ABSTRACT
Vitamin D has shown to play a role in multiple diseases due to its skeletal and extraskeletal actions. Furthermore, vitamin D deficiency has become a worldwide health issue. Few supplementation guidelines mention calcifediol treatment, despite being the direct precursor of calcitriol and the biomarker of vitamin D status. This 1‐year, phase III–IV, double‐blind, randomized, controlled, multicenter clinical trial assessed the efficacy and safety of calcifediol 0.266 mg soft capsules in vitamin D–deficient postmenopausal women, compared to cholecalciferol. Results reported here are from a prespecified interim analysis, for the evaluation of the study's primary endpoint: the percentage of patients with serum 25‐hydroxyvitamin D (25(OH)D) levels above 30 ng/ml after 4 months. A total of 303 patients were enrolled, of whom 298 were included in the intention‐to‐treat (ITT) population. Patients with baseline levels of serum 25(OH)D <20 ng/ml were randomized 1:1:1 to calcifediol 0.266 mg/month for 12 months, calcifediol 0.266 mg/month for 4 months followed by placebo for 8 months, and cholecalciferol 25,000 IU/month for 12 months. At month 4, 35.0% of postmenopausal women treated with calcifediol and 8.2% of those treated with cholecalciferol reached serum 25(OH)D levels above 30 ng/ml (p < 0.0001). The most remarkable difference between both drugs in terms of mean change in serum 25(OH)D levels was observed after the first month of treatment (mean ± standard deviation change = 9.7 ± 6.7 and 5.1 ± 3.5 ng/ml in patients treated with calcifediol and cholecalciferol, respectively). No relevant treatment‐related safety issues were reported in any of the groups studied. These results thus confirm that calcifediol is effective, faster, and more potent than cholecalciferol in raising serum 25(OH)D levels and is a valuable option for the treatment of vitamin D deficiency. © 2021 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: CALCIFEDIOL, CHOLECALCIFEROL, VITAMIN D DEFICIENCY, MENOPAUSE, CLINICAL TRIALS
Introduction
Vitamin D deficiency is a worldwide public health issue affecting more than one billion people.( 1 ) However, prevalence rates for this condition vary depending on the defined thresholds, because there is no consensus on optimal concentrations, not even for its principal effect on skeletal tissue, for which the benefits of vitamin D have been clearly demonstrated.( 2 )
Vitamin D can be obtained from food, dietary supplements, or synthesized in response to sunlight. In the skin, solar ultraviolet‐B radiation converts 7‐dehydrocholesterol to previtamin D3, which is then rapidly converted to vitamin D3. Afterward, vitamin D3 is metabolized in the liver to 25‐hydroxyvitamin D (calcifediol or calcidiol; 25OHD3 or 25(OH)D), a reliable marker of vitamin D status.( 3 ) This compound is metabolized mainly in the kidneys to its active form, 1,25‐dihydroxyvitamin D (calcitriol), by the enzyme 25‐hydroxyvitamin D‐1α‐hydroxylase (CYP27B1).
Vitamin D status is measured as total serum 25(OH)D levels. However, some authors suggest that free 25(OH)D should be the vitamin D status biomarker because it is not bound to serum proteins and shows high biological activity. In most subjects, this activity is strongly correlated with total serum 25(OH)D levels and inversely related to intact parathyroid hormone (iPTH) concentration.( 4 , 5 ) Under some circumstances, when vitamin D–binding protein and albumin levels are altered (i.e., liver disease, inflammatory diseases, or pregnancy),( 6 ) it might offer a better indication of vitamin D status. In some studies it also presents a positive correlation with bone mineral density, unlike total 25(OH)D levels.( 7 , 8 , 9 )
Vitamin D has been found to have skeletal and extraskeletal actions (such as immunomodulation). However, whereas extraskeletal actions are yet under study, skeletal ones have been broadly described, including rickets, osteoporosis, and osteomalacia. It has been associated with an increased risk of osteoporotic fractures and inadequate response to antiresorptive treatment.( 10 )
There is a strong consensus that 25(OH)D levels <25 nmol/L (10 ng/ml) reflect severe vitamin D deficiency. However, there is no consensus about the threshold that should be reached to be within optimal levels. Some societies recommend 50 nmol/L (20 ng/ml), whereas others recommend 75 nmol/L (30 ng/ml).( 11 , 12 )
There are several alternatives for vitamin D deficiency treatment: cholecalciferol (D3), ergocalciferol (D2), or calcifediol (25‐hydroxycholecalciferol). However, few supplementation guidelines mention the latter, because it is not broadly available worldwide.
Routine monitoring of 25(OH)D levels in patients supplemented with vitamin D is not necessary in the general population according to guidelines. However, it is recommended in the high‐risk subgroups and for specific conditions.( 13 , 14 ) Postmenopausal osteoporosis is considered one of these conditions, because antiresorptive and anabolic treatments should be accompanied by vitamin D and calcium supplements.( 15 , 16 ) The most commonly recommended standard dose for vitamin D in this population is 800 IU/day of vitamin D3 (cholecalciferol).( 17 )
In Spain, calcifediol has been available as a prescription drug and widely used for more than 40 years. The efficacy of calcifediol oral solution has been demonstrated in various population groups and different studies. Moreover, these small‐scale trials have shown that calcifediol is more potent and faster than cholecalciferol( 18 ) in terms of raising 25(OH)D levels.
The aim of the present study was to assess the efficacy and safety of calcifediol in the correction and maintenance of 25(OH)D levels in postmenopausal women. It also compared calcifediol 0.266 mg soft capsules with cholecalciferol treatment, at doses recommended by current guidelines.( 13 , 14 , 15 , 16 ) Results reported here are from a prespecified interim analysis for the evaluation of the study's primary endpoint: the percentage of patients with 25(OH)D levels above 30 ng/ml after 4 months. Additional analyses were done on the change in 25(OH)D levels according to subgroups.
Patients and Methods
Trial design
This was a 1‐year phase III–IV, double‐blind, randomized, controlled, multicenter, international, superiority clinical trial; registered at the European Union Drug Regulating Authorities Clinical Trials Database (EudraCT number: 2017‐004028‐31; https://www.clinicaltrialsregister.eu/ctr-search/search?query=2017-004028-31). The study took place at 10 centers in Spain and Italy. Patients were stratified according to their history of osteoporosis, and randomized at a 1:1:1 ratio into three groups (Figure 1):
FIGURE 1.
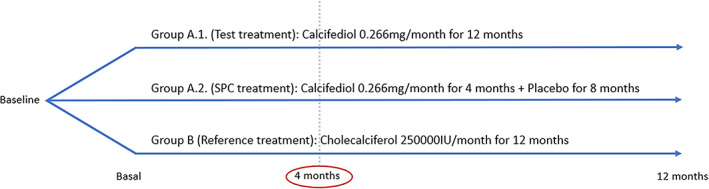
Schematic representation of the clinical trial design
Group A.1: Test. Calcifediol treatment group: monthly administration of one calcifediol 0.266 mg soft capsule (Hidroferol®; Faes Farma, Leioa, Spain), for 12 months.
Group A.2: SmPC. Treatment approved in Summary of Product Characteristics (SmPC), or Prescribing Information, by the Spanish Agency for Medicinal Products and Medical Devices (AEMPS) for Hidroferol® 0.266 mg soft capsules: monthly administration of one calcifediol 0.266 mg soft capsule for 4 months. After this time, the treatment was withdrawn, and for the next 8 months, a monthly placebo soft capsule was administered.
Group B: Reference. Cholecalciferol (Dibase®; Abiogen Pharma, Pisa, Italy) treatment group: monthly administration of one cholecalciferol 25,000 IU single‐dose container, for 12 months. This is the reference treatment defined in guidelines as cholecalciferol 800 IU/day ≈ 25,000 IU/month (0.625 mg/month).( 19 , 20 )
The women received active medication as well as the placebo matching the other treatment arm. For both medicinal products, the placebos and active formulations looked identical.
