Abstract
Pediatric gastrointestinal endoscopy has been established as safe and effective for diagnosis and management of many pediatric gastrointestinal diseases. Nevertheless, certain patient and procedure factors should be recognized that increase the risk of intra- and/or postprocedural adverse events (AEs). AEs associated with endoscopic procedures can broadly be categorized as involving sedation-related physiological changes, bleeding, perforation, and infection. Factors which may increase patient risk for such AEs include but are not limited to, cardiopulmonary diseases, anatomical airway or craniofacial abnormalities, compromised intestinal luminal wall integrity, coagulopathies, and compromised immune systems. Examples of high-risk patients include patients with congenital heart disease, craniofacial abnormalities, connective tissues diseases, inflammatory bowel disease, and children undergoing treatment for cancer. This clinical report is intended to help guide clinicians stratify patient risks and employ clinical practices that may minimize AEs during and after endoscopy. These include use of CO2 insufflation, endoscopic techniques for maneuvers such as biopsies, and endoscope loop-reduction to mitigate the risk of such complications such as bleeding and intestinal perforation. Endoscopic infection risk and guidance regarding periprocedural antibiotics are also discussed.
Keywords: adverse events, bleeding, endoscopy, pediatric
Pediatric gastrointestinal (GI) endoscopy is a well-established and integral approach to the diagnosis and management of digestive disorders in children. Published data from the Pediatric Clinical Outcomes Research Initiative (PEDS-CORI) suggest the overall rate of complications during upper GI procedures is 2.3%, including a specific risk of respiratory issues (1.5%) and bleeding (0.3%) (1). The rates of complications during colonoscopy in this database were also reported at 1.1%, with the highest rates of adverse events (AEs) during polypectomy (2). Nevertheless, a number of patient and procedure factors may increase the risk of intra- and/or postprocedural AEs. The goals of this clinical practice statement from the North American Society of Gastroenterology Hepatology and Nutrition (NASPGHAN) are to define high-risk pediatric patients undergoing GI procedures; discuss preoperative preparation as a means to mitigate risk; and identify practices, which may increase safety during endoscopy in children considered to be at high risk.
The American Society of Gastrointestinal Endoscopy (ASGE) has defined a lexicon of major and minor AEs related to GI procedures (3). A preoperative assessment or checklist may be useful in evaluating these factors. Postprocedural events may be recorded and often involve concerns for late-onset complications of physiological effects of sedation, bleeding, perforation, and infection. For example, 1 recent large pediatric-referral center study reported 249 of 9577 (2.6%) endoscopic procedures to involve reports of postprocedure AEs (4). The most common events in this study presented concerns for procedurally related infection and/or perforation, and included fever, abdominal pain, chest, and throat pain.
Most AEs occurring during or after pediatric endoscopy can be broadly classified as involving cardiopulmonary compromise, bleeding, perforation, and infection (3). Patients at high risk for such intraprocedural AEs can be defined as children with primary or secondary comorbidities that may place them at increased risk for these known complications across all or a subset of endoscopic procedures.
CARDIOPULMONARY AND SEDATION-RELATED EVENTS
Patients at High Risk for Cardiopulmonary and Sedation-related Events
Most cardiopulmonary AEs associated with pediatric endoscopy are related to procedural sedation and anesthesia (5). Despite a shifting landscape in sedation practices over the past few decades to anesthesiologist-assisted approaches, sedation complications in modern practice are still common, and may account for up to ~60% of all AEs that occur during pediatric GI procedures (1,5,6). Cardiopulmonary events during pediatric endoscopy can range from minor to major complications, and include transient oxygen desaturation, aspiration, respiratory arrest, shock, and myocardial infarction (7). Patients at high risk for cardiopulmonary events include those with compromised cardiopulmonary function, including decreased forced expiratory volumes (as measured by Forced Expiratory Volume-1) (3). Specific examples of patients at high risk for sedation complications include infants younger than 1 year, infants and children with congenital heart disease, pulmonary hypertension, cystic fibrosis, muscular dystrophy and obesity, and children with acute upper respiratory illnesses (1,5,6).
Airway Assessment
The Mallampati score has been proposed as a standardized way to identify patients who may be at risk for difficult planned or unanticipated endotracheal intubation (8) (Fig. 1). Generally speaking, a Mallampati score of grades I and II, where the soft palate and uvula are visible, is associated with the likelihood that endotracheal intubation for airway protection can be performed for elective or rescue purposes without difficulty. Patients who are scored as either grades III or IV, where the soft palate and uvula are less visible, should be recognized to be at increased risk of difficult endotracheal intubation. In adults, there are known relationships between obesity, neck circumference, and higher Mallampati scores (10). The relationship between obesity and sedation risks during pediatric GI procedures has not been well elucidated (5).
FIGURE 1.
