Abstract
Psoriasis is a chronic inflammatory skin disease without cure. Systemic and biological therapies are the most effective treatments for patients with severe psoriasis. However, these drugs can cause serious side effects from extended use. Safe and effective topical drugs are needed to decrease psoriatic plaques and reduce the risk of adverse effects. Amygdalin analogues are stable small molecules that showed benefits in psoriasis xenografts to immune‐deficient mice by systemic application. However, whether topical application of these amygdalin analogues could reduce the progression of the psoriatic phenotype in an immune‐competent organism is unknown. Here, we analyse the efficiency of topical application of an amygdalin analogue cream on a well‐established genetic and immune‐competent mouse model of psoriasis. Topical application of an amygdalin analogue cream ameliorates psoriasis‐like disease in mice, reduces epidermal hyperplasia and skin inflammation. Amygdalin analogue treatment leads to reduced expression of local pro‐inflammatory cytokines, but systemic pro‐inflammatory cytokines that are highly expressed in psoriasis patients such as IL‐17A, IL6 or G‐CSF are also decreased. Furthermore, expression of important mediators of psoriasis initiation and epidermal hyperplasia, such as TNFa, S100A9 and TSLP, is decreased in lesional epidermis after amygdalin analogue treatment. In conclusion, we show that amygdalin analogue reduces the proliferative capacity of psoriasis‐like stimulated keratinocytes and their inflammatory response in vivo and in vitro. These results suggest that topical application of amygdalin analogues may represent a safe and effective treatment for psoriasis.
Keywords: amygdalin analogues, antimicrobial peptides, GEMMs, hyperplasia, inflammation, preclinical mouse models, pro‐inflammatory cytokines, psoriasis, topical application, thymic stromal lymphopoietin
1. INTRODUCTION
Psoriasis is one of the most common chronic inflammatory skin diseases affecting ~3% of the world population, with a significant variability between populations and countries. 1 The vast majority of patients develop a chronic “plaque‐type” psoriasis referred to as psoriasis vulgaris, and approximately one‐third of patients with psoriasis develop psoriatic arthritis (PsA). 2 Histologically, there is a considerable thickening of the epidermis due to increased proliferation of keratinocytes with a dense dermal infiltrate of immune cells, such as T cells and dendritic cells, as well as neutrophil recruitment in the epidermis. 3 Keratinocytes, the main non‐immune cells in the skin, recruit and activate immune cells in the inflamed skin by secreting soluble mediators, such as TNFα (tumor‐necrosis factor) or IL‐23. 4 , 5 One of the cytokines that is rapidly induced in keratinocytes under stress is Thymic stromal lymphopoietin (TSLP), 6 , 7 and high expression levels of TSLP are found in psoriatic patients. 8 Recently, we have shown that TSLP promotes proliferation and inflammatory response not only in keratinocytes, but also in epidermal stem cells during early stages of psoriasis promoting psoriasis progression. 9 These keratinocyte mediators act on immune cells producing cytokines in the inflamed skin and induce the activation and proliferation of keratinocytes, which in psoriasis generates a complex inflammatory loop.
Despite its considerable effect on quality of life, psoriasis is underdiagnosed and undertreated. 10 , 11 Based on the extent of involvement and affection on the quality of life, the treatment of psoriasis is individualized with either topical therapy with or without phototherapy for mild‐to‐moderate cases, 12 to systemic treatments involving small molecules or biologics for moderate‐to‐severe cases. 13 Clinical studies of selective biologics that target the immune system have demonstrated that blockade of cytokines such as TNFα, IL‐23 and IL‐17A results in almost complete disease recovery, 13 although extended use can cause serious side effects. The majority of patients with psoriasis have limited disease that can be managed with topical therapies alone. Thus, topical therapy plays a very important role in the therapeutic armamentarium of psoriasis. 14 The strategy of combining topical therapy with systemic and phototherapy results in additional and immediate symptom relief, reduction in the dose of systemic medications and better psychological comfort to patients. The various topical therapeutic agents used in psoriasis include emollients, keratolytic agents such as salicylic acid, coal tar, dithranol, corticosteroids, vitamin D analogues, retinoids and immunomodulators such as tacrolimus. 15 Despite their proven efficacy and safety, there is a continuous need for more effective and better tolerated agents with different mechanisms of action for the treatment of recalcitrant lesions and for improved patient adherence.
Amygdalin (D‐mandelonitrile‐β‐D‐gentiobioside), also known as vitamin B17, is the major component of the seeds of rosaceous stone fruits. The pharmacological profile of amygdalin comprises anti‐inflammatory, 16 anti‐nociceptive 17 and neurotrophic effects. 18 Amygdalin exhibits certain level of toxicity (LD10 > 500 mg/kg in rats) associated with the release of hydrogen cyanide in vivo. 19 , 20 To reduce its toxicity diverse, structural modifications were introduced, including the removal or substitution of the cyanide moiety, producing a series of amygdalin analogues. 21 These amygdalin analogues are small molecule compounds designed as peptidomimetics of peptide T, 21 , 22 a HIV entry inhibitor discovered in 1986. 23 A remarkable improvement in the histopathological score and epidermal thickness was seen in more than 50% of psoriatic patients treated systematically with peptide T in a limited clinical study. 24 While peptide T is an unstable small molecule with a low pharmacokinetic profile, amygdalin analogues exhibit a better pharmacokinetic profile. 25 Preliminary studies of systemic administration of amygdalin analogues in a xenograft transplantation model of psoriasis skin onto immunodeficient mice showed a significantly reduction of several psoriatic indicators, such as the semi‐quantitative clinical psoriasis score, epidermal thickness, parakeratosis or Munro's abscesses score. 25 In addition, these amygdalin analogues inhibited IFN‐γ signalling in human epidermal keratinocytes in vitro. 26 , 27 Further investigations are required in immune‐competent mouse models of psoriasis to understand how the epidermal compartment but also the immune system respond to amygdalin analogue treatment. In order to reduce any systemic side effects, the amygdalin analogue was prepared in cream formulation for topical application. Previous studies on the parent compound demonstrated that amygdalin exhibits a good skin penetration profile, making it suitable for topical administration. 28
In the present work, we sought to evaluate the therapeutic efficacy of the amygdalin analogue FIB‐116 and its mechanism of action as novel topical treatment in psoriasis. Different mouse models have been generated to mimic various aspects of the disease. 29 We have previously generated an inducible double knock‐out mouse model by epidermal deletion of c‐Jun and JunB using the K5 promoter (referred as DKO*). 30 Mutant mice develop a psoriasis‐like disease within 2 weeks after induction, exhibiting several psoriatic hallmarks including hyper‐ and parakeratosis, inflammatory infiltrate, elevated levels of cytokines/chemokines, increased subepidermal vascularization, and co‐morbidities like bone loss and arthritic joints. 30 Using this well‐established mouse model for psoriasis, we demonstrate that topical treatment with FIB‐116 ameliorated most of psoriatic hallmarks in these DKO* mice. Importantly, we show for the first time that FIB‐116 amygdalin analogue cream penetrated the epidermis and reduced the proliferation and inflammatory response of inflamed keratinocytes, suggesting that topical treatment using this amygdalin analogue could be a safe and efficient therapeutic agent to treat mild‐moderate psoriatic plaques and as adjuvant for severe psoriatic patients.
