Abstract
We examined the relationship between polarized growth and division site selection, two fundamental processes important for proper development of eukaryotes. Diploid Saccharomyces cerevisiae cells exhibit an ellipsoidal shape and a specific division pattern (a bipolar budding pattern). We found that the polarity genes SPA2, PEA2, BUD6, and BNI1 participate in a crucial step of bud morphogenesis, apical growth. Deleting these genes results in round cells and diminishes bud elongation in mutants that exhibit pronounced apical growth. Examination of distribution of the polarized secretion marker Sec4 demonstrates that spa2Δ, pea2Δ, bud6Δ, and bni1Δ mutants fail to concentrate Sec4 at the bud tip during apical growth and at the division site during repolarization just prior to cytokinesis. Moreover, cell surface expansion is not confined to the distal tip of the bud in these mutants. In addition, we found that the p21-activated kinase homologue Ste20 is also important for both apical growth and bipolar bud site selection. We further examined how the duration of polarized growth affects bipolar bud site selection by using mutations in cell cycle regulators that control the timing of growth phases. The grr1Δ mutation enhances apical growth by stabilizing G1 cyclins and increases the distal-pole budding in diploids. Prolonging polarized growth phases by disrupting the G2/M cyclin gene CLB2 enhances the accuracy of bud site selection in wild-type, spa2Δ, and ste20Δ cells, whereas shortening the polarized growth phases by deleting SWE1 decreases the fidelity of bipolar budding. This study reports the identification of components required for apical growth and demonstrates the critical role of polarized growth in bipolar bud site selection. We propose that apical growth and repolarization at the site of cytokinesis are crucial for establishing spatial cues used by diploid yeast cells to position division planes.
Cellular morphogenesis and division plane selection are two fundamental processes common to both unicellular and multicellular organisms. Morphological changes enable cells to establish unique shapes and specialized functions. Polarized cell division allows cells to assume higher-order organization, which is essential for the development of complex multicellular organs and organisms (reviewed in references 12, 15, and 71). Coordination of cell division with changes in cell shape is likely to be important for ensuring the proper segregation of cytoplasmic components. How cells undergo morphogenesis, determine division patterns, and coordinate the position of the division plane with cellular morphogenesis is not well understood.
The budding yeast Saccharomyces cerevisiae is an excellent model for studying cell shape and division plane selection. S. cerevisiae exists in different forms, each with a specific cell morphology and division pattern (12, 15, 47, 55, 61). During growth in rich media, both haploid and diploid yeast cells are ellipsoid and select budding sites according to their MAT locus and pedigree (10, 23, 33, 68). Haploid MATa and MATα cells use an axial budding pattern in which cells form new buds adjacent to the preceding site of cytokinesis. Diploid MATa/MATα cells exhibit a bipolar budding pattern: mother cells bud either distal or proximal to the birth site, whereas daughter cells bud almost exclusively at distal poles. It is thought that future bud sites are marked by bud site tags that serve as cues for the initiation of new buds (9, 10, 22, 69). When nitrogen sources are limited, diploid cells undergo pseudohyphal differentiation: cells become extremely elongated and preferentially bud distal to their birth site (26, 60). A similar mode of growth has also been observed in certain haploid strains grown on solid media (59). These features are thought to help cells spread efficiently across a surface to gain access to nutrients (26, 47).
The morphology of yeast cells is determined during bud growth. Buds emerge at late G1 of the yeast cell cycle. Early bud growth is restricted to the bud tip and is termed apical growth (42, 73). As the bud enlarges, growth enters a second step, the isotropic phase, during which growth is still restricted to the bud but deposited over the entire bud surface. After nuclear division, a repolarization phase begins, and new materials are directed toward the mother-bud neck to prepare for cytokinesis (septation). Understanding apical growth and its regulation and identifying the molecules that participate in this growth process will provide insight into how morphogenesis is controlled and coordinated with other cellular events.
A number of components important for bud growth have been identified and characterized (12, 15, 32, 55). The actin cytoskeleton is essential for bud growth and is thought to mediate directional transport of secretory vesicles (1, 15, 35, 49, 52, 57). Both cortical actin patches and components of the secretory apparatus localize to sites of growth (1, 21, 35).
The nonessential polarity proteins Spa2, Pea2, Bud6, and Bni1 also participate in yeast morphogenesis (2, 19, 25, 36, 68, 69, 74, 78). spa2Δ cells appear rounder than wild-type cells and have defects in cytokinesis, mating projection formation, and pseudohyphal growth (25, 48, 60, 68, 69). Similar phenotypes have also been reported for pea2, bud6, and bni1 mutants (2, 19, 36, 48, 74, 78). At the onset of apical growth, Spa2, Pea2, Bud6, and Bni1 concentrate at the incipient bud site and remain at the bud tip during the apical growth phase (2, 19, 25, 68, 69, 74). In large budded cells undergoing isotropic growth, they either become more dispersed or are not detectable. These abundant proteins have been proposed to function together as a complex (the polarisome) that regulates the actin cytoskeleton and/or concentrates cortical actin at the bud tip, thereby restricting growth to that site (24, 66).
Another protein that concentrates at the bud tip is Ste20 (40, 54). Ste20 is homologous to the mammalian p21-activated kinase (39, 64), and it functions upstream of a mitogen-activated protein kinase (MAPK) signaling pathway required for both mating and pseudohyphae formation (38, 44, 51, 59). Both of these morphogenetic processes also involve Spa2 function (25, 48, 60). Like Spa2, Ste20 is at the tips of both emerging and small buds and later disperses as buds enlarge (40, 54). The temporal and spatial localization patterns of Spa2, Pea2p, Bud6, Bni1, and Ste20 raise the possibility that they may function in apical growth. However, direct evidence of such a role for these proteins has not been investigated.
The timing of the apical growth phase is controlled by the cell cycle machinery (42). The onset of apical growth and actin polarization is induced by the G1 cyclins Cln1 and Cln2, which activate the cyclin-dependent kinase Cdc28 (13, 50). Later, activation of Cdc28 by the G2 cyclins Clb1 and Clb2 triggers the switch from apical to isotropic growth and the redistribution of the cortical actin patches over the entire bud surface (42, 58, 72). Finally, repolarization of growth materials and the actin cytoskeleton to the mother-bud neck prior to cytokinesis requires inactivation of Cdc28 through destruction of the G2 cyclins. How the cell cycle machinery affects the localization of growth is unclear.
Many genes that function in cell morphogenesis in yeast are also required for bipolar bud site selection. Deletion of SPA2, PEA2, BUD6, or BNI1 and some mutations that affect the actin cytoskeleton and secretory pathway cause a random-budding defect that specifically affects the bipolar budding program but not the axial pattern (7, 16, 20, 29, 67, 68, 74, 77, 78). Despite the large number of proteins that have been identified, it is still not clear how these gene products can simultaneously affect cell morphogenesis and bipolar bud site selection.
Here we demonstrate that SPA2, PEA2, BUD6, BNI1, and STE20 are crucial for the apical growth phase of bud growth. Mutations in any of these genes lead to rounder cells (68, 78) (see below). These mutants also have bipolar bud site selection defects, suggesting that the altered budding pattern in these mutants is related to the apical growth defect. Indeed, we found that the fidelity of bipolar bud site selection strongly correlates with the length of the apical growth phase. Moreover, bud site selection defects resulting from inappropriate apical growth, as observed in spa2 and ste20 mutants, can be partially suppressed by extending the duration of apical growth. We propose that directional growth processes are crucial for establishing bipolar bud site selection tags at distal and proximal poles. This model explains why many mutants known to affect morphogenesis (such as cell cycle regulators, signaling proteins, components of the exocytic pathway, proteins involved in polarized growth, etc.) also affect bipolar bud site selection.
MATERIALS AND METHODS
Yeast strains and media.
The S. cerevisiae strains used in this study are listed in Table 1. All strains are congenic derivatives of S288C. Growth media and genetic manipulation were as described previously (28, 65).
TABLE 1.
