Abstract
Trajectories of youth antisocial behavior (ASB) are characterized by both continuity and change. Twin studies have further indicated that genetic factors underlie continuity, while environmental exposures unique to each child in a given family underlie change. However, most behavioral genetic studies have examined continuity and change during relatively brief windows of development (e.g., during childhood but not into adolescence). It is unclear whether these findings would persist when ASB trajectories are examined across multiple stages of early development (i.e., from early childhood into emerging adulthood). Our study sought to fill this gap by examining participants assessed up to five times between the ages of 3 and 22 years using an accelerated longitudinal design in the Michigan State University Twin Registry (MSUTR). We specifically examined the etiologies of stability and change via growth curve modeling and a series of univariate and bivariate twin analyses. While participants exhibited moderate-to-high rank-order stability, mean levels of ASB decreased linearly with age. Genetic and nonshared environmental influences that were present in early childhood also contributed to both stability and change across development, while shared environmental contributions were negligible. In addition, genetic and nonshared environmental influences that were not yet present at the initial assessment contributed to change over time. Although ASB tended to decrease in frequency with age, participants who engaged in high levels of ASB during childhood generally continued to do so throughout development. Moreover, the genetic and nonshared environmental contributions to ASB early in development also shaped the magnitude of the decrease with age.
Keywords: Youth antisocial behavior, Trajectories, Behavioral genetics
Childhood antisocial behavior (ASB) predicts a myriad of poor outcomes in adolescence and young adulthood, such as substance use, poor physical health, and internalizing pathology, as well as continued engagement in delinquent activities (e.g., Odgers et al. 2008). In children, ASB is characterized by persistent aggression, deceitfulness, property destruction, and/or rule violations (American Psychiatric Association 2013). One of the defining features of ASB is its relatively high level of rank-order stability, with the same individuals typically exhibiting the highest levels of delinquent behavior across development. Despite this stability, mean levels of ASB decline throughout the first twenty or so years of life (e.g., Monahan et al. 2009), and there is considerable individual variation in the magnitude of this decline (Burt 2012; Martino et al. 2008). In short, extant research has clearly indicated that youth ASB trajectories are characterized by both stability and change, and that these patterns vary from person to person.
To date, however, it is less clear what etiologic mechanisms underlie individual differences in these patterns of continuity and change. There are several competing a priori possibilities. The relatively high rank-order stability of ASB could be due to continuity in underlying genetic influences, while change over time could stem from specific environmental exposures (e.g., environmental risk factors could predict escalating behavior problems while environmental protective factors predict desistance). Alternatively, genetic contributions could change over time as different genes become (de)activated, while environmental factors could contribute to stability. One method for evaluating these competing hypotheses is the twin design, which compares monozygotic (MZ) and dizygotic (DZ) twins to disambiguate the genetic and environmental contributions to a given phenotype. By examining these contributions across multiple timepoints, twin researchers can clarify the origins of continuity and change.
Prior longitudinal twin studies have begun to evaluate these possibilities. These studies have consistently implicated genetic factors as a major source of rank-order stability in ASB (Bartels et al. 2004; Burt et al. 2007; Eley et al. 2003; Lacourse et al. 2014; Pingault et al. 2015; Porsch et al. 2016; van Beijsterveldt et al. 2003). As an example, Burt et al. (2007) used latent growth curve modeling to examine the etiologic trajectory of ASB from late adolescence through early adulthood (approximately ages 17–25) in 626 twin pairs from the Minnesota Twin Family Study and found the same genetic factors to be present over time. These factors explained a moderate-to-large proportion of the variance in ASB at each timepoint and were largely responsible for trait stability. Nonshared environmental influences (or experiences that serve to differentiate children raised in the same family; e.g., peer groups) were found to underlie change over time. Shared environmental influences (experiences common to children raised in the same family; e.g., similar parenting) did not contribute to ASB at baseline or over time, consistent with research indicating that shared environmental influences on ASB become less salient (and nonshared more salient) with age (e.g., Tuvblad et al. 2011). Child and adolescent twin studies using liability threshold analyses, simplex modeling, or Cholesky decomposition modeling have reported somewhat similar results, finding that non-shared environmental factors largely contribute to change over time, while genetic influences contribute to stability (Bartels et al. 2004; Eley et al. 2003; van Beijsterveldt et al. 2003). However, these studies have also found evidence that shared environmental influences contribute to stability over time. In other words, shared environmental influences have been found to impact ASB development in younger samples, but its effects appear to be negligible by late adolescence.
Despite these consistencies in results, several questions remain unanswered. First, it is unclear whether the genetic factors contributing to adolescent and young adult ASB are the same as those contributing to child ASB. Although the studies discussed above found largely continuous genetic effects, most assessed continuity and change during a relatively brief window of development: early childhood through pre-adolescence, or middle childhood through early adolescence, or late adolescence through early adulthood. The most comprehensive etiologic study of ASB development (Pingault et al. 2015) began in early childhood (twins were assessed beginning at age 4 through 16 years), but did not assess participants in late adolescence or emerging adulthood, two key developmental periods in the transition to adult social and occupational roles (Alink and Egeland 2013). Indeed, no study to date has examined the etiologies of continuity and change in ASB across all of early development (i.e., the first 20 or so years of life). As such, we do not know to what extent genetic factors underlie stability in ASB from the preschool years through emerging adulthood, nor is it clear how shared and nonshared environmental factors differentially affect stability and change over this time period.
The latter uncertainty is particularly important, given that the shared environment has been identified as an important etiologic source of stability in ASB during childhood and early adolescence (Bartels et al. 2004; Eley et al. 2003; van Beijsterveldt et al. 2003), but does not appear to affect the trajectory of ASB during late adolescence (Burt et al. 2007). By contrast, nonshared environmental influences appear to be transient and idiosyncratic prior to adulthood, with non-shared environmental correlations for positive and negative affect and interpersonal warmth each decreasing monotonically in a matter of minutes or days (Burt et al. 2015). At some point during adolescence, however, these influences appear to become more enduring. Although a few studies have reported this increased stability of the non-shared environment as early as age 7 (e.g., van Beijsterveldt et al. 2003), most studies place it sometime in late adolescence (e.g., Hopwood et al. 2011). As such, results from studies of children identifying the nonshared environment as a source of change may not persist to older samples. In short, much is unknown about the developmental origins of ASB because no study of its etiologic trajectory has spanned early childhood through emerging adulthood.
The aim of the present study was to address these gaps in the literature by examining the origins of stability and change in youth ASB from preschool through to emerging adulthood. We used up to five waves of data from the Michigan State University Twin Registry (MSUTR; Burt and Klump 2019) collected across ages 3 to 22 years using an accelerated longitudinal design. We applied multilevel growth curve modeling, in which measurements were nested within participants, to estimate participants’ baseline levels of ASB (i.e., intercepts) and rates of change with age (i.e., slopes). We subsequently used classical twin modeling to quantify the genetic, shared environmental, and nonshared environmental influences on the intercept and slope, respectively. Based on prior research, we hypothesized that genetic factors would underlie stability and nonshared environmental factors would underlie change. Given that shared environmental effects have been found to decrease with age, we did not expect them to significantly impact participants’ trajectories into emerging adulthood.
