Abstract
The protein phosphatase calcineurin is a critical mediator of calcium signals during T-cell activation. One substrate of calcineurin is the transcription factor NFATc1, which is retained in the cytoplasm of quiescent cells. NFATc1 activation requires the translocation of the transcription factor into the nucleus, a process that is mediated by calcineurin. This interaction with calcineurin requires a targeting domain (PxIxIT motif) located in the NH2-terminal region of NFATc1. Here we demonstrate that the calcineurin targeting domain of NFATc1 is phosphorylated and inactivated by the c-Jun NH2-terminal kinase (JNK). This disruption of calcineurin targeting inhibits the nuclear accumulation and transcription activity of NFATc1 and accounts for the observation that Jnk1−/− T cells exhibit greatly increased NFATc1-dependent nuclear responses.
The c-Jun NH2-terminal kinase (JNK) group of mitogen-activated protein kinases is activated by treatment of cells with cytokines or exposure to environmental stress (19). Gene disruption studies indicate that JNK protein kinases are required for multiple biological processes (15, 23, 32, 41, 42). However, the molecular mechanisms that account for the phenotypes displayed by JNK knockout mice remain unclear. For example, the disruption of the Jnk1 gene causes a severe defect in the response of CD4+ helper T (TH) cells to antigen-presenting cells (15). Wild-type naive TH cells can differentiate to TH1 or TH2 effector cells, which mediate inflammatory and humoral responses, respectively. In contrast, Jnk1−/− CD4 TH cells preferentially differentiate to TH2 effector cells and secrete large amounts of the TH2 cytokines, including interleukin-4 (IL-4). As a consequence, these mice are highly susceptible to infection with Leishmania. These effects of Jnk1 gene disruption were associated with increased nuclear accumulation of the NFATc1 transcription factor (15), which is known to act at the IL-4 promoter and is essential for TH2 responses (30, 44). The phenotype of Jnk1−/− mice suggests that NFATc1 is negatively regulated by JNK1 (15). However, this phenotype could be a direct or an indirect consequence of Jnk1 gene disruption.
Transcription factor NFAT was first identified as an important regulator of IL-2 gene expression (16, 20). More recently, it has been established that NFAT contributes to the expression of several cytokines and participates in multiple physiological processes (12, 31). In resting T cells, NFAT is restricted to the cytoplasm (12, 31). Following T-cell activation, a sustained increase in intracellular calcium (37) activates the phosphatase calcineurin (11, 21). The activated calcineurin dephosphorylates NFAT and leads to increased nuclear accumulation (12, 31). The nuclear NFAT transcription factor then increases the expression of target genes, including IL-2, IL-4, and Fas ligand.
The interaction of calcineurin with NFAT is a critical element in the signal transduction pathway that leads to increased NFAT-dependent gene expression. Interestingly, this interaction is mediated by a targeting domain (PxIxIT motif) that is present in the NH2-terminal region of the NFAT transcription factor (1, 2, 8). This targeting domain is required for efficient NFAT activation in vivo. Furthermore, ectopic expression of the targeting domain causes profound and specific inhibition of NFAT-mediated gene expression in cultured cells (2, 8). Studies of transgenic mice also demonstrate inhibition of NFAT-mediated gene expression caused by expression of the calcineurin targeting domain (8). Together, these data indicate that the calcineurin targeting domain is a critical component of the regulatory mechanism that controls NFAT activity.
The purpose of this study was to examine the role of JNK1 in NFATc1 regulation. Since the phenotype of Jnk1−/− mice suggests a correlation between the JNK1 signaling pathway and NFATc1 (15), we tested whether NFATc1 is directly regulated by JNK1. We report that JNK1 binds and phosphorylates NFATc1 on sites located near the PxIxIT calcineurin targeting domain. This phosphorylation inhibits the interaction of calcineurin with the targeting domain and blocks nuclear accumulation. This observation provides a molecular mechanism for the observation of increased NFATc1 nuclear accumulation in Jnk1−/− mice.
MATERIALS AND METHODS
Reagents.
The IL-4 luciferase reporter plasmid (36) and the expression vectors for calcineurin (28), NFATc1 (18), NFATc1-α (25), dominant negative NFAT (dnNFAT) (8), and JNK signaling pathway components (7, 40) have been described elsewhere. The NFATc1-β expression vector was constructed by cloning a PCR-derived cDNA in the BamHI site of pSRα. Recombinant NFAT proteins were expressed using the vector pGEX-5X1 (Amersham-Pharmacia Biotech) by cloning cDNA fragments in the BamHI and NotI sites. Deletion and point mutations were constructed by PCR and sequenced with an Applied Biosystems machine. Bacterially expressed CRM1 was purified by glutathione (GSH) affinity chromatography (43). The phosphospecific NFATc1 antibody prepared by immunization of rabbits with ovalbumin conjugated with glutaraldehyde to the synthetic phosphopeptide Ala-Pro-Ala-Leu-Glu-Ser(P)-Pro-Arg-Ile-Glu-Ile-Thr-Ser-Cys-Leu. The phosphospecific NFATc1 antibody was affinity purified from the rabbit serum using standard techniques (17).
Binding assays.
Cell extracts prepared using Triton-lysis buffer (20 mM Tris [pH 7.4], 137 mM NaCl, 2 mM EDTA, 1% Triton X-100, 25 mM β-glycerophosphate, 1 mM sodium vanadate, 2 mM sodium pyrophosphate, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml) were incubated (5 h at 4°C) with recombinant proteins (5 μg) prebound to 20 μl of GSH-Sepharose. After three washes with Triton-lysis buffer, the bound proteins were detected by protein immunoblot analysis.
Kinase assays.
Hemagglutinin epitope (HA)-tagged JNK1 was coexpressed together with and without MLK3 in COS cells. Cell extracts were prepared with Triton-lysis buffer 48 h after transfection. JNK1 immunocomplex kinase assays were performed with recombinant NFATc1 proteins (1 μg) as the substrate (7).
Immunofluorescence assays.
BHK cells were transfected using Lipofectamine (Life-Technologies Inc.) according to the manufacturer's protocol. Immunofluorescence analysis was performed using cells transfected with an NFATc1-α expression vector (1 μg) together with and without expression vectors for activated calcineurin (0.2 μg), MKK7 (0.2 μg) plus JNK1 (0.2 μg), dnJNK1 (0.5 μg), or JIP-1 (0.3 and 0.7 μg). NFATc1 proteins were detected using monoclonal antibody 7A6 (1:200; Affinity Bioreagents). The secondary antibody was Texas red-conjugated anti-mouse immunoglobulin antibody (1:100; Jackson ImmunoResearch). Nuclei were visualized using 4′,6-diamidino-2-phenylindole (Sigma).
