Abstract
Background
No simple staging system has emerged for basal cell carcinomas (BCCs), since they do not follow the TNM process, and practitioners failed to agree on simple clinical or pathological criteria as a basis for a classification. Operational classification of BCCs is required for decision‐making, trials and guidelines. Unsupervised clustering of real cases of difficult‐to‐treat BCCs (DTT‐BCCs; part 1) has demonstrated that experts could blindly agree on a five groups classification of DTT‐BCCs based on five patterns of clinical situations.
Objective
Using this five patterns to generate an operational and comprehensive classification of BCCs.
Method
Testing practitioner's agreement, when using the five patterns classification to ensure that it is robust enough to be used in the practice. Generating the first version of a staging system of BCCs based on pattern recognition.
Results
Sixty‐two physicians, including 48 practitioners and the 14 experts who participated in the generation of the five different patterns of DTT‐BCCs, agreed on 90% of cases when classifying 199 DTT‐BCCs cases using the five patterns classification (part 1) attesting that this classification is understandable and usable in practice. In order to cover the whole field of BCCs, these five groups of DTT‐BCCs were added a group representing the huge number of easy‐to‐treat BCCs, for which sub‐classification has little interest, and a group of very rare metastatic cases, resulting in a four‐stage and seven‐substage staging system of BCCs.
Conclusion
A practical classification adapted to the specificities of BCCs is proposed. It is the first tumour classification based on pattern recognition of clinical situations, which proves to be consistent and usable. This EADO staging system version 1 will be improved step by step and tested as a decision tool and a prognostic instrument.
Introduction
There is a need for a staging or at least a categorization of basal cell carcinomas (BCCs), in order to compare different therapeutic strategies in homogeneous subgroups, to discuss cases in tumour boards, to design clinical trials, and to elaborate guidelines.
The TNM classification is not operational since most BCCs are easy to manage, and although most severe forms do not metastasize in the nodes and distant metastases are exceptional, they can, however, kill the patient. Even the T staging for skin (T0–T4) used for melanoma and squamous cell carcinoma is not relevant for BCCs. As a consequence, TNM system is never used for BCCs and is thus not advised in most guidelines in which BCCs are usually only classified in not clearly defined subgroups of low risk and high risk. 1 , 2 , 3 , 4 , 5
We were able to generate a consensual categorization of the BCCs for which there are therapeutic problems into five patterns, using an original methodology based on independent clustering of real cases by experts. 6
The EADO objective was to check that this categorization was understandable and usable by practitioners and, if so, to use it as the core for a complete staging system for BCCs for the daily practice.
Methods
Testing the operability of the resulting classification of DTT–BCCs
The classification of 199 difficult‐to‐treat BCCs (DTT‐BCCs) based on clustering of real cases by 14 experts resulted in five consensual groups representative of five different patterns of clinical situations. 6 The methods and results have been described in detail in the part 1. 6 It resulted in a classification in five consensual groups.
In order to check that these groups were meaningful, understandable and usable by other clinicians, and that cases are consistently and homogeneously classified by any practitioners, a validation phase was organized. It involved 48 physicians familiar with BCCs, different from the 14 involved in the generation of the groups. They were provided a template of the classification in five groups with illustrative pictures (see part 1), and they were asked to use it to classify the 199 cases (minus those used as illustration). This test was performed on in a dedicated website. The 14 experts involved in the initial clustering were also asked to do the same exercise.
Their agreement was evaluated by the percentage of cases remaining in the cluster they have been initially assigned to, according to the classification resulting from the agreement procedure. In this context, the contrast criterion was used to characterize the distribution of votes on the clusters for each case. Contrast is maximal if all observers allocate the case to the same cluster, and it is minimal if votes are equally distributed over the five groups. In particular, contrast allows easily spotting borderline cases that received ambiguous assignations.
Building a staging system for all BCCs
A dedicated meeting of the European Association for Dermato‐Oncology EADO was organized (i) to decide whether the five consensual groups of DT‐BCCs identified by clustering could be considered a relevant basis for a comprehensive classification of BCCs, in other words whether a cognitive recognition of five patterns of situation was superior to any other criteria so far available (like TNM, concept of advanced BCC, classification of common BCCs in low and high risk as presented in several guidelines 1 , 2 , 3 , 4 , 7 ); (ii) in case of agreement, to integrate these five groups in a more comprehensive classification of BCCs, including the cases excluded from the initial clustering experiment, i.e. the highly prevalent easy‐to‐treat BCCs and the very rare metastatic BCCs; (iii) to determine whether other classical criteria (pathology, clinical) should be added to the pattern recognition, and (iv) to see whether the scientific board could come to an agreement for a comprehensive classification of all BCCs in stages ranging from I to IV like in most solid tumours.
