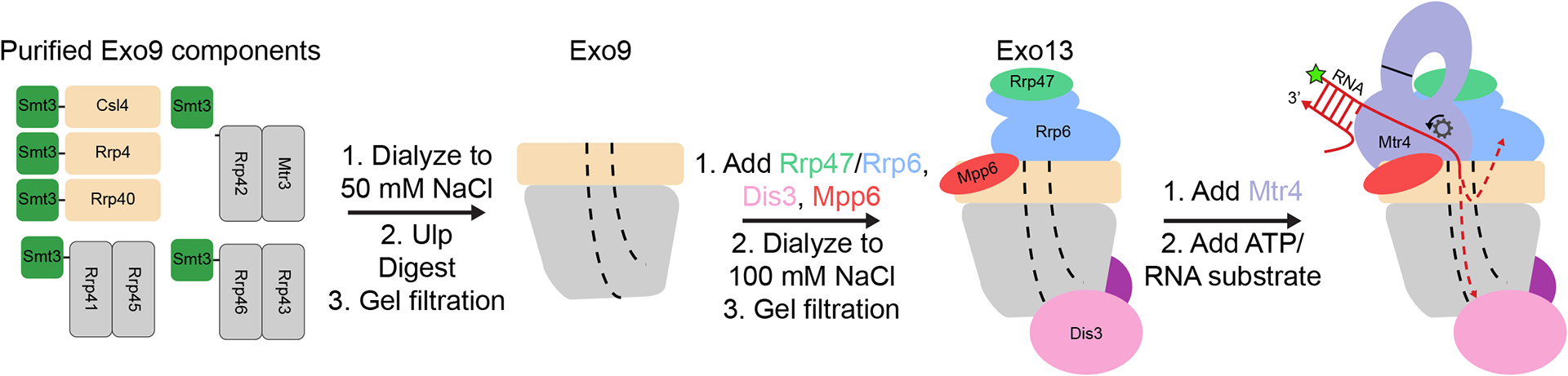
Side views are shown with the central channel indicated by dashed lines. RNA is depicted as a red arrow pointed towards the 3′ end and the 5′ fluorescein label is indicated with a green star.
2. Materials
2.1. Protein Expression, and Purification.
ELGA Purelab Ultra 18.2 MΩ-cm ultrapure water or equivalent for all solutions.
Plasmids: pRSFduet and pET28b (Novagen) with S. cerevisiae RNA exosome components cloned as N-terminal His6Smt3 fusions (see Note 1, Table 1).
50 mL and 15 mL conical tubes.
1.5 mL microcentrifuge tubes.
200 μL thin-walled PCR tubes.
Refrigerated microcentrifuge capable of 20,000 × g force (e.g. Eppendorf 5424r)
Luria broth (LB)-agar pellets.
Super broth (SB) pellets.
50 mg/mL kanamycin, in water
30 mg/mL chloramphenicol, in 70% (v/v) ethanol.
Bacterial strains for protein expression: E. coli BL21 (DE3) RIL Codon Plus (Agilent) and SoluBL21 (DE3) Codon Plus (Genlantis).
Shaking incubators (18–37°C).
2 L baffled shaking flasks.
Autoclavable 12 L fermentation vessel and apparatus capable of agitation, aeration, and temperature control (e.g. New Brunswick Scientific BioFlo 3000).
Peristaltic pump (e.g. Millipore Masterflex).
Antifoam 204.
Beta-mercaptoethanol (BME).
Sumo protease (Invitrogen or purified in-house (see Note 2)).
1 M Tris-HCl pH 8.0 (see Note 3).
5 M NaCl.
1 M isopropyl β-D-thiogalactopyranoside (IPTG).
100 mM phenylmethylsuldonyl fluoride (PMSF) in dry isopropanol.
0.5 M MgSO4.
2.5 M imidazole (adjusted to pH 8.0 with concentrated HCl).
10 % (v/v) IGEPAL ca-630.
100 mM adenosine triphosphate (ATP, adjusted to pH 7.0 with NaOH).
2.5 M KCl (see Note 4).
Lysozyme and DNAse I at 10 mg/mL in 20 mM Tris-HCl (pH 8.0), 50 mM NaCl aliquoted, flash frozen in liquid nitrogen and stored at −20°C.
Tris-sucrose buffer: 50 mM Tris-Cl pH 8.0, 20% (w/v) sucrose (0.2 μm filtered, stored at 4°C).
Branson 450 watt digital sonifer equipped with ½ inch disrupter horn and acoustic enclosure.
Beckman Coulter Avanti J-26XP (or similar) equipped with JLA 8.1 and JA-20 rotors.
Beckman Coulter Allegra X-22R (or similar) equipped with SX4250 swinging bucket rotor.
10 mL and 1 mL plastic syringes.
0.2 μm syringe filters.
0.2 μm bottle top filters.
FPLC Buffer T150: 20 mM Tris-Cl pH 8.0, 150 mM NaCl, 1.4 mM BME (all FPLC buffers are 0.2 μm filtered, degassed, and stored at 4°C).
FPLC Buffer T300: 20 mM Tris-Cl pH 8.0, 300 mM NaCl, 1.4 mM BME.
FPLC Buffer T1000: 20 mM Tris-Cl pH 8.0, 1 M NaCl, 1.4 mM BME.
Ni-NTA agarose resin (Qiagen) (see Note 5).
Empty, reusable columns (Bio-Rad Econo-Pac or similar).
Wash buffer: 20 mM Tris-Cl pH 8.0, 300 mM NaCl, 1.4 mM BME, 10 mM imidazole.
Chaperone buffer: 20 mM Tris-Cl pH 8.0, 300 mM NaCl, 1.4 mM BME, 10 mM imidazole, 50 mM KCl, 10 mM MgSO4, 2 mM ATP.AKTA-FPLC (GE Healthcare) equipped 10 mL and 0.5 mL injection loops, gel filtration columns (HiLoad 26/60 Superdex 75, HiLoad 26/60 Superdex 200, Superdex 200 Increase 10/300 GL), and heparin affinity column (HiTrap Heparin HP 5 mL).
Amicon Ultra concentration devices (Millipore) with 10K and 30K molecular weight cutoffs (0.5 mL and 15 mL capacities).
5x Bradford reagent (Bio-Rad).
