Abstract
Bronchopulmonary dysplasia (BPD) still carries a heavy burden of morbidity and mortality in survivors of extreme prematurity. The disease is characterized by simplification of the alveolar structure, involving a smaller number of enlarged alveoli due to decreased septation and a dysmorphic pulmonary microvessel growth. These changes lead to persistent abnormalities mainly affecting the smaller airways, lung parenchyma, and pulmonary vasculature, which can be assessed with lung function tests and imaging techniques. Several longitudinal lung function studies have demonstrated that most preterm‐born subjects with BPD embark on a low lung function trajectory, never achieving their full airway growth potential. They are consequently at higher risk of developing a chronic obstructive pulmonary disease‐like phenotype later in life. Studies based on computer tomography and magnetic resonance imaging, have also shown that in these patients there is a persistence of lung abnormalities like emphysematous areas, bronchial wall thickening, interstitial opacities, and mosaic lung attenuation also in adult age. This review aims to outline the current knowledge of pulmonary and vascular growth in survivors of BPD and the evidence of their lung function and imaging up to adulthood.
Keywords: bronchopulmonary dysplasia, lung growth, lung structure, preterm birth, pulmonary function
1. INTRODUCTION
Rates of preterm birth (gestational age < 37 weeks) have increased globally and now account for 11% of live births. 1 Thanks to remarkable advances in perinatal care, more than 95% of subjects born preterm survive and reach adulthood. 2 The improved survival rate may come at the expense of future health risks, however, including bronchopulmonary dysplasia (BPD), which is the most common complication of prematurity.
BPD affects between 10% and 89% of preterm infants and about 45% of those born at less than 29 weeks of gestation (WG), depending on the countries and the definition used, 3 , 4 , 5 remains a major cause of mortality and long‐term respiratory consequences in these populations. 5 , 6 , 7 , 8
The lungs of survivors of prematurity with and without BPD face a deranged parenchymal and vascular growth, and with frequent respiratory infections in the first 2 years of life. In addition, several of these patients fail to reach the optimal peak of lung function in early adulthood. 9
BPD and prematurity are clear examples of how a perinatal insult can be associated with a functional impairment that persists into adult age. 10 Pulmonary disease is just one of the multiple morbidities experienced by individuals born preterm. In parallel with aberrant lung development, preterm birth also interrupts the development and maturation of several other organ systems. In fact, it has been suggested that prematurity be considered a chronic condition itself, given its adverse consequences in terms of growth failure, neurodevelopmental sequelae, systemic hypertension, pulmonary hypertension, chronic kidney disease, type 1 and type 2 diabetes, ischemic heart disease in mid‐adulthood, and mortality rate. 11 Subjects born prematurely require early assessment, long‐term follow‐up, and preventive action to reduce the risk of multiple chronic diseases later in life.
In this review, we describe the effects of BPD on lung growth, function, and structure in infants who survive the disease.
2. LUNG AND VASCULAR GROWTH IN SURVIVORS OF BPD
In term‐born healthy infants, multiplication of the alveoli and maturation of the microvasculature in the lung start in utero and continue in postnatal life. 12 , 13 Alveolarization persists throughout childhood and adolescence, and even into adulthood, 14 resulting in a 20‐fold increase in the surface area from birth (0–50 million alveoli) to adulthood (>300 million). Similarly, intra‐acinar arteries and veins continue to develop after birth by angiogenesis as long as the alveoli increase in number and size. 15 Blood vessel formation and alveolar growth are inter‐related processes, with the former actively promoting the latter, and contributing to maintaining the alveolar structures for the rest of an individual's life. 16 , 17 , 18 , 19
Extremely low gestational age newborns (i.e., <28 WG) are born before the alveoli precursors (saccules, alveolar ducts, alveolar air sacs) have formed, the capillary bed has increased, 12 , 17 , 20 and a sufficient amount of surfactant has been produced by Type II cell (AT2 cells). 13 , 16 In addition to this picture of lung immaturity, BPD is also a clinical syndrome in which alveolarization and microvascular development are disrupted, resulting in abnormal gas exchange and lung mechanics. 13
Most of our current knowledge of BPD histology comes from autoptic findings in infants who died of severe BPD (which happened more frequently in the last century than in recent years, which have seen a reduction in BPD‐related mortality). The oldest evidence comes from the pre‐surfactant era, when generalized emphysematous changes were found in mechanically ventilated extremely premature infants who died of BPD. 21 These findings were also confirmed in the post‐surfactant era by Husain et al. 22 and classically described what is called “old” BPD.
Premature infants born in more recent years, since the introduction of early rescue surfactant treatment, antenatal glucocorticoids, and more gentle ventilation techniques, show a modified BPD histology or the so‐called “new” BPD. This form of the disease is characterized by less severe injury in very immature lungs. The picture is dominated by a simplified alveolar and a smaller number of enlarged alveoli due to reduced septation, associated with a dysmorphic pulmonary microvessel growth that leads to a decreased surface area for alveolar‐capillary gas exchange. Histological specimens from infants up to 3 years of age 13 and imaging studies in BPD survivors reveal areas of reduced alveolarization, cystic emphysema, fibrosis, and some airway alterations like trachea‐ and bronchomalacia, and subglottic stenosis. 23 The small airways may be affected as well, with mucus gland hyperplasia, epithelial edema, and smooth muscle cell proliferation causing bronchoconstriction. Recent animal studies suggest that immune cells, such as macrophages, may be causally implicated in the disruption of postnatal lung organogenesis. 24 , 25
It is still unclear how these abnormalities evolve, however, as lung specimens of grown‐up BPD patients are rare. Some information may be inferred indirectly from lung function studies assessing pulmonary gas exchange, which confirm a decreased alveolar‐capillary membrane function by showing a lower carbon monoxide diffusing capacity in BPD subjects. 26 , 27 One of the few studies exploring the histopathology of grown‐up BPD survivors was recently published by Galderisi et al., 28 who analyzed endobronchial biopsy specimens from three adolescents with reduced lung function and recurrent wheezing exacerbations. They revealed a lymphocytic infiltrate pointing to an ongoing active inflammatory process in the airways and a prominent bronchial vascular density reflecting increased angiogenesis. 29
