Abstract
A major challenge for the development of a wearable artificial kidney (WAK) is the removal of urea from the spent dialysate, as urea is the waste solute with the highest daily molar production and is difficult to adsorb. Here we present results on glucose degradation products (GDPs) formed during electrooxidation (EO), a technique that applies a current to the dialysate to convert urea into nitrogen, carbon dioxide, and hydrogen gas. Uremic plasma and peritoneal effluent were dialyzed for 8 hours with a WAK with and without EO‐based dialysate regeneration. Samples were taken regularly during treatment. GDPs (glyoxal, methylglyoxal, and 3‐deoxyglucosone) were measured in EO‐ and non‐EO‐treated fluids. Glyoxal and methylglyoxal concentrations increased 26‐ and 11‐fold, respectively, in uremic plasma (at [glucose] 7 mmol/L) and 209‐ and 353‐fold, respectively, in peritoneal effluent (at [glucose] 100 mmol/L) during treatment with EO, whereas no change was observed in GDP concentrations during dialysate regeneration without EO. EO for dialysate regeneration in a WAK is currently not safe due to the generation of GDPs which are not biocompatible.
Keywords: 3‐Deoxyglucosone, artificial kidney, biocompatibility, electrooxidation, glucose degradation products, glyoxal, hemodialysis, methylglyoxal, peritoneal dialysis, urea
Electrooxidation (EO) is currently not safe for dialysate regeneration in a wearable artificial kidney due to the generation of glucose degradation products (GDPs) which are not biocompatible. Glyoxal and methylglyoxal concentrations increased 26‐ and 11‐fold, respectively, in uremic plasma (at [glucose] 7 mmol/L) and 209‐ and 353‐fold, respectively, in peritoneal effluent (at [glucose] 100 mmol/L) during treatment with EO in vitro, whereas no change was observed in GDP concentrations during dialysate regeneration without EO (Figure 1).
![]()
Figure 1. Concentrations of GDPs (µmol/L) in regenerated uremic plasma (A) and peritoneal effluent (B). GO, glyoxal; MGO, methylglyoxal; 3‐DG, 3‐deoxyglucosone. Solid line: EO on; dashed line: EO off. The mean ± SD of 3 experiments is presented.
1. INTRODUCTION
The availability of a wearable artificial kidney (WAK) for patients with end‐stage kidney disease (ESKD) has been long‐awaited. Such a device would facilitate more frequent or continuous dialysis outside the hospital, improving patient's health and quality of life. 1 Miniaturization of the hemodialysis (HD) machine and peritoneal dialysis (PD) technology is based on the continuous regeneration of a small volume of dialysate in a closed‐loop system. 2 The major challenge is the removal of urea from the spent dialysate, as urea is the waste solute with the highest daily molar production (240‐470 mmol/d 3 , 4 ) and is difficult to adsorb. 5 Currently, no efficient urea removal strategy is available that allows for the realization of a WAK <2.0 kg that can easily be carried by patients for a number of hours per day. 5 Several miniature artificial kidney devices for HD and PD are under development which is based on enzymatic hydrolysis of urea by urease or urea adsorption by activated carbon (AC). 6 However, these systems are still relatively large (>5 kg including the daily amount of sorbents and dialysate) and do not allow for further miniaturization. Therefore, electrooxidation (EO) is being explored as an alternative strategy to efficiently remove urea from dialysate. 2
EO is a technique that applies a current to the dialysate to oxidize urea into nitrogen, carbon dioxide, and hydrogen gas, either directly at the anode or indirectly in the bulk solution via hypochlorite that is produced by oxidation of chloride ions. 7 , 8 EO offers the prospect of reducing the size and weight of a WAK to wearable proportions, as the electrodes are small, lightweight, stackable, and can be reused. We previously demonstrated that clinically relevant urea removal could be achieved in vitro and in vivo when using EO for the regeneration of spent dialysate. 2 , 9 However, a disadvantage of EO is the formation of toxic oxidative by‐products, such as free chlorine (hypochlorous acid and hypochlorite) and bound chlorine (chloramines and chlorinated organic compounds), ammonium (due to hydrolysis of nitrogenous organic compounds), and other (potentially toxic) unknown compounds. We previously showed that free and bound chlorine release could be reduced to levels below the maximum acceptable levels (as defined by the Association for the Advancement of Medical Instrumentation [AAMI] standards for dialysate in HD) by using graphite electrodes combined with AC at a current of 3 A. 9 , 10 Ammonium release could be reduced by the placement of AC and a cation exchanger downstream of the EO unit to capture ammonium. 9 However, it is unknown whether other potentially toxic compounds are formed that are not removed by AC.
