Abstract
ABO‐incompatible (ABOi) transplantation requires preemptive antibody reduction; however, the relationship between antibody‐mediated rejection (AMR) and ABO‐antibodies, quantified by hemagglutination (HA), is inconsistent, possibly reflecting variable graft resistance to AMR or HA assay limitations. Using an ABH‐glycan microarray, we quantified ABO‐A antigen‐subtype (A‐subtype)‐specific IgM and IgG in 53 ABO‐O recipients of ABO‐A kidneys, before and after antibody removal (therapeutic plasma exchange [TPE] or ABO‐A‐trisaccharide immunoadsorption [IA]) and 1‐year posttransplant. IgM binding to all A‐subtypes correlated highly (R 2 ≥ .90) and A‐subtype antibody specificities was reduced equally by IA versus TPE. IgG binding to the A‐subtypes (II–IV) expressed in kidney correlated poorly (.27 ≤ R 2 ≤ .69). Reduction of IgG specific to A‐subtype‐II was equivalent for IA and TPE, whereas IgG specific to A‐subtypes‐III/IV was not as greatly reduced by IA (p < .005). One‐year posttransplant, IgG specific to A‐II remained the most reduced antibody. Immunostaining revealed only A‐II on vascular endothelium but A‐subtypes II‐III/IV on tubular epithelium. These results show that ABO‐A‐trisaccharide is sufficient for IgM binding to all A‐subtypes; this is true for IgG binding to A‐II, but not subtypes‐III/IV, which exhibits varying degrees of specificity. We identify A‐II as the major, but importantly not the sole, antigen relevant to treatment and immune modulation in adult ABO‐A‐incompatible kidney transplantation.
Keywords: ABO incompatibility, antibody biology, antigen biology, clinical research/practice, glycomics, histocompatibility, kidney transplantation/nephrology, translational research/science
Short abstract
Using a novel ABH‐glycan microarray, the authors demonstrate the fine specificities of ABO IgG and IgM antibodies in serum of patients before and after ABO‐incompatible kidney transplantation and their differential modulation by plasmapheresis versus immunoadsorption, and also demonstrate the glycan subtype targets in biopsies.
Abbreviations
- ABOc
ABO‐compatible
- ABOi
ABO‐incompatible
- AMR
antibody‐mediated rejection
- BSA
bovine serum albumin
- Gal
galactose
- GalNAc
N‐acetylgalactosamine
- HA
hemagglutination
- IA
immunoadsorption
- TPE
therapeutic plasma exchange
1. INTRODUCTION
Live donor kidney transplantation is not straightforward in all donor pairs due to a significant proportion of potential donor–recipient pairs being blood group‐incompatible (ABOi). While kidney donor exchange programs help, there is a patient survival benefit from ABOi kidney transplantation if no ABO‐compatible (ABOc) match is found. 1 The immunological barrier in ABOi transplantation can be overcome by undertaking pretransplant extracorporeal antibody removal therapy (EART), but there are risks associated with ABOi transplantation including early graft loss, postoperative bleeding, and infection. 2 , 3 , 4 , 5 , 6 These complications may relate to the presence of donor‐directed antigen‐specific antibody, the treatments required to remove antibody and inhibit its resynthesis or by using excessively heavy immunosuppression because of presumed risks of ABOi transplantation.
Several different forms of EART have been used with success, all guided by hemagglutination (HA)‐based antibody detection and quantification. The HA assay is not well‐standardized, exhibiting significant intracenter and intercenter, as well as intra‐observer and interobserver, variability, leading to incomplete risk assessment and inconsistent clinical management. 7 , 8 , 9 Furthermore, the precise relationship between HA titers and biological activity relevant to organ transplantation is ill‐defined. The return of ABO antibodies following ABOi transplantation can result in antibody‐mediated rejection (AMR) but often is not associated with obvious adverse effects. 10 The latter situation is termed “accommodation,” which can be broadly defined as absence of allograft injury despite the presence of alloantibody and alloantigen. The mechanisms underlying accommodation are incompletely understood and apparently multifaceted. 11
Blood groups A and B oligosaccharides are defined by a core Fuc‐α1‐2‐Gal disaccharide structure. Unmodified, this terminal disaccharide defines the H‐antigen found in blood group O individuals. Modification of the core disaccharide with a terminal N‐acetylgalactosamine (GalNAc) or galactose (Gal) in α1‐3 linkage generates the terminal trisaccharide antigens of blood groups A and B, respectively, which decorate glycoproteins or glycolipids of cells and are classified as ABH‐subtypes I‐VI 12 (Figure 1), of which subtypes I–IV are known to be expressed in humans. 13 , 14 , 15 We previously demonstrated that subtype II is the only ABO‐A or ABO‐B glycan present in heart (on vascular endothelium). 16 Breimer et al. demonstrated the distribution of A/B antigens on kidney biopsies, but the wider kidney distribution of subtype antigens has not been described. 17 Holgersson reported the presence of subtype structures in ABO‐A and ABO‐B kidneys but without specific locations. 18 , 19 Demonstration of the histological location of subtype structures in renal tissue is important given implications for antibody/antigen interaction.
FIGURE 1.
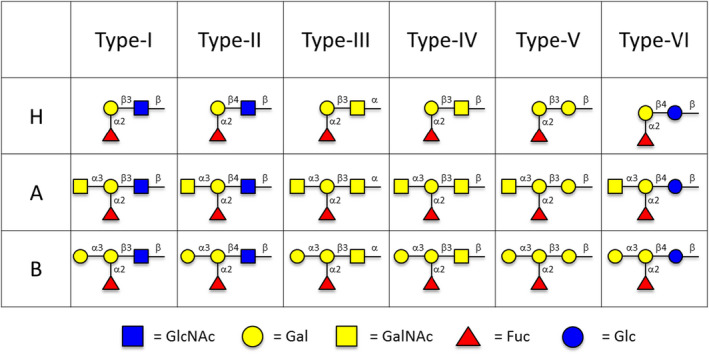
ABO subtype antigens: A‐subtype I–VI, B‐subtype I–VI, and H‐subtype I–VI (Symbol Nomenclature for Glycans 36 )
We additionally reported persistent deficiency of “natural” antibodies specific solely for donor A/B‐subtype II structures in patients receiving ABOi heart transplants as infants, whereas production of antibodies with other specificities developed normally. 16 These results are evidence of specific immune tolerance to the only nonself ABH glycotope in the heart graft but not to other donor subtype antigens, a distinction that is not evident in antibody detection with the HA assay. These initial studies were performed in pediatric heart transplant recipients, where immunological naivety is acknowledged, 20 whereas an adult kidney transplant population has a mature immune system; thus, the question of tolerance or accommodation is an important distinction in this study from our previous published work.
The aim of this study was to investigate in adult ABO‐O kidney transplant recipients the presence of A‐subtype antigen‐specific antibodies in plasma from patients receiving ABO‐A‐incompatible transplants. Antibody profiles were compared in ABOi kidney transplant recipients at baseline, after antibody removal (immediately prior to kidney transplantation), and late in the first year posttransplant in order to understand the balance of tolerance and accommodation in ABOi transplantation. In addition, we sought to demonstrate the location of renal expression of ABH subtype antigens in order to confirm donor specificity of antibodies to these structures.