NanoDrop 2000 Spectrophotometer (Thermo Scientific) or equivalent.
Power supply (e.g. Bio-Rad PowerPac Basic).
XCell SureLock Mini-Cell electrophoresis system (Thermo-Scientific).
MOPS-SDS running buffer.
NuPAGE 12% and 4–12% gradient polyacrylamide Bis-Tris gels (Thermo-Scientific).
4x LDS sample dye (Thermo-Scientific, adjusted to 70 mM BME prior to use).
Benchmark protein ladder (Thermo-Scientific).
Gel staining boxes.
Coomassie stain solution: 40% (v/v) methanol, 10% (v/v) glacial acetic acid, 0.1% (w/v) Coomassie R250.
Destain solution: 10% (v/v) methanol, 10% (v/v) glacial acetic acid.
SYPRO Ruby stain (Bio-Rad).
Gel imaging apparatus with UV and white light illumination (e.g. Bio-Rad Geldoc).
Table 1.
Expression vectors for S. cerevisiae nuclear exosome components.
| Component | Vector Backbone | 1st MCS | 2nd MCS |
|---|---|---|---|
| Smt3-Rrp41/Rrp45 | pRSFduet-Smt3 | Rrp41 | Rrp45 |
| Smt3-Rrp42/Mtr3 | pRSFduet-Smt3 | Rrp42 | Mtr3 |
| Smt3-Rrp46/Rrp43 | pRSFduet-Smt3 | Rrp46 | Rrp43 |
| Smt3-Csl4 | pET28b-Smt3 | Csl4 | N/A |
| Smt3-Rrp4 | pET28b-Smt3 | Rrp4 | N/A |
| Smt3-Rrp40 | pET28b-Smt3 | Rrp40 | N/A |
| Smt3-Dis3 | pET28b-Smt3 | Dis3 | N/A |
| Smt3-Mtr4 | pET28b-Smt3 | Mtr4 | N/A |
| Smt3-Mpp6 | pET28b-Smt3 | Mpp6 | N/A |
| Smt3-Rrp47/Rrp6 | pRSFduet-Smt3 | Rrp47 | Rrp6 |
2.2. Core Exosome Reconstitution
0.5 M MgCl2.
Dialysis cassettes (3–12 mL volume, 3500 kDa cutoff, e.g. Thermo-Fisher Slide-A-Lyzer).
Buffer R1: 20 mM Tris-Cl pH 8.0, 50 mM NaCl, 0.1 mM MgCl2, 10 mM dithiothreitol (DTT, powder added immediately prior to use).
2.3. Exo13 Reconstitution
0.5 M tris(2-carboxyethyl)phosphine-HCl (TCEP-HCl) (stored at −20°C for no longer than 1yr).
Dialysis cassettes (0.1–0.5 mL volume, 3500 kDa cutoff, e.g. Thermo-Fisher Slide-A-Lyzer).
Buffer R2: 20 mM Tris-Cl pH 8.0, 100 mM NaCl, 0.1 mM MgCl2, 0.5 mM TCEP-HCl.
2.4. Substrates for Exo13/Mtr4 Activity Assay
0.5 M EDTA (adjusted to pH 8.0 with NaOH).
Thermocycler with a hot lid.
RNA top strand (5′ AAG UGA UGG UGG UGG GG 3′, synthesized and HPLC purified by Integrated DNA Technologies [IDT]).
RNA bottom strand (5′ FAM CCC CAC CAC CAU CAC UUA AAA AAA AAA 3′, synthesized and HPLC purified by IDT).
Annealing buffer: 10 mM Tris-Cl pH 8.0, 100 mM KCl, 0.5 mM EDTA pH 8.0.
2.5. Exo13/Mtr4 Biochemical Reconstitution
0.5 M magnesium acetate.
2.5 M potassium acetate.
1 M DTT (made fresh on the day of the experiment).
1 M HEPES-KOH pH 7.5.
50 mM AMP-PNP (adjusted to pH 7.0 with KOH if necessary).
50 mM ATP-KOH pH 7.0.
40 U/μL RNAse inhibitor from human placenta (New England Biolabs [NEB]).
10% (w/v) SDS.
50% (v/v) glycerol.
800 units/mL proteinase K (NEB).
10x Tris-Borate/EDTA (TBE) buffer.
Thermomixer equipped with block for 1.5 mL microcentrifuge tubes (e.g. Eppendorf Thermomixer C).
RNA decay buffer: 20 mM HEPES-KOH pH 7.5, 50 mM potassium acetate, 1.1 mM magnesium acetate, 2.5 mM DTT.
3x stop solution: 15% (v/v) glycerol, 0.3% (w/v) SDS, 30 mM EDTA, 3 U/mL proteinase K.
Novex 4–20% polyacrylamide TBE gels (Thermo-Scientific).
Typhoon FLA-9500 scanner (GE Healthcare).
3. Methods
3.1. Protein Expression
To facilitate rapid isolation and high yield of the target proteins from the bacterial expression host, we favor a strategy that employs an N-terminal hexa-histidine (His6) sequence linked to yeast Sumo (Smt3). This His6Smt3 tag can improve expression and solubility [20], enables rapid isolation from soluble lysates by nickel affinity chromatography, and is efficiently removed by incubation with Ubiquitin-like protease (Ulp) at 4°C [21]. Cap subunits, Dis3, Mtr4, and Mpp6 can be expressed alone while PH-like subunits and Rrp6/Rrp47 are expressed as heterodimers (Table 1 and Fig. 1). Tag placement for the heterodimers has been optimized to maximize stoichiometry and yield. Similar strategies for cloning and expression have been described previously (see Note 6) [8, 10, 19].
3.1.1. Cell Growth and Expression of Rrp4, Csl4 Rrp40, Mpp6, Mtr4, Dis3, Rrp42/Mtr3, Rrp46/Rrp43 and Rrp41/Rrp45
Transform the plasmids into E. coli BL21 (DE3) RIL Codon Plus cells and plate on LB-agar supplemented with 30 μg/mL chloramphenicol and 50 μg/mL kanamycin.
Inoculate 80 mL of LB containing antibiotics with the transformant from a single or several colonies, and grow at 37°C overnight (~16 hours) with shaking.