RESULTS
JNK inhibits the nuclear accumulation of NFATc1-α.
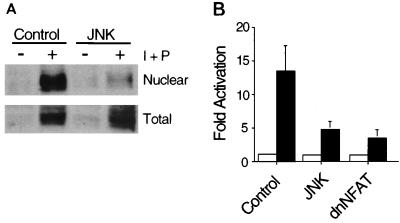
To test whether JNK1 regulates NFATc1, we examined the effect of activated JNK1 in transfection assays using cultured Jurkat T cells. Treatment with phorbol ester and ionomycin caused a transient increase in JNK protein kinase activity which returned to basal levels within 1 h (24, 35). At later times, a large increase in the expression of NFATc1 was detected (Fig. 1A). Cell fractionation studies demonstrated the presence of NFATc1 in the nucleus. Expression of activated JNK1 did not alter the expression of NFATc1 but did cause a marked reduction in the amount of nuclear NFATc1 (Fig. 1A). In contrast, expression of dnJNK1 increased the amount of nuclear NFATc1 caused by anti-CD3 stimulation (data not shown). Transfection assays were performed to examine the effect of JNK1 on transcription activity using an IL-4 promoter reporter gene. Activated JNK1, like dnNFAT, strongly inhibited reporter gene expression (Fig. 1B). Since constitutively nuclear mutant NFATc1 caused greatly increased reporter gene expression (data not shown), it is likely that the effect of activated JNK1 to inhibit reporter gene expression (Fig. 1B) was the consequence of the decreased amount of nuclear NFATc1 (Fig. 1A). Together, these data demonstrate that JNK1 functions as an inhibitor of the endogenous NFATc1 transcription factor expressed by Jurkat T cells.
FIG. 1.
JNK1 inhibits NFAT activity in T cells. (A) Activation of JNK1 inhibits NFATc1 nuclear localization. Jurkat T cells were transfected without (empty expression vector; Control) and with MKK7 plus JNK1 (JNK). After 16 h, the cells were stimulated without (−) and with (+) ionomycin (I; 2 μM) plus PMA (P; 100 nM) for 6 h. Nuclear extracts were isolated (14), and NFATc1 was examined by protein immunoblot analysis using monoclonal antibody 7A6 (Affinity Bioreagents). Similar data were obtained in three independent experiments. (B) Activated JNK inhibits NFAT transcription activity. Jurkat T cells were transfected with an IL-4 promoter reporter gene (firefly luciferase) plasmid together without (Control) and with MKK7 plus JNK1 (JNK) or dnNFAT. Transfection efficiency was monitored by measurement of Renilla luciferase activity. The cells were stimulated without (□) and with (■) ionomycin (2 μM) plus PMA (100 nM) for 6 h. The data are presented as fold activation by in treated compared to untreated cells (mean ± standard deviation; n = 4).
Several alternatively spliced variant forms of NFATc1 have been described (9, 10, 18, 25, 26, 33). The major isoform expressed by T cells has been identified as NFATc1-α (25, 33). We therefore performed further analysis of the regulation of NFATc1 by JNK using the NFATc1-α isoform.
JNK phosphorylates NFATc1-α.
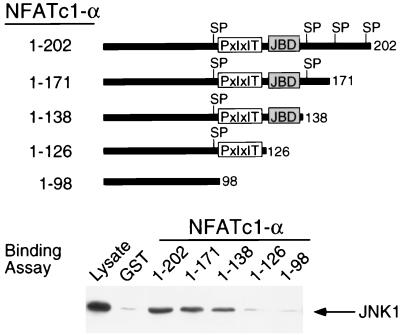
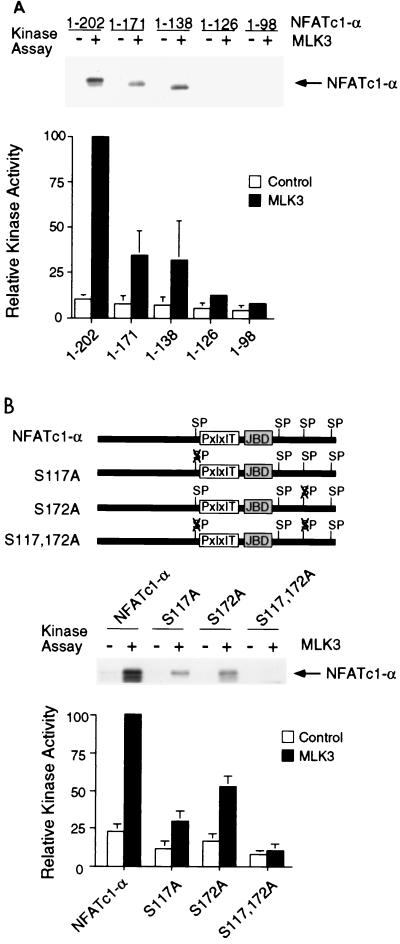
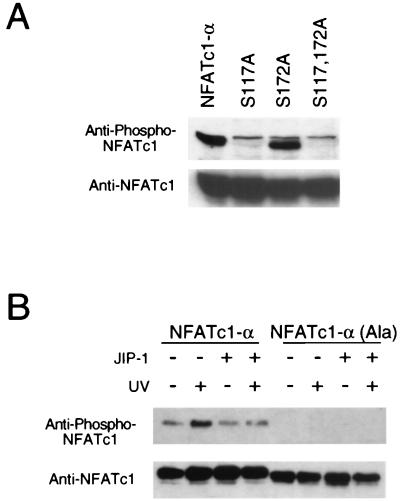
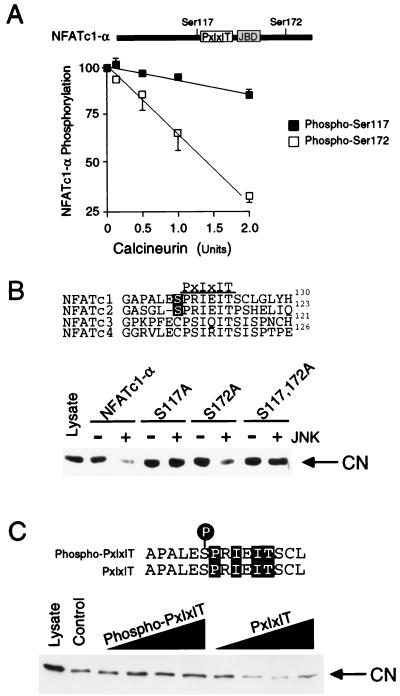
The JNK1 protein kinase binds and phosphorylates its substrates (19). Deletion analysis and in vitro binding assays indicated that NFATc1-α residues 126 to 138 are required for the binding of NFATc1-α to JNK1 (Fig. 2). In contrast, NFATc1-α phosphorylation by JNK1 was reduced by truncation at residue 171 and was eliminated by truncation at residues 126 (Fig. 3A). Phosphoamino acid analysis demonstrated the presence of phosphoserine (data not shown). Four potential JNK1 phosphorylation sites (Ser-Pro motifs) were identified. Mutational analysis indicated that both Ser117 and Ser172 were phosphorylated by JNK1. Replacement of either Ser117 or Ser172 with Ala reduced phosphorylation by JNK1, while the replacement of both Ser117 and Ser172 eliminated phosphorylation by JNK1 (Fig. 3B). Together, these data demonstrate that JNK1 binds NFATc1-α and phosphorylates both Ser117 and Ser172 in vitro. The JNK1 phosphorylation site Ser172, but not Ser117, is conserved in the related transcription factor NFATc3 (7). Comparative tryptic phosphopeptide mapping of NFATc1-α isolated from [32P]phosphate-labeled cells indicated that two phosphopeptides present in maps of wild-type NFATc1-α were absent in maps of mutated [Ala117 Ala172] NFATc1-α, suggesting that Ser117 and Ser172 may be phosphorylated in vivo (data not shown). To test this hypothesis, we prepared a phospho-NFATc1 antibody and performed immunoblot analysis of NFATc-α. The phospho-NFATc1 antibody specifically detected phosphorylation on Ser117 (Fig. 4A). Activation of endogenous JNK caused increased NFATc1-α phosphorylation (Fig. 4B). Overexpression of the scaffold protein JIP-1 causes profound inhibition of JNK (13, 39) and inhibited NFATc1-α phosphorylation (Fig. 4B). Together, these data suggest that NFATc1-α is a JNK substrate in vivo.