2. METHODS
2.1. Cohorts
To examine the influence of EART and ABOi transplantation on the presence of antibodies against ABH subtype antigens, two groups were assessed: (1) recipients of ABO‐A‐incompatible kidney transplants studied pre‐EART / rituximab (timepoint 1), post‐EART / pre‐transplant (timepoint 2), and 12 months posttransplant (timepoint 3) and (2) seven recipients of ABO‐O compatible kidney transplants studied pretransplant and 12 months posttransplant as controls. For the ABOi cohort, there were 53 ABO‐O recipients and 12 ABO‐B recipients who received an ABO‐A donor kidney included in this study with samples available at these timepoints as outlined in Figure 2. In this observational multicenter study approved by South Birmingham Research and Ethics Committee, UK (08 H1207 293; clinical and demographic characteristics of the entire cohort previously reported 5 ), each center performed EART as indicated per local protocol. This was with therapeutic plasma exchange (TPE) or with antigen‐specific immunoadsorption (IA; GlycoSorb ABO™, Glycorex Transplantation AB) before transplantation, where the treatment number depended on reaching a local target titer of 1:8. All patients received tacrolimus and mycophenolate mofetil posttransplant with prednisone, having received rituximab and/or basiliximab for induction. No IVIg was used post‐EART. Plasma samples from these different groups were assessed for antibody binding to A type I‐VI, B type I‐VI, H type I‐VI, and Gal‐α1,3‐Gal 21 xenoantigen (αGal), a blood group‐like antigen not expressed in humans, using a glycan microarray as described below. Kidney biopsies obtained from grafts in eight ABOi recipients at 1–3 months posttransplant were stained for expression of ABH subtype structures.
FIGURE 2.
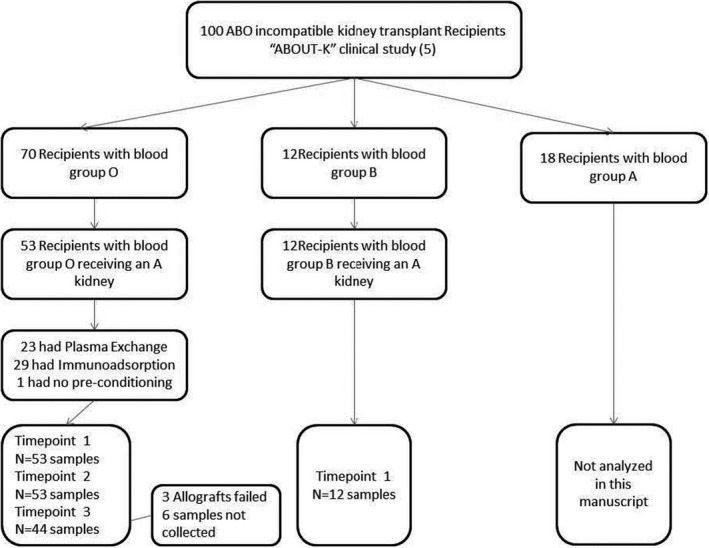
Flowsheet of ABO‐incompatible patient cohort included in current study with sample correlations. “ABOUT‐K” study population with inclusion criteria for this study, where recipient is ABO‐O and donor kidney is ABO‐A and available sera at each timepoint. ABO‐B recipient data are reported in supporting information figures
2.2. Detection of ABH subtype‐specific IgG and IgM antibodies by glycan microarray
Detailed chemical synthesis and characterization of blood group antigens A and B subtype I–VI and αGal, and the bovine serum albumin (BSA) conjugates used in the generation of the glycan array were previously described. 13 , 14 , 15 , 22 Microarray slides were printed at Engineering Arts LLC. Plasma samples were diluted (100 μl at 1:100) in blocking buffer and incubated for 30 min at 37°C. Bound antibodies were detected using fluorochrome‐conjugated goat antihuman IgM or IgG secondary antibodies (109‐505‐008 DyLight 549 AffiniPure Goat Anti‐Human IgG, Fc (γ) Fragment Specific; 109‐495‐129 Dylight 649 Affinipure Goat Anti‐human IgM, Fc (5 µ) Fragment Specific, Jackson Immunoresearch) at predetermined dilutions (0.3 μg/ml) in blocking buffer. These affinity‐chromatography purified secondary antibodies have been reported to bind specifically to the Fc fragment of the heavy chain of human IgG (all subclasses) and IgM. 23 Microarray slides were scanned using Nimblegen MS200 (Roche) at 5‐μm resolution and analyzed using ImaGene software (Biodiscovery). Normalized mean fluorescence intensities (MFI) were derived by subtracting local background fluorescence for individual spots and BSA‐only spots; averages of triplicates were reported. The MFI of binding of IgG and IgM varied linearly with plasma dilution over a wide range of MFI from >6000 to <25. Microarray printing protocols for dispensing BSA conjugates of ABH subtype I‐VI antigens and αGal were optimized based on spot morphology and antigen density using monoclonal antibodies specific for A and B subtype structures and αGal as previously described. The MFI of IgG and IgM detection was linearly related to plasma dilution, so that changes in subtype‐specific binding reflect equivalent changes in antibody concentration shown in previously published supplemental data. 16
2.3. Immunohistochemistry
In order to demonstrate the location of ABH‐subtype antigens, formalin‐fixed paraffin‐embedded sections were studied from eight kidney tissue blocks from biopsies of ABOi transplants (4 from ABO‐A1 and 4 from ABO‐B donors) that had been previously obtained per clinical indication. 5 Detection of ABH‐subtype antigens in cardiac/splenic tissue by immunohistochemistry has been described elsewhere. 22 Briefly, paraffin sections were processed for staining by deparaffinization (3 × 5 min in Toluene) and rehydration (3 × 100%, 1 × 95%, 1 × 70% ethanol, and distilled water). After quenching peroxidase activity, slides were blocked with 3% BSA/PBS then incubated with monoclonal antibodies at predetermined dilutions in blocking buffer. Monoclonal antibody specificities were Z2B‐1: A‐I, II, III/IV (Virogen Corp); 89F: B‐I, II, IV (Virogen Corp); JTL‐2: A‐III/IV, B‐III/IV, H‐III/IV 22 ; JTL‐4: A‐II and B‐II. 22 Secondary staining was performed with biotinylated antimouse IgM or IgG (Bethyl Laboratories) and streptavidin‐HRP (Jackson ImmunoResearch Labs, Inc.) in blocking buffer; color development was performed using ImmPACT DAB (Vector Laboratories). Slides were counterstained with Mayer's hematoxylin (Sigma–Aldrich), dehydrated and mounted with Entellen mounting media (Electron Microscopy Sciences). Bright‐field microscopy images were obtained using NikonEclipse E400 microscope fitted with Spot idea camera (SPOT Imaging Solutions) and processed using SPOT software.
2.4. ABO antibody measurement by hemagglutination assay in central laboratory
Plasma samples (EDTA) were taken at pre‐EART (timepoint 1), post‐EART immediately prior to kidney transplantation (timepoint 2), and 12 months posttransplant (timepoint 3) and stored (−40°C) at the central laboratory (NHSBT laboratory). Following final follow‐up at 1 year, the samples were thawed and assayed in batches at the central laboratory by a single operator using a technique based on that developed for use in NHS Blood and Transplant laboratories as previously reported. 5 In brief, reagent erythrocytes were obtained from NHSBT Supplies; A1rr (product code PR014); Brr (product code PR035) and OR1r (product code PR045), in a Cell Stab suspension (0.8 ± 0.2% concentration). For the total hemagglutination titer, referred to as an IgM titer, DiaMed gel cards (Id‐n°: 50520‐ NaCl, Enzyme Test and Cold Agglutinins BioRad) were used at 20°C. For reporting a titer as IgG, DiaMed gel cards, containing antihuman globulin (Id‐n°: 50531‐ LISS/Coombs, BioRad) were used at 37°C. The plasma used for the IgG titer was treated with 0.1‐M dithiothreitol (DTT, Sigma) for 30 min at 37°C prior to serial dilutions. Serial doubling dilutions of the plasma sample were performed, and after centrifugation, a weak positive reaction was taken as the endpoint; titer was recorded as the reciprocal of the dilution.