Optional: Make a glycerol stock by adding 1 mL of saturated overnight culture to 0.5 mL of 60% (v/v) glycerol in a cryovial, flash freezing in liquid nitrogen, and storing at −80°C (see Note 7). These can be plated and used to inoculate overnight cultures without the need to transform a new aliquot of competent cells.
Inoculate each of 4 × 1 L of Superbroth containing antibiotics in 2 L baffled shaking flasks with 10 mL of overnight culture and grow with shaking at 37°C.
When the OD600 of the culture reaches 1.0–1.5, transfer the cultures to an ice bath and induce with 400 μM IPTG.
After ~30 min on ice, transfer the cultures back into the shaking incubator and grow overnight (~16 hrs) at 18°C.
Optional: add 1 drop of Antifoam 204 to each of the flasks.
Transfer cultures to 1 L JLA 8.1 bottles.
Collect cells by centrifugation (4000 × g, 10 min).
Suspend cell pellets in Tris-sucrose buffer (1 mL of buffer per g of wet cell mass).
Flash freeze in liquid nitrogen and store at −80°C for future use. Typical yields range from 5–10 g of wet cell weight per L of culture.
3.1.2. Cell Growth and Expression of Rrp47/Rrp6
Transform the plasmids into E. coli SoluBL21 (DE3) RIL cells and plate on LB-agar supplemented with 30 μg/mL chloramphenicol and 50 μg/mL kanamycin.
Inoculate 330 mL of SB containing antibiotics with several colonies, and grow at 37°C for ~20 hours with shaking (see Note 8).
Inoculate 10 L of Superbroth containing antibiotics and ~1 mL Antifoam 204 in a 12 L fermenter with the entire overnight culture and incubate at 37°C with vigorous agitation and aeration.
When the culture reaches OD600 = 1.0, cool it to 18°C, add 200 mL ethanol, and induce by adding 0.95 g IPTG powder (final concentration 400 μM).
Continue growth with agitation and aeration overnight (~16 hrs) at 18°C.
Transfer cultures to 1L JLA 8.1 bottles using a peristaltic pump.
Collect cells by centrifugation (4000 × g, 10 min).
Suspend cell pellets in Tris-sucrose buffer (1 mL of buffer per g of wet cell mass).
Split into 40 mL aliquots in 50 ml conical tubes, flash freeze in liquid nitrogen, and store at −80°C for future use. Typical yield is around 220 g wet cell weight.
3.2. Protein purification
Below are protocols for preparation of 5–20 mg (final yield) of purified exosome components except for the Rrp47/Rrp6 heterodimer, where yields will be closer to 1–2.5 mg (see Note 9). To minimize local heating from sonication, excess lysozyme is used and sonication is performed at low power with constant stirring on ice (see Note 10). All protocols can be scaled, though if >50 mg is desired, the amount of Ni resin and, accordingly, the volume of elution buffer used will have to be increased. The 10 mL elution volume from the Ni resin enables injection onto the FPLC directly after elution and avoids additional concentration steps. Likewise, salt gradients for the heparin affinity chromatography step have been optimized to elute the desired protein in a volume of <10 mL.
3.2.1. Purification of Exo9 Components Rrp4, Csl4, Rrp40, Rrp42/Mtr3, Rrp46/Rrp43 and Rrp41/Rrp45
Thaw 40 mL cell of suspension in room temperature water. On ice with stirring, add (in this order) 35 mL Tris-Sucrose buffer, 4.5 mL 5 M NaCl (300 mM), 300 μL 2.5 M imidazole (10 mM), 7.5 μL BME (1.4 mM), 750 μL 100 mM PMSF (1 mM), 750 μL 10% (v/v) IGEPAL ca-630 (0.1% [v/v]), 75 μL 10 mg/mL DNase I (10 μg/mL), and 150 μL of 10 μg/mL lysozyme (20 μg/mL).
On ice/water slurry with rapid stirring, sonicate at 40% output for 1.5 minutes with 20% duty cycle (e.g. 1 s on, 4 s off, 90 repeats) to disrupt cells.
Transfer the lysed cells to JA-20 tubes.
Pellet cell debris by centrifugation at 44,000 × g for 30 minutes.
Equilibrate 2 mL of Ni-NTA resin with Wash buffer.
Transfer resin to 50 mL conical tubes and apply supernatant.
Rotate for 10–30 min.
Transfer the resin to a disposable column and allow the lysate to pass by gravity flow.
Wash column with 10 column volumes (20 mL) of Wash buffer.
Wash column with 5 column volumes of Chaperone buffer.
Wash the column again with 5 column volumes of Wash buffer.
Elute protein with 10 mL of Buffer T300 plus 250 mM imidazole and measure protein concentration (see Note 11).
Filter the eluate through 0.2 μm and inject over a HiLoad 26/60 Superdex 200 (for Rrp42/Mtr3, Rrp46/Rrp43, Rrp41/Rrp45, and Rrp4) or HiLoad 26/60 Superdex 75 (for Csl4 and Rrp40) column that has been equilibrated with buffer T300 (see Note 12). Collect 5 mL fractions.
Pool the peak (typically 4 or 5 fractions) and concentrate to ca. 10 mg/mL (see Note 11) in a 15 mL capacity Amicon YM-10 (3000 × g spins, 10 min). If there is any ambiguity as to which fractions should be pooled, analyze the fractions in question by SDS-PAGE (see Note 13).
For Rrp42/Mtr3, add 1:1000 molar ratio Ulp and incubate at 4°C overnight.
Concentrate Superdex eluates to >10 mg/mL in a 15 mL capacity Amicon YM-30 (3,000 × g, 15 min spins in a hanging bucket rotor).
Aliquot, flash freeze, and store at −80°C for later use.
3.2.2. Purification of Dis3, Mpp6, and Mtr4
Perform steps 1–11 of Subheading 3.2.1
Elute protein with 10 mL of Buffer T150 plus 250 mM imidazole
Filter the eluate through 0.2 μm and inject over a 5 mL HiTrap Heparin HP column that has been equilibrated with buffer T150.
Elute with a linear gradient to 100% Buffer T1000 over 15 column volumes.
Collect 5 mL fractions for the flowthrough and 3 mL fractions for the gradient elution (for SDS-PAGE analysis, see Note 13).
Collect peak fractions (Fig. 2A–C) and add 1:1000 ratio Ulp. Concentrate sample to 10 mL in an Amicon YM-30 if necessary.