2. MATERIAL AND METHODS
2.1. Cream preparation
Cream formulation requires mixing of an oily phase, an aqueous phase and the active compound phase. The oily phase contains the following ingredients: PEG‐8 beeswax (10%), light liquid paraffin (6%), white mineral oils and wax and paraffin (10%), cetyl alcohol (4%), hydrogenated palm/palm kernel oil PEG‐6 Esters (3%), lauroyl PEG‐32 glycerides (3%) and glyceril capryl caprate (6%). The aqueous phase consists of a solution of disodium EDTA (1%). Finally, the active compound phase consists of a solution of the active compound (3%), dissolved in a mixture of benzyl alcohol (1%) and diethylene glycol monoethyl ether (5%). The preparation is completed with purified water.
Preparation of the cream is carried out by adding the oily phase into the aqueous phase to form an emulsion to which the active compound phase is subsequently added. Specifically, the components of the oily phase are first mixed gently at 75–80ºC until complete melting to get a homogeneous mixture. The blend is added into the aqueous phase that is also heated at 75–80ºC, being the mixture subjected to high stirring conditions for 15 min. Once the emulsion is formed, it is cooled to room temperature (RT) at low shear mixing. When the emulsion reaches ~30ºC, the active compound phase is added and subsequently subject to a high shear mixing for 5 min. Finally, the mixture is cooled to RT under slow shear mixing. The vehicle cream formulation is carried out in the same way, but without adding the active ingredient into that phase.
2.2. Skin inflammation model and Treatment
All mouse experiments were performed in accordance with local and institutional regulations/licences. The generation of the DKO* psoriasis‐like mouse model has previously been described. 30 Briefly, mice carrying the floxed JunB allele (JunBf / f ) and floxed c‐Jun allele (c‐Junf / f ) were crossed with transgenic mice expressing the Cre recombinase‐oestrogen receptor fusion under the control of the basal keratinocyte specific K5 promoter (K5‐Cre‐ERT) to obtain JunBf / f c‐Junf / f K5‐Cre‐ERT mice. 8‐week‐old mutant mice were injected daily (intraperitoneal), four times with 2 mg tamoxifen (Sigma) to induce the deletion of floxed JunB and c‐Jun alleles in DKO* mice. Mice showing the first signs of the psoriasis‐like phenotype in the ears (6–8 days after the last tamoxifen injection) were topically treated in a blinded experiment with amygdalin analogue or vehicle cream (3 μg active compound = 100 μl of cream per ear) every day during 16 days. This amount was selected based on a previous assay performed in a xenograft transplantation model of psoriasis, in which 300 μg/kg of amygdalin analogue was systemically administrated (Perez et al. 2013) without toxic effects. In this study, we applied the same total amount of 300 μg/kg by topical application. The psoriasis‐like severity in DKO* mice was evaluated two weeks later and was photographed and scored semiquantitatively according to the following clinical signs: inflammation, areas of skin affected and psoriatic‐like plaque size (large or small). The parameters were scored using a 5‐point scale: 0 = Lack of cutaneous inflammation and psoriatic‐like plaques; 1 = inflammation on feet; 2 = inflammation of feet and ears; 3 = inflammation on feet and small psoriatic‐like plaques on the ears; 4 = inflammation on feet and large psoriatic‐like plaques on feet and ears; 5 = inflammation and large psoriatic‐like plaques on feet, ears, tail and back skin. Skin samples from the ears and blood serum were harvested for further analyses.
2.3. Histology and Immunofluorescence
Serial sections from mouse ears embedded in formalin‐fixed paraffin were processed for H&E staining and for immunofluorescence. Paraffin sections were processed for their deparaffinization and antigen retrieval. Primary antibodies specific for Ki67 (Master Diagnostica, clone SP6, dilution 1:5), Keratin 5 (Biolegend, ref # 905901, dilution 1:1000), Involucrin (Covance, ref # PRB‐14℃, dilution 1:1000), S100A9 (Santa Cruz, ref # SC8115, dilution 1:100), Ly6b (BioRad, ref # MCA771GA, dilution 1:200) and S. aureus (Abcam, ref #ab20920, dilution 1:200) were incubated overnight (O/N) at 4ºC. Sections were then washed three times with PBS and incubated with secondary antibodies conjugated to the appropriate fluorophores (Life Technologies) for 1 h at RT. Sections were then washed three times with PBS and mounted with gel mount to be analysed by inverted fluorescence microscope (Nikon) or confocal microscope (TCS‐SP5, Leica Microsystems).
2.4. RNA isolation and qRT‐PCRs
A piece of ear from DKO* mice in the different conditions or primary keratinocytes were collected in Trizol‐LS (Thermofisher Scientific) for further RNA isolation as described manufacturer´s instructions. RNA extraction was followed by reverse transcription using Promega kit (A2801). qPCR analyses were carried out using SYBR Green master mix kit (Qiagen, 204143). Expression levels were compared to housekeeping gene RPL4. The primer sequences are shown in Table S1.
2.5. ELISA Immunoassay
Blood sera from DKO*, DKO*‐vehicle and DKO*‐Amygdalin‐treated mice were harvested to quantify soluble cytokines according to the manufacturer's instructions (Mouse IL‐17A Quantikine ELISA Kit, Ref # M1700, mouse IL6 Quantikine ELISA Kit, Ref # M6000B, mouse G‐CSF Quantikine ELISA Kit, Ref # MCS00, R&D Systems).
2.6. Primary keratinocyte cultures and treatments
To obtain fresh dissociated epidermal cells from ear and tail skin of WT mice, the epidermis was separated from the dermis by trypsin digestion (0.75%) for 1 h at 37ºC. After mechanical dissociation, the cell suspension was filtered through a 70 μm cell strainer and centrifuged 5 min at 450 g. Pellet was re‐suspended in culture medium for further in vitro studies.
To recombinant TSLP and amygdalin analogue treatment, fresh isolated epidermal cells were cultured in keratinocyte growth medium (KGM, Invitrogen) in a 24‐well culture plate treated with coating matrix kit (Cascade Biologicals) in a concentration of 25.000 cells/cm. 2 Next day, mouse recombinant TSLP (eBioscence, ref # 14–8498–80) was added at 20 μg/μl during 72 h. Amygdalin analogue (10 nM) was added in the correspondent wells one day later than mouse recombinant TSLP. 5‐Ethynyl‐2’‐deoxyuridine (EdU) was added to the cultures in the end of the experiment in a concentration of 10 μM during 4 h at 37ºC. EdU staining was performed, and random confocal images were captures. EdU+keratinocytes were quantified by Image J. Three independent experiments were performed for statistical analysis.
2.7. Statistical analyses
For studies including animals, n is the number of total animals tested, and for in vitro studies, the number of independent experimental replicates is indicated in figure legends, with n representing number of repeats with different sets of cells. Statistical analyses were performed using Prism5 (GraphPad) software. When comparing two groups, a two‐tailed Student´s t test was used, and to compare three or more groups, a one‐ or two‐way ANOVA and Bonferroni post‐test were implemented. p values were calculated with a confidence interval of 95% to indicate the statistical significance between groups. Statistically significant differences between groups are noted in figures with asterisks.