FIG. 2.
Interaction of JNK1 with NFATc1-α. Lysates were prepared from COS cells transfected with HA-JNK1, and the binding of HA-JNK1 to immobilized recombinant NFATc1-α was examined. The effect NFATc1-α deletions is shown. The location of the JNK binding domain (JBD) and PxIxIT motif on NFATc1-α are illustrated schematically. Potential JNK phosphorylation sites (SP) are indicated.
FIG. 3.
Phosphorylation of NFATc1-α by JNK1 in vitro. (A) JNK1 phosphorylates NFATc1-α. Immunocomplex kinase assays were performed using JNK1 activated without (Control; −) or with (+) MLK3 using recombinant NFATc1-α as the substrate. Phosphorylated NFATc1-α was detected by autoradiography and quantitated by PhosphorImager (Molecular Dynamics) analysis. (B) JNK phosphorylates NFATc1-α on Ser117 and Ser172. Wild-type and mutant NFATc1-α proteins (residues 1 to 202) were phosphorylated by JNK1 in vitro. The effects of JNK1 activation by MLK3 and the replacement of Ser117 and Ser172 with Ala were examined.
FIG. 4.
Phosphorylation of NFATc1-α in vivo. (A) NFATc1-α was coexpressed with MKK7 plus JNK1 in COS cells. The effect of replacement of Ser117 and Ser172 with Ala residues was investigated. Cell lysates were examined by protein immunoblot analysis by sequentially probing with anti-phospho-NFATc1-α and anti-NFATc1. A nonspecific band was detected by the phospho-NFATc1-α antibody immediately above the NFATc1-α band. (B) NFATc1-α was coexpressed without (−) and with (+) the JNK inhibitor JIP-1 in COS cells. The cells were treated without (−) and with (+) UV-C radiation (80 J/m2) and harvested after 30 min. The effect of replacement of the JNK phosphorylation sites (Ser117 and Ser172) with Ala was examined. The NFATc1-α proteins were detected by protein immunoblot analysis by sequentially probing with anti-phospho-NFATc1-α and anti-NFATc1.
Phosphorylation by JNK inhibits the nuclear accumulation of NFATc1-α.
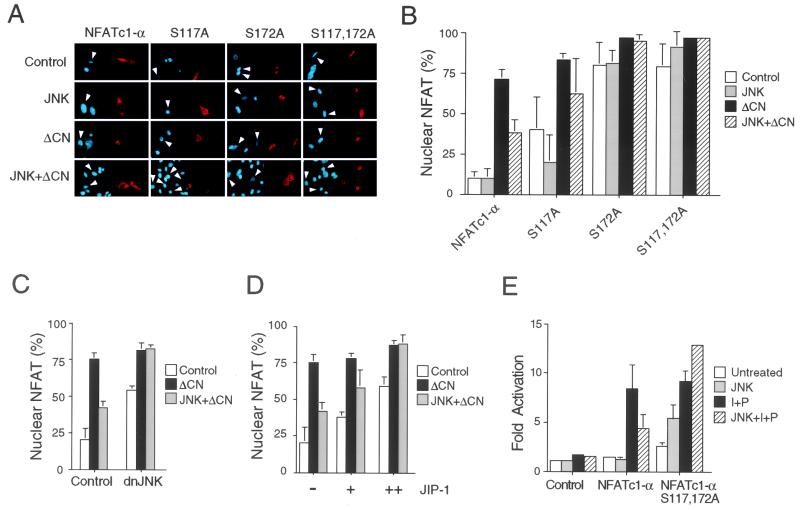
Studies of Jurkat T cells demonstrated that activated JNK1 inhibited the calcium-stimulated nuclear accumulation of NFATc1 (Fig. 1A). This observation was confirmed by immunofluorescence analysis of the subcellular distribution of NFATc1-α. Activated JNK1 inhibited the calcineurin-stimulated nuclear accumulation of NFATc1-α (Fig. 5A and B). This effect of activated JNK1 to inhibit NFATc1-α nuclear accumulation was blocked by overexpression of proteins that can inhibit JNK signaling, including dnJNK1 (Figure 5C) and the scaffold protein JIP-1 (Fig. 5D). Together, these data demonstrate that activated JNK1 regulates NFATc1-α nuclear accumulation.
FIG. 5.