Incubate at 4°C overnight.
Filter through 0.2 μm and inject over a HiLoad 26/60 Superdex 200 (for Dis3 and Mtr4) or HiLoad 26/600 Superdex 75 (for Mpp6) column that has been equilibrated with buffer T300. Collect 5 mL fractions.
Perform SDS-PAGE analysis (omitting the ‘flowthrough’ sample) if desired (Figs. 3A–C, see Note 13).
Pool the peak fractions and concentrate to >10 mg/mL in a 15 mL capacity Amicon YM-30 (3,000 × g, 15 min spins in a hanging bucket rotor).
Aliquot, flash freeze, and store at −80 °C for later use.
Figure 2. Purification of nuclear exosome components by heparin affinity chromatography.

Purification of His6Smt3 tagged (A) Dis3, (B) Mtr4, (C) Mpp6, and (D) Rrp47/Rrp6 enzymes directly after Nickel elution. (Top) Traces show absorbance at 280 nm (A280) and conductivity. Conductivity values at the maxima of the absorption peaks are indicated. (Bottom) 4–12% polyacrylamide Bis-Tris gels were stained with Coomassie (panels A through C) or SYPRO Ruby (panel D) are shown below. Lanes labeled ‘FT’ contained samples from the flowthrough fractions. Fractions in the vicinity of the main peak of the elution were analyzed.
Figure 3. Purification of nuclear exosome components by gel filtration chromatography.

Gel filtration of (A) Dis3, (B) Mtr4, (C) Mpp6, and (D) Rrp47/Rrp6 after heparin affinity chromatography and Ulp digestion (except for Rrp47/Rrp6 in panel D, which is not Ulp digested). A 320 mL Superdex200 column was used for all proteins except Mpp6, where a 320 mL Superdex75 column was used. (Top) Traces show absorbance at 280 nm, and elution volumes for the peak maxima are indicated. (Bottom) 4–12% polyacrylamide Bis-Tris gels stained with Coomassie (panels A through C) or SYPRO Ruby (panel D) are shown below. Fractions in the vicinity of the main peak of the elution were analyzed.
3.2.3. Purification of Rrp47/Rrp6
Thaw 120 mL (3 × 40 ml) cell suspension. On ice with stirring, add (in this order) 55 mL of Tris-sucrose buffer, 10.5 mL 5 M NaCl, (300 mM), 700 μL 2.5 M imidazole (10 mM), 17.5 μL BME (1.4 mM), 1.75 mL 100 mM PMSF (1 mM), 1.75 mL 10% (v/v) IGEPAL ca-630 (0.1%[v/v]), 175 μL 10 mg/mL DNase I (10 μg/mL), and 350 μL 20 mg/mL lysozyme (20 μg/mL).
On ice/water slurry with rapid stirring, sonicate at 40% output for 2 minutes with 20% duty cycle (e.g. 1 s on, 4 s off, 120 repeats) to disrupt cells.
Perform steps 3 through 12 of Subheading 3.2.1, omitting the chaperone wash from the protocol.
Filter the eluate through 0.2 μm and inject over a 5 mL HiTrap Heparin HP column that has been equilibrated with buffer T300. Collect 5 mL fractions for the flowthrough and 3 mL fractions for the gradient elution.
Elute with a linear gradient to 75% Buffer T1000 over 15 column volumes (for SDS-PAGE analysis, see Note 14).
Collect peak fractions (Fig. 2D) and concentrate in an Amicon YM-30 to 10 mL if necessary.
Filter through 0.2 μm and inject over a HiLoad 26/60 Superdex 200 that has been equilibrated with buffer T300. Collect 5 mL fractions.
Perform SDS-PAGE analysis (omitting the ‘flowthrough’ sample) if desired (Fig. 3D, see Note 14).
Pool peak fractions and concentrate to 5–10 mg/mL in a 15 mL capacity Amicon YM-30 (2,000 × g, 15 min spins in a hanging bucket rotor).
Aliquot, flash freeze, and store at −80°C for later use.
3.3. Exo9 Reconstitution
Prior to the addition of the RNase subunits and Mpp6 cofactor, we generate the non-catalytic Exo9 core [19]. Purified His6Smt3-tagged components are mixed, dialyzed to low salt, and their tags removed by Ulp to generate Exo9 at high yields (Figs. 1 and 4). The resulting complex is stable, apparently stoichiometric by SDS-PAGE, and elutes as a mono-disperse peak in gel filtration that can be separated from excess subunits and their cleaved His6Smt3 tags (Fig. 4).
Figure 4. Reconstitution of Exo9.

(A) A280 trace and (B) Coomassie stained 4–12% polyacrylamide Bis-Tris gel of fractions from gel filtration (320 mL column) for Exo9 reconstitution. The elution volume of the peak maximum is indicated. (C) Coomassie stained 12% polyacrylamide Bis-Tris gel showing individual, Ulp-cleaved Exo9 components and purified Exo9 complex.
Thaw aliquots of purified Rrp4, Rrp40, Csl4, Rrp42/Mtr3, Rrp46/Rrp43 (see Note 15), and Rrp41/45 proteins and place on ice.
Centrifuge proteins at 20,000 × g for 3 min to pellet any precipitated material.
Measure the protein concentration of the supernatants.
Mix proteins, Ulp, and Buffer R1 in a conical tube on ice according to the example protocol in Table 2. Note that molecular weights include the extra 12 kDa from the His6Smt3 tags. ‘Multiplier’ refers to the molar excess of the given component used.
Transfer to a 3–12 mL dialysis cassette and dialyze into 1–2 L Buffer R1 overnight.
Filter through 0.2 μm and inject over a HiLoad 26/60 Superdex 200 column that has been equilibrated with Buffer R1. Collect 5 mL fractions.
Perform SDS-PAGE analysis with Coomassie staining (Fig. 4B, see Note 13).
Pool the peak fractions (Fig. 4) and concentrate in a 15 mL capacity Amicon YM-10 (2,000 × g, 15 min spins in a hanging bucket rotor) to >5 mg/mL.
Aliquot, flash freeze, and store at −80°C for later use.
The final yield for the protocol in Table 2 (and Fig. 4) was 9 mg of Exo9, or 75% theoretical yield.