Methods
Participants
Participants were drawn from the Twin Study of Behavioral and Emotional Development in Children (TBED-C), a sample within the population-based Michigan State University Twin Registry (MSUTR; Burt and Klump 2019). The TBED-C includes both a population-based subsample (n = 528 families) and an independent ‘at risk’ subsample of twin families residing in impoverished Census tracts (n = 502 families). When combined, the overall sample thus comprised 1030 twin pairs: 224 MZ male pairs, 211 DZ male pairs, 202 MZ female pairs, 207 DZ female pairs, and 186 DZ opposite-sex pairs. Mean household income at the middle childhood assessment was $76,329 (SD = $45,650) in the population-based sample and $55,652 (SD = $31,088) in the at-risk sample. Other recruitment details are detailed at length in prior publications (e.g., Burt and Klump 2019). Families across the two samples collectively identified as White (non-Latinx): 81%, Black: 10%, Latinx: 1%, Asian: 1%, Indigenous: 1%, and multiracial: 6%. These proportions are largely consistent with those for the population of the State of Michigan, based on data from the U.S. Census Bureau (http://www.Census.gov/) (e.g., White: 79%, Black: 14%). For all studies, parents provided informed consent, children provided informed assent, and families were compensated for their time.
Behavioral and emotional data relevant to the current study were collected at as many as five time points. All 1030 twin families were assessed once in middle childhood (ages 6–11) as part of the TBED-C. Those TBED-C twins residing in modestly-to-severely disadvantaged neighborhoods are currently being reassessed in-person as adolescents up to two times, 18-months apart, through the Michigan Twin Neurogenetics Study (MTwiNS). The first of the MTwiNS assessments was conducted approximately 4–6 years after participation in TBED-C (ages 7–19; currently available for 354 families), while the second adolescent assessment was 5–7 years after participation in TBED-C (ages 10–19; currently available for 188 families). TBED-C families with twins between ages 11 and 22 were also recently recruited for an online assessment of youth psychopathology (N = 637 families completed the online assessment). Finally, we were also able to link to data collected on TBED-C families as part of the population-based Michigan Twins Project (MTP), an ongoing study of approximately 12,000 Michigan-born child and adolescent twin pairs (93.3% of TBED-C families were recruited out of the MTP). See Table 1 for additional details about the sample at each age, including sample sizes at each assessment.
Table 1.
Descriptive statistics: youth ASB scores by age
| Age | Total N | MTP early | TBED-C | MTP late | MTwiNS | Online | Mean ASB (SD) | Range ASB |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 3 | 288 | 288 | 0 | 0 | 0 | 0 | 1.91 (1.63) | 0–8 |
| 4 | 284 | 284 | 0 | 0 | 0 | 0 | 1.65 (1.49) | 0–8 |
| 5 | 286 | 286 | 0 | 0 | 0 | 0 | 1.74 (1.55) | 0–7 |
| 6 | 858 | 196 | 600 | 62 | 0 | 0 | 1.49 (1.62) | 0–10 |
| 7 | 584 | 132 | 408 | 38 | 6 | 0 | 1.39 (1.54) | 0–10 |
| 8 | 682 | 254 | 344 | 66 | 18 | 0 | 1.37 (1.54) | 0–10 |
| 9 | 556 | 128 | 338 | 78 | 12 | 0 | 1.15 (1.42) | 0–9 |
| 10 | 476 | 34 | 312 | 92 | 38 | 0 | 1.17 (1.47) | 0–7 |
| 11 | 118 | 0 | 58 | 8 | 36 | 16 | 1.01 (1.37) | 0–5 |
| 12 | 116 | 0 | 0 | 14 | 58 | 44 | 1.09 (1.43) | 0–6 |
| 13 | 200 | 0 | 0 | 4 | 62 | 134 | 0.88 (1.31) | 0–6 |
| 14 | 290 | 0 | 0 | 2 | 146 | 142 | 1.00 (1.56) | 0–10 |
| 15 | 312 | 0 | 0 | 0 | 150 | 162 | 0.83 (1.42) | 0–9 |
| 16 | 260 | 0 | 0 | 4 | 96 | 160 | 0.70 (1.26) | 0–9 |
| 17 | 254 | 0 | 0 | 0 | 78 | 176 | 0.95 (1.43) | 0–9 |
| 18 | 146 | 0 | 0 | 0 | 18 | 128 | 0.75 (1.09) | 0–5 |
| 19 | 156 | 0 | 0 | 0 | 2 | 154 | 0.86 (1.39) | 0–9 |
| 20 | 116 | 0 | 0 | 0 | 0 | 116 | 0.72 (1.15) | 0–5 |
| 21–22 | 44 | 0 | 0 | 2 | 0 | 42 | 0.57 (1.07) | 0–5 |
Of note, the MTP assessments were not completed in the same order or at the same ages across participating TBED-C families. For example, while most families were first assessed as part of the MTP (73.9%), others were first assessed as part of TBED-C and were assessed only later as part of the MTP (26.1%). Follow-up MTP assessments are also on-going. A portion of TBED-C families (N = 637; 61.8%; 56 of these were assessed twice) were re-assessed approximately 5–8 years after their original participation in the MTP. To account for these irregularities in the ordering of data collection across the TBED-C/MTwiNS and the MTP, data were organized chronologically by age for each participating twin. As such, each assessment wave in the current study includes MTP and either TBED-C or MTwiNS assessments (see Table 2). A total of 677 pairs (66% of the sample) completed at least three of the assessments, while 96% (N = 989) completed at least two. The highest number of assessments completed by any single participant was five.
Table 2.
Sample sizes and correlations in ASB over time
| Time 2 r | Time 3 | Time 4 | Time 5 | Mean age (SD) | Mean ASB (SD) | N | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Time 1 | 0.49* | 0.31* | 0.30* | 0.20 | 6.57 (2.09) | 1.56 (1.61) | 1996 |
| Time 2 | – | 0.40* | 0.36* | 0.46* | 8.63 (2.60) | 1.28 (1.49) | 1978 |
| Time 3 | – | 0.56* | 0.55* | 15.29 (3.14) | 0.87 (1.34) | 1354 | |
| Time 4 | – | 0.53* | 16.61 (2.16) | 0.87 (1.41) | 551 | ||
| Time 5 | – | 16.79 (0.86) | 0.48 (0.87) | 56 | |||
Bold font and asterisk indicate that the correlation was significantly different than zero at p < 0.05. Time points indicate assessment waves in chronological order, which varies across participants (e.g., Time 1 was MTP 1 for some participants, TBED-C for others)
Recruitment
Because birth records are confidential in Michigan, we collaborated with the Michigan Department of Health and Human Services (MDHHS; formerly known as the Michigan Department of Community Health) to recruit families for all MSUTR twin studies (including the MTP and all waves of the TBED-C/MTwiNS). The MDHHS is the agency in charge of all vital records in the State of Michigan and thus has direct access to individual SSNs, full names, and birth dates. The MDHHS identifies twin pairs residing in lower Michigan who meet age criteria for a given study and whose addresses or parents’ addresses (for twins who are minors) can be located either using driver’s license information obtained from the State of Michigan or the proprietary search engine used by police. Twins indicating interest in participation via pre-stamped postcards or e-mails/calls to the MSUTR project office are then contacted by study staff to determine study eligibility and to schedule their assessments.