JNK1 inhibits the nuclear accumulation of NFATc1-α. (A and B) Subcellular distribution of NFATc1-α, examined by immunofluorescence analysis in transfected BHK cells. The NFAT proteins (red; right) and nucleus (blue; left) were visualized (A). The effects of expression of a constitutively activated calcineurin (ΔCN) and MKK7 plus JNK1 (JNK) were examined. Arrowheads indicate the nuclei of cells expressing transfected proteins. The data were quantitated (B) following examination of 300 transfected cells and are presented as the percentage of cells with nuclear NFATc1-α (mean ± standard deviation [SD]; n = 3). (C) Effect of dnJNK on the subcellular distribution of NFATc1-α, examined by immunofluorescence analysis. The effects of expression of constitutively activated calcineurin (ΔCN) and MKK7 plus JNK1 (JNK) were examined. The percentage of cells with nuclear NFATc1-α is presented (mean ± SD; n = 3). (D) Effect of JIP-1 on the subcellular distribution of NFATc1-α, examined by immunofluorescence analysis. The percentage of cells with nuclear NFATc1-α is presented (mean ± SD; n = 3). The effects of increasing amounts of JIP-1 expression vector (0.3 and 0.7 μg) and the expression of constitutively activated calcineurin (ΔCN) and MKK7 plus JNK1 (JNK) were examined. (E) Mutational removal of Ser117 and Ser172 potentiates NFATc1-α transcription activity on the IL-4 promoter. Wild-type and [Ala117 Ala172] NFATc1-α were cotransfected with an IL-4 reporter plasmid in the absence (Untreated) and presence of MKK7 plus JNK1 (JNK). The cells were stimulated without (Untreated) and with PMA (P; 100 nM) plus ionomycin (I; 2 μM) for 16 h. The data are presented as fold activation compared to an untreated control (cells transfected without an NFATc1-α expression vector).
To test whether the phosphorylation of NFATc1-α is mechanistically relevant to the regulation of NFATc1-α nuclear accumulation by JNK1, we examined the effect of the replacement of the NFATc1-α phosphorylation sites with Ala residues. The subcellular distribution of the wild-type and mutated NFATc1-α proteins was examined by immunofluorescence analysis. Mutations at both phosphorylation sites (Ser117 and Ser172) altered the subcellular distribution of NFATc1-α. The mutated [Ala117 Ala172] NFATc1-α protein was found to be present in the nucleus under basal conditions and was not regulated by activated JNK1 (Fig. 5A and B). Mutation at either Ser117 or Ser172 was sufficient to increase the nuclear accumulation of NFATc1-α. To examine whether the altered nuclear accumulation of the phosphorylation-defective NFATc1-α proteins was relevant to transcription activity, we performed transfection assays of Jurkat T cells with an IL-4 promoter reporter plasmid (Fig. 5E). Expression of wild-type NFATc1-α using Jurkat T cells caused a large increase in phorbol myristate acetate (PMA)-ionomycin-stimulated IL-4 promoter reporter gene expression, which was inhibited by coexpression of activated JNK1. In contrast, the [Ala117 Ala172] NFATc1-α protein caused increased reporter gene expression in the absence of PMA-ionomycin stimulation and was not inhibited by activated JNK1 in the presence or absence of PMA-ionomycin (Fig. 5E). Together, these data demonstrate that the JNK1 phosphorylation sites (Ser117 and Ser172) are required for the regulation of both nuclear accumulation and transcription activity of NFATc1-α by JNK1.
JNK selectively regulates NFATc1 isoforms.
We have previously reported that recombinant NFATc1 is not regulated by JNK1 (7). This conclusion markedly differs from the results obtained from the analysis of endogenous NFATc1 expressed by Jurkat T cells (Fig. 1). Several alternatively spliced variant forms of NFATc1 have been described, including molecules with three distinct COOH-terminal domains (9, 10) and three distinct NH2-terminal domains (18, 25, 26, 33). The isoforms with distinct NH2-terminal domains correspond to NFATc1 (18), NFATc1-α (25), and NFATc1-β (26, 33). NFATc1-α is the major isoform expressed by T cells (25, 33).
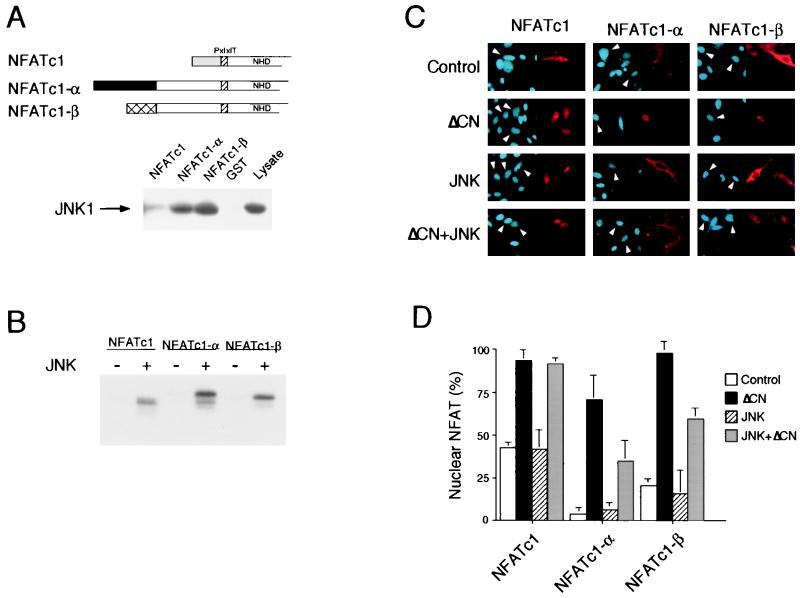
To test whether JNK may differentially interact with NFATc1 isoforms, we expressed the NH2-terminal region of NFATc1, NFATc1-α, and NFATc1-β in bacteria as glutathione S-transferase (GST) fusion proteins. The purified GST-NFAT proteins were immobilized on GSH-agarose. Control experiments demonstrated that JNK1 did not bind to immobilized GST (Fig. 6A). However, strong binding of JNK1 to NFATc1-α and NFATc1-β was detected. In contrast, NFATc1 bound poorly to JNK1. In vitro immune complex protein kinase assays using epitope-tagged JNK1 demonstrated that there was greater phosphorylation of NFATc1-α and NFATc1-β than of NFATc1 (Fig. 6B). These data suggest that JNK1 may be selectively targeted to NFATc1-α and NFATc1-β.
FIG. 6.
