Abstract
Background:
Disease-modifying therapies (DMTs) can reduce the risk of disability worsening in patients with relapsing forms of multiple sclerosis (RMS). High-efficacy DMTs can lead to confirmed or sustained disability improvement (CDI and SDI).
Objective and Methods:
Post hoc analyses of data from the TRANSFORMS, FREEDOMS, and FREEDOMS II trials and their extensions assessed the effects of fingolimod (0.5–1.25 mg/day) on stabilizing or improving disability over ⩽8 years in participants with RMS. CDI and SDI rates were compared between participants initially randomized to fingolimod, interferon (IFNβ-1a), or placebo.
Results:
At 8 years’ follow-up in TRANSFORMS, 35.1% (95% confidence interval [CI], 28.2%–43.1%) of assessed participants in the IFNβ-1a–fingolimod switch group and 41.9% (36.6%–47.6%) on continuous fingolimod experienced CDI; disability did not worsen in approximately 70%. Similar results were seen in the combined FREEDOMS population. Proportionally fewer TRANSFORMS participants achieved SDI in the IFNβ-1a–fingolimod switch group than on continuous fingolimod (5.4% [3.0%–9.5%] vs 14.2% [10.8%–18.4%], p = 0.01).
Conclusion:
CDI and SDI are outcomes of interest for clinical trials and for long-term follow-up of participants with RMS. Monitoring CDI and SDI in addition to disability worsening may facilitate understanding of the therapeutic benefit of RMS treatments.
Keywords: Disease-modifying therapies, fingolimod, multiple sclerosis, outcome measurement, relapsing/remitting, confirmed disability improvement
Introduction
Disease-modifying therapies (DMTs) may help restore function over time in multiple sclerosis (MS) patients. One measure of restoration of function is confirmed disability improvement (CDI), defined by a specific decrease in the Expanded Disability Status Scale (EDSS) score, confirmed over a specific time period (e.g. 3 or 6 months).1–4 Therapies with CDI might afford improved quality of life and better prognosis.3,4
CDI may be a complementary endpoint to confirmed disability worsening (CDW) in clinical trials.3,5–9 Typically, relapsing multiple sclerosis (RMS) trials last no more than 2 years, during which CDW and CDI changes are relatively uncommon; this impedes assessment of the impact of DMTs on long-term disability outcomes. Moreover, recent trials include participants with mild MS, characterized by relatively stable EDSS scores. 10 Nonetheless accurate measurement of changes in EDSS score can characterize a treatment’s impact on evolution of long-term disability. 11
Using data from the active-controlled TRANSFORMS trial, 12 the placebo-controlled FREEDOMS 13 and FREEDOMS II 14 trials, and their long-term extensions up to 8 years,15–17 the impact of fingolimod on MS disability over time was evaluated. Relationships between clinical and magnetic resonance imaging (MRI) outcomes and EDSS category were evaluated throughout. To assess disability improvement with subsequent stabilization free from further worsening, sustained disability improvement (SDI) was defined as an extension of CDI over a longer time frame. SDI was defined by CDI that was maintained at all subsequent assessments in participants with baseline EDSS score ⩾2.
Methods
Analysis population
In TRANSFORMS, data were analyzed from participants randomized to oral fingolimod 0.5 or 1.25 mg or to intramuscular interferon beta-1a (IFNβ-1a) in the 12-month core phase, and to fingolimod 0.5 or 1.25 mg in the open-label extension.12,15,17 Participants randomized to IFNβ-1a were re-randomized to fingolimod 0.5 or 1.25 mg for the extension. Following a protocol amendment in November 2009, any participants receiving fingolimod 1.25 mg in the extension switched to fingolimod 0.5 mg after 1–2 years (Appendix Figure 1S). Two treatment groups are considered within the overall study population: fingolimod (participants continuously receiving either fingolimod dose) and IFNβ-1a/fingolimod (participants randomized to IFNβ-1a then switching to fingolimod 0.5 mg). Although only the 0.5-mg dose is used clinically, participants who received fingolimod 1.25 mg were included within the fingolimod treatment arm because no difference in therapeutic benefit was seen between the two doses in the original trial.
In the FREEDOMS trials, participants were randomized to fingolimod 0.5 or 1.25 mg or placebo during the 24-month core phases, and to either fingolimod dose in the open-label extensions.13,14,16 Participants randomized to placebo were re-randomized to either fingolimod dose for the extensions. After protocol amendment, all participants on fingolimod 1.25 mg switched to fingolimod 0.5 mg (Appendix Figure 1S). Data from two treatment groups were analyzed: participants receiving fingolimod (either dose) continuously or participants receiving placebo then fingolimod (either dose). The protocols for the TRANSFORMS and FREEDOMS studies were approved by each site’s institutional review board; participants gave written informed consent before the studies.12–14
Analyses
Analyses were conducted in the full analysis set (FAS; individuals with EDSS scores at baseline, month (M)12 (TRANSFORMS only), 24, 48, and/or 96), the completer subgroup (CS; individuals with EDSS scores at all times), and the non-completer subgroup (NCS; individuals in the FAS but not in the CS). A trained and certified neurologist determined EDSS scores at each 3-monthly visit. Baseline demographic and disease characteristics were reported descriptively in the FAS and compared between the CS and the NCS. Categorical variables were compared using the χ2 test. Continuous variables were compared using the Wilcoxon rank-sum test. Participants in the FAS and CS were classified as having one of three disability patterns based on EDSS score changes over 96 months: “improving,” “stable or fluctuating,” or “worsening” (Table 1).
Table 1.
Disability patterns definitions.
| Improving | Decreases of ⩾1.0 or ⩾0.5 points from baseline EDSS score if baseline ⩽5.0 or >5.0, respectively, assessed at a scheduled or unscheduled visit, confirmed at 6 months at a scheduled visit in the absence of relapses |
| Stable or fluctuating | Stable: increases or decreases of 0.5 points from baseline EDSS score if baseline score ⩽5.5, or no change in score if baseline score >5.5. Fluctuating: unsustained changes in EDSS score that did not meet the definitions of the worsening, stable, or improving categories. |
| Worsening | Increases of ⩾1.0 or ⩾0.5 points from baseline EDSS score if baseline ⩽5.0 or >5.0, respectively, assessed at a scheduled or unscheduled visit, confirmed at 6 months at a scheduled visit in the absence of relapses |
EDSS: Expanded Disability Status Scale.