Four recruitment mailings were used to ensure optimal twin participation. Overall, response rates across studies (56–85%) are on par with or better than those of other twin registries that use similar types of anonymous recruitment mailings and have thus far yielded largely representative samples. Families of the naturally-conceived twins in the large-scale MTP, for example, closely resemble families across the State of Michigan (Burt and Klump 2013). The proportion of MTP families that identify as White, non-Hispanic (81.0%) is very similar to the 80.2% indicated in state-wide Census data. Mean family incomes are also quite comparable ($75,940 in the MTP versus $73,373 in the Census), as are the proportion of families with graduate or professional degrees (10.3% in the MTP versus 9.6% in the Census).
Because 90 + % of TBED-C families were recruited out of the MTP, we were able to use the MTP data to compare families who chose to participate in TBED-C with those who were recruited but did not participate. TBED-C families were generally representative of recruited but non-participating families. As compared to non-participating twins, participating twins reported similar levels of conduct problems, emotional symptoms, and hyperactivity (d ranged from − 0.08 to 0.01 in the population-based sample and 0.01 to 0.09 in the at-risk sample; all ns). Participating families also did not differ from non-participating families in paternal felony convictions (d = − 0.01 and 0.13 for the population-based and the at-risk samples, respectively), rate of single parent homes (d = 0.10 and − 0.01 for the population-based and the at-risk samples, respectively), paternal years of education (both d ≤ 0.12), or maternal and paternal alcohol problems (d ranged from 0.03 to 0.05 across the two samples). However, participating mothers in both samples reported slightly more years of education (d = 0.17 and 0.26, both p < 0.05) than non-participating mothers. Maternal felony convictions differed across participating and non-participating families in the population-based sample (d = − 0.20; p < 0.05) but not in the at-risk sample (d = 0.02). In short, our recruitment procedures thus appear to yield samples that are representative of both recruited families and the general population of the State of Michigan.
Procedure
Some of the assessments, specifically the MTP assessments and the “online” assessment, were completed remotely by the twins’ primary caregiver, nearly always their mother. The TBED-C and MTwiNS assessments were completed in-person. For TBED-C, twins were assessed either at our East Lansing-based laboratories or at the family’s home. Questionnaires did not vary across the laboratory-based and family home assessments. For MTwiNS, the twins and their parent(s) completed an in-person assessment lasting 4–8 hours at either the East Lansing or Ann Arbor-based laboratories.
Measures
Youth antisocial behavior
Youth ASB was assessed via maternal report at all ages. At the MTP assessments, participating twins’ mothers completed the Strengths and Difficulties Questionnaire (SDQ; Goodman 2001), a 25-item measure in which parents rate the extent to which a series of statements describe the child’s behavior over the past six months using a three-point scale (0 = not true to 2 = certainly true). For these analyses, we focused on the Conduct Problems subscale (5 items: hot temper, obedient (reverse-scored), fights, lies or cheats, steals; α = 0.60, 0.63, and 0.66 at MTP assessments 1, 2, and 3, respectively). Psychometric studies have found the SDQ to have satisfactory test–retest reliability (r > 0.85 for the Conduct Problems subscale) and to be highly correlated with other parent-report measures, including the Child Behavior Checklist (CBCL) (e.g., Muris et al. 2003). In addition, studies in samples spanning childhood and adolescence support the use of the parent-report SDQ as a screening measure that adequately distinguishes between community and clinical populations across age groups (Becker et al. 2004; He et al. 2013).
At the in-person TBED-C and MTwiNS assessments, the twins’ mothers completed the CBCL (Achenbach and Rescorla 2001), rating the extent to which a series of statements described the child’s behavior during the past six months on a three-point scale (0 = never to 2 = often/mostly true). To maximize comparability with the SDQ, we constructed a scale using 5 items on the CBCL that were analogous to those on the SDQ: hot temper, disobedient at home, fights, lies or cheats, steals from home (α = 0.66 in the TBED-C and 0.69 and 0.60, respectively, at the two MTwiNS assessments). While these items screen for behaviors that may manifest differently at different ages (e.g., lying, hot temper), all are relatively common across early development. As such, we believe that they adequately assess ASB across the broad age range included in our sample.
Zygosity
Zygosity was established using physical similarity questionnaires administered to the twins’ primary caregiver (Peeters et al. 1998). On average, the physical similarity questionnaires used by the MSUTR have accuracy rates of at least 95% when compared to DNA.
Disadvantage
Family socioeconomic disadvantage was assessed using the Area Deprivation Index (ADI), a composite measure comprising 17 indices of Census-tract disadvantage (e.g., poverty rate, income disparity). We recreated Kind & Buckingham’s index of disadvantage in our sample, as assessed via Census data collected from 2008 to 2012. The measures were weighted according to the factor loadings identified by Kind and Buckingham (2018), and weighted variables were summed to create a deprivation index score for each Census tract. Families were assigned a percentile indicating the level of deprivation in their Census tract relative to that of all Census tracts in Michigan. The mean ADI was 42.51 (SD = 26.17) and ranged from 1 to 100.
Data analyses
Phenotypic analyses
All analyses were conducted using Mplus 8.0 (Muthén and Muthén 2019). To examine phenotypic changes in ASB over time, we used a three-level growth curve model in which occasions of measurement (Level 1) were nested within participants (Level 2) who were nested within families (Level 3). These models capture both the average rate of change in ASB over the course of the study, as well as individual variability in change via random intercept and slope terms. Age was used as the index of time in these models and was centered at three years old, the youngest age in our sample. The intercept can thus be interpreted as the level of ASB at age 3.
We initially estimated an unconditional growth model with a random linear slope, which allowed for interindividual variation in the rate of change over time. Models with non-linear slopes encountered serious convergence difficulties. Moreover, prior research consistently indicates that mean levels of ASB decline steadily throughout development (e.g., Monahan et al. 2009). We subsequently estimated a conditional growth model. In this model, questionnaire type (SDQ or CBCL) was added as a time-varying covariate on level 1 with a random slope. We included sex, ethnicity, and ADI as covariates of the random intercept and slope on level 2.
Full-information maximum likelihood estimation was used to account for missing data, as prior simulations have shown it to be robust to at least 50% missing data (Enders and Bandalos 2001). Moreover, accelerated longitudinal designs such as ours, which have planned missing data, have been found to have robust power despite small sample sizes at certain ages (Rhemtulla and Hancock 2016). Data were log-transformed prior to analysis to better approximate normality. Random intercept and slope factor scores were generated for subsequent biometric analyses using maximum a posteriori (MAP) scoring (MacCallum 2009). The individual factor scores were obtained from an unconditional, two-level model in which measurement occasions were nested within participants, as biometric twin models account for clustering within families. Sex, ethnicity, and ADI were regressed out of the factor scores prior to running the twin analyses (McGue and Bouchard 1984).
