Abstract
Background:
Knowledge on immunity after SARS-CoV-2 infection in patients with multiple sclerosis (pwMS) and the impact of disease-modifying treatment (DMT) is limited.
Objective:
To evaluate degree, duration and potential predictors of specific humoral immune response in pwMS with prior COVID-19.
Methods:
Anti-SARS-CoV-2 antibody testing was performed in pwMS with PCR-confirmed diagnosis of symptomatic COVID-19 from a nation-wide registry. Predictors of seropositivity were identified by multivariate regression models.
Results:
In 125 pwMS (mean age = 42.4 years (SD = 12.3 years), 70% female), anti-SARS-CoV-2 antibodies were detected in 76.0% after a median of 5.2 months from positive PCR. Seropositivity rate was significantly lower in patients on IS-DMT (61.4%, p = 0.001) than without DMT or immunomodulatory DMT (80.6%; 86.0%, respectively). In multivariate analysis, IS-DMT was associated with reduced probability of seropositivity (odds ratio (OR): 0.51; 95% confidence interval (95% CI): 0.17–0.82; p < 0.001). Predefined subgroup analyses showed marked reduction of seropositivity in pwMS on rituximab/ocrelizumab (OR 0.15; 95% CI: 0.05–0.56; p < 0.001). Rate of seropositivity did not change significantly over 6 months.
Conclusions:
Humoral immunity is stable after SARS-CoV-2 infection in MS, but is reduced by immunosuppressive DMT, particularly anti-CD20 monoclonal antibodies. This provides important evidence for advising pwMS as well as for planning and prioritizing vaccination.
Keywords: Multiple sclerosis, COVID-19, SARS-CoV-2, humoral response, antibody, seropositivity, disease-modifying treatment
Introduction
The pandemic spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has not only directly affected 174 million with confirmed infections (as by 10 June 2021) and 3.8 million dead from the virus-associated respiratory disease (CoronaVirus-Disease 2019, COVID-19), but also disrupted every aspect of daily life. The global struggle for returning to life once termed ‘normal’ and dealing with the tremendous social and economic consequences of the pandemic hinders to a large part on developing protective immunity both as societies and individuals. 1 Besides the all-important role of vaccination, knowledge on the quality and duration of immunity after experienced SARS-CoV-2 infection is essential for advising patients as well as planning and prioritizing vaccination.
SARS-CoV-2 infection induces an immune response with activation of the innate and adaptive immune systems, leading to viral clearance and, consequently, production of virus-specific antibodies by plasma cells, usually several days to several weeks after infection. 2 In the general population immune response is high and mostly stable for at least 6–9 months.3,4
However, in multiple sclerosis (MS), there is particular concern whether immunomodulatory or immunosuppressive disease-modifying treatments (DMT) may alter immune response to SARS-CoV-2 infection. Although currently available evidence suggests that clinical course and severity of COVID-19 are not significantly increased in patients receiving DMT, the degree or duration of protective immunity against SARS-CoV-2 could be impaired.5,6 So far, data regarding SARS-CoV-2 antibodies in patients with MS is scarce.7,8
Thus, the objective of this study was to test for SARS-CoV-2 antibodies in a large cohort of pwMS with confirmed symptomatic COVID-19 to evaluate the degree, the duration and potential predictors of humoral immunological response.
Methods
Patients and data collection
The Austrian MS-COVID-19 registry (AUT-MuSC), a collaboration of MS centres certified by the Austrian Neurological Society (ÖGN) adhering to a common and controlled quality standard of excellence, documents epidemiological, clinical and paraclinical data of pwMS suffering a SARS-CoV-2 infection, as described in detail elsewhere. 9
In this nation-wide multicenter prospective cohort study, all patients included in the AUT-MuSC registry with (a) an established diagnosis of MS according to McDonald criteria and (b) a history of symptomatic COVID-19 confirmed by positive SARS-CoV-2 real-time polymerase chain reaction (PCR), were invited to participate in SARS-CoV-2 antibody testing.10 –12 Database was closed on 31 May 2021.
Exclusion criteria were treatment for COVID-19 with reconvalescent plasma, SARS-CoV-2 vaccination, or multiple SARS-CoV-2 infections (defined as another positive SARS-CoV-2 PCR occurring ⩾4 weeks after Covid-19 or after a negative SARS-CoV-2 PCR had already been obtained).
The study was designed and conducted in accordance with the Declaration of Helsinki, the General Data Protection Regulation and the Strengthening Reporting of Observational Studies in Epidemiology (STROBE) guidelines and was approved by the ethics committee of the Medical University Vienna (ethical approval number: EK 1338-2020). Written informed consent was obtained from all participants.
SARS-CoV2-antibody testing
Antibody testing was performed using the commercially available Anti-SARS-CoV-2-QuantiVac-ELISA (IgG) (Euroimmun Medizinische Labordiagnostika AG, Lübeck, Germany) according to the manufacturer’s instructions for use. Briefly, diluted samples were incubated in the coated wells of the kits for 60 min at 37°C, followed by enzyme conjugate incubation for 30 min at 37°C and substrate incubation for 30 min at room temperature. In between washing steps were performed and after reactions were stopped by addition of the stopping solution, absorbance was measured at 450 nm. The ELISA detects IgGs (neutralizing antibodies) against the S1 domain of the spike protein of SARS-CoV2. The kit correlates with the WHO reference material ‘First WHO International Standard for anti-SARS-CoV-2 immunoglobulin’ (NIBSC code: 20/136), enabling declaration of results in standardized units – binding antibody units per millilitre (BAU/ml). Usage of a 6-point calibration curve allows quantitative determination of antibody titers in patient’s serum or plasma within a range from 3.2 to 384 BAU/mL. A value of 35.2 BAU/mL marks the cut-off for positive samples, while results between 25.6 and 35.2 BAU/ml are considered borderline.