Preliminary biocompatibility studies suggest that oxidative status, cell viability, and cell migration are not impaired after exposure to uremic plasma that has been dialyzed against electrochemically regenerated dialysate in vitro 11 (Supporting Information: wound closure assay, Figure S1A). However, a decline in endothelial cell migration was observed after exposure to peritoneal effluent that was treated with EO (Figure S1B). Since glucose concentrations in peritoneal effluent are considerably higher (~10‐15 fold) compared with plasma, we hypothesized that the formation of glucose degradation products (GDPs) may be responsible for this. GDPs are highly reactive carbonyl compounds that are associated with oxidative stress, progression of atherosclerosis and cardiovascular disease, and mortality in patients with diabetes 12 , 13 , 14 , 15 and could theoretically be formed during the oxidation of carbohydrates in the dialysate.
The aim of this study was to measure three prototypical GDPs (glyoxal [GO], methylglyoxal [MGO], and 3‐deoxyglucosone [3‐DG]) in EO‐treated uremic plasma and EO‐treated peritoneal effluent.
2. METHODS
2.1. Materials
A prototype wearable dialysis device was designed, consisting of a blood and dialysate circuit as described. 2 The dialysate circuit contained an EO unit with 13 graphite electrodes with a cumulative surface area of 900 cm2, 150 g of poly(styrene‐divinylbenzene) (PS‐DVB) sulfonate beads, and 75 g of iron oxide hydroxide beads for respective removal of potassium and phosphate. The EO units with sorbents, active carbon, and a degassing unit were provided by Nanodialysis (Oirschot, The Netherlands). The ion exchangers were equilibrated at physiological calcium (1.20 mmol/L) and magnesium (0.45 mmol/L) concentrations and a low sodium (122 mmol/L) concentration, to prevent calcium and magnesium adsorption and sodium release by the cation exchanging PS‐DVB sulfonate, respectively, as previously demonstrated. 15 FX CorDiax 50 dialyzers (1.0 m2) were purchased from Fresenius Medical Care (Bad Homburg, Germany). Thomas peristaltic pumps were obtained from Gardner Denver Thomas (Sheboygan, WI). Human uremic plasma was obtained by therapeutic plasmapheresis of a patient with ESKD. Peritoneal effluent (Extraneal 7.5%, Baxter, McGaw Park, Illinois) was obtained from 3 different patients after an intraperitoneal dwell time of ~12 hours. Ethical approval for this study was waived by the medical research ethics committee Utrecht because the use of anonymized leftover bodily materials for scientific purposes does not comply with the Medical Research Involving Human Subjects Act (WMO) if patients’ consent is obtained.
2.2. Experimental setup: Electrooxidation of uremic plasma
A dialysis circuit was built consisting of a plasma and dialysate circuit separated by a high‐flux dialyzer (FX CorDiax 50), a reservoir with 2 L of human uremic plasma, and 2 pumps (Figure 1). The dialysate circuit contained an EO unit, 150 g of PS‐DVB sulfonate beads, and 75 g of iron oxide hydroxide beads. At the start of the experiment, the dialysate circuit contained 200 mL of dialysate ([Na+] 100 mmol/L, [Mg2+] 0.45 mmol/L, [Ca2+] 1.10 mmol/L, [] 28 mmol/L, and [Cl−] 75 mmol/L). A current of 3 A was applied per unit, corresponding with a current density of 3.3 mA/cm2, based on our previous observation that this resulted in efficient urea degradation and acceptable chlorine release. 2 The applied voltage was 3.2 V. AC (150 g) was placed downstream of the EO unit in series as this was shown to significantly reduce chlorine concentrations to levels below the maximum allowable level as defined in the AAMI standards for dialysate used in standard HD. 2 EO was turned on after the first hour and applied for 7 hours, in total 21 Ah for 2 L plasma. A degassing unit was placed downstream of the EO unit (before the AC) to allow gasses formed during EO to escape.
FIGURE 1.