2.5. Statistical analysis
Statistical analysis was undertaken with Prism‐7 (GraphPad Software) or JMP® Version 14 (SAS Institute Inc.). Continuous variables are expressed as median and interquartile range or mean and standard deviation if normally distributed. Probability of values (p) less than .05 is described as significant. The significance of difference between MFI of antibody binding between cohorts and between timepoints was defined by the Mann–Whitney–Wilcoxon test.
3. RESULTS
3.1. Study population and treatment
Plasma samples were obtained from 53 ABO‐O and 12 ABO‐B live donor kidney transplant recipients who received a kidney from an ABO‐A donor. Clinical and demographic characteristics of the entire cohort were previously reported. 5 In this cohort, Table 1 describes the clinical characteristics of these patients including blood group, immunosuppression, induction and type of EART ([TPE] or A‐antigen‐specific immunoadsorption [IA] using GlycoSorb ABO™ columns). In this study, we report the results for plasma samples from ABO‐O (n = 53) patients analyzed for IgG and IgM antibodies specific for A‐subtype antigens obtained prior to antibody removal (timepoint 1), after antibody removal (timepoint 2), and 1 year after transplant (timepoint 3); analyses from ABO‐B recipients are additionally included in Figure S1.
TABLE 1.
Demographics of study cohort describing the donor–recipient clinical details, induction agents, antibody removal techniques, and early clinical outcomes
| Total | O | B | |
|---|---|---|---|
| Number | 65 | 53 | 12 |
| Recipient sex (female, n [%]) | 26 (40%) | 20 (37.8%) | 6 (50%) |
| Recipient age mean (SD) | 47.8 (13.1) | 48.0 (13.1) | 47.2 (13.7) |
| Recipient ethnicity (Caucasian) n (%) | 61 (93.8%) | 51 (96.3%) | 10 (83.3%) |
| Recipient CMV positive n (%) | 23 (37.8%) | 17 (33.3%) | 6 (60%) |
| Calculated reaction frequency = 0% | 62 (95.3%) | 50 (94.3%) | 12 (100%) |
| HLA ABDR mismatch median (Q1–Q3) | 3 (2–4) | 3 (2–4) | 4(3–5) |
| Pre‐emptive n (%) | 29 (44.6%) | 23 (43.4%) | 6 (50%) |
| Retransplants n (%) | 9 | 9 | 0 |
| Donor sex (female, n [%]) | 35 (53.8%) | 26 (49.1%) | 9 (75%) |
| Donor age mean (SD) | 48.7 (11.3) | 48.3 (11.5) | 50.7 (10.7) |
| Donor ethnicity (Caucasian) n (%) | 61 (93.8%) | 51 (96.3%) | 10 (83.3%) |
| Donor CMV positive n (%) | 31 (47.7%) | 24 (45.3%) | 7 (58.3%) |
| Relationship | |||
| Spouse n (%) | 26 (40%) | 18 (34.0%) | 8 (66.7%) |
| Extracorporeal antibody removal (EART) | |||
| Therapeutic plasma exchange n (%) | 23 (35.4%) | 23 (43.4%) | 1 (8.3%) |
| Immunoadsorption n (%) | 37 (56.9%) | 29 (54.7%) | 8 (66.7%) |
| No antibody removal n (%) | 4 (6.2%) | 1 (1.9%) | 3 (25%) |
| Rituximab n (%) | 57 (87.7%) | 47 (88.7%) | 10 (83.3%) |
| No lymphocyte depletion n (%) | 5 (7.7%) | 3 (5.7%) | 2 (16.7%) |
| Alemtuzumab n (%) | 3 (4.6%) | 3 (5.7%) | 0 (0%) |
| Basiliximab n (%) | 33 (50.8%) | 24 (45.3%) | 9 (75%) |
| Combined | |||
| Rituximab and basiliximab n (%) | 25 (38.5%) | 18 (34.0%) | 7 (58.3%) |
| Alemtuzumab and basiliximab n (%) | 3 (4.6%) | 3 (5.7%) | 0 (0%) |
| eGFR (MDRD, ml/min/BSA) | |||
| 3‐month mean (SD) | 49.6 (17.9) | 50.5 (18.6) | 46.0 (15.1) |
| 12‐month mean (SD) | 53 (18.3) | 55.4 (18.8) | 44.5 (13.7) |
| Graft failure n (%) | 5 (7.7%) | 5 (9.4%) | 0 (0%) |
| Biopsy‐proven rejection first month | 12 (18.5%) | 8 (15.1%) | 4 (33.3%) |
| Antibody‐mediated rejection n (%) | 3 (4.6%) | 2 (3.8%) | 1 (8.3%) |
| Cellular n (%) | 9 (13.8%) | 6 (11.3%) | 3 (25%) |
| Banff 1A n (% of rejections a ) | 5 (55.6%) | 4 (66.7%) | 1 (33.3%) |
| Banff 1B n (% of rejections a ) | 3 (33.3%) | 2 (33.3%) | 1 (33.3%) |
| Banff 2A n (% of rejections a ) | 1 (11.1%) | 0 | 1 (33.3%) |
Calculated Reaction Frequency is the UK equivalent of CPRA—degree of sensitization; eGFR (estimated Glomerular Filtration Rate using MDRD equation).
Percentage of cellular rejection.
3.2. A‐subtype‐specific IgG and IgM antibodies at timepoint 1 and comparison with anti‐A HA titers
Before EART, in sera from ABO‐O individuals the MFI of IgG binding to all A‐subtype antigens was significantly higher than in ABO‐B individuals (Figure S1); in contrast, IgM binding was not different between ABO‐O and ABO‐B, with IgM MFIs lower than IgG MFIs across all A‐subtype specificities. Given this significant difference in IgG binding between ABO‐O and ABO‐B, further comparisons were made using only ABO‐O recipients' sera (n = 53). In our standardized central laboratory HA assay, 5 IgG and IgM titers in ABO‐O patients were higher than ABO‐B patients (median anti‐A titer in O vs. B: IgG 1:32 vs. 1:4 [p < .001]; IgM 1:32 vs. 1:16 [p = .06]).
The correlation of HA with the microarray is shown in Figure 3 comparing both IgG and IgM titers to each subtype antigen for ABO‐O recipients (Figure 3A,B). These data demonstrate a correlation R 2 for IgG of .572, .309, and .357 for subtypes II, III, and IV, respectively. Correlation was poor with IgM titers in ABO‐O recipients, demonstrating the polyvalent nature of IgM and the HA method. The data for microarray and IgG titers in ABO‐B recipients are shown in Figure S2A,B.
FIGURE 3.