Results
Testing the operability of the resulting classification of DTT–BCCs
The agreement between the 14 initial experts in classifying the cases was rather high: a majority of experts agree on 90% of cases. However, 16 cases received distributed votes, nearly equally spread over two groups (we call them ‘borderline cases’) or three groups (we call them ‘complex cases’). Out of these 16 cases, agreement raised to 98%. The agreement between the 48 other practitioners was 84%. Disagreement essentially lied in 26 cases (‘borderline’ or ‘complex’), and out of them, agreement also rises to 98%. All 62 physicians together, the agreement was 85%, while 30 cases received percentage of votes comprised in the 20–50% interval for at least two different groups, and can be subsequently considered as ‘ambiguous’ cases with respect to this classification. The most frequent ambiguities were between groups 3 and 4 (eight cases lying on the borderline), and between groups 3 and 5 (five cases on the borderline).
Agreement on a staging system for all BCCs
The board of EADO acknowledged that the 5 patterns of situation defined by clustering could serve as a basis for a classification, and were more relevant to the practice than any other available criteria. The group agreed that common and easy‐to‐treat BCCs which account for the majority of the BCCs did not need a detailed classification, since there was no operational need for their simple management. Finally, the board of EADO agreed on a final classification in four stages (Table 1). EADO‐stage I includes most of the BCCs which are easy to treat, and low risk, and constitute the majority of cases. EADO‐stage II includes EADO‐stage IIA, and IIB which correspond to DTT‐BCC group 1, and 2 identified by the expert clustering experiment, respectively (see part 1 and Table 2); EADO‐stage IIA thus includes common BCCs, which differ from EADO‐stage I since they are somewhat difficult to treat for any reasons linked to the tumour (location, uncertain limits, prior recurrences) or the patient (poor status, comorbidities, unwillingness to cope with the treatment choice), while EADO‐stage IIB includes BCCs considered difficult to treat mainly because of the high number of BCCs, and not because of individual BCC characteristics. EADO‐stages IIIA, IIIB and IIIC correspond to the consensual DTT‐BCC groups 3, 4 and 5 of the clustering experiment, respectively (see part 1 and Table 2). EADO‐stage III thus includes large or destructive BCCs, sub‐classified in EADO‐stage IIIA, IIIB and IIIC, depending on their location out of (EADO‐stage IIIA), or on critical/functional areas (EADO‐stage IIIB), while extremely destructive situations are classified separately (EADO‐stage IIIC). EADO‐stage IV is created for the very rare cases with distant metastases, which were not studied in the clustering experiment (see part 1).
Table 1.
EADO staging system for BCC
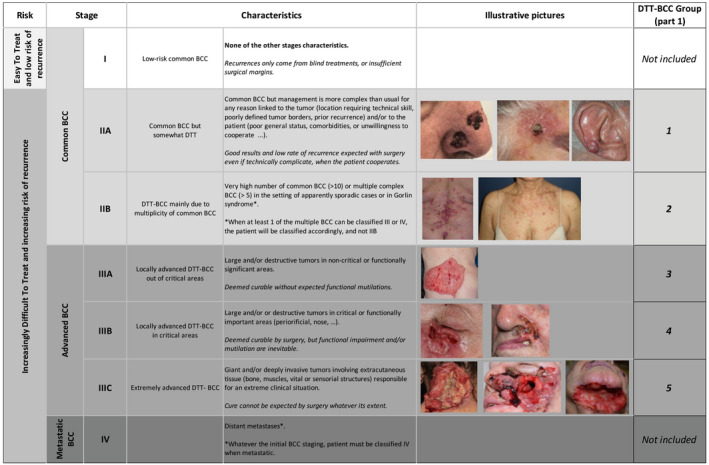
BCC, basal cell carcinoma.
Table 2.
Correspondence between EADO staging system for BCC, and the 5 consensual groups of clinical patterns identified in part 1 by a clustering experiment
| BCC‐EADO stages | Consensual groups or clusters identified in the clustering analysis (Part1) |
|---|---|
| IIA | 1 |
| IIB | 2 |
| IIIA | 3 |
| IIIB | 4 |
| IIIC | 5 |
Discussion
A consensual classification of DTT‐BCC into five clinical patterns or scenarios was generated by case clustering by experts (see part 1). Herein, we showed that this classification is usable by other clinicians, with a rather good overall agreement. The scientific board of EADO came to a consensus that these five patterns constitute the best core for a classification of all BCCs.
A classification into patterns is quite different from regular cancer staging systems, which are usually based on a list of clinical and pathological criteria. It is based on pattern recognition which derives from human brain natural ability to perceive similarity between objects or situations. Such a process, also formalized as ‘expertize’, is unconsciously used by everyone and is of paramount importance in the medical field.