3. RESULTS
3.1. Topical application of the amygdalin analogue FIB‐116 cream ameliorates psoriasis‐like disease in DKO* mice
To determine whether topical application of amygdalin analogue FIB‐116 (structure in Figure S1A) cream is effective for the clearance of psoriatic plaques on the skin of a well‐established immune‐competent psoriasis‐like mouse model, 30 we treated the ears of DKO* mice with FIB‐116 or vehicle cream in a blinded experiment (see Methodology). The psoriasis‐like phenotype in DKO* mice was induced by genetic deletion of c‐Jun and JunB floxed alleles in K5+ basal keratinocytes after tamoxifen administration. When mutant mice showed the first symptoms of skin inflammation and psoriatic‐like phenotype, 6–7 days after the last tamoxifen injection, we applied FIB‐116 or vehicle cream in both ears every day during 15 days (Figure 1A). Two weeks later, psoriatic‐like mice were visually classified according to their psoriasis‐like severity score (Figure S1B). Amygdalin analogue‐treated DKO* mice showed a significant reduction in the psoriasis‐like severity (6 of 9 animals with score 2, 2 animals with score 1 and one animal with score 3), whereas untreated DKO* or vehicle‐treated DKO* mice were prone to develop a moderate‐severe psoriasis‐like phenotype including psoriasis‐like arthritis in paws (7 of 9 mice with score 3–5; Figure 1B). Macroscopic analyses showed a clear reduction of skin inflammation and swelling after two weeks of amygdalin cream treatment in comparison with untreated DKO* and vehicle‐treated DKO* mice, whereas vehicle cream exacerbates psoriasis‐like features in these DKO* mice (Figure S1C). Macroscopic psoriasis‐like arthritis symptoms in paws were also decreased after amygdalin analogue treatment. Haematoxylin‐and‐eosin‐stained skin sections show the typical histopathological signs of the psoriasis‐like phenotype in untreated DKO* and vehicle‐treated DKO* mice, such as acanthosis (thickened epidermis), hyperkeratosis (thickening of the stratum corneum), parakeratosis (retention of nuclei in the stratum corneum), microabscess formation and immune cell infiltrates (Figure 1C). Topical application of amygdalin analogue cream led to a clear reduction in the psoriasis‐like histological features. Measurement of total ear and epidermis thickness confirms a reduction of almost 50% in amygdalin analogue‐treated DKO* mice in comparison with untreated DKO* and vehicle‐treated mice (Figure 1D,E). Overall, these results indicate that topical application of amygdalin analogue cream reduces the psoriasis‐like phenotype in DKO* mice.
FIGURE 1.
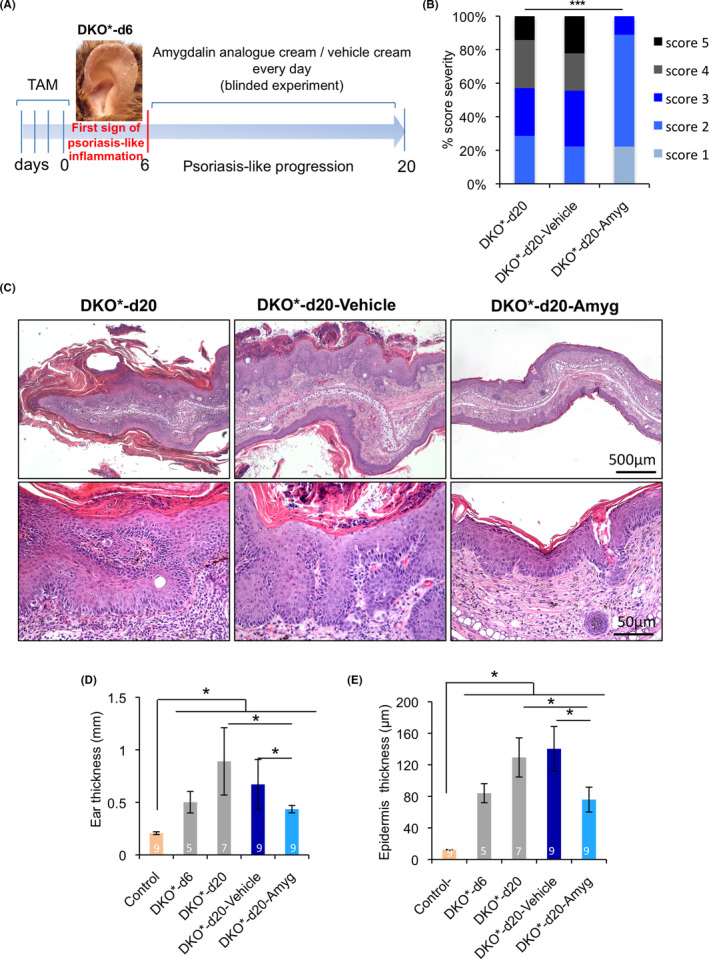
Topical application of amygdalin analogue cream ameliorates psoriasis‐like severity in DKO* mice. (A) Experimental timeline to induce psoriasis‐like disease in 8‐week‐old mice with four consecutive doses of tamoxifen injections (2 mg/dose). Six days later after psoriasis‐like induction, mice were visually evaluated and only mice with an initial psoriasis‐like severity score = 2 were used. One hundred microlitre of amygdalin analogue and vehicle creams was blindly applied in both ears every day during 14 days in randomly chosen DKO* mice. Wild‐type (WT) and DKO* mice without cream were used as control groups. n > 7 per group. (B) DKO* mice classified according to the psoriasis‐like severity score (1 minimum–5 maximum) after treatment with amygdalin analogue or vehicle cream at day 20 after disease induction. DKO* without cream as control without treatment. n = 7 for DKO* mice and n = 9 for each vehicle and amygdalin‐treated mice. Statistical significance ***p < 0.0001. p value was determined by χ 2 statistics. (C) Representative images of haematoxylin and eosin (H&E) staining of ear sections from DKO* without treatment, DKO* treated with amygdalin cream and DKO* mice treated with vehicle cream at day 20 after psoriasis‐like induction. (D, E) Ear and epidermis thickness measurement in control (WT), DKO* before treatment at day 6, DKO* without treatment at day 20 and DKO* mice treated with amygdalin analogue and vehicle creams at day 20. n > 5 per group. Statistical significance *p < 0.05 (t‐student two‐tailed test relative to controls and vehicle group)
3.2. FIB‐116 treatment decreases epidermal cell proliferation and restores epidermal architecture in DKO* mice
To determine how topical amygdalin analogue treatment reduces psoriasis‐like disease progression, we first analysed the epidermal architecture and the proliferation rate of epidermal keratinocytes in amygdalin analogue and vehicle‐treated DKO* mice, untreated DKO* mice and control littermate mice after 15 days of treatment (Figure 2). The cytokeratin 5 (K5) is normally expressed in the basal epidermal layer, whereas involucrin is expressed in the cornified layers. During psoriatic hyper‐proliferation of keratinocytes, K5 is widely expressed in suprabasal layers, whereas involucrin expression is disrupted in the stratum corneum and untypically over‐expressed in the lower suprabasal layers (Figure 2A). These features were also observed in vehicle‐treated DKO* mice, whereas mice treated with amygdalin analogue cream normalized the K5 expression and the overexpression of involucrin was limited to the cornified layers. We next quantified the number of Ki67+ proliferative keratinocytes (Figure 2B) and found decreased proliferative activity in epidermal keratinocytes after amygdalin analogue cream treatment on ear skin. Therefore, amygdalin analogue cream treatment decreases epidermal hyperplasia in the DKO* mouse model by reducing keratinocyte proliferation.