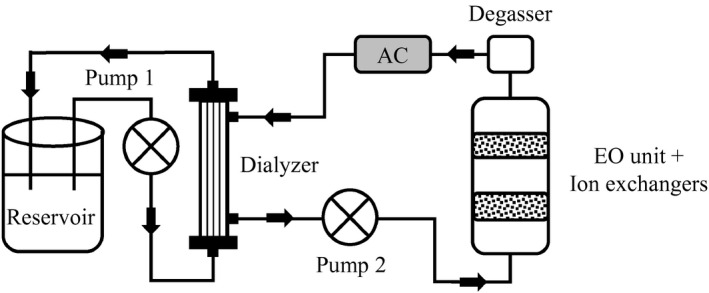
Experimental setup for uremic plasma experiments. Human uremic plasma was continuously recirculated for 8 hours from a reservoir through a high‐flux dialyzer, and dialysate was circulated in countercurrent direction through a circuit containing an electrooxidation (EO) unit (containing iron oxide hydroxide and poly(styrene‐divinylbenzene) sulfonate beads for phosphate and potassium removal) in series with a degasser downstream and activated carbon (AC) (downstream of the degasser). During EO experiments, the EO unit was turned on after 1 hour
2.3. Experimental setup: Electrooxidation of peritoneal effluent
Two dialysis setups, similar to the dialysis circuit used for uremic plasma, were built for the treatment of 5 L of peritoneal effluent with EO on (Figure 2A) and EO off (Figure 2B). To achieve clinically relevant urea, potassium and phosphate removal, both systems were upscaled. The new EO‐on system comprised 2 EO units with a cumulative surface area of 2093 cm2, 300‐400 g of PS‐DVB sulfonate beads, 100‐160 g of iron oxide hydroxide beads, and 240 and 720 g of AC up‐ and downstream of the EO units in series, respectively. A current of 3 A was applied per unit, corresponding with a current density of 2.9 mA/cm2 to treat the dialysate, again for 7 hours, in total 42 Ah for 5 L dialysate. The applied voltage was 3.2 V per unit (connected in parallel). The sorbent cartridge of the new EO‐off system comprised 300‐400 g of PS‐DVB sulfonate beads, 100‐160 g of iron oxide hydroxide beads, and 1730‐3000 g of AC to achieve comparable urea removal as with the EO‐on system. At the start of the experiment, the dialysate circuit of both setups contained 600 mL of dialysate ([Na+] 100 mmol/L, [Mg2+] 0.45 mmol/L, [Ca2+] 1.10 mmol/L, [] 28 mmol/L, [Cl−] 75 mmol/L, and glucose 278 mmol/L). This high glucose concentration (5%) was used to preload AC with glucose to establish glucose release into the dialysate to obtain a high osmolality of the peritoneal dialysate, required to remove excess water via osmosis from the patient during PD in vivo. Since EO was switched on after 1 hour, dialysate glucose concentrations during EO were lower (max ~100 mmol/L, see Figure 3D).
FIGURE 2.
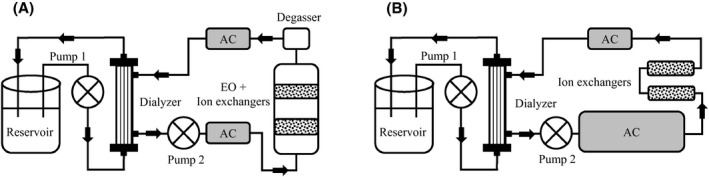
Experimental setup for peritoneal effluent experiments. Peritoneal effluent was continuously recirculated from a reservoir through a high‐flux dialyzer for 8 hours. In setup A (electro‐oxidation [EO] on), peritoneal effluent was recirculated in countercurrent direction through a circuit containing an EO unit (containing iron oxide hydroxide and poly(styrene‐divinylbenzene) sulfonate beads for phosphate and potassium removal) in series with a degasser and activated carbon (AC) (downstream of the degasser). In setup B (EO off), peritoneal effluent was recirculated in countercurrent direction through a circuit containing AC, iron oxide hydroxide beads, and poly(styrene‐divinylbenzene) sulfonate beads. In setup A, the EO unit was turned on after 1 hour
FIGURE 3.