Biometric twin analyses
A series of biometric twin models were then run to estimate the relative genetic and environmental influences on variability in the estimated intercept and slope factor scores. We used these factor score estimates rather than running a full biometric latent growth curve model due to the large variation in participant age at each timepoint (time is typically based on assessment schedule in biometric latent growth curve models). Time can be easily modelled via participants’ chronological age at a given time point in multilevel growth models, however, making it better-suited for study designs that involve an uneven schedule of assessments (Hox and Stoel 2014), such as this one. Although each analytic framework has its practical advantages, structural equation and multilevel growth models are conceptually analogous.
Classical twin models leverage the difference in the proportion of segregating genes shared between identical (MZ) twins, who share 100% of their genes, and fraternal (DZ) twins, who share an average of 50% of their segregating genes to estimate the relative contributions of genetic and environmental influences to the variance within observed behaviors (phenotypes). Phenotypic variance is decomposed into three variance components: additive genetic (A), shared environmental (C), and nonshared environmental (E). More information on twin modeling is provided elsewhere (Neale and Cardon 1992). For the present study, we first computed the A, C, and E estimates for the random intercept and slope factors, respectively. We then used a bivariate twin model to clarify the extent to which the etiologies of the slope and the intercept overlapped.
Results
Descriptive statistics
Descriptive statistics by age are shown in Table 1, while Table 2 contains correlations across assessment waves (operationalized here in person-specific chronological order because of the irregularities in when specific assessments were administered). Participants evidenced moderate-to-high rank-order stability in their reported ASB over time, with correlations ranging from 0.20 to 0.56. Paired-sample t tests further indicated that, within persons, ASB decreased significantly from the first assessment to the second (t(1913) = − 7.97, p < 0.001), and from the second assessment to the third (t(1333) = − 9.55, p < 0.001). Changes across subsequent assessments were not significant. In addition, mean ASB scores decreased steadily with age (see Fig. 1). Collectively, these findings indicate that, while participants who exhibited high levels of ASB during childhood largely continued to do so during adolescence, the absolute level of ASB decreased significantly over time. Although not shown in Table 1, males had slightly higher ASB scores than females at the first two timepoints (Cohen’s d = 0.21 and 0.22, respectively; both p < 0.001), whereas there were no significant sex differences at assessments three, four, or five. Lastly, ADI was significantly correlated with ASB at the first two timepoints (r = 0.17 and 0.11, respectively, both p < 0.001), but not at the last three.
Fig. 1.
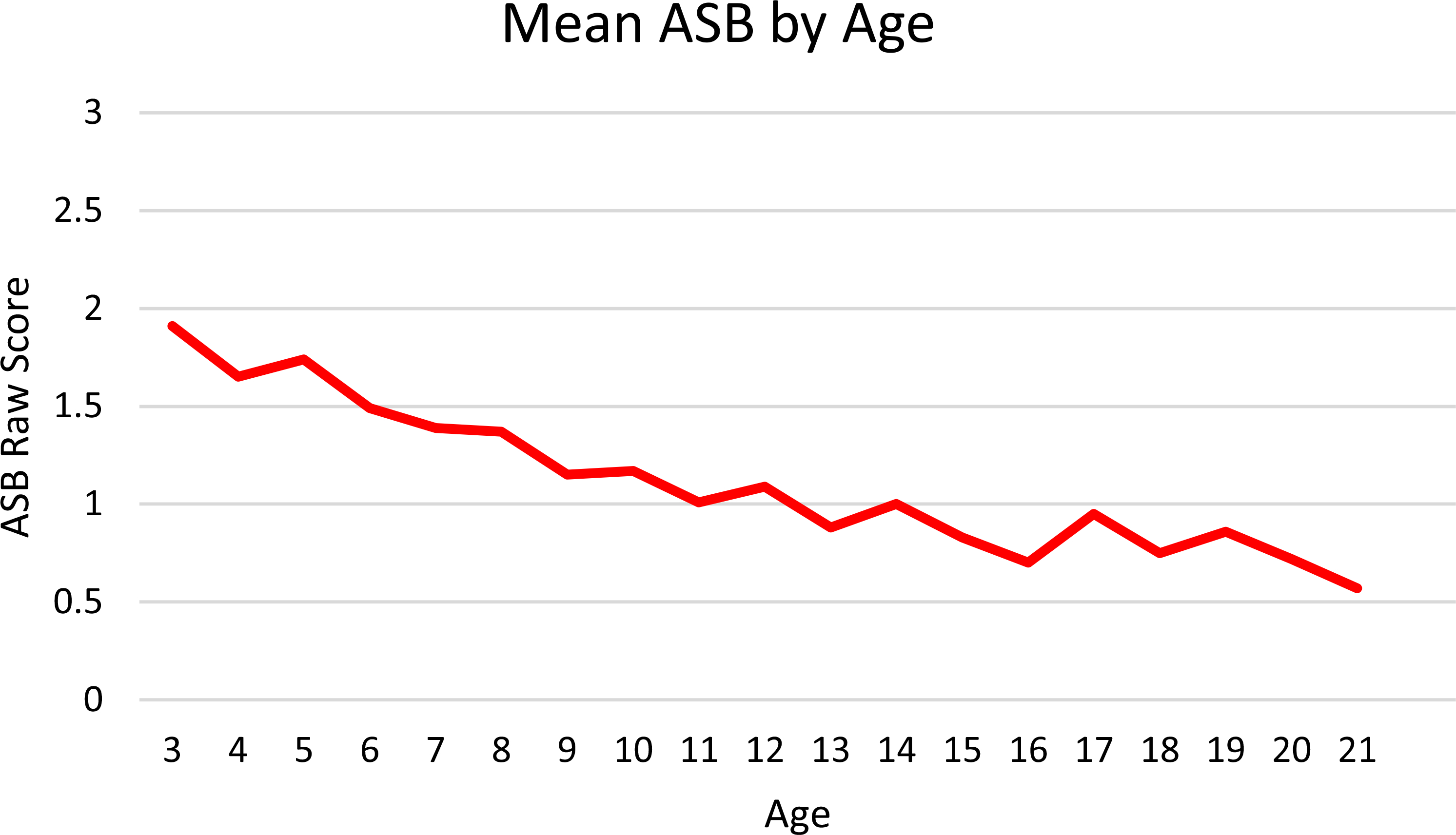
Age-related change in ASB
Multilevel modeling
Phenotypic results
In the baseline unconditional growth model, ASB decreased significantly over time (slope mean = − 0.03, p < 0.001), although there was significant interindividual variation in the magnitude of the decline (slope variance = 0.001, p < 0.001). The covariance between the intercept and the slope was negative, meaning that participants with higher ASB scores at baseline tended to exhibit a more rapid decline in ASB over time. We next fitted a conditional growth model. Results are shown in Table 3. Sex significantly predicted both intercept and slope, such that male participants had higher ASB scores at baseline and displayed a more rapid decline with age. ADI was also a significant predictor of both the intercept and slope, with participants from more impoverished neighborhoods exhibiting higher levels of ASB at baseline and declining more rapidly over time. By contrast, race (a socially constructed category coded as white/non-white given the composition of our sample) did not significantly predict the intercept or the slope.