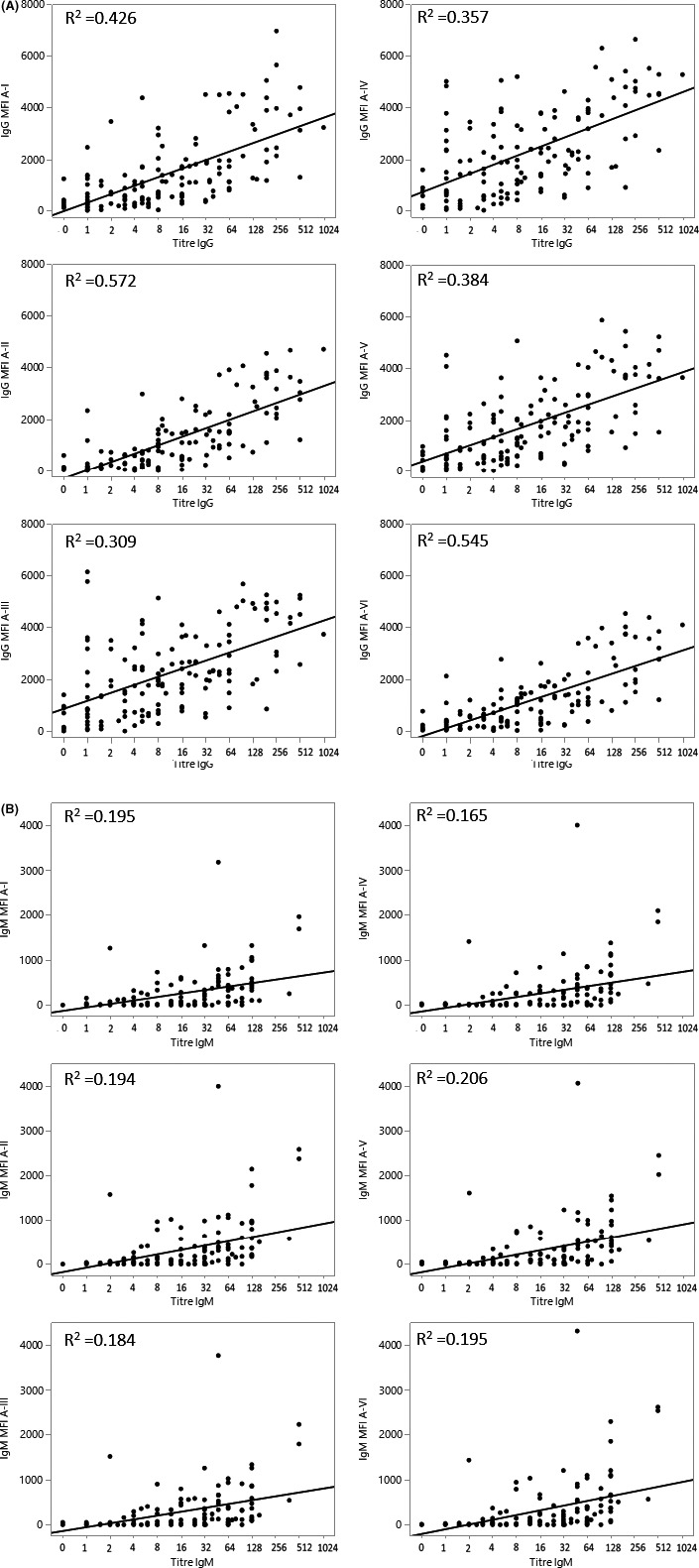
Anti‐A IgG and IgM hemagglutination titers at any timepoint in ABO‐O recipients of ABO‐A kidneys against microarray for each A‐subtype I, II, III, IV, V, and VI. (A) IgG anti‐A titers compared to IgG binding to A‐antigen subtypes. The correlation of hemagglutination IgG titers against A red blood cells in Blood Group O recipients compared to IgG binding to each subtype I, II, III, IV, V, and VI with R 2 .426, .572, .309, .357, .384, and .545, respectively, for each subtype. (B) IgM anti‐A titers compared to IgM binding to A‐antigen subtypes. The correlation of hemagglutination IgM titers against A red blood cells in Blood Group O recipients compared to IgM binding to each subtype I, II, III, IV, V and VI with R 2 .195, .194, .184, .165, .206, and.195, respectively, for each subtype
3.3. Anti‐A‐II IgM strongly correlates with IgM to all other A‐subtypes, whereas anti‐A‐II IgG strongly correlates only with anti‐A‐VI
At timepoint 1, the MFI of IgG binding to A‐subtype II, shown to be the sole A‐subtype expressed on vascular endothelium, correlated strongly with IgG specific to A‐VI (R 2 = .94, Figure 4A). In contrast, IgG specific to A‐II correlated poorly with A‐III, IV, and V (particularly III and IV) (.27 < R 2 < .53). A‐III‐specific IgG correlated strongly with A‐V (R 2 = .89); A‐III and A‐IV correlated moderately (R 2 = .69) and A‐IV and A‐V moderately (R 2 = .65). The MFI of IgM binding to A‐II strongly correlated with binding to all other A‐subtypes (R 2 > .90) (Figure 4B). Taken together, these data highlight the different binding properties of IgG anti‐A subtype‐specific antibodies.
FIGURE 4.
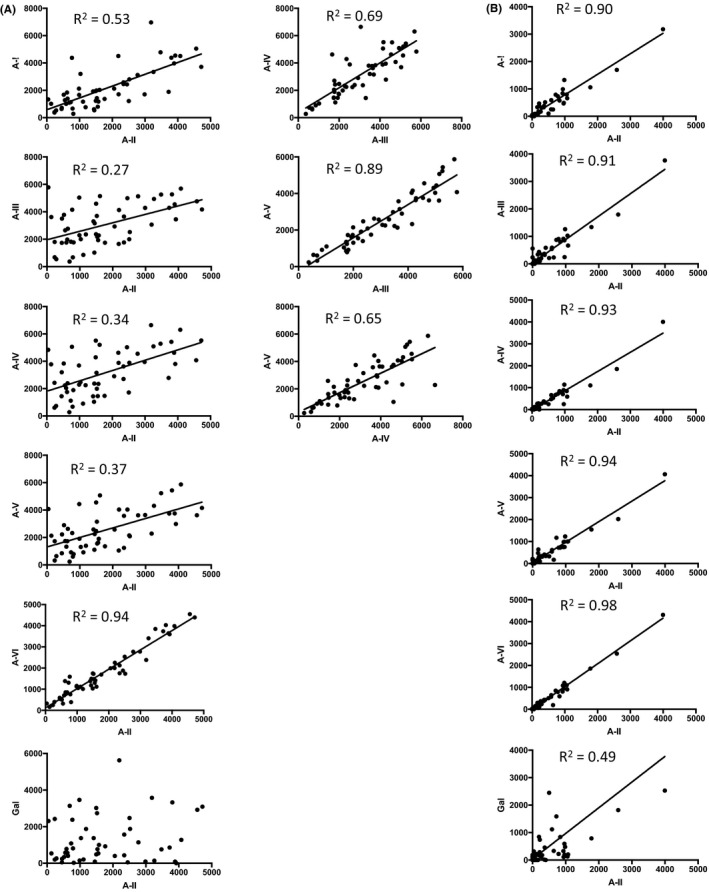
Correlation of antibody binding to different A antigen subtypes in pre‐transplant sera (timepoint 1). (A) Correlation of IgG binding. Prior to EART there was a significant correlation of IgG to all A‐subtypes (p < .001) but not to αGal. The relationships between binding to A‐II and other A‐subtypes are shown to the left, and between A‐subtypes III, IV, and V to the right. IgG binding to A‐I did not correlate any more closely with binding to other A‐subtypes than with binding to A‐II. (B) Correlation of IgM binding. Prior to EART there was a highly significant correlation of IgM to all A‐subtypes and to αGal (p < .001). The relationship between binding to A‐II and to other A‐subtypes is shown
3.4. EART with A‐antigen‐specific IA columns is associated with subtype‐dependent differences in antibody reduction
EART was undertaken to reduce anti‐A antibodies to a pre‐specified titer in the HA assays used in each center recruiting to the study. In the retrospectively analyzed central laboratory assay, there was no difference in anti‐A HA titer between the groups treated with TPE and with IA, at either timepoint 1 or following EART (timepoint 2). 5 With the microarray, binding of IgG and IgM to all A‐subtypes and to αGal was not significantly different at timepoint 1 between the groups subsequently treated with TPE versus IA (Figure 5A,B and Table S2). In sera from timepoint 2, microarray analysis showed that TPE had significantly reduced IgG binding to all A‐subtypes I‐VI and to αGal (p < 10−3). IA had also reduced IgG to all A‐subtypes (p < 10−3) but not to αGal (Figure 5A). Although the reduction in IgG to A‐I, II, and VI was similar between the IA and TPE techniques, removal of IgG specific to A‐III, IV, and V was significantly less effective by IA than TPE (p < .001). With regard to IgM, TPE significantly reduced IgM specific to all A‐subtypes (p < .001) and αGal (p < .001); IA significantly reduced IgM binding to all A‐subtypes (p < .001) but not to αGal (Figure 5B), and IA was equally effective to TPE.