FIGURE 2.
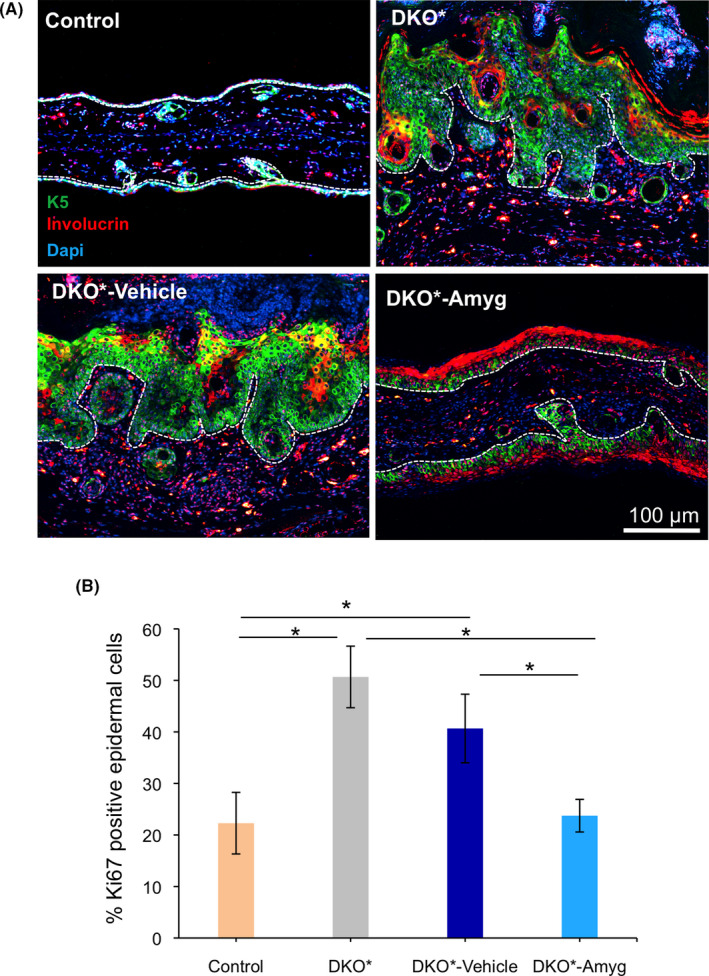
Hyperplasia reduction and epidermal architecture recovery after FIB‐116 application on ear skin of DKO* mice. (A) Representative immunofluorescence images of ear section from control (WT), DKO* without treatment, DKO*‐Amygdalin, DKO*‐Vehicle mice after 14 days of treatment. Green (Keratin 5), Red (Involucrin) and blue (Dapi). Aberrant expression of the basal layer protein K5 in suprabasal epidermal layers in DKO* and DKO*‐vehicle mice, whereas animals treated with amygdalin cream rescue the expression of K5 mainly in the basal layer. Involucrin, a corneal layer protein, increases in animals treated with amygdalin cream while its expression is disrupted in DKO* and DKO‐vehicle mice. n = 3 per group. Dotted lines separate epidermis and dermis. (B) Quantification analysis of proliferative marker Ki67 in ear skin of control (WT), DKO* without treatment, DKO*‐Amygdalin, DKO*‐Vehicle mice after 14 days of treatment. n = 3 per group. Statistical significance *p < 0.05 (t‐student two‐tailed test relative to controls and vehicle group)
3.3. Reduced local and systemic pro‐inflammatory cytokine expression after FIB‐116 application in DKO* mice
Hyperproliferative keratinocytes in psoriasis are driven by a feedforward loop of cytokines secreted by activated resident immune cells, T cells, dendritic cells and cells of the innate immune system, as well as the keratinocytes themselves. A large number of inflammatory cytokines were shown to be elevated in lesional psoriatic skin, and serum concentrations of a subset of these cytokines also correlate with psoriasis disease severity. We have evaluated in psoriatic skin and serum of DKO* mice the expression of relevant pro‐inflammatory cytokines in psoriasis such as Il17a, Il1α, Il1β, Il6, Infγ, Tnfα and G‐CSF (Figure 3A), and the pro‐inflammatory cytokine Il22 and the anti‐inflammatory cytokine Il10 from the IL‐10 family member (Figure S2). Untreated DKO* mice showed increased expression of all pro‐inflammatory cytokines and reduction of the anti‐inflammatory cytokine Il10 in lesional skin, whereas treated amygdalin analogue cream DKO* mice reduced the expression of Il17a, Il1α, Infγ, Tnfα and G‐CSF (Figure 3A). However, the expression of Il1β, Il6 and Il22 was not significantly reduced in comparison with untreated DKO* mice and Il10 was not increased to basal levels as in control mice (Figures 3A and S2). Interestingly, vehicle‐treated mice had significantly increased expression of all pro‐inflammatory cytokines, in particular Il17a, Il1β, Infγ, Il22 and Tnfα, suggesting that vehicle cream exacerbated some pathological features of psoriasis‐like diseases in DKO* mice. We also measured the amount of IL17‐A, IL6 and G‐CFS in the serum of DKO* mice, and all were reduced in mice treated with amygdalin analogue cream (Figure 3B‐D). In conclusion, topical application of amygdalin analogue reduced the production of Th1/Th17 pro‐inflammatory cytokines, the therapeutic targets in psoriasis.
FIGURE 3.