Yeast strains used in this study
| Strain(s) | Genotype | Source |
|---|---|---|
| Y2017, Y2018, Y2019 | MATa/MATα leu2Δ98/leu2Δ98 ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 | This study |
| Y555 | MATa/MATα spa2Δ::TRP1/spa2Δ::TRP1 ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 trp1-Δ1/trp1-Δ1 | This laboratory |
| Y2020 | MATa/MATα spa2(1-2, 116-1466)::HA/spa2(1-2, 116-1466)::HA leu2Δ98/leu2Δ98 ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 | This study |
| Y2021 | MATa/MATα spa2(1-410, 531-1466)::HA/spa2(1-410, 531-1466)::HA leu2Δ98/leu2Δ98 ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 | This study |
| Y2022 | MATa/MATα pea2Δ::HIS3/pea2Δ::HIS3 leu2Δ98/leu2Δ98 ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 | This study |
| Y2023 | MATa/MATα bud6Δ::HA/bud6Δ::HA leu2Δ98/leu2Δ98 ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 | This study |
| Y2024 | MATa/MATα bni1Δ::HIS3/bni1Δ::HIS3 leu2Δ98/leu2Δ98 ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 TRP1/trp1-Δ1 | This study |
| Y2025 | MATa/MATα swe1Δ::LEU2/swe1Δ::LEU2 leu2Δ98/leu2Δ98 ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 trp1-Δ1/trp1-Δ1 | This study |
| Y2026 | MATa/MATα clb2Δ::TRP1/clb2Δ::TRP1 leu2Δ98/leu2Δ98 ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 trp1-Δ1/trp1-Δ1 | This study |
| Y2027 | MATa/MATα spa2Δ3::URA3/spa2Δ3::URA3 leu2Δ98/leu2Δ98 ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 trp1-Δ1/trp1-Δ1 | This study |
| Y2028 | MATa/MATα clb2Δ::TRP1/clb2Δ::TRP1 spa2Δ3::URA3/spa2Δ3::URA3 leu2Δ98/leu2Δ98 ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 trp1-Δ1/trp1-Δ1 | This study |
| Y270 | MATa/MATα ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 trp1-Δ1/trp1-Δ1 | This laboratory |
| Y2029 | MATa/MATα ste20Δ::URA3/ste20Δ::TRP1 ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/ his3-Δ200 trp1-Δ1/trp1-Δ1 | This study |
| Y2030 | MATa/MATα clb2Δ::TRP1/clb2Δ::TRP1 ste20Δ::URA3/ste20Δ::HA leu2Δ98/leu2Δ98 ura3-52/ura3-52 lys2-801 /lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 trp1-Δ1/trp1-Δ1 | This study |
| Y2031 | MATa/MATα grr1Δ::hisG/grr1Δ::hisG ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 | This study |
| Y2032 | MATa cdc34-2 leu2Δ98 ura3-52 lys2-801 ade2-101 his3-Δ200 | This study |
| Y2033 | MATa cdc34-2 spa2Δ3::URA3 leu2Δ98 ura3-52 lys2-801 ade2-101 his3-Δ200 | This study |
| Y2034 | MATa cdc34-2 pea2Δ::HIS3 leu2Δ98 ura3-52 lys2-801 ade2-101 his3-Δ200 | This study |
| Y2035 | MATa cdc34-2 bud6Δ::HA leu2Δ98 ura3-52 lys2-801 ade2-101 his3-Δ200 | This study |
| Y2036 | MATa cdc34-2 bni1Δ::HIS3 leu2Δ98 ura3-52 lys2-801 ade2-101 his3-Δ200 | This study |
| Y2037 | MATa cdc34-2 ste20Δ::URA3 leu2Δ98 ura3-52 lys2-801 ade2-101 his3-Δ200 | This study |
| Y2038 | MATa cdc34-2 ste11Δ::HA leu2Δ98 ura3-52 lys2-801 ade2-101 his3-Δ200 | This study |
| Y2039 | MATa cdc34-2 ste7Δ::HA leu2Δ98 ura3-52 lys2-801 ade2-101 his3-Δ200 | This study |
| Y2040 | MATa cdc12-1 leu2Δ98 ura3-52 lys2-801 ade2-101 his3-Δ200 | B. Santos |
| Y2041 | MATa cdc12-1 spa2Δ3::URA3 leu2Δ98 ura3-52 lys2-801 ade2-101 his3-Δ200 | B. Santos |
| Y2042 | MATa bud3Δ::TRP1 ura3-52 lys2-801 ade2-101 trp1-Δ1 his3-Δ200 | This study |
| Y2043 | MATa bud3Δ::TRP1 ste20Δ::URA3 ura3-52 lys2-801 ade2-101 trp1-Δ1 his3-Δ200 | This study |
| Y2044 | MATa bud3Δ::TRP1 ste11Δ::HA ura3-52 lys2-801 ade2-101 trp1-Δ1 his3-Δ200 | This study |
| Y2045 | MATa bud3Δ::TRP1 ste7Δ::HA ura3-52 lys2-801 ade2-101 trp1-Δ1 his3-Δ200 | This study |
| Y2046 | MATa bud3Δ::TRP1 spa2Δ::HIS3 ura3-52 lys2-801 ade2-101 trp1-Δ1 his3-Δ200 | This study |
| Y2047 | MATa bud3Δ::TRP1 spa2Δ::HIS3 ste20Δ::URA3 ura3-52 lys2-801 ade2-101 trp1-Δ1 his3-Δ200 | This study |
The null alleles bni1Δ::HIS3, bud3Δ::TRP1, clb2Δ::TRP1, pea2Δ::HIS3, and spa2Δ::HIS3 lack the entire protein-coding regions and were constructed using the strategy described by Baudin et al. (6). The null alleles bud6Δ::HA, ste7Δ::HA, ste11Δ::HA, and ste20Δ::HA were created by replacing the entire protein-coding sequence on the chromosome by a cassette encoding three copies of the hemagglutinin epitope (62). For alleles spa2(1-2, 116-1466)::HA and spa2(1-410, 531-1466)::HA, a region of the SPA2 gene on the chromosome was deleted and replaced with the hemagglutinin epitope by using the same method. The spa2Δ3::URA3 allele was created by replacing the SacI-SphI fragment of the SPA2 coding sequence with the URA3 gene. This construct removes residues 41 to 1205 of Spa2. The ste20Δ::URA3 and ste20Δ::TRP1 alleles were generated using the disruption plasmids pEL45 (38) and pDH104 (76), respectively. swe1Δ::LEU2 and grr1Δ::hisG were as described previously (43, 46). All deletions were confirmed by PCR. The cdc12-1 and cdc34-2 allele have been described previously (27, 30).
Examination of cell morphology.
Mid-log-phase cells were treated as indicated and fixed for 1 h with 3.7% formaldehyde. Fixed cells were then washed and resuspended in phosphate-buffered saline (PBS) solution. Cells were examined by phase-contrast and Nomarski microscopy. In some cases, the samples were stained with fluorescent brightener 28 (2 μg/ml; Calcofluor White; Sigma), which stains chitin in the cell wall, and examined by fluorescence microscopy.
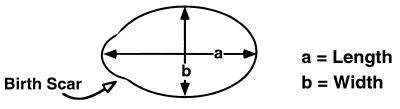
To measure the length and the width of yeast cells, random fields of cells for each sample were recorded using a photometric Sensys charge-coupled device camera and cell diameters were measured (in pixels) by using Imagepoint Lab Spectrum software (Signal Analytics Corporation). All unbudded and budded mother cells and unseparated daughters larger than one-half the size of mother cells were measured. The length is the distance of a cell's long axis, determined relative to the position of its birth pole (illustration in Table 2). The width is defined as the maximum measurement perpendicular to the long axis. Indicated numbers of samples were selected for further analysis according to the order of entry (Table 2). For distribution graphs, the length/width ratios of these samples were sorted in an ascending order and plotted (Fig. 1B; see Fig. 5B).
TABLE 2.
Length/width ratios of yeast strains
| Strain | Length/width ratio (mean ± SD)a |
|---|---|
| n = 100 | |
| Wild type | 1.43 ± 0.15 |
| Wild type | 1.36 ± 0.15 |
| Wild type | 1.36 ± 0.13 |
| spa2Δ | 1.07 ± 0.10 |
| spa2(1-2, 116-1466)::HA | 1.07 ± 0.11 |
| spa2(1-410, 531-1466)::HA | 1.11 ± 0.10 |
| pea2Δ | 1.08 ± 0.11 |
| bud6Δ | 1.08 ± 0.11 |
| bni1Δ | 1.00 ± 0.11 |
| n = 150 | |
| Wild type | 1.42 ± 0.15 |
| swe1Δ | 1.25 ± 0.11 |
| clb2Δ | 1.66 ± 0.29 |
| spa2Δ | 1.07 ± 0.09 |
| clb2Δ spa2Δ | 1.14 ± 0.14 |
| n = 125 | |
| Wild type | 1.37 ± 0.13 |
| ste20Δ | 1.23 ± 0.10 |
| clb2Δ | 1.73 ± 0.32 |
| clb2Δ ste20Δ | 1.37 ± 0.20 |
The measurement of the length/width ratios was performed as described in Materials and Methods.
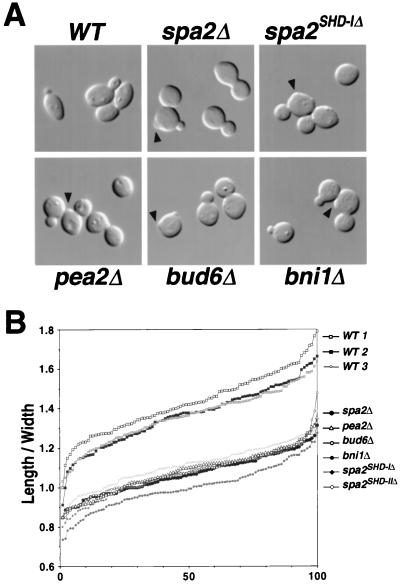
FIG. 1.
spa2Δ, pea2Δ, bud6Δ, and bni1Δ polarity mutants are rounder than wild-type cells. (A) Cell shapes of wild-type, spa2Δ, spa2(1-2, 116-1466) (i.e., spa2SHD-IΔ), pea2Δ, bud6Δ, and bni1Δ cells in mid-log phase. Abnormal cell wall protrusions in mutant cells are indicated with arrowheads. (B) The length/width ratios of 100 individual mid-log-phase cells from wild-type (WT) spa2Δ, spa2(1-2, 116-1466), spa2(1-410, 531-1466) (i.e., spa2SHD-IIΔ), pea2Δ, bud6Δ, and bni1Δ cultures. The ratios for individual cells are plotted in ascending order.
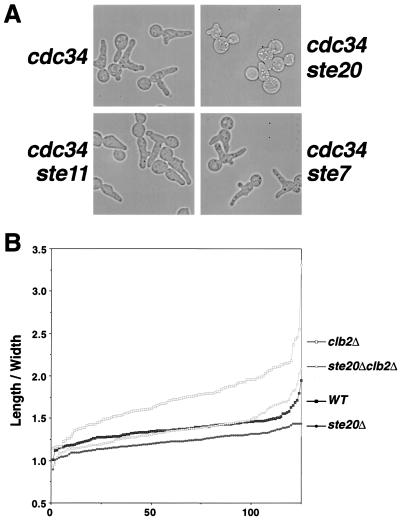
FIG. 5.
Ste20 is important for apical growth. (A) Morphology of cdc34, cdc34 ste20Δ, cdc34 ste11Δ, and cdc34 ste7Δ cells after incubation at 37°C for 6 h. The formation of elongated buds in the cdc34 mutant is hindered by ste20Δ but not by ste11Δ or ste7Δ mutations. Cells that failed to form elongated buds in the cdc34 ste20Δ culture are shown. (B) Length/width ratios of 125 individual cells from mid-log-phase wild-type (WT) ste20Δ, and clb2Δ cultures plotted in ascending order. ste20Δ cells are rounder than wild-type cells, and clb2Δ ste20Δ cells are not as elongated as clb2Δ cells.
FITC-ConA labeling.
Log phase cultures (1.5 ml) were collected, washed once with a solution containing 50 mM Tris-HCl (pH 7.5) and 100 mM NaCl, and sonicated to separate clumps. Fluorescein isothiocyanate (FITC)-concanavalin A (ConA) (Polysciences Inc., Warrington, Pa.), which binds tightly to glycoproteins of the cell wall, was added to the sonicated cell suspension to a final concentration of 100 μg/ml, and cells were incubated in the dark for 10 min at 25°C. Cells were then washed once and resuspended in 3 ml of yeast extract-peptone-adenine-dextrose (YPAD) medium to resume growth. After 45 min at 30°C, cells were fixed in 3.7% formaldehyde at 30°C, washed with PBS, and resuspended in PBS solution. Labeled cells were observed using fluorescence microscopy. The percentages of buds or new cells with apical and isotropic growth patterns were determined. Buds or new cells with weaker staining at their tips than at their bases were categorized as undergoing apical growth, while those with uniform staining were scored as undergoing isotropic growth. New cells were distinguished by the lack of FITC-ConA labeling at their birth scars.