FIGURE 5.
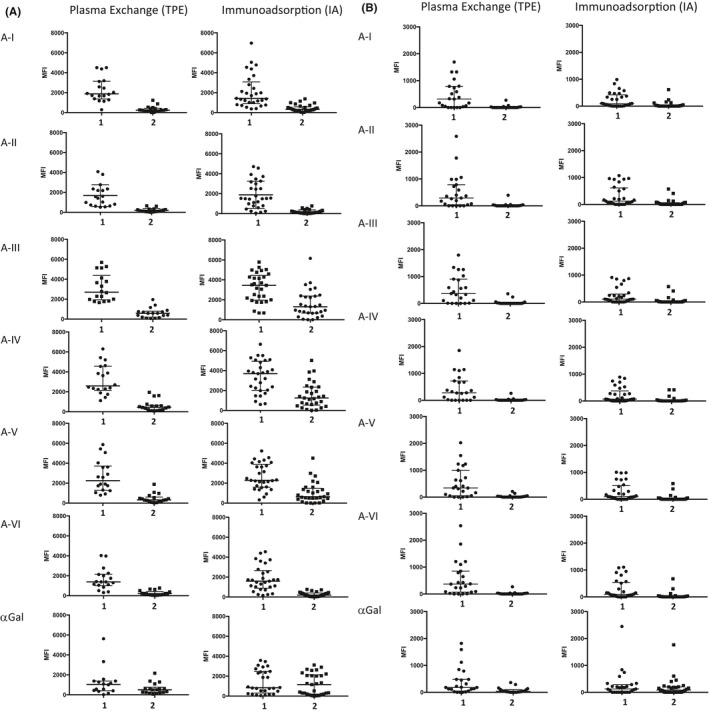
A‐subtype‐specific antibodies in ABO‐O patients undergoing plasma exchange or immunoadsorption measured at timepoints 1 and 2. (A) IgG antibodies. At timepoint 1, IgG binding to any A‐subtype was not significantly different between the cohorts that subsequently underwent EART by plasma exchange and by antigen‐specific immunoadsorption. At timepoint 2 there was a significant reduction in IgG binding to all A‐subtypes (p < .001). IgG binding to αGal was reduced only by plasma exchange. There was no significant difference between plasma exchange and antigen‐specific immunoadsorption with respect to IgG binding to A‐I, II and VI at timepoint 2 (p > .3), whereas IgG binding to A‐III, IV, and V was significantly higher in the group treated with immunoadsorption (p ≤ .006). Data points on individual patients, and lines showing median and interquartile range in this and all subsequent dotplots. (B) IgM antibodies. At timepoint 1, IgM binding to A‐subtypes was not significantly different in the plasma exchange group except for A‐III which reached statistical significance (p = .04). There was no significant difference between plasma exchange and antigen‐specific immunoadsorption with respect to IgM binding to any A‐subtypes at timepoint 2 (p ≥ .4)
The difference in subtype‐specific IgG reduction achieved by IA compared to TPE was not associated with any difference in outcome with respect to AMR, graft survival, or eGFR at 1 year. With respect to subtype‐specific IgM at timepoint 2, there was one high outlier in the TPE group and two in the IA group, one of whom developed AMR (treated with IA).
3.5. A‐subtype‐specific antibodies, most notably anti‐A‐II IgG, remain low 1‐year posttransplant
We performed a paired analysis of sera from timepoint 1 (pre‐treatment) and timepoint 3 (late posttransplant) to assess the re‐accumulation of A‐subtype‐specific antibodies. There were 44 available sera at this late timepoint.
IgG with all A‐subtype‐specificities remained significantly lower at timepoint 3 than at timepoint 1 (Figure 6; p < .001 for all subtypes), although there was partial re‐accumulation compared with timepoint 2 (Figure 5). The greatest relative persistent reduction was anti‐A‐II IgG, for which the median MFI was 19% of timepoint 1 (Figure 6 and Table S2). There were six patients in whom anti‐A‐II IgG at timepoint 3 were outliers, however, showing re‐accumulation of anti‐A‐II equivalent to the median MFI at timepoint 1. All six retained good allograft function (mean eGFR = 60.7 ml/min, not significantly different to the overall cohort), with no history of AMR. Similarly, IgM with all A‐subtype specificities remained significantly lower at timepoint 3 than at timepoint 1 (Figure 6A; p < .001 for all subtypes), with significant return of antibody compared with timepoint 2.
FIGURE 6.
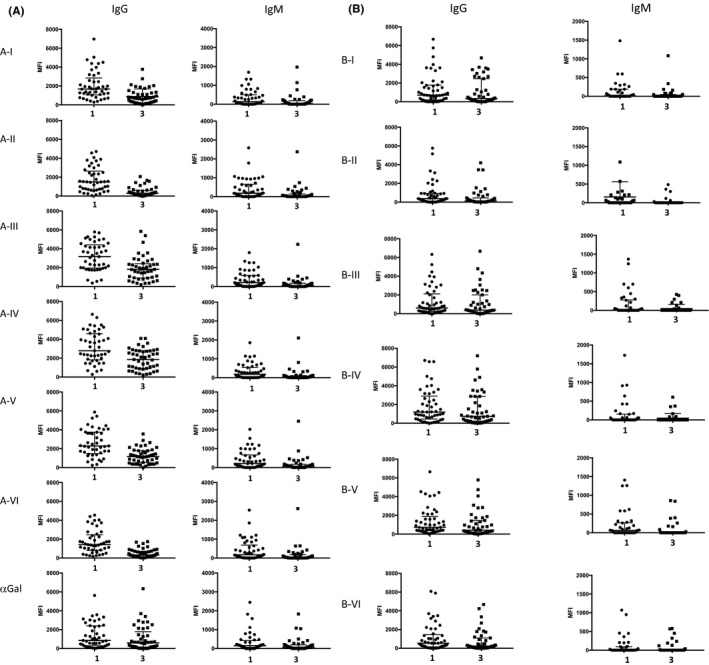
Serum antibodies binding of A‐into‐O recipients at timepoints 1 and 3. (A) Binding to A‐subtype antigens at timepoints 1 and 3. MFI of antibody binding to A‐subtypes at timepoints 1 and 3 (data shown for paired samples only) was significantly lower for both IgM (p < .005) and IgG (p < .0005) to all A‐subtypes at timepoint 3. There was no significant difference in IgG or IgM binding to αGal across the cohort at these timepoints. (B) Serum antibodies binding to B‐subtype antigens at timepoints 1 and 3. IgG binding to B‐I and III‐VI was not significantly different at timepoint 3 compared to timepoint 1, but it was lower for B‐II binding (p = .04). IgM binding to each B‐subtype was not significantly different at timepoint 3 compared to timepoint 1
No significant difference was detected in IgG or IgM binding to αGal between timepoints 1 and 3, suggesting an unaffected systemic immunoglobulin status. Furthermore, no significant reduction was seen in IgG binding to B‐subtypes (third‐party) at timepoint 3 (Figure 6B), except a reduction in binding to subtype II (p = .04). B‐subtype‐specific IgM was low at timepoint 1 and unchanged at timepoint 3. In contrast, for seven ABO‐O recipients of ABOc transplants, there was no significant reduction in A (or B) subtype‐specific IgG and IgM between timepoints 1 and 3 (Figure S3), suggesting that immunosuppression was not influencing the reduction in anti‐A antibodies observed in the 1‐year posttransplant follow‐up.