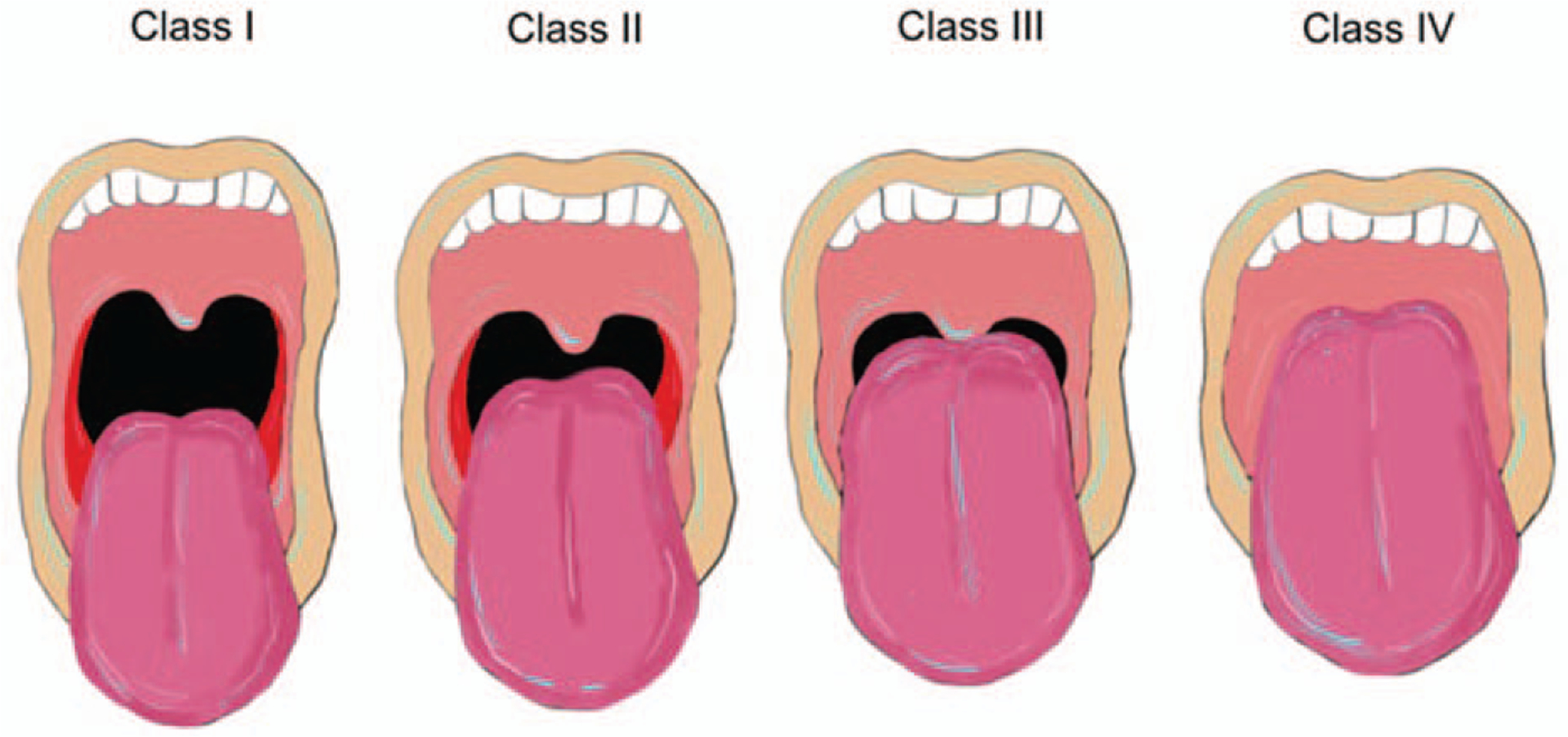
Mallampati scoring of airways (8,9). Modified from Mallampati Classification. Samsoon GL, Young Jr. Difficult tracheal intubation: a retrospective study. Anaesthesia 1987;42:487–490; Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J 1985;32 (4):429–434. Image courtesy of Dr. Mark Mazziotti.
Nevertheless, all patients with difficult airways should be recognized to be at risk for sedation-related complications. Pediatric populations at highest risk for having difficult airways are those with craniofacial congenital abnormalities, including a large tongue, a highly arched or narrow palate, a short, thick neck, and prominent overbite (11). Furthermore, all children with limited range of motion of their necks are considered to have difficult airways, including Down syndrome patients with overlapping airway concerns and atlanto-occipital instability risk (11). Specific patient diagnoses that should elicit concern for increased cardiopulmonary risks due to their challenging airways during endoscopy include: Pierre Robin syndrome; Treacher Collins syndrome, and patients with laryngeal atresia.
Patients with history of lung disease, including chronic aspiration or other aerodigestive disease, reactive airways, pulmonary hypertension, and cystic fibrosis may be at particular risk of ventilatory compromise during endoscopy (12). Patients may also be taking certain medications, which can potentiate cardiopulmonary effects of sedation, includingantiseizure, psychotropic, and pain medicines(12).Certainly, patients taking benzodiazepines or opioids on a chronic basis may be at risk for complications of sedation and anesthesia, and should be identified during preprocedure patient preparation to be at high risk for cardiopulmonary events during endoscopy (13).