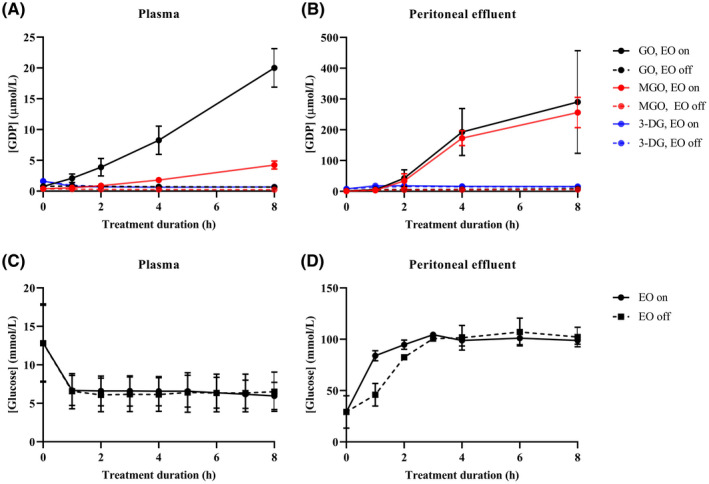
Concentrations of glucose degradation products (GDPs) (µmol/L; A, B) and glucose (mmol/L; C, D) in regenerated uremic plasma (A, C) and peritoneal effluent (B, D). GO, glyoxal; MGO, methylglyoxal; 3‐DG, 3‐deoxyglucosone. Solid line: EO on; dashed line: EO off. The mean ± SD of 3 experiments is presented. Of note, glucose concentration in uremic plasma (C) decreased during the first hour due to glucose adsorption by activated carbon, after which equilibration occurred at a glucose concentration of ~6 mmol/L, whereas in peritoneal effluent (D), glucose concentration increased due to glucose release by activated carbon which was preloaded with glucose at 278 mmol/L [Color figure can be viewed at wileyonlinelibrary.com]
Human uremic plasma or peritoneal effluent was recirculated for 8 hours at a flow rate of ~140 mL/min and ~125 mL/min, respectively, through a high‐flux dialyzer. Of note, these were the maximum flow rates that could be achieved with the Thomas pump which were used at the same settings for both experiments. The lower flow rate during the peritoneal effluent may be due to higher flow resistance in the dialyzer resulting from fibrin deposition or other small changes in the fluidic circuit, that is, the length or the diameter of the connecting tubing. A higher fluidic resistance requires a higher pressure drop to maintain the same flow rate. In this case, it is likely that the pump could not provide the necessary pressure, resulting in a different flow rate between the experiments. Dialysate was circulated in countercurrent direction at a flow rate of 70‐80 mL/min over the EO unit and sorbents with EO on (n = 3) or EO off (n = 3) for comparison. Urea concentration in uremic plasma and peritoneal effluent at the start of the experiment was 20 ± 2.0 mmol/L. The EO unit was turned on after 1 hour to allow for saturation of AC upstream of the EO unit with urea, and urea was spiked every 1‐2 hours into the reservoir to prevent depletion of urea at the electrode and therewith avoid side reactions. This approach resulted in relatively stable urea concentrations (Figure S2). Samples were taken from the plasma or peritoneal effluent reservoir before the start and hourly during treatment for the wound closure assay and for the measurement of urea, glucose, GO, MGO, and 3‐DG. Samples were stored at −80℃ until measurement of GDPs.
2.4. Measurement of glucose degradation products
Samples for analysis of GDPs were stored at −80℃ until analyses. Ultraperformance LC‐tandem MS (UPLC‐MS/MS) was used to measure concentrations of GO, MGO, and 3‐DG in plasma and peritoneal effluent as described. 16
2.5. Statistical analysis
One‐way ANOVA for repeated measures with post‐hoc Tukey test for multiple comparisons was used to analyze the difference in cell migration capacity and the concentration of GDPs in samples during treatment with EO on and EO off. A P value of <.05 was considered to be significant. Analyses were performed with GraphPad Prism 7.04 (GraphPad Software, La Jolla, California).
3. RESULTS
3.1. Formation of glucose degradation products
GO and MGO concentrations increased 26‐ and 11‐fold, respectively, in uremic plasma (at [glucose] 7 mmol/L) and 209‐ and 353‐fold, respectively, in peritoneal effluent (at [glucose] 100 mmol/L) during treatment with EO, whereas no change was observed in GO and MGO concentrations in plasma or peritoneal effluent that was circulated through a dialysate circuit without EO (Figure 3). No change in 3‐DG concentrations was observed in uremic plasma or peritoneal effluent during treatment with or without EO.