Definitions and endpoints
The primary endpoint was defined as presence of anti-SARS-CoV-2 antibodies (seropositivity). Secondary endpoint was the titre of anti-SARS-CoV-2 antibodies.
Predefined potential predictors of seropositivity were age, sex, obesity (body-mass-index ⩾30), smoking status, comorbidities (cardiovascular disease (coronary heart disease and/or ischemic heart failure and/or cardiac valve disease), chronic pulmonary disease (asthma, obstructive pulmonary disease or pulmonary fibrosis), diabetes mellitus, chronic kidney disease and current malignancy), MS course, MS disease duration, expanded disability status scale (EDSS), DMT status, time on DMT, lymphopenia and lymphopenia ⩾ grade 3 and COVID-19 severity. 13
Time to antibody testing was defined as the duration from the first positive SARS-CoV-2 PCR test result to the date of blood sample drawing in months.
DMT status at the time of COVID-19 was grouped as either no DMT (N-DMT); immunomodulating DMT (IM-DMT) comprising dimethyl fumarate, glatiramer acetate, interferon-beta preparations, natalizumab and teriflunomide; or immunosuppressive DMT (IS-DMT) comprising alemtuzumab, cladribine, fingolimod, ocrelizumab or rituximab.5,14
Time on DMT was defined as the duration from first application of the DMT concurrent at the first positive SARS-CoV-2 PCR test to the date of blood sample drawing in years and categorized in month 1–3 (M1–3), month 4–6 (M4–6) and >6 months (>M6).
Lymphocyte count was only considered if available within a maximum of 3 months and a minimum of 2 weeks before positive SARS-CoV-2 PCR test result.
COVID-19 severity was classified as the clinical status at the most severe point of COVID-19 course: (1) mild course (no pneumonia or mild pneumonia without hospitalization); (2) severe course requiring hospitalization and fulfilling at least one of five criteria (breathing rate > 30/min, SpO2 ⩽ 93%, PaO2/FiO2-Ratio < 300, Pulmonary infiltrate > 50% within 24–48 h); and (3) critical course requiring admission to an intensive care unit or requirement of noninvasive ventilation, mechanical ventilation or extracorporeal membrane oxygenation.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 (SPSS Inc, Chicago, IL). Categorical variables were expressed in frequencies and percentages. Continuous variables were tested for normal distribution by Lilliefors-test and expressed as mean and standard deviation (SD) or median and inter-quartile-range (IQR) or range (minimum-maximum) as appropriate. Univariate group comparisons were conducted by t-test, ANOVA, Mann–Whitney U test, Kruskal–Wallis test, or chi-square test as appropriate. Univariate correlations were performed by Pearson or Spearman test as appropriate.
To determine predictors of seropositivity, we calculated multivariate binary logistic regression models with seropositivity as the dependent variable adjusting for time to antibody testing and step-wise including all predefined potential predictors of seropositivity as independent variables showing a univariate association with a p value < 0.2. The model’s goodness of fit was tested by omnibus-test of fit and Nagelkerke R 2 was used to assess explanation of variance within the overall model.
Then, to specifically evaluate the independent impact of DMT status on seropositivity, we performed multivariate logistic regression models with seropositivity as the dependent variable and DMT categories (reference category: N-DMT) as the independent variable adjusting for all predictors of seropositivity identified by the regression models described above. Predefined subgroup analyses were conducted comparing patients on anti-CD20 monoclonal antibodies (anti-CD20 mAbs: rituximab/ocrelizumab) and fingolimod to patients with N-DMT/ IM-DMT.
Analogously, we conducted multivariate linear regression models with anti-SARS-CoV-2 antibody titre as the dependent variable and potential predictors of seropositivity as independent variables.
We tested all variables for collinearity by variance inflation factor (VIF) and excluded all variables from the regression analysis if the VIF was >2.0, corresponding to an R2 of 0.60. Robustness of the statistically significant differences to unidentified confounders not accounted for was quantified with Rosenbaum sensitivity test for Hodges–Lehmann Γ. 15 Missing values were handled by multiple (20 times) imputation using the missing not at random (MNAR) approach with pooling of estimates according to Rubin’s rules. 16 A two-sided p value <0.05 was considered statistically significant.
Data availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request by a qualified researcher and upon approval by the ethics committee of the Medical University Vienna since data contain potentially sensitive information.
Results
Of 183 patients in the AUT-MuSC registry, 125 patients were available for antibody testing and included in the present study. Characteristics of the study cohort are given in Table 1.
Table 1.