Food and Drug Administration Warning Related to Anesthetics
As an additional sedation risk, the United States Food and Drug Administration has recently called attention to concerns that almost all common sedatives may pose a risk of neurotoxicity in the developing brain (14). In 2016, the Food and Drug Administration released a black box warning for a number of common anesthetic agents used to induce sedation for pediatric GI procedures, including midazolam, fentanyl, propofol, and inhalational anesthetics. Although the warning as written specifically pertains to children younger than 3 years receiving general anesthesia for multiple sessions or for >3 hours of duration, it is critical for providers to be comfortable discussing it with their patients. Pediatric endoscopists should also be familiar with nonsedation approaches to GI procedures, including unsedated transnasal endoscopy for the appropriate clinical indications and in the appropriate setting (15).
BLEEDING-RELATED EVENTS
Patients at High Risk for Bleeding During Pediatric Endoscopy
Significant bleeding is a rare AE of endoscopic procedures in children (1). When it does occur, bleeding may be intraluminal, intramural, and, more rarely, extraluminal into the peritoneum, retroperitoneum, or chest. In terms of the last, massive pulmonary hemorrhage should be recognized to be a rare, but well described complication of endoscopy in older patients with cystic fibrosis, and may represent another way that this patient population should be recognized to be at high risk (16,17).
Limited large data sets exist detailing endoscopic bleeding risks in the pediatric population. PEDS-CORI data from 2008 regarding >8000 procedures has provided the only direct estimation of bleeding risks of colonoscopy in children to be 0.43%. In terms of upper GI procedures, PEDS-CORI data on >10,000 esophagogastroduodenoscopy identified a 0.3% reported rate of bleeding (1). These data are now more than 10 years old, and does not report on postprocedure bleeding, therefore possibly underestimating the complication rate. Furthermore, the PEDS-CORI data do not differentiate between only mild bleeding requiring no intervention and cases of more significant hemorrhage. Kramer and Narkewicz reported a postprocedural bleeding rate of 0.11% across all procedures, including rebleeding in patients who underwent procedures for hemostasis; 75% of bleeding cases resulted in at least a referral to the emergency department or unanticipated evaluation by a physician (4).
Data pooled from adult studies suggest a lower bleeding risk than the PEDS-CORI reports, ranging 0.03% to 0.14% for EGD and 0.008% to 0.03% for colonoscopy (18). Another study of colonoscopy in adults reported an adverse bleeding event in 0.1% to 0.6% of procedures (19). The bleeding risk during colonoscopy may depend on whether or not polypectomy is also performed (20).
During diagnostic procedures, bleeding can result from endoscope manipulation or mucosal biopsy (21). Endoscope advancement, especially around blind or angulated turns may result in mucosal shearing or tearing and subsequent bleeding. The sigmoid colon may be at particular risk of intraluminal or intramural bleeding, resulting in a hematoma, during colonoscope advancement and loop reduction (22). The risk of bleeding following mucosal biopsy is generally related to patient-specific risk factors (ie, inflammation, coagulopathy, hemophilia) or the site of biopsy rather than the total number of biopsies obtained during the procedure (18,21,23). Additional instrumentation and therapeutic procedures increase the risk of bleeding. In a large review of endoscopic retrograde cholangiopancreatography (ERCP) in children, Enestvedt et al (24) reported serious bleeding AEs to occur in 1.4% of procedures.
Intramural hematomas mostly occur in the duodenum post EGD with mucosal biopsy. The incidence of EGD with biopsy-related duodenal hematoma in children has been reported to be 1 in 1922 procedures (25). Unique features of the third portion of the duodenum have been hypothesized to account for the vulnerability of this anatomic location: relatively fixed retroperitoneal position, adjacency to the lumbar spine, lack of well-developed retroperitoneal serosal layer, and rich submucosal vascular plexus susceptible to shearing forces during biopsy acquisition (26,27). It has also been theorized that one may be able to decrease the risk of a duodenal hematoma by avoiding extension of the biopsy forceps >2 to 3 cm beyond the endoscope tip, thereby decreasing any stripping of the mucosa from the immobile bowel wall behind it (28,29).
Certain comorbidities may increase the risk of duodenal hematoma, which typically presents as abdominal pain and/or vomiting within 72 hours of the EGD (25). Children with leukemia and recipients of hematopoietic bone marrow transplants seem to be at particular risk based on available case reports (29–31). Underlying coagulation disorders may also predispose to intramural duodenal hematoma (32). Once a hematoma occurs, it generally requires weeks to resolve, unless surgical evacuation is performed.