Table 3.
Key parameter estimates from conditional multilevel growth curve model of ASB development
| Estimate (S.E.) | |
|---|---|
|
| |
| Level 1 (within-person) | |
| Intercept | |
| Mean | 0.667* (0.069) |
| Variance | 0.179* (0.015) |
| Slope | |
| Mean | −0.018* (0.007) |
| Variance | 0.001* (0.000) |
| Residual variance | 0.152* (0.006) |
| Level 2 (between-person) | |
| Intercept (DV) | |
| Sex → intercept | 0.170* (0.032) |
| Ethnicity → intercept | −0.035 (0.054) |
| ADI → intercept | 0.004* (0.001) |
| Slope (DV) | |
| Sex → slope | −0.008* (0.003) |
| Ethnicity → slope | 0.001 (0.006) |
| ADI → slope | −0.0002* (0.000) |
| Level 3 (between-family) | |
| Intercept-slope covariance | −0.010* (0.001) |
Bold font and asterisk indicate that the estimate was significantly different than zero at p < 0.05. Questionnaire type was included as a random, time-varying covariate
Twin model results
Univariate ACE models were first run using intercept and slope factor scores from the unconditional growth model, in order to estimate the genetic, shared environmental, and nonshared environmental contributions to stability and change in ASB. Standardized univariate variance estimates are presented in Table 4. For both the intercept and the slope, there were significant genetic and nonshared environmental contributions, but no significant shared environmental influences, although the estimated magnitude of the shared environmental variance for the intercept was non-zero (0.10).
Table 4.
Standardized variance estimates from univariate ACE model
| Variance estimates |
|||
|---|---|---|---|
| a | c | e | |
|
| |||
| Univariate | |||
| Intercept | 0.53* | 0.10 | 0.37* |
| Slope | 0.45* | 0.03 | 0.52* |
Bold font and asterisk indicate that the estimate was significantly different than zero at p < 0.05
The bivariate ACE model (Fig. 2) indicated both significant unique and overlapping genetic and nonshared environmental influences on the intercept and the slope factors. Path estimates are shown in Fig. 2. More than one-third (38%) of the genetic variance in the slope factor was shared with the intercept. Thus, the genetic etiology of ASB development was due to both genetic influences that had emerged during the preschool years, as well as to novel genetic influences emerging later in development. Interestingly, one-third of the nonshared environmental variance in the slope was also present at baseline, indicating that our estimates of E did not represent solely transient, time-specific influences, but rather exhibited a fair amount of stability across development. Consistent with the univariate results, shared environmental contributions were not significant. Taken together, the genetic and nonshared environmental influences on ASB in early childhood also appear to contribute to its stability across development.
Fig. 2.
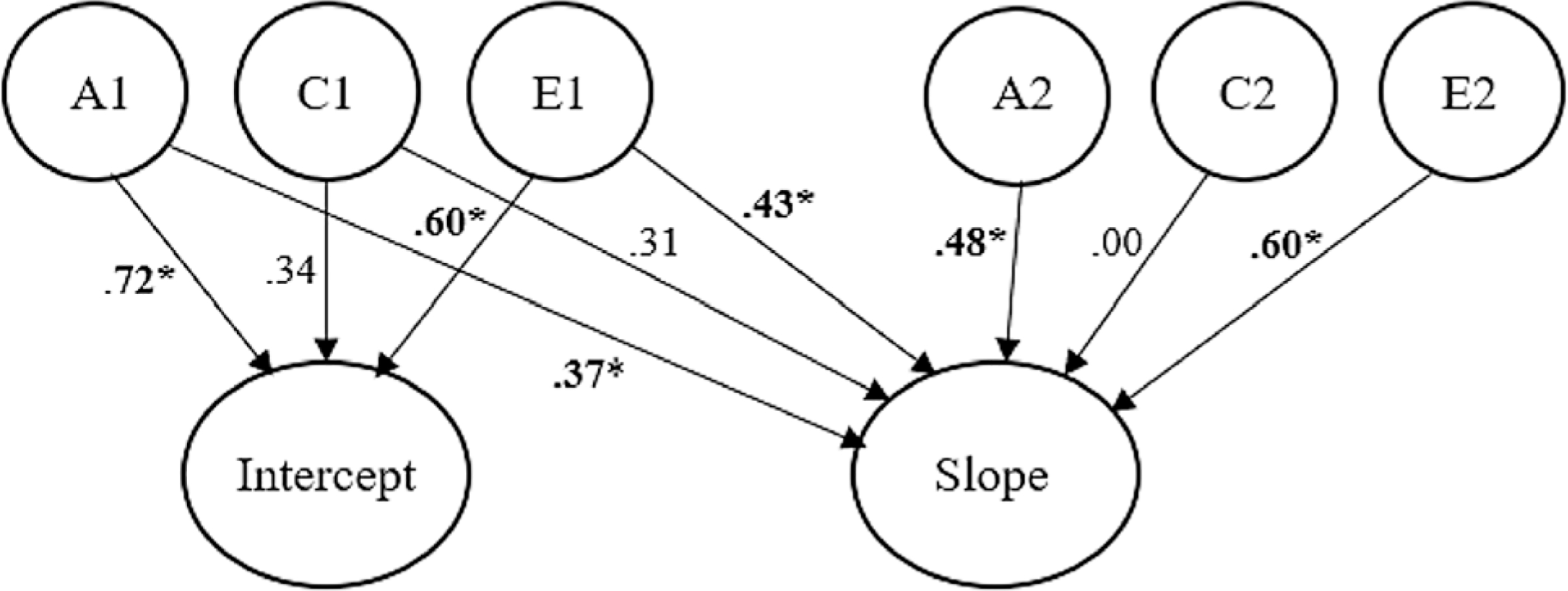
Path diagram of a bivariate twin model. The variance in the intercept and the slope is partitioned into additive genetic effects (A1 and A2), shared environmental effects (C1 and C2), and nonshared environmental effects (E1 and E2). For ease of presentation, this path diagram represents one twin in a pair. Standardized path estimates are squared to represent the proportion of variance accounted for
Discussion
The aim of the present study was to elucidate genetic and environmental contributions to continuity and change in ASB from early childhood into emerging adulthood. To do so, we obtained estimates of participants’ baseline level of ASB and change over time via multilevel growth curve modeling. We then made use of a series of classical twin models to illuminate genetic, shared environmental, and nonshared environmental contributions to the intercept and the slope of ASB, as estimated via factor scores generated in the prior analyses. The results indicate that initial levels and change over time in ASB were due to both genetic and nonshared environmental influences, some of which overlapped. Neither initial level of ASB nor change over time were subject to significant shared environmental influences.