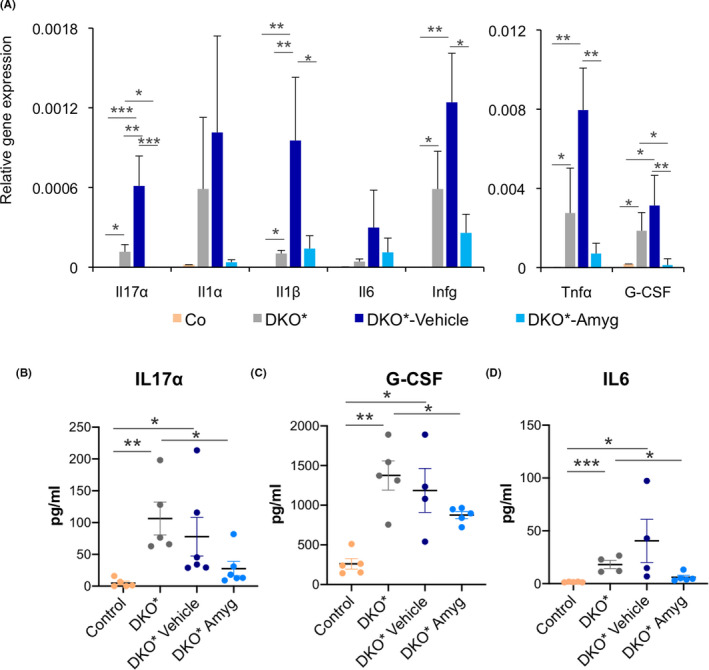
Amygdalin cream reduces local and systemic pro‐inflammatory cytokine expression, whereas vehicle exacerbates it. (A) Gene expression of the interleukins Il17α, Il1α, Il1β, Il6 and Ifnγ and the cytokines Tnfα and G‐CSF of ear samples from control (WT), DKO* without treatment, DKO*‐Amygdalin, DKO*‐Vehicle mice after 14 days of treatment. n > 5 per group. Statistical significance *p < 0.05, **p < 0.01, ***p < 0.001 (ANOVA, one‐tailed test). (B‐D) Quantification of IL17α, IL6 and G‐CSF production in sera of control (WT), DKO* without treatment, DKO*‐Amygdalin, DKO*‐Vehicle mice after 14 days of treatment. n > 4 per group. Statistical significance *p < 0.05 (t‐student two‐tailed test relative to controls and vehicle group).
3.4. FIB‐116 application reduces neutrophil recruitment and expression of cytokine‐based antimicrobial peptides in DKO* mice
A significant reduction of microabscess formation in DKO* mice treated with amygdalin analogue cream was observed (Figure 1D‐F). Thus, we next analysed the main cellular source of microabscesses in the stratum corneum of psoriatic patients, the neutrophils. We analysed gene expression of specific chemokine‐mediated neutrophil trafficking in lesional skin of DKO* mice, such as Ccl2, Cxcl1 and Cxcl2 (Figure 4A). These chemokines were over‐expressed in untreated and vehicle‐treated DKO* mice; in contrast, the expression of Cxcl1 and Cxcl2 was significantly down‐regulated in mice treated with amygdalin analogue cream. We also analysed the expression of cytokine‐based antimicrobial peptides, highly expressed in epithelial cells and neutrophils in DKO* mice. Interestingly, Lcn2, S100a9 and Tslp were significantly down‐regulated in amygdalin analogue‐treated DKO* mice in comparison with untreated and vehicle‐treated mice, in which these antimicrobial peptides were highly expressed (Figure 4A). Immunofluorescence of affected ear skin for S100A9 and Ly6B (neutrophils) confirmed the co‐expression of S100A9 and Ly6B in neutrophils infiltrated in the dermis and epidermis of affected skin, including in microabscesses of untreated and vehicle‐treated DKO* mice (Figure 4B). In addition, epidermal cells upregulated S100A9 in untreated and vehicle‐treated DKO* mice as previously reported. 31 Interestingly, DKO* mice treated with amygdalin analogue cream displayed significantly reduced number of neutrophils infiltrating the dermis and epidermis (Figure 4C) and the expression of S100A9 was significantly down‐regulated in the epidermis of the treated mice (Figure 4B). However, big microabcesses of neutrophils expressing S100A9 were observed in vehicle‐treated DKO* mice, characteristic of microbial infections. Therefore, we analysed whether vehicle cream exacerbates psoriasis‐like severity by a typical S. aureus colonization similar to that in AD (Figure 4D). Immunofluorescence analyses showed positive staining for S. aureus in vehicle‐treated DKO* mice, whereas in untreated DKO* mice S. aureus colonization was absent. This result suggests that vehicle cream may be harmful for psoriasis leading to bacterial infections and an increase of the inflammatory response. Our data indicate that amygdalin analogue cream reduced neutrophil recruitment and cytokine‐based antimicrobial peptide expression, such as TSLP and S100A9, important epidermal secreted cytokines in the pathology of psoriasis.
FIGURE 4.
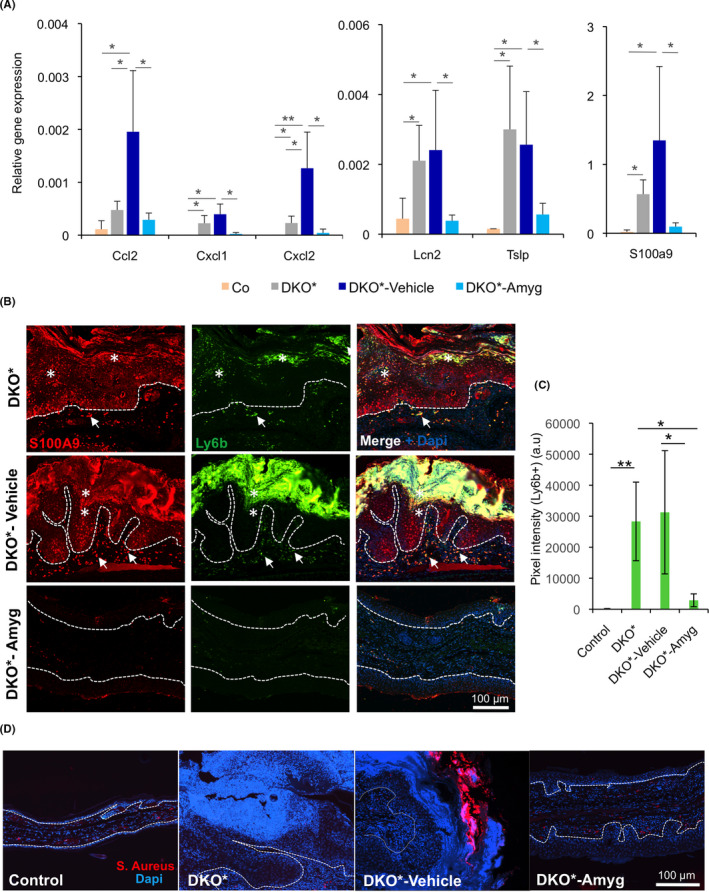
FIB‐116 cream reduces neutrophil recruitment in skin and reduces antimicrobial peptides towards baseline expression in DKO* treated mice. (A) Gene expression of neutrophil recruitment chemokines Ccl2, Cxcl1 and Cxcl2 and antimicrobial peptides Lcn2, S100a9 and Tslp of the ear samples from control (WT), DKO* without treatment, DKO*‐Amygdalin, DKO*‐Vehicle mice after 14 days of treatment. n > 5 per group. Statistical significance *p < 0.05, **p < 0.01, ***p < 0.001 (ANOVA, one‐tailed test). (B and C) Representative immunofluorescence images of ear section from DKO* without treatment, DKO*‐Amygdalin, DKO*‐Vehicle mice after 14 days of treatment. Green (Ly6b), Red (S100A9) and blue (Dapi). Epidermal overexpression of S100A9 in DKO* and DKO*‐vehicle mice, whereas animals treated with amygdalin cream reduce its expression. Neutrophil infiltration (Ly6b+ S100A9+) increases in the dermis (arrows) and epidermis (asterisks) of animals DKO* and DKO*‐vehicle mice, whereas the number of neutrophils in mice treated with amygdalin cream reduces dramatically (C, a.u. = arbitrary unit). n = 3 per group. Dotted lines separate epidermis and dermis. (D) Representative immunofluorescence images for Staphylococcus aureus of ear section from control (WT), DKO* without treatment, DKO*‐Amygdalin, DKO*‐Vehicle mice after 14 days of treatment. Red (S. Aureus) and blue (Dapi). n = 3 per group. Dotted lines separate epidermis and dermis
3.5. FIB‐116 treatment neutralizes pro‐inflammatory cytokine expression and hyper‐proliferation of thymic stromal lymphopoietin (TSLP)‐stimulated keratinocytes
TSLP mediates the proliferation and inflammatory response of keratinocytes and epidermal stem cells in DKO* psoriasis‐like mice. 9 To test whether FIB‐116 can neutralize the pro‐inflammatory effect of TSLP in keratinocytes in vitro, recombinant TSLP (20 μg/ml) was added to primary wild‐type keratinocytes during 3 days (Figure 5). Non‐toxic concentration of amygdalin analogue FIB‐116 (10 nM) (Figure S3A) was added 24 h later to recombinant TSLP induction during the next 48 h (Figure 5A), and keratinocyte proliferation was analysed by EdU assay. Gene expression analysis confirmed increased expression of TSLP in keratinocytes after recombinant TSLP treatment and its reduction after amygdalin treatment (Figure S3B). We observed a significant reduction in keratinocyte proliferation after amygdalin analogue treatment, while recombinant TSLP increased the proliferation rate as expected (Figure 5B‐C). This high proliferation rate was confirmed by the expression of the proliferation promoting cyclin CYCA2, which was increased in TSLP‐induced keratinocytes and reduced after FIB‐116 treatment (Figure 5D).