Hematologic Parameters
To some extent, risks of bleeding may be of more concern in patients who are anemic before a procedure, and severe anemia can affect cardiovascular stability during endoscopy. Nevertheless, there is no data to support a hemoglobin threshold below which endoscopy should not be performed in children. Compelling data around optimal hemoglobin levels in adults and triggers to institute blood transfusions to address anemia suggest that a restrictive strategy (transfusion for hemoglobin <7 g/dL) may be preferred to a liberal strategy (transfusion for hemoglobin <9 g/dL) (33). Similar data in patients with portal hypertension suggest that portal pressure may be increased with more liberal transfusions. Recent international guidelines suggest conservative target hemoglobin levels in portal hypertensive bleeding between 7 and 8 g/dL, taking into account hemodynamic status and ongoing bleeding (34).
Patient risk factors for bleeding during endoscopy include thrombocytopenia, poor platelet function, coagulopathy, and use of certain medications. Pediatric patient populations at increased risk of bleeding complications during endoscopic procedures (and who often experience the above risk factors) include those with bone marrow failure or hematologic malignancies, history of hematopoietic stem cell transplant (HSCT), end-stage liver disease, disorders of coagulation, and those taking antithrombotic medications (Table 1). Antithrombotic agents carry varying degrees of bleeding risks, and include anticoagulants (heparin, low-molecular-weight heparin, and warfarin) and antiplatelet medications (nonsteroidal anti-inflammatory drugs, aspirin, clopidogrel, ticlopidine, and glycoprotein IIb/IIIa inhibitors).
TABLE 1.
Bleeding risk: risk factors for bleeding during endoscopy and examples of conditions more commonly seen in the pediatric patient
| Thrombocytopenia |
| Lack of production: bone marrow failure, post bone marrow transplant |
| Sequestration: portal hypertension and hypersplenism |
| Destruction: hemolytic uremic syndrome, autoimmune diseases |
| Coagulopathy |
| Congenital disorders of coagulation: factor deficiencies, von Willebrand’s |
| Hepatobiliary disease: factor synthesis dysfunction, vitamin K deficiency |
| Hematologic malignancies or chemotherapy related |
| Disseminated intravascular coagulation |
| Vitamin K deficiency (malnutrition, cholestasis, exocrine pancreatic insufficiency, short bowel syndrome, renal disease) |
| Platelet dysfunction: uremia |
| Medications |
| Anticoagulants: heparin, low-molecular-weight heparin, warfarin |
| Antiplatelet: nonsteroidal anti-inflammatory drugs, aspirin, clopidogrel, ticlopidine, glycoprotein IIb/IIIa inhibitors |
| Prolonged antibiotic use (vitamin K deficiency) |
| Cholestyramine (vitamin K deficiency) |
A platelet count threshold for the performance of GI endoscopy has not been determined. One report of 191 children with a history of HSCT undergoing endoscopy suggested that the platelet count was <50 × 103 μL in 10 of 13 (77%) patients who experienced a serious bleeding AE, defined as duodenal hematoma or acute anemia requiring blood transfusion (35). However, in a study of adult patients with cancer undergoing EGD without biopsy, Chu et al (36) described no adverse bleeding events in patients with platelet counts ≤20,000. The Standards of Practice Committee of the ASGE recommends a minimum platelet threshold >20 × 103 μL if performing endoscopy with mucosal inspection, and 50 × 103 μL if obtaining mucosal biopsies, respectively (37).Nevertheless, these oft-quoted thresholds may not fully reflect the role of platelet dysfunction as much as platelet count in overall bleeding risk (38). There are no readily available tests to easily assess platelet function. Yet, it is reasonable to assume that comorbidities of uremia, hypoalbuminemia, or recent bleeding event may confer additional risk of bleeding related to platelet dysfunction (39–41).
Antithrombotic Medications
Another setting in which an altered hemostatic milieu exists is in patients using antithrombotic medications. The ASGE Standards of Practice Committee has issued guidelines for the management of antithrombotic medications at the time of endoscopic procedures in adult patients (42). It is recommended to consider the medication being used, the degree of procedure urgency, and balancing the risks of both bleeding from the procedure and thromboembolic events from medication discontinuation in deciding to proceed with or to defer a procedure (42). Although the generalizability of these guidelines to children has not been rigorously examined, it seems reasonable that elective procedures may be deferred for patients on time-limited chronic anticoagulation, such as treatment for a deep vein thrombosis, until treatment is completed. Furthermore, anticoagulation should be stopped in patients with acute bleeding until achieving hemostasis. In patients receiving long-term anticoagulation who require endoscopic procedures, undergoing a change in antithrombotic therapy to low-molecular-weight heparin in the periendoscopy period is advised. According to the ASGE, an international normalized ratio (INR) of 1.4 to 1.7 in adult patients for therapeutic endoscopic procedures is acceptable (42). No data support or refute this statement in children.