Proportions of participants in each category were reported descriptively at M12, M24, M48, and M96. Comparison between treatment groups used the Mantel–Haenszel χ2 test; comparisons across EDSS categories used the Jonckheere Terpstra test, a nonparametric test similar to the Kruskal–Wallis H test but with greater statistical power for temporally ordered samples.
The proportion of participants achieving CDI was determined in the FAS in the subgroup of participants with baseline EDSS score ⩾2.0 because participants are not considered to have disability below this threshold (only minimal signs in one or more functional systems). CDI was defined as decrease in EDSS score of ⩾1.0 point if baseline EDSS score was 2.0–5.5, or of ⩾0.5 points if baseline score was ⩾6.0, sustained for ⩾166 days (the 6-month point). SDI was defined as CDI maintained at all EDSS assessments after CDI was confirmed for the first time. Cumulative probabilities of achieving CDI/SDI in each group were estimated using Kaplan–Meier time-to-event analyses (event day: first observed decrease in EDSS score). Hazard ratios were calculated with a Cox regression model adjusted for sex, age, MS disease duration since first symptom, and baseline EDSS score was used for the analyses of time to CDI and time to SDI.
Number of relapses and annualized relapse rate (ARR; clinical outcomes) and annualized rate of brain atrophy, number of new/enlarging T2 lesions and change in T2 lesion volume (MRI outcomes) were assessed from baseline (M0) to core study end (TRANSFORMS, M12; FREEDOMS, M24), M0 to extension study end (M96), and from M12/M24 to M96 in the FAS and CS, using the Jonckheere Terpstra test.
Results
Baseline participant demographic and disease characteristics
In TRANSFORMS, the FAS included 1280 participants with a mean age of 36.1 years; 67.3% were women. In the overall population, 49.9% of participants (428/857) randomized to fingolimod and 47.6% of participants (207/435) randomized to IFNβ-1a had previously received IFNβ-1a and had experienced a relapse within the previous year, or two relapses within the previous 2 years. 12 Baseline demographic and disease characteristics in the TRANSFORMS CS and NCS are summarized in Table 2. Proportionally fewer participants in the CS than the NCS had received MS treatment before enrollment (p = 0.01). EDSS scores in the CS were slightly lower than in the NCS (p = 0.008), but the median and range of scores were the same in both groups. There were no other significant between-group differences in baseline demographics and disease characteristics.
Table 2.
Baseline demographics and disease characteristics in the completer and non-completer subgroups of TRANSFORMS and of the combined FREEDOMS populations.
| TRANSFORMS | FREEDOMS and FREEDOMS II combined | |||
|---|---|---|---|---|
| CS (n = 544) | NCS (n = 736) | CS (n = 505) | NCS (n = 1051) | |
| Participant demographics | ||||
| Women, n (%) | 354 (65.1) | 507 (68.9) | 357 (70.7) | 800 (76.1) |
| p value a | 0.151 | 0.022 | ||
| Age, years | ||||
| Mean ± SD | 35.9 ± 8.16 | 36.3 ± 8.76 | 37.9 ± 8.39 | 38.8 ± 8.87 |
| Median (range) | 36 (18–55) | 37 (18–55) | 38 (18–55) | 39 (18–55) |
| p value a | 0.424 | 0.064 | ||
| Prior MS treatment, n (%) | ||||
| Yes | 294 (54.0) | 451 (61.3) | 215 (42.6) | 658 (62.6) |
| p value a | 0.01 | <0.0001 | ||
| Duration of MS since first symptom, years | ||||
| Mean ± SD | 7.2 ± 5.97 | 7.5 ± 6.31 | 8.3 ± 6.71 | 9.6 ± 7.50 |
| Median (range) | 5.8 (0–33) | 6.0 (0–40) | 6.7 (0–32) | 7.9 (0–49) |
| p value a | 0.577 | 0.001 | ||
| Clinical disease characteristics | ||||
| Relapses in the 2 years before baseline, n | ||||
| Mean ± SD | 2.2 ± 1.99 | 2.3 ± 1.25 | 2.1 ± 1.20 | 2.2 ± 1.34 |
| Median (range) | 2 (1–40) | 2 (1–12) | 2 (1–11) | 2 (1–14) |
| p value a | 0.266 | 0.976 | ||
| EDSS score | ||||
| Mean ± SD | 2.10 ± 1.275 | 2.29 ± 1.311 | 2.31 ± 1.205 | 2.43 ± 1.346 |
| Median (range) | 2.00 (0.0–5.5) | 2.00 (0.0–5.5) | 2.00 (0.0–5.5) | 2.00 (0.0–6.5) |
| p value a | 0.008 | 0.068 | ||
| MRI disease characteristics | ||||
| Gadolinium-enhancing lesions, n | ||||
| Mean ± SD | 1.0 ± 3.54 | 1.3 ± 3.60 | 1.3 ± 3.19 | 1.4 ± 4.30 |
| Median (range) | 0.0 (0–66) | 0.0 (0–36) | 0.0 (0–37) | 0.0 (0–84) |
| p value a | 0.468 | 0.672 | ||
| T2 lesion volume, mm3 | ||||
| Mean ± SD | 4806.5 ± 5847.24 | 5253.2 ± 6322.96 | 6115.5 ± 7537.45 | 5710.2 ± 7646.82 |
| Median (range) | 2640.7 (0–46 020) | 2932.7 (0–46 280) | 3136.0 (0–47 148) | 2941.4 (0–69 203) |
| p value a | 0.333 | 0.065 | ||
| Normalized brain volume, cm3 | ||||
| Mean ± SD | 1527.8 ± 71.30 | 1524.3 ± 85.19 | 1520.6 ± 80.83 | 1519.9 ± 85.85 |
| Median (range) | 1533.4 (1245–1716) | 1526.7 (1185–1862) | 1525.6 (1171–1733) | 1527.7 (1144–1756) |
| p value a | 0.397 | 0.291 | ||
CS: completer subgroup; EDSS: Expanded Disability Status Scale; NCS: non-completer subgroup; SD: standard deviation.