Table 2:
Sample Exo9 reconstitution
| Component | Molecular weight (Da) | mg/mL | μM | Multiplier | nmol needed | mL needed |
|---|---|---|---|---|---|---|
| Smt3-Rrp41/Rrp45 | 74,000 | 17 | 230 | 1.0 | 42 | 0.19 |
| Smt3-Rrp42/Mtr3 | 81,000 | 10 | 140 | 1.0 | 42 | 0.29 |
| Smt3-Rrp46/Rrp43 | 80,000 | 7.6 | 95 | 3.0 | 130 | 1.4 |
| Smt3-Csl4 | 44,000 | 26 | 590 | 3.0 | 130 | 0.22 |
| Smt3-Rrp4 | 51,000 | 11 | 220 | 1.0 | 42 | 0.2 |
| Smt3-Rrp40 | 39,000 | 14 | 360 | 1.8 | 74 | 0.21 |
| Ulp | 26,000 | 2.0 | 77 | 0.010 | 0.4 | 0.005 |
| Buffer R1 | 7.5 |
3.4. Exo13 Reconstitution
Addition of excess RNase subunits (Dis3 and Rrp47/Rrp6) (see Notes 16–19) and Mpp6 to Exo9 produces a thirteen-component complex (Exo13) after dialysis. Exo13 can be purified from the excess subunits via gel filtration (see Note 18).
Thaw aliquots of purified Exo9, Csl4, Mpp6, Dis3, and Rrp47/Rrp6 and place on ice.
Centrifuge proteins at 20,000 × g for 3 min.
Measure the protein concentration of the supernatants.
Mix proteins, Ulp, and Buffer T150 in a conical tube on ice according to the example protocol in Table 3. Components can be mixed in any order except Rrp47/Rrp6, which is added last. This protocol can be scaled according to the investigators’ needs, but may require a larger column if >5 mg of complex is desired.
Transfer to a 0.1–0.5 mL dialysis cassette and dialyze into 500 mL Buffer R2 overnight.
Transfer dialysate to a microcentrifuge tube and pellet any insoluble material by centrifuging at 20,000 × g for 3 min.
Inject supernatant over a Superdex 200 Increase 10/300 GL column that has been equilibrated with buffer R2. Collect 0.5 mL fractions.
Perform SDS-PAGE analysis with Coomassie staining (Fig. 5B, see Note 13).
Collect the peak fractions and concentrate in a 0.5 mL capacity Amicon YM-10 (5,000 × g, 10 min per spin) to >5 mg/ml.
Aliquot, flash freeze, and store at −80°C for later use.
Table 3:
Sample Exo13 reconstitution
| Component | Molecular weight (Da) | mg/mL | μM | Multiplier | nmol needed | μL needed |
|---|---|---|---|---|---|---|
| Exo9 | 280,000 | 7.6 | 27 | 1.0 | 3.3 | 120 |
| Dis3 | 110,000 | 16 | 150 | 1.5 | 5.0 | 34 |
| Mpp6 | 22,000 | 4.6 | 210 | 2.5 | 8.3 | 40 |
| Smt3-Rrp47/Rrp6 | 130,000 | 7.5 | 58 | 2.5 | 8.3 | 140 |
| Smt3-Csl4 | 44,000 | 26 | 590 | 1.0 | 3.3 | 5.6 |
| Ulp | 26,000 | 2.0 | 77 | 1.0 | ||
| Buffer T150 | 150 |
Fig. 5. Reconstitution of Exo13.

(A) A280 trace and (B) Coomassie stained 4–12% polyacrylamide Bis-Tris gel of fractions from gel filtration (24 mL column) for Exo13 reconstitution. Elution volume for the peak maximum is indicated.
3.5. Exo14 Biochemical Reconstitution
While Mtr4-containing RNA exosome complexes from S. cerevisiae can be obtained, we favor reconstitution of helicase-dependent RNA decay in biochemical assays to show recruitment of Mtr4 to the RNA exosome [13]. To observe this activity, we use a dsRNA with 10 nt poly(A) 3′ overhang (ds17A10). In the absence of Mtr4 and ATP, this overhang can be trimmed by the distributive RNase activity of Rrp6 but not degraded by the processive activity of Dis3 (Fig. 6B), presumably because its 3′ overhang is too short to span Exo9’s central channel to reach Dis3 [18]. When both Mtr4 and ATP are present, the duplex region of this RNA can be unwound, resulting in a 27 nt ssRNA that can reach Dis3’s exonuclease site by threading through the central channel (Figs. 1 and 6B).
Figure 6. Reconstitution of helicase-dependent RNA decay by Exo13 and Mtr4.

(A) SYPRO Ruby stained 4–12% polyacrylamide Bis-Tris gel showing Exo13 and Exo13 + Mtr4 enzyme solutions. (B) Activity assay for Exo13 in the presence and absence of Mtr4 on a double-stranded RNA with 3′ overhang (upper gels) or single-stranded RNA (lower gels). RNA is represented as an arrow pointing towards the 3′ end, and the location of the 5′ FAM is indicated with a green star. The gels are 4–20% polyacrylamide TBE and are imaged for fluorescence.
3.5.1. Preparation of ds17A10 and ss17A10 Substrates
Dilute the top strand and labeled bottom strand to 110 and 100 μM, respectively, in annealing buffer (see Subheading 2.4 for sequences).
Mix equal volumes of these solutions together in a thin-walled PCR tube.
In a thermocycler with a hot lid, heat the solution to 95°C for 2 min, then cool to 60°C for 5 min, then hold at 20°C.
Dilute this solution 1:50 in annealing buffer to achieve a 1 μM stock of ds17A10 substrate (see Note 20).
Repeat steps 1–4 to prepare the ss17A10 substrate, except use Annealing buffer in place of the 110 μM top strand solution for step 1.
Aliquot, flash freeze, and store at −80°C for later use.
3.5.2. Helicase-Coupled RNA Degradation Assay
Thaw aliquots of purified Exo13, Mtr4, ds17A10, and ss17A10 and place on ice.
Centrifuge proteins at 20,000 × g for 3 min.
Measure the protein concentration of the supernatants.
Prepare 4 μM Exo13 and 4.4 μM Mtr4 stock solutions by diluting the concentrated stocks in RNA decay buffer.
Mix equal volumes of 4 μM Exo13 and 4.4 μM Mtr4 for the 100x Exo14 stock.