The vascular aspect of the “new” form of BPD is particularly intriguing, since angiogenesis and vessel branching drive alveolar growth. 17 , 19 The alveolar‐capillary membrane grows considerably between 22 and 32 weeks of gestation, and that is why prematurity and lung injury during the neonatal period impair the growth, branching, and distribution of the pulmonary vasculature. 30 , 31 , 32 The vascular abnormalities typical of BPD include dysmorphic growth and an altered vascular remodeling, tone, and reactivity, with a higher risk of pulmonary hypertension beyond the first few months of life. Various animal models of BPD and autopsy studies on humans who died of BPD have consistently shown a reduction in the number of small arteries, and an abnormal distribution of vessels in the distal lung. 33 The current working hypothesis, the so‐called “vascular hypothesis,” 34 is that disrupted angiogenesis interferes with alveolarization, while preserved vascular growth and endothelial survival promote growth and sustain the architecture of the distal airspace. 19 Vascular endothelial growth factor (VEGF) seems to be the most critical angiogenic growth factor for both vascular and alveolar development. Interestingly, among the avenue of promising future therapies for BPD are mesenchymal stem/stromal cells (MSCs) or their effectors, the extracellular vesicles (EV‐MSCs), which proved encouraging results in preclinical studies and are now under testing in Phase 1 and Phase 2 trials. These new drugs have shown clear histological improvements in animal models of BPD, 35 with evidence of larger numbers of smaller‐sized alveoli, and a reduction in medial arteriolar thickness compared with sham‐treated animals. These effects are thought to stem from the paracrine effects of MSC‐derived humoral factors, such as interleukin (IL)‐6, IL‐8, VEGF, collagen, and elastin. 36 , 37
3. RESPIRATORY OUTCOMES AND LUNG FUNCTION IN INFANCY AND CHILDHOOD
Being born preterm predisposes infants to a high risk of respiratory sequelae in both the short term and the long term. 9
Children with BPD have a hospitalization rate of as high as 50% during the first 2 years of life. 38 , 39 This figure varies among studies, and shows no significant difference between preterm infants with and without BPD. 40 Respiratory syncytial virus (RSV) and rhinovirus may increase the risk of hospital admissions in this age group, as they frequently precipitate pulmonary exacerbations. 41 This may be due to the infants' immature humoral and adaptive immunity, which increases the risk of severe viral respiratory infections in the first 2 years of life, 42 and of earlier and more frequent respiratory symptoms compared to their term counterparts. 43 , 44 Respiratory infections and exacerbations in infancy and childhood may worsen an already impaired lung structure, giving rise to frequent coughing and recurrent wheezing disorders. 40 , 45 Furthermore, neonates with BPD and tracheobronchomalacia, a common comorbidity of BPD, are more likely to be mechanically ventilated upon discharge and longer hospitalized compared to their peers without tracheobronchomalacia. 46
Lung function in infants and pre‐school age can be assessed using tidal flow–volume loops, multiple breath washout, whole‐body plethysmography, single‐breath occlusion, rapid thoracho‐abdominal compression technique (RVRTC) or forced oscillation technique. Studies on neonatal lung function have mostly failed to predict BPD development or duration of mechanical ventilation and supplemental oxygen, 47 but lung mechanics measurements reflecting severity of neonatal disease have shown a correlation with a reduced lung function in BPD toddlers. 48
Studies applying the raised volume rapid thoraco‐abdominal compression technique and the measurement of the maximal expiratory flow at functional residual capacity (VmaxFRC) on preterm infants with and without BPD have shown reduced expiratory flows up to 2 years of age, 43 , 49 , 50 , 51 , 52 with infants having lower respiratory system compliance at 10–20 days of life demonstrating lower forced expiratory flows at the 2‐year follow‐up. 51 Ventilation with high‐frequency oscillatory ventilation (HFOV) during neonatal hospitalization, in combination with surfactant treatment, may lessen the neonatal lung injury with positive effects on later lung function. 53 In children with BPD, the need for supplemental oxygen or ventilation at 2 years has been associated with a worse VʹmaxFRC/FRC. 54
Respiratory morbidity at the time of follow‐up may better predict airway function and respiratory compliance than BPD severity at 36 weeks postmenstrual age (PMA), as demonstrated by a longitudinal cohort of preterm infants where those with BPD and respiratory symptoms had lower VʹmaxFRC, forced mid‐expiratory flows (MEF50) and respiratory system compliance (C rs) compared to those without symptoms at 6 and 18 months of postnatal age. 55
Whereas lung compliance seems to improve with increasing age, lung volumes and FRC measured with dilution techniques 51 , 56 and plethysmography 55 , 57 , 58 tend to normalize or even rise by 1 year, suggesting obstructive airway disease with air trapping. Parameters of airway function (forced expiratory volume in 0.5 s (FEV0.5), forced expiratory flow at 75% (FEF75) and FEF25–75), instead, tend to remain low up to 3 years of age. 59 Interestingly, Sheperd and colleagues proposed a classification of pulmonary function in BPD survivors based on infant pulmonary function testing (PFT) at approximately 52 weeks PMA, recognizing an obstructive, a restrictive, and a mixed phenotype according to functional residual volume in 0.5 s (FRV0.5), forced vital capacity (FVC), and total lung capacity (TLC). Patients with characteristics of obstruction were about a half (51%) of the total and were also more responsive to bronchodilators (74%). 60
The airflow limitation, however, affects infants born preterm without BPD as well, although infants with a previous diagnosis of BPD show lower z scores of airflow parameters. Ethnic differences should be taken into account when conducting PFT in nonwhite infants, as differences in expiratory parameters may derive from ethnicity and not to worse lung function or disease, as occurs in older subjects. 61
Expiratory airflow limitation persists at school age and beyond in former preterm infants with and without BPD 43 compared with their term‐born peers, 62 with no apparent catch‐up growth in the former's lung function. On the other hand, structural imaging studies at school age (10–14 years) show that, despite their lower forced expiratory volume in 1 s (FEV1), children born extremely preterm have comparable alveolar dimensions to those of term‐born and mildly preterm children. 63 This may be partly explained by the concept of dysanapsis.
4. RESPIRATORY OUTCOMES AND LUNG FUNCTION IN ADOLESCENCE AND ADULTHOOD
Several longitudinal studies following up cohorts of preterm‐born infants with and without BPD demonstrate tracking of lung function over time, as measured by FEV1—a powerful spirometric parameter of flow limitation—regardless of whether subjects were born before or after the introduction of surfactant. 64 , 65 , 67 According to a review and meta‐analysis on patients aged 5–23 years, the %FEV1 deficit in those born preterm with and without BPD was about 16.8% and 7.2%, respectively. 67 Some reports reveal a gradual decline in lung function with age, raising concern that children with BPD are at higher risk of early‐onset chronic obstructive pulmonary disease (COPD). 18 , 68 In a recent Australian study, Simpson et al. found that preterm infants had a worrying decline in spirometric values from 4 to 12 years of age (0.1 z score per year for FEV1, FEF25–75, and FEV1/FVC), with the poorest trajectories in BPD survivors. Children with bronchial wall thickening on chest computed tomography (CT) (suggestive of inflammation), those exposed to tobacco smoke, born at earlier gestational age, or requiring more supplemental oxygen after birth showed a faster decline in FEV1 (Figure 1). 69 Another study conducted by Doyle et al. demonstrated a reduction in all lung function variables reflecting airflow in a BPD group at a mean age of 18.9 years. In 42.4% of this sample, the FEV1/FVC ratio was <75% (the recognized threshold for airway obstruction). This value deteriorated more between 8 and 18 years old than in previous years. 70
Figure 1.