Indirect immunofluorescence of Sec4.
Mid-log-phase cells from wild-type and mutant cultures were prepared for indirect immunofluorescence microscopy as described previously (75). Cells were fixed in 3.7% formaldehyde at 30°C for 1 h, spheroplasted, and attached to polylysine-coated multiwell microslides. Cells were then treated with 0.5% sodium dodecyl sulfate in PBS for 5 min, washed, and blocked with 0.1% bovine serum albumin (BSA) in PBS (PBS-BSA) for 30 min at room temperature. Cells were then incubated with a 1:4 dilution of monoclonal antibody against Sec4 (a generous gift from P. Novick's laboratory) overnight at 4°C. After washes with PBS-BSA, cells were incubated with a 1:200 dilution of preabsorbed Cy3-conjugated goat anti-mouse antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) at room temperature for 1.5 h, washed, mounted, and visualized by fluorescence microscopy.
Budding pattern analysis.
The cells used for budding pattern analysis were maintained in log phase for at least six consecutive generations. Mid-log-phase cells were then fixed in 3.7% formaldehyde at 30°C, washed with PBS, and resuspended in PBS. Calcofluor White was added to a final concentration of 2 μg/ml, and bud scars and birth scars were visualized by fluorescence microscopy (31). Birth scars are chitin-poor regions in the cell wall where cells separated from their mother. Bud scars are chitin-rich ring structures that mark the region where mother cells separated from their daughter cells. A bud site is defined as proximal when a bud or a bud scar resides in the third (portion) of the cell closest to the birth scar, as distal when it is in the third of the cell opposite to the birth scar and as medial when it is in the middle third of the cell (22, 78). Bud positions for first, second, and third bud sites were scored as described elsewhere (78). A total of 200 to 600 cells were scored for each division event of each sample.
To quantify the fidelity of bipolar bud site selection, only cells that had experienced at least three budding events were examined; cells with medial budding sites were scored as random budding. The percentage of random budding cells was then determined for each sample. For each sample, 100 cells were counted from at least four independent fields.
RESULTS
Spa2, Pea2, Bud6, and Bni1 are important for apical growth.
Wild-type diploid cells are normally ellipsoidal. However, microscopic examination of spa2Δ, pea2Δ, bud6Δ, and bni1Δ cells as well as of two other spa2 mutants, spa2(1-2, 116-1466) and spa2(1-410, 531-1466), which lack two conserved domains, SHD-I and -II (60), respectively, reveals that these cells are rounder than wild-type cells (Fig. 1A). To quantify this defect, we measured the length and the width of vegetatively grown wild-type and mutant cells relative to their birth sites (see Materials and Methods and illustration in Table 2). The ratio of length to width is expected to be 1 for round cells and >1 for both ellipsoidal and elongated cells. The length/width ratios, plotted by ascending value, for 100 randomly selected cells from three independent wild-type strains and several mutants are shown in Fig. 1B. Unlike wild-type cells, which have an average length/width ratio of 1.4, the average length/width ratios for the polarity mutants spa2Δ, spa2(1-2, 116-1466), spa2(1-410, 531-1466), pea2Δ, bud6Δ, and bni1Δ are closer to 1.0 (Table 2). Thus, all three spa2 mutants and pea2Δ, bud6Δ, and bni1Δ cells are quantitatively rounder than wild-type cells. Since yeast cell elongation occurs primarily during the apical growth phase, these results suggest roles for Spa2, Pea2, Bud6, and Bni1 in apical growth.
To investigate further the importance of SPA2, PEA2, BUD6, and BNI1 in apical growth, we used several yeast strains that form highly elongated buds due to pronounced apical growth. CDC34 encodes a component of the ubiquitin-dependent degradation machinery that targets the cyclin-dependent kinase inhibitor Sic1 for destruction (27, 63). Sic1 inhibits the activation of Clb-Cdc28p kinase (63); hence its stabilization results in failure to switch from apical to isotropic growth. After prolonged incubation at the restrictive temperature (37°C), temperature-sensitive cdc34 mutant cells form multiple buds that are thin and elongated (Fig. 2A). cdc34 spa2Δ cells also form multiple buds at 37°C. However, the buds are never as pointed and elongated as those of cdc34 single mutants; instead, they are much more rounded. Similar results are obtained for cdc34 pea2Δ, cdc34 bud6Δ, and cdc34 bni1Δ cells (Fig. 2A). cdc34 bni1Δ cells display the strongest defect, whereas cdc34 bud6Δ cells exhibit a less-severe defect in apical growth. Thus, bud elongation in cdc34 mutants requires the functions of SPA2, PEA2, BUD6, and BNI1.
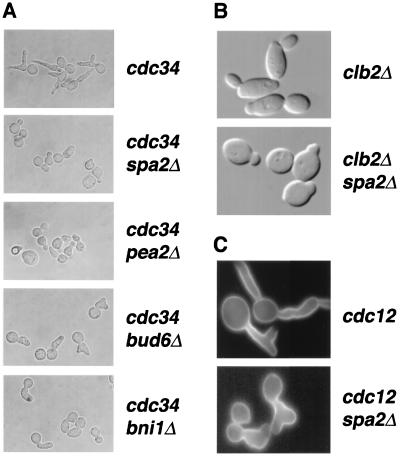
FIG. 2.
Spa2, Pea2, Bud6, and Bni1 participate in apical growth. (A) Spa2, Pea2, Bud6, and Bni1 are required for the elongated bud morphology of the cdc34 mutant grown at restrictive temperature. (B) Spa2 is required for the elongated cell shape of clb2Δ mutant cells. (C) Spa2 is required for the elongated bud morphology of the cdc12 mutant at 37°C. Mid-log-phase cultures were transferred from room temperature to 37°C for 6 h and fixed at 37°C. The morphologies of these cells were visualized by Nomarski microscopy (A and B) or fluorescence microscopy with Calcofluor White staining (C).
We also demonstrated a role for Spa2 in bud and cell elongation using two other mutant strains. Clb2 is the major cyclin that controls the switch from apical to isotropic growth and prevents actin repolarization to the mother-bud neck prior to cytokinesis (42). Deletion of CLB2 yields elongated cells as a result of a delayed apical-to-isotropic switch. As reported previously (42, 58, 72), we found that clb2Δ cells are more elongated, with an average length/width ratio of about 1.7 (Table 2), than wild-type cells. In contrast, spa2Δ clb2Δ double mutants are not elongated and have an average length/width ratio of 1.1, slightly higher than that of the spa2Δ mutant (Fig. 2B and Table 2). Thus, SPA2 is required for the elongated cell shape of the clb2Δ cells. At nonpermissive temperatures, cdc12 mutants form elongated buds due to the activation of a septin-dependent checkpoint that prevents the switch from apical to isotropic growth (5). As shown in Fig. 2C, SPA2 is required for the elongated bud phenotype of the cdc12 mutant at 37°C; cdc12 spa2Δ cells form buds that are much less elongated. In summary, analysis of cell morphology and the genetic studies using cdc34, cdc12, and clb2Δ mutants strongly indicate that SPA2, PEA2, BUD6, and BNI1 are important for apical growth.
spa2Δ mutants fail to confine cell wall expansion to a small region at the bud tip during apical growth.
We also analyzed apical growth and isotropic growth directly by using FITC-ConA labeling experiments (42, 73). Vegetatively growing cells were collected, labeled briefly over their entire surface with FITC-ConA, and then returned to growth in the absence of FITC-ConA. In wild-type cells, buds undergoing apical growth lack FITC-ConA staining at the bud tip but maintain strong staining at the base (Fig. 3A). This pattern indicates a tightly confined area of cell surface growth at the apical tip of the bud. Buds undergoing isotropic growth expand uniformly and have a uniformly faded staining on their surface. We found that the percentages of buds or cells with an apical growth pattern are very similar in wild-type, spa2Δ, pea2Δ, and bud6Δ cells (48, 45, 46, and 47%, respectively [Fig. 3B and data not shown]). However, the quality of apical growth in spa2Δ mutants is different from that in wild-type cells. Approximately 60% of spa2Δ buds with apical growth patterns show a gradient of FITC-ConA staining that is stronger at the base and gradually diminishes at the tip; the staining at these bud tips is weak but apparent (Fig. 3A, arrowheads). Only about 30% of wild-type buds undergoing apical growth show this pattern. The ConA-staining patterns for pea2Δ, bud6Δ, and bni1Δ cells are similar to that of spa2Δ cells (data not shown). Interestingly, bni1Δ cells also appear to have less apical growth (28%) than spa2Δ, pea2Δ, and bud6Δ cells; bni1Δ mutants have most severe polarity defects among these polarity mutants. In conclusion, the staining pattern in spa2Δ, pea2Δ, bud6Δ, and bni1Δ mutants suggests that, in these cells, apical growth is not as tightly confined at bud tips as in wild-type cells.
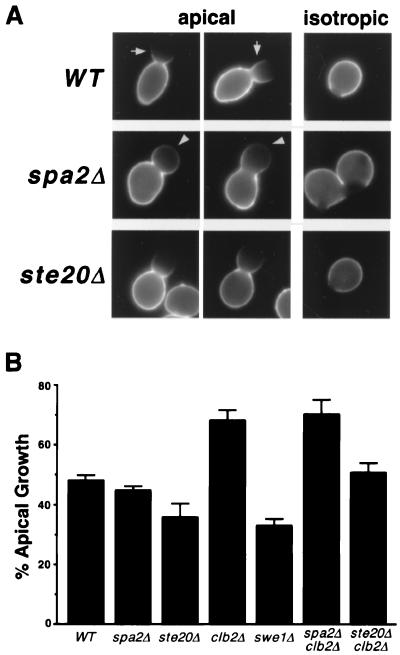
FIG. 3.
Cell wall growth in wild type and mutants analyzed by FITC-ConA labeling experiments. (A) Wild-type, spa2Δ, and ste20Δ cells were labeled on their cell surface with FITC-ConA and then returned to the growth medium lacking ConA. For each strain, two cells exhibiting apical growth and one undergoing isotropic growth are shown. Arrows indicate the strong staining at the base of wild-type buds. Arrowheads indicate the weak but apparent staining at the bud tip of spa2Δ mutants. (B) Percentages of vegetative cells with the apical growth pattern in wild-type (WT) and mutant cells. One hundred cells were examined in each scoring, and each strain was scored at least three times. Error bars, standard errors.