Characteristics of the AUT-MuSC-19 antibody study cohort.
| n = 125 | |
|---|---|
| Female a | 87 (69.6) |
| Age (years) b | 42.4 (12.3) |
| BMI b | 24.9 (4.6) |
| Smokers a | 18 (14.5) |
| Ethnicity a | |
| Caucasian a | 124 (99.2) |
| Other a | 1 (0.8) |
| No of relevant comorbiditiesc,d | 0 (0–4) |
| Cardiovascular disease a | 14 (11.2) |
| Chronic pulmonary disease a | 3 (2.4) |
| Diabetes mellitus a | 5 (4.0) |
| Chronic kidney disease a | 2 (1.6) |
| Current malignancy a | 0 (0) |
| Disease duration (years) b | 9.7 (7.3) |
| Disease course a | |
| RRMS a | 97 (77.6) |
| SPMS a | 20 (16.0) |
| PPMS a | 8 (6.4) |
| EDSS c | 2.0 (0–8.5) |
| On DMT at SARS-CoV2-infection a | 94 (75.2) |
| Time on DMT at SARS-CoV2-infection (years) c | 2.6 (0–17) |
| IM-DMT a | 50 (40.0) |
| Dimethyl fumarate a | 15 (12.0) |
| Glatiramer acetate a | 9 (7.2) |
| Interferon-beta a | 9 (7.2) |
| Natalizumab a | 11 (8.8) |
| Teriflunomide a | 6 (4.8) |
| IS-DMT a | 44 (35.2) |
| Alemtuzumab a | 2 (1.6) |
| Cladribine | 4 (3.2) |
| Fingolimod a | 16 (12.8) |
| Ocrelizumab a | 12 (9.6) |
| Rituximab a | 10 (8.0) |
| Lymphopenia at last lab before SARS-CoV2 infectiona,e | 18 (17.5) |
| Grade 3 or higher a | 7 (5.6) |
| Covid-19 course | |
| Mild | 116 (92.8) |
| Severe | 7 (5.6) |
| Critical | 2 (1.6) |
BMI: body mass index; DMT: disease modifying treatment; EDSS: Expanded Disability Status Scale; IM-DMT: Immunomodulating DMT: dimethyl fumarate, glatiramer acetate, interferon beta preparations, natalizumab and teriflunomide; IS-DMT: Immunosuppressive DMT: alemtuzumab, cladribine, fingolimod, ocrelizumab or rituximab; MS: multiple sclerosis; PPMS: primary progressive MS; RRMS: relapsing-remitting MS; SPMS: secondary progressive MS.
Absolute number and percentage.
Mean and standard deviation.
Median and minimum-maximum range.
Defined as cardiovascular diseases, chronic obstructive pulmonary disease, chronic kidney disease, diabetes and concurrent malignancy.
Available from 103 patients.
After a median of 5.2 months (IQR 1.7; minimum 1.5, maximum 13.7) from positive PCR to antibody testing, anti-SARS-CoV-2 antibodies were detected in 95 (76.0%) patients with a median quantity (anti-SARS-CoV-2 antibody titre) of 194 BAU/ml (IQR 331).
Neither seropositivity nor anti-SARS-CoV-2 antibody titers were significantly associated with age, sex, obesity, smoking status, ethnicity, number or type of comorbidities, MS course, MS disease duration, EDSS or severity of COVID-19 (see Supplemental Tables 1 and 2).
In the 103 patients with lymphocyte count available, 17.4% (4/23) of non-converters had lymphopenia ⩾ grade 3 compared to 3.8% (3/80) of seroconverters (p = 0.043), while there was no significant difference in seropositivity between patients with and without lymphopenia of any grade. Patients with lymphopenia ⩾ grade 3 also had lower median anti-SARS-CoV-2 antibody titers (9 BAU/ml (IQR 267) vs 200 BAU/ml (IQR 314), p = 0.035).
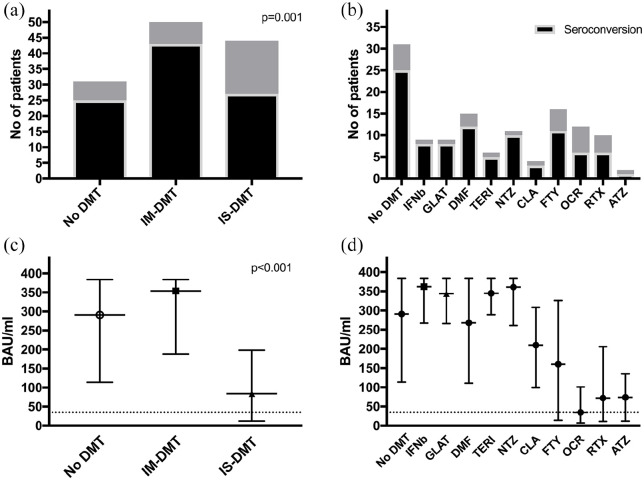
Looking at DMT status, patients without DMT and patients on immunomodulatory DMT (IM-DMT) displayed anti-SARS-CoV-2 antibodies significantly more often (80.6% (25/31) and 86.0% (43/50) respectively) than patients on immunosuppressive DMT (IS-DMT; 61.4% (27/44) p = 0.001, Figure 1(a)). Observed seropositivity rates were lowest in patients treated with ocrelizumab (50%, 6/12), alemtuzumab (50%, 1/2), rituximab (60%, 6/10), fingolimod (68.8%, 11/16) and cladribine (75%, 3/4), while seropositivity was at or above 80% in patients on dimethyl fumarate (80%, 12/15), teriflunomide (83%, 5/6), interferon beta (88.9%, 8/9), glatiramer acetate (88.9%, 8/9) and natalizumab (90.9%, 10/11, Figure 1(b)).
Figure 1.