There was a strong agreement in the cases classification between the initial 14 experts who contributed to the generation of the classification, and 48 other clinicians, showing that the classification was self‐explanatory for the naïves. The disagreement in the allocation of cases was more frequent between groups 3 and 4, probably due to a different interpretation of the limits of ‘functional areas’, and between groups 3 and 5 probably due to a different interpretation of cases in the grey zone.
We can try to summarize the advantages and limitation of this new classification based on pattern recognition. The most important advantages of this classification are: (i) the independent clustering methodology ensured that the different categories of BCCs are meaningful to the practice, since they are extracted from real‐life expertise (see part 1); (ii) it is per se consensual since consensus was mathematically derived from blind contributions, and not biased by a conflicting debate between different point of views. No other classification of BCCs could offer such advantages, for several reasons: TNM is not adapted to BCCs. It is difficult to select among hundreds of possible criteria for classification, none being relevant by itself (pathological subtypes, location, size, operability, recurrences, prior treatments, patient desires, etc.) but all contributing to characterize a situation. It is quite difficult to conciliate different point of views (dermatologists, surgeons, radiotherapists, oncologists, etc.) and to obtain a consensual classification by a panel discussion, which will be immediately questioned by another board.
As a limitation, it may be considered that a pattern recognition of situations is less stringent than criteria like pathological subtypes, tumour thickness, measure of inflammation, sentinel node status, etc., which are used in other solid tumours classification. In fact, the presence of some ‘ambiguous’ cases, which are not classified exactly the same by all clinicians, is inherent in any classification with cut‐off. At the border of each stage, a patient may be in a grey overlap zone between the upper and lower stages. For instance, in AJCC melanoma, tumours around the 1mm, 2 or 4 mm tumour thickness cut‐offs can be classified in the upper or lower stage just by chance. The kappa statistics as to the interpretation of ulceration or the measure of Breslow lead to an ‘agreement’ far to be perfect, 8 , 9 which is, however, easily accepted because clinicians are used to it. Second, back to the BCCs classification, the most frequent ambiguous situation leading to apparent inconsistency is among large and/or destructive tumours, depending whether they are considered in ‘critical or functionally significant areas (periorificial, nose, other, etc.)’ and classified EADO‐stage IIIB, or in ‘non‐critical or functionally significant areas’ and classified EADO‐stage IIIA. We can easily figure out that some cases not that far from the eye, or the ear, for instance, can be in the grey zone of whether they are considered in ‘critical or functionally areas’, yes or no. This will lead to classify the case either in stage IIIA or IIIB, without shocking any expert, and more importantly without misinterpretation of the overall clinical situation. A second potential limitation for a classification based on pattern recognition is the lack of demonstrated prognostic value. In part 1, 6 we have shown that DTT‐score, which is an evaluation by each expert on a Likert scale of the difficulty of treatment, increases from group 1 to 5. This confirms that this categorization is tightly connected to the difficulty of treatment, and therefore probably to prognosis. Indeed, the difficulty in the treatment of a BCC, whatever its cause, is not only a major source of recurrence per se, but is also often responsible for some compromise in the treatment, which in turn increases the risk of recurrence. The prognostic value of this new EADO BCC classification will, however, have to be assessed prospectively, although we have to be conscious that OS and PFS which are usually used to assess prognostic in solid tumours are not relevant to BCCs.
This version 1 of this EADO staging system is the first operational staging system adapted to the specificities of BCCs and encompassing the full spectrum of BCCs (Table 1). The limitations of such classification based on a cognitive approach are similar to those of any staging system and do not compromise the high relevance of pattern recognition in a complex system like BCCs. This version 1 of the classification will be improved step after step like all staging system, integrating returns from real‐life practice in terms of therapeutic decisions and prognostic value.
This work was presented during the 15th EADO Congress 24‐27 April 2019, Paris, France.