The dose of calcifediol was chosen based on the recommendations of AEMPS, taking into account the higher potency of this supplement reported in other studies. In our study, the dose of cholecalciferol was 2.35 times higher than the dose of calcifediol, within the range that has been reported in a position statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the International Osteoporosis Foundation (IOF).( 21 )
The randomization sequence was created by a statistician using PROC PLAN, available in SAS® version 9.4 (SAS Institute, Inc., Cary, NC, USA), using a block size of three. After obtaining the consent of the subject, the investigator introduced the data in the electronic case report form; patients were stratified according to the presence or absence of osteoporosis, and finally the treatment number for that patient was then received using an interactive web response system. All the investigators, staff, and participants were blinded to the allocation.
The study began when the first patient was randomized (March 27, 2018). The last visit of the last patient for month 4 took place on October 25, 2019. Overall, individual participation in the study lasted approximately 12 months. The trial included six onsite visits (screening, baseline, and after 1, 4, 8, and 12 months) and a final telephone follow‐up visit.
This trial was conducted in accordance with the principles set out in the Declaration of Helsinki and followed good clinical practice guidelines. Informed consent was obtained from the participants, and the study protocol was formally approved by the appropriate institutional human research committee and regulatory authorities.
Study participants
A total of 303 postmenopausal women with 25(OH)D levels <20 ng/ml were randomly assigned to different treatment groups. A sample size of 300 patients was estimated as necessary for superiority testing, using a two‐group chi‐square test with a 0.05 two‐sided significance level. This would give 80% power to detect a proportion difference of more than 20% between the groups in favor of group A, with a 20% loss rate.
In addition to serum 25(OH)D levels <20 ng/ml, inclusion criteria were as follows: being postmenopausal (defined as amenorrhea for >6 months or follicle‐stimulating hormone [FSH] levels >30 IU/L with estradiol <30 pg/ml); signing of the informed consent; and understanding of the study procedures. Exclusion criteria included concomitant use of drugs that can modify vitamin D levels such as long‐term corticosteroids, orlistat and cholestyramine, or any nutritional supplement such as vitamin complexes. Other exclusion criteria were history of malabsorption, nephrolithiasis, primary hyperparathyroidism, hyperthyroidism, hypercalcemia, creatinine clearance <30 ml/min, neoplastic disease within the last 5 years, history of any conditions or circumstances that could alter the conduct of the study, or allergy to any of the ingredients of the medication. Subjects under treatment with an investigational drug (including investigational vaccines), having used an invasive investigational medical device within 30 days before the screening, or already enrolled in an investigational interventional study were also excluded.
The collection of blood samples was performed by qualified health professionals in each of the centers during visits, and later shipped to the central laboratory. Clinical visits were scheduled during the morning, and patients had to fast 8 h before the sample collection.
Serum 25(OH)D levels
Serum 25(OH)D concentrations were determined in the central laboratory (Synlab, Barcelona, Spain) using an automated chemiluminescence system (LIAISON® XL; DiaSorin, Saluggia, Italy). The lower detection limit was <4 pg/ml. The intraassay coefficient of variation (CV) was 2.34%, and the interassay coefficient of variation was 5.60%. The 25(OH)D levels were examined at the screening visit and months 1, 4, 8, and 12.
Serum free 25(OH)D
Serum free 25(OH)D was also determined at the central laboratory. A competitive enzyme‐linked immunosorbent assay (ELISA) assay using DIAsource ImmunoAssays® S.A. kits (DIAsource, Louvain‐la‐Neuve, Belgium) was employed, based on patented monoclonal antibodies, which allows direct measurement. The intraassay CV was <5.5% and the interassay CV was <6.3%. This assay has been validated analytically, in terms of precision, accuracy, sensitivity, and specificity. Additional validation work is being conducted, including a multicenter reproducibility study.( 22 ) Blood aliquots were obtained from blood samples collected at baseline and after 4 and 12 months.
Bone mineral metabolism parameters and bone remodeling markers
Concentrations of total serum calcium (tCa), albumin, phosphorus, iPTH, and total alkaline phosphatase were obtained in the central laboratory using an automated analyzer (AU5800; Beckman Coulter, Brea, CA, USA). The samples were collected at the screening visit, and after 1, 4, 8, and 12 months.
Serum concentrations of procollagen type 1 N‐terminal propeptide (P1NP) and β‐isomerized C‐terminal telopeptides (β‐CTx) were also measured in the central laboratory using an automated chemiluminescence system (Cobas E411; Roche Diagnostics GmbH, Mannheim, Germany). The lower detection limit of P1NP was <5 ng/ml (reference range 5–1200 ng/ml); the intraassay and interassay CVs were 2.06% and 3.23%, respectively. The lower limit of detection for β‐CTX was <0.01 ng/ml (reference range 0.01–6 ng/ml)( 23 , 24 ); the intraassay and interassay CVs were 3.10% and 3.35%, respectively. P1NP and β‐CTX levels were measured in the group of patients not receiving treatment with drugs that could affect bone metabolism, at baseline and after 4, 8, and 12 months.
Other assessments
Dietary calcium consumption at baseline, 4, 8, and 12 months was assessed using an adapted version of a validated questionnaire.( 25 ) Physical examination was performed at screening, baseline, and at 4 and 12 months. Safety and tolerability were also examined throughout the study.
Statistical analysis
Efficacy endpoints were analyzed for the intention‐to‐treat (ITT) population. For the present interim analysis, the two calcifediol groups (A.1. and A.2) were pooled.
The comparison for the primary efficacy analysis was performed using the chi‐square test (without continuity correction) and the corresponding 95% asymptotic (Wald) confidence interval (CI) for the proportion difference, using the last observation carried forward (LOCF) imputation for missing data. Superiority was demonstrated by a difference between groups greater than 20% (minimum effect). The lower limit of 95% asymptotic CI for the proportion difference was more than 0%, based on the ITT population.
The baseline value was defined as the last valid assessment before the first administration of the studied drug and applied for all efficacy and safety parameters analyzed unless specified otherwise. To guarantee the blinding of the study during the analysis at month 4, any of the planned subgroups (e.g., division by baseline 25(OH)D levels, body mass index [BMI], age groups) that were too small (i.e., <10 observations) were not reported, including the safety analysis due to the low incidence of adverse events.
Serum 25(OH)D levels and changes from baseline were summarized in terms of the number of observations, mean, standard deviation (SD), 95% CI of mean, median, range, and interquartile range. The statistical significance of differences between groups (pairwise comparisons) was obtained using Student's t test or Mann‐Whitney test.
Multivariate regression (correlation) between total 25(OH)D and free 25(OH)D at month 4 was performed and adjusted by BMI, age, 25(OH)D, treatment group, and osteoporosis diagnosis (yes/no) at the baseline.
Results were considered statistically significant when p was <0.05. All statistical tests performed were two‐sided, and the reported CIs used a significance level of 5%. All statistical analyses were conducted using SAS® (version 9.4; SAS Institute Inc.) in a secure and validated environment.
Results
Initially, 303 patients were randomized (Figure 2). From these, 298 were included in the present ITT population (266 without and 32 subjects with osteoporosis: 89.3% and 10.7%, respectively).
FIGURE 2.