4. DISCUSSION
Our study shows that electrochemical dialysate regeneration with the current setup using graphite electrodes combined with AC is not safe due to the formation of GDPs which are not biocompatible. GO and MGO concentrations increased considerably in regenerated peritoneal effluent at supraphysiological glucose concentrations during dialysis against electrochemically regenerated dialysate and to a lesser extent in uremic plasma at physiological glucose concentrations.
Little is known about the safety of EO. Only 2 studies report on the short‐term safety of EO in vivo, while data on the composition of EO‐treated dialysate and biocompatibility in vitro are scarce. Schueneman et al found that daily intraperitoneal injection of human hemofiltration regenerated by AC combined with EO, in rats (n = 18) for 20 d, was well tolerated without histopathological signs of organ toxicity. 17 In addition, hemofiltration of 3 minipigs (n = 3 sessions in 4 d), using EO‐regenerated hemofiltration or Ringer's lactate as a substitution fluid, did not result in a change in vital parameters (blood pressure, heart rate, pO2, and pCO2) or blood hematology and biochemistry (white blood count, hemoglobin levels, alkaline phosphatase, aspartate aminotransferase, and total protein). 17 Although bicarbonate and pH tended to decline with EO‐regenerated hemofiltration, this could be attributed to not‐EO‐related bicarbonate removal during dialysate regeneration. Our group evaluated a miniature dialysis device, identical to the device used in the present study for plasma experiments, with continuous dialysate regeneration using EO in combination with AC and ion exchangers in vitro and healthy goats. 9 , 11 In vitro, treatment of dialysate or uremic plasma with this device did not affect total antioxidant capacity, intracellular ROS generation, cell viability, or cell proliferation compared to treatment with AC without EO. For the in vivo evaluation, 16 dialysis experiments of 3 hours were performed (~2/mo) in healthy goats. 9 Although vital parameters remained stable during dialysis and no changes in blood hematology or biochemistry occurred that were related to EO, ammonium was released and chlorine concentrations exceeded acceptable levels at higher dialysate flow rates. GDPs were not measured. Thus, no manifest toxicity was observed during these preliminary safety studies. However, the duration of exposure to EO‐treated fluids was short. Insufficient evidence exists on degradation products and (long term) safety of electrochemical dialysate regeneration.
GO (58 Da) and MGO (72 Da) are dicarbonyl compounds that can be formed during oxidative degradation of glucose. In patients with ESKD, GO, and MGO plasma concentrations are ~3‐fold higher compared with healthy controls 18 and have been associated with endothelial dysfunction, inflammation, and deterioration of CKD. 19 , 20 Thus, the observation that GO and MGO are formed during EO of uremic plasma and dialysate is a concern. In comparison, concentrations of GO and MGO in EO‐treated uremic plasma were ninefold and fourfold higher, respectively, than plasma concentrations in dialysis patients treated with HD. 18 Furthermore, to achieve clinically relevant urea removal, continuous treatment (24 h/d) with 2 EO units or intermittent treatment with 6 EO units for 8 h/d is required, 9 further increasing GDP formation. GO and MGO concentrations in EO‐treated peritoneal effluent were ~220‐660‐fold and ~27‐46‐fold higher, respectively, than concentrations in commercial PD fluids containing glucose 1.5%‐4.25%. 21 , 22 GDPs in glucose‐containing PD fluids are formed during heat sterilization and storage and are associated with functional and morphological changes of the peritoneal membrane in vitro and in vivo. 23 , 24 , 25 , 26 , 27 , 28 , 29 Theoretically, glucose oxidation is likely to occur because glucose is abundantly present in the dialysate, in particular in peritoneal dialysate, and glucose may be oxidized at a relatively low redox potential. In accordance with this, several studies observed substantial glucose degradation during electrochemical dialysate treatment 30 , 31 , 32 and suggest that glucose was oxidized to gluconic acid, a nontoxic monocarboxylic acid. 30 , 31 Electrocatalytic oxidation of glucose in pH neutral solutions on catalytic surfaces (eg, platinum, gold, or copper electrodes) yielded gluconic acid, gluconolactone, carbon monoxide, and carbon dioxide at potentials <1.4 V versus a Reversible Hydrogen reference Electrode (RHE) and glucaric acid, oxalic acid, formic acid, tartaric acid, and glycolic acid at higher potentials up to 1.8 V versus RHE. 33 , 34 Because our EO device is current‐driven, it is difficult to compare reaction products in our setup with potential‐driven EO experiments in the literature. However, it is likely that at the relatively high voltage of ~3.2 V, as applied in the present study, glucose and its reaction products are oxidized at the electrode interface to form GO and MGO. 35
As GDPs were not removed by AC downstream of the EO unit, other methods must be explored to prevent their release into the patient. For example, the addition of a dicarbonyl “scavenger” such as 2‐hydroxybenzylamine or an antioxidant, for example, ascorbic acid or N‐acetylcysteine, downstream of the EO unit may remove or neutralize dicarbonyl compounds. 30 , 36 , 37 , 38 Because GO and MGO formation was much lower during EO of uremic plasma compared with peritoneal effluent due to lower glucose concentration in plasma, it may initially be more feasible to reduce the formation or neutralize the effects of GO and MGO in an HD‐based WAK than in a PD‐based WAK.