Genetic influences were found to make important contributions to ASB in early life, as well as to change in ASB across development. Furthermore, more than one-third of the genetic contributions to change over time were already present at baseline (i.e., during the preschool years), indicating a fair amount of continuity in genetic influences throughout early development. These findings are consistent with those of other studies that have found prominent genetic influences on continuity in youth ASB, albeit during much shorter windows of development (Bartels et al. 2004; Burt et al. 2007; Eley et al. 2003; Porsch et al. 2016) or over longer periods that did not include late adolescence/emerging adulthood (Pingault et al. 2015). Our study extends these findings by indicating that genetic influences contribute to continuity in ASB across all of early development (i.e., the first 20 or so years of life). Genetic influences were also found to underlie change in ASB, with nearly two-thirds of the genetic variance in the slope representing novel influences that were not present at baseline. The emergence of novel genetic influences over the course of our study is unsurprising, given the broad age range (3 to 22 years) represented in the sample. In addition, this pattern of results is consistent with those of other studies of children and adolescents that have found genetic influences to contribute to both continuity and change in ASB (Bartels et al. 2004), particularly for nonaggressive rule-breaking (Eley et al. 2003).
That said, neither continuity nor change in ASB were due solely to genetic influences. Nonshared environmental variance played a considerable role in continuity across development in our sample, with fully one-third of the nonshared environmental contributions to the slope already present at baseline. Such findings stand in contrast to those of prior longitudinal studies of youth ASB, which have found the nonshared environment to exert largely transient effects on change over time that were specific to each assessment wave (Bartels et al. 2004; Burt et al. 2007; Eley et al. 2003). Because nonshared environmental influences tend to become more stable with age (Burt et al. 2015; Hopwood et al. 2011), it is possible that our study was better positioned to detect stability in the nonshared environment compared to those conducted in samples of children and young adolescents (e.g., Bartels et al. 2004; Eley et al. 2003). The possibility of nonshared environmental influences contributing increasingly to stability with age is also consistent with developmental theories of canalization, which posit that, as youth begin to shape their own environments, their range of potential outcomes typically narrows. In other words, individuals increasingly follow idiosyncratic trajectories in accordance with both genetic predispositions and environmental exposures that they themselves may seek out (e.g., Turkheimer and Gottesman 1991). For example, a twin who is parented more harshly during preschool may experience difficulty regulating his/her emotions throughout childhood and adolescence and increasingly choose to spend time with peers who have similar difficulties, further differentiating the child from his/her co-twin. That said, this interpretation is not consistent with the findings of Burt et al. (2007), which also identified transient effects of the nonshared environment between late adolescence and early adulthood. However, Burt and colleagues examined diagnostic symptom counts of Antisocial Personality Disorder, a more extreme phenotype than the more dimensional ASB assessment examined here (Lahey et al. 2005).
What might be the specific non-shared environmental experiences that underlie stability in ASB? One possible non-shared environmental influence is deviant peer affiliation, which has been found to predict growth in ASB throughout adolescence (Eamon 2002; Gardner et al. 2008). That said, prior twin work has suggested that twin differences in deviant peer affiliation appear to be a consequence, rather than a cause, of differences in their ASB (Burt et al. 2009). Another possibility centers on aspects of the family environment that, while objectively shared by siblings, impact each child in idiosyncratic ways (e.g., siblings respond differently to parental divorce) (Goldsmith 1993). Such familial influences could have an enduring impact on ASB development throughout childhood and adolescence. A final possibility is differential parenting, which may represent a relatively continuous influence that stably differentiates children in the same family. Such considerations are consistent with theoretical work positing that “proximal processes”, or reciprocal interactions between the individual and his/her immediate environment, play a critical role in shaping behavioral development (Bronfenbrenner 1988), and empirical work identifying harsh parenting as a risk factor for child, adolescent, and young adult ASB in particular (Beauchaine et al. 2005; Conger et al. 1994; Gard et al. 2017). Furthermore, studies of within-family differences in parental harshness significantly predicted within-pair differences in monozygotic twins’ ASB, both cross-sectionally (Burt et al. 2021) and over time (Burt et al. 2006). Such findings point to parenting as a particularly promising target for subsequent studies of the environmental etiology of ASB.
Of note, however, only environmental exposures unique to each child in a given family appeared to impact change in ASB across development, as shared or common family-level environmental influences were negligible. While the shared environment has previously been found to contribute to continuity in ASB during childhood and early adolescence (Bartels et al. 2004; Eley et al. 2003), it has not been found to impact ASB development during emerging adulthood (Burt et al. 2007). While our inclusion of emerging adulthood could conceivably contribute to our null findings for shared environmental influences, we also note that ASB was assessed using a 5-item screening measure of youth behavior problems. Brief measures often have lower reliabilities than do longer measures, an especially salient point here since increased measurement error would increase estimates of nonshared environmental effects (see Burt (2009) for a discussion of factors affecting detection of shared environmental effects). Consistent with the latter, supplemental analyses using participants’ scores on the full CBCL Conduct Problems scale (17 items) across the TBED-C and MTwiNS administrations indicated that there were significant shared environmental contributions to baseline ASB during middle childhood (C variance estimate = 0.20, p < 0.05), although shared environmental influences on rate of change remained non-significant (C variance estimate = 0.11). Reassuringly, however, there were also significant, and partially overlapping, genetic and nonshared environmental contributions to both intercept and slope for CBCL scores, consistent with our results for the 5-item measure (see Table SI and Figure SI).
There are several other limitations to keep in mind when interpreting the results of the present study. First, our analyses are not able to clarify the exact duration of nonshared environmental contributions to ASB. While the significant overlap in these contributions at baseline and over time indicates some degree of continuity, it is unclear whether the influences that do not overlap represent transient effects lasting minutes or days, or more enduring effects that contribute to systematic change. Second, there was a drop in sample size at ages 11–12 and 20–22. As our intercept and slope estimates were based on growth curves, however, there is relatively little impact of ASB estimates at one particular age on participants’ overall trajectories.
Next, the SDQ does not delineate aggressive and nonaggressive rule-breaking sub-types of ASB. This is potentially problematic since these two dimensions of ASB have been shown to exhibit distinct etiologies and developmental trajectories (Burt 2012). Indeed, our finding that ASB decreased linearly across development likely indicates that our measure was unable to capture the spike in rule-breaking typically seen in studies spanning adolescence (e.g., Bongers et al. 2004; Windle 2000). There is thus a need for subsequent research on the development of rule-breaking and aggression as separate phenotypes from childhood into emerging adulthood, particularly using developmentally sensitive measures that capture differences in symptom presentation by age (i.e., heterotypic continuity). However, the items included on the SDQ, and in our abbreviated scale from the CBCL, screen for behaviors that are typically present, to some degree, throughout early development (e.g., lying, disobedience). Moreover, scores on the 17-item Conduct Problems scale on the CBCL also declined linearly across the three TBED-C/MTwiNS assessments, which spanned ages 6 to 19, indicating that the brevity of our measure likely did not prevent it from capturing age-related trends in ASB development in our sample.
In addition, child sex was entered as a covariate in models including male and female participants. Some longitudinal twin studies (e.g., Burt et al. 2007; Eley et al. 2003) have found models allowing for sex differences in the etiology of ASB development to fit better than models constraining parameters to be equal across sex. However, this pattern of results is generally the exception rather than the rule (Burt et al. 2019; Jacobson et al. 2002). Moreover, we note that Burt et al. (2007) found few differences between male and female participants in standardized parameter estimates. As such, while males evidenced higher levels of ASB at baseline and somewhat more decline over time relative to females in our study, we do not expect our overall conclusions to differ in models allowing for sex differences in parameter estimates.