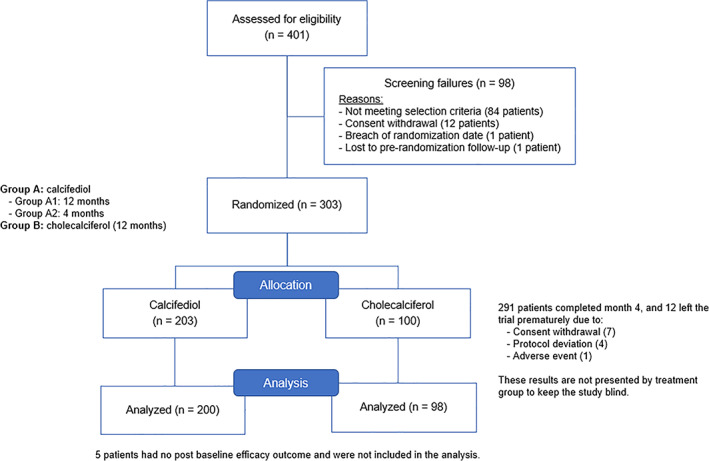
Flowchart of patient disposition
Table 1 summarizes baseline characteristics for the two treatment groups, showing a homogenous population. The mean age of the participants was 63.4 ± 8.2 years, 98.3% were white, and risk factors for osteoporosis and dietary calcium consumption in the two treatment groups were similar. The mean serum 25(OH)D level at the screening was 13.0 ± 3.9 ng/ml, mean free 25(OH)D concentration 3.9 ± 1.1 pg/ml, and the mean iPTH level was 60.1 ± 25.5 pg/ml.
TABLE 1.
Demographic and other baseline characteristics of participants
| Overall (n = 298) | Calcifediol (n = 200) | Cholecalciferol (n = 98) | p | |
|---|---|---|---|---|
| Age (years), mean ± SD | 63.4 ± 8.2 | 63.3 ± 8.0 | 63.6 ± 8.9 | 0.7638 |
| White ethnicity, n (%) | 292 (98.3) | 196 (98) | 96 (98) | 0.6663 |
| Age at menarche (years), mean ± SD | 12.6 ± 1.7 | 12.5 ± 1.6) | 12.6 ± 1.8 | 0.5865 |
| Age at menopause (years), mean ± SD | 48.5 ± 5.7 | 48.6 ± 5.7 | 48.4 ± 5.5 | 0.7340 |
| Osteoporosis diagnosis, n (%) | 32 (10.7) | 21 (10.5) | 11 (11.2) | 0. 8442 |
| Season of baseline assessment, n (%) | 0.3601 | |||
| Spring | 135 (45.3) | 93 (46.5) | 42 (42.9) | |
| Summer | 64 (21.5) | 45 (22.5) | 19 (19.4) | |
| Autumn | 24 (8.1) | 14 (7) | 10(10.2) | |
| Winter | 75 (25.2) | 48 (24) | 27 (27.6) | |
| Patients by location, n (%) | 1.0000 | |||
| North (north of latitude 40 degrees) | 256 (85.9) | 172 (86) | 84 (85.7) | |
| South (south of latitude 40 degrees) | 42 (14.1) | 28 (14) | 14 (14.3) | |
| Body mass index (kg/m2), mean ± SD a | 29.3 ± 6.0 | 29.0 ± 6.3 | 29.8 ± 5.4 | 0.2525 |
| Body mass index, n (%) | 0.0698 | |||
| Normal weight (18.5–24.9 kg/m2) | 75 (25.2) | 58 (29.0) | 17 (17.3) | |
| Overweight (25.9–29.9 kg/m2) | 97 (32.6) | 59 (29.5) | 38 (38.8) | |
| Obese (>30 kg/m2) | 123 (41.3) | 80 (40.0) | 43 (43.9) | |
| Abdominal circumference (cm), mean ± SD | 96.2 ± 14.0 | 95.8 ± 14.4 | 97.1 ± 13.1 | 0.4686 |
| Current smoking, n (%) | 46 (15) | 30 (15.0) | 16 (16.3) | 0.8646 |
| Alcohol consumption, n (%) | 37 (12.42) | 26 (13.0) | 11 (11.23) | 0.7127 |
| Daily calcium consumption (mg/day), mean ± SD | 866.2 ± 361.2 | 863.9 ± 376.9 | 871.0 ± 328.9 | 0.8725 |
| Total 25(OH)D (ng/ml), mean ± SD b | 13.0 ± 3.9 | 12.8 ± 3.9 | 13.2 ± 3.7 | 0.4135 |
| 25(OH)D level ≤10 ng/ml, n (%) | 74 (24.8) | 54 (27.0) | 20 (20.4) | |
| 25(OH)D level >10 to 20 ng/ml, n (%) | 224 (75.2) | 146 (73.0) | 78 (79.6) | |
| Free 25(OH)D concentration (pg/ml), mean ± SD | 3.9 ± 1.1 | 3.8 ± 1.1 | 4.0 ± 1.1 | 0 .2959 |
| Total serum calcium (mg/dl), mean ± SD | 9.6 ± 0.4 | 9.6 ± 0.4 | 9.6 ± 0.4 | 0 .0852 |
| Phosphate (mg/dl), mean ± SD | 3.5 ± 0.5 | 3.5 ± 0.5 | 3.5 ± 0.5 | 0.7950 |
| Intact parathormone (pg/ml), mean ± SD | 60.1 ± 25.5 | 59.0 ± 27.4 | 62.3 ± 21.2 | 0.2587 |
| Total alkaline phosphatase (IU/L), mean ± SD | 87.4 ± 23.5 | 86.8 ± 23.9 | 88.6 ± 23.0 | 0.5367 |
| β‐CTX (μg/L), mean ± SD (n = 257) c | 0.5 ± 0.3 | 0.5 ± 0.4 | 0.5 ± 0.2 | 0.7988 |
| P1NP (ng/ml), mean ± SD (n = 257) | 51.1 ± 20.3 | 51.5 ± 19.4 | 50.1 ± 22.3 | 0.5932 |
Notes: The table includes baseline characteristics for the intention‐to‐treat population. Values of p are not statistically significant overall, demonstrating a homogeneous population at baseline.
Abbreviations: 25(OH)D, 25‐hydroxyvitamin D; β‐CTX, β‐isomerized C‐terminal telopeptides; ODS, Office of Dietary Supplements; P1NP, procollagen type 1 N‐terminal propeptide; SD, standard deviation.
Underweight patients (n = 3, 1%) are not represented on this table, to keep data blind.
25(OH)D: 1 ng/ml = 2.5 nmol/L (ODS, National Institutes of Health, updated on October 9, 2020).
Assessed only in non‐osteoporotic patients.
At month 4, the analysis of the primary endpoint of this study showed that 35.0% (95% CI, 25.4% to 42.0%) of participants treated with calcifediol reached 25(OH)D levels >30 ng/ml, whereas only 8.2% (95% CI, 3.6% to 15.5%) in the cholecalciferol group did (Figure 3). The proportion difference between both groups was of 26.8% (95% CI, 18.3% to 35.4%). This difference in efficacy (in terms of reaching 25(OH)D levels >30 ng/ml) was already present at month 1, where 13.5% (95% CI, 9.1%; to 19.0%) of the patients in the calcifediol arm and none of those on cholecalciferol arm (95% CI, 0.0% to 3.7%) achieved the target level. Both results were statistically significant, with p < 0.0001.
FIGURE 3.
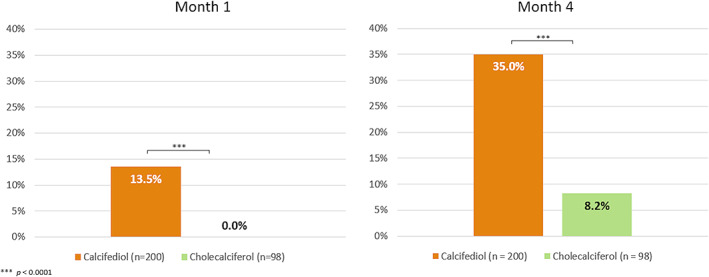
Percentage of subjects with 25(OH)D levels >30 ng/ml at months 1 and 4, per treatment group. ***p < 0.0001. Abbreviation: 25(OH)D, 25‐hydroxyvitamin D.