It is important to consider the preprocedural preparation for patients at higher risk of bleeding. Laboratory assessments may include a complete blood count, partial thromboplastin time, and prothrombin time/INR to assess for thrombocytopenia and coagulopathy, and a type and crossmatch for blood products to be available. Routine pre-endoscopy testing of partial thromboplastin time and prothrombin time is not indicated. Testing should, however, likely be performed in patients with risk factors for coagulopathy or a suspicion of a coagulation disorder based on history and physical examination (43).
Preprocedure correction of thrombocytopenia and coagulopathy with blood products may be considered if there are patient-specific risk factors, accounting for the type of endoscopic procedure planned. Consultation with a hematologist should be considered for reversal of antithrombotic medications and management of patients with factor deficiencies or other disorders of coagulation. Clear communication with in-hospital surgical colleagues to be available in circumstances of uncontrolled bleeding is also advisable. Depending on the clinical condition of the patient, blood products such as packed red blood cells, fresh frozen plasma, platelets, cryoprecipitate, and recombinant activated factor VII should be available in the blood bank or in the operating room during the procedure.
PERFORATION-RELATED EVENTS
Patients at High Risk for Perforation
Perforation during endoscopy can be defined as instrumental injury leading to evidence of air or luminal contents outside the GI tract (3). Incidence of perforation during endoscopy ranges from 0.06% to 0.3% (44), and is generally classified as large or small. Perforation is rare during diagnostic endoscopy, but has been described (45,46). Certain endoscopic maneuvers and patient comorbidities can increase the risk of perforation (47). Therefore, in patients with high risk for perforation, it is important to use endoscopic techniques to reduce the risk of instrumentation trauma.
Large perforations usually result from injury from the shaft of the endoscope. Risk factors include large intracolonic loops, particularly when formed in the rectosigmoid region (48). This area is more prone to large endoscope perforation due to sharp angulation at the rectosigmoid junction or the sigmoid-descending junction. The antimesenteric side of the bowel also has a higher risk of perforation due to the overextension of the bowel by the shaft of the endoscope during advancement.
If the spectre of perforation is raised during or after endoscopy, prompt imaging with an abdominal x-ray is recommended. Ideally at least 2 views should be obtained, including a left lateral decubitus film. Abdominal x-rays or computerized tomography scans are likely to demonstrate extraluminal free air. Presentation of large perforations is immediate, and patients will likely have peritoneal signs.
To minimize perforation risks, it is important to minimize large loop formation. In the sigmoid colon, reduction is most commonly required of an “alpha loop,” which can typically be performed with clockwise torque on the insertion tube and pulling back on the endoscope, whereas a “reverse alpha” loop typically requires counterclockwise torque. This will straighten the colonoscope shaft, and decrease the tension on the lumen (47). Abdominal pressure and changes in patient position can also be used to help facilitate advancement of the colonoscope more safely (47). In patients with tortuous colons where standard colonoscopy has failed, use of a balloon enteroscope in lieu of a colonoscope may be helpful to reduce large colonic loops and minimize risk of perforation (49–51).
Small perforations usually occur due to the endoscope tip (48). During colonoscopy, these can occur when advancing through a turn with a “sliding by” technique. Therapeutic maneuvers, such as hot snare polypectomy and sphincterotomy, can also result in small focal perforations. Presentation of this complication can be delayed by hours to days with nonspecific abdominal pain and tenderness (48). Postpolypectomy syndrome, defined as fever, abdominal pain with peritoneal signs, and leukocytosis related to thermal injury postcauterization, may also present with symptoms similar to intestinal perforation without radiographic signs of extraluminal air (52,53).
Although not described in children, excessive air insufflation can also result in perforation (54). This occurs more commonly in the left colon than the more proximal colon. Advancing in a fully distended colon also increases the length of endoscope required to reach the cecum. Distention with air may increase the technical difficulty of loop reduction and create a taut, less flexible mucosa. These 2 factors can occur iatrogenically or be related to patient comorbidity; in either case, they increase the risk of perforation.
Use of carbon dioxide (CO2) for insufflation during endoscopic procedures, in lieu of air, has been postulated but not proven to increase safety during endoscopy (55). CO2 is absorbed across the intestines considerably more rapidly than air, and does mitigate patient discomfort after procedure (55). As an inert gas, CO2 has also been suggested to lower risks associated with combustion during use of electrocautery (56).
Generally speaking, patients with intestinal strictures may be at higher risk for perforation. This may be due to intermittent obstruction that occurs mechanically when a colonoscope traverses a stricture, or simply due to distension with air that cannot easily diffuse. In addition, patients with both primary and secondary pseudo-obstruction associated with massive dilated bowel (eg, spinal muscular atrophy, metabolic disorders, Hirschsprung disease) should be recognized to be at increased risk of perforation during endoscopic procedures (57). The increased risk for perforation during therapeutic decompression of a pseudo-obstruction should be mitigated by employing CO2, performing intermittent suctioning to decompress the intestinal lumen, or utilizing water immersion throughout the procedure (55,57). Similarly, argon plasma coagulation has been described to be associated with over-insufflation and perforation due to the differential diffusion of inert argon gas (58).