Sec4 is more dispersed in spa2Δ, pea2Δ, bud6Δ, and bni1Δ mutants.
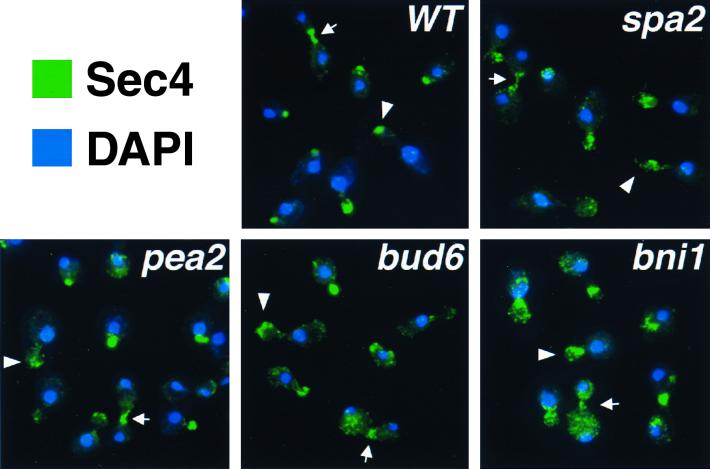
If apical growth is defective in spa2Δ, pea2Δ, bud6Δ, and bni1Δ cells, we might expect polarized secretion to be disturbed in these mutants. To address this possibility, the localization of a polarized marker, Sec4, which is involved in late secretory steps, was examined in spa2Δ, pea2Δ, bud6Δ, and bni1Δ mutants. In wild-type cells, Sec4 is tightly concentrated at bud tips during apical growth and at the mother-bud neck during cytokinesis (75) (Fig. 4). In spa2Δ, pea2Δ, bud6Δ, and bni1Δ mutants, despite a generally polarized localization pattern, Sec4 staining is more diffuse than in the wild-type cells. In small and medium budded cells, it is often in patches throughout the bud (Fig. 4, arrowheads). Likewise, in large budded cells undergoing cytokinesis, it is also less concentrated at the neck (Fig. 4, arrows). The Sec4 staining pattern is least concentrated in the bni1Δ mutant, which exhibits the most-severe defects in both morphogenesis and bipolar bud site selection. Taken together, these results suggest that Spa2, Pea2, Bud6, and Bni1 function to help concentrate polarized growth and secretion not only at bud tips but also at mother-bud necks.
FIG. 4.
Indirect immunofluorescence staining of Sec4 in wild-type and polarity mutants. Green represents the anti-Sec4 staining pattern, and blue corresponds to nuclei stained by DAPI (4′,6′-diamidino-2-phenylindole). Arrowheads and arrows indicate Sec4 staining at buds or bud tips and mother-bud necks, respectively.
Consistent with the role of Spa2, Pea2, Bud6, and Bni1 at the neck region, spa2Δ, spa2(1-2, 116-1466), pea2Δ, bud6Δ, and bni1Δ cells also exhibit abnormalities at the site of septation or cytokinesis. The mother-bud necks of these mutants are less constricted (wider) than those in wild-type cells; similar observations for spa2, bud6, and bni1 mutants have been described (2, 36, 69, 78). We also observed cell wall protrusions on the mother cells under Nomarski microscopy (Fig. 1A, arrow heads); when stained with Calcofluor white and visualized by fluorescence microscopy, these structures correspond to bud scars. Together, these observations suggest that Spa2, Pea2, Bud6, and Bni1 are important for septation.
Ste20 plays a role in apical growth.
Ste11 and Ste7 were previously identified as Spa2-interacting proteins by the yeast two-hybrid system (66). Ste11 and Ste7 are the MAPK kinase kinase and MAPK kinase, respectively, of the Ste MAPK pathway and are required for mating and pseudohypha formation (18, 44, 59, 70). Both of these processes also involve Spa2 (25, 48, 60). We therefore investigated whether Ste11, Ste7, and their upstream activator, Ste20, affect bud elongation of the temperature-sensitive cdc34 mutant. As shown in Fig. 5A, the cdc34 ste11Δ and cdc34 ste7Δ double mutants, like the cdc34 single mutant, form multiple elongated buds after 6 h at 37°C. Thus, STE11 and STE7 are not required for apical growth. Under the same conditions, cdc34 ste20Δ double mutants also form multiple buds, but most of these buds are not as elongated as those of the cdc34 mutant (Fig. 5A), although some cells do make elongated buds (see Discussion). Moreover, ste20Δ/ste20Δ diploid cells are less elongated, with an average length/width ratio of 1.23, compared to 1.37 for the isogenic wild-type cells (n = 125 cells) (Fig. 5B and Table 2). The difference is even more pronounced in a clb2Δ background, in which the length/width ratios are 1.37 for ste20Δ/ste20Δ clb2Δ/clb2Δ cells, compared to 1.73 for clb2Δ/clb2Δ cells (Fig. 5B). Thus, like Spa2, Ste20 also plays a role in apical growth.
We also directly examined apical growth in the ste20Δ mutant using FITC-ConA labeling (Fig. 3). Whereas 48% of wild-type buds undergo apical growth, only 36% of the buds exhibit apical growth in the ste20Δ mutant (Fig. 3B). Thus, ste20Δ mutants may have a shorter apical growth phase compared with wild-type cells. Growth at the bud tip in ste20Δ mutants appears to be concentrated, similar to that in wild-type cells (Fig. 3A), but unlike spa2Δ, bud6Δ, and bni1Δ mutants.
ste20Δ is defective in choosing distal budding sites.
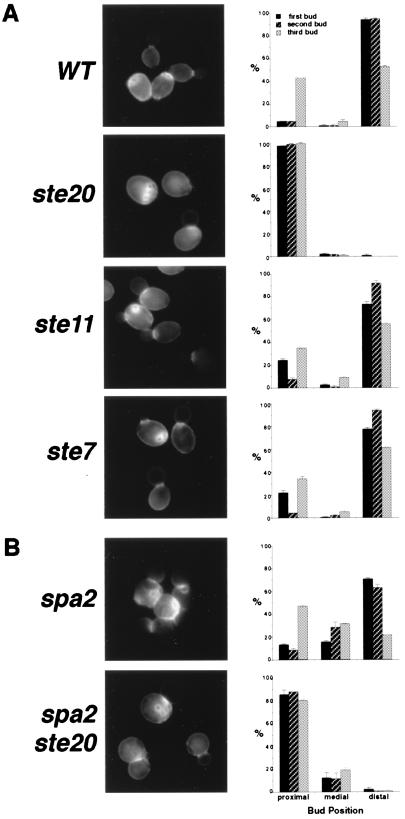
Since Ste20 is also involved in the control of apical growth, we examined whether Ste20 is required for bipolar bud site selection as is the case for Spa2, Pea2, Bud6, and Bni1. Haploid bud3Δ strains, which normally bud in a bipolar pattern, were used for this analysis; bud3Δ strains have been used successfully by Zahner et al. (78) to identify genes involved in bipolar bud site selection. The ste11Δ and ste7Δ mutations had little, if any, effect on the bipolar pattern (Fig. 6A). In contrast, the ste20Δ mutant displayed a unipolar budding pattern, with bud scars clustered adjacent to the birth scar, at the proximal pole (Fig. 6A). In these cells, individual bud sites can be observed adjacent to, overlapping, or within the birth scar and in the vicinity of other bud scars; however, these bud scars are not always immediately adjacent to the bud site of the previous cell cycle. This budding pattern is typical of the bipolar pattern at the proximal pole and is distinct from the axial budding pattern used by wild-type haploid cells. In the axial pattern, new buds form immediately adjacent to the preceding division site, producing a continuous chain of chitin rings (10, 22).
FIG. 6.
Evaluation of bud position for the first three budding events in wild-type (WT) and mutant cells (right panels). All strains used for this analysis are derivatives of MATa bud3Δ haploids. Bud scar staining is presented for each sample (left panels). Error bars, standard errors. (A) ste20Δ mutants bud almost exclusively at the proximal pole. (B) Ste20 is required for spa2Δ cells to bud at the distal pole; Spa2 is required for ste20Δ to bud precisely at the proximal pole.
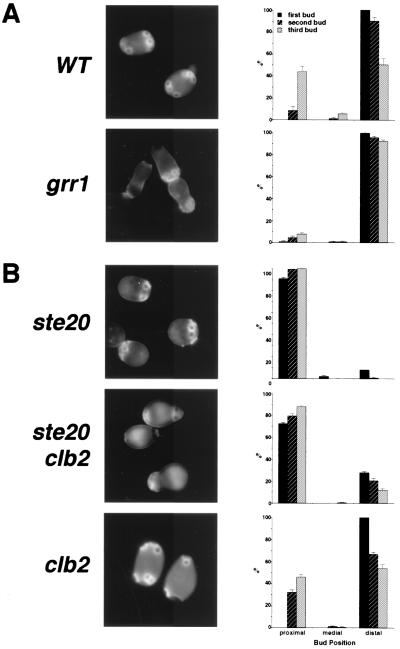
To ensure that the bud site selection defect of ste20Δ cells is independent of bud3Δ, we analyzed diploid mutants. ste20Δ/ste20Δ diploid cells are strongly biased toward choosing the proximal budding site and only rarely bud at the distal site (8% of the first buds, 0.7% of the second buds, and 0% of the third buds of ste20Δ/ste20Δ cells form at distal sites, compared to 97% of the first, 83% of the second, and 41% of the third buds for wild-type cells [Fig. 7]). The budding pattern of heterozygous ste20Δ/STE20 cells is similar to that of isogenic wild-type cells but is slightly biased towards the selection of proximal budding sites (data not shown). Thus, ste11Δ and ste7Δ mutants, which do not have a detectable defect in apical growth, display normal budding patterns, whereas ste20Δ mutants, which have defects in apical growth, are defective in distal bud site selection.
FIG. 7.
(A) Deletion of GRR1 enhances distal budding. (B) Deletion of CLB2 partially restores distal bud site selection of ste20 mutants. Bud positions were scored for the first three budding events in diploid wild-type (WT) grr1Δ/grr1Δ, ste20Δ/ste20Δ, clb2Δ/clb2Δ, and ste20Δ/ste20Δ clb2Δ/clb2Δ cells. Error bars, standard errors.