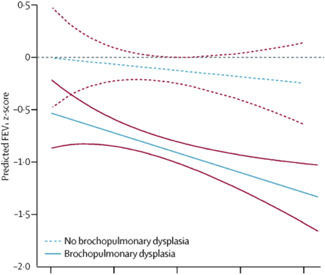
Trajectories of FEV1 through childhood in very preterm children with and without bronchopulmonary dysplasia. 69 Slopes in preterm groups represent the rate of lung function decline relative to the term group and show significant declines in lung function trajectory throughout childhood. Solid red and dashed red lines show the 95% CIs for the respective group. FEV1, forced expiratory volume in 1 s. Reprinted from Simpson et al., 69 copyright (2018), with permission from Elsevier [Color figure can be viewed at wileyonlinelibrary.com]
Other authors, however, did not report such an early decline of airflow parameters. Hurst et al. 71 documented impaired FEV1 z score and higher bronchodilator reversibility at 19 years of age in subjects born before 26 GW compared to controls, but with similar differences compared to a previous evaluation at 11 years. Similarly, Vollsæter et al. 72 found a significant reduction in z scores for FEV1, FEF25–75, and FEV1/FVC in subjects born extremely preterm (GA ≤ 28 GW) only from 18 to 25 years of age, but these lung function changes were similar in the term‐born group and there were no trends related to BPD. Children born preterm in 1999–2000 compared to those born in the early 1990s apparently show better pulmonary outcomes, suggesting positive effects of antenatal steroids and surfactant treatment. 73
Although the decline of lung function and the time at which it may occur are still a matter of debate, it is clear that preterm infants with and without BPD do not catch up to the lung function of their matched term peers, failing to reach the normal peak at 20–25 years of age and being therefore at risk for early chronic obstructive lung disease. 18
Factors contributing to chronic lung dysfunction in children with BPD may include nonsynchronous increases in lung size and airway caliber, chronic airway inflammation, air trapping, and emphysematous changes. 13 , 74 , 75 The concept of “dysanaptic growth,” meaning a disproportionate growth between lung size and airway caliber, may explain the difference in expiratory flows between individuals with BPD and term‐born ones despite similar lung sizes. 76 , 77 Dysanapsis may also explain other spirometric alterations characteristic of adult survivors of very preterm birth, with and without BPD, such as a significantly higher average slope ratio throughout the effort‐independent portion of the maximal expiratory flow–volume curve compared with adult controls. This higher slope ratio during early expiration may indeed be due to structural and mechanical properties of the airways, 78 and to the persistence of active airway inflammation, as demonstrated by studies on exhaled breath condensate and nitric oxide. 79 , 80
Available data show that most preterm‐born infants and BPD patients embark on a lower than normal lung function trajectory with a higher risk of developing a COPD‐like phenotype later in life. 20 , 54 , 55 , 56 , 57 To better understand the evolution of pulmonary function in preterm‐born subjects with and without BPD, clinicians and researchers should be encouraged to follow‐up these patients longitudinally into adult age. More insight on this topic could come from the new ERS Clinical Collaboration on Chronic Airway Diseases Early Stratification (CADSET). 81
Lung function should be considered as a global health biomarker. Poor lung development early in life can point to a poor development of other organ systems too, possibly predisposing to non‐communicable diseases later in life. 82
5. EXERCISE PERFORMANCE
Preterm‐born children with and without BPD may show a higher prevalence of exercise intolerance and reduced physical activity at school‐age (8–12 years) compared to their term‐matched peers, 83 , 84 although some follow‐up studies do not report exercise limitation in this population. 85 , 86 Several studies demonstrate that impaired exercise tolerance is predominant in survivors of moderate‐severe BPD 87 , 88 , 89 and may stem from different pathophysiological changes, from expiratory flow limitation, 83 , 84 , 89 , 90 to decreased peak oxygen consumption and anaerobic threshold, 87 , 91 , 92 to altered respiratory mechanics. 88 , 90 The lower aerobic exercise capacity, derived from the impaired and inadequate respiratory and cardiopulmonary systems to the challenge, may persist into adolescence and adulthood. 93 Nevertheless, prematurity regardless of BPD appears to be related to a more sedentary behavior as monitored by accelerometry 87 despite the still contrasting results. 85 , 92
Finally, the few studies conducted in the post‐surfactant era have produced conflicting results regarding the diffusing capacity of the lung for carbon monoxide (DLCO) in BPD survivors. Some found a significantly lower DLCO in infants and children born very preterm with BPD, while others showed no difference. 94 Nevertheless, despite a decrease in DLCO with the reduction in both membrane component of diffusion capacity (D m) and pulmonary capillary blood volume (V c) in infants with BPD, Chang et al. 95 found a constant D m/V c, indicating both impaired alveolar development and reduced pulmonary vascular bed.
6. IMAGING
Various imaging methods have been used to shed light on BPD ever since the first description published by Northway et al. 96 They have initially involved chest radiography (CR), classically supported by CT, but magnetic resonance imaging (MRI) and lung ultrasound (LU) have also recently gained in importance.
All these imaging techniques can at least partially visualize the structural changes that occur in the lung of patients with BPD, and monitor their evolution over time. They are currently used as descriptive tools, but there is increasing evidence to support their application for predictive and prognostic purposes as part of the BPD diagnostic package.
7. CHEST RADIOGRAPHY
CR has demonstrated its capability to predict the evolution of BPD in preterm infants at high risk. Hyödynmaa et al. 97 showed that most (89.5%) of the patients who would develop moderate‐to‐severe BPD at 36 weeks PMA had one of the two radiological patterns of leaky lung syndrome (LLS) with hazy/opaque lungs, or cystic BPD (cBPD) with bubbly lung changes. Oxygen demand at discharge is an independent risk factor for cBPD (odds ratio OR 3). 98 So far, CR performed at 7 days of life has been tested as a predictor of BPD or death before 36 weeks PMA, 99 with satisfactory results: an interstitial pneumonia pattern had a positive predictive value of 89% and a negative predictive value of 66%.
8. COMPUTED TOMOGRAPHY
CT has been used to assess former preterm infants at various ages, from discharge home after birth up to adulthood. 100 When Boechat et al. 101 applied a scoring system based on High‐resolution computed tomography (HRCT) abnormalities to the youngest infants, they found it was able to identify those likely to develop respiratory morbidities in the first year of life. Other studies confirmed a good correlation between CT abnormalities and clinical scores at 36 weeks PMA, duration of oxygen therapy, and risk of hospitalization for respiratory tract infections. 102 , 103 , 104 HRCT abnormalities correlate better with BPD than CR. 98 , 105
Chest CT frequently shows bronchial wall thickening, hypoattenuation, emphysematous areas and linear or triangular opacities in preterm‐born infants and toddlers with BPD, 104 , 106 and these signs tend to decrease with age. 103 , 107
An interesting feature that can be analyzed with CT is airway cross‐sectional area, which has been found larger in the upper airways and smaller in the lower airways of patients with BPD compared with controls. 108 , 109
Some studies examined the correlation between CT scoring outcomes and lung function results. Mahut et al. 104 reported an inverse correlation between CT score and FRC, while Sarria et al. 107 did not found such relationships between CT scores and forced expiratory flows or pulmonary diffusing capacity.
Regarding school age, Simpson et al. 94 described the persistence of an extensive damage in preterm‐born infants with BPD compared with controls. Comparing these data with functional test results, the authors found that infants with increased subpleural opacities, bronchial wall thickening, and hypoattenuated lung areas had more signs of obstructive lung disease, as expressed by decreased FEV1, FEV1/FVC, and FEV25–75 z scores. Lower z scores for residual volume were associated with areas of collapse and consolidation, while a higher TLC was characteristic of infants with bronchiectasis. The same group recently suggested that infants with CT changes reflecting inflammation (bronchial wall thickening, subpleural opacities or hypoattenuation on inspiratory scans) have the poorest respiratory trajectories, and may be at greater risk of chronic lung disease in elder life. 69 Similarly, Ronkainen et al. 110 confirmed the inverse relationship between structural CT lung abnormalities and FEV1 on spirometry in school children with a history of BPD.