A number of other factors may increase a patient’s risk for perforation. These include using a larger endoscope in small patients, poor or compromised endoscopic visualization, and impaired bowel wall strength (54). It is important to recognize that performance of diagnostic and therapeutic procedures in small children may require larger endoscopes, with larger working channels and greater availability of endoscopic accessories (59). For example, pediatric therapeutic endoscopists performing ERCP generally prefer a standard therapeutic duodenoscope in patients who weigh >10 kg, although such patients may still be relatively small (59). In addition, to control GI bleeding in neonates and young infants, a larger gastroscope than standard neonatal gastroscopes may be required to use accessories such as bipolar electrocautery and endoclips (59). Also, to successfully perform colonoscopy in infants and small children, gastroscopes may be sometimes be used, which may be stiff and relatively large for a small child’s intestinal lumen (5). Commercial colonoscopes are now available with the same outer diameter as some gastroscopes. Although patient-endoscope mismatch may be unavoidable, it is still important to recognize the increased risks of perforation and proceed accordingly.
Poor endoscopic visualization also poses an increased risk for perforation and is generally due to poor bowel preparation (60,61). Poor bowel preparation is a common factor which leads to higher rates of incomplete colonoscopy (62). Visualization may be limited and more technically challenging when using duodenoscopes and echoendoscopes, because they present an altered endoscopic side or oblique-view, compared with standard forward-viewing endoscopes. Use of a side-viewing scope risks poor visualization of pharyngeal-esophageal pathology, as de facto the esophagus must be intubated blindly with a duodenoscope for ERCP (63).
Disease-specific Risks for Perforation
Patients with inflammatory bowel disease may be at higher risk for perforation due to intestinal strictures and decreased mucosal wall strength. Two large pediatric series have described risks of perforation in patients with IBD (45,64). This risk can be compounded when patients are on high-dose steroids, due to decreased bowel wall thickness and strength (65). High-dose steroid use can also mask and delay the onset of peritoneal symptoms (66). Therefore one must have a high suspicion for bowel perforation in patients receiving high-dose steroids who develop persistent abdominal pain after endoscopy.
Distinct genetic conditions may place patients more at risk for endoscopic perforation, including recessive dystrophic epidermolysis bullosa (RDEB) and type 4 (vascular type) Ehlers-Danlos syndrome (EDS) (67,68). In children with severe generalized RDEB, epithelial and mucosal scarring due to even minimal manipulation or abrasion is a significant risk, and can occur with taping of the skin or with establishment of a secure airway (69,70). Ideally, specially designed adhesives should be used exclusively, and careful decision making about airway management should occur before the procedure (70). When performing upper endoscopy in children with RDEB, it is particularly important to recognize that esophageal scarring can lead to esophageal stricturing, along with consequent malnutrition (71). Stents are not an option in this disease due to risks of abrasion damage. Similarly, endoscopic dilation is associated with increased risks of perforation, in addition to rescarring due to fibrosis and the possibility of creating a false track. Any dilation of strictures in RDEB should be conservatively performed (68). At least 1 report has examined prevention of subsequent refibrosis postdilation in RDEB by application of topical mitomycin C, with limited success (72). Another article has described a dual approach to dilation in this population, with the initial dilation occurring peroral associated with a push gastrostomy insertion, and subsequent esophageal balloon dilation occurring via the gastrostomy and gastroesophageal junction, which may have the advantage of preventing traction trauma to the proximal esophagus (71).
Patients with EDS have disease of the joints, skin, and connective tissue. In particular patients with EDS type IV (vascular type) have a have an extremely high risk of bowel perforation and GI bleeding during endoscopy (67). EDS type IV accounts for 5% of EDS prevalence and has an autosomal dominant inheritance pattern. The endoscopic complications have been shown to occur both spontaneously and iatrogenically with higher rates in children and teenagers. Perforation usually occurs in the colon which contains a high amount of collagen (67,73–75). Therefore endoscopic and surgical procedures should be undertaken in type IV EDS only with extreme caution.
Acquired patient risk factors for endoscopic perforation include certain types of ingestions, history of prior perforation, prolonged procedures, and HSCT (45). In particular, compromised mucosal wall strength may increase risks of esophageal perforation in patients with caustic ingestion, disk battery ingestion, and tracheal-esophageal atresia surgical anastomotic sites (45,76).
Warning signs associated with perforation include pain out of proportion to examination, persistent tachycardia, atypical use of pain medication, and fever and pain lasting beyond a few hours. Any of these should alert the team of a possible perforation event (45). Initial imaging should include a full abdominal x-ray and left lateral decubitus radiograph to look for air under the diaphragm, which may require having the patient on their side for 5 to 10 minutes to adequately demonstrate air. If perforation is suspected and plain radiography does not confirm this, a computerized tomography scan with water-soluble contrast should be considered (77).