Seropositivity and antibody titre levels after COVID-19 differ between DMT categories (panels a and c) and substances (panels b and d).
DMT: disease modifying treatment; IM-DMT: Immunomodulating DMT: dimethyl fumarate, glatiramer acetate, interferon beta preparations, natalizumab and teriflunomide; IS-DMT: Immunosuppressive DMT: alemtuzumab, cladribine, fingolimod, ocrelizumab or rituximab; IFNb: interferon beta preparations; GLAT: glatiramer acetate; DMF: dimethyl fumarate; TERI: teriflunomide; NTZ: natalizumab; CLA: cladribine; FTY: fingolimod; OCR: ocrelizumab; RTX: rituximab; ATZ: alemtuzumab.
P values calculated by Chi-square test (panel A) and Kruskal–Wallis test (panel C).
Median anti-SARS-CoV-2 antibody titers levels were significantly lower in the IS-DMT group (84 BAU/ml (IQR 191), p < 0.001) compared to the IM-DMT group (354 BAU/ml (IQR 198)) and patients without DMT (291 BAU/ml (IQR 181), Figure 1(c)).
We found the lowest median titre levels in patients on ocrelizumab (35 BAU/ml), rituximab (72 BAU/ml), alemtuzumab (74 BAU/ml), fingolimod (160 BAU/ml) and cladribine (210 BAU/ml, Figure 1(d)). While median time on DMT did not significantly differ between seroconverters and non-converters (2.9 years (IQR: 4.6) vs 1.6 years (IQR: 2.4), p = 0.267) in the whole cohort, it did in the subgroup of patients on ocrelizumab/rituximab (0.5 years (IQR: 1.9) in seroconverters vs 2.3 years (IQR: 1.8) in non-converters, p = 0.011).
Predictors of seropositivity and antibody titre
Of all predefined potential predictors of seropositivity investigated, only lymphopenia ⩾ grade 3 remained significant through the step-wise inclusion process in the multivariate regression model. When including DMT groups, the model revealed IS-DMT to be significantly associated with a reduction of the probability of seropositivity (odds ratio (OR): 0.51; 95% confidence interval (95%CI): 0.17–0.82; p < 0.001) with reference to no DMT, while IM-DMT was not (Table 2). After inclusion of DMT, lymphopenia ⩾ grade 3 marginally lost statistical significance. In the predefined subgroup analyses, anti-CD20 mAbs were associated with a reduced probability of seropositivity (OR 0.15; 95%CI: 0.05–0.56; p < 0.001) compared to N-DMT/M-DMT, but fingolimod was not.
Table 2.
Predictors of anti-SARS-CoV2 seropositivity and antibody titre.
| Seropositivity a | Antibody titer b | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | B | 95% CI | p value | |
| Lymphopenia ⩾ grade 3 | 0.22 | 0.03–1.05 | 0.056 | –93.4 | –198.9 to 12.1 | 0.082 |
| DMT c | ||||||
| IM–DMT | 1.77 | 0.42– 7.5 | 0.439 | 34.1 | –60.4 to 107.8 | 0.374 |
| IS–DMT | 0.51 | 0.17 to 0.82 | < 0.001 | –113.1 | –164.4 to −61.8 | < 0.001 |
| R square 0.421; p < 0.001 | R square 0.475; p < 0.001 | |||||
| Subgroup analyses | ||||||
| FTY vs. N–DMT/IM–DMT d | 0.81 | 0.31 to 1.49 | 0.319 | –31.8 | –109.1 to 45.4 | 0.414 |
| OCR/ RTX vs. N–DMT/IM–DMT e | 0.15 | 0.05 to 0.56 | < 0.001 | –157.0 | –216.3 to −97.6 | < 0.001 |
IM-DMT: Immunomodulating DMT: dimethyl fumarate, glatiramer acetate, interferon beta preparations, natalizumab and teriflunomide; IS-DMT: Immunosuppressive DMT: alemtuzumab, cladribine, fingolimod, ocrelizumab or rituximab; B: regression coefficient; OR: odds ratio; 95% CI: confidence interval.
Calculated by multivariate binary regression models with seropositivity as the dependent variable adjusted for age, sex and time to antibody testing.
Calculated by multivariate linear regression models with anti-SARS-CoV-2 antibody titer as the dependent variable adjusted for age, sex and time to antibody testing.
Per month.
Reference category: no DMT.
Predefined subgroup analyses comparing patients on ocrelizumab/rituximab and fingolimod to patients with N-DMT/IM-DMT.
IS-DMT was also associated with lower antibody titre levels (b = −113; 95% CI: −164 to −0.62; p < 0.001) in the linear multivariate regression model. Anti-CD20 mAbs (b = −157; 95% CI: −216 to −97; p < 0.001) but not fingolimod were significant predictors of lower antibody titre in subgroup analyses.
Stability of humoral immune response
Categorizing patients according to time to antibody testing, rate of anti-SARS-CoV-2 antibody seropositivity did not significantly differ between M1-3 (84.6%, 22/26), M4-6: (75.0%, 36/48) and > M6 (72.6%, 37/51, p = 0.492) in the whole cohort. Differentiating by DMT groups, change of seropositivity rates was similar in patients without DMT (M1-3: 100%; M4-6: 87%; > M6: 71%), patients on IM-DMT (M1-3: 100%; M4-6: 84%; > M6: 78%) and patients on IS-DMT (M1-3: 64%; M4-6: 53%; > M6: 49%; Figure 2(a)).