A few studies focused on chest CT in adult BPD patients. Wong et al. described the common presence of triangular, linear opacities and gas trapping, 111 and more importantly reported that the extent of radiological emphysema was inversely related to the FEV1 z score (Figure 2). 112
Figure 2.
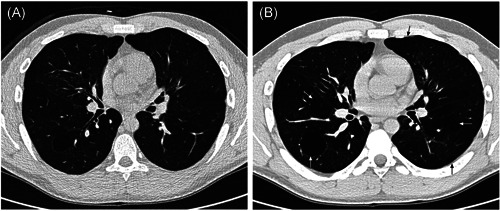
Thin‐section (A) inspiratory and (B) expiratory computed tomography scans of a 25‐yr‐old nonsmoking male, born weighing 1100 g at 29 weeks gestation, dependent upon supplementary oxygen until 60 days postpartum. The forced expiratory volume in 1 s z score was −4.75. There was moderate‐to‐severe emphysema (arrows; voxel index 46.7%). 112 Reproduced with permission of the © ERS 2021: Wong et al. 112
The main issue related to CT is the use of ionizing radiation, limiting the chance to repeat the exam. Another reported problem is the possible need for sedation in young children, increasing the procedural risk. Modern pos‐processing techniques are particularly well suited to demonstrate previously unevaluable areas of low attenuation alternated with others of higher attenuation (variegate mosaic attenuation) seen in patients with BPD with small airways obstruction components. 113
9. MAGNETIC RESONANCE IMAGING
The evaluation of lung parenchyma with MRI has the advantage of using a nonionizing technique, allowing repeated exams. One limitation, however, is the lower spatial resolution compared with CT (0.86 mm3 vs. 0.2 mm3). Additionally, the low proton density of lung tissue with many air–tissue interfaces can determine extremely low levels of rapidly decaying signal, resulting in the formation of extremely low‐resolution images of the lung parenchyma. 113 Also, different relaxation times between fibrotic and inflamed tissues and normal and hypodense ones (alveolar simplification, emphysema, cysts) could limit MRI lung evaluation. These issues are partially addressed using ultrashort echo times, as shown by Higano et al. 23 Many authors have explored the use of MRI as a diagnostic tool for BPD in recent years. Hahn et al. 114 identified a significantly higher signal from the lung parenchyma of BPD patients compared with controls, and attributed this to a greater degree of lung disease (fibrosis, edema, and atelectasis). Walkup et al. 115 reported instead that the most severe cases had a drop of signal that they attributed to alveolar simplification. Forster et al. 116 found a longer lung T2 relaxation time and a shorter T1 relaxation time as indicators of BPD. When scores similar to those used for CT were applied to the lung MRI findings in BPD patients, Walkup et al. 115 described higher scores in the BPD group than in controls, and Higano et al. 23 described a good correlation between MRI scores and the duration of respiratory support. Yoder et al. 117 demonstrated that MRI can quantify hyperinflation in BPD patients and that their lung volume parameters (FRC, tidal volume, and minute ventilation) increased consistently with disease severity.
Narayanan et al. 63 used MRI to study older patients (10–14 years). When they analyzed alveolar size, they found similar results for survivors of extreme prematurity and term‐born children, suggesting a catch‐up of alveolarization. Contrasting results were published by Flors et al., 118 who described a significantly greater apparent diffusion coefficient (a surrogate of average alveolar dimensions) in patients with BPD, but same lung volumes as age‐matched healthy controls, consistently with BPD patients' alveoli being fewer in number and larger in size.
10. LUNG ULTRASOUND
Ultrasound interaction with the highly reflective pleura produces different artifact patterns which correlate with pulmonary aeration. 119 LU does not expose the preterm infant to ionizing radiation or require a transfer from the neonatal intensive care unit.
When studied with LU, BPD appears as a nonhomogeneous disease with coalescent B‐lines interspersed with intact areas, and always accompanied by multiple different‐sized subpleural consolidations, as well as thickened pleural lines. LU has also proved capable of detecting coexisting diseases like atelectasis, pulmonary edema, or consolidations. This suggests that LU could also be of preventive value, guiding treatment decisions and reducing the risk of ventilator‐induced lung injury. 120 , 121
A few studies tested LU as a predictor of BPD development. It seems promising as a way to identify infants that will develop the disease using a semi‐quantitative score (with a different sensibility and specificity depending on the cutoff applied and the time of the assessment). 122 , 123 A recently published large multicenter study has corroborated previous evidence of the predictive power of LU. The method has revealed a good capacity to monitor lung aeration and function in extremely preterm infants, with LU scores correlating significantly with oxygenation metrics and work of breathing. Scores adjusted for gestational age can also significantly predict the occurrence of BPD, starting from the seventh day of life. 124
Figure 3 represents the pulmonary structure–function relationship in survivors of BPD and preterm birth.
Figure 3.
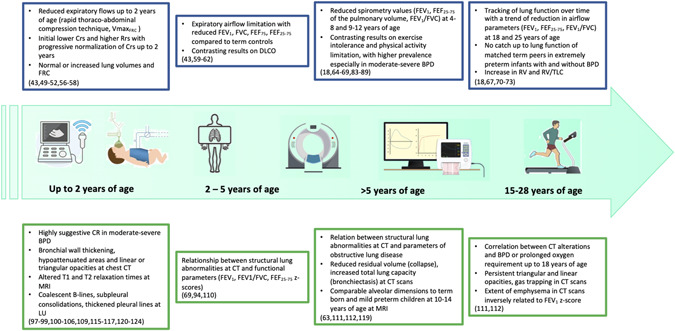
Pulmonary structure–function relationship in survivors of BPD and preterm birth. Relationship between lung function (blue rectangles on the top) and lung structure as shown by imaging techniques (green rectangles on the bottom) from infancy till early adult age. The large green arrow from left to right depicts the pulmonary function tests and imaging methods that have been used in previous studies at different ages (i.e., lung ultrasound, rapid thoracoabdominal compression technique, chest CT, etc.). BPD, bronchopulmonary dysplasia; CT, computed tomography. Icons from Macrovector (freepik.com) and VectorStock [Color figure can be viewed at wileyonlinelibrary.com]
11. CONCLUSION
Several longitudinal lung function studies have demonstrated that most preterm newborn and BPD patients embark on a low lung function trajectory, never achieve their full airway growth potential, and are at a higher risk of developing a COPD‐like phenotype later in life.
To improve the care of BPD survivors and prevent long‐term respiratory morbidities, it is fundamental to develop a structured and standardized cardiopulmonary follow‐up, and to identify early predictive biomarkers to guide treatment decisions. Lung function and imaging techniques are essential surrogates for monitoring survivors of prematurity with and without BPD from infancy to adult age. 125 , 126 The long‐term surveillance and treatment of these subjects should be strongly promoted. 127 Not only pediatricians, but also chest physicians, internists, and family doctors should be aware of their potential for developing a “novel” chronic obstructive lung disease and the multiple associated adverse sequelae.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Laura Moschino: Resources (equal); visualization (equal); writing—original draft (equal). Luca Bonadies: Resources (equal); visualization (equal); writing—original draft (equal).