FIGURE 5.
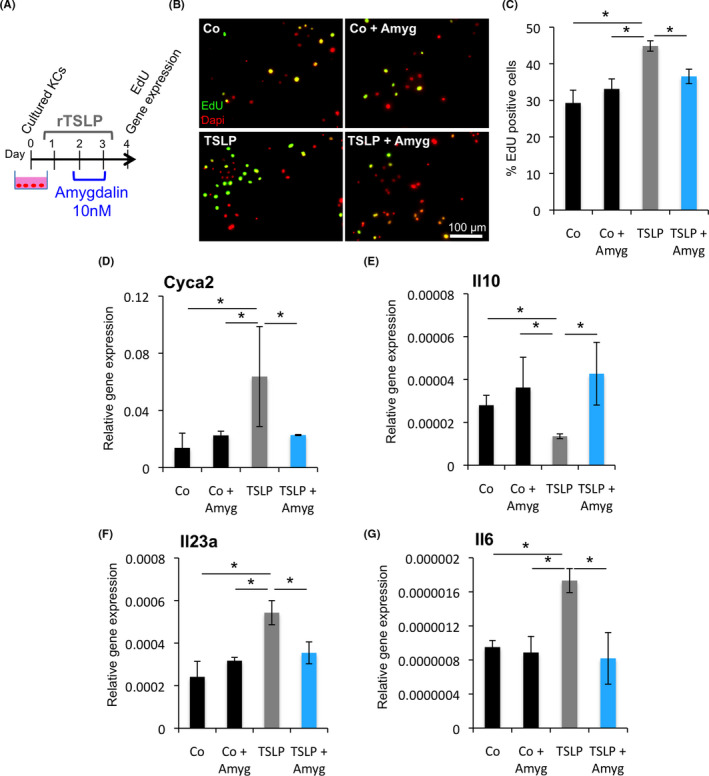
FIB‐116 inhibits the proliferative effect and pro‐inflammatory response of TSLP in cultured keratinocytes. (A) Experimental design for induction of primary murine keratinocytes with recombinant TSLP and treatment with amygdalin analogue in vitro. (B) Representative immunofluorescence images for EdU (green) of primary murine keratinocytes in the different treatments. Nuclei (Dapi) in red. (C) Percentage of EdU+keratinocytes after treating with recombinant TSLP and Amygdalin analogue. Statistical significance *p < 0.05 (t‐student two‐tailed test). (D‐G) Gene expression of Cyca2, Il10, Il23a and Il6 in TSLP‐induced keratinocytes treated with amygdalin analogue. Statistical significance *p < 0.05 (t‐student two‐tailed test)
We also analysed gene expression profiles for anti‐inflammatory and pro‐inflammatory cytokines altered in keratinocytes in psoriasis (Figure 5 and Figure S3). A significant reduction in the expression of the anti‐inflammatory cytokine Il10 was observed in keratinocytes treated with recombinant TSLP, while its expression was normalized after FIB‐116 treatment (Figure 5E). Pro‐inflammatory cytokines, such as Il23a and Il6, were highly expressed in keratinocytes treated with recombinant TSLP, whereas their expression was reduced after amygdalin analogue FIB‐116 treatment (Figure 5F,G). However, expression of other pro‐inflammatory mediators expressed in inflamed keratinocytes in psoriasis, such as S100a9, Tnfα and Vegfα, was not significantly changed after FIB‐116 treatment (Figure S3C‐E). Therefore, FIB‐116 acts directly on keratinocytes reducing their proliferation rate and inflammatory response induced by TSLP.
4. DISCUSSION
In this study, we demonstrate that topical application of amygdalin analogue FIB‐116 significantly reduces psoriasis‐like disease in the skin of a genetically engineering mouse model (GEMM) for psoriasis referred as DKO*. The DKO* mouse model serves as a preclinical model for human psoriatic lesions, since it exhibits similar characteristics including erythema, epidermal thickening, scaling, immune cell infiltration and vascular proliferation. 29 This murine model has been widely used to evaluate diverse therapeutic approaches for the treatment of psoriasis and to understand the diverse mechanisms underlying the disease, such as the use of anti‐VEGF, 32 miR‐21 treatments 33 or anti‐TSLP. 9 Thus, the use of DKO* mice has allowed to test the effectiveness and safety of topical application of amygdalin analogues in the presence of both, innate and adaptive immune response, an important limitation of xenotransplant mouse models. 34 Amygdalin exhibits a good skin penetration profile 28 and a short lifetime in blood of mice (30 min) with low toxic effects even in high amounts. 25 These positive and safety properties make amygdalin analogue FIB‐116 a promising candidate for preclinical and clinical trials.
The reduction of psoriatic‐like skin inflammation in DKO* mice treated with FIB‐116 cream was accompanied by decreased epidermal cell proliferation, a reduction of local and systemic pro‐inflammatory cytokine production and a notable decline of neutrophil recruitment. Importantly, vehicle cream exacerbated psoriasis‐like development and induced colonization by S. aureus, a common bacterial infection in patients with severe psoriasis and AD. 35 We suggest that vehicle cream formulation to carry out the amygdalin analogue is suboptimal and hypoallergenic transdermally. The cream formulation used in our experiments left an oily film on ear skin after application that could affect the transepidermal hydration and facilitate microbial infections in DKO* mice. 36 Even with these limitations, the beneficial effects of FIB‐116 cream were evident in DKO* mice. Therefore, delivery of topical amygdalin analogues for psoriasis treatment should be considered in future preclinical studies, including hydrogels, micro and nanoemulsions or aerosol foams for an easy and optimal topical application. 37 , 38
Three specific psoriasis‐like symptoms were evaluated in DKO* mice after the treatment with FIB‐116 or vehicle cream: local inflammation, systemic inflammation and epidermal architecture/hyperplasia. Cross‐talk between different cytokines produced by keratinocytes and immune cells is key regulators in the pathogenesis of psoriatic disease. 5 Amygdalin analogue cream reduced not only the local expression of several of these cytokines in the skin, but also decreased significantly the systemic production of IL17‐A, IL6 and G‐CSF in sera of treated mice. These data highlight that amygdalin analogue compound penetrates efficiently the skin leading to a beneficial systemic response in the immune‐competent psoriasis‐like mouse model.