Results were also analyzed by using 20 ng/ml as threshold. Likewise, calcifediol was also superior to cholecalciferol at month 1, with 59.0% and 34.0% (95% CI, 51.8% to 65.9%; and 95% CI, 24.4% to 43.9%, respectively) of patients achieving this threshold (p < 0.0001). At month 4, the results for these groups were 81.0% and 72.4% (95% CI, 74.9% to 86.2% and 62.5% to 81.0%, respectively; p > 0.05).
Table 2 shows the increase of 25(OH)D levels after supplementation with calcifediol and cholecalciferol at baseline, months 1 and 4, and by baseline 25(OH)D levels. The mean serum 25(OH)D levels were higher for calcifediol than for cholecalciferol both at month 1 (22.6 ± 7.8 versus 18.4 ± 4.0, p < 0.0001) and month 4 (27.8 ± 9.0 versus 23.1 ± 5.4, p < 0.0001). At month 1, the mean increase in serum 25(OH)D levels from baseline was 9.7 ± 6.7 ng/ml in the calcifediol group and 5.1 ± 3.5 ng/ml in the cholecalciferol group. At month 4, the mean increases were 14.9 ± 8.1 ng/ml and 9.9 ± 5.7 ng/ml, respectively. Both results were statistically significant (p < 0.0001).
TABLE 2.
Effect of treatment on mean serum 25(OH)D levels at months 1 and 4 by baseline 25(OH)D levels
| Calcifediol treatment | Cholecalciferol treatment | |||||
|---|---|---|---|---|---|---|
| Time period | Baseline serum 25(OH)D ≤10 ng/ml (n = 54) | Baseline serum 25(OH)D >10 to 20 ng/ml (n = 146) | p | Baseline serum 25(OH)D ≤10 ng/ml (n = 20) | Baseline serum 25(OH)D >10 to 20 ng/ml (n = 78) | p |
| Baseline | 7.7 ± 1.8 | 14.7 ± 2.5 | <0.0001 | 7.8 ± 1.5 | 14.6 ± 2.7 | <0.0001 |
| Month 1 | 17.1 ± 5.4 | 24.6 ± 7.6 | <0.0001 | 15.1 ± 4.2 | 19.2 ± 3.5 | <0.0001 |
| Month 4 | 22.6 ± 8.0 | 29.7 ± 8.6 | <0.0001 | 20.4 ± 6.0 | 23.8 ± 5.0 | 0.0110 |
Notes: Values are mean ± SD 25(OH)D levels in ng/ml. Mean serum 25(OH)D levels that are reached at months 1 and 4 are statistically significantly different when comparing both baseline 25(OH)D levels subgroups, and for both treatments.
Abbreviations: 25(OH)D, 25‐hydroxyvitamin D; SD, standard deviation.
The major difference in the mean change after treatment with both drugs was observed at month 1 (Figure 4), due to a faster increase of 25(OH)D levels with calcifediol. This difference remained almost constant until month 4, being p < 0.0001 at both time points.
FIGURE 4.
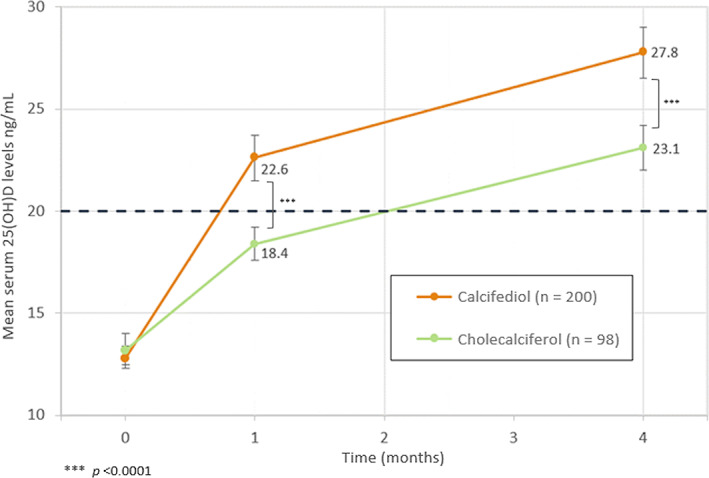
Evolution of mean 25(OH)D levels (ng/mL) at months 1 and 4 per treatment group. Mean serum 25(OH)D levels are represented with their 95% CI. ***p < 0.0001. The horizontal dashed line represents the 25(OH)D threshold of 20 ng/ml. Abbreviation: 25(OH)D, 25‐hydroxyvitamin D; CI, confidence interval.
When interpreting the results by 25(OH)D basal levels, there are statistically significant differences between calcifediol and cholecalciferol for patients with basal 25(OH)D levels between 10 and 20 ng/ml. For patients with basal levels <10 ng/ml, there are differences between both treatments; however, this difference is not statistically significant. However, when expressing the results by increase in 25(OH)D levels per microgram of drug administered in this subgroup of patients, the increment at month 1 is superior with calcifediol 0.0354 ng/ml (95% CI, 0.0297 to 0.0410) when compared to cholecalciferol 0.0118 ng/ml (95% CI, 0.0085 to 0.0151), p < 0.0001.
Free 25(OH)D concentrations displayed a similar pattern after 4 months of treatment, with levels of 7.6 ± 2.5 pg/ml in the calcifediol treatment arm versus 6.5 ± 1.6 pg/ml in cholecalciferol arm (p < 0.0001). The mean change was 3.8 ± 2.4 pg/ml and 2.5 ± 1.8 pg/ml, respectively (p < 0.0001). A positive correlation was found between total 25(OH)D and free 25(OH)D concentrations (r = 0.83, p < 0.0001) after adjusting for BMI, age, baseline 25(OH)D levels, treatment group, and osteoporosis.
Regarding calcium and phosphate metabolism, no differences were observed after administering calcifediol or cholecalciferol (Table 3). Similarly, no changes iPTH levels or in bone remodeling markers were found with any treatment.
TABLE 3.
Effect of treatment on bone mineral metabolism parameters
| Baseline | Month 4 | |||||
|---|---|---|---|---|---|---|
| Parameter | Calcifediol (n = 200) | Cholecalciferol (n = 97) | p | Calcifediol (n = 200) | Cholecalciferol (n = 97) | p |
| Total serum calcium (mg/dl) | 9.6 ± 0.4 | 9.6 ± 0.4 | 0.0852 | 9.5 ± 0.5 | 9.6 ± 0.5 | 0.1329 |
| Phosphate (mg/dl) | 3.5 ± 0.5 | 3.5 ± 0.5 | 0.7950 | 3.6 ± 0.5 | 3.6 ± 0.5 | 0.2544 |
| Intact parathormone (pg/ml) | 59.0 ± 27.4 | 62.3 ± 21.2 | 0.2587 | 54.9 ± 24.6 | 58.4 ± 22.3 | 0.2423 |
| Baseline | Month 4 | |||||
|---|---|---|---|---|---|---|
| Calcifediol (n = 175) | Cholecalciferol (n = 82) | p | Calcifediol (n = 179) | Cholecalciferol (n = 87) | p | |
| β‐CTX (μg/L) a | 0.47 ± 0.4 | 0.46 ± 0.2 | 0.7870 | 0.43 ± 0.2 | 0.43 ± 0.2 | 0.9013 |
| P1NP (ng/ml) a | 51.5 ± 19.4 | 50.1 ± 22.3 | 0.5932 | 51.3 ± 19.0 | 49.9 ± 20.2 | 0.5588 |
Note: There were no statistically significant changes in bone mineral metabolism parameters values after 4 months of treatment, when compared to baseline for both treatment groups.
Abbreviations: 25(OH)D, 25‐hydroxyvitamin D; β‐CTX, β‐isomerized C‐terminal telopeptides; P1NP, procollagen type 1 N‐terminal propeptide; SD, standard deviation.
Assessed only in non‐osteoporotic patients.