Moschino L, Bonadies L, Baraldi E. Lung growth and pulmonary function after prematurity and bronchopulmonary dysplasia. Pediatric Pulmonology. 2021;56:3499–3508. 10.1002/ppul.25380
REFERENCES
- 1. Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162‐2172. [DOI] [PubMed] [Google Scholar]
- 2. Raju TNK, Pemberton VL, Saigal S, Blaisdell CJ, Moxey‐Mims M, Buist S. Long‐term healthcare outcomes of preterm birth: an executive summary of a conference sponsored by the National Institutes of Health. J Pediatr. 2017;181:309‐318. [DOI] [PubMed] [Google Scholar]
- 3. Higgins RD, Jobe AH, Koso‐Thomas M, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. 2018;197:300‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Isayama T, Lee SK, Canadian Neonatal Network and Canadian Neonatal Follow‐Up Network Investigators , et al. Revisiting the definition of bronchopulmonary dysplasia: effect of changing panoply of respiratory support for preterm neonates. JAMA Pediatr. 2017;171(3):271‐279. [DOI] [PubMed] [Google Scholar]
- 5. Siffel C, Kistler KD, Lewis JFM, Sarda SP. Global incidence of bronchopulmonary dysplasia among extremely preterm infants: a systematic literature review. J Matern Fetal Neonatal Med. 2019;9:1‐11. [DOI] [PubMed] [Google Scholar]
- 6. Stoll BJ, Hansen NI, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network , et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314(10):1039‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Marter LJ. Epidemiology of bronchopulmonary dysplasia. Semin Fetal Neonatal Med. 2009;14:358‐366. [DOI] [PubMed] [Google Scholar]
- 8. Davidson LM, Berkelhamer SK. Bronchopulmonary dysplasia: chronic lung disease of infancy and long‐term pulmonary outcomes. J Clin Med. 2017;6(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Priante E, Moschino L, Mardegan V, Manzoni P, Salvadori S, Baraldi E. Respiratory outcome after preterm birth: a long and difficult journey. Am J Perinatol. 2016;33:1040‐1042. [DOI] [PubMed] [Google Scholar]
- 10. Martinez FD. Early‐life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016;375:871‐878. [DOI] [PubMed] [Google Scholar]
- 11. Crump C, Sundquist J, Winkleby MA, Sundquist K. Gestational age at birth and mortality from infancy into mid‐adulthood: a national cohort study. Lancet Child Adolesc Health. 2019;3:408‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delacourt C, Hadchouel A. Regulation of alveolarization. In: Polin R, Abman S, Rowitch D, Benitz W, Fox W, eds. Fetal and Neonatal Physiology. 5th ed. Philadelphia: Elsevier; 2017:642‐646. [Google Scholar]
- 13. Thébaud B, Goss KN, Laughon M, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers. 2019;5(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Butler JP, Loring SH, Patz S, Tsuda A, Yablonskiy DA, Mentzer SJ. Evidence for adult lung growth in humans. N Engl J Med. 2012;367(3):244‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plosa E, Guttentag SH. Lung development. In: Gleason CA, Juul SE, eds. Avery's Diseases of the Newborn. 10th ed. Philadelphia: Elsevier; 2018:586‐599. [Google Scholar]
- 16. Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015;385(9971):899‐909. [DOI] [PubMed] [Google Scholar]
- 17. Shojaie S, Post M. Molecular mechanisms of lung development and lung branching morphogenesis. In: Polin R, Abman S, Rowitch D, Benitz W, Fox W, eds. Fetal and Neonatal Physiology. 5th ed. Philadelphia: Elsevier; 2017:658‐666. [Google Scholar]
- 18. Moschino L, Baraldi E. Prematurity and intra‐uterine insults. Encyclopedia of Respiratory Medicine (2nd ed.). 10.1016/B978-0-08-102723-3.00024-X [DOI] [Google Scholar]
- 19. Thébaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007;175(10):978‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357(19):1946‐1955. [DOI] [PubMed] [Google Scholar]
- 21. Hislop AA, Wigglesworth JS, Desai R, Aber V. The effects of preterm delivery and mechanical ventilation on human lung growth. Early Hum Dev. 1987;15(3):147‐164. [DOI] [PubMed] [Google Scholar]
- 22. Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29(7):710‐717. [DOI] [PubMed] [Google Scholar]
- 23. Higano NS, Spielberg DR, Fleck RJ, et al. Neonatal pulmonary magnetic resonance imaging of bronchopulmonary dysplasia predicts short‐term clinical outcomes. Am J Respir Crit Care Med. 2018;198(10):1302‐1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kalymbetova TV, Selvakumar B, Rodríguez‐Castillo JA, et al. Resident alveolar macrophages are master regulators of arrested alveolarization in experimental bronchopulmonary dysplasia. J Pathol. 2018;245(2):153‐159. [DOI] [PubMed] [Google Scholar]
- 25. Surate Solaligue DE, Rodríguez‐Castillo JA, Ahlbrecht K, Morty RE. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2017;313(6):L1101‐L1153. [DOI] [PubMed] [Google Scholar]
- 26. Hakulinen AL, Järvenpää AL, Turpeinen M, Sovijärvi A. Diffusing capacity of the lung in school‐aged children born very preterm, with and without bronchopulmonary dysplasia. Pediatr Pulmonol. 1996;21(6):353‐360. [DOI] [PubMed] [Google Scholar]
- 27. Satrell E, Røksund O, Thorsen E, Halvorsen T. Pulmonary gas transfer in children and adolescents born extremely preterm. Eur Respir J. 2013;42(6):1536‐1544. [DOI] [PubMed] [Google Scholar]
- 28. Galderisi A, Calabrese F, Fortarezza F, Abman S, Baraldi E. Airway histopathology of adolescent survivors of bronchopulmonary dysplasia. J Pediatr. 2019;211:215‐218. [DOI] [PubMed] [Google Scholar]
- 29. Baker CD, Abman SH. Impaired pulmonary vascular development in bronchopulmonary dysplasia. Neonatology. 2015;107:344‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Paepe ME, Mao Q, Powell J, et al. Growth of pulmonary microvasculature in ventilated preterm infants. Am J Respir Crit Care Med. 2006;173(2):204‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coalson J. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003;8(1):73‐81. [DOI] [PubMed] [Google Scholar]
- 32. Stocks J, Hislop A, Sonnappa S. Early lung development: Lifelong effect on respiratory health and disease. Lancet Respir Med. 2003;1(9):728‐742. [DOI] [PubMed] [Google Scholar]
- 33. Coalson JJ. Pathology of chronic lung disease of early infancy. In: Bland R, Coalson JJ, eds. Lung Biology in Health and Disease: Chronic Lung Disease in Early Infancy. New York: Marcel Dekker; 2000:85‐124. [Google Scholar]
- 34. Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis”. Am J Respir Crit Care Med. 2001;164:1755‐1756. [DOI] [PubMed] [Google Scholar]