3.6. ABH‐subtype antigens are expressed differentially in A and B kidneys
In order to demonstrate the clinical relevance of measuring subtype‐specific antibodies, we stained kidney biopsy tissues with ABH‐subtype‐specific monoclonal antibodies for expression of subtype structures. This was done in tissue from ABO‐A1 and ABO‐B donor kidneys in ABO‐O recipients that had been obtained in the first 3 months posttransplant for clinically indicated biopsies. Figure 7 is representative of ABO‐A1 (n = 4) and ABO‐B (n = 4) kidney grafts and demonstrates the presence and location of specific A‐ and B‐subtype structures. Vascular endothelial cells (glomeruli, peritubular capillaries) express only subtype II A or B antigens. Tubular epithelial cells of ABO‐A kidneys express A‐subtypes II, III/IV, whereas in ABO‐B kidneys tubular epithelial cells express only B‐subtype II antigens.
FIGURE 7.
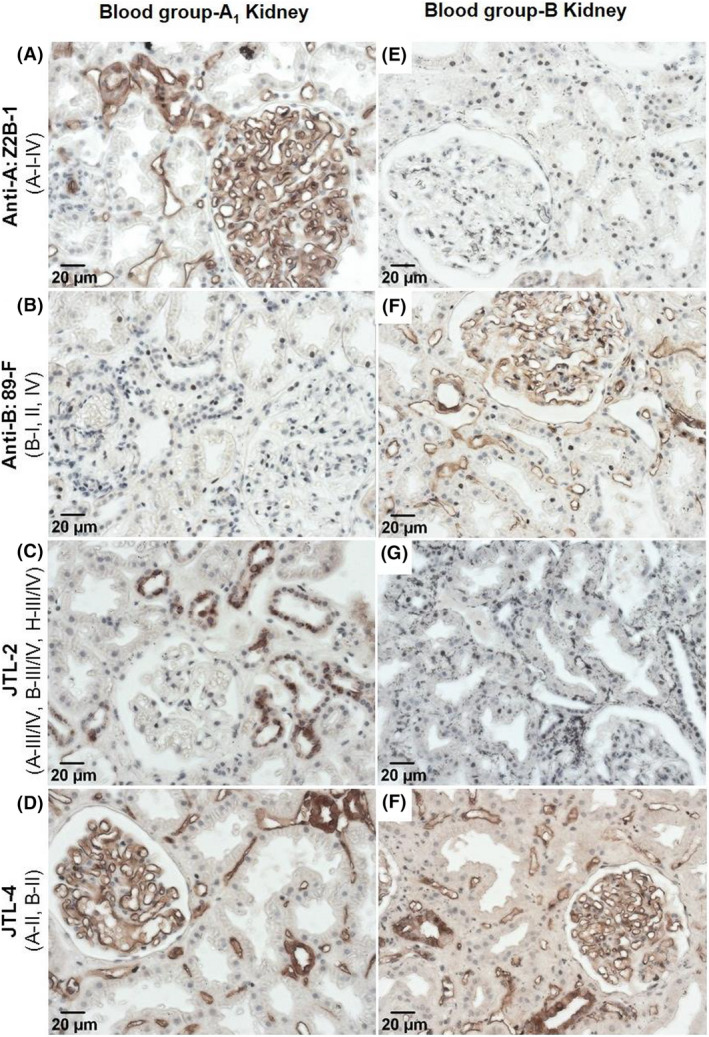
A‐subtype and B‐subtype expression in renal vascular and tubular compartments. Biopsies obtained from blood group A1 (panels A–D) and blood group B (panels E–H) kidney grafts in ABO‐O recipients at 1–3 months posttransplant were stained for expression of A‐ and B‐subtype structures. Vascular endothelial cells (glomeruli and peritubular capillaries) express only subtype II A or B antigens (panels D and H, respectively). Tubular epithelial cells of ABO‐A kidneys express A‐subtypes II, III/IV (positive staining in panels C and D), whereas in ABO‐B kidneys tubular epithelial cells express only B‐subtype II antigens (negative staining in panel G, positive staining in panel H). Representative images are of immunohistochemistry staining of ABO‐A1 (n = 4) and ABO‐B (n = 4) kidney grafts. Monoclonal antibody specificities 22 : Z2B‐1: A‐I, II, III/IV. 89F: B‐I, II, IV. JTL‐2: A‐III/IV, B‐III/IV, H‐III/IV. JTL‐4: A‐II and B‐II
4. DISCUSSION
This study demonstrates specific antibody binding differences to ABO‐A subtype antigens during ABOi kidney transplantation, raising the potential that a blood group subtype assay may give greater insight into clinical management of ABOi transplantation. The current standard HA assay for detecting and quantifying ABO antibodies is semiquantitative, with well‐recognized intracenter and intercenter variation in performance. 5 , 7 , 8 , 9 HA does not allow identification of antibody subtype‐specificity. This factor is particularly relevant in ABOi organ transplantation where variable tissue expression of antigen subtypes makes defining antibody donor specificity of key importance. Furthermore, red cells express not only ABH antigens but also non‐ABH blood group and other cell surface antigens and adsorbed circulating plasma antigens. Thus, antibodies detected by the HA test can be misleading for many reasons, which may impact decision‐making regarding success of antibody removal strategies and eligibility for ABOi transplantation. After ABOi transplantation, HA assay variability contributes to discrepant treatment exposure, 5 hinders comparisons between centers and limits research on clinical outcomes and the biology of ABO‐incompatibility in transplantation. 5 , 7 , 24 Advances in HLA‐histocompatibility laboratories in recent years have improved the characterization of anti‐HLA alloantibodies, where single‐antigen bead Luminex techniques are typically used together with the cell‐based cross‐match to guide clinical decision‐making. While there is still progress to be made, this has widely improved clinical transplantation management, importantly reducing early loss associated with antidonor antibodies, which were less well‐defined in earlier eras. 25 , 26 A similar approach to enhance antibody assays for ABO‐histocompatibility would be an important addition to assessing risk and predictability of ABOi transplantation.
In our study using an ABH antigen subtype microarray, we have demonstrated a change in A‐II‐specific antibodies after transplantation, both in response to IA with preferential removal and with persistently reduced re‐accumulation late after transplant. This is in contrast to anti‐A‐III/IV re‐accumulation in follow‐up. The importance of anti‐A‐II removal preferentially by IA suggests a biological significance of directed antibody removal compared to TPE, which removes all A‐subtype antibodies. This is due to A‐II antigen expression on endothelial cells, and thus, clinical practice may also benefit from A‐subtype‐specific antibody measurement to assess IA efficacy in antibody removal; this is not possible to measure using HA for ABO antibodies. Previous work demonstrated ineffective removal of blood group antibodies by IA but lacked the ability to differentiate subtype‐specific antibodies by HA; the authors hypothesized that it may be important to measure subtype‐specific antibodies in order to explain these findings. 27 Lindberg et al demonstrated this to be significant in vitro by reporting the variability of blood group‐specific antibodies in which A‐subtype antigen‐specific binding was measured following pre‐adsorption on Sepharose‐linked A‐trisaccharide and A‐subtype I‐IV tetrasaccharides. They demonstrated that IgM to A‐subtypes I‐IV was efficiently removed by trisaccharide alone, but in contrast, IgG to A‐I, III, and IV was incompletely removed by trisaccharide, similar to findings in our study. IgG to A‐II was effectively removed by trisaccharide alone with no further reduction following adsorption with A‐I‐IV tetrasaccharides. 28 These findings imply that IgG binding does not differentiate between trisaccharide and subtype‐II tetrasaccharide, whereas antibodies to A‐subtypes I‐III‐IV include some clones specific for the tetrasaccharide. Previous work by Breimer et al in 1987 reported different subtype‐specific IgG responses at the time of rejection; however, measuring circulating subtype‐specific antibodies is difficult and has not been widely adopted. 29
The strong correlation of IgM binding to different A‐subtype antigens seen in our data is similarly consistent with trisaccharide demonstrating the polyvalency of IgM binding. In contrast, the different correlation of IgG binding to A‐subtypes is consistent with binding to the different subtype saccharides, a finding in keeping with class‐switch recombination. Furthermore, the closer correlation of IgG binding to A‐II and A‐VI is attributed to these structures having equivalent glycotopes, in contrast to IgG specific to A‐I, III, IV, and V, which correlated poorly with IgG to A‐II. This implies the presence of additional glycotopes bound by IgG in the presence of core chain subtype I, III, IV, and V saccharides.