The main limitation of this study is that we only measured 3 GDPs, while other glucose oxidation products or other unknown potentially toxic oxidative by‐products may be formed. Extensive biocompatibility testing of the EO technique will be performed after the GDP issues have been solved.
In conclusion, EO is currently not safe for dialysate regeneration in a WAK for either HD or PD due to glucose oxidation yielding toxic compounds. Future studies must focus on strategies to prevent GDP formation or, once formed, to neutralize their toxic effects.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: van Gelder, Vollenbroek, Lentferink, Besseling, Simonis, Hazenbrink, Joles, Gerritsen
Data curation: van Gelder, Vollenbroek, Lentferink, Simonis
Formal analysis: van Gelder
Funding acquisition: Simonis, Bajo Rubio, Selgas, Cappelli, Joles, Gerritsen
Investigation: van Gelder, Vollenbroek, Lentferink, Besseling, Simonis, Hazenbrink, Giovanella, Ligabue, Bianchini, Joles, Gerritsen
Methodology: van Gelder, Vollenbroek, Lentferink, Besseling, Simonis, Hazenbrink, Joles, Gerritsen
Project administration: Simonis, Bajo Rubio, Selgas, Cappelli, Joles, Gerritsen
Supervision: Simonis, Joles, Gerritsen
Visualization: van Gelder
Writing—original draft: van Gelder, Vollenbroek
Writing – review & editing: Besseling, Simonis, Giovanella, Ligabue, Bianchini, Hazenbrink, Bajo Rubio, Selgas, Cappelli, Joles, Verhaar, Gerritsen
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We gratefully thank Prof. CG Schalkwijk and Drs. MPH van de Waarenburg (Department of Internal Medicine, MUMC, Maastricht, the Netherlands) for the measurement of GDPs. This study was supported by the European Union (WEAKID, Horizon 2020 Research and Innovation Program, grant agreement no. 733169) and by the Dutch Kidney Foundation (grant no. NT12.05). All authors acknowledge the financial support of the Strategic Alliance of the University of Twente,University of Utrecht and University Medical Center Utrecht.
van Gelder MK, Vollenbroek JC, Lentferink BH, Hazenbrink DHM, Besseling PJ, Simonis F, et al. Safety of electrooxidation for urea removal in a wearable artificial kidney is compromised by formation of glucose degradation products. Artif. Organs. 2021;45:1422–1428. 10.1111/aor.14040
REFERENCES
- 1. Kooman JP, Joles JA, Gerritsen KG. Creating a wearable artificial kidney: where are we now? Expert Rev Med Devices. 2015;12:373–6. [DOI] [PubMed] [Google Scholar]
- 2. Wester M, Simonis F, Lachkar N, Wodzig WK, Meuwissen FJ, Kooman JP, et al. Removal of urea in a wearable dialysis device: a reappraisal of electro‐oxidation. Artif Organs. 2014;38:998–1006. [DOI] [PubMed] [Google Scholar]
- 3. Shinaberger JH, Shear L, Barry KG. Increasing efficiency of peritoneal dialysis: experience with peritoneal‐extracorporeal recirculation dialysis. Trans Am Soc Artif Intern Organs. 1965;11:76–82. [DOI] [PubMed] [Google Scholar]
- 4. Weiner ID, Mitch WE, Sands JM. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin J Am Soc Nephrol. 2015;10:1444–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Gelder MK, Jong JAW, Folkertsma L, Guo Y, Blüchel C, Verhaar MC, et al. Urea removal strategies for dialysate regeneration in a wearable artificial kidney. Biomaterials. 2020;234:119735. [DOI] [PubMed] [Google Scholar]
- 6. van Gelder MK, Mihaila SM, Jansen J, Wester M, Verhaar MC, Joles JA, et al. From portable dialysis to a bioengineered kidney. Expert Rev Med Devices. 2018;15:323–36. [DOI] [PubMed] [Google Scholar]