The mean change for iPTH from baseline to month 4 was −4.1 pg/ml (95% CI, −6.5 to −1.7 pg/ml) for calcifediol, and −3.9 pg/ml (95% CI, −7.9 to −0.0 pg/ml; p = 0.9469) for cholecalciferol. However, women with 25(OH)D levels >30 ng/ml at month 4 showed a decrease in iPTH levels of −4.4 ± 16.7 pg/ml (95% CI, −8.2 to −0.6 pg/ml; p = 0.0227). In these patients, only those treated with calcifediol showed a statistically significant decrease, −5.0 ± 16.5 pg/ml (95% CI, −9.0 to −1.1 pg/ml; p = 0.0131) compared to the cholecalciferol group, 1.1 ± 18.5 pg/ml (95% CI −14.3 to 16.6 pg/ml; p = 0.8683). Women with 25(OH)D levels >20 ng/ml at month 4 showed a decrease in iPTH levels of −4.5 ± 17.2 pg/ml (95% CI, −6.8 to −2.3 pg/ml; p < 0.0001). Correlation between iPTH and 25(OH)D levels in the overall population is inverse and statistically significant at baseline, (r = −0.21, p = 0.0003), at month 1 (r = −0.18, p = 0.0023) and at month 4 (r = −0.13, p = 0.0258). When dividing the population by treatment groups, this correlation was only observed at month 1, in women treated with calcifediol (r = −0.18, p = 0.0127). When dividing the population by iPTH quartiles (Figure 5A,B ), women in the fourth quartile had the lowest 25(OH)D levels, associated to the highest iPTH levels, higher than the upper limit of normality. In this subgroup, the decrease in iPTH levels after treatment administration was statistically significant for both calcifediol (month 1 and month 4) and cholecalciferol (month 4).
FIGURE 5.
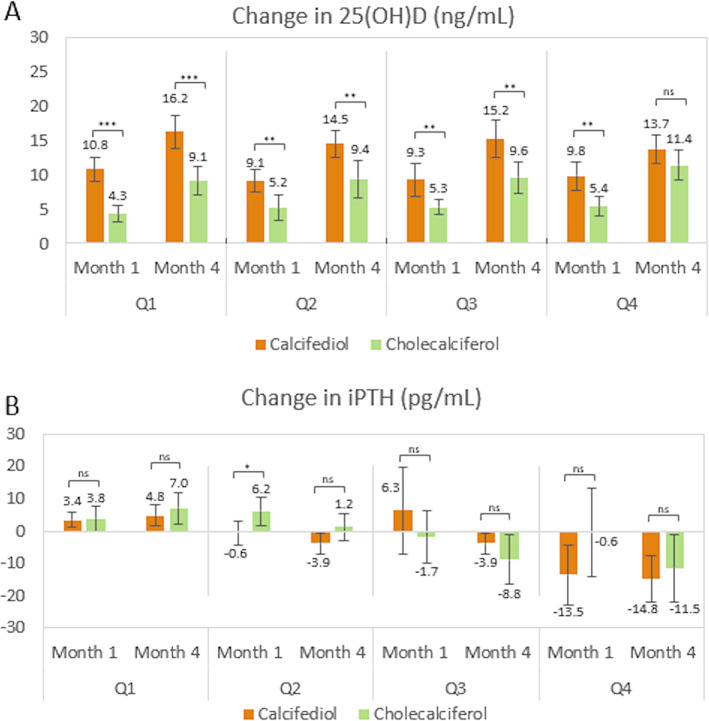
Change in serum 25(OH)D levels and iPTH levels, per iPTH quartiles, at months 1 and 4. (A) Mean change in serum 25(OH)D levels with their 95% CI, per each quartile of iPTH values at baseline for both treatment groups at months 1 and 4. (B) Mean change in serum iPTH levels with their 95% CI, per each quartile of iPTH values at baseline for both treatment groups at months 1 and 4. The number of patients per quartile is: Calcifediol Q1 (53), Q2 (55), Q3 (46), Q4 (46); Cholecalciferol Q1 (18), Q2 (26), Q3 (26), Q4 (27). ***p ≤ 0.001; **p ≤ 0.01; *p ≤ 0.05. Abbreviation: 25(OH)D, 25‐hydroxyvitamin D; CI, confidence interval; iPTH, intact parathyroid hormone; ns, not significant; Q, quartile.
Results were also analyzed by BMI, comparing obese and non‐obese patients. Obese patients treated with calcifediol show higher 25(OH)D levels than those treated with cholecalciferol, and the difference was statistically significant at month 1 (p = 0.0060), and at month 4 (p = 0.0088). The same was also observed for non‐obese patients at month 1 (p < 0.0001), and at month 4 (p < 0.0001).
In these first 4 months of treatment, 72 of the 303 (23.8%) patients enrolled reported at least one adverse event (AE). Only one AE related to the treatment was reported by one (0.3%) patient, and only one (0.3%) subject had an AE leading to her withdrawal from the study. Eight (2.6%) patients reported at least one serious adverse event (SAE). No safety issues were associated with the present analysis; there were no deaths, no SAEs attributable to either of the two study drugs nor any other significant AEs.
Discussion
Our study shows that calcifediol soft capsules in a monthly dosage effectively increases serum 25(OH)D levels. These results show that calcifediol is faster and more potent than cholecalciferol in terms of increasing 25(OH)D levels. Calcifediol increases the concentration of 25(OH)D in a steady, consistent manner, independent of baseline 25(OH)D levels, whereas the increments brought about by cholecalciferol administration are variable. For patients with baseline values >10 ng/ml treated with cholecalciferol, the increase in 25(OH)D levels is weaker than for those receiving calcifediol. When dividing the population by BMI, in obese (BMI > 30 kg/m2) and non‐obese patients, serum 25(OH)D levels showed a statistically significant increase in calcifediol when compared to cholecalciferol, at months 1 and 4.
Few studies have compared the efficacy of calcifediol and cholecalciferol in increasing serum 25(OH)D levels and have reported similar findings. However, none of them compared monthly doses of both drugs. Rossini et al.( 26 ) studied 271 postmenopausal women with either osteoporosis or osteopenia, using similar cholecalciferol doses but larger doses of calcifediol (calcifediol 14 μg/day vs. 9 μg/day in our study). They reported comparable increments in 25(OH)D concentration and a superior (1.66 times higher) calculated relative potency of calcifediol versus cholecalciferol. Bischoff‐Ferrari et al.( 27 ) performed a similar comparison with a low number of subjects (20 postmenopausal women between the ages of 50 and 70 years). They compared daily administration of 20 μg calcifediol and 20 μg (800 IU) cholecalciferol and weekly administration of 140 μg calcifediol and 140 μg (5600 IU) cholecalciferol. The group treated with calcifediol showed a larger increment in 25(OH)D concentration and a significant decrease in iPTH levels. However, the sample size in that study was small and doses used were greater than ours. Interestingly, an additional analysis, performed by Meyer et al.,( 28 ) reported that all women treated with calcifediol in that study achieved 25(OH)D levels >30 ng/ml, in comparison with 50% of women taking cholecalciferol. In the study of Shieh et al.,( 29 ) large cholecalciferol doses (60 μg/day or 2400 IU/day) and 20 μg/day of calcifediol were used in a group of 35 adults with vitamin D deficiency (25(OH)D < 20 ng/ml). The authors have reported that 87.5% of the subjects reached 25(OH)D levels ≥30 ng/ml in the calcifediol treatment arm at 4 weeks, compared to 23.1% in the cholecalciferol arm (p = 0.001). They also showed that the free 25(OH)D concentration increased more after calcifediol than after cholecalciferol administration, whereas the iPTH level was not significantly reduced by either of the supplements used.