INFECTION-RELATED EVENTS
Patients at High Risk for Endoscopy-related Infections
Infectious events are infrequent, but can occur after flexible GI endoscopy (78). They may result from either exposing patients to infectious agents through the use of contaminated equipment (exogenous transmission) or by creating a portal of entry through which host flora may set up infection (endogenous transmission) (78,79). Thus, rates of postprocedural infectious events are dependent on the fidelity of postprocedural equipment processing, the presence of patient specific risk factors such as a compromised immune system, and the nature of the procedure being performed.
Exogenous Infection Transmission
During endoscopy, the external portion of the endoscope, its channels, and the used accessories are inevitably contaminated with bodily fluids, organic debris, and the microorganism milieu of the patient (78,79). The complex design and long internally located channels of endoscopy equipment renders them difficult to clean and disinfect thus predisposing them to colonization and biofilm formation. An accurate estimate of exogenous transmission of infection following endoscopy remains elusive. The most often quoted estimated rate of transmission is 1 out of 1.8 million procedures (80), although this estimate was developed before widespread adoption of the rigorous cleaning and disinfectant standards currently used today (81).
To minimize the risk of transmitting infectious agents, multisocietal guidelines currently recommend that scopes should be thoroughly cleaned and undergo high-level disinfection before reuse or storage (81). In addition all reusable accessories designed to breach the GI mucosa should be completely sterilized. A high index of suspicion should be maintained, as meticulous adherence to high-level disinfection techniques does not completely eliminate the risk of transmitting infection. Most recently, New Delhi metallo-β-lactamase Escherichia coli and carbapenem-resistant Enterobacteriaceae were recognized to be transmitted via well-processed duodenoscopes in adult patients in the United States (82). Infectious transmission of any organisms, however, remains an extremely rare event in the setting of appropriately implemented endoscopy equipment processing standards. Information regarding duodenoscope reprocessing is available at www.fda.gov (83).
Patient Risk Factors for Endogenous Infection Transmission
Several patient-related factors should be considered when assessing a patient’s risk for developing a postprocedural infectious event. Patients with cardiovascular disease have long been considered to be at high risk for developing endocarditis after GI endoscopy (84). This risk is thought to be secondary to the transient bacteremia that may result from the mucosal tears that occur during the procedure that could subsequently lead to seeding of cardiac tissue and synthetic materials. Rate of transient bacteremia after diagnostic EGD or colonoscopy with biopsy in adult patients is felt to be around 4% (85). Similarly, small pediatric series suggest that transient bacteremia after routine GI endoscopy with biopsy is uncommon (86,87). In adult patients, the mean reported rates of transient bacteremia associated with therapeutic maneuvers such as esophageal stricture dilation (22%), sclerotherapy (14.6%), and variceal ligation (8.8%) are higher (88,89). Such rates of bacteremia are not dissimilar to the rates of bacteremia associated with activities of daily living such as chewing food (7%–51%) or flossing and tooth brushing (20%–40%) (90). In addition, despite the hundreds of thousands of endoscopic procedures performed annually in the United States, case reports of infectious endocarditis remain rare and anecdotal in nature. No study has demonstrated that administration of antibiotic prophylaxis prevents development of infectious endocarditis associated with endoscopy (5,85). Despite potential increased rates of bacteremia the ASGE does not recommend antibiotics solely for the prevention of bacteremia. Periprocedure antibiotics are, however, recommended in circumstances such as sclerotherapy and variceal band ligation for acute GI bleeding. This is due to the benefit seen in cirrhotic patients with acute bleeding, rather than prevention of bacteremia.
The American Heart Association and the American Society of Gastrointestinal Endoscopy no longer recommend antibiotic prophylaxis specifically to prevent infectious endocarditis in patients with cardiovascular risk factors (85). Nevertheless, both societies recognize that there are specific cardiac conditions that may increase risks of infectious endocarditis (85) (Table 2). In children with specific cardiac conditions with increased risk for infectious endocarditis, consider prophylactic antibiotic coverage against enterococci (typically with amoxicillin or ampicillin), in children with specific cardiac conditions, as a common cause of infectious endocarditis in these special populations. This is particularly true for patients who have documented infections of the GI tract in which enterococci may be a part of the infectious milieu (eg, cholangitis) or who are undergoing procedures associated with relatively high rates of bacteremia and infectious events such as ERCP and endoscopic ultrasound-fine needle aspiration (EUS-FNA) (see discussion below) (85). At this time, definitive studies showing that antibiotic prophylaxis is or is not helpful in preventing endocarditis in pediatric patients with congenital heart disease are lacking. When performing endoscopy in children with cardiac conditions, a conversation with the patient’s cardiologist and consideration of unique patient factors that may influence decision-making is prudent.
TABLE 2.