No differences were observed between white participants and those identifying with marginalized races/ethnicities in either baseline ASB or change over time in our sample. Given the demographics of the State of Michigan, however, there were not sufficient numbers of those who identified with any specific marginalized race or ethnicity to model these groups separately. There was a significant effect of neighborhood disadvantage, with youth from impoverished neighborhoods exhibiting higher levels of ASB at baseline and more rapid decline over time. That less privileged youth had higher initial levels of ASB is consistent with a large body of research demonstrating that familial and neighborhood disadvantage increases risk for nearly all youth psychiatric disorders (e.g., Kupersmidt et al. 1995; Leventhal and Brooks-Gunn 2000). Our finding that these youth also desisted more quickly suggests that discrepancies in behavioral outcomes by socioeconomic status may decrease with age. Regardless, there is a need for further research examining disadvantage in the broader context (e.g., neighborhoods, schools), and inequitable structural characteristics (e.g., differences in policing, housing policies) in particular, as a predictor of ASB development over time in racially, ethnically, and socioeconomically diverse samples.
Despite these limitations, the present study is the first to examine the genetic and environmental etiology of ASB over time in a sample spanning nearly all of childhood, adolescence, and emerging adulthood. The key strength of such a study, when incorporating a twin design, is its potential to elucidate the genetic and environmental factors contributing to human development across multiple stages of the life course. Our study yielded two important conclusions. First, genetic factors contributed significantly to both continuity and change in ASB. Given the broad age range under study, the genetic contributions to continuity are perhaps more noteworthy. Nearly 40% of genetic influences on change throughout development were already present at the baseline assessment, which was conducted as early as age 3 in some participants. Such findings underscore the importance of genetic influences in shaping ASB trajectories. While the specific genetic factors underlying continuity and change are unknown, one possibility is that genetic contributions to improved behavioral and emotional regulation are activated as youth progress through adolescence, resulting in fewer problem behaviors over time. On the other hand, genetic factors underlying dimensions of temperament that are known to be predictive of ASB, including negative emotionality and disinhibition, may be among those contributing to continuity in ASB, as temperament is both heritable and moderately stable throughout development (Ganiban et al. 2008). Future work should seek to contrast and test these two possibilities.
Second, the nonshared environmental influences on ASB reflected not only transient person-specific environmental influences, but also more enduring influences that overlapped across assessment waves. Put another way, environmental influences unique to each child within a given family, rather than shared exposures affecting the entire family, were found to be important for both stability and change in ASB across early development. Such findings are consistent with research indicating the importance of the nonshared environment to behavioral outcomes (Plomin and Daniels 1987). Subsequent studies should seek to identify specific nonshared environmental influences that persist over time prior to adulthood.
Supplementary Material
Funding
This project was supported by Grant Nos. R01-MH081813, UG3/UH3 MH114249, and UH3-MH114249-04S1 from the National Institute of Mental Health (NIMH) and R01-HD066040, F31HD102094, and R01-HD093334 from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH, NICHD, or the National Institutes of Health.
Footnotes
Conflict of interest Sarah L. Carroll, D. Angus Clark, Luke W. Hyde, Kelly L. Klump, and S. Alexandra Burt declare no conflicts of interest.
Ethical approval The described study has been approved by the Michigan State University IRB.
Consent to participate Parents provided written consent for their children to participate (if under 18), and adult twins provided written consent for their own participation.
Code availability Mplus code is available from the authors upon request.
Human and animal rights and informed consent All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Participants were provided with information about the study, including their rights as participants. Parents provided informed consent, and children provided informed assent. This article does not include any studies with animals performed by any of the authors.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10519-021-10066-8.
References
- Achenbach TM, Rescorla LA (2001) Manual for the ASEBA school-age forms & profiles. University of Vermont Research Centre for Children, Youth and Families, Burlington [Google Scholar]
- Alink LR, Egeland B (2013) The roles of antisocial history and emerging adulthood developmental adaptation in predicting adult antisocial behavior. Aggress Behav 39(2):131–140 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Association, Washington [Google Scholar]
- Bartels M, Van den Oord EJCG, Hudziak JJ, Rietveld MJH, Van Beijsterveldt CEM, Boomsma DI (2004) Genetic and environmental mechanisms underlying stability and change in problem behaviors at ages 3, 7, 10, and 12. Dev Psychol 40(5):852. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Webster-Stratton C, Reid MJ (2005) Mediators, moderators, and predictors of 1-year outcomes among children treated for early-onset conduct problems: a latent growth curve analysis. J Consult Clin Psychol 73(3):371. [DOI] [PubMed] [Google Scholar]
- Becker A, Woerner W, Hasselhorn M, Banaschewski T, Rothenberger A (2004) Validation of the parent and teacher SDQ in a clinical sample. Eur Child Adolesc Psychiatry 13(2):ii11–ii16 [DOI] [PubMed] [Google Scholar]
- Bongers IL, Koot HM, Van Der Ende J, Verhulst FC (2004) Developmental trajectories of externalizing behaviors in childhood and adolescence. Child Dev 75(5):1523–1537 [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U (1988) Interacting systems in human development. Research paradigms: Present and future. Pers Context 2:25–49 [Google Scholar]
- Burt SA (2009) Rethinking environmental contributions to child and adolescent psychopathology: a meta-analysis of shared environmental influences. Psychol Bull 135(4):608. [DOI] [PubMed] [Google Scholar]
- Burt SA (2012) How do we optimally conceptualize the heterogeneity within antisocial behavior? An argument for aggressive versus non-aggressive behavioral dimensions. Clin Psychol Rev 32(4):263–279 [DOI] [PubMed] [Google Scholar]