Funding sources
This work was supported by 2 unrestricted grants from Roche and Sun Pharma. The funders had no role in the design and conduct of the study, collection, management, analysis and interpretation of the data, preparation, review or approval of the manuscript; and decision to submit the manuscript for publication
Conflicts of interest
Dr. Grob reports personal fees from BMS, personal fees from MSD, personal fees from Novartis, personal fees from Roche, personal fees from Amgen, personal fees from Pierre Fabre, personal fees from Sanofi, personal fees from Merck, personal fees from Pfizer, personal fees from Sun Pharma, outside the submitted work. Dr. Guminski reports non‐financial support from Sun Pharma, during the conduct of the study; non‐financial support from Sun Pharma, personal fees from Regeneron, personal fees from BMS, personal fees from Merck KGaA, personal fees from Sanofi, outside the submitted work; Dr. Malvehy reports grants and personal fees from Sun Pharma corp., grants from Roche, outside the submitted work.Dr. Basset‐Seguin reports personal fees from Sun Pharma, during the conduct of the study; personal fees from Novartis, personal fees from BMS, personal fees from Galderma, personal fees from Leo Pharma, personal fees from Pierre Fabre, outside the submitted work. Dr. Bertrand has nothing to disclose. Dr. Fernandez‐Penas reports personal fees from Roche, personal fees from Sun Pharma, during the conduct of the study; personal fees from Sanofi, personal fees from Lilly, personal fees from Janssen, personal fees from Novartis, personal fees from AbbVie, personal fees from UCB, personal fees from Merck, personal fees from Amgen, personal fees from MSD, personal fees from Leo, outside the submitted work. Dr. Kaufmann reports grants from Janssen, Lilly, MSD, Novartis, Regeneron, and from Roche, outside the submitted work. Dr. Zalaudek reports personal fees and other from Sun Pharma, personal fees and other from Novartis Oncology, personal fees and other from MSD, personal fees and other from Sanofi Genzyme, outside the submitted work. Dr. Gaudy‐Marqueste reports personal fees from BMS, non‐financial support from Janssen, non‐financial support from BMS, non‐financial support from Pierre Fabre, personal fees from Roche, outside the submitted work. Dr. Fargnoli reports personal fees from Roche, personal fees from Sun Pharma, during the conduct of the study; grants and personal fees from Almirall, grants and personal fees from Leo Pharma, personal fees from Janssen, grants and personal fees from Novartis, personal fees from Lilly, grants and personal fees from Sanofi, personal fees from UCB, grants and personal fees from AbbVie, personal fees from Celgene, personal fees from Pierre Fabre, grants and personal fees from Galderma, personal fees from Mylan, personal fees from Medac Pharma, outside the submitted work. Dr. Tagliaferri reports a patent TIMER applicator pending. B. Fertil has nothing to disclose. Dr. Del Marmol has nothing to disclose. Professor Stratigos reports personal fees and/or research support from Novartis, Roche, BMS, AbbVie, Sanofi, Regeneron, Genesis Pharma outside the submitted work. Dr. Garbe reports grants and personal fees from BMS, personal fees from MSD, grants and personal fees from NeraCare, grants and personal fees from Novartis, personal fees from Philogen, grants and personal fees from Roche, grants and personal fees from Sanofi, outside the submitted work. Dr. Peris reports personal fees from Roche, personal fees from Sun Pharma, during the conduct of the study; personal fees from AbbVie, personal fees from Almirall, personal fees from Biogen, personal fees from Lilly, personal fees from Celgene, personal fees from Galderma, personal fees from Leo Pharma, personal fees from Novartis, personal fees from Pierre Fabre, personal fees from Sanofi, personal fees from Sandoz, personal fees from Janssen, outside the submitted work.
References
- 1. Dandurand M, Petit T, Martel P, Guillot B; ANAES . Management of basal cell carcinoma in adults Clinical practice guidelines. Eur J Dermatol 2006; 16: 394–401. [PubMed] [Google Scholar]
- 2. Telfer NR, Colver GB, Morton CA; British Association of Dermatologists . Guidelines for the management of basal cell carcinoma. Br J Dermatol 2008; 159: 35–48. [DOI] [PubMed] [Google Scholar]
- 3. Trakatelli M, Morton C, Nagore E et al. Update of the European guidelines for basal cell carcinoma management. Eur J Dermatol 2014; 24: 312–329. [DOI] [PubMed] [Google Scholar]
- 4. Bichakjian CK, Olencki T, Aasi SZ et al. Basal cell skin cancer, version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 14: 574–597. [DOI] [PubMed] [Google Scholar]
- 5. Peris K, Licitra L, Ascierto PA et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Future Oncol 2015; 11: 703–712. [DOI] [PubMed] [Google Scholar]
- 6. Grob JJ, Guminski A, Malvehy J et al. Position statement on classification of basal cell carcinomas. Part 1: unsupervised clustering of experts as a way to build an operational classification of advanced basal cell carcinoma based on pattern recognition. J Eur Acad Dermatol Venereol 2021; 35: 1949–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peris K, Fargnoli MC, Garbe C et al. Diagnosis and treatment of basal cell carcinoma: European consensus‐based interdisciplinary guidelines. Eur J Cancer 2019; 118: 10–34. [DOI] [PubMed] [Google Scholar]
- 8. Lock‐Andersen J, Hou‐Jensen K, Hansen JP, Jensen NK, Søgaard H, Andersen PK. Observer variation in histological classification of cutaneous malignant melanoma. Scand J Plast Reconstr Surg Hand Surg 1995; 29: 141–148. [DOI] [PubMed] [Google Scholar]
- 9. Berger DMS, Wassenberg RM, Jóźwiak K et al. Inter‐observer variation in the histopathology reports of head and neck melanoma; a comparison between the seventh and eighth edition of the AJCC staging system. Eur J Surg Oncol 2019; 45: 235–241. [DOI] [PubMed] [Google Scholar]