Comparison of the completer and non-completer subgroups. p values for binary outcomes were obtained using a chi-square test; p values for ordinal outcomes were obtained using a Mantel–Haenszel chi-square test; p values for quantitative variables were obtained using a Wilcoxon rank-sum test.
In the combined FREEDOMS populations, the 1556 participants in the FAS had a mean age of 38.5 years, and 74.4% were women. Baseline demographic and disease characteristics in the CS and NCS are summarized in Table 2. At baseline, there were proportionally fewer women (p = 0.022) and previously treated participants (p < 0.0001) in the CS than the NCS, and participants in the CS had on average a shorter disease duration (p = 0.001).
Disability patterns
Participant baseline demographic and disease characteristics by EDSS category (improving, stable or fluctuating, worsening) in TRANSFORMS and in the combined FREEDOMS populations are reported in Table 3. At 96 months, participants in the improving category had a higher mean baseline EDSS score than did those in the worsening category, both in TRANSFORMS (p < 0.0001) and in the combined FREEDOMS populations (p = 0.014). In FREEDOMS, other significant differences among the EDSS categories were also seen: compared with those who worsened, those improving were younger (p <0.001), with shorter disease duration (p = 0.008), larger baseline total brain volume (p = 0.001) and smaller T2 lesion volume (p = 0.001); proportionally fewer were women (p = 0.042).
Table 3.
TRANSFORMS and combined FREEDOMS completer subgroup baseline demographics and disease characteristics by EDSS category at 96 months.
| TRANSFORMS | FREEDOMS and FREEDOMS II combined | |||||
|---|---|---|---|---|---|---|
| Improving (n = 162) | Stable or fluctuating (n = 213) | Worsening (n = 169) | Improving (n = 143) | Stable or fluctuating (n = 209) | Worsening (n = 153) | |
| Participant demographics | ||||||
| Women, n (%) | 95 (58.6) | 156 (73.2) | 103 (60.9) | 90 (62.9) | 154 (73.7) | 113 (73.9) |
| p value a | 0.692 | 0.042 | ||||
| Age, years | ||||||
| Mean ± SD | 35.2 ± 7.52 | 35.5 ± 8.86 | 37.0 ± 7.73 | 36.3 ± 8.68 | 37.4 ± 8.49 | 39.9 ± 7.57 |
| Median (range) | 35.5 (19–52) | 36 (18–55) | 37.0 (19–54) | 36.0 (18–53) | 37.0 (18–54) | 40.0 (20–55) |
| p value a | 0.54 | <0.001 | ||||
| Prior MS treatment, n (%) | ||||||
| Yes | 93 (57.4) | 108 (50.7) | 93 (55.0) | 64 (44.8) | 77 (36.8) | 74 (48.8) |
| p value a | 0.677 | 0.500 | ||||
| Duration of MS since first symptom, years | ||||||
| Mean ± SD | 6.9 ± 5.73 | 7.20 ± 6.30 | 7.6 ± 5.78 | 7.4 ± 5.89 | 8.0 ± 6.73 | 9.7 ± 7.21 |
| Median (range) | 6.0 (0–33) | 5.2 (0–30) | 6.0 (0–30) | 6.0 (0–27) | 6.1 (0–32) | 8.2 (1–32) |
| p value a | 0.153 | 0.008 | ||||
| Clinical disease characteristics | ||||||
| Number of relapses in the 2 years before baseline | ||||||
| Mean ± SD | 2.2 ± 1.30 | 2.2 ± 2.76 | 2.2 ± 1.27 | 2.2 ± 1.15 | 2.0 ± 1.25 | 2.1 ± 1.16 |
| Median (range) | 2.0 (1–8) | 2.0 (1–40) | 2.0 (1–11) | 2.0 (1–8) | 2.0 (1–11) | 2.0 (1–8) |
| p value a | 0.996 | 0.374 | ||||
| EDSS score | ||||||
| Mean ± SD | 2.60 ± 1.165 | 1.95 ± 1.114 | 1.82 ± 1.425 | 2.63 ± 1.075 | 2.12 ± 1.160 | 2.26 ± 1.323 |
| Median (range) | 2.50 (1.0–5.5) | 1.50 (0.0–5.5) | 1.50 (0.0–5.5) | 2.50 (1.0–5.5) | 2.00 (0.0–5.5) | 2.00 (0.0–5.5) |
| p value a | <0.001 | 0.014 | ||||
| MRI disease characteristics | ||||||
| Gadolinium-enhancing lesions, n | ||||||
| Mean ± SD | 1.4 ± 5.67 | 1.1 ± 2.47 | 0.6 ± 1.23 | 1.1 ± 2.31 | 1.3 ± 2.47 | 1.4 ± 4.53 |
| Median (range) | 0.0 (0–66) | 0.0 (0–26) | 0.0 (0–7) | 0.0 (0–13) | 0.0 (0–14) | 0.0 (0–37) |
| p value a | 0.103 | 0.835 | ||||
| T2 lesion volume, mm3 | ||||||
| Mean ± SD | 5578.5 ± 6390.39 | 4796.6 ± 6406.53 | 4075.1 ± 4289.76 | 5119.9 ± 6523.87 | 5638.1 ± 7297.59 | 7716.5 ± 8490.96 |
| Median (range) | 3052.8 (0–33 027) | 2260.3 (55–46 020) | 2557.8 (17–26 888) | 2603.7 (0–32 012) | 3021.5 (0–43 706) | 4480.4 (0–47 148) |
| p value a | 0.150 | 0.001 | ||||
| Normalized brain volume, cm3 | ||||||
| Mean ± SD | 1523.4 ± 72.62 | 1527.7 ± 74.57 | 1532.1 ± 65.77 | 1537.1 ± 74.19 | 1522.1 ± 84.52 | 1503.0 ± 78.49 |
| Median (range) | 1524.8 (1245–1714) | 1531.0 (1305–1716) | 1538.1 (1339–1663) | 1540.0 (1327–1733) | 1522.6 (1171–1723) | 1513.8 (1285–1684) |
| p value a | 0.325 | 0.001 | ||||
EDSS: Expanded Disability Status Scale; SD: standard deviation.