Cardiac conditions in which periprocedural antibiotic prophylaxis may be considered because of high risk for poor outcome from infective endocarditis (90)
| Previous history of infectious endocarditis |
| Cardiac valve repair utilizing prosthetic materials |
| Cardiac transplantation recipients who develop cardiac valvulopathy |
| Congenital heart disease (CHD) with any of the following: |
| Unrepaired cyanotic CHD, including palliative shunts and conduits |
| Completely repaired CHD when prosthetic material or device during the first 6 months following placement. |
| Repaired CHD when residual defects at the site or adjacent to the site of a prosthetic patch or device |
Patient-specific decision-making around antibiotic prophylaxis for endoscopic procedures may also be reasonable to pursue in patients who possess congenital or acquired defects in their immune response. Examples of such patients include those with a diagnosed solid or liquid malignancy, those on immunosuppressive medications, those with absolute or functional neutropenia, and those with hyposplenism or asplenism (91–93). The primary concern in immunocompromised patients is they may not be able to clear transient bacteremia that may occur during endoscopic procedures which could consequently result in deep seeded infections or sepsis. For patients not on a daily prophylactic antibiotic already (eg, recently splenectomized patients), antibiotic prophylaxis is likely unnecessary and is not routinely recommended by the ASGE (85). In contrast, the British Society of Gastroenterology does recommend offering antibiotic prophylaxis for all profoundly patients with neutropenia (absolute neutrophil count <0.5 × 109/L) undergoing GI endoscopic procedures known to be associated with higher rates of bacteremia (ie, sclerotherapy, dilation, ERCP) as a matter of expert opinion (93). The British Society of Gastroenterology nonetheless currently recommends against routine prophylaxis in other immunocompromised patient groups (eg, biologic therapies for inflammatory bowel disease).
At this time, data evaluating infectious risks associated with endoscopy in immunocompromised children are lacking. One small pediatric study showed that upper and lower endoscopy was associated with low rates of infectious events in children with cancer, although neutropenic patients (absolute neutrophil count <1.0 × 109/L) were routinely given antibiotic prophylaxis with cefuroxime (91). Infectious complications attributable to endoscopy have also not been observed in pediatric patients undergoing upper and lower endoscopy after bone marrow transplantation despite 1 study in adults that found that clinically significant bacteremia is more commonly encountered in adult bone marrow transplantation recipients, particularly when undergoing evaluation for graft-versus-host disease or receiving prednisolone therapy (92). It remains reasonable that appropriateness of antibiotic prophylaxis be considered in each patient on a case-by-case basis, specifically in procedures with potential increase risk of bacteremia such as stricture dilation, sclerotherapy, and esophageal banding. It may also be prudent to delay elective procedures for patients with transient insults to the immune system (ie, chemotherapy with time-dependent effects on platelets or neutrophils).
As another population that may be at high risk for infection associated with endoscopic procedures, pediatric patients with advanced cirrhosis may present with GI hemorrhage precipitating the need for endoscopic intervention. At this time, pediatric specific studies that precluding recommendation on the use of prophylactic antibiotics in such situations are lacking (94). Current adult literature, however, supports the initiation of intravenous antibiotics (ceftriaxone or equivalent) upon admission in such patients, with significantly decreased rates of infectious complications and all-cause mortality (95). Despite the limited pediatric data, the writing group of this document concurs that it is prudent to place all pediatric patients with known cirrhosis presenting with GI bleeding on antibiotic therapy regardless of whether or not endoscopy is pursued (5,85).
Risk Factors for Procedural-related Infections
Certain procedural techniques and interventions may place patients at higher risk of experiencing a postprocedural infectious event. As mentioned above, diagnostic EGD and colonoscopy with biopsy are considered low-risk procedures and antibiotic prophylaxis during either is rarely recommended (85). The same medical societies have, however, recognized that certain therapeutic interventions, such as stricture dilation, sclerotherapy, and esophageal banding, are associated with increased rates bacteremia. In turn, prophylaxis during these procedures in certain high-risk patient groups (ie, neutropenia, etc) is recommended (85,93) (Table 3). At this point, most advanced procedures (ie, ERCP, EUS, etc) are not as frequently performed in children, and data specifically regarding postprocedural infectious events are limited (4). As such, pediatric endoscopists are generally well advised to adopt adult-based guidelines.
TABLE 3.
Antibiotic prophylaxis recommendations for endoscopic patients stratified based on patient and procedural contexts (85,89)
| Patient Context: | Procedural Context |
|---|---|
|
ENDO-A: GI endoscopy associated with low rates of bacteremia (EGD or colonoscopy with biopsy) |
| ENDO-B: GI endoscopy associated with high rates of bacteremia (dilation, sclerotherapy, banding, ERCP). | |
| PEG: Percutaneous endoscopic gastrostomy | |
| ERCP-A: ERCP with anticipated complete biliary drainage (eg, simple choledocholithiasis) | |
| ERCP-B: ERCP with anticipated incomplete biliary drainage (ex: PSC, hilar tumors) or management of biliary complications in the setting previous liver transplantation | |
| ERCP-C: ERCP in which pancreatic cyst or pseudocyst manipulation is anticipated | |
| EUS-A: EUS with biopsy of