Besides cytokines, chemoattractant molecules such as S100a9, Lcn2 and Tslp are increased in DKO* mice and in patients. 9 , 31 , 39 , 40 , 41 These molecules are considered antimicrobial peptides, and one of the multiple functions is to attract immune cells towards lesional skin, such as neutrophils to kill pathogens. 42 Interestingly, neutrophils were highly recruited in psoriasis plaques and other neutrophil‐associated chemokines, such as Cxcl1 and Cxcl2, were also increased in DKO* mice. Topical amygdalin treatment significantly reduced the number of neutrophils infiltrated in lesional skin and the expression of antimicrobial peptides. Little is known about the role of neutrophils in the pathogenesis of psoriasis. Recent studies demonstrated the development of psoriasis by continuous neutrophil infiltration into the epidermis. 43 Future experiments should determine how amygdalin cream impacts on neutrophil trafficking and immune responses in psoriasis.
The dysregulation of epidermal differentiation and hyper‐proliferation of basal keratinocytes leads to epidermal hyperplasia and skin swelling in psoriasis. 2 Amygdalin cream treatment reduced the proliferation of basal keratinocytes and hyperplasia. Studies in vitro confirmed that FIB‐116 acts directly on stimulated keratinocytes inhibiting their proliferation and pro‐inflammatory response associated with TSLP, an important mediator in psoriasis. Interestingly, epidermal TSLP is also a trigger factor for other skin pathologies, such as atopic dermatitis (AD), suggesting that amygdalin analogue cream could also be considered for AD treatment.
In conclusion, amygdalin cream application is efficient to reduce psoriasis‐like hallmarks in a preclinical mouse model and further investigations are required to determine its direct role in the immune cell response with the possibility to implement FIB‐116 as a potential novel drug for clinical trials.
CONFLICT OF INTEREST
C.L.A is an employee of Ferrer Advanced Biotherapeutics company. The remaining authors declare no competing interests.
AUTHOR CONTRIBUTIONS
N.G.L, J.J.P and E.F.W. conceived the project. N.G.L designed and performed the experiments, and wrote the article. C.L.A. generated vehicle and amygdalin analogue FIB‐116 creams. E.F.W. and J.J.P supervised the project, provided funding and edited the article.
Supporting information
Relative to Fig. 1. FIB‐116 and psoriasis‐like severity scores
Relative to Fig. 3. Gene expression of Il22 and Il10 in the skin of DKO* mice treated with amygdalin analogue cream.
Relative to Fig. 5. Amygdalin analogue treatment in keratinocytes in vitro.
List of primers for qPCR
ACKNOWLEDGEMENTS
We are very grateful to Drs. L. Mellor, L. Bakiri, M. Palomo‐Irgoyen and members of the Wagner laboratory for critical reading of the manuscript and valuable suggestions. We thank V. Bermeo, A. Guio, L. Diez, L. Mellor and G. Medrano for technical help and IT support. N.G.L received funding from the People programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme (FP7/2007‐2013) under REA grant agreement n 608765. J.J. Pérez was funded by the “Fundación Española para la Ciencia y la Tecnología (FECYT)” to investigate the potential of amygdalin analogues for the treatment of psoriasis. The Wagner laboratory is supported by an ERC‐AdG 2016 CSI‐Fun‐741888, and a H2020‐MSCA‐ITN 2019‐859860‐CANCERPREV grant and the Medical University of Vienna (MUV).
Gago‐López N, Lagunas Arnal C, Perez JJ, Wagner EF. Topical application of an amygdalin analogue reduces inflammation and keratinocyte proliferation in a psoriasis mouse model. Exp Dermatol. 2021;30:1662–1674. 10.1111/exd.14390
Contributor Information
Nuria Gago‐López, Email: ngago@cnio.es, Email: erwin.wagner@meduniwien.ac.at.
Erwin F. Wagner, Email: erwin.wagner@meduniwien.ac.at.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are included in the manuscript and available in the supplementary material of this article.
REFERENCES
- 1. Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):205‐212. [DOI] [PubMed] [Google Scholar]
- 2. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496‐509. [DOI] [PubMed] [Google Scholar]
- 3. Dainichi T, Kitoh A, Otsuka A, et al. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat Immunol. 2018;19(12):1286‐1298. [DOI] [PubMed] [Google Scholar]
- 4. Albanesi C, Madonna S, Gisondi P, Girolomoni G. The interplay between keratinocytes and immune cells in the pathogenesis of psoriasis. Front Immunol. 2018;9:1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014;14(5):289‐301. [DOI] [PubMed] [Google Scholar]
- 6. Varricchi G, Pecoraro A, Marone G, et al. Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Front Immunol. 2018;9:1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Volpe E, Pattarini L, Martinez‐Cingolani C, et al. Thymic stromal lymphopoietin links keratinocytes and dendritic cell‐derived IL‐23 in patients with psoriasis. J Allergy Clin Immunol. 2014;134(2):373‐381. [DOI] [PubMed] [Google Scholar]
- 8. El‐Ghareeb MI, Helmy A, Al Kazzaz S, Samir H. Serum TSLP is a potential biomarker of psoriasis vulgaris activity. Psoriasis (Auckl). 2019;9:59‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gago‐Lopez N, Mellor LF, Megias D, et al. Role of bulge epidermal stem cells and TSLP signaling in psoriasis. EMBO Mol Med. 2019;11(11):e10697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Augustin M, Alvaro‐Gracia JM, Bagot M, et al. A framework for improving the quality of care for people with psoriasis. J Eur Acad Dermatol Venereol. 2012;26(Suppl 4):1‐16. [DOI] [PubMed] [Google Scholar]
- 11. Augustin M, Radtke MA. Quality of life in psoriasis patients. Expert Rev Pharmacoecon Outcomes Res. 2014;14(4):559‐568. [DOI] [PubMed] [Google Scholar]
- 12. Verallo‐Rowell K, Evangelista D. Review update on topical therapy for psoriasis. Curr Derm Rep. 2018;7(1):24–36. [Google Scholar]
- 13. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58(6):649‐658. [DOI] [PubMed] [Google Scholar]
- 14. Wu JJ, Lynde CW, Kleyn CE, et al. Identification of key research needs for topical therapy treatment of psoriasis ‐ a consensus paper by the International Psoriasis Council. J Eur Acad Dermatol Venereol. 2016;30(7):1115‐1119. [DOI] [PubMed] [Google Scholar]