Comparison between EDSS categories. p values for binary outcomes were obtained using a chi-square test; p values for ordinal outcomes were obtained using a Mantel–Haenszel chi-square test; p values for quantitative variables were obtained using a Wilcoxon rank-sum test.
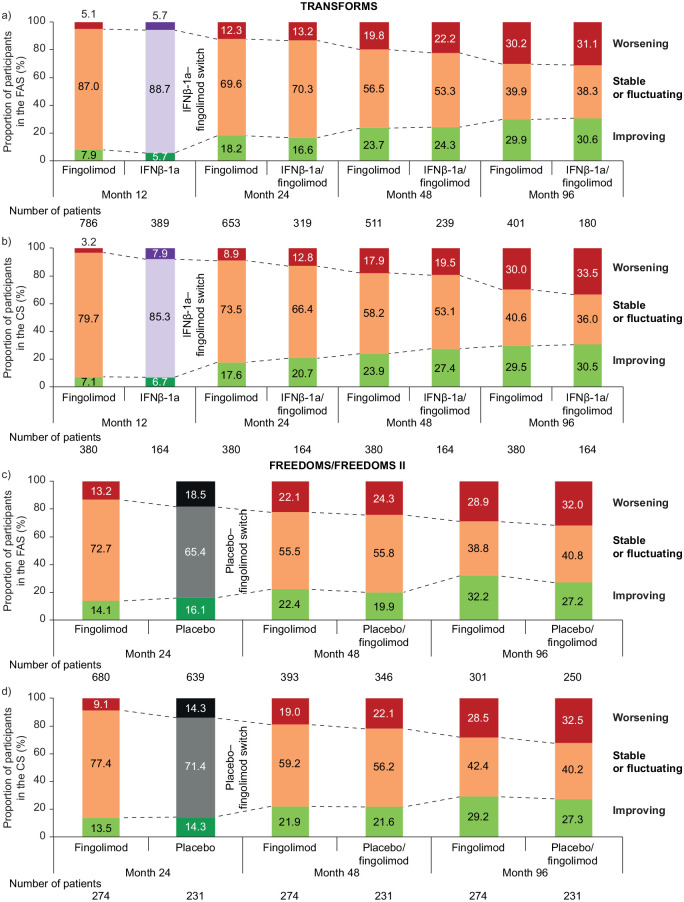
In both analysis sets in TRANSFORMS (Figure 1(a) and (b); Appendix Figures 2Sa, 2Sb) and in the combined FREEDOMS cohort (Figure 1(c) and (d); Appendix Figures 3Sa, 3Sb), the proportions of participants who were stable or fluctuating decreased over time in both treatment groups whereas the proportions whose disability either improved or worsened increased. At M12 in TRANSFORMS (the period before treatment switch) there were proportionally slightly more participants with disability improvement and slightly fewer with disability worsening on fingolimod than on IFNβ-1a (in both the FAS and CS), but these differences were non-significant. At M24 in FREEDOMS (the period before treatment switch), there were proportionally fewer participants with disability improvement but also fewer with disability worsening on fingolimod than on placebo (in both the FAS and CS; non-significant). At each time point thereafter, in both study populations and in both analysis sets, there were essentially no between-treatment differences in the proportions of participants in each EDSS category. A between-treatment difference that was apparent in the combined FREEDOMS population when separating the “stable or fluctuating” participants into two categories was that proportionally more participants were improving or stable at month 96 on continuous fingolimod (55.1%) than in the switch group (44.0%) (Appendix Figure 3Sa). This difference was not seen in TRANSFORMS (49.1% vs 47.8%, respectively). At 8 years, and irrespective of whether they had received fingolimod continuously or had switched to fingolimod after IFNβ-1a or placebo, approximately 70% of participants had either improved or were stable. Of those randomized to fingolimod in TRANSFORMS who were categorized as improving between baseline and M12, 14.8% continued to improve during M12–M96, compared with 18.2% of those randomized to IFNβ-1a.
Figure 1.
EDSS score trends over time by treatment group in TRANSFORMS (a) FAS, (b) CS, and in the FREEDOMS/FREEDOMS II populations (c) FAS, (d) CS.
CS: completer subgroup; EDSS: Expanded Disability Status Scale; FAS: full analysis set; IFNβ-1a: interferon beta-1a.
Comparisons were made using the Mantel–Haenszel χ2 test for trends.
Improving: decreases of ⩾1.0 or ⩾0.5 points from baseline EDSS score if baseline ⩽5.0 or >5.0, respectively, assessed at a scheduled or unscheduled visit, confirmed at 6 months at a scheduled visit in the absence of relapses.
Worsening: increases of ⩾1.0 or ⩾0.5 points from baseline EDSS score if baseline ⩽5.0 or >5.0, respectively, assessed at a scheduled or unscheduled visit, confirmed at 6 months at a scheduled visit in the absence of relapses.
Stable: increases or decreases of 0.5 points from baseline EDSS score if baseline score ⩽5.5, or no change in score if baseline score >5.5.
Fluctuating: all other EDSS patterns not meeting any other definitions.