The present interim analysis shows that total 25(OH)D levels after calcifediol or cholecalciferol treatment in postmenopausal women correlate well (r = 0.82, p < 0.0001) with free 25(OH)D levels after adjusting for BMI, age, baseline 25(OH)D levels, treatment group, and osteoporosis. This positive correlation has also been shown by additional studies in different populations.( 4 ) For many years, this assessment relied on calculations, which overestimate the results when compared to directly measuring free 25(OH)D.( 30 ) The use of an ELISA assay, as in the present study, has some limitations because the antibodies have a decreased affinity for the D2 form of the hormone, resulting in an underestimation of the true concentration of free 25(OH)D2, and the assay is prone to interference with lipids, hemoglobin, and bilirubin. Nonetheless, in our study no vitamin D2 was used and samples were not prone to interfering substances.
Cashman et al.( 31 ) studied 56 vitamin D–deficient patients supplemented with cholecalciferol 20 μg/day, calcifediol 7 μg/day, calcifediol 20 μg/day, or placebo during 10 weeks of winter. The authors showed that cholecalciferol and lower calcifediol doses did not reduce the iPTH levels, although 25(OH)D concentration increased. In contrast, calcifediol administered at 20 μg/day significantly increased the levels of 25(OH)D and reduced the iPTH levels at weeks 5 and 10. Navarro‐Valverde et al.,( 18 ) in a more recent study, used different doses of calcifediol and different dosing intervals (20 μg/day, 266 μg/week, and 266 μg/2 weeks); their cholecalciferol dose was 800 IU/day, similar to ours (25,000 IU/month). Although their sample size was small (40 postmenopausal women), they obtained significantly higher 25(OH)D levels (after 6 and 12 months) in all three calcifediol treatment arms compared to cholecalciferol treatment, showing a three to six times higher potency of calcifediol over cholecalciferol. Remarkably, iPTH levels decreased significantly after 12 months. In a study performed in an elderly population (≥75 years of age) by Ruggiero et al.,( 32 ) calcifediol rapidly increased serum 25 (OH)D levels and reached the optimal target threshold (defined as 22–30 ng/ml). This was particularly marked among those with comorbidity, taking multiple drugs and showing low muscle strength. Minisola et al.,( 33 ) using the calcifediol doses of 20 μg/day, 40 μg/day, and 125 μg/week, achieved 25(OH)D concentrations >30 ng/ml from day 14 onward. Olmos et al.( 34 ) conducted a real‐world study with 156 osteoporotic patients, 23 men and 133 women, employing calcifediol 266 μg in fortnightly and monthly administrations. They obtained the largest increases in 25(OH)D levels using the fortnightly dose, with 92% of the subjects reaching 25(OH)D concentrations >30 ng/ml. In both groups, reductions in the concentration of iPTH and bone remodeling markers were observed at least a year after starting treatment (mean 15 ± 3 months). It should be noted that all the patients received antiresorptive treatment.
The superior speed of action and potency observed for calcifediol can be attributed to several mechanisms. One explanation could be its high absorption rate, close to 100% compared to only 70% for cholecalciferol.( 35 ) Due to its lower lipophilicity, calcifediol is less trapped by adipose tissue. The fact that the hepatic 25‐hydroxylation is not required( 36 ) might also contribute to the superiority of calcifediol in raising 25(OH)D levels. Moreover, calcifediol has a higher affinity to the transporter (megalin), allowing efficient internalization in the cells using the megalin‐cubilin endocytic receptor system.( 37 ) In previous studies, daily or weekly doses have been used, meanwhile our study used monthly doses. Other studies( 38 , 39 ) have shown that monthly doses reach similar 25(OH)D concentrations to daily or weekly doses, and allow a better treatment adherence.
When the population was divided by baseline 25(OH)D levels, we found differences in the increase in serum 25(OH)D levels in subjects with baseline levels <10 ng/ml, between both treatment groups, albeit this difference was not statistically significant. This was probably due to the reduced number of participants in this subgroup, given that the evaluation of this threshold was not one of the main objectives for this study. This may be also due to a more rapid conversion of cholecalciferol into 25(OH)D in patients with severe vitamin D deficiency.( 40 ) Cholecalciferol seems to have a biphasic behavior: with higher baseline serum 25(OH)D concentrations, it shows slower increases, whereas this is not the case for calcifediol.
Vitamin D deficiency can result in a rise in PTH levels, or secondary hyperparathyroidism, increasing bone remodeling. This, in turn, causes a deterioration in the quality and quantity of bone, reducing bone strength and increasing the risk of fracture.( 41 ) However, most vitamin D–deficient patients can have normal PTH levels,( 29 ) which was also noted in our study. Age, sex, obesity, and basal 25(OH)D levels can affect this relationship. We observed that increasing 25(OH)D did not modify iPTH levels or alter bone remodeling parameters during the studied period. The fact that the basal iPTH levels were within normal range could explain this absence of treatment effect. Another important observation was that after 4 months of treatment, the mean 25(OH)D levels were 27.8 ± 9.0 ng/ml and 23.1 ± 5.4 ng/ml in the calcifediol and cholecalciferol groups, respectively. It is possible that higher concentrations of 25(OH)D are required for iPTH suppression and normalization of bone remodeling.
Indeed, baseline iPTH was highest in the most severely deficient patients, and in patients with the highest quartile of iPTH both cholecalciferol and calcifediol decreased iPTH (Figure 5B ).
In the present study, no relevant safety issues were encountered for the analyzed drugs. Furthermore, maximum 25(OH)D levels reached were 60 ng/ml, and vitamin D–related toxicity has been reported for concentrations >100 ng/ml.( 42 )
The clinical significance of achieving optimal 25(OH)D levels in postmenopausal women, especially in osteoporotic ones, has previously been described. Vitamin D deficiency is one of the causes of inadequate response to osteoporosis treatment.( 43 ) Therefore, a rapid increase in 25(OH)D concentration to optimal levels could facilitate a correct therapeutic response. This is especially important in cases of an imminent risk of fracture.( 44 ) Moreover, calcifediol could be a good option for osteoporotic women treated with alendronate( 45 ) and the preferred option for obese women with malabsorption syndrome or hepatic insufficiency.( 40 , 46 ) It is also important to note that serum 25(OH)D is considered by some authors as a negative acute phase reactant.( 47 ) This could be a better alternative because of the speed of action for patients with systemic inflammatory conditions, such as the hyperinflammatory phase of coronavirus disease 2019 (COVID‐19) infection, associated with vitamin D deficiency.( 48 , 49 )
The principal strength of this study is the sample size, with 298 postmenopausal women, which gives sufficient statistical power to confirm significant differences between treatments. The homogeneity of the study population and the centralization of the laboratory analyses are also important factors. In addition, our study used monthly doses and allowed an analysis in subjects with a wide range of BMI. Furthermore, the doses used here have been recommended by clinical guidelines for vitamin D supplementation and the dosage recommended for calcifediol in the SmPC for the general population (0.266 mg once a month). Following this posology, we found that calcifediol 0.266 mg/month has a potency approximately four times higher than cholecalciferol 25,000 IU/month. The main limitation of this study, however, is the dose of the study drugs used, which could be considered insufficient for the pursued objectives. The criteria for selecting these therapeutic regimes were based on the recommendations of clinical practice guidelines and the SmPC available at the time of study design, given the lack of international consensus on optimal treatment schemes.
In conclusion, this study compares the efficacy of calcifediol and cholecalciferol to correct serum 25(OH)D in a cohort of vitamin D–deficient postmenopausal women (osteoporotic and non‐osteoporotic). Calcifediol is faster and more potent than cholecalciferol in obtaining 25(OH)D levels >30 ng/ml after 4 months of treatment. This indicates that calcifediol is an effective and safe treatment to reach optimal 25(OH)D levels in postmenopausal vitamin D–deficient patients.