The phenotype of ste20Δ cells is very similar to that described for bud8 mutants (78), suggesting that these two mutants function in the same pathway. Consistent with this hypothesis, ste20Δ bud8Δ double mutants display an identical budding pattern to those of single mutants, suggesting that they function in the same pathway for bud site selection (data not shown; see Discussion).
Spa2 and Ste20 perform different functions in bipolar bud site selection.
ste20Δ and spa2Δ have different phenotypes in apical growth and bud site selection. ste20Δ cells are defective only in distal budding, whereas spa2Δ mutants fail to bud precisely at both poles; we found that in spa2Δ mutants, even though the budding events may be categorized as either proximal or distal, they often are near, but not at, the pole of the cell (wild-type cells usually use the poles). This is consistent with a previous observation that the first two bud sites in spa2 and bud6 cells were generally not directly adjacent to each other (78). To further decipher the relationship between SPA2 and STE20 in the process of bipolar bud site selection, we analyzed the budding pattern of a spa2Δ ste20Δ double mutant in the bud3Δ background and compared it to isogenic bud3Δ spa2Δ and bud3Δ ste20Δ mutants. spa2Δ ste20Δ double mutants exhibit a combination of the bud site selection phenotypes observed in spa2Δ and ste20Δ single mutants. Similar to ste20Δ mutants, the double mutants use distal bud sites very infrequently (Fig. 6B). This is in contrast to spa2Δ mutants, which prefer distal sites for the first two budding events (68, 78). Thus, spa2Δ mutants are still capable of distinguishing the proximal pole from the distal pole, and STE20 is required for both wild-type and spa2Δ cells to bud at distal sites. In the spa2Δ ste20Δ double mutant, most budding events occur within the proximal half of the cells, similar to the budding pattern of the ste20Δ mutant. However, the bud scars of the ste20Δ mutant are tightly clustered near the birth scar while the bud scars of the spa2Δ ste20Δ double mutant are more loosely distributed within the proximal hemisphere (Fig. 6B), indicating a role of Spa2 in confining budding within a small area at proximal poles. These data also indicate that Spa2 and Ste20 perform different functions in bipolar bud site selection.
Genes controlling cell cycle progression influence bipolar bud site selection.
ste20Δ cells and polarity mutants spa2Δ, pea2Δ, bud6Δ, and bni1Δ display defects in both apical growth and bipolar bud site selection. The strong correlation between these two processes prompted us to hypothesize that the apical growth phase is crucial for bipolar bud site selection. This idea was tested further using several yeast mutants. We first investigated whether extending bud tip growth can promote budding at distal poles. grr1Δ cells are elongated because apical growth is enhanced in these cells due to accumulation of high levels of G1 cyclins (3, 4). Diploid grr1Δ/grr1Δ cells bud in a bipolar pattern. However, these cells display a stronger-than-normal bias towards using distal bud sites; this bias is especially apparent in the later budding events (Fig. 7A). For example, in a vegetatively growing grr1Δ culture, 92% of the third buds form at distal poles, compared to only 51% of the third buds for wild-type cells (Fig. 7A). In addition, we also observed a decreased frequency of nonpolar (i.e., medial) budding in grr1Δ mutants for the first three budding events (Fig. 7A). Thus, grr1Δ mutants, which have increased apical growth at bud tips, preferentially bud at distal poles and display less random budding.
We next analyzed several strains that differ in their timing of the apical-to-isotropic switch: the fidelity of bipolar bud site selection was examined for wild-type, clb2Δ, and swe1Δ vegetative cells with three or more bud scars. Clb2-Cdc28 triggers the switch from apical growth to isotropic growth and prevents entry into cytokinesis (42, 72). Deletion of CLB2 results in an extended apical growth phase (42) and a more elongated cell shape (42, 58, 72). SWE1 is homologous to the Schizosaccharomyces pombe wee1 gene and encodes a kinase that negatively regulates the activity of the Clb1, 2-Cdc28 kinase (8). Using FITC-ConA labeling experiments, we found that swe1Δ cells exhibit less apical growth (Fig. 3B). In addition, the shape of swe1Δ cells is less elongated, with a length/width ratio of 1.25, than that of wild-type cells, with a ratio of 1.42 (Table 2). Decreased apical growth and shorter cell length of the swe1Δ mutant indicate that this mutant switches to isotropic growth earlier.
In our analysis, 6% of wild-type cells displayed a random budding pattern (for definition, see Materials and Methods). In contrast, only 1% of clb2Δ mutant cells, which exhibit more apical growth, budded randomly, whereas swe1Δ mutants, with reduced apical growth, showed a random bud scar distribution in 18% of the cells (Fig. 8). Inspection of individual cells in the swe1Δ and wild-type populations reveals that most of the random-budding cells are rounder than their bipolar counterparts in the same culture. Together, these observations demonstrate that cells with enhanced apical growth use bipolar bud sites with higher fidelity, whereas cells with less apical growth display a reduced fidelity of bipolar bud site selection.
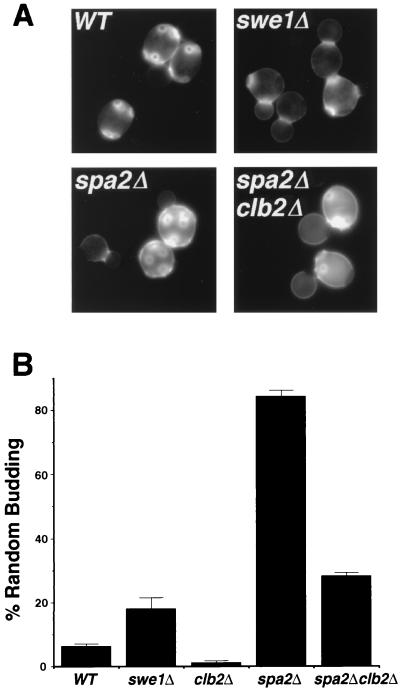
FIG. 8.
The fidelity of bipolar bud site selection is affected by the timing of the switch from apical to isotropic growth. (A) Bud scar staining of wild-type (WT) swe1Δ, spa2Δ, and clb2Δ spa2Δ cells. Although most of the swe1Δ cells exhibit a bipolar budding pattern, random-budding cells from the swe1Δ culture are shown here. Defects in bipolar bud site selection of the spa2Δ mutant are partially suppressed by clb2Δ. (B) Percentage of random-budding cells in the mid-log-phase wild-type (WT) swe1Δ, clb2Δ, spa2Δ, and clb2Δ spa2Δ cultures. Error bars, standard errors.
The bud site selection defect of spa2Δ and ste20Δ cells can be suppressed by deletion of CLB2.
We reasoned that if insufficient or defective apical growth is responsible for the random budding phenotype of the spa2Δ mutant, this phenotype might be corrected by extending the period of apical growth in spa2Δ cells. To test this idea, we examined and compared the budding patterns of diploid spa2Δ mutants and spa2Δ clb2Δ double mutants, which exhibit more apical growth (70%) than spa2Δ single mutants (45%), as judged from the FITC-ConA labeling experiment (Fig. 3B). Approximately 84% of diploid spa2Δ cells that bud three or more times exhibit random budding, whereas this percentage decreases significantly to approximately 28% in the spa2Δ clb2Δ double mutant (Table 2). However, in some spa2Δ clb2Δ double mutant cells that display bipolar budding, the bud scars are not tightly concentrated at the poles as in wild-type cells (Fig. 8A), which presumably reflects the role of Spa2 in concentrating polarized growth. Nevertheless, deletion of CLB2, which extends the duration of polarized growth, can partially suppress the bud site selection defect of spa2Δ cells.
We also investigated whether deletion of CLB2 can suppress the inability of ste20 cells to choose distal sites, which we speculate is due, in part, to insufficient apical growth in these cells. Deletion of CLB2 extended the apical growth phase of ste20 mutants (36% for ste20Δ/ste20Δ and 51% for ste20Δ/ste20Δ clb2Δ/clb2Δ double mutants [Fig. 3B]). ste20Δ/ste20Δ clb2Δ/clb2Δ double mutants display a bipolar budding pattern and an increased usage of distal budding sites (27.7% of the first buds, 20.5% of the second buds, and 11.7% of the third buds are placed distally [Fig. 7B]) compared to ste20Δ/ste20Δ cells (7.7% of the first buds, 0.7% of the second buds, and 0% of the third buds are placed distally). We do not expect restoration of distal budding in ste20Δ/ste20Δ clb2Δ/clb2Δ double mutants to a wild-type level since deletion of CLB2 is expected to initiate repolarization earlier and is likely to cause a longer repolarization phase, which we think might also promote budding at the proximal pole (see Discussion). Thus, although proximal bud sites of ste20Δ/ste20Δ clb2Δ/clb2Δ double mutants are still more preferred than distal sites compared to wild-type cells, increasing apical growth can partially restore distal site usage in ste20Δ mutants.
DISCUSSION
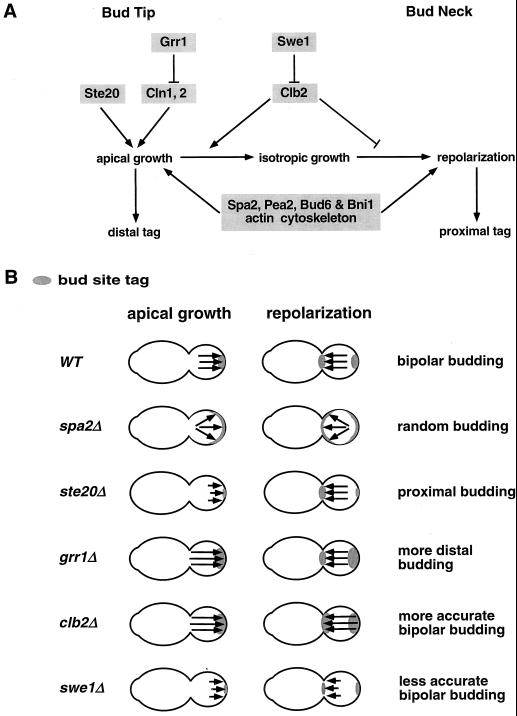
In this study, we demonstrated that SPA2, PEA2, BUD6, BNI1, and STE20 are required for the proper execution of apical growth and that STE20, like SPA2, PEA2, BUD6, and BNI1, is required for bipolar bud site selection. The intimate relationship between apical growth and bipolar bud site selection was further demonstrated by analyzing cell cycle mutants that exhibit altered durations of polarized growth phases. We propose that apical growth at bud tips is important for establishing the bud site tags at distal poles, and that repolarization of growth materials at mother-bud necks prior to cytokinesis is a crucial process for marking proximal poles (Fig. 9A). This model is similar to but more detailed than one of the possibilities suggested by Chant and Pringle (10). Since apical growth is also an important determinant of cell shape, this model suggests a means by which the establishment of a cell division plane can be coupled to cell shape, as is evident during pseudohyphal differentiation in budding yeast.