Mix equal volumes of 4 μM Exo13 and buffer for the 100x Exo13 stock.
Incubate on ice for 30 min.
Dilute these stocks 1:10 in RNA decay buffer to obtain 10x enzyme stocks (200 nM Exo13, 220 nM or 0 nM Mtr4).
Optional: Prepare samples for SDS-PAGE analysis by mixing 15 μL of 10x enzyme mix with 5 μL of 4x LDS sample solution. Run 2–10 μL of this on a 4–12% polyacrylamide Bis-Tris gel and stain with SYPRO Ruby as previously (Fig. 6A, see Note 14).
Prepare four 1.1x RNA stocks by mixing buffer, RNA and ATP or AMP-PNP as indicated in Table 4. Final concentrations will be 10 nM RNA substrate, 1 mM ATP or AMP-PNP, and 0.5 U/μL RNase inhibitor.
Place 90 μL of these mixes in a thermomixer at 20°C.
Aliquot 5 μL of 3x stop mix into each of 48 tubes.
Add 9 μL of the RNA mixes and 1 μL of RNA decay buffer to four of the stop mix aliquots. These are the 0 min time points (Fig. 6B).
Initiate the reaction by adding 9 μL of 10x Exo13 (final concentration = 20 nM) and mix well by gently flicking. Stagger initiation of the four reactions by ~15 seconds each to allow for accurate time points.
Take time points at 1, 5, and 25 min by removing 10 μL from the reaction and adding it to 5 μL of stop mix. Mix well by flicking the tube several times.
Repeat steps 10–15 for Exo14.
Centrifuge the tubes at 20,000 × g for 30 seconds and incubate at 37°C for 60 min to digest the protein components of the reaction. This step is critical for PAGE analysis.
Load 3.5 μL sample/lane and run on a 4–20% polyacrylamide TBE (room temperature, 160 V limiting, 45 min, 0.5x TBE as running buffer).
Image gel using the FITC setting of a Typhoon FLA9500 scanner (600V).
Adjust levels and convert to 8-bit with ImageJ image processing software (NIH) (Fig. 6B).
Table 4:
Substrate mixes for Exo14 biochemical reconstitutions
| Component | Stock concentration (mM) | ds17A10, AMP-PNP | ds17A10, ATP | ss17A10, AMP-PNP | ss17A10, ATP |
|---|---|---|---|---|---|
| AMP-PNP | 50 | 4.4 μL | 0 μL | 4.4 μL | 0 μL |
| ATP | 50 | 0 μL | 4.4 μL | 0 μL | 4.4 μL |
| ds17A10 RNA | 0.001 | 2.2 μL | 2.2 μL | 0 μL | 0 μL |
| ss17A10 RNA | 0.001 | 0 μL | 0 μL | 2.2 μL | 2.2 μL |
| RNase inhibitor | 40 U/μL | 2.5 μL | 2.5 μL | 2.5 μL | 2.5 μL |
| RNA decay buffer | 191 μL | 191 μL | 191 μL | 191 μL | |
| Total | 200 μL | 200 μL | 200 μL | 200 μL |
4. Notes
pET28b-Smt3 and pRSFduet-Smt3 can be generated by amplifying residues 1–98 of Smt3 from S. cerevisiae genomic DNA and appending a sequence encoding six histidines plus a Gly-Ser linker to the 5′ end, then inserting it between the NcoI (ATG used as start codon) and BamHI sites of pRSFduet and pET28b vectors. The BamHI site encodes Gly-Ser, so the Gly98 codon of Smt3 can be mutated from GGT to GGA to make up the first half of the restriction site [21]. Smt3’s internal EcoRI site should be removed by site directed mutagenesis prior to use. To minimize extra N-terminal amino acids after tag cleavage, we use the BamHI or EcoRI as the 5′ restriction site for the first MCS, and NdeI as the 5′ restriction site if a gene is to be cloned into the second restriction site.
S. cerevisiae Ulp residues 403–621 can be purchased commercially or generated in house by following the cloning and purification protocol from reference 21. Ulp cleaves N-terminal Smt3 tags after Gly98 of Smt3 [21].
For buffers and nucleotide stocks, all pH measurements were made at room temperature (22°C).
Unless otherwise specified, all chemicals were from Sigma-Aldrich and were >98% pure.
To conserve costs, we routinely strip and regenerate our nickel resin according to the manufacturer’s protocols. Prior to stripping, we store the used resin in 20% (v/v) ethanol at 4°C.
While we have historically used restriction enzyme protocols to generate vectors for expressing the exosome subunits, Gibson assembly could easily be applied to achieve constructs that would not contain extra amino acids after Ulp cleavage. This strategy would also avoid complications that arise from internal restriction sites in the genes. Additionally, synthetic genes optimized for bacterial expression could be used in place of genomically amplified DNA if greater yields are required. This would also enable use of other expression strains of E. coli such as BL21 Star (Thermo-Scientific) and Arctic Express (Agilent) which may improve protein behavior and/or yield in some cases.
All expression strains described here, except those for expressing Mtr4 and the Rrp47/Rrp6 heterodimer, store well over long periods of time (>3 yr) at −80°C as glycerol stocks. Mtr4 and Rrp47/Rrp6 expression strains must be freshly transformed before each expression.
Rrp47/Rrp6 can be grown in flasks rather than a fermenter for expression, but this dramatically reduces final yields. We favor leaving the His6Smt3 tag on the Rrp47/Rrp6 heterodimer prior to reconstitution because it increases its solubility and is not predicted to interfere with its incorporation into the exosome [18].
The C-terminal 100 amino acids of Rrp6, while important for RNA binding and decay activity in vitro [22], can be omitted for certain applications such as crystallography where large quantities of material are needed [12, 18, 23]. Rrp6 constructs lacking the N-terminal 128 residues (Rrp6ΔN) cannot bind Rrp47 [24] but express at much higher levels than the Rrp47/Rrp6 heterodimer [22]. Constructs lacking these residues can be cloned into pET28b-Smt3 and expressed as standalone enzymes. Expressing full-length Rrp6 without Rrp47 is not advisable as the protein displays poor expression, activity, and solubility as well as weak homo-dimerization [13, 25]. Consistent with this, full length but not Rrp6ΔN is unstable in the absence of its binding partner Rrp47 in vivo [26].