- 7. Koster K, Wendt H, Gallus J, Krisam G, Lehmann HD. Regeneration of hemofiltrate by anodic oxidation of urea. Artif Organs. 1983;7:163–8. [DOI] [PubMed] [Google Scholar]
- 8. Grinval'd VM, Leshchinskii GM, Rodin VV, Strelkov SI, Iakovleva AA. Unit of electrochemical oxidation of hemodialysis products–development and research. Med Tekh. 2003;2:3–7. [PubMed] [Google Scholar]
- 9. Wester M, van Gelder MK, Joles JA, Simonis F, Hazenbrink DHM, van Berkel TWM, et al. Removal of urea by electro‐oxidation in a miniature dialysis device: a study in awake goats. Am J Physiol Renal Physiol. 2018;315:F1385–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Association for the Advancement of Medical Instrumentation (AAMI) . Water for hemodialysis and related therapies. ANSI/AAMI; 13959: 2014. [Google Scholar]
- 11. Gremmels H, Hazenbrink DH, Simonis F, Otten ML, Wester M, Boer WH, et al. Dialysate regeneration by electro‐oxidation and activated carbon does not increase oxidative stress or endothelial cytotoxicity. [ABSTRACT] Nephrol Dial Transplant. 2014;29:213–4. [Google Scholar]
- 12. Maessen DE, Stehouwer CD, Schalkwijk CG. The role of methylglyoxal and the glyoxalase system in diabetes and other age‐related diseases. Clin Sci. 2015;128:839–61. [DOI] [PubMed] [Google Scholar]
- 13. Hanssen NM, Stehouwer CD, Schalkwijk CG. Methylglyoxal and glyoxalase I in atherosclerosis. Biochem Soc Trans. 2014;42:443–9. [DOI] [PubMed] [Google Scholar]
- 14. Schalkwijk CG, Stehouwer CDA. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age‐related diseases. Physiol Rev. 2020;100:407–61. [DOI] [PubMed] [Google Scholar]
- 15. Niwa T, Tsukushi S. 3‐deoxyglucosone and AGEs in uremic complications: inactivation of glutathione peroxidase by 3‐deoxyglucosone. Kidney Int Suppl. 2001;78:S37–41. [DOI] [PubMed] [Google Scholar]
- 16. Scheijen JL, Schalkwijk CG. Quantification of glyoxal, methylglyoxal and 3‐deoxyglucosone in blood and plasma by ultra performance liquid chromatography tandem mass spectrometry: evaluation of blood specimen. Clin Chem Lab Med. 2014;52:85–91. [DOI] [PubMed] [Google Scholar]
- 17. Schuenemann B, Quellhorst E, Kaiser H, Richter G, Mundt K, Weidlich E, et al. Regeneration of filtrate and dialysis fluid by electro‐oxidation and absorption. Trans Am Soc Artif Intern Organs. 1982;28:49–53. [PubMed] [Google Scholar]
- 18. Martens RJH, Broers NJH, Canaud B, Christiaans MHL, Cornelis T, Gauly A, et al. Advanced glycation endproducts and dicarbonyls in end‐stage renal disease: associations with uraemia and courses following renal replacement therapy. Clin Kidney J. 2020;13:855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martens RJH, Broers NJH, Canaud B, Christiaans MHL, Cornelis T, Gauly A, et al. Relations of advanced glycation endproducts and dicarbonyls with endothelial dysfunction and low‐grade inflammation in individuals with end‐stage renal disease in the transition to renal replacement therapy: a cross‐sectional observational study. PLoS ONE. 2019;14:e0221058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tezuka Y, Nakaya I, Nakayama K, Nakayama M, Yahata M, Soma J. Methylglyoxal as a prognostic factor in patients with chronic kidney disease. Nephrology. 2019;24:943–50. [DOI] [PubMed] [Google Scholar]
- 21. Mittelmaier S, Niwa T, Pischetsrieder M. Chemical and physiological relevance of glucose degradation products in peritoneal dialysis. J Ren Nutr. 2012;22:181–5. [DOI] [PubMed] [Google Scholar]
- 22. Zeier M, Schwenger V, Deppisch R, Haug U, Weigel K, Bahner U, et al. Glucose degradation products in PD fluids: do they disappear from the peritoneal cavity and enter the systemic circulation? Kidney Int. 2003;63:298–305. [DOI] [PubMed] [Google Scholar]