FIG. 9.
(A) A model to explain how components involved in cell morphogenesis (shaded) contribute to the establishment of bipolar bud site tags in yeast. (B) Budding patterns of wild-type, spa2Δ, ste20Δ, grr1Δ, clb2Δ, and swe1Δ cells interpreted by the model proposed above. Apical growth and repolarization are indicated by arrows. The relative length of each growth period corresponds to the length of the arrow. Growth direction is represented by the direction of the arrowhead. Parallel arrows indicate confined growth patterns, while dispersed arrows indicate diffuse growth patterns. The distribution of bipolar bud site tags is indicated by gray-shaded areas. See Discussion for more details.
The role of Spa2, Pea2, Bud6, and Bni1 in polarized growth and bipolar bud site selection.
We demonstrated that Spa2, Pea2, Bud6, and Bni1 are all required for elongation of cells and buds in wild-type and cdc34 strains and that Spa2 is also required for cell and bud elongation in clb2Δ and cdc12 cells. Thus, Spa2, Pea2, Bud6, and Bni1 control cell shape by participating in apical growth. Since the length of the apical growth phase is similar in wild type and spa2Δ cells (Fig. 3B), we presume that the quality of apical growth is affected in the mutants (see below).
Spa2, Pea2, Bud6, and Bni1 may serve to confine growth to a restricted region rather than to establish polarized growth directly. Unlike mutations in polarity establishment genes, such as cdc24 and cdc42 (15, 55), spa2Δ, pea2Δ, bud6Δ, and bni1Δ cells are still able to undergo polarized growth to form buds or mating projections. These mutants also exhibit polarized distribution of the actin cytoskeleton and a secretion marker, Sec4. However, unlike wild-type cells, these mutants form buds that are quite round and fail to concentrate Sec4 at sites of polarized growth. Furthermore, the mating-projection tip of the spa2Δ mutant is not pointed and contains actin patches that are less concentrated (25) (data not shown). Given that Spa2, Pea2, Bud6, and Bni1 are able to interact with one another and colocalize at the tips of buds and mating projections (2, 19, 24, 25, 66, 68, 74), we suggest that these polarisome proteins confine growth by forming a multiprotein complex that helps concentrate the actin cytoskeleton and/or exocytic vesicles at growth sites.
Prior to cytokinesis, a similar complex containing Spa2, Pea2, Bud6, and Bni1 may form at the mother-bud neck to help concentrate growth materials to this region. This idea is consistent with the following observations. First, Spa2, Pea2, Bud6, and Bni1 localize to the mother-bud neck just before and during septation (2, 34, 68, 69, 74). Second, Sec4 is less concentrated in this region in spa2Δ, pea2Δ, bud6Δ, and bni1Δ mutants (Fig. 4). Third, we have observed structural abnormalities at neck regions and in bud scars in these mutants (Fig. 1), consistent with previous observations that spa2Δ, bud6Δ, and bni1Δ cells have cytokinesis defects (2, 36, 69, 78). It is thus likely that Spa2, Pea2, Bud6, and Bni1 concentrate the actin cytoskeleton and secretory vesicles at growth sites during both apical growth and septation.
How might apical growth and septation defects in the spa2Δ mutant account for its random budding pattern? Two observations indicate that the two poles are probably still marked in spa2 mutants despite the random budding phenotype. First, the first budding event in spa2 mutants is generally positioned at the distal pole, suggesting that a functional bud site selection signal exists there. Second, in the ste20Δ background, which buds proximally, deletion of SPA2 does not simply lead to random budding, as would be expected for mutants completely lacking bud site tags. Instead, spa2Δ ste20Δ double mutants bud within the proximal hemisphere, although their bud scars are not tightly clustered (Fig. 6B); this indicates that the proximal poles are marked in these cells. Thus, in spa2Δ mutants, the bud site tags are probably still directed to both poles of the daughter cell. However, as discussed earlier, spa2Δ mutants fail to concentrate growth at both poles, and hence bipolar tags are deposited in a less-compacted fashion (Fig. 9B). As a result, diploid spa2Δ cells fail to choose bud sites with precision and therefore display a “random-like” budding pattern. The random budding pattern observed in diploid pea2Δ, bud6Δ, and bni1Δ mutants may be due to the same reason. A large number of other mutants affecting the actin cytoskeleton and secretory pathway disrupt bipolar bud site selection similar to spa2Δ (7, 16, 20, 29, 67, 77). We speculate that these mutants also affect apical growth as well as repolarization to the mother-bud neck and that defects in these processes lead to their diploid-specific random budding phenotypes.
Role of Ste20 in apical growth and bipolar bud site selection.
We also demonstrated that Ste20 is involved in both apical growth and bipolar bud site selection. Disruption of STE20 results in cell elongation defects in wild-type, cdc34, and clb2Δ strains (Fig. 5 and Table 2) and shortens the apical growth phase (Fig. 3B). Ste20 is also required for proper bipolar bud site selection: diploid ste20Δ mutants rarely bud at distal poles (Fig. 7B). We suggest that the distal poles of ste20Δ mutants are not properly marked due, at least in part, to insufficient apical growth (Fig. 9B). In addition, the Ste20 kinase may affect bud site selection by mechanisms independent of its role in apical growth, for example, by directly phosphorylating the distal tag (perhaps Bud8; see below).
Ste20 and Spa2 contribute differently in the processes of apical growth, cytokinesis, and bipolar bud site selection. Ste20 does not seem to be required for restricting growth to a small region as does Spa2; the shape of buds in cdc34 ste20Δ double mutants is more pointed, unlike that of the rounder cdc34 spa2Δ buds, and ste20Δ mutants can still confine cell wall expansion to a small region during apical growth (Fig. 3A). Furthermore, the machinery that facilitates apical growth may still be functional, because some of the cdc34 ste20Δ buds are able to elongate. Finally, since ste20Δ mutants exhibit less apical growth (Fig. 3B), Ste20 may be required for timely activation or for maintenance of the activity of the apical growth machinery rather than being a component of the machinery itself. Ste20 has been shown to localize to bud tips like Spa2, but unlike Spa2, it has not been found at sites of cytokinesis (40, 54). In addition, we do not observe septation defects in ste20Δ cells. Thus, Ste20 is probably not critical for septation and therefore, as predicted by our model, does not affect budding at the proximal pole (Fig. 9B).
The role of Ste20 in apical growth and bipolar bud site selection is unique among its associated signaling components, including its downstream kinases, Ste11 (70) and Ste7 (18), and another p21-activated kinase, Cla4 (14). We have found that Ste11 and Ste7 are not required for apical growth and these two kinases and their scaffolding protein Ste5 (11, 56) are not important for bipolar bud site selection (Fig. 6; data not shown). Consistent with our finding, it was reported that Ste20, but not Cla4 or Ste7, is required for cell elongation and polarization of the actin cytoskeleton in the cdc28-4 mutant arrested at 37°C (17). These observations suggest that either Ste20 has additional targets that control these functions independently of Ste11 and Ste7 or that a redundant pathway substitutes for Ste11 and Ste7 functions. We also found that deletion of CLA4 does not result in the same morphological abnormality and budding pattern alteration in diploid cells as the ste20Δ mutation (data not shown). Hence, Ste20 and Cla4 have nonredundant vegetative functions.
How might Ste20 exert its vegetative function in apical growth? During apical growth, Ste20 may transmit signals from the polarity establishment protein Cdc42 (reviewed in references 12 and 15), a Rho-type GTPase that interacts with Ste20 (79), to components that execute or maintain apical growth. Proper localization of Ste20 in both vegetative and mating cells requires its ability to bind Cdc42 (40, 54). Thus, the functions of Ste20 during apical growth may be controlled by Cdc42, similar to that observed during the mating response and filamentous differentiation. Given the localization of the Ste20 kinase to bud tips, it may phosphorylate and activate key components at bud tips to initiate and/or maintain apical growth that leads to proper establishment of distal bud site tags.
The identical budding pattern of diploid ste20Δ, bud8Δ, and ste20Δ bud8Δ double mutants indicates that Ste20 and Bud8 function in the same pathway to promote budding at the distal pole. Bud8 is thought to be the distal pole marker or involved in proper positioning of the marker (78). Thus, Ste20 might play a role in phosphorylating the potential distal tag in addition to its role in apical growth. Alternatively, Bud8 may function in apical growth like Ste20. Perhaps the Ste20 kinase phosphorylates and regulates Bud8 function, or perhaps Bud8 regulates the kinase activity of Ste20.
Regulation of cell morphogenesis and budding pattern by the cell cycle machinery.
We found that the cell cycle machinery, which regulates bud morphogenesis (41), also influences bipolar bud site selection. In diploid grr1Δ mutants, which stabilize G1 cyclins (3), apical growth is enhanced and buds are preferentially formed at distal poles (Fig. 7A). Deletion of SWE1 shortens the apical growth phase and results in a rounder cell shape, and these cells also show a decreased fidelity in bipolar bud site selection (Fig. 8B). In contrast, deletion of CLB2, which results in a longer apical growth phase, leads to the formation of elongated cells (42, 58, 72) and improves the fidelity of bipolar bud site selection (Fig. 8B). The analyses of clb2Δ, swe1Δ, and grr1Δ cells further indicate a strong link between the control of cell morphogenesis and bipolar bud site selection.
Quantification of individual budding events shows that the clb2Δ mutant does not simply increase its distal site usage as does the diploid grr1Δ/grr1Δ mutant (Fig. 7); rather, it reduces the number of randomly budding cells (Fig. 8B). Given that Clb2 also has a negative role in repolarization of actin to septation sites after nuclear division (42), this situation can be explained by our model: the extended duration of both the apical growth phase and the repolarization phase would allow both distal and proximal tags to be reinforced in clb2Δ cells (Fig. 9B). The fact that deletion of CLB2 can partially suppress the bud site selection defect of spa2Δ and restore distal bud site usage of ste20Δ mutants further supports this idea. The enhancement of bud site tags in both poles may be part of the reason why deleting CLB2 in the ste20Δ mutant does not restore distal budding to a wild-type level. In contrast to clb2Δ cells, swe1Δ cells may enter the repolarization phase later and therefore have a shorter duration for this process and weaker proximal tags. Together with the shorter apical growth phase, swe1Δ cells probably have weaker bipolar bud site tags at both poles and hence decreased fidelity of bipolar bud site selection (Fig. 9B). Therefore, the length of apical growth and repolarization affects not only cell shape but also the fidelity of bipolar budding.