- 35. Bonadies L, Zaramella P, Porzionato A, Perilongo G, Muraca M, Baraldi E. Present and future of bronchopulmonary dysplasia. J Clin Med. 2020;9(5):1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Porzionato A, Zaramella P, Dedja A, et al. Intratracheal administration of clinical‐grade mesenchymal stem cell‐derived extracellular vesicles reduces lung injury in a rat model of bronchopulmonary dysplasia. Am J Physiol Cell Mol Physiol. 2019;316(1):L6‐L19. [DOI] [PubMed] [Google Scholar]
- 37. Bonadies L, Zaramella P, Porzionato A, Muraca M, Baraldi E. Bronchopulmonary dysplasia: what's new on the horizon? Lancet Child Adolesc Health. 2018;2(8):549‐551. [DOI] [PubMed] [Google Scholar]
- 38. Resch B, Kurath‐Koller S, Eibisberger M, Zenz W. Prematurity and the burden of influenza and respiratory syncytial virus disease. J Pediatr. 2016;12(1):8‐18. [DOI] [PubMed] [Google Scholar]
- 39. Ralser E, Mueller W, Haberland C, et al. Rehospitalization in the first 2 years of life in children born preterm. Acta Paediatr (Stockholm). 2011;101(1):e1‐e5. [DOI] [PubMed] [Google Scholar]
- 40. Pramana I, Latzin P, Schlapbach L, et al. Respiratory symptoms in preterm infants: burden of disease in the first year of life. Eur J Med Res. 2011;16(5):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Townsi N, Laing IA, Hall GL, Simpson SJ. The impact of respiratory viruses on lung health after preterm birth. Eur Clin Respir J. 2018;5(1):1487214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martinez F. Childhood asthma inception and progression. Immunol Allergy Clin North Am. 2019;39(2):141‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the short‐ and long‐term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;192(2):134‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kallapur SG, Ikegami M. Physiological consequences of intrauterine insults. Paediatr Respir Rev. 2006;7(2):110‐116. [DOI] [PubMed] [Google Scholar]
- 45. Been JV, Lugtenberg MJ, Smets E, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta‐analysis. PLOS Med. 2014;11(1):e1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hysinger EB, Friedman NL, Children's Hospitals Neonatal Consortium , et al. Tracheobronchomalacia is associated with increased morbidity in bronchopulmonary dysplasia. Ann Am Thorac Soc. 2017;14(9):1428‐1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ren CL, Feng R, Davis SD, et al. Tidal breathing measurements at discharge and clinical outcomes in extremely low gestational age neonates. Ann Am Thorac Soc. 2018;15(11):1311‐1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bhandari A, Panitch HB. Pulmonary outcomes in bronchopulmonary dysplasia. Semin Perinatol. 2006;30(4):219‐226. [DOI] [PubMed] [Google Scholar]
- 49. Sanchez‐Solis M, Perez‐Fernandez V, Bosch‐Gimenez V, Quesada JJ, Garcia‐Marcos L. Lung function gain in preterm infants with and without bronchopulmonary dysplasia. Pediatr Pulmonol. 2016;51(9):936‐942. [DOI] [PubMed] [Google Scholar]
- 50. Lum S, Hülskamp G, Merkus P, Baraldi E, Hofhuis W, Stocks J. Lung function tests in neonates and infants with chronic lung disease: forced expiratory maneuvers. Pediatr Pulmonol. 2006;41(3):199‐214. [DOI] [PubMed] [Google Scholar]
- 51. Baraldi E, Filippone M, Trevisanuto D, Zanardo V, Zacchello F. Pulmonary function until two years of life in infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 1997;155(1):149‐155. [DOI] [PubMed] [Google Scholar]
- 52. Friedrich L, Stein RT, Pitrez PM, Corso AL, Jones MH. Reduced lung function in healthy preterm infants in the first months of life. Am J Respir Crit Care Med. 2006;173(4):442‐447. [DOI] [PubMed] [Google Scholar]
- 53. Hofhuis W, Huysman MW, van der Wiel EC, et al. Worsening of V'maxFRC in infants with chronic lung disease in the first year of life: a more favorable outcome after high‐frequency oscillation ventilation. Am J Respir Crit Care Med. 2002;166(12, pt 1):1539‐1543. [DOI] [PubMed] [Google Scholar]
- 54. Talmaciu I, Ren CL, Kolb SM, Hickey E, Panitch HB. Pulmonary function in technology‐dependent children 2 years and older with bronchopulmonary dysplasia. Pediatr Pulmonol. 2002;33(3):181‐188. [DOI] [PubMed] [Google Scholar]
- 55. Thunqvist P, Gustafsson P, Norman M, Wickman M, Hallberg J. Lung function at 6 and 18 months after preterm birth in relation to severity of bronchopulmonary dysplasia. Pediatr Pulmonol. 2015;50(10):978‐986. [DOI] [PubMed] [Google Scholar]
- 56. Gerhardt T, Hehre D, Feller R, Reifenberg L, Bancalari E. Serial determination of pulmonary function in infants with chronic lung disease. J Pediatr. 1987;110(3):448‐456. [DOI] [PubMed] [Google Scholar]
- 57. Robin B, Kim YJ, Huth J, et al. Pulmonary function in bronchopulmonary dysplasia. Pediatr Pulmonol. 2004;37(3):236‐242. [DOI] [PubMed] [Google Scholar]
- 58. Wauer RR, Maurer T, Nowotny T, Schmalisch G. Assessment of functional residual capacity using nitrogen washout and plethysmographic techniques in infants with and without bronchopulmonary dysplasia. Intensive Care Med. 1998;24(5):469‐475. [DOI] [PubMed] [Google Scholar]
- 59. Filbrun AG, Popova AP, Linn MJ, McIntosh NA, Hershenson MB. Longitudinal measures of lung function in infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 2011;46(4):369‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shepherd EG, Clouse BJ, Hasenstab KA, et al. Infant pulmonary function testing and phenotypes in severe bronchopulmonary dysplasia. Pediatrics. 2018;141(5):e20173350. [DOI] [PubMed] [Google Scholar]
- 61. Hoo AF, Gupta A, Lum S, et al. Impact of ethnicity and extreme prematurity on infant pulmonary function. Pediatr Pulmonol. 2014;49(7):679‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lombardi E, Fainardi V, Calogero C, et al. Lung function in a cohort of 5‐year‐old children born very preterm. Pediatr Pulmonol. 2018;53(12):1633‐1639. [DOI] [PubMed] [Google Scholar]
- 63. Narayanan M, Beardsmore CS, Owers‐Bradley J, et al. Catch‐up alveolarization in ex‐preterm children: evidence from (3)He magnetic resonance. Am J Respir Crit Care Med. 2013;187(10):1104‐1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Moschino L, Stocchero M, Filippone M, Carraro S, Baraldi E. Longitudinal assessment of lung function in survivors of bronchopulmonary dysplasia from birth to adulthood. The Padova BPD Study. Am J Respir Crit Care Med. 2018;198(1):134‐137. [DOI] [PubMed] [Google Scholar]
- 65. Fortuna M, Carraro S, Temporin E, et al. Mid‐childhood lung function in a cohort of children with “new bronchopulmonary dysplasia”. Pediatr Pulmonol. 2016;51(10):1057‐1064. [DOI] [PubMed] [Google Scholar]
- 66. Kotecha SJ, Edwards MO, Watkins WJ, et al. Effect of preterm birth on later FEV1: a systematic review and meta‐analysis. Thorax. 2013;68(8):760‐766. [DOI] [PubMed] [Google Scholar]