Figure 2.
Seropositivity and level of anti-SARS-CoV2 antibodies. Panel (a) proportion of seropositivity according to DMT groups and time from infection to antibody testing, Panel (b) antibody levels according to DMT groups and time from infection to antibody testing, Panel (c) distribution of antibody levels in single patients according to DMT groups and time from infection to antibody testing, Panel (d) distribution of antibody levels in single patients according to DMT substances and time from infection to antibody testing after infection depend on DMT status and slightly decrease over time.
BAU/ml: binding antibody units per millilitre; DMT: disease modifying treatment; IM-DMT: Immunomodulating DMT: dimethyl fumarate, glatiramer acetate, interferon beta preparations, natalizumab and teriflunomide; IS-DMT: Immunosuppressive DMT: alemtuzumab, cladribine, fingolimod, ocrelizumab or rituximab; IFNb: interferon beta preparations; GLAT: glatiramer acetate; DMF: dimethyl fumarate; TERI: teriflunomide; NTZ: natalizumab; CLA: cladribine; FTY: fingolimod; OCR: ocrelizumab; RTX: rituximab; ATZ: alemtuzumab.
While anti-SARS-CoV-2 antibody titre was weakly inversely correlated to time to antibody testing (rho = −0.232, p = 0.009), there was no significant decrease in median titre levels when comparing time to antibody testing categories (M1-3: 266 BAU/ml (IQR 352); M4-6: 174 BAU/ml (IQR 343); > M6: 206 BAU/ml (IQR 295), p = 0.541) in the whole cohort. Again, there was no significant difference in change of antibody levels between DMT groups (Figure 2(b)).
Figure 2(c) and (d) depicts the relationship of antibody levels with DMT groups/ substances and time to antibody testing.
Discussion
In this nation-wide population-based study of humoral immune response after PCR-confirmed symptomatic SARS-CoV-2 infection in pwMS, we report three key findings: (1) more than three-quarters of pwMS (76%) have anti-SARS-CoV-2 antibodies at a level considered protective, (2) immunosuppressive DMT about halves the probability of developing anti-SARS-CoV-2 antibodies (OR = 0.51) with anti-CD20 mAbs decreasing the likelihood of seropositivity even six-fold (OR = 0.15) and (3) seropositivity is stable for at least 6 months, although antibody levels seem to slightly decrease over time independent of DMT.
Currently, data on humoral response to SARS-CoV-2 infection in pwMS is limited. Reported rates of seropositivity range from 41% to 89% in small samples.7,17 –20 Encouragingly, the observed rate of seropositivity in the whole cohort is high, although lower than in general population (>90%) after a comparable interval to infection.3,4 Our results are in line with another recently published study, where 76.8% overall and 78.9% of patients without DMT were seropositive after a median of 75 days duration from symptom onset to antibody testing. 8 While we only included PCR confirmed cases, Sormani et al. 8 did not find a significant difference in seropositivity between patients with PCR confirmed SARS-CoV-2 infection compared to patients with negative or unavailable PCR (73.5% vs 80.4% and 81%, respectively).
We did not find any differences in rate of seropositivity or antibody levels for sex, age, or severity of COVID-19 course, which is in line with data in healthy populations and MS.3,8
Immunosuppressive DMT was identified as significant predictors of not developing immunity against SARS-CoV-2 after suffering infection. As the development of SARS-CoV-2-specific antibodies depends on B cells and consequently plasma cells, a negative impact of immunosuppressive DMT and specifically anti-CD20 B-cell depleting antibodies is to be expected and has already been indicated in some smaller case series and a recent Italian cohort study.2,17,21 However, observed seropositivity rates, although significantly lower than in pwMS untreated or on immunomodulatory DMT, are not as dramatically low as in earlier case series (16%–27%).7,17 –20 While this may in part be due to higher sample size in our cohort and consequent regression to mean, the statistical correction for presence of lymphopenia plays an important role, which is highly likely to have an independent influence on development of antibodies but was not adjusted for in those case series.
Of note, treatment with fingolimod was not associated with decreased seropositivity rates compared to no DMT and immunomodulatory DMT in a predefined subgroup analysis. This is in line with other recent reports of humoral response after COVID-19 during fingolimod treatment, but strikingly discrepant to a recent report on response to mRNA-based vaccination, where only 1 of 26 (3.8%) MS patients treated with fingolimod developed antibodies.7,18,22 With all due caution when interpreting these results stemming from cohorts with limited sample size, this raises questions whether there might be a difference in immunological response to SARS-Cov2 infection as opposed to vaccination specifically relevant under the influence of sphingosline-1-phosphate-receptor modulators. 23
If a patient has developed humoral immunity after SARS-Cov2 infection, estimating duration and stability of humoral immunity is essential for both timing and prioritizing subsequent vaccination. In the general population, levels of antibodies decrease over time but stabilize at 6 months with a protective level prevailing in most patients. 3 While our study did not include repeated antibody measurement, the heterogeneity of time from infection to antibody testing in our cohort enables extrapolation on a group level to a limited degree. As expected, antibody levels slightly decreased in correlation with elapsed time from infection to testing, but antibody positivity rates did not differ between time to testing categories. Thus, humoral immunity against SARS-CoV2 seems to be stable in MS with no indication of earlier loss than in the general population and comparable to response to other viral infections.24,25 Reassuringly, we did not find a signal towards accelerated loss of seropositivity in patients treated with DMT overall or immunosuppressive DMT. Still, large-scale longitudinal studies with serial antibody testing will be needed to answer this question sufficiently.