Role of apical growth in morphogenesis and bud site selection in filamentous differentiated cells.
Cells undergoing filamentous differentiation, such as pseudohyphal and haploid invasive growth, alter both their shape and budding pattern. Filamentous growing cells are extremely elongated and bud more frequently at distal poles (26, 59, 60). How are these two features simultaneously achieved? A possible mechanism is by prolonging or enhancing apical growth, which would facilitate bud elongation and strengthen distal bud site tags. Consistent with this idea, components involved in apical growth play important roles in filamentous differentiation. Spa2, Pea2, and Bni1 are required for formation of elongated filamentous cells, and Spa2 is essential for bud site selection during this process (48, 60). Ste20 is also essential for signaling during filamentous differentiation (45, 59). Contrary to our observation in vegetatively growing cells, it has been reported that Ste20 is not required for the budding pattern of filamentous cells (44, 59). This discrepancy might be explained by a longer apical growth phase that helps restore the usage of distal bud sites in filamentous differentiating ste20 cells. Thus, the defect of filamentous differentiation in spa2, pea2, and bni1 mutants and perhaps part of the filamentous growth defect in ste20 mutants are likely due to defects in apical growth.
Cell cycle regulators involved in apical growth are also crucial for filamentous differentiation. Cln1 and Cln2 are required for filamentous differentiation (4, 53); these G1 cyclins are partially stabilized in pseudohyphal cells (4). In addition, the clb2Δ mutant is more sensitive to pseudohyphal induction (37). The involvement of Cln1, Cln2, and Clb2 in filamentous growth suggests that the cell cycle machinery may help promote this differentiation process by prolonging the duration of apical growth. Therefore, filamentous differentiation of yeast cells demonstrates that cell elongation and budding pattern alteration can be coordinated by promoting apical growth.
ACKNOWLEDGMENTS
We thank E. Leberer and B. Santos for generous gifts of plasmids and strains. We also thank P. Novick for kindly providing the Sec4 monoclonal antibody. We thank C. Costigan, B. Manning, G. Michaud, B. Santos, and S. Vidan for critical comments on the manuscript. We also thank the G. S. Roeder laboratory for use of the microscope and the charge-coupled device camera.
This research was supported by National Institute of Health grant GM36494.
REFERENCES
- 1.Adams A, Pringle J. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amberg D C, Zahner J E, Mulholland J W, Pringle J R, Botstein D. Aip3p/Bud6p, a yeast actin-interacting protein that is involved in morphogenesis and the selection of bipolar budding sites. Mol Biol Cell. 1997;8:729–753. doi: 10.1091/mbc.8.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barral Y, Jentsch S, Mann C. G1 cyclin turnover and nutrient uptake are controlled by a common pathway in yeast. Genes Dev. 1995;9:399–409. doi: 10.1101/gad.9.4.399. [DOI] [PubMed] [Google Scholar]
- 4.Barral Y, Mann C. G1 cyclin degradation and cellular differentiation of Saccharomyces cerevisiae. C R Acad Sci Ser III. 1995;318:43–50. [PubMed] [Google Scholar]
- 5.Barral Y, Parra M, Bidlingmaier S, Snyder M. Nim1-related kinases coordinate cell cycle progression with organization of the peripheral cytoskeleton. Genes Dev. 1999;13:176–187. doi: 10.1101/gad.13.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baudin A, Ozier-Kalogeropoulos O, Denouel A, Lacroute F, Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer F, Urdaci M, Aigle M, Crouzet M. Alteration of a yeast SH3 protein leads to conditional viability with defects in cytoskeletal and budding patterns. Mol Cell Biol. 1993;13:5070–5084. doi: 10.1128/mcb.13.8.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booher R N, Deshaies R J, Kirschner M W. Properties of Saccharomyces cerevisiae wee1 and its differential regulation of p34CDC28 in response to G1 and G2 cyclins. EMBO J. 1993;12:3417–3426. doi: 10.1002/j.1460-2075.1993.tb06016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chant J, Corrado K, Pringle J R, Herskowitz I. Yeast BUD5, encoding a putative GDP-GTP exchange factor, is necessary for bud site selection and interacts with bud formation gene BEM1. Cell. 1991;65:1213–1224. doi: 10.1016/0092-8674(91)90016-r. [DOI] [PubMed] [Google Scholar]
- 10.Chant J, Pringle J R. Patterns of bud-site selection in the yeast Saccharomyces cerevisiae. J Cell Biol. 1995;129:751–765. doi: 10.1083/jcb.129.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi K-Y, Satterberg B, Lyons D M, Elion E A. Ste5 tethers multiple protein kinases in the MAP kinase cascade required for mating in S. cerevisiae. Cell. 1994;78:499–512. doi: 10.1016/0092-8674(94)90427-8. [DOI] [PubMed] [Google Scholar]
- 12.Costigan C, Snyder M. Cell polarity in the budding yeast, Saccharomyces cerevisiae. Adv Mol Cell Biol. 1998;26:1–66. [Google Scholar]
- 13.Cross F R. Starting the cell cycle: what's the point? Curr Opin Cell Biol. 1995;7:790–797. doi: 10.1016/0955-0674(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 14.Cvrčková F, De Virgilio C, Manser E, Pringle J, Nasmyth K. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 1995;9:1817–1830. doi: 10.1101/gad.9.15.1817. [DOI] [PubMed] [Google Scholar]
- 15.Drubin D G, Nelson W J. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 16.Durrens P, Revardel E, Bonneu M, Aigle M. Evidence for a branched pathway in the polarized cell division of Saccharomyces cerevisiae. Curr Genet. 1995;27:213–216. doi: 10.1007/BF00326151. [DOI] [PubMed] [Google Scholar]
- 17.Eby J J, Holly S P, van Drogen F, Grishin A V, Peter M, Drubin D G, Blumer K J. Actin cytoskeleton organization regulated by the PAK family of protein kinases. Curr Biol. 1998;8:967–970. doi: 10.1016/s0960-9822(98)00398-4. [DOI] [PubMed] [Google Scholar]
- 18.Errede B, Gartner A, Zhou Z, Nasmyth K, Ammerer G. MAP kinase-related FUS3 from S. cerevisiae is activated by STE7 in vitro. Nature. 1993;362:261–264. doi: 10.1038/362261a0. [DOI] [PubMed] [Google Scholar]
- 19.Evangelista M, Blundell K, Longtine M S, Chow C J, Adames N, Pringle J R, Peter M, Boone C. Bni1p, a yeast formin linking Cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–122. doi: 10.1126/science.276.5309.118. [DOI] [PubMed] [Google Scholar]
- 20.Finger F P, Novick P. Sec3p is involved in secretion and morphogenesis in Saccharomyces cerevisiae. Mol Biol Cell. 1997;8:647–662. doi: 10.1091/mbc.8.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finger F P, Novick P. Spatial regulation of exocytosis: lessons from yeast. J Cell Biol. 1998;142:609–612. doi: 10.1083/jcb.142.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flescher E G, Madden K, Snyder M. Components required for cytokinesis are important for bud site selection in yeast. J Cell Biol. 1993;122:373–386. doi: 10.1083/jcb.122.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freifelder D. Bud position in Saccharomyces cerevisiae. J Bacteriol. 1960;124:511–523. doi: 10.1128/jb.80.4.567-568.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujiwara T, Tanaka K, Mino A, Kikyo M, Takahashi K, Shimizu K, Takai Y. Rho1p-Bni1p-Spa2p interactions: implication in localization of Bni1p at the bud site and regulation of the actin cytoskeleton in Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:1221–1233. doi: 10.1091/mbc.9.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gehrung S, Snyder M. The SPA2 gene of Saccharomyces cerevisiae is important for pheromone-induced morphogenesis and efficient mating. J Cell Biol. 1990;111:1451–1464. doi: 10.1083/jcb.111.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gimeno C J, Ljungdahl P O, Styles C A, Fink G R. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 27.Goebl M G, Yochem J, Jentsch S, McGrath J P, Varshavsky A, Byers B. The yeast cell cycle gene CDC34 encodes a ubiquitin-conjugating enzyme. Science. 1988;241:1331–1335. doi: 10.1126/science.2842867. [DOI] [PubMed] [Google Scholar]
- 28.Guthrie C, Fink G R, editors. Methods in enzymology. 194. Guide to yeast genetics and molecular biology. San Diego, Calif: Academic Press; 1991. [PubMed] [Google Scholar]
- 29.Haarer B K, Corbett A, Kweon Y, Petzold A S, Silver P, Brown S S. SEC3 mutations are synthetically lethal with profilin mutations and cause defects in diploid-specific bud-site selection. Genetics. 1996;144:495–510. doi: 10.1093/genetics/144.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartwell L H. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971;69:265–276. doi: 10.1016/0014-4827(71)90223-0. [DOI] [PubMed] [Google Scholar]
- 31.Hayashibe M, Katohda S. Initiation of budding and chitin-ring. J Gen Appl Microbiol. 1973;19:23–39. [Google Scholar]
- 32.Herskowitz I, Park H-O, Sanders S, Valtz N, Peter M. Programming of cell polarity in budding yeast by endogenous and exogenous signals. Cold Spring Harbor Symp Quant Biol. 1995;60:717–727. doi: 10.1101/sqb.1995.060.01.078. [DOI] [PubMed] [Google Scholar]
- 33.Hicks J B, Strathern J N, Herskowitz I. Interconversion of yeast mating types. III. Action of the homothallism (HO) gene in cells homozygous for the mating type locus. Genetics. 1977;85:395–405. doi: 10.1093/genetics/85.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jansen R P, Dowzer C, Michaelis C, Galova M, Nasmyth K. Mother cell-specific HO expression in budding yeast depends on the unconventional myosin Myo4p and other cytoplasmic proteins. Cell. 1996;84:687–697. doi: 10.1016/s0092-8674(00)81047-8. [DOI] [PubMed] [Google Scholar]