Differential regulation of NFATc1 isoforms by JNK. (A) The NH2-terminal regions of NFATc1 (18), NFATc1-α (25), and NFATc1-β (26, 33) are illustrated schematically. The PxIxIT motif (hatched box), the NFAT homology domain (NHD), and the alternative NFATc1 NH2 termini are indicated. Immobilized GST, GST-NFATc1 (residues 1 to 126), GST–NFATc1-α (residues 1 to 202), and GST–NFATc1-β (residues 1 to 189) was incubated with extracts prepared from COS cells transfected with HA-JNK1. The binding of HA-JNK1 was examined by immunoblot analysis. (B) Comparison of the phosphorylation of NFATc1 isoforms by JNK1 in vitro. Immunocomplex kinase assays were performed using Flag epitope-tagged JNK1 activated without (−) and with (+) MLK3, using recombinant NFATc1 isoforms as the substrate. (C and D) Subcellular distribution of NFATc1 isoforms, examined by immunofluorescence analysis in transfected BHK cells. The NFAT proteins (red; right) and nucleus (blue; left) were visualized (C). The effect of constitutively activated calcineurin (ΔCN) or MKK7 plus JNK1 (JNK) was examined. Arrowheads indicate the nuclei of cells expressing transfected proteins. The data were quantitated (D) following examination of 300 transfected cells and are presented as the percentage of cells with nuclear NFATc1 (mean ± standard deviation; n = 3).
We compared the subcellular distribution of NFATc1, NFATc1-α, and NFATc1-β by immunofluorescence analysis (Fig. 6C). In control cells, NFATc1-α and NFATc1-β were located predominantly in the cytoplasm, but a large fraction of NFATc1 was found in the nucleus. Expression of activated JNK1 caused no significant change in the subcellular distribution of these NFATc1 isoforms. In contrast, activated calcineurin caused increased nuclear localization of all three NFATc1 isoforms. Activated JNK1 suppressed the calcineurin-stimulated nuclear accumulation of NFATc1-α and NFATc1-β but not the NFATc1 isoform.
Taken together, these data indicate that JNK1 negatively regulates the NFATc1-α and NFATc1-β isoforms but not NFATc1. It is likely that the markedly decreased binding of JNK1 to NFATc1 (Fig. 6A) compared to the other NFATc1 isoforms contributes to this differential regulation. As NFATc1-α is the major isoform expressed by T cells (25, 33), these data indicate that JNK1 may function as an inhibitor in T cells. The phenotype of Jnk1−/− mice is consistent with the hypothesis (15).
Phosphorylation by JNK inhibits targeting of NFATc1-α by the phosphatase calcineurin.
JNK1 phosphorylates NFATc1-α on Ser117 and Ser172 (Fig. 3). Ser117 of NFATc1-α is located adjacent to the previously identified calcineurin targeting domain (PxIxIT motif) (1, 2, 8). We hypothesized that phosphorylation on Ser117 may regulate the targeting of NFATc1-α to calcineurin. However, the proximity of Ser117 to the calcineurin targeting domain suggests that Ser117 may represent a potential substrate for dephosphorylation by calcineurin. We therefore examined the kinetics of calcineurin-mediated dephosphorylation of JNK1-phosphorylated NFATc1-α. This analysis demonstrated that Ser172 (and not Ser117) was preferentially dephosphorylated by calcineurin in vitro (Fig. 7A). Thus, the PxIxIT targeting motif that binds calcineurin facilitates the dephosphorylation of distal (e.g., Ser172) but not proximal (e.g., Ser117) NFAT phosphorylation sites. These data support the hypothesis that Ser117 may regulate the targeting function of the NFATc1-α PxIxIT motif. To test this hypothesis, we examined the effect of NFATc1-α phosphorylation by JNK1 on the interaction of NFATc1-α with calcineurin. Phosphorylation caused a marked reduction in the binding of calcineurin to NFATc1-α (Fig. 7B). Mutational analysis demonstrated that the phosphorylation of Ser117, but not Ser172, was required for the regulation of calcineurin binding by JNK1. To confirm that phosphorylation on Ser117 regulates calcineurin binding, we performed competition assays using a synthetic PxIxIT peptide with either Ser117 or phospho-Ser117 (Fig. 7C). Binding assays confirmed that the Ser117 PxIxIT peptide disrupted calcineurin binding in a dose-dependent manner. In contrast, calcineurin binding to NFATc1-α was not inhibited by the phospho-Ser117 PxIxIT synthetic peptide. Together, these data demonstrate that Ser117 phosphorylation inhibits the function of the calcineurin targeting domain of NFATc1-α.
FIG. 7.
JNK1 inhibits the binding of calcineurin to NFATc1-α. (A) Comparison of calcineurin-stimulated dephosphorylion of Ser117 and Ser172. Recombinant NFATc1-α (residues 1 to 202) was phosphorylated by JNK1 in vitro and incubated (30 min at 30°C) in 50 mM HEPES (pH 7.4)–2 mM MnCl2–0.5 mM EDTA–15 mM 2-mercaptoethanol–0.1 mg of bovine serum albumin per ml together with calmodulin (250 U) and indicated amounts of calcineurin (Sigma). The phosphorylated NFATc1-α was detected after sodium dodecyl sulfate-polyacrylamide gel electrophoresis by autoradiography and was quantitated by PhosphorImager (Molecular Dynamics) analysis. Dephosphorylation at Ser117 and Ser172 was examined using [Ala172] and [Ala117] NFATc1-α, respectively. (B) Phosphorylation of NFATc1-α by JNK1 inhibits the binding of NFATc1-α to the phosphatase calcineurin. Recombinant NFATc1-α (residues 1 to 202) was phosphorylated by JNK1 in vitro. The NFATc1-α proteins were immobilized on GSH-Sepharose, incubated with cell extracts, and washed; bound calcineurin was detected by protein immunoblot analysis. The effect of replacement of the NFATc1-α phosphorylation sites (Ser117 and Ser172) with Ala was examined. (C) Phosphorylation of Ser117 inhibits calcineurin binding to NFATc1-α. Immobilized recombinant NFATc1-α (residues 1 to 202) was incubated with cell extracts, and the binding of calcineurin was examined by protein immunoblot analysis. Competition analysis was performed by investigating the effects of various concentrations (0, 0.7, 1.4, 7, and 14 μM) of a synthetic peptide corresponding to the PxIxIT motif which mediates the targeting of calcineurin to NFATc1-α. The effect of the phosphorylation of the synthetic peptide on Ser117 was examined.
CRM1 interacts with dephosphorylated NFATc1-α.
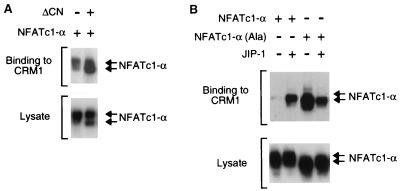
The export of NFAT from the nucleus is mediated, in part, by a mechanism that involves the GTPase Ran and the exportin CRM1 (22). One CRM1 binding site on NFATc3 is located adjacent to the calcineurin targeting domain (45). Indeed, binding of NFATc3 to calcineurin competes with the binding to CRM1 (45). Since NFATc1-α phosphorylation inhibits the binding of calcineurin (Fig. 7), we examined whether phosphorylation affected the interaction of NFATc1-α with CRM1. Binding assays were performed using immobilized GST-CRM1 and lysates prepared from COS cells expressing NFATc1-α (Fig. 8). We found that NFATc1-α bound to CRM1. The binding to CRM1 was increased when the NFATc1-α was coexpressed with activated calcineurin (Fig. 8A). This observation suggests that calcineurin-mediated dephosphorylation of NFATc1-α increases binding to CRM1.