Strengths and limitations
The main strengths of this study are its population-based approach, the sample size and the detailed characterization of the study cohort provided by the high-quality data from certified specialized MS centres. As reported elsewhere, the AUT-MuSC-19 registry includes most of pwMS with symptomatic SARS-CoV-2 infections in Austria and is likely representative of a central European, primarily Caucasian MS population. 9
We have to acknowledge some potential limitations inherent to the study design. As already noted, we did not have serial antibody testing available limiting the possibilities to measure change of antibody levels intra-individually. Also, the present study is not sufficiently powered to determine the effect of single DMT substances on seropositivity or antibody levels. Therefore, we did not perform any significance tests on single DMT level. By grouping DMTs with similar degree of immunomodulating and immunosuppressive impact, as well as by conducting predefined subgroup analyses comparing the substances of particular interest to the cohort, power issues were mitigated. There may also be confounders influencing seropositivity in pwMS unaccounted for in our study. To manage potential sources of bias, a standardized and strictly statistically modelled step-wise process including a pre-defined set of potential predictors was applied, which is underlined by Rosenbaum bounds indicating only a small potential impact of hidden bias not accounted for in the multivariable models. 15
Conclusion
Our study shows that humoral immunity is stable after SARS-CoV-2 infection in MS, but is reduced by immunosuppressive DMT, particularly anti-CD20 monoclonal antibodies. This provides important evidence for advising pwMS as well as planning and prioritizing vaccination.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585211049391 for Humoral immune response after COVID-19 in multiple sclerosis: A nation-wide Austrian study by Gabriel Bsteh, Sophie Dürauer, Hamid Assar, Harald Hegen, Bettina Heschl, Fritz Leutmezer, Franziska Di Pauli, Christiane Gradl, Gerhard Traxler, Gudrun Zulehner, Paulus Rommer, Peter Wipfler, Michael Guger, Romana Höftberger, Christian Enzinger and Thomas Berger in Multiple Sclerosis Journal
Acknowledgments
We thank all the AUT-MuSC-19 investigators, clinical research staff and especially the patients for helping to collect these data. The named individuals were not compensated for their help. A complete list of the members of the AUT-MuSC investigators is given as in alphabetical order: Aigner, Doris (LKH Bruck, Bruck, Austria); Assar, Hamid (Kepler University Hospital, Linz, Austria); Berger, Thomas (Medical University of Vienna, Vienna, Austria); Böck, Klaus (Kepler University Hospital, Linz, Austria); Bsteh, Christian (Private practice neurologist, Salzburg, Austria); Bsteh, Gabriel (Medical University of Vienna, Vienna, Austria); Di Pauli, Franziska (Medical University of Innsbruck, Innsbruck, Austria); Enzinger, Christian (Medical University of Graz, Graz, Austria); Gradl, Christiane (Medical University of St. Pölten, St. Pölten, Austria); Gruber, Elisabeth (Hospital Diakonissen Schladming, Schladming, Austria); Guger, Michael (Med Campus III, Kepler University Hospital GmbH, Linz, Austria); Hegen, Harald (Medical University of Innsbruck, Innsbruck, Austria); Heschl, Bettina (Medical University of Graz, Graz, Austria); Hiller, Marie-Sophie (Barmherzige Brüder Hospital Eisenstadt, Eisenstadt, Austria); Kornek, Barbara (Medical University of Vienna, Vienna, Austria); Lemmerer, Heidi (Barmherzige Brüder Hospital Graz, Graz, Austria); Leutmezer, Fritz (Medical University of Vienna, Vienna, Austria); Lex, Camillo (Hietzing Hospital, Vienna, Austria); Mayr, Markus (District Hospital Kufstein, Kufstein, Austria); Morgenstern, Gabriele (Private practice neurologist, Lienz, Austria); Oel, Dirk (Klinikum Wels-Grieskirchen, Wels-Grieskirchen, Austria); Rommer, Paulus (Medical University of Vienna, Vienna, Austria); Salhofer-Polanyi, Sabine (Private practice neurologist, Vienna, Austria); Schnabl, Peter (Private practice neurologist, Velden, Austria); Schneider-Koch, Gabriela (Ottakring Hospital, Vienna, Austria); Schrotter, Gabriele (LKH West Graz, Graz, Austria); Traxler, Gerhard (Clinic for Neurology 2, Med Campus III, Kepler University Hospital GmbH, Linz, Austria); Wipfler, Peter (Paracelsus Medical University of Salzburg, Salzburg, Austria); Zulehner, Gudrun (Medical University of Vienna, Vienna, Austria); Zrzavy, Tobias (Medical University of Vienna, Vienna, Austria).