- 67. Vollsæter M, Røksund OD, Eide GE, Markestad T, Halvorsen T. Lung function after preterm birth: development from mid‐childhood to adulthood. Thorax. 2013;68(8):767‐776. [DOI] [PubMed] [Google Scholar]
- 68. Hirata K, Nishihara M, Kimura T, et al. Longitudinal impairment of lung function in school‐age children with extremely low birth weights. Pediatr Pulmonol. 2017;52(6):779‐786. [DOI] [PubMed] [Google Scholar]
- 69. Simpson SJ, Turkovic L, Wilson AC, et al. Lung function trajectories throughout childhood in survivors of very preterm birth: a longitudinal cohort study. Lancet Child Adolesc Health. 2018;2(5):350‐359. [DOI] [PubMed] [Google Scholar]
- 70. Doyle LW, Faber B, Callanan C, Freezer N, Ford GW, Davis NM. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics. 2006;118(1):108‐113. [DOI] [PubMed] [Google Scholar]
- 71. Hurst JR, Beckmann J, Ni Y, et al. Respiratory and cardiovascular outcomes in survivors of extremely preterm birth at 19 years. Am J Respir Crit Care Med. 2020;202(3):422‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vollsæter M, Clemm HH, Satrell E, et al. Adult respiratory outcomes of extreme preterm birth: a regional cohort study. Ann Am Thorac Soc. 2015;12(3):313‐322. [DOI] [PubMed] [Google Scholar]
- 73. Vollsæter M, Skromme K, Satrell E, et al. Children born preterm at the turn of the millennium had better lung function than children born similarly preterm in the early 1990s. PLOS One. 2015;10(12):e0144243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vom Hove M, Prenzel F, Uhlig HH, Robel‐Tillig E. Pulmonary outcome in former preterm, very low birth weight children with bronchopulmonary dysplasia: a case–control follow‐up at school age. J Pediatr. 2014;164(1):40‐45. [DOI] [PubMed] [Google Scholar]
- 75. Urs R, Kotecha S, Hall GL, Simpson SJ. Persistent and progressive long‐term lung disease in survivors of preterm birth. Paediatr Respir Rev. 2018;28:87‐94. [DOI] [PubMed] [Google Scholar]
- 76. Ioan I, Gemble A, Hamon I, et al. Expiratory flow—vital capacity: airway—lung dysanapsis in 7 year olds born very preterm? Front Physiol. 2018;9:650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis. 1980;121:339‐342. [DOI] [PubMed] [Google Scholar]
- 78. Molgat‐Seon Y, Dominelli PB, Peters CM, et al. Analysis of maximal expiratory flow–volume curves in adult survivors of preterm birth. Am J Physiol Regul Integr Comp Physiol. 2019;317(4):R588‐R596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Baraldi E, Bonetto G, Zacchello F, Filippone M. Low exhaled nitric oxide in school‐age children with bronchopulmonary dysplasia and airflow limitation. Am J Respir Crit Care Med. 2005;171(1):68‐72. [DOI] [PubMed] [Google Scholar]
- 80. Carraro S, Giordano G, Pirillo P, et al. Airway metabolic anomalies in adolescents with bronchopulmonary dysplasia: new insights from the metabolomic approach. J Pediatr. 2015;166(2):234‐239. [DOI] [PubMed] [Google Scholar]
- 81. Agusti A, Faner R, Donaldson G, et al. on behalf of the CADSET Clinical Research Collaboration. Current members of the CADSET Clinical Research Collaboration Chronic Airway Diseases Early Stratification (CADSET): a new ERS Clinical Research Collaboration. Eur Respir J. 2019;53(3):1900217. [DOI] [PubMed]
- 82. Agustí A, Noell G, Brugada J, Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med. 2017;5(12):935‐945. [DOI] [PubMed] [Google Scholar]
- 83. Baraldi E, Carraro S. Exercise testing and chronic lung diseases in children. Paediatr Respir Rev. 2006;7(suppl 1):S196‐S198. [DOI] [PubMed] [Google Scholar]
- 84. O'Dea CA, Logie K, Maiorana A, et al. Increased prevalence of expiratory flow limitation during exercise in children with bronchopulmonary dysplasia. ERJ Open Res. 2018;4(4):00048‐02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Clemm H, Røksund O, Thorsen E, Eide GE, Markestad T, Halvorsen T. Aerobic capacity and exercise performance in young people born extremely preterm. Pediatrics. 2012;129(1):e97‐e105. [DOI] [PubMed] [Google Scholar]
- 86. Clemm HH, Vollsæter M, Røksund OD, Eide GE, Markestad T, Halvorsen T. Exercise capacity after extremely preterm birth. Development from adolescence to adulthood. Ann Am Thorac Soc. 2014;11(4):537‐545. [DOI] [PubMed] [Google Scholar]
- 87. Ruf K, Thomas W, Brunner M, Speer CP, Hebestreit H. Diverging effects of premature birth and bronchopulmonary dysplasia on exercise capacity and physical activity—a case control study. Respir Res. 2019;20(1):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. MacLean JE, DeHaan K, Fuhr D, et al. Altered breathing mechanics and ventilatory response during exercise in children born extremely preterm. Thorax. 2016;71(11):1012‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Praprotnik M, Stucin Gantar I, Lučovnik M, Avčin T, Krivec U. Respiratory morbidity, lung function and fitness assessment after bronchopulmonary dysplasia. J Perinatol. 2015;35(12):1037‐1042. [DOI] [PubMed] [Google Scholar]
- 90. Lovering AT, Elliott JE, Laurie SS, et al. Ventilatory and sensory responses in adult survivors of preterm birth and bronchopulmonary dysplasia with reduced exercise capacity. Ann Am Thorac Soc. 2014;11(10):1528‐1537. [DOI] [PubMed] [Google Scholar]
- 91. Santuz P, Baraldi E, Zaramella P, Filippone M, Zacchello F. Factors limiting exercise performance in long‐term survivors of bronchopulmonary dysplasia. Am J Respir Crit Care Med. 1995;152(4, pt 1):1284‐1289. [DOI] [PubMed] [Google Scholar]
- 92. Welsh L, Kirkby J, EPICure Study Group , et al. The EPICure study: maximal exercise and physical activity in school children born extremely preterm. Thorax. 2010;65(2):165‐172. [DOI] [PubMed] [Google Scholar]
- 93. Duke JW, Lovering AT. Respiratory and cardiopulmonary limitations to aerobic exercise capacity in adults born preterm. J Appl Physiol (1985). 2020;129(4):718‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Simpson SJ, Logie KM, O'Dea CA, et al. Altered lung structure and function in mid‐childhood survivors of very preterm birth. Thorax. 2018;72(8):702‐711. [DOI] [PubMed] [Google Scholar]
- 95. Chang DV, Assaf SJ, Tiller CJ, Kisling JA, Tepper RS. Membrane and capillary components of lung diffusion in infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2016;193(7):767‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Northway WH, Jr. , Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline‐membrane disease Bronchopulmonary dysplasia. N Engl J Med. 1967;276(7):357‐368. [DOI] [PubMed] [Google Scholar]
- 97. Hyödynmaa E, Korhonen P, Ahonen S, Luukkaala T, Tammela O. Frequency and clinical correlates of radiographic patterns of bronchopulmonary dysplasia in very low birth weight infants by term age. Eur J Pediatr. 2012;171(1):95‐102. [DOI] [PubMed] [Google Scholar]