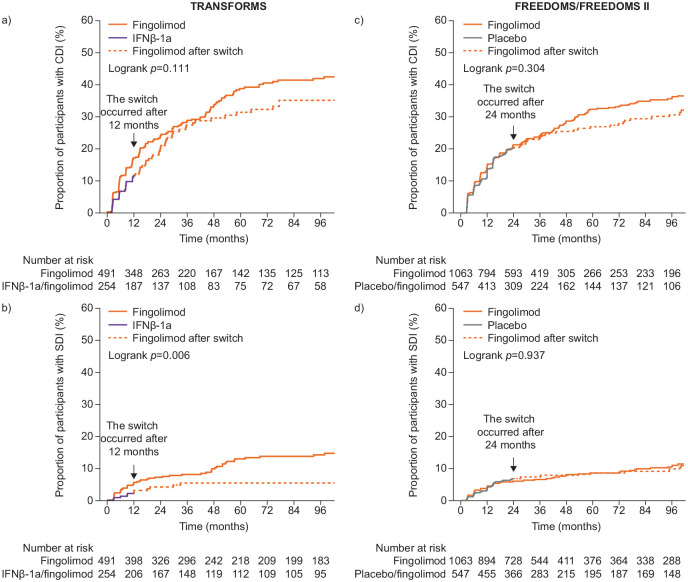
CDI and SDI
In TRANSFORMS, Kaplan–Meier time-to-event analysis over 8 years showed a trend toward a greater cumulative probability of achieving CDI with continuous fingolimod than with IFNβ-1a/fingolimod (Figure 2(a)). Kaplan–Meier estimates (95% confidence intervals (CI)) for CDI were 41.9% (36.6%–47.6%) for fingolimod (152/491) and 35.1% (28.2%–43.1%) for IFNβ-1a/fingolimod (63/254); p = 0.13. The cumulative probability of achieving SDI was greater with continuous fingolimod than with IFNβ-1a/fingolimod (Figure 2(b)). Kaplan–Meier estimates (95% CI) were 14.2% (10.8%–18.4%) for the fingolimod group (51/491) and 5.4% (3.0%–9.5%) for the IFNβ-1a/fingolimod group (11/254; p = 0.01). These differences were confirmed by Cox regression analysis (hazard ratios (HRs) (95% CI) for fingolimod versus IFNβ-1a/fingolimod groups: CDI, 1.26 (0.94–1.69) SDI, 2.34 (1.22–4.48).
Figure 2.
Kaplan–Meier time-to-event analysis by treatment group in TRANSFORMS, (a) CDI, (b) SDI, and in the combined FREEDOMS/FREEDOMS II populations, (c) CDI, (d) SDI.
CDI: confirmed disability improvement; IFN β-1a: interferon β-1a; SDI: sustained disability improvement.
CDI was calculated for participants whose baseline EDSS score was ⩾2.0. SDI was defined as CDI maintained at all EDSS assessments after CDI was confirmed for the first time.
In the analysis of combined FREEDOMS trial data, there was also a trend toward greater cumulative probability of achieving CDI over 8 years for participants receiving fingolimod continuously than for those who switched from placebo to fingolimod (Kaplan–Meier estimates (95% CI): 35.9% (32.1%–39.9%) for fingolimod (269/1063) and 30.7% (26.0%–36.1%) for placebo/fingolimod (125/547), p = 0.25; Figure 2(c)). However, there was no between-group difference in the proportion of participants who experienced SDI (Kaplan–Meier estimates (95% CI): 10.8% (8.6%–13.6%) for fingolimod (80/1063) and 9.9% (7.2%–13.5%) for placebo/fingolimod (41/547) p = 0.87; Figure 2(d)).
Relationship between relapses, MRI outcomes, and disability status
In TRANSFORMS over the study duration (M0–M12 in FAS; M12–M96 and M0–M96 in CS), there were consistently more relapses and a greater ARR in the worsening than in the stable/fluctuating or improving EDSS categories (number of relapses: M0–M12, p < 0.05; M0–M96 and M12–M96, p < 0.01; ARR: M0–M12, p < 0.05; M0–M96 and M12–M96, p < 0.01; Figure 3(a)). There were some concurrent associations between relapses or ARR and EDSS category, by treatment, for both fingolimod (number of relapses: M0–M12 and M12–M96, p < 0.05; M0–M96, non-significant; ARR: M0–M12 and M12–M96, both p < 0.05; M0–M96, non-significant) and IFNβ-1a (number of relapses: M0–M12; non-significant; M12–M96 and M0–M96, p < 0.05, ARR: M0–M12, non-significant; M12–M96 and M0–M96, p < 0.05).
Figure 3.
Association between EDSS category and ARR, overall and by treatment group in the full analysis set (M0–M12) and the completer subgroup (M0–M96, M12–M96) in TRANSFORMS (a) and combined FREEDOMS/FREEDOMS II populations (b).
ARR: annualized relapse rate; EDSS: Expanded Disability Status Scale; IFNβ-1a: interferon beta-1a; M: month.
p values from Jonckheere Terpstra test.
aParticipants received IFNβ-1a for first 12 months, then fingolimod; M0–M12: full analysis set; M12–M96 and M0–M96: completer subgroup.
bParticipants received placebo for first 24 months, then fingolimod; M0–M12: full analysis set; M12–M96 and M0–M96: completer subgroup.
Improving: decreases of ⩾1.0 or ⩾0.5 points from baseline EDSS score if baseline ⩽5.0 or >5.0, respectively, assessed at a scheduled or unscheduled visit, confirmed at 6 months at a scheduled visit in the absence of relapses.
Worsening: increases of ⩾1.0 or ⩾0.5 points from baseline EDSS score if baseline ⩽5.0 or >5.0, respectively, assessed at a scheduled or unscheduled visit, confirmed at 6 months at a scheduled visit in the absence of relapses.
Stable: increases or decreases of 0.5 points from baseline EDSS score if baseline score ⩽5.5, or no change in score if baseline score >5.5.
Fluctuating: all other EDSS patterns not meeting any other definitions.
There were no significant concurrent associations between MRI outcomes and EDSS category in TRANSFORMS. The annualized rate of brain atrophy was numerically greatest in the EDSS worsening category in the FAS and CS in each study period. There were no significant longitudinal associations between EDSS categories from M0–M96 and other clinical or MRI outcomes between M0 and M12, but the worsening category was generally associated with a higher relapse rate and increased T2 lesion volume than the other categories during M0–M12. Notably, no between-category differences in annualized rate of brain atrophy were seen during M0–M12.
In the combined FREEDOMS population, there were significant concurrent associations between ARR and EDSS categories for M0–M24 and M0–M96 (Figure 3(b)). A greater proportion of participants had relapses in the worsening than the stable/fluctuating or improving categories. Similar but non-significant associations were observed for M24–M96.