Footnotes
Author Contributions: G.B. had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. G.B. contributed to the study concept and design, patient recruitment, acquisition of data, statistical analysis and interpretation of data, drafting of manuscript. S.D. contributed to patient recruitment, acquisition and management of data, performance of antibody testing and critical revision of manuscript for intellectual content. H.A. contributed to patient recruitment, acquisition of data and critical revision of manuscript for intellectual content. H.H. contributed to patient recruitment, acquisition of data, critical revision of manuscript for intellectual content and drafting of manuscript. B.H. contributed to patient recruitment, acquisition of data and critical revision of manuscript for intellectual content. F.L. contributed to patient recruitment, acquisition of data and critical revision of manuscript for intellectual content. F.D.P. contributed to patient recruitment, acquisition of data and critical revision of manuscript for intellectual content. C.G. contributed to patient recruitment, acquisition of data and critical revision of manuscript for intellectual content. G.T. contributed to patient recruitment, acquisition of data and critical revision of manuscript for intellectual content. G.Z. contributed to patient recruitment, acquisition of data and critical revision of manuscript for intellectual content. P.R. contributed to patient recruitment, acquisition of data and critical revision of manuscript for intellectual content. P.W. contributed to patient recruitment, acquisition of data and critical revision of manuscript for intellectual content. M.G. contributed to patient recruitment, acquisition of data and critical revision of manuscript for intellectual content. R.H. contributed to patient recruitment, critical revision of manuscript for intellectual content and supervision of antibody testing. C.E. contributed to patient recruitment, acquisition of data and critical revision of manuscript for intellectual content. T.B. contributed to study concept and design, patient recruitment, interpretation of data, critical revision of manuscript for intellectual content and study supervision.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: G.B. has participated in meetings sponsored by and received speaker honoraria or travel funding from Biogen, Celgene/BMS, Merck, Novartis, Roche, Sanofi-Genzyme and Teva and received honoraria for consulting Biogen, Roche and Teva. S.D. has nothing to disclose. H.A. has participated in meetings sponsored by and received honoraria (advisory boards, consultations) or travel funding from Biogen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. H.H. has participated in meetings sponsored by and received speaker honoraria or travel funding from Bayer, Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, Siemens and Teva and received honoraria for consulting Biogen, Novartis and Teva. B.H. has nothing to disclose. F.L. has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Bayer, Biogen, Celgene/BMS, MedDay, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. F.D.P. has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) or travel funding from Bayer, Biogen, Celgene/BMS, Merck, Novartis, Sanofi-Genzyme, Roche and Teva. C.G. has participated in meetings sponsored by and received honoraria (lectures, consultations) and/or travel funding from Biogen, D-Pharma, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. G.T. has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) or travel funding from Biogen, Celgene/BMS, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. G.Z. has participated in meetings sponsored by or received travel funding from Biogen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. P.R. has received honoraria for consultancy/speaking from AbbVie, Allmiral, Alexion, Biogen, Merck, Novartis, Roche, Sandoz and Sanofi Genzyme and has received research grants from Amicus, Biogen, Merck and Roche. P.W. has received funding for travel and honoraria (lectures, advisory boards) from Bayer, Biogen, Celgene/BMS, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. M.G. has received support and honoraria for research, consultation, lectures and education from Almirall, Bayer, Biogen, Celgene/BMS, Genzyme, MedDay, Merck, Novartis, Octapharma, Roche, Sanofi-Genzyme, Shire and Teva. R.H. has nothing to disclose. C.E. has received funding for travel and speaker honoraria from Bayer, Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, Shire and Teva and has received research support from Biogen, Celgene/BMS, Merck and Teva and is serving on scientific advisory boards for Bayer, Biogen, Celgene/BMS, Merck, Novartis, Roche and Teva. T.B. has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Bayer, Biogen, Bionorica, Celgene/BMS, GSK, Janssen-Cilag, MedDay, Merck, Novartis, Octapharma, Roche, Sanofi-Genzyme and Teva. His institution has received financial support in the past 12 months by unrestricted research grants (Bayer, Biogen, Celgene/BMS, Merck, Novartis, Sanofi Aventis, Teva) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Celgene/BMS, Merck, Novartis, Octapharma, Roche, Sanofi-Genzyme and Teva.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by a research grant from the Austrian Multiple Sclerosis Society and was partly supported by the Medical Scientific Fund of the Mayor of the City of Vienna, Project COVID038.
ORCID iDs: Gabriel Bsteh  https://orcid.org/0000-0002-0825-0851
https://orcid.org/0000-0002-0825-0851
Paulus Rommer  https://orcid.org/0000-0001-5209-6647
https://orcid.org/0000-0001-5209-6647
Michael Guger  https://orcid.org/0000-0001-8219-781X
https://orcid.org/0000-0001-8219-781X
Thomas Berger  https://orcid.org/0000-0001-5626-1144
https://orcid.org/0000-0001-5626-1144
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Gabriel Bsteh, Department of Neurology, Medical University of Vienna, Waehringer Guertel 18-20, 1090 Vienna, Austria; Department of Neurology, Medical University of Vienna, Vienna, Austria.
Sophie Dürauer, Division of Neuropathology and Neurochemistry, Department of Neurology, Medical University of Vienna, Vienna, Austria.
Hamid Assar, Department of Neurology, Kepler University Hospital, Linz, Austria.
Harald Hegen, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Bettina Heschl, Department of Neurology, Medical University of Graz, Graz, Austria.
Fritz Leutmezer, Department of Neurology, Medical University of Vienna, Vienna, Austria.
Franziska Di Pauli, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Christiane Gradl, Department of Neurology, Medical University of St. Pölten, St. Pölten, Austria.
Gerhard Traxler, Department of Neurology 2, Med Campus III, Kepler University Hospital GmbH, Linz, Austria.
Gudrun Zulehner, Department of Neurology, Medical University of Vienna, Vienna, Austria.