- 35.Kilmartin J V, Adams A E M. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984;98:922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohno H, Tanaka K, Mino A, Umikawa M, Imamura H, Fujiwara T, Fujita Y, Hotta K, Qadota H, Watanabe T, Ohya Y, Takai Y. Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 1996;15:6060–6068. [PMC free article] [PubMed] [Google Scholar]
- 37.Kron S J, Gow N A R. Budding yeast morphogenesis: signalling, cytoskeleton and cell cycle. Curr Opin Cell Biol. 1995;7:845–855. doi: 10.1016/0955-0674(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 38.Leberer E, Dignard D, Harcus D, Thomas D Y, Whiteway M. The protein kinase homologue Ste20p is required to link the yeast pheromone response G-protein beta gamma subunits to downstream signalling components. EMBO J. 1992;11:4815–4824. doi: 10.1002/j.1460-2075.1992.tb05587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leberer E, Thomas D Y, Whiteway M. Pheromone signalling and polarized morphogenesis in yeast. Curr Opin Genet Dev. 1997;7:59–66. doi: 10.1016/s0959-437x(97)80110-4. [DOI] [PubMed] [Google Scholar]
- 40.Leberer E, Wu C, Leeuw T, Fourest-Lieuvin A, Segall J E, Thomas D Y. Functional characterization of the Cdc42p binding domain of yeast Ste20p protein kinase. EMBO J. 1997;16:83–97. doi: 10.1093/emboj/16.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lew D, Reed S. Cell cycle control of morphogenesis in budding yeast. Curr Opin Genet Dev. 1995;5:17–23. doi: 10.1016/s0959-437x(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 42.Lew D J, Reed S I. Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J Cell Biol. 1993;120:1305–1320. doi: 10.1083/jcb.120.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li F N, Johnston M. Grr1 of Saccharomyces cerevisiae is connected to the ubiquitin proteolysis machinery through Skp1: coupling glucose sensing to gene expression and the cell cycle. EMBO J. 1997;16:5629–5638. doi: 10.1093/emboj/16.18.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu H, Styles C A, Fink G R. Elements of the yeast pheromone response pathway required for filamentous growth of diploids. Science. 1993;262:1741–1744. doi: 10.1126/science.8259520. [DOI] [PubMed] [Google Scholar]
- 45.Liu S C, Derick L H, Agre P, Palek J. Alteration of the erythrocyto-membrane skeletal ultrastructure in hereditary spherocytosis, hereditary elliptocytosis, and pyropoikilocytosis. Blood. 1990;76:198–205. [PubMed] [Google Scholar]
- 46.Ma X J, Lu Q, Grunstein M. A search for proteins that interact genetically with histone H3 and H4 amino termini uncovers novel regulators of the Swe1 kinase in Saccharomyces cerevisiae. Genes Dev. 1996;10:1327–1340. doi: 10.1101/gad.10.11.1327. [DOI] [PubMed] [Google Scholar]
- 47.Madden K, Costigan C, Snyder M. Cell polarity and morphogenesis in Saccharomyces cerevisiae. Trends Cell Biol. 1992;2:22–29. doi: 10.1016/0962-8924(92)90140-i. [DOI] [PubMed] [Google Scholar]
- 48.Mösch H-U, Fink G R. Dissection of filamentous growth by transposon mutagenesis in Saccharomyces cerevisiae. Genetics. 1997;145:671–684. doi: 10.1093/genetics/145.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mulholland J, Preuss D, Moon A, Wong A, Drubin D, Botstein D. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J Cell Biol. 1994;125:381–391. doi: 10.1083/jcb.125.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nasmyth K. At the heart of the budding yeast cell cycle. Trends Genet. 1996;12:405–412. doi: 10.1016/0168-9525(96)10041-x. [DOI] [PubMed] [Google Scholar]
- 51.Neiman A M, Herskowitz I. Reconstitution of a yeast protein kinase cascade in vitro: activation of the yeast MEK homologue STE7 by STE11. Proc Natl Acad Sci USA. 1994;91:3398–3402. doi: 10.1073/pnas.91.8.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Novick P, Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985;40:405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- 53.Oehlen L J W M, Cross F R. Potential regulation of Ste20 function by the Cln1-Cdc28 and Cln2-Cdc28 cyclin-dependent protein kinases. J Biol Chem. 1998;273:25089–25097. doi: 10.1074/jbc.273.39.25089. [DOI] [PubMed] [Google Scholar]
- 54.Peter M, Neiman A M, Park H-O, van Lohuizen M, Herskowitz I. Functional analysis of the interaction between the small GTP binding protein Cdc42 and the Ste20 protein kinase in yeast. EMBO J. 1996;15:7046–7059. [PMC free article] [PubMed] [Google Scholar]
- 55.Pringle J, Bi E, Harkins H, Zahner J, De Virgilio C, Chant J, Corrado K, Fares H. Establishment of cell polarity in yeast. Cold Spring Harbor Symp Quant Biol. 1995;60:729–744. doi: 10.1101/sqb.1995.060.01.079. [DOI] [PubMed] [Google Scholar]
- 56.Printen J A, Sprague G F., Jr Protein-protein interactions in the yeast pheromone response pathway: Ste5p interacts with all members of the MAP kinase cascade. Genetics. 1994;138:609–619. doi: 10.1093/genetics/138.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pruyne D W, Schott D H, Bretscher A. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J Cell Biol. 1998;143:1931–1945. doi: 10.1083/jcb.143.7.1931. [DOI] [PubMed] [Google Scholar]
- 58.Richardson H, Lew D J, Henze M, Sugimoto K, Reed S I. Cyclin-B homologs in Saccharomyces cerevisiae function in S phase and in G2. Genes Dev. 1992;6:2021–2034. doi: 10.1101/gad.6.11.2021. [DOI] [PubMed] [Google Scholar]
- 59.Roberts R, Fink G R. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 1994;8:2974–2985. doi: 10.1101/gad.8.24.2974. [DOI] [PubMed] [Google Scholar]
- 60.Roemer T, Vallier L, Sheu Y-J, Snyder M. The Spa2p-related protein, Sph1p, is important for polarized growth in yeast. J Cell Sci. 1998;111:479–494. doi: 10.1242/jcs.111.4.479. [DOI] [PubMed] [Google Scholar]
- 61.Roemer T, Vallier L, Snyder M. Selection of polarized growth sites in yeast. Trends Cell Biol. 1996;6:434–441. doi: 10.1016/s0962-8924(96)10039-8. [DOI] [PubMed] [Google Scholar]
- 62.Schneider B L, Seufert W, Steiner B, Yang Q H, Futcher A B. Use of PCR epitope tagging for protein tagging in Saccharomyces cerevisiae. Nucleic Acids Res. 1995;11:1265–1274. doi: 10.1002/yea.320111306. [DOI] [PubMed] [Google Scholar]
- 63.Schwob E, Bohm T, Mendenhall M D, Nasmyth K. The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae. Cell. 1994;79:233–244. doi: 10.1016/0092-8674(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 64.Sells M A, Chernoff J. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol. 1997;7:162–167. doi: 10.1016/S0962-8924(97)01003-9. [DOI] [PubMed] [Google Scholar]
- 65.Sherman F, Fink G, Hicks J. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 66.Sheu Y-J, Santos B, Fortin N, Costigan C, Snyder M. Spa2p interacts with cell polarity proteins and signaling components involved in yeast cell morphogenesis. Mol Cell Biol. 1998;18:4053–4069. doi: 10.1128/mcb.18.7.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sivadon P, Bauer F, Aigle M, Crouzet M. Actin cytoskeleton and budding pattern are altered in the yeast rvs161 mutant: the Rvs161 protein shares common domains with the brain protein amphiphysin. Mol Gen Genet. 1995;246:485–495. doi: 10.1007/BF00290452. [DOI] [PubMed] [Google Scholar]
- 68.Snyder M. The SPA2 protein of yeast localizes to sites of cell growth. J Cell Biol. 1989;108:1419–1429. doi: 10.1083/jcb.108.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Snyder M, Gehrung S, Page B D. Studies concerning the temporal and genetic control of cell polarity in Saccharomyces cerevisiae. J Cell Biol. 1991;114:515–532. doi: 10.1083/jcb.114.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stevenson B J, Rhodes N, Errede B, Sprague G F., Jr Constitutive mutants of the protein kinase STE11 activate the yeast pheromone response pathway in the absence of G protein. Genes Dev. 1992;6:1293–1304. doi: 10.1101/gad.6.7.1293. [DOI] [PubMed] [Google Scholar]
- 71.Strome S. Determination of cleavage planes. Cell. 1993;72:3–6. doi: 10.1016/0092-8674(93)90041-n. [DOI] [PubMed] [Google Scholar]
- 72.Surana U, Robitsch H, Price C, Schuster T, Fitch I, Futcher B, Nasmyth K. The role of CDC28 and cyclins during mitosis in the budding yeast S. cerevisiae. Cell. 1991;65:145–161. doi: 10.1016/0092-8674(91)90416-v. [DOI] [PubMed] [Google Scholar]
- 73.Tkacz J S, Lampen J O. Wall replication in Saccharomyces species: use of fluorescein-conjugated concanavalin A to reveal the site of mannan insertion. J Gen Microbiol. 1972;72:243–247. doi: 10.1099/00221287-72-2-243. [DOI] [PubMed] [Google Scholar]
- 74.Valtz N, Herskowitz I. Pea2 protein of yeast is localized to sites of polarized growth and is required for efficient mating and bipolar budding. J Cell Biol. 1996;135:725–739. doi: 10.1083/jcb.135.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walch-Solimena C, Collins R N, Novick P J. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu C, Whiteway M, Thomas D Y, Leberer E. Molecular characterization of Ste20p, a potential mitogen-activated protein or extracellular signal-regulated kinase kinase (MEK) kinase kinase from Saccharomyces cerevisiae. J Biol Chem. 1995;270:15984–15992. doi: 10.1074/jbc.270.27.15984. [DOI] [PubMed] [Google Scholar]
- 77.Yang S, Ayscough K R, Drubin D G. A role for the actin cytoskeleton of Saccharomyces cerevisiae in bipolar bud-site selection. J Cell Biol. 1997;136:111–123. doi: 10.1083/jcb.136.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zahner J E, Harkins H A, Pringle J R. Genetic analysis of the bipolar pattern of bud site selection in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:1857–1870. doi: 10.1128/mcb.16.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao Z, Leung T, Manser E, Lim L. Pheromone signalling in Saccharomyces cerevisiae requires the small GTP-binding protein Cdc42p and its activator CDC24. Mol Cell Biol. 1995;15:5246–5257. doi: 10.1128/mcb.15.10.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]