Discussion
CDW, the most commonly used disability measure in RMS clinical trials, does not address potential improvement in function. Here, CDI and SDI were assessed over time to evaluate whether long-term fingolimod treatment could reverse some disability in RMS. The definition of CDI used in many studies incorporates improved EDSS score even if the improvement is not sustained or subsequently worsens. Therefore, we assessed SDI, defined as CDI sustained to the end of study; SDI may represent maintained reversal of disability, a potentially clinically important target.
CDI was reported with several highly effective treatments. CDI was first described in a post hoc analysis of the AFFIRM trial: natalizumab increased the probability of 3-month CDI over 2 years by 69% compared with placebo (29% versus 18%; HR, 1.69; p = 0.006). 1 In a post hoc analysis of CAREMS II, RMS alemtuzumab-treated participants were more than twice as likely to experience 3 month CDI compared with the IFNβ-1a group (34.7% versus 19.4%; HR, 2.13; p < 0.001). 3 In a pooled analysis of the OPERA studies, there was a 33% higher rate of CDI at 12 weeks with ocrelizumab (20.7%) than with IFNβ-1a (15.6%; p = 0.02). 18 In a propensity score–matched cohort, fingolimod increased the probability of 3-month CDI compared with IFNβ or glatiramer acetate (GA) (HR, 2.75; p < 0.001). 7 A retrospective study showed that 29% of participants switching from GA or IFN-β1a to natalizumab experienced a rapid and confirmed improvement in EDSS scores over 44 weeks. 19
Clinical trials are increasingly including CDI and SDI, in addition to CDW and confirmed disability progression, as prespecified endpoints. A phase 1a trial investigating ATA188, an allogeneic Epstein–Barr virus-targeted T-cell therapy, for the treatment of progressive forms of MS included CDI (defined as improvement in EDSS, or time to 25-foot walk (T25FW) at two consecutive time points) as a secondary outcome. Data were available for 24 patients at 6 months and for 17 patients at 12 months. CDI was achieved by 6 patients at month 6 and by five patients at month 12. All patients who achieved CDI at month 6 maintained it at month 12. 20 A phase 3 placebo-controlled study of high-dose biotin in progressive MS (SPI) had CDI as the primary outcome (defined as EDSS or T25FW improvement at 12 months, confirmed at 15 months) but found no significant treatment effect. 21 Assessing CDI and SDI outcomes prospectively instead of post hoc in randomized clinical trials could shift the goal of treatment from slowing or halting disability worsening or progression to patient recovery, in terms of disability reversal.
In TRANSFORMS and both FREEDOMS studies, approximately one-third of participants experienced CDI, and about one-third of these individuals had SDI at 8 years in the continuously treated fingolimod groups. This is notable because the proportion of participants with SDI is expected to decline with increasing duration of follow-up. More participants receiving continuous fingolimod achieved CDI than did those initially randomized to either IFNβ-1a or placebo. In TRANSFORMS a similar difference was seen with SDI possibly suggesting that SDI could be a more sensitive outcome than CDI. In TRANSFORMS the SDI survival curves for the IFNβ-1a and fingolimod groups began to separate at month 12 with an apparently greater effect occurring after month 48. The increase in SDI in the fingolimod-treated group after month 48 could be due to either a longer-term beneficial effect of continuous fingolimod treatment or alterations in the composition of study participants enriching for those with more favorable outcomes. Why the switched group did not similarly improve is not clear. Possibly, IFNβ-1a followed by fingolimod is less effective at restoring function over time than starting fingolimod early. In contrast, the survival curves in the pooled FREEDOMS studies never separated and the proportion of participants achieving SDI was similar at 8 years. The reasons why initial fingolimod treatment was significantly associated with SDI in TRANSFORMS but not in FREEDOMS is not immediately apparent and presumably is related to an unidentified intrinsic difference between the study populations at baseline. Nevertheless, in FREEDOMS, the different proportions of participants in the continuous fingolimod and switch groups who were improving or stable suggests a general benefit associated with early, higher efficacy treatment. It is likely that treatments with higher efficacy than fingolimod will be associated with a greater effect on long-term SDI.
Somewhat counterintuitively, long-term disability improvement was inversely associated with baseline disability: the improving category at M96 had a higher median baseline EDSS score than the other categories. Perhaps higher baseline EDSS scores in RMS patients reflect sequelae of recent relapsing activity and could be amenable to reversion to lower scores after starting more effective treatment. No other significant baseline associations with long-term improvement were seen in TRANSFORMS but several characteristics indicative of less-advanced disease at baseline were associated with long-term improvement in FREEDOMS; the statistical underrepresentation of women among the FREEDOMS improvers is likely to be a chance finding and not clinically significant. Differences at baseline between non-completers and completers did not appear to substantially influence outcomes. For TRANSFORMS, non-completers had, on average, higher mean EDSS values at baseline than did completers (2.29 versus 2.10, p = 0.008), although the median EDSS scores for both groups was 2.0. The small difference in mean EDSS scores seems unlikely to account for study outcomes.
The effect of relapsing activity on disability category varied between studies. In TRANSFORMS, relapse activity in the FAS was significantly associated with changes in EDSS score in the first year and in the subsequent 7 years. While the association between relapse activity and EDSS category was not significant in the IFNβ-1a group for the first year of TRANSFORMS, this association was significant in the 7 years following the switch to fingolimod. By contrast, a significant association was seen in the fingolimod group during TRANSFORMS at 1 year and the subsequent 7-year follow-up. Moreover, the greater ARR with IFNβ-1a than with fingolimod in all three EDSS categories in TRANSFORMS, suggests a weaker effect of IFNβ-1a on inflammatory disease activity that could lead to a less-pronounced effect on long-term disability.