FIG. 8.
NFATc1-α binding to CRM1 is regulated by phosphorylation. (A) Activated calcineurin increases the binding of NFATc1-α to CRM1. NFATc1-α was expressed without (−) or with (+) activated calcineurin (ΔCN) in COS cells. Recombinant CRM1 was immobilized on GSH-Sepharose, incubated with the COS cell extracts, and washed; bound NFATc1-α was detected by protein immunoblot analysis. (B) JNK phosphorylation inhibits the binding of NFATc1-α to CRM1. The effect of replacement of the JNK phosphorylation sites (Ser117 and Ser172) on the binding of NFATc1-α to CRM1 was examined. The effect of JNK inhibition was investigated by overexpression of the scaffold protein JIP-1.
To test whether phosphorylation of NFATc1-α on the JNK sites altered CRM1 binding, we examined the effect of replacement of Ser117 and Ser172 with Ala residues. A marked increase in NFATc1-α binding to CRM1 was detected for the mutated, phosphorylation-defective NFATc1-α protein (Fig. 8B). These data suggested that phosphorylation on Ser117 and Ser172 may inhibit binding to CRM1. To test this hypothesis, we examined the effect of inhibiting JNK signaling on the binding of NFATc1-α to CRM1. Overexpression of the scaffold protein JIP-1 causes a profound inhibition of JNK signaling (13, 39). JIP-1 increased the binding of CRM1 to wild-type [Ser117 and Ser172] NFATc1-α but not to the mutated phosphorylation-defective [Ala117 and Ala172] NFATc1-α. These data indicate that phosphorylation regulates the interaction between CRM1 and NFATc1-α.
DISCUSSION
In this study we demonstrate that the JNK signaling pathway plays a key role as a negative regulator of substrate targeting by the phosphatase calcineurin. The phosphorylation of NFATc1 by JNK provides a mechanism whereby NFATc1-driven responses can be terminated, which can set a threshold for a given response. This is likely to be important for normal cellular physiology. Indeed, we have demonstrated that Jnk1−/− mice displayed enhanced TH2 responses due to increased nuclear localization of NFATc1 (15). Conversely, activation of the JNK1 signaling pathway inhibits IL-4 gene promoter activity (Fig. 1). Thus, JNK1 functions in TH2 cells to suppress the expression of cytokines that enhance TH2 responses (e.g., IL-4) which would otherwise lead to the generation of an unbalanced TH cell immune response to pathogens. Together, the genetic evidence and biochemical analysis that we present strongly support the hypothesis that JNK is a physiologically relevant regulator of NFATc1 function.
Recent studies have established that the formation of protein complexes is a critical aspect of signal transduction mechanisms that ensures both efficiency and specificity in vivo (27, 40). An example of the formation of signaling complexes is the requirement of targeting domains for the interaction of protein kinases and protein phosphatases with their substrates. We demonstrate that such interactions are subjected to regulation by phosphorylation. Our results indicate that phosphorylation of NFATc1 by JNK inhibits targeting of the phosphatase calcineurin. Since calcineurin functions to induce the nuclear accumulation of NFAT, phosphorylation by JNK inhibits NFATc1 activation. Inhibition of phosphatase targeting is a novel mechanism of regulation by a mitogen activated protein kinase. However, this regulatory mechanism may also be applicable to other signaling systems that are regulated by phosphorylation.
Our study further establishes the importance of the PxIxIT motif in the regulation of calcineurin targeting to NFAT. Previous studies demonstrated that the PxIxIT motif was required for efficient activation of NFAT by calcineurin (1) and that ectopic expression of the PxIxIT motif inhibits NFAT-mediated gene expression in both cultured cells (2, 8) and transgenic mice (8). Here we demonstrate that the function of the PxIxIT targeting motif can be regulated by phosphorylation. Our data suggest that regulated substrate targeting represents a potential approach for the design of novel therapeutic agents.
Coordination of NFAT binding to calcineurin and CRM1.
It is established that both the phosphatase calcineurin and the exportin CRM1 bind to NFAT (22, 45). Studies of NFATc3 indicate that one of the binding sites for CRM1 is located adjacent to the calcineurin binding site (45). Binding to calcineurin is calcium dependent and competes with the binding to CRM1. The binding affinity of NFATc3 for calcineurin/Ca2+ is higher than that for CRM1 (45). These observations have led to the conclusion that calcineurin acts, in part, to induce nuclear accumulation of NFATc3 by suppressing the CRM1-dependent export pathway (45). It is likely that other NFAT proteins are regulated by similar mechanisms.
Our studies of NFATc1-α indicate that phosphorylation by JNK causes inhibition of calcineurin binding (Fig. 7). This inhibited interaction with calcineurin may account for the effect of activated JNK to prevent calcium-mediated nuclear accumulation of NFATc1-α (Fig. 5). Since calcineurin and CRM1 compete for binding to NFAT, we considered that the effect of JNK to decrease calcineurin binding might lead to increased CRM1 binding. This potential mechanism suggests that increased CRM1-mediated export of NFATc1-α from the nucleus may contribute to the effect of JNK signaling to inhibit nuclear accumulation of NFATc1-α. However, measurement of CRM1 binding demonstrated that JNK phosphorylation inhibited the binding of both CRM1 and calcineurin (Fig. 7 and 8). These data suggest that the primary action of JNK to prevent the nuclear accumulation of NFATc1-α is inhibition of calcineurin binding.
Regulation of NFAT subcellular distribution by phosphorylation.
The NFAT transcription factors are phosphoproteins that are located in the cytosol of resting cells. Upon dephosphorylation by the phosphatase calcineurin, NFAT accumulates in the nucleus (12, 31). Conversely, phosphorylation of NFAT isoforms opposes nuclear accumulation. NFAT phosphorylation has been shown to be mediated by many different protein kinases (3, 5–7, 29, 34, 46). The relative importance and physiological significance of each of the protein kinases remains to be established.