Disclosures
José Luis Pérez‐Castrillón is a consultant for Farmalider, Faes Farma, and Gedeon Richter, and has received research support from Faes Farma, Farmalider, and Pfizer. Antonio Dueñas‐Laita has received research support from Faes Farma and Farmalider. Maria Luisa Brandi is a consultant and has received research support from Alexion, Abiogen, Amgen, Bruno Farmaceutici, Faes Farma, Eli Lilly, MSD, NPS, Shire, SPA, and Servier. Esteban Jódar is a consultant for Amgen, AstraZeneca, FAES, GSK, Helios‐Fresenius, Italfármaco, Lilly, MSD, Mundipharma, Novo Nordisk, Shire, and UCB; has received lecture fees from Amgen, Asofarma, AstraZeneca, Boehringer, FAES, Lilly, MSD, Mundipharma, Novartis, Novo Nordisk, Tecnofarma, and UCB; has received research support from Amgen, Boehringer, AstraZeneca, FAES, GSK, Janssen, Lilly, MSD, Novo Nordisk, Pfizer, Sanofi, Shire, and UCB; and holdings from SICAM SL, HnB & Cajal PME. Javier del Pino‐Montes has received research support from Bristol, Faes Farma, Roche, and is a consultant for Gedeon Richter. José Manuel Quesada‐Gómez is a consultant for Amgen, Faes Farma, Gebro, Gedeon Richter, Quesper, Shire, Theramex, and UCB; has received lecture fees from Amgen, Faes Farma, Gebro, Gedeon Richter, Quesper, and Theramex and research support from Amgen, Faes Farma, Lilly, and Quesper. Fernando Cereto Castro has received research support from Faes Farma. Carlos Gómez‐Alonso is a consultant and has received research support from Alexion, Amgen, Faes Farma, Gebro Pharma, Kiowa‐Kirin, and UCB. Laura Gallego López has nothing to declare. José Manuel Olmos Martínez is a recipient of lecture fees from Amgen and Stada and research support from Amgen and Gedeon Richter through Instituto de Investigación M. Valdecilla (IDIVAL). María Rosa Alhambra Expósito has received research support from Faes Farma. Bernat Galarraga has received research support from Faes Farma. Jesús González‐Macías has nothing to declare. Roger Bouillon is a consultant in Faes Farma and a recipient of lecture fees from Ceres (Belgium), Procter & Gamble (Belgium), Abiogen (Italy). Gonzalo Hernández‐Herrero, Nieves Fernández‐Hernando, Paula Arranz‐Gutiérrez, and Sandra P. Chinchilla are employees of Faes Farma.
AUTHOR CONTRIBUTIONS
José Luis Pérez‐Castrillón: conceptualization, investigation, supervision, validation, writing‐original draft, writing‐review and editing Antonio Dueñas‐Laita: conceptualization, investigation, supervision, validation, writing‐original draft, writing‐review and editing Maria Luisa Brandi: investigation, writing‐review and editing Esteban Jódar: investigation, writing‐review and editing Javier del Pino‐Montes: investigation, writing‐review and editing José Manuel Quesada‐Gómez: conceptualization, investigation, writing‐review and editing Fernando Cereto Castro: investigation, writing‐review and editing Carlos Gómez‐Alonso: investigation, writing‐review and editing Laura Gallego López: investigation, writing‐review and editing José Manuel Olmos Martínez: investigation, writing‐review and editing María Rosa Alhambra Expósito: investigation, writing‐review and editing Bernat Galarraga: investigation, writing‐review and editing Jesús González‐Macías: conceptualization, writing‐review and editing Roger Bouillon: writing‐review and editing Gonzalo Hernández‐Herrero: conceptualization, methodology, writing‐review and editing Nieves Fernández‐Hernando: conceptualization, methodology, writing original draft, writing‐review and editing Paula Arranz‐Gutiérrez: visualization, writing original draft, writing‐review and editing Sandra P. Chinchilla: visualization, writing original draft, writing‐review and editing
Acknowledgments
This study was funded by Faes Farma S.A. and Bruno Farmaceutici S.p.A. We thank the study participants, research staff, the secondary investigators of the Osteoferol study group, and the Faes Farma clinical research team: Lorena Elgezabal González and Mariana Frau Usoz.
References
- 1. Hilger J, Friedel A, Herr R, et al. A systematic review of vitamin D status in populations worldwide. Br J Nutr. 2014;111(1):23‐45. 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 2. Cashman KD, Vitamin D. Deficiency: defining, prevalence, causes, and strategies of addressing. Calcif Tissue Int. 2020;106(1):14‐29. 10.1007/s00223-019-00559-4. [DOI] [PubMed] [Google Scholar]
- 3. Cashman KD, van den Heuvel EG, Schoemaker RJ, Prévéraud DP, Macdonald HM, Arcot J. 25‐Hydroxyvitamin D as a biomarker of vitamin D status and its modeling to inform strategies for prevention of vitamin D deficiency within the population. Adv Nutr. 2017;8(6):947‐957. 10.3945/an.117.015578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bikle DD, Malmstroem S, Schwartz J. Current controversies: are free vitamin metabolite levels a more accurate assessment of vitamin D status than total levels? Endocrinol Metab Clin North Am. 2017;46(4):901‐918. 10.1016/j.ecl.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Altieri B, Cavalier E, Bhattoa HP, et al. Vitamin D testing: advantages and limits of the current assays. Eur J Clin Nutr. 2020;74(2):231‐247. 10.1038/s41430-019-0553-3. [DOI] [PubMed] [Google Scholar]
- 6. Bikle DD, Schwartz J. Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front Endocrinol. 2019;10:317. 10.3389/fendo.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allison RJ, Farooq A, Cherif A, Hamilton B, Close GL, Wilson MG. Why don't serum vitamin D concentrations associate with BMD by DXA? A case of being “bound” to the wrong assay? Implications for vitamin D screening. Br J Sports Med. 2018;52(8):522‐526. 10.1136/bjsports-2016-097130. [DOI] [PubMed] [Google Scholar]
- 8. Powe CE, Ricciardi C, Berg AH, et al. Vitamin D‐binding protein modifies the vitamin D‐bone mineral density relationship. J Bone Miner Res. 2011;26(7):1609‐1616. 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chhantyal K, He L, Mo J, et al. Free vitamin D correlate better with bone mineral density and thoracolumbar junction osteoporotic vertebral fractures than serum vitamin D. BMC Musculoskelet Disord. 2020;21(1):164. 10.1186/s12891-020-3179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bouillon R, Marcocci C, Carmeliet G, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev. 2019;40(4):1109‐1151. 10.1210/er.2018-00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bouillon R, Carmeliet G. Vitamin D insufficiency: definition, diagnosis and management. Best Pract Res Clin Endocrinol Metab. 2018;32(5):669‐684. 10.1016/j.beem.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 12. Institute of Medicine . Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press; 2011. 10.17226/13050. [DOI] [PubMed] [Google Scholar]
- 13. Aspray TJ, Bowring C, Fraser W, et al. National Osteoporosis Society Vitamin D Guideline Summary. Age and Ageing. 2014;43(5):592‐595. 10.1016/j.maturitas.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 14. Holick MF, Binkley NC, Bischoff‐Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911‐1930. 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 15. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359‐2381. 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. González‐Macías J, del Pino‐Montes J, Olmos J, Noguñes X. Clinical practice guidelines for postmenopausal, glucocorticoid‐induced and male osteoporosis. Spanish Society for Research on Bone and Mineral Metabolism (3rd updated version 2014). Rev Clínica Española. 2014;215(9):515‐526. 10.1016/j.rceng.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 17. Rizzoli R, Boonen S, Brandi ML, et al. Vitamin D supplementation in elderly or postmenopausal women: a 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Curr Med Res Opin. 2013;29(4):305‐313. 10.1185/03007995.2013.766162. [DOI] [PubMed] [Google Scholar]