As is the case with many proteins derived from mesophilic organisms, exposure to temperatures above 4°C can be harmful to exosome component activity and/or solubility and thus should be minimized. All handling, including centrifugation steps, should be performed at 4°C or on ice unless otherwise specified.
All protein concentrations in these protocols are obtained using a standard Bradford assay.
For the His6Smt3 tagged Exo9 components, the major peaks should be readily apparent. Their approximate absorption maxima should be close to the following elution volumes: Csl4: 170 mL, Rrp4: 225 mL, Rrp40: 165 mL, Rrp41/45: 210 mL, Rrp42/Mtr3: 220 mL, Rrp46/43: 220 mL (one column volume is 320 mL). Note that Csl4 and Rrp40 are purified over a Superdex 75 rather than a Superdex 200 and thus elute earlier than the other components despite having lower molecular weights.
SDS-PAGE analysis with Coomassie Blue staining: Prepare a ‘Load’ sample by mixing 6 μL input, 24 μL water, and 10 μL 4x LDS loading dye. For analysis of heparin eluates, prepare a ‘Flowthrough’ sample by mixing 15 μL of each of the two flowthrough fractions (which should come out between ~5–15 mL) with 10 μL of 4x LDS loading dye. Prepare ‘Fractions’ samples by mixing 30 μL of each fraction with 10 μL of 4x LDS loading dye. Choose fractions surrounding the main peak for analysis. Run 2–15 μL of each sample on a 12-well, 4–12% polyacrylamide Bis-Tris gel in MOPS-SDS running buffer (180 V limiting, 60 min, room temp). Transfer the gel to a staining box and stain by gently shaking in Coomassie stain solution for 30 min. Decant the stain solution into an appropriate waste receptacle (or a container for re-use; stain solutions can be used 2–3 times), rinse the gel with ultrapure water, and fill ~1/3 with destain solution. Shake for 2–3 hrs with 1–3 kimwipes in the box and image.
SDS-PAGE analysis with SYPRO Ruby staining: Prepare ‘Load,’ ‘Flowthrough’ (for heparin columns), and ‘Fractions’ samples as in Note 13. Run 2–15 μL on a 12-well, 4–12% polyacrylamide Bis-Tris gel in MOPS-SDS running buffer (180 V limiting, 60 min, room temp). Transfer the gel to a staining box and fix in Destain solution by shaking for 15–30 min. Decant the Destain solution into a waste receptacle and stain by gently shaking in SYPRO Ruby for 30 min. Decant the stain solution in a storage receptacle (the stain solution can be used at least three times), rinse the gel, and fill ~1/3 with Destain solution. Shake for 2–3 hrs or overnight then image using a Geldoc or Typhoon scanner.
We have observed poor behavior of the isolated Rrp46/Rrp43 heterodimer when stored at −80°C for >2 weeks, though it seems to be stable for long periods of time (>4 yrs) at −80°C in whole cell suspensions or when incorporated into the exosome. We therefore recommend purifying it within 2 weeks of the Exo9 reconstitution. Additionally, we have had success using Rrp46/Rrp43 heterodimer directly after it was eluted from the nickel resin in Exo9 reconstitutions.
RNase subunits can be rendered inert by introducing a D238N mutation to Rrp6 and D171N/D551N mutations to Dis3 (for its endoribonuclease and exoribonuclease activities, respectively). Note that Dis3’s endonuclease activity is not observed except when non-physiological concentrations of zinc or manganese are included in the reaction buffer [10, 27]. We observe greatly increased yield of the mutants relative to their wild type counterparts.
Dis3 and Mtr4 purify with minor contaminants (Fig. 3) that do not significantly associate with the exosome. If needed, these components can be further purified by dialyzing into 20 mM Tris-Cl 8.0, 100 mM NaCl, 1.4 mM BME after Superdex elution, injecting the dialysate over a MonoQ (GE) column, and eluting with a gradient from 100 to ~400 mM NaCl in 20 mM Tris-Cl pH 8.0, 1.4 mM BME over 25 column volumes.
Any combination of Rrp47/Rrp6, Dis3, and Mpp6 can be omitted from the Exo13 reconstitution (Fig. 5) to achieve different complexes if desired. Note that in cases where Rrp47/Rrp6 are omitted complexes should be dialyzed to 50 mM NaCl and Csl4 may be sub-stoichiometric. Csl4 is the most weakly associated of the Exo9 core components and is often substoichiometric in exosome reconstitutions [11] as well as TAP-tag purified complexes from yeast cells [28]. Because it runs very close to Rrp45 in 4–12% polyacrylamide Bis-Tris gels, we use 12% polyacrylamide Bis-Tris gels run for longer periods of time (e.g. 70 minutes at 180 V limiting) to resolve the doublet (Fig. 4). Residues 530–630 of Rrp6 tether the enzyme to Exo9 and interact extensively with Mtr3 and Rrp43 as well as Csl4, which helps stabilize Csl4 on the complex [11]. In light of this, we include additional Csl4 at the time of Exo13 reconstitution to achieve a stoichiometric complex (Table 3). In the cytoplasm, Rrp6 is absent and the cofactor Ski7 binds Exo9 using the same surface as Rrp6’s C-terminus [29, 30], suggesting it could stabilize Csl4 on the cytoplasmic exosome. Protocols for isolating cytoplasmic RNA exosomes using TAP-based purification from yeast or reconstitution from recombinantly expressed proteins can be found in references 29 and 30, respectively.
Many of the components of the exosome, most notably Rrp6, can stick to surfaces in the FPLC and contaminate other preparations. For this reason, we routinely inject 10 mL of 4 M guanidine-HCl over our columns during the equilibration step to clean them.
Previously we have reported a strategy to generate the ds17A10 substrate that uses 1.5-fold excess top strand in the annealing reaction and purifying it away via HPLC using DEAE chromatography [13]. The protocol presented here generates a substrate that behaves identically in helicase-coupled decay assays and is far less costly to generate.
Acknowledgements
This work was supported in part by GM065872 and GM118080 (NIH/NIGMS, C.D.L) and P30CA008748 (NIH/National Cancer Institute). The content is the authors’ responsibility and does not represent the official views of the NIH. C.D.L is a Howard Hughes Medical Institute Investigator.