Paulus Rommer, Department of Neurology, Medical University of Vienna, Vienna, Austria.
Peter Wipfler, Department of Neurology, Paracelsus Medical University of Salzburg, Salzburg, Austria.
Michael Guger, Department of Neurology 2, Med Campus III, Kepler University Hospital GmbH, Linz, Austria.
Romana Höftberger, Division of Neuropathology and Neurochemistry, Department of Neurology, Medical University of Vienna, Vienna, Austria.
Christian Enzinger, Department of Neurology, Medical University of Graz, Graz, Austria.
Thomas Berger, Department of Neurology, Medical University of Vienna, Vienna, Austria.
References
- 1. Mofijur M, Fattah IMR, Alam MA, et al. Impact of COVID-19 on the social, economic, environmental and energy domains: Lessons learnt from a global pandemic. Sustain Prod Consum 2020; 26: 343–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: Current State of the Science. Immunity 2020; 52: 910–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Achiron A, Gurevich M, Falb R, et al. SARS-COV-2 antibody dynamics and B-cell memory response over-time in COVID-19 convalescent subjects. Clin Microbiol Infect 2021; 27(9): 1349.e1–1349.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deisenhammer F, Borena W, Bauer A, et al. 6-month SARS-CoV-2 antibody persistency in a Tyrolian COVID-19 cohort. Wien Klin Wochenschr 2021; 133: 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol 2020; 77: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: A review of 873 published cases. J Clin Med 2020; 9: 4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kempen ZLE, van Strijbis EMM, Al MMCT, et al. SARS-CoV-2 antibodies in adult patients with multiple sclerosis in the Amsterdam MS Cohort. Jama Neurol 2021; 78: 880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sormani MP, Schiavetti I, Landi D, et al. SARS-CoV-2 serology after COVID-19 in multiple sclerosis: An international cohort study. Mult Scler J. Epub ahead of print 30 July 2021. DOI: 10.1177/13524585211035318. [DOI] [PubMed] [Google Scholar]
- 9. Bsteh G, Assar H, Hegen H, et al. COVID-19 severity and mortality in multiple sclerosis are not associated with immunotherapy: Insights from a nation-wide Austrian registry. PLoS ONE 2021; 16: e0255316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 11. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69(2): 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald Criteria’. Ann Neurol 2005; 58(6): 840–846. [DOI] [PubMed] [Google Scholar]
- 13. Bsteh G, Bitschnau C, Hegen H, et al. Multiple sclerosis and COVID-19: How many are at risk? Eur J Neurol. Epub ahead of print 25 September 2020. DOI: 10.1111/ene.14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brownlee W, Bourdette D, Broadley S, et al. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology 2020; 94: 949–952. [DOI] [PubMed] [Google Scholar]
- 15. Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc 1984; 79: 516–524. [Google Scholar]
- 16. Council NR. The prevention and treatment of missing data in clinical trials. Washington, DC: National Academies Press, 2010. [PubMed] [Google Scholar]
- 17. Sharifian-Dorche M, Sahraian MA, Fadda G, et al. COVID-19 and disease-modifying therapies in patients with demyelinating diseases of the central nervous system: A systematic review. Mult Scler Relat Disord 2021; 50: 102800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zabalza A, Cárdenas-Robledo S, Tagliani P, et al. COVID-19 in multiple sclerosis patients: Susceptibility, severity risk factors and serological response. Eur J Neurol. Epub ahead of print 19 December 2020. DOI: 10.1111/ene.14690. [DOI] [PubMed] [Google Scholar]
- 19. Conte WL. Attenuation of antibody response to SARS-CoV-2 infection in patients with multiple sclerosis on ocrelizumab: A case-control study. Mult Scler Relat Disord 2021; 52: 103014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wallach AI, Picone MA. The presence of SARS CoV2 antibodies in MS patients. Mult Scler Relat Disord 2021; 50: 102793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baker D, Roberts CAK, Pryce G, et al. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin Exp Immunol 2020; 202(2): 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Dis 2021; 14: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rommer PS, Bsteh G, Berger T, et al. SARS-CoV-2 antibodies in multiple sclerosis patients depending on the vaccine mode of action? Mult Scler J. Epub ahead of print 13 August 2021. DOI: 10.1177/13524585211039128. [DOI] [PubMed] [Google Scholar]
- 24. Nguyen J, Hardigan P, Kesselman MM, et al. Immunogenicity of the influenza vaccine in multiple sclerosis patients: A systematic review and meta-analysis. Mult Scler Relat Dis 2020; 48: 102698. [DOI] [PubMed] [Google Scholar]
- 25. Berger JR, Brandstadter R, Bar-Or A. COVID-19 and MS disease-modifying therapies. Neurol Neuroimmunol Neuroinflammation 2020; 7: e761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585211049391 for Humoral immune response after COVID-19 in multiple sclerosis: A nation-wide Austrian study by Gabriel Bsteh, Sophie Dürauer, Hamid Assar, Harald Hegen, Bettina Heschl, Fritz Leutmezer, Franziska Di Pauli, Christiane Gradl, Gerhard Traxler, Gudrun Zulehner, Paulus Rommer, Peter Wipfler, Michael Guger, Romana Höftberger, Christian Enzinger and Thomas Berger in Multiple Sclerosis Journal
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request by a qualified researcher and upon approval by the ethics committee of the Medical University Vienna since data contain potentially sensitive information.