The analysis of polyclonal antibody binding cannot exclude the possibility that proximal carbohydrate residues also inhibit antibody binding to trisaccharide. Indeed, in solid phase assays, the absence of a core chain saccharide has been reported to permit antibody binding to autologous blood group trisaccharide, which has been attributed to the revelation of otherwise cryptic glycotopes. 30 This necessarily complicates direct comparison of trisaccharide and tetrasaccharide binding and the interpretation of trisaccharide‐based solid phase assays. A recent analysis of murine monoclonal antibody specificities suggests that this inhibition could differ between subtypes. 31 For example, A‐III and IV fail to bind a monoclonal antibody (87‐G) that binds to A‐trisaccharide and A‐tetrasaccharide‐subtypes II and VI. In human polyclonal antibody preparations, our data, and those of Lindberg and colleagues suggest that inhibition is a less common determinant of subtype‐associated variability in IgG binding than the generation of additional tetrasaccharide type‐specific epitopes. The latter is exemplified by the binding of other monoclonal antibodies such as one that solely binds to A‐III and IV (HE‐10). The impact of “nonbiologic” conformation of saccharides on a solid phase platform may represent a potential limitation of the microarray assay when compared to a cell‐based assay such as erythrocyte agglutination. However, despite the possible greater biologic relevance of a cell‐based assay, the many individual differences between cells, cell preparations, and assay conditions add to the previously described lack of standardization of the HA assay; in contrast, the microarray is rigorously standardized. The potential limitations of each assay could be circumvented by exploration of their combined use, as noted above in the case of HLA antibodies where solid phase assays are typically used together with the lymphocyte‐based cross‐match.
Our findings are relevant to the clinical practice of ABOi organ transplantation. Given our observation that A‐II is the dominant antigen on vascular endothelium of ABO‐A kidneys and is the antibody specificity most efficiently removed by trisaccharide‐based IA, strategies specifically identifying this component of the antibody profile could improve targeting of therapy. For example, in some patients, “antigen‐specific IA” inadequately removes antigen‐specific IgG as measured by HA. 32 In our study, removal of A‐II‐specific IgG by the same columns is reliable, but removal of A‐III‐ and IV‐specific IgG is not as efficient. It is therefore possible that patients who apparently fail IA have high levels of IgG specific for A‐III and IV. These antigens are both expressed on the red cell surface, and such antibody specificities may therefore contribute to erythrocyte agglutination in the HA assay without affecting transplant outcome. The physiologic significance of subtype‐specific antibodies might, however, depend upon clinical context, for example, A‐III antigen expression is reportedly increased in vascular endothelium at sites of inflammation, 33 which may be relevant if there is delayed graft function or rejection.
The pattern of subtype‐specific antibodies in peripheral blood at 1‐year posttransplant is relevant to understanding tolerance and accommodation in ABOi transplantation in adult recipients. In the ABOi and not the ABOc cohort, despite similar immunosuppression, a similar “tolerant” phenotype as seen in infant ABOi heart transplant recipients is demonstrated; this is supported by the finding of no reduction of anti‐B antibodies in ABO‐O recipients of A‐incompatible kidneys. The reduction in IgG binding to A‐antigens was not consistent across subtypes, being greater for A‐II than for A‐III and IV. Even though IgG binding to A‐II remains significantly low at 1 year, in some individuals, it does not fall to undetectable or to the very low levels observed in ABO‐A individuals or reported (by HA titers) in pediatric recipients of A‐incompatible cardiac transplants. 16 , 34 , 35 This suggests that in adult kidney transplant recipients, the mechanisms underlying long‐term engraftment are multifaceted and seemingly involve both accommodation as well as modulation of IgM and IgG antibody production (i.e., tolerance phenotype) to antigens expressed on vascular endothelium. 16 These findings are then consistent with the literature on both the role of accommodation and antigen‐specific immune tolerance and provide more precise information on the glycotope specificities that underlie these mechanisms.
The practical implication of these data are to help to (1) understand the nature of blood group antibody specificities, (2) understand how antibody removal techniques affect different subtype specificities which is important in pre‐conditioning for transplant, and (3) suggest a role for immune tolerance with the re‐emergence of some subtype‐specific antibodies but persistently reduced production of others, in this instance anti‐A‐II. Further investigation is needed, with prospective studies including clinical management and clinical outcomes to differentiate the impact of these subtype antibodies.
In conclusion, we have demonstrated that anti‐A‐II antibodies, but not anti‐A‐III/IV, are significantly reduced following blood group‐specific IA and that circulating anti‐A‐II antibodies do not return to similar levels as the other A‐subtype‐specific (and anti‐B) antibodies after ABO‐A‐incompatible kidney transplantation. We detected A‐II antigens expressed on renal vascular endothelium and tubular epithelium but longitudinal histological studies are needed to determine the ongoing expression post‐transplant. Detailed characterization of isotypes and subtype‐specificities of ABO antibodies using “solid phase” assays such as the ABH glycan microarray used here, information that is not provided by HA, may direct antibody removal strategies and determination of transplant eligibility and improve precision of assessment of relevant antidonor ABO antibodies, thereby diminishing the clinical unpredictability in ABOi transplantation.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This work was supported through the Canadian Institutes of Health Research / Canadian Donation and Transplantation Research Program, the Kidney Foundation of Canada, and the Women and Children's Health Research Institute / Stollery Children's Hospital Foundation.
Bentall A, Jeyakanthan M, Braitch M, et al. Characterization of ABH‐subtype donor‐specific antibodies in ABO‐A‐incompatible kidney transplantation. Am J Transplant. 2021;21:3649–3662. 10.1111/ajt.16712
Andrew Bentall and Mylvaganam Jeyakanthan are co‐first authors.
Lori J. West and Simon Ball are co‐senior authors.