Interestingly, no significant concurrent associations were seen between EDSS category and MRI outcomes in any of the three analysis periods in TRANSFORMS. Over some intervals, relatively small sample sizes may have hindered the analysis; however, concurrent associations between MRI and disability changes are inherently weak.22,23 A previous analysis of associations between brain volume loss and changes in various disease parameters found a weakly correlated association between study changes in brain volume and EDSS score in FREEDOMS, but not in FREEDOMS II or TRANSFORMS. 24 In contrast, significant longitudinal associations between baseline MRI parameters or early changes in brain volume and subsequent disability progression were reported in the FREEDOMS population. 25
This study has several limitations. The analyses of CDI and SDI are post hoc and are not anchored by a priori hypotheses; therefore, the observations should be considered as hypothesis generating rather than confirmatory. As is typical with long-term studies, a substantial proportion of study participants either discontinued treatment or were lost to follow-up raising the possibility that informative censoring biased the results: study participants experiencing either CDI or SDI would be more likely to be retained in the study than participants whose disability worsened. Given the study duration, it was important to evaluate if the treatment effect among the dropouts was lower, potentially introducing a responder bias. Indeed, participants who discontinued the study tended to have a higher disease activity while on-study compared with completers. However, EDSS trends were similar in the FAS and among the completers, supporting the notion that findings at 8 years were not necessarily biased by the majority of participants (57.5%) who discontinued treatment or who were lost to follow-up before 8 years. Furthermore, the EDSS was the only disability outcome used and limitations of this score are well recognized. The impact of prior IFNβ therapy was not addressed in this study. It is possible that between-treatment differences in CDI rates would change if participants on prior IFN treatment were excluded from the analysis. In TRANSFORMS, 50.8% randomized to fingolimod 0.5 mg and 47.6% randomized to IFNβ-1a were previously treated with an IFNβ. Participants previously treated with IFNβ-1a who experienced ongoing disease activity on that therapy and who entered TRANSFORMS and were randomized to IFNβ-1a might not respond to treatment. A sufficiently large proportion of such participants entering the study could bias the results against IFNβ-1a. Finally, improvement in function may be part of MS natural history. An analysis of the British Columbia MS database found that about 8% of treatment-naïve patients experienced improvement in EDSS scores. 26 This magnitude of innate improvement is substantial in the comparison to the effect sizes reported in the present manuscript. However, innate disability improvement should be similar across study groups and therefore is unlikely to solely account for this study’s observations on disability improvement.
Conclusion
In conjunction with CDW, CDI and SDI are clinically relevant outcomes in controlled clinical trials and long-term studies in RMS. Monitoring these parameters may clarify whether DMTs reverse disability, informing treatment selection, and helping to define long-term disability evolution in MS.
Supplemental Material
Supplemental material, sj-pdf-1-msj-10.1177_13524585211000280 for Disability improvement as a clinically relevant outcome in clinical trials of relapsing forms of multiple sclerosis by Bruce AC Cree, Jeffrey A Cohen, Anthony T Reder, Davorka Tomic, Diego Silva, Daniela Piani Meier, Annik K Laflamme, Shannon Ritter, David Leppert and Ludwig Kappos in Multiple Sclerosis Journal
Acknowledgments
The authors are grateful to all participants in the TRANSFORMS and FREEDOMS studies. Medical writing assistance was provided by Jenny Charlton, Alex Gavin, and Angela Pozo Ramajo (Oxford PharmaGenesis, Oxford, UK). Liz Hambly, Jenny Thorp, and Allie Gonzato (Oxford PharmaGenesis, Oxford, UK) copyedited and styled the manuscript as per journal requirements.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: B.A.C.C. has received personal compensation for consulting for Alexion, Atara, Autobahn, EMD Serono, Novartis, Sanofi, TG Therapeutics, and Therini, and received research support from Genentech. J.A.C. has received personal compensation for consulting for Convelo, Population Council; speaking for Mylan; and serving as an Editor of Multiple Sclerosis Journal. A.T.R. has consulted recently for Abbvie, Bayer, Biogen, Genentech, Mylan, Novartis, Merck Serono, and TG Therapeutics. D.T., D.S., and A.K.L. were employees of Novartis Pharma AG at the time of writing. D.P.M. is an employee of Novartis Pharma AG. S.R. is an employee of Novartis Pharmaceuticals Corporation. D.L. was a Novartis employee until 2019/1; he has received travel reimbursement and personal compensation for consulting from Sanofi, Novartis, Quanterix, and Roche; he is Chief Medical Officer of GeNeuro. L.K.’s institution, the University Hospital Basel, has received payments that were used exclusively for research support for L.K.’s activities as principal investigator and member or chair of planning and steering committees or advisory boards for trials sponsored by Actelion, Addex, Almirall, Bayer HealthCare Pharmaceuticals, CLC Behring, F. Hoffmann-La Roche Ltd and Genentech, Inc., GeNeuro SA, Genzyme, Merck Serono, Mitsubishi Pharma, Novartis, Octapharma, Ono Pharmaceutical, Pfizer, Receptos, Sanofi, Santhera, Siemens, Teva, UCB, and XenoPort; has received license fees for Neurostatus products; and has received research grants from the European Union, Gianni Rubatto Foundation, Novartis Research Foundation, Roche Research Foundation, Swiss Multiple Sclerosis Society, and Swiss National Research Foundation and Innosuisse.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Medical writing and editorial assistance for the development of this manuscript was funded by Novartis.
ORCID iDs: Bruce AC Cree  https://orcid.org/0000-0001-7689-2533
https://orcid.org/0000-0001-7689-2533
Jeffrey A Cohen  https://orcid.org/0000-0001-9245-9772
https://orcid.org/0000-0001-9245-9772
Ludwig Kappos  https://orcid.org/0000-0003-4175-5509
https://orcid.org/0000-0003-4175-5509
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Bruce AC Cree, UCSF Weill Institute for Neurosciences, Department of Neurology, University of California San Francisco, San Francisco, CA, USA.
Jeffrey A Cohen, Department of Neurology, Mellen Center for Multiple Sclerosis Treatment and Research, Neurological Institute, Cleveland Clinic, Cleveland, OH, USA.
Anthony T Reder, Department of Neurology, University of Chicago, Chicago, IL, USA.
Davorka Tomic, Novartis Pharma AG, Basel, Switzerland.
Diego Silva, Novartis Pharma AG, Basel, Switzerland.
Daniela Piani Meier, Novartis Pharma AG, Basel, Switzerland.
Annik K Laflamme, Novartis Pharma AG, Basel, Switzerland.