- 23. Wieslander AP, Nordin MK, Kjellstrand PT, Boberg UC. Toxicity of peritoneal dialysis fluids on cultured fibroblasts, L‐929. Kidney Int. 1991;40:77–9. [DOI] [PubMed] [Google Scholar]
- 24. Inagi R, Miyata T, Yamamoto T, Suzuki D, Urakami K‐I, Saito A, et al. Glucose degradation product methylglyoxal enhances the production of vascular endothelial growth factor in peritoneal cells: role in the functional and morphological alterations of peritoneal membranes in peritoneal dialysis. FEBS Lett. 1999;463:260–4. [DOI] [PubMed] [Google Scholar]
- 25. Schwenger V, Morath C, Salava A, Amann K, Seregin Y, Deppisch R, et al. Damage to the peritoneal membrane by glucose degradation products is mediated by the receptor for advanced glycation end‐products. J Am Soc Nephrol. 2006;17:199–207. [DOI] [PubMed] [Google Scholar]
- 26. Witowski J, Wisniewska J, Korybalska K, Bender TO, Breborowicz A, Gahl GM, et al. Prolonged exposure to glucose degradation products impairs viability and function of human peritoneal mesothelial cells. J Am Soc Nephrol. 2001;12:2434–41. [DOI] [PubMed] [Google Scholar]
- 27. Witowski J, Jörres A, Korybalska K, Ksiazek K, Wisniewska‐Elnur J, Bender TO, et al. Glucose degradation products in peritoneal dialysis fluids: do they harm? Kidney Int Suppl. 2003;63:S148–51. [DOI] [PubMed] [Google Scholar]
- 28. Park MS, Lee HA, Chu WS, Yang DH, Hwang SD. Peritoneal accumulation of AGE and peritoneal membrane permeability. Perit Dial Int. 2000;20:452–60. [PubMed] [Google Scholar]
- 29. Park SH, Lee EG, Kim IS, Kim YJ, Cho DK, Kim YL. Effect of glucose degradation products on the peritoneal membrane in a chronic inflammatory infusion model of peritoneal dialysis in the rat. Perit Dial Int. 2004;24:115–22. [PubMed] [Google Scholar]
- 30. Fels M. Recycle of dialysate from the artificial kidney by electrochemical degradation of waste metabolites: continuous reactor investigations. Med Biol Eng Comput. 1982;20:257–63. [DOI] [PubMed] [Google Scholar]
- 31. Yao SJ, Wolfson SK Jr, Joyce M, Tokarsky K, Ahn BK. De‐ureation by electrochemical oxidation. Bioelectrochem Bioenerg. 1974;1:180–6. [Google Scholar]
- 32. Yao SJ, Wolfson SK, Krupper MA, Wu KJ. Controlled‐potential controlled‐current electrolysis: in vitro and in vivo electrolysis of urea. Bioelectrochem Bioenerg. 1984;13:15–24. [Google Scholar]
- 33. De Mele MFL, Videla HA, Arvia AJ. The electrooxidation of glucose on platinum electrodes in buffered media. Bioelectrochem Bioenerg. 1982;10:469–87. [Google Scholar]
- 34. Blaesi AH, Cullen RB. Characterization of the electrochemical oxidation of glucose on Pt nanoparticle catalysts in pH neutral phosphate buffer solution. ECS Trans. 2011;33:41–8. [Google Scholar]
- 35. Allaman I, Belanger M, Magistretti PJ. Methylglyoxal, the dark side of glycolysis. Front Neurosci. 2015;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tao H, Huang J, Yancey PG, Yermalitsky V, Blakemore JL, Zhang Y, et al. Scavenging of reactive dicarbonyls with 2‐hydroxybenzylamine reduces atherosclerosis in hypercholesterolemic Ldlr(−/−) mice. Nat Commun. 2020;11:4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhitkovich A. N‐Acetylcysteine: antioxidant, aldehyde scavenger, and more. Chem Res Toxicol. 2019;32:1318–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guerra BA, Bolin AP, Otton R. Carbonyl stress and a combination of astaxanthin/vitamin C induce biochemical changes in human neutrophils. Toxicol in Vitro. 2012;26:1181–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