REFERENCES
- 1. Massie AB, Orandi BJ, Waldram MM, et al. Impact of ABO‐incompatible living donor kidney transplantation on patient survival. Am J Kidney Dis. 2020;76:616‐623. [DOI] [PubMed] [Google Scholar]
- 2. de Weerd AE , van Agteren M , Leebeek FW, Ijzermans JN, Weimar W, Betjes MG. ABO‐incompatible kidney transplant recipients have a higher bleeding risk after antigen‐specific immunoadsorption. Transpl Int. 2015;28:25‐33. [DOI] [PubMed] [Google Scholar]
- 3. Montgomery JR, Berger JC, Warren DS, James NT, Montgomery RA, Segev DL. Outcomes of ABO‐incompatible kidney transplantation in the United States. Transplant. 2012;93:603‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Becker LE, Siebert D, Susal C, et al. Outcomes following ABO‐Incompatible kidney transplantation performed after desensitization by nonantigen‐specific immunoadsorption. Transplant. 2015;99:2364‐2371. [DOI] [PubMed] [Google Scholar]
- 5. Bentall A, R. Barnett AN, Braitch M, et al. Clinical outcomes with ABO antibody titer variability in a multicenter study of ABO‐incompatible kidney transplantation in the United Kingdom. Transfusion. 2016;56:2668‐2679. [DOI] [PubMed] [Google Scholar]
- 6. Bentall A, Neil D, Sharif A, Ball S. ABO‐Incompatible kidney transplantation is a novel risk factor for BK nephropathy. Transplant. 2015;99:e8‐e9. [DOI] [PubMed] [Google Scholar]
- 7. Bentall A, Regan F, White J, et al. No progress in ABO titer measurement: time to aim for a reference? Transplant. 2014;97:e19‐e21. [DOI] [PubMed] [Google Scholar]
- 8. AuBuchon JP, de Wildt‐Eggen J , Dumont LJ. Reducing the variation in performance of antibody titrations. Arch Pathol Lab Med. 2008;132:1194‐1201. [DOI] [PubMed] [Google Scholar]
- 9. Kumlien G, Wilpert J, Safwenberg J, Tyden G. Comparing the tube and gel techniques for ABO antibody titration, as performed in three European centers. Transplant. 2007;84:S17‐S19. [DOI] [PubMed] [Google Scholar]
- 10. Bentall A, Herrera LP, Cornell LD, et al. Differences in chronic intragraft inflammation between positive crossmatch and ABO‐incompatible kidney transplantation. Transplant. 2014;98:1089‐1096. [DOI] [PubMed] [Google Scholar]
- 11. Lynch RJ, Platt JL. Accommodation in organ transplantation. Curr Opin Organ Transplant. 2008;13:165‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clausen H, Hakomori S. ABH and related histo‐blood group antigens; immunochemical differences in carrier isotypes and their distribution. Vox Sang. 1989;56:1‐20. [DOI] [PubMed] [Google Scholar]
- 13. Meloncelli PJ, Lowary TL. Synthesis of ABO histo‐blood group type I and II antigens. Carbohyd Res. 2010;345:2305‐2322. [DOI] [PubMed] [Google Scholar]
- 14. Meloncelli PJ, Lowary TL. Synthesis of ABO histo‐blood group type V and VI antigens. Aust J Chem. 2009;62:558‐574. [Google Scholar]
- 15. Meloncelli PJ, West LJ, Lowary TL. Synthesis and NMR studies on the ABO histo‐blood group antigens: synthesis of type III and IV structures and NMR characterization of type I‐VI antigens. Carbohyd Res. 2011;346:1406‐1426. [DOI] [PubMed] [Google Scholar]
- 16. Jeyakanthan M, Meloncelli PJ, Zou L, et al. ABH‐Glycan microarray characterizes ABO subtype antibodies: fine specificity of Immune tolerance after ABO‐incompatible transplantation. Am J Transplant. 2016;16:1548‐1558. [DOI] [PubMed] [Google Scholar]
- 17. Breimer ME, Molne J, Norden G, Rydberg L, Thiel G, Svalander CT. Blood group A and B antigen expression in human kidneys correlated to A1/A2/B, Lewis, and secretor status. Transplant. 2006;82:479‐485. [DOI] [PubMed] [Google Scholar]
- 18. Holgersson J, Clausen H, Hakomori S, Samuelsson BE, Breimer ME. Blood group A glycolipid antigen expression in kidney, ureter, kidney artery, and kidney vein from a blood group A1Le(a‐b+) human individual. Evidence for a novel blood group A heptaglycosylceramide based on a type 3 carbohydrate chain. J Biol Chem. 1990;265:20790‐20798. [PubMed] [Google Scholar]
- 19. Holgersson J, Jovall PA, Samuelsson BE, Breimer ME. Blood group type glycosphingolipids of human kidneys. Structural characterization of extended globo‐series compounds. Glycoconj J. 1991;8:424‐433. [DOI] [PubMed] [Google Scholar]
- 20. John M, Bailey LL. Neonatal heart transplantation. Ann Cardiothorac Surg. 2018;7:118‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Macher BA, Galili U. The Galalpha 1,3Galbeta1,4GlcNAc‐R (alpha‐Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta. 2008;1780:75‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jeyakanthan M, Tao K, Zou L, et al. Chemical basis for qualitative and quantitative differences between ABO blood groups and subgroups: implications for organ transplantation. Am J Transplant. 2015;15:2602‐2615. [DOI] [PubMed] [Google Scholar]
- 23. Muthana SM, Xia L, Campbell CT, Zhang Y, Gildersleeve JC. Competition between serum IgG, IgM, and IgA anti‐glycan antibodies. PLoS One. 2015;10:e0119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thorpe SJ, Fox B, Sharp G, White J, Milkins C. A WHO reference reagent to standardize haemagglutination testing for anti‐A and anti‐B in serum and plasma: international collaborative study to evaluate a candidate preparation. Vox Sang. 2016;111:161‐170. [DOI] [PubMed] [Google Scholar]
- 25. Tinckam KJ. 2012. Basic histocompatibility testing methods. In: Chandraker A, Sayegh MH, Singh AK, eds. Core Concepts in Renal Transplantation. Springer; 2012:21‐42. [Google Scholar]
- 26. Schinstock CA, Gandhi MJ, Stegall MD. Interpreting Anti‐HLA antibody testing data: a practical guide for physicians. Transplant. 2016;100:1619‐1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rydberg L, Bengtsson A, Samuelsson O, Nilsson K, Breimer ME. In vitro assessment of a new ABO immunosorbent with synthetic carbohydrates attached to sepharose. Transpl Int. 2005;17:666‐672. [DOI] [PubMed] [Google Scholar]
- 28. Lindberg L, Theinert K, Liu J, Holgersson J. Adsorption of chain type‐specific ABO antibodies on Sepharose‐linked A and B tetrasaccharides. Transfusion. 2012;52:2356‐2367. [DOI] [PubMed] [Google Scholar]
- 29. Breimer ME, Brynger H, Le Pendu J, et al. Blood group ABO‐incompatible kidney transplantation biochemical and immunochemical studies of blood group A glycolipid antigens in human kidney and characterization of the antibody response (antigen specificity and antibody class) in O recipients receiving A2 grafts. Transpl Proc. 1987;19:226‐230. [PubMed] [Google Scholar]
- 30. Obukhova P, Korchagina E, Henry S, Bovin N. Natural anti‐A and anti‐B of the ABO system: allo‐ and autoantibodies have different epitope specificity. Transfusion. 2012;52:860‐869. [DOI] [PubMed] [Google Scholar]
- 31. Gildersleeve JC, Wright WS. Diverse molecular recognition properties of blood group A binding monoclonal antibodies. Glycobiol. 2016;26:443‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Genberg H, Kumlien G, Wennberg L, Tyden G. The efficacy of antigen‐specific immunoadsorption and rebound of anti‐A/B antibodies in ABO‐incompatible kidney transplantation. Nephrol Dial Transplant. 2011;26:2394‐2400. [DOI] [PubMed] [Google Scholar]
- 33. Nosaka M, Ishida Y, Tanaka A, et al. Aberrant expression of histo‐blood group A type 3 antigens in vascular endothelial cells in inflammatory sites. J Histochem Cytochem. 2008;56:223‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. West LJ, Pollock‐Barziv SM, Dipchand AI, et al. ABO‐incompatible heart transplantation in infants. N Engl J Med. 2001;344:793‐800. [DOI] [PubMed] [Google Scholar]
- 35. Fan X, Ang A, Pollock‐Barziv SM, et al. Donor‐specific B‐cell tolerance after ABO‐incompatible infant heart transplantation. Nat Med. 2004;10:1227‐1233. [DOI] [PubMed] [Google Scholar]
- 36. Varki A, Cummings RD, Aebi M, et al. Symbol nomenclature for graphical representations of glycans. Glycobiol. 2015;25:1323‐1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