Shannon Ritter, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
David Leppert, Novartis Pharma AG, Basel, Switzerland.
Ludwig Kappos, Research Center for clinical Neuroimmunology and Neuroscience Basel (RC2NB) and MS Center, Departments of Medicine, Clinical Research, Biomedicine, and Biomedical Engineering, University Hospital, University of Basel, Basel, Switzerland.
References
- 1. Phillips JT, Giovannoni G, Lublin FD, et al. Sustained improvement in Expanded Disability Status Scale as a new efficacy measure of neurological change in multiple sclerosis: Treatment effects with natalizumab in patients with relapsing multiple sclerosis. Mult Scler 2011; 17(8): 970–979. [DOI] [PubMed] [Google Scholar]
- 2. Ghezzi A, Chitnis T, K-Laflamme A, et al. Long-term effect of immediate versus delayed fingolimod treatment in young adult patients with relapsing-remitting multiple sclerosis: Pooled analysis from the FREEDOMS/FREEDOMS II trials. Neurol Ther 2019; 8(2): 461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giovannoni G, Cohen JA, Coles AJ, et al. Alemtuzumab improves preexisting disability in active relapsing-remitting MS patients. Neurology 2016; 87: 1985–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ziemssen T, Lang M, Tackenberg B, et al. Real-world persistence and benefit-risk profile of fingolimod over 36 months in Germany. Neurol Neuroimmunol Neuroinflamm 2019; 6(3): e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coles AJ, Fox E, Vladic A, et al. Alemtuzumab versus interferon beta-1a in early relapsing-remitting multiple sclerosis: Post-hoc and subset analyses of clinical efficacy outcomes. Lancet Neurol 2011; 10: 338–348. [DOI] [PubMed] [Google Scholar]
- 6. Kalincik T, Brown JWL, Robertson N, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: A cohort study. Lancet Neurol 2017; 16(4): 271–281. [DOI] [PubMed] [Google Scholar]
- 7. Alsop JC, Bergvall N, Cornelissen C, et al. Confirmed disability improvement in patients with active multiple sclerosis treated with fingolimod versus brace: A matched comparison of treatments from the Pangaea and Pearl Registry studies. Value Health 2015; 18: A750. [Google Scholar]
- 8. Laroni A, Gandoglia I, Solaro C, et al. Clinical baseline factors predict response to natalizumab: Their usefulness in patient selection. BMC Neurol 2014; 14: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Pesch V, Bartholome E, Bissay V, et al. Safety and efficacy of natalizumab in Belgian multiple sclerosis patients: Subgroup analysis of the natalizumab observational program. Acta Neurol Belg 2014; 114(3): 167–178. [DOI] [PubMed] [Google Scholar]
- 10. Steinvorth SM, Rover C, Schneider S, et al. Explaining temporal trends in annualised relapse rates in placebo groups of randomised controlled trials in relapsing multiple sclerosis: Systematic review and meta-regression. Mult Scler 2013; 19(12): 1580–1586. [DOI] [PubMed] [Google Scholar]
- 11. Liu C, Li Wan Po A, Blumhardt LD. “Summary measure” statistic for assessing the outcome of treatment trials in relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiat 1998; 64: 726–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362: 402–415. [DOI] [PubMed] [Google Scholar]
- 13. Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387–401. [DOI] [PubMed] [Google Scholar]
- 14. Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13(6): 545–556. [DOI] [PubMed] [Google Scholar]
- 15. Khatri B, Barkhof F, Comi G, et al. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: A randomised extension of the TRANSFORMS study. Lancet Neurol 2011; 10(6): 520–529. [DOI] [PubMed] [Google Scholar]
- 16. Kappos L, O'Connor P, Radue EW, et al. Long-term effects of fingolimod in multiple sclerosis: The randomized FREEDOMS extension trial. Neurology 2015; 84: 1582–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen JA, Khatri B, Barkhof F, et al. Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: Results from the extension of the randomised TRANSFORMS study. J Neurol Neurosurg Psychiatry 2016; 87(5): 468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017; 376: 221–234. [DOI] [PubMed] [Google Scholar]
- 19. Belachew S, Phan-Ba R, Bartholome E, et al. Natalizumab induces a rapid improvement of disability status and ambulation after failure of previous therapy in relapsing-remitting multiple sclerosis. Eur J Neurol 2011; 18(2): 240–245. [DOI] [PubMed] [Google Scholar]
- 20. Pender MP, Hodgkinson SJ, Broadley S, et al. Phase I study of ATA188, an off-the-shelf, allogeneic Epstein-Barr virus-targeted T-cell immunotherpy for progressive forms of multiple sclerosis. Mult Scler 2020; 26: S3228. [Google Scholar]
- 21. Cree BAC, Cutter G, Wolinsky JS, et al. Safety and efficacy of MD1003 (high-dose biotin) in patients with progressive multiple sclerosis (SPI2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2020; 19(12): 988–997. [DOI] [PubMed] [Google Scholar]
- 22. University of California San Francisco MS-EPIC Team; Cree BA, Gourraud PA, et al. Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol 2016; 80(4): 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. University of California San Francisco MS-EPIC Team; Cree BAC, Hollenbach JA, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol 2019; 85(5): 653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Radue EW, Barkhof F, Kappos L, et al. Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurology 2015; 84: 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jeffery DR, Di Cantogno EV, Ritter S, et al. The relationship between the rate of brain volume loss during first 24 months and disability progression over 24 and 48 months in relapsing MS. J Neurol 2016; 263(2): 299–305. [DOI] [PubMed] [Google Scholar]
- 26. Tremlett H, Zhu F, Petkau J, et al. Natural, innate improvements in multiple sclerosis disability. Mult Scler 2012; 18: 1412–1421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-msj-10.1177_13524585211000280 for Disability improvement as a clinically relevant outcome in clinical trials of relapsing forms of multiple sclerosis by Bruce AC Cree, Jeffrey A Cohen, Anthony T Reder, Davorka Tomic, Diego Silva, Daniela Piani Meier, Annik K Laflamme, Shannon Ritter, David Leppert and Ludwig Kappos in Multiple Sclerosis Journal