The role of NFAT phosphorylation is unclear, but it is likely that different sites of phosphorylation will regulate different biological functions. Here we report that the phosphorylation of NFATc1-α by JNK causes inhibition of calcineurin targeting to the PxIxIT motif (Fig. 7). Previous studies indicate that phosphorylation on the Ser-rich region facilitates intramolecular interaction with the COOH-terminal nuclear localization sequence (NLS2) of NFATc1 (4). Phosphorylation-dependent intramolecular interaction within the NFAT homology region has also been proposed to regulate NFATc3 subcellular distribution (46). Furthermore, NFAT phosphorylation facilitates intermolecular interaction with 14-3-3 adapter proteins (6). Binding to 14-3-3 masks the NH2-terminal NLS1 and may regulate NFAT subcellular distribution. These data indicate that phosphorylation regulates multiple NFAT intermolecular and intramolecular interactions that contribute to the shuttling of NFAT isoforms between the nuclear and cytoplasmic compartments of the cell. Further studies of NFAT phosphorylation are required before we can obtain a complete understanding of the mechanism of regulation of NFAT nuclear accumulation.
Role of JNK-regulated NFATc1-α activity.
NFATc1-α and NFATc2 represent the major NFAT isoforms expressed by T cells. NFATc1-α (Fig. 1), but not NFATc2 (7), is negatively regulated by JNK. This observation raises an important question concerning the physiological function of JNK as an NFAT inhibitor. Studies of primary naive peripheral T cells indicate that NFATc1-α is expressed at very low levels, but the expression of NFATc1-α is induced following T-cell activation (20, 33). In contrast, NFATc2 is expressed at high levels both before and after T-cell activation (20). This pattern of expression suggests that JNK is not a significant regulator of NFAT activity in naive T cells or in T cells at early times following activation. However, a role for JNK as an inhibitor of NFAT activity at late times following T-cell activation is implicated.
The possible role for JNK as an NFAT inhibitor at late times following T-cell activation is consistent with the results of a recent report that examined JNK expression by T cells (38). JNK1 and JNK2 were found to be expressed at very low levels in naive peripheral CD4+ T cells. Similarly, the JNK activators MKK4 and MKK7 were found to be expressed at low levels. Activation mediated by the T-cell receptor plus the CD28 coreceptor caused a marked induction of mRNA and protein expression of JNK1, JNK2, MKK4, and MKK7 that peaked at approximately 24 h following T-cell activation. The peak of expression correlated with increased JNK activity in the activated T cells. Together, these data indicate that the JNK signaling pathway in T cells is induced during T-cell activation. This temporal pattern of expression of the JNK signaling pathway components is similar to that of NFATc1-α. The extremely low level of expression of NFATc1-α and JNK pathway components in naive peripheral CD4+ T cells strongly argues against a role for JNK during the early phase of T-cell activation.
The considerations outlined above indicate that the function of JNK to inhibit NFATc1-α most likely occurs at late times following T-cell activation (e.g., 24 h). We propose that JNK functions to provide a tonic inhibitory signal that opposes NFATc1-α activation and expression of downstream target genes (e.g., IL-4). JNK may therefore provide a threshold for the maintenance of T-cell responses by requiring higher levels of stimulatory signals. This mechanism is consistent with the observation that Jnk1−/− CD4+ T cells are hyperresponsive to stimulatory signals, including enhanced production of IL-4 (15). This role of JNK in setting a threshold for T-cell activation may contribute to tolerance and the initiation of an immune response.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
We thank G. R. Crabtree, J. S. Gutkind, T. Hoey, S. Kornbluth, K. M. Murphy, and T. Soderling for providing reagents; C. Turck for peptide synthesis; T. Barrett, A. Quail, and J. Stein for technical assistance; and K. Gemme for administrative assistance.
C.-W. Chow is an Arthritis Foundation fellow. This work was supported in part by grants CA65861 and CA72009 and by the Howard Hughes Medical Institute.
REFERENCES
- 1.Aramburu J, Garcia-Cozar F, Raghavan A, Okamura H, Rao A, Hogan P G. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol Cell. 1998;1:627–637. doi: 10.1016/s1097-2765(00)80063-5. [DOI] [PubMed] [Google Scholar]
- 2.Aramburu J, Yaffe M B, Lopez-Rodriguez C, Cantley L C, Hogan P G, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 3.Avots A, Buttmann M, Chuvpilo S, Escher C, Smola U, Bannister A J, Rapp U R, Kouzarides T, Serfling E. CBP/p300 integrates Raf/Rac-signaling pathways in the transcriptional induction of NF-ATc during T cell activation. Immunity. 1999;10:515–524. doi: 10.1016/s1074-7613(00)80051-5. [DOI] [PubMed] [Google Scholar]
- 4.Beals C R, Clipstone N A, Ho S N, Crabtree G R. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev. 1997;11:824–834. doi: 10.1101/gad.11.7.824. [DOI] [PubMed] [Google Scholar]
- 5.Beals C R, Sheridan C M, Turck C W, Gardner P, Crabtree G R. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1934. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- 6.Chow C-W, Davis R J. Integration of calcium and cyclic AMP signaling pathways by 14-3-3. Mol Cell Biol. 2000;20:702–712. doi: 10.1128/mcb.20.2.702-712.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow C W, Rincon M, Cavanagh J, Dickens M, Davis R J. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science. 1997;278:1638–1641. doi: 10.1126/science.278.5343.1638. [DOI] [PubMed] [Google Scholar]
- 8.Chow C W, Rincon M, Davis R J. Requirement for transcription factor NFAT in interleukin-2 expression. Mol Cell Biol. 1999;19:2300–2307. doi: 10.1128/mcb.19.3.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuvpilo S, Avots A, Berberich-Siebelt F, Glockner J, Fischer C, Kerstan A, Escher C, Inashkina I, Hlubek F, Jankevics E, Brabletz T, Serfling E. Multiple NF-ATc isoforms with individual transcriptional properties are synthesized in T lymphocytes. J Immunol. 1999;162:7294–7301. [PubMed] [Google Scholar]
- 10.Chuvpilo S, Zimmer M, Kerstan A, Glockner J, Avots A, Escher C, Fischer C, Inashkina I, Jankevics E, Berberich-Siebelt F, Schmitt E, Serfling E. Alternative polyadenylation events contribute to the induction of NF-ATc in effector T cells. Immunity. 1999;10:261–269. doi: 10.1016/s1074-7613(00)80026-6. [DOI] [PubMed] [Google Scholar]