- 15. Rendon A, Schakel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hwang HJ, Lee HJ, Kim CJ, Shim I, Hahm DH. Inhibitory effect of amygdalin on lipopolysaccharide‐inducible TNF‐alpha and IL‐1beta mRNA expression and carrageenan‐induced rat arthritis. J Microbiol Biotechnol. 2008;18(10):1641‐1647. [PubMed] [Google Scholar]
- 17. Hwang HJ, Kim P, Kim CJ, et al. Antinociceptive effect of amygdalin isolated from Prunus armeniaca on formalin‐induced pain in rats. Biol Pharm Bull. 2008;31(8):1559‐1564. [DOI] [PubMed] [Google Scholar]
- 18. Yang C, Zhao J, Cheng Y, Li X, Rong J. Bioactivity‐guided fractionation identifies amygdalin as a potent neurotrophic agent from herbal medicine Semen Persicae extract. Biomed Res Int. 2014;2014:306857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cressey P, Reeve J. Metabolism of cyanogenic glycosides: a review. Food Chem Toxicol. 2019;125:225‐232. [DOI] [PubMed] [Google Scholar]
- 20. Carter JH, McLafferty MA, Goldman P. Role of the gastrointestinal microflora in amygdalin (laetrile)‐induced cyanide toxicity. Biochem Pharmacol. 1980;29(3):301‐304. [DOI] [PubMed] [Google Scholar]
- 21. Araya E, Rodriguez A, Rubio J, et al. Synthesis and evaluation of diverse analogs of amygdalin as potential peptidomimetics of peptide T. Bioorg Med Chem Lett. 2005;15(5):1493‐1496. [DOI] [PubMed] [Google Scholar]
- 22. Filizola M, Centeno NB, Perez JJ. Computational study of the conformational domains of peptide T. J Pept Sci. 1997;3(2):85‐92. [DOI] [PubMed] [Google Scholar]
- 23. Pert CB, Hill JM, Ruff MR, et al. Octapeptides deduced from the neuropeptide receptor‐like pattern of antigen T4 in brain potently inhibit human immunodeficiency virus receptor binding and T‐cell infectivity. Proc Natl Acad Sci USA. 1986;83(23):9254‐9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marcusson JA, Talme T, Wetterberg L, Johansson O. Peptide T a new treatment for psoriasis? A study of nine patients. Acta Derm Venereol. 1991;71(6):479‐483. [PubMed] [Google Scholar]
- 25. Perez JJ. Amygdalin analogs for the treatment of psoriasis. Future Med Chem. 2013;5(7):799‐808. [DOI] [PubMed] [Google Scholar]
- 26. Baroni A, Paoletti I, Greco R, et al. Immunomodulatory effects of a set of amygdalin analogues on human keratinocyte cells. Exp Dermatol. 2005;14(11):854‐859. [DOI] [PubMed] [Google Scholar]
- 27. Paoletti I, De Gregorio V, Baroni A, Tufano MA, Donnarumma G, Perez JJ. Amygdalin analogues inhibit IFN‐gamma signalling and reduce the inflammatory response in human epidermal keratinocytes. Inflammation. 2013;36(6):1316‐1326. [DOI] [PubMed] [Google Scholar]
- 28. Kong H, Qu H, Qu B, et al. Correlation between the transdermal characteristics of pseudoephedrine and amygdalin in majiepingchuan in vitro. J Tradit Chin Med. 2016;36(2):238‐242. [DOI] [PubMed] [Google Scholar]
- 29. Wagner EF, Schonthaler HB, Guinea‐Viniegra J, Tschachler E. Psoriasis: what we have learned from mouse models. Nat Rev Rheumatol. 2010;6(12):704‐714. [DOI] [PubMed] [Google Scholar]
- 30. Zenz R, Eferl R, Kenner L, et al. Psoriasis‐like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437(7057):369‐375. [DOI] [PubMed] [Google Scholar]
- 31. Schonthaler HB, Guinea‐Viniegra J, Wculek SK, et al. S100A8‐S100A9 protein complex mediates psoriasis by regulating the expression of complement factor C3. Immunity. 2013;39(6):1171‐1181. [DOI] [PubMed] [Google Scholar]
- 32. Schonthaler HB, Huggenberger R, Wculek SK, Detmar M, Wagner EF. Systemic anti‐VEGF treatment strongly reduces skin inflammation in a mouse model of psoriasis. Proc Natl Acad Sci USA. 2009;106(50):21264‐21269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guinea‐Viniegra J, Jimenez M, Schonthaler HB, et al. Targeting miR‐21 to treat psoriasis. Sci Transl Med. 2014;6(225):225re1. [DOI] [PubMed] [Google Scholar]
- 34. Schon MP, Manzke V, Erpenbeck L. Animal models of psoriasis‐highlights and drawbacks. J Allergy Clin Immunol. 2021;147(2):439‐455. [DOI] [PubMed] [Google Scholar]
- 35. Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science. 2014;346(6212):954‐959. [DOI] [PubMed] [Google Scholar]
- 36. Surber C, Smith EW. The mystical effects of dermatological vehicles. Dermatology. 2005;210(2):157‐168. [DOI] [PubMed] [Google Scholar]
- 37. Benson HAE, Grice JE, Mohammed Y, Namjoshi S, Roberts MS. Topical and transdermal drug delivery: from simple potions to smart technologies. Curr Drug Deliv. 2019;16(5):444‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paul C, Stein Gold L, Cambazard F, et al. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy vs. gel in patients with psoriasis vulgaris: randomized, controlled PSO‐ABLE study. J Eur Acad Dermatol Venereol. 2017;31(1):119‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aochi S, Tsuji K, Sakaguchi M, et al. Markedly elevated serum levels of calcium‐binding S100A8/A9 proteins in psoriatic arthritis are due to activated monocytes/macrophages. J Am Acad Dermatol. 2011;64(5):879‐887. [DOI] [PubMed] [Google Scholar]
- 40. Wang D, Fang L, Pan G. Association of serum lipocalin‐2 concentrations with psoriasis and psoriatic arthritis: an updated meta‐analysis. Dis Markers. 2019;2019:7361826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Suwarsa O, Dharmadji HP, Sutedja E, et al. Skin tissue expression and serum level of thymic stromal lymphopoietin in patients with psoriasis vulgaris. Dermatol Reports. 2019;11(1):8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chiricozzi A, Romanelli P, Volpe E, Borsellino G, Romanelli M. Scanning the immunopathogenesis of psoriasis. Int J Mol Sci. 2018;19(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boehncke WH, Brembilla NC. Unmet needs in the field of psoriasis: pathogenesis and treatment. Clin Rev Allergy Immunol. 2018;55(3):295‐311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative to Fig. 1. FIB‐116 and psoriasis‐like severity scores
Relative to Fig. 3. Gene expression of Il22 and Il10 in the skin of DKO* mice treated with amygdalin analogue cream.
Relative to Fig. 5. Amygdalin analogue treatment in keratinocytes in vitro.
List of primers for qPCR
Data Availability Statement
The data that supports the findings of this study are included in the manuscript and available in the supplementary material of this article.