- Burt SA, Klump KL (2013) The Michigan state university twin registry (MSUTR): an update. Twin Res Hum Genet 16(1):344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Klump KL (2019) The Michigan State University Twin Registry (MSUTR): 15 years of twin and family research. Twin Res Hum Genet 22(6):741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, McGue M, Iacono WG, Krueger RF (2006) Differential parent-child relationships and adolescent externalizing symptoms: cross-lagged analyses within a monozygotic twin differences design. Dev Psychol 42(6):1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, McGue M, Carter LA, Iacono WG (2007) The different origins of stability and change in antisocial personality disorder symptoms. Psychol Med 37(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, McGue M, Iacono WG (2009) Nonshared environmental mediation of the association between deviant peer affiliation and adolescent externalizing behaviors over time: results from a cross-lagged monozygotic twin differences design. Dev Psychol 45(6):1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Klahr AM, Klump KL (2015) Do non-shared environmental influences persist over time? An examination of days and minutes. Behav Genet 45(1):24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Slawinski BL, Carsten EE, Harden KP, Hyde LW, Klump KL (2019) How should we understand the absence of sex differences in the genetic and environmental origins of antisocial behavior? Psychol Med 49(10):1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Clark DA, Gershoff ET, Klump KL, Hyde LW (2021) Twin differences in harsh parenting predict youth’s antisocial behavior. Psychol Sci 32(3):395–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger RD, Ge X, Elder GH Jr, Lorenz FO, Simons RL (1994) Economic stress, coercive family process, and developmental problems of adolescents. Child Dev 65(2):541–561 [PubMed] [Google Scholar]
- Eamon MK (2002) Poverty, parenting, peer, and neighborhood influences on young adolescent antisocial behavior. J Soc Serv Res 28(1):1–23 [Google Scholar]
- Eley TC, Lichtenstein P, Moffitt TE (2003) A longitudinal behavioral genetic analysis of the etiology of aggressive and nonaggressive antisocial behavior. Dev Psychopathol 15(2):383–402 [DOI] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL (2001) The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equ Model 8(3):430–457 [Google Scholar]
- Ganiban JM, Saudino KJ, Ulbricht J, Neiderhiser JM, Reiss D (2008) Stability and change in temperament during adolescence. J Pers Soc Psychol 95(1):222. [DOI] [PubMed] [Google Scholar]
- Gard AM, Waller R, Shaw DS, Forbes EE, Hariri AR, Hyde LW (2017) The long reach of early adversity: parenting, stress, and neural pathways to antisocial behavior in adulthood. Biol Psychiatry 2(7):582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner TW, Dishion TJ, Connell AM (2008) Adolescent self-regulation as resilience: resistance to antisocial behavior within the deviant peer context. J Abnorm Child Psychol 36(2):273–284 [DOI] [PubMed] [Google Scholar]
- Goldsmith HH (1993) Nature–nurture issues in the behavioral genetic context: overcoming barriers to communication. In: Plomin R (ed) Nature, nurture, and psychology. American Psychological Association, Washington, pp 325–339 [Google Scholar]
- Goodman R (2001) Strengths and Difficulties Questionnaire (SDQ). Youthinmind, London [Google Scholar]
- He JP, Burstein M, Schmitz A, Merikangas KR (2013) The Strengths and Difficulties Questionnaire (SDQ): the factor structure and scale validation in US adolescents. J Abnorm Child Psychol 41(4):583–595 [DOI] [PubMed] [Google Scholar]
- Hopwood CJ, Donnellan MB, Blonigen DM, Krueger RF, McGue M, Iacono WG, Burt SA (2011) Genetic and environmental influences on personality trait stability and growth during the transition to adulthood: a three-wave longitudinal study. J Pers Soc Psychol 100(3):545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hox J, Stoel RD (2014) Multilevel and SEM approaches to growth curve modeling. Wiley, Hoboken [Google Scholar]
- Jacobson KC, Prescott CA, Kendler KS (2002) Sex differences in the genetic and environmental influences on the development of antisocial behavior. Dev Psychopathol 14(2):395–416 [DOI] [PubMed] [Google Scholar]
- Kind AJ, Buckingham WR (2018) Making neighborhood-disadvantage metrics accessible—the neighborhood atlas. N Engl J Med 378(26):2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersmidt JB, Griesler PC, DeRosier ME, Patterson CJ, Davis PW (1995) Childhood aggression and peer relations in the context of family and neighborhood factors. Child Dev 66(2):360–375 [DOI] [PubMed] [Google Scholar]
- Lacourse E, Boivin M, Brendgen M, Petitclerc A, Girard A, Vitaro F, Tremblay RE (2014) A longitudinal twin study of physical aggression during early childhood: evidence for a developmentally dynamic genome. Psychol Med 44(12):2617. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Loeber R, Burke JD, Applegate B (2005) Predicting future antisocial personality disorder in males from a clinical assessment in childhood. J Consult Clin Psychol 73(3):389. [DOI] [PubMed] [Google Scholar]
- Leventhal T, Brooks-Gunn J (2000) The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol Bull 126(2):309. [DOI] [PubMed] [Google Scholar]
- MacCallum RC (2009) Factor analysis. In: Millsap RE, Maydeu-Olivares A (eds) The SAGE handbook of quantitative methods in psychology. SAGE Publications Ltd, London, pp 123–147 [Google Scholar]
- Martino SC, Ellickson PL, Klein DJ, McCaffrey D, Edelen MO (2008) Multiple trajectories of physical aggression among adolescent boys and girls. Aggress Behav 34(1):61–75 [DOI] [PubMed] [Google Scholar]
- McGue M, Bouchard TJ (1984) Adjustment of twin data for the effects of age and sex. Behav Genet 14(4):325–343 [DOI] [PubMed] [Google Scholar]
- Monahan KC, Steinberg L, Cauffman E (2009) Affiliation with antisocial peers, susceptibility to peer influence, and antisocial behavior during the transition to adulthood. Dev Psychol 45(6):1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Meesters C, van den Berg F (2003) The strengths and difficulties questionnaire (SDQ). Eur Child Adolesc Psychiatry 12(1):1–8 [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén B (2019) Mplus. The comprehensive modelling program for applied researchers: user’s guide 5 [Google Scholar]
- Neale MC, Cardon LR (1992) Methodology for genetic studies of twins and families. Boston, MA: Kluwer Academic [Google Scholar]
- Odgers CL, Moffitt TE, Broadbent JM, Dickson N, Hancox RJ, Harrington H, Caspi A (2008) Female and male antisocial trajectories: from childhood origins to adult outcomes. Dev Psychopathol 20(2):673. [DOI] [PubMed] [Google Scholar]
- Peeters H, Van Gestel S, Vlietinck R, Derom C, Derom R (1998) Validation of a telephone zygosity questionnaire in twins of known zygosity. Behav Genet 28(3):159–163 [DOI] [PubMed] [Google Scholar]
- Pingault JB, Rijsdijk F, Zheng Y, Plomin R, Viding E (2015) Developmentally dynamic genome: evidence of genetic influences on increases and decreases in conduct problems from early childhood to adolescence. Sci Rep 5(1):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Daniels D (1987) Why are children in the same family so different from one another? Behav Brain Sci 10(1):1–16 [Google Scholar]
- Porsch RM, Middeldorp CM, Cherny SS, Krapohl E, Van Beijsterveldt CE, Loukola A, Bartels M (2016) Longitudinal heritability of childhood aggression. Am J Med Genet B Neuropsychiatr Genet 171(5):697–707 [DOI] [PubMed] [Google Scholar]
- Rhemtulla M, Hancock GR (2016) Planned missing data designs in educational psychology research. Educ Psychol 51(3–4):305–316 [Google Scholar]
- Turkheimer E, Gottesman II (1991) Individual differences and the canalization of human behavior. Dev Psychol 27:18–22 [Google Scholar]
- Tuvblad C, Narusyte J, Grann M, Sarnecki J, Lichtenstein P (2011) The genetic and environmental etiology of antisocial behavior from childhood to emerging adulthood. Behav Genet 41(5):629–640 [DOI] [PubMed] [Google Scholar]
- Van Beijsterveldt CEM, Bartels M, Hudziak JJ, Boomsma DI (2003) Causes of stability of aggression from early childhood to adolescence: a longitudinal genetic analysis in Dutch twins. Behav Genet 33(5):591–605 [DOI] [PubMed] [Google Scholar]
- Windle M (2000) A latent growth curve model of delinquent activity among adolescents. Appl Dev Sci 4(4):193–207 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