- 11.Clipstone N A, Crabtree G R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 12.Crabtree G R. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 13.Dickens M, Rogers J S, Cavanagh J, Raitano A, Xia Z, Halpern J R, Greenberg M E, Sawyers C L, Davis R J. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277:693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- 14.Dignam J D, Martin P L, Shastry B S, Roeder R G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- 15.Dong C, Yang D D, Wysk M, Whitmarsh A J, Davis R J, Flavell R A. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–2095. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- 16.Durand D B, Shaw J P, Bush M R, Replogle R E, Belagaje R, Crabtree G R. Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol Cell Biol. 1988;8:1715–1724. doi: 10.1128/mcb.8.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1988. [Google Scholar]
- 18.Hoey T, Sun Y L, Williamson K, Xu X. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity. 1995;2:461–472. doi: 10.1016/1074-7613(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 19.Ip Y T, Davis R J. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 20.Jain J, Loh C, Rao A. Transcriptional regulation of the IL-2 gene. Curr Opin Immunol. 1995;7:333–342. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 21.Jain J, McCaffrey P G, Miner Z, Kerppola T K, Lambert J N, Verdine G L, Curran T, Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993;365:352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- 22.Kehlenbach R H, Dickmanns A, Gerace L. Nucleocytoplasmic shuttling factors including Ran and CRM1 mediate nuclear export of NFAT in vitro. J Cell Biol. 1998;141:863–874. doi: 10.1083/jcb.141.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuan C Y, Yang D D, Samanta Roy D R, Davis R J, Rakic P, Flavell R A. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 24.Li B, Tournier C, Davis R J, Flavell R A. Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. EMBO J. 1999;18:420–432. doi: 10.1093/emboj/18.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Northrop J P, Ho S N, Chen L, Thomas D J, Timmerman L A, Nolan G P, Admon A, Crabtree G R. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature. 1994;369:497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- 26.Park J, Takeuchi A, Sharma S. Characterization of a new isoform of the NFAT (nuclear factor of activated T cells) gene family member NFATc. J Biol Chem. 1996;271:20914–20921. doi: 10.1074/jbc.271.34.20914. [DOI] [PubMed] [Google Scholar]
- 27.Pawson T, Scott J D. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 28.Perrino B A, Fong Y L, Brickey D A, Saitoh Y, Ushio Y, Fukunaga K, Miyamoto E, Soderling T R. Characterization of the phosphatase activity of a baculovirus-expressed calcineurin A isoform. J Biol Chem. 1992;267:15965–15969. [PubMed] [Google Scholar]
- 29.Porter C M, Havens M A, Clipstone N A. Identification of amino acid residues and protein kinases involved in the regulation of NFATc subcellular localization. J Biol Chem. 2000;275:3543–3551. doi: 10.1074/jbc.275.5.3543. [DOI] [PubMed] [Google Scholar]
- 30.Ranger A M, Hodge M R, Gravallese E M, Oukka M, Davidson L, Alt F W, de la Brousse F C, Hoey T, Grusby M, Glimcher L H. Delayed lymphoid repopulation with defects in IL-4-driven responses produced by inactivation of NF-ATc. Immunity. 1998;8:125–134. doi: 10.1016/s1074-7613(00)80465-3. [DOI] [PubMed] [Google Scholar]
- 31.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 32.Sabapathy K, Hu Y, Kallunki T, Schreiber M, David J P, Jochum W, Wagner E F, Karin M. JNK2 is required for efficient T-cell activation and apoptosis but not for normal lymphocyte development. Curr Biol. 1999;9:116–125. doi: 10.1016/s0960-9822(99)80065-7. [DOI] [PubMed] [Google Scholar]
- 33.Sherman M A, Powell D R, Weiss D L, Brown M A. NF-ATc isoforms are differentially expressed and regulated in murine T and mast cells. J Immunol. 1999;162:2820–2828. [PubMed] [Google Scholar]
- 34.Shibasaki F, Price E R, Milan D, McKeon F. Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NF-AT4. Nature. 1996;382:370–373. doi: 10.1038/382370a0. [DOI] [PubMed] [Google Scholar]
- 35.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 36.Szabo S J, Gold J S, Murphy T L, Murphy K M. Identification of cis-acting regulatory elements controlling interleukin-4 gene expression in T cells: roles for NF-Y and NF-ATc. Mol Cell Biol. 1993;13:4793–4805. doi: 10.1128/mcb.13.8.4793. . (Erratum, 13:5928.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Timmerman L A, Clipstone N A, Ho S N, Northrop J P, Crabtree G R. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature. 1996;383:837–840. doi: 10.1038/383837a0. [DOI] [PubMed] [Google Scholar]
- 38.Weiss L, Whitmarsh A J, Yang D D, Rincon M, Davis R J, Flavell R A. Signal transduction by activated MAP kinase regulated by selective expression in differentiated cells. J Exp Med. 2000;191:139–146. doi: 10.1084/jem.191.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitmarsh A J, Cavanagh J, Tournier C, Yasuda J, Davis R J. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science. 1998;281:1671–1674. doi: 10.1126/science.281.5383.1671. [DOI] [PubMed] [Google Scholar]
- 40.Whitmarsh A J, Davis R J. Structural organization of MAP-kinase signaling modules by scaffold proteins in yeast and mammals. Trends Biochem Sci. 1998;23:481–485. doi: 10.1016/s0968-0004(98)01309-7. [DOI] [PubMed] [Google Scholar]
- 41.Yang D D, Conze D, Whitmarsh A J, Barrett T, Davis R J, Rincon M, Flavell R A. Differentiation of CD4+ T cells to Th1 cells requires MAP kinase JNK2. Immunity. 1998;9:575–585. doi: 10.1016/s1074-7613(00)80640-8. [DOI] [PubMed] [Google Scholar]
- 42.Yang D D, Kuan C Y, Whitmarsh A J, Rincon M, Zheng T S, Davis R J, Rakic P, Flavell R A. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 43.Yang J, Bardes E S, Moore J D, Brennan J, Powers M A, Kornbluth S. Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1. Genes Dev. 1998;12:2131–2143. doi: 10.1101/gad.12.14.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida H, Nishina H, Takimoto H, Marengere L E, Wakeham A C, Bouchard D, Kong Y Y, Ohteki T, Shahinian A, Bachmann M, Ohashi P S, Penninger J M, Crabtree G R, Mak T W. The transcription factor NF-ATc1 regulates lymphocyte proliferation and Th2 cytokine production. Immunity. 1998;8:115–124. doi: 10.1016/s1074-7613(00)80464-1. [DOI] [PubMed] [Google Scholar]
- 45.Zhu J, McKeon F. NF-AT activation requires suppression of Crm1-dependent export by calcineurin. Nature. 1999;398:256–260. doi: 10.1038/18473. [DOI] [PubMed] [Google Scholar]
- 46.Zhu J, Shibasaki F, Price R, Guillemot J C, Yano T, Dotsch V, Wagner G, Ferrara P, McKeon F. Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase I and MEKK1. Cell. 1998;93:851–861. doi: 10.1016/s0092-8674(00)81445-2. [DOI] [PubMed] [Google Scholar]