- 98. Arai H, Ito T, Ito M, Ota S, Takahashi T. Impact of chest radiography‐based definition of bronchopulmonary dysplasia. Pediatr Int. 2019;61(3):258‐263. [DOI] [PubMed] [Google Scholar]
- 99. Kim HR, Kim JY, Yun BLa, Lee B, Choi CW, Kim BIl. Interstitial pneumonia pattern on day 7 chest radiograph predicts bronchopulmonary dysplasia in preterm infants. BMC Pediatr. 2017;17(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. van Mastrigt E, Logie K, Ciet P, et al. Lung CT imaging in patients with bronchopulmonary dysplasia: a systematic review. Pediatr Pulmonol. 2016;51(9):975‐986. [DOI] [PubMed] [Google Scholar]
- 101. Boechat MCB, de Mello RR, da Silva KS, et al. A computed tomography scoring system to assess pulmonary disease among premature infants. Sao Paulo Med J. 2010;128(6):328‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shin SM, Kim WS, Cheon JE, et al. Bronchopulmonary dysplasia: new high resolution computed tomography scoring system and correlation between the high resolution computed tomography score and clinical severity. Korean J Radiol. 2013;14(2):350‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Spielberg DR, Walkup LL, Stein JM, et al. Quantitative CT scans of lung parenchymal pathology in premature infants ages 0–6 years. Pediatr Pulmonol. 2018;53(3):316‐323. [DOI] [PubMed] [Google Scholar]
- 104. Mahut B, De Blic J, Emond S, et al. Chest computed tomography findings in bronchopulmonary dysplasia and correlation with lung function. Arch Dis Child Fetal Neonatal Ed. 2007;92(6):F459‐F464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li R, Zhang J. Diagnostic value of chest CT combined with x‐ray for premature infants with bronchopulmonary dysplasia. Med (Baltimore). 2018;97(9):e9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tonson La Tour A, Spadola L, Sayegh Y, et al. Chest CT in bronchopulmonary dysplasia: clinical and radiological correlations. Pediatr Pulmonol. 2013;48(7):693‐698. [DOI] [PubMed] [Google Scholar]
- 107. Sarria EE, Mattiello R, Rao L, et al. Computed tomography score and pulmonary function in infants with chronic lung disease of infancy. Eur Respir J. 2011;38(4):918‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sarria EE, Mattiello R, Rao L, et al. Quantitative assessment of chronic lung disease of infancy using computed tomography. Eur Respir J. 2012;39(4):992‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. van Mastrigt E, Kakar E, Ciet P, et al. Structural and functional ventilatory impairment in infants with severe bronchopulmonary dysplasia. Pediatr Pulmonol. 2017;52(8):1029‐1037. [DOI] [PubMed] [Google Scholar]
- 110. Ronkainen E, Perhomaa M, Mattila L, Hallman M, Dunder T. Structural pulmonary abnormalities still evident in schoolchildren with new bronchopulmonary dysplasia. Neonatology. 2018;113(2):122‐130. [DOI] [PubMed] [Google Scholar]
- 111. Wong P, Murray C, Louw J, French N, Chambers D. Adult bronchopulmonary dysplasia: computed tomography pulmonary findings. J Med Imaging Radiat Oncol. 2011;55(4):373‐378. [DOI] [PubMed] [Google Scholar]
- 112. Wong PM, Lees AN, Louw J, et al. Emphysema in young adult survivors of moderate‐to‐severe bronchopulmonary dysplasia. Eur Respir J. 2008;32(2):321‐328. [DOI] [PubMed] [Google Scholar]
- 113. Semple T, Akhtar MR, Owens CM. Imaging bronchopulmonary dysplasia—a multimodality update. Front Med (Lausanne). 2017;4:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hahn AD, Higano NS, Walkup LL, et al. Pulmonary MRI of neonates in the intensive care unit using 3D ultrashort echo time and a small footprint MRI system. J Magn Reson Imaging. 2017;45(2):463‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Walkup LL, Tkach JA, Higano NS, et al. Quantitative magnetic resonance imaging of bronchopulmonary dysplasia in the neonatal intensive care unit environment. Am J Respir Crit Care Med. 2015;192(10):1215‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Förster K, Ertl‐Wagner B, Ehrhardt H, et al. Altered relaxation times in MRI indicate bronchopulmonary dysplasia. Thorax. 2019;75(2):184‐187. [DOI] [PubMed] [Google Scholar]
- 117. Yoder LM, Higano NS, Schapiro AH, et al. Elevated lung volumes in neonates with bronchopulmonary dysplasia measured via MRI. Pediatr Pulmonol. 2019;54(8):1311‐1318. [DOI] [PubMed] [Google Scholar]
- 118. Flors L, Mugler JP, Paget‐Brown A, et al. Hyperpolarized helium‐3 diffusion‐weighted magnetic resonance imaging detects abnormalities of lung structure in children with bronchopulmonary dysplasia. J Thorac Imaging. 2017;32(5):323‐332. [DOI] [PubMed] [Google Scholar]
- 119. Singh Y, Tissot C, Fraga MV, et al. International evidence‐based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care. 2020;24:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Liu J, Chen SW, Liu F, et al. BPD, not BPD, or iatrogenic BPD: findings of lung ultrasound examinations. Medicine (Baltimore). 2014;93(23):e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bonadies L, Donà D, Baraldi E. Lung ultrasound is used in neonatology for diagnostics, monitoring and prognostics, but also for prevention. Pediatr Pulmonol. 2021;56(2):333‐334. [DOI] [PubMed] [Google Scholar]
- 122. Alonso‐Ojembarrena A, Lubián‐López SP. Lung ultrasound score as early predictor of bronchopulmonary dysplasia in very low birth weight infants. Pediatr Pulmonol. 2019;54(9):1404‐1409. [DOI] [PubMed] [Google Scholar]
- 123. Abdelmawla M, Louis D, Narvey M, Elsayed Y. A lung ultrasound severity score predicts chronic lung disease in preterm infants. Am J Perinatol. 2019;36(13):1357‐1361. [DOI] [PubMed] [Google Scholar]
- 124. Loi B, Vigo G, Baraldi E, et al. LUSTRE (Lung UltraSound to pReterm nEonates) study group. Lung ultrasound to monitor extremely preterm infants and predict BPD: multicenter longitudinal cohort study [published online ahead of print December 22, 2020]. Am J Respir Crit Care Med. 10.1164/rccm.202008-3131OC [DOI] [PubMed] [Google Scholar]
- 125. Katz SL, Luu TM, Nuyt AM, et al. Long‐term follow‐up of cardiorespiratory outcomes in children born extremely preterm: recommendations from a Canadian consensus workshop. Paediatr Child Health. 2017;22:75‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. den Dekker HT, Sonnenschein‐van der Voort AMM, de Jongste JC, et al. Early growth characteristics and the risk of reduced lung function and asthma: a meta‐analysis of 25,000 children. J Allergy Clin Immunol. 2016;137(4):1026‐1035. [DOI] [PubMed] [Google Scholar]
- 127. Duijts L, van Meel ER, Moschino L, et al. European Respiratory Society guideline on long term management of children with bronchopulmonary dysplasia. Eur Respir J. 2020;55(1):1900788. [DOI] [PubMed] [Google Scholar]