- 18. Navarro‐Valverde C, Sosa‐Henríquez M, Alhambra‐Expósito MR, Quesada‐Gómez JM. Vitamin D3 and calcidiol are not equipotent. J Steroid Biochem Mol Biol. 2016;164:205‐208. 10.1016/j.jsbmb.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 19. Kanis JA, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3‐44. 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pludowski P, Holick MF, Grant WB, et al. Vitamin D supplementation guidelines. J Steroid Biochem Mol Biol. 2018;175:125‐135. 10.1016/j.jsbmb.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 21. Cianferotti L, Cricelli C, Kanis JA, et al. The clinical use of vitamin D metabolites and their potential developments: a position statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the International Osteoporosis Foundation (IOF). Endocrine. 2015;50(1):12‐26. 10.1007/s12020-015-0606-x. [DOI] [PubMed] [Google Scholar]
- 22. Heureux N, Lindhout E, Swinkels L. A direct assay for measuring free 25‐hydroxyvitamin D. J AOAC Int. 2017;100(5):1318‐1322. 10.5740/jaoacint.17-0084. [DOI] [PubMed] [Google Scholar]
- 23. Garnero P, Vergnaud P, Hoyle N. Evaluation of a fully automated serum assay for total N‐terminal propeptide of type I collagen in postmenopausal osteoporosis. Clin Chem. 2008;54(1):188‐196. 10.1373/clinchem.2007.094953. [DOI] [PubMed] [Google Scholar]
- 24. Schmidt‐Gayk H, Spanuth E, Kötting J, et al. Performance evaluation of automated assays for β‐CrossLaps, N‐MID‐Osteocalcin and intact parathyroid hormone (BRIOROSE Multicenter Study). Clin Chem Lab Med. 2004;42(1):90‐95. 10.1515/CCLM.2004.017. [DOI] [PubMed] [Google Scholar]
- 25. Montomoli M, Gonnelli S, Giacchi M, et al. Validation of a food frequency questionnaire for nutritional calcium intake assessment in Italian women. Eur J Clin Nutr. 2002;56(1):21‐30. 10.1038/sj.ejcn.1601278. [DOI] [PubMed] [Google Scholar]
- 26. Rossini M, Viapiana O, Gatti D. The long term correction of vitamin D deficiency: comparison between different treatments with vitamin D in clinical practice. Minerva Med. 2005;96:1‐7. Italian.15827537 [Google Scholar]
- 27. Bischoff‐Ferrari HA, Dawson‐Hughes B, Stöcklin E, et al. Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J Bone Miner Res. 2012;27(1):160‐169. 10.1002/jbmr.551. [DOI] [PubMed] [Google Scholar]
- 28. Meyer O, Dawson‐Hughes B, Sidelnikov E, et al. Calcifediol versus vitamin D3 effects on gait speed and trunk sway in young postmenopausal women: a double‐blind randomized controlled trial. Osteoporos Int. 2014;26(1):373‐381. 10.1007/s00198-014-2949-1. [DOI] [PubMed] [Google Scholar]
- 29. Shieh A, Ma C, Chun RF, et al. Effects of cholecalciferol vs calcifediol on total and free 25‐hydroxyvitamin D and parathyroid hormone. J Clin Endocrinol Metab. 2017;102(4):1133‐1140. 10.1210/jc.2016-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malmstroem S, Rejnmark L, Imboden JB, Shoback DM, Bikle DD. Current assays to determine free 25‐hydroxyvitamin D in serum. J AOAC Int. 2017;100(5):1323‐1327. 10.5740/jaoacint.17-0085. [DOI] [PubMed] [Google Scholar]
- 31. Cashman KD, Seamans KM, Lucey AJ, et al. Relative effectiveness of oral 25‐hydroxyvitamin D3 and vitamin D3 in raising wintertime serum 25‐hydroxyvitamin D in older adults. Am J Clin Nutr. 2012;95(6):1350‐1356. 10.3945/ajcn.111.031427. [DOI] [PubMed] [Google Scholar]
- 32. Ruggiero C, Baroni M, Bini V, et al. Effects of weekly supplementation of cholecalciferol and calcifediol among the oldest‐old people: findings from a randomized pragmatic clinical trial. Nutrients. 2019;11(11):2778. 10.3390/nu11112778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Minisola S, Cianferotti L, Biondi P, et al. Correction of vitamin D status by calcidiol: pharmacokinetic profile, safety, and biochemical effects on bone and mineral metabolism of daily and weekly dosage regimens. Osteoporos Int. 2017;28(11):3239‐3249. 10.1007/s00198-017-4180-3. [DOI] [PubMed] [Google Scholar]
- 34. Olmos JM, Arnaiz F, Hernández JL, Olmos‐Martínez JM, González MJ. Calcifediol mensual frente a calcifediol quincenal en el tratamiento de pacientes osteoporóticos. Estudio en la vida real. [Monthly versus biweekly calcifediol in the treatment of osteoporotic patients. Study in real life]. Rev Osteoporos Metab Miner. 2018;10(2):89‐94. [Google Scholar]
- 35. Quesada‐Gomez JM, Bouillon R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos Int. 2018;29(8):1697‐1711. 10.1007/s00198-018-4520-y. [DOI] [PubMed] [Google Scholar]
- 36. Brandi ML, Minisola S. Calcidiol [25(OH)D3]: from diagnostic marker to therapeutical agent. Curr Med Res Opin. 2013;29(11):1565‐1572. 10.1185/03007995.2013.838549. [DOI] [PubMed] [Google Scholar]
- 37. Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144(Part A):132‐137. 10.1016/j.jsbmb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ish‐Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab. 2008;93(9):3430‐3435. 10.1210/jc.2008-0241. [DOI] [PubMed] [Google Scholar]
- 39. Binkley N, Gemar D, Engelke J, et al. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab. 2011;96(4):981‐988. 10.1210/jc.2010-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heaney RP, Armas LAG, Shary JR, Bell NH, Binkley N, Hollis BW. 25‐Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008;87(6):1738‐1742. 10.1093/ajcn/87.6.1738. [DOI] [PubMed] [Google Scholar]
- 41. Sohl E, de Jongh RT, Heymans MW, van Schoor NM, Lips P. Thresholds for serum 25(OH)D concentrations with respect to different outcomes. J Clin Endocrinol Metab. 2015;100(6):2480‐2488. 10.1210/jc.2015-1353. [DOI] [PubMed] [Google Scholar]
- 42. Lee J, Tansey M, Jetton J, Krasowski M. Vitamin D toxicity: a 16‐year retrospective study at an academic medical center. Lab Med. 2018;49(2):123‐129. 10.1093/LABMED/LMX077. [DOI] [PubMed] [Google Scholar]
- 43. Díez‐Pérez A, Olmos JM, Nogués X, et al. Risk factors for prediction of inadequate response to antiresorptives. J Bone Miner Res. 2012;27(4):817‐824. 10.1002/jbmr.1496. [DOI] [PubMed] [Google Scholar]
- 44. Johansson H, Siggeirsdóttir K, Harvey NC, et al. Imminent risk of fracture after fracture. Osteoporos Int. 2017;28(3):775‐780. 10.1007/s00198-016-3868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Giampà E, Di Bonito M, Ferretti V, et al. Effects of alendronate and calcifediol compared to alendronate and cholecalciferol in osteoporotic patients. Minerva Endocrinol. 2019;44(4):344‐350. 10.23736/S0391-1977.19.03052-9. [DOI] [PubMed] [Google Scholar]
- 46. Michaud J, Naud J, Ouimet D, et al. Reduced hepatic synthesis of calcidiol in uremia. J Am Soc Nephrol. 2010;21(9):1488‐1497. 10.1681/ASN.2009080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Waldron JL, Ashby HL, Cornes MP, et al. Vitamin D: a negative acute phase reactant. J Clin Pathol. 2013;66(7):620‐622. 10.1136/jclinpath-2012-201301. [DOI] [PubMed] [Google Scholar]
- 48. Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID‐19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Quesada‐Gomez JM, Entrenas‐Castillo M, Bouillon R. Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS‐CoV‐2 infections. J Steroid Biochem Mol Biol. 2020;202:105719. 10.1016/j.jsbmb.2020.105719. [DOI] [PMC free article] [PubMed] [Google Scholar]