References
- 1.Kilchert C, Wittmann S, Vasiljeva L (2016) The regulation and functions of the nuclear RNA exosome complex. Nat Rev Mol Cell Biol 17:227–239 [DOI] [PubMed] [Google Scholar]
- 2.Łabno A, Tomecki R, Dziembowski A (2016) Cytoplasmic RNA decay pathways - Enzymes and mechanisms. Biochim Biophys Acta 1863:3125–3147 [DOI] [PubMed] [Google Scholar]
- 3.Houseley J, Tollervey D (2009) The Many Pathways of RNA Degradation. Cell 136:763–776 [DOI] [PubMed] [Google Scholar]
- 4.Zinder JC, Lima CD (2017) Targeting RNA for processing or destruction by the eukaryotic RNA exosome and its cofactors. Genes Dev 31:88–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaCava J, Houseley J, Saveanu C et al. (2005) RNA Degradation by the Exosome Is Promoted by a Nuclear Polyadenylation Complex. Cell 121:713–724 [DOI] [PubMed] [Google Scholar]
- 6.Wyers F, Rougemaille M, Badis G et al. (2005) Cryptic Pol II Transcripts Are Degraded by a Nuclear Quality Control Pathway Involving a New Poly(A) Polymerase. Cell 121:725–737 [DOI] [PubMed] [Google Scholar]
- 7.Vanacova S, Wolf J, Martin G et al. (2005) A New Yeast Poly(A) Polymerase Complex Involved in RNA Quality Control. PLoS Biol 3:e189–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Q, Greimann JC, Lima CD (2006) Reconstitution, Activities, and Structure of the Eukaryotic RNA Exosome. Cell 127:1223–1237 [DOI] [PubMed] [Google Scholar]
- 9.Bonneau F, Basquin J, Ebert J et al. (2009) The Yeast Exosome Functionsas a Macromolecular Cage to Channel RNA Substrates for Degradation. Cell 139:547–559 [DOI] [PubMed] [Google Scholar]
- 10.Wasmuth EV, Lima CD (2012) Exo- and endoribonucleolytic activities of yeast cytoplasmic and nuclear RNA exosomes are dependent on the noncatalytic core and central channel. Mol Cell 48:133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makino DL, Baumgärtner M, Conti E (2013) Crystal structure of an RNA-bound 11-subunit eukaryotic exosome complex. Nature 495:70–75 [DOI] [PubMed] [Google Scholar]
- 12.Wasmuth EV, Januszyk K, Lima CD (2014) Structure of an Rrp6-RNA exosome complex bound to poly(A) RNA. Nature 511:435–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wasmuth EV, Zinder JC, Zattas D et al. (2017) Structure and reconstitution of yeast Mpp6-nuclear exosome complexes reveals that Mpp6 stimulates RNA decay and recruits the Mtr4 helicase. Elife 6:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuch B, Feigenbutz M, Makino DL et al. (2014) The exosome-binding factors Rrp6 and Rrp47 form a composite surface for recruiting the Mtr4 helicase. EMBO J 33:2829–2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falk S, Bonneau F, Ebert J et al. (2017) Mpp6 Incorporation in the Nuclear Exosome Contributes to RNA Channeling through the Mtr4 Helicase. Cell Rep 20:2279–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weick EM, Puno MR, Januszyk K et al. (2018) Helicase-dependent RNA decay illuminated by a cryo-EM structure of a human nuclear RNA exosome-MTR4 complex. Cell 173:1663–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuller JM, Falk S, Fromm L et al. (2018) Structure of the nuclear exosome captured on a maturing preribosome. Science 360:219–222 [DOI] [PubMed] [Google Scholar]
- 18.Makino DL, Schuch B, Stegmann E et al. (2015) RNA degradation paths in a 12-subunit nuclear exosome complex. Nature 524:54–58 [DOI] [PubMed] [Google Scholar]
- 19.Greimann JC, Lima CD (2008) Reconstitution of RNA exosomes from human and Saccharomyces cerevisiae cloning, expression, purification, and activity assays. Meth Enzymol 448:185–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marblestone JG, Edavettal SC, Lim Y et al. (2006) Comparison of SUMO fusion technology with traditional gene fusion systems: enhanced expression and solubility with SUMO. Protein Sci 15:182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mossessova E, Lima CD (2000) Ulp1-SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Mol Cell 5:865–876. [DOI] [PubMed] [Google Scholar]
- 22.Wasmuth EV, Lima CD (2017) The Rrp6 C-terminal domain binds RNA and activates the nuclear RNA exosome. Nucleic Acids Res 45:846–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zinder JC, Wasmuth EV, Lima CD (2016) Nuclear RNA Exosome at 3.1 Å Reveals Substrate Specificities, RNA Paths, and Allosteric Inhibition of Rrp44/Dis3. Mol Cell 64:734–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stead JA, Costello JL, Livingstone MJ, Mitchell P (2007) The PMC2NT domain of the catalytic exosome subunit Rrp6p provides the interface for binding with its cofactor Rrp47p, a nucleic acid-binding protein. Nucleic Acids Res 35:5556–5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dedic E, Seweryn P, Jonstrup AT et al. (2014) Structural analysis of the yeast exosome Rrp6p-Rrp47p complex by small-angle X-ray scattering. Biochem Biophys Res Commun 450:634–640 [DOI] [PubMed] [Google Scholar]
- 26.Feigenbutz M, Garland W, Turner M, Mitchell P (2013) The exosome cofactor Rrp47 is critical for the stability and normal expression of its associated exoribonuclease Rrp6 in Saccharomyces cerevisiae. PLoS ONE 8:e80752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebreton A, Tomecki R, Dziembowski A, Séraphin B (2008) Endonucleolytic RNA cleavage by a eukaryotic exosome. Nature 456:993–996 [DOI] [PubMed] [Google Scholar]
- 28.Wang H-W, Wang J, Ding F et al. (2007) Architecture of the yeast Rrp44 exosome complex suggests routes of RNA recruitment for 3’ end processing. Proc Natl Acad Sci USA 104:16844–16849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J-J, Niu C-Y, Wu Y et al. (2016) CryoEM structure of yeast cytoplasmic exosome complex. Cell Res 26:822–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kowalinski E, Kögel A, Ebert J et al. (2016) Structure of a Cytoplasmic 11-Subunit RNA Exosome Complex. Mol Cell 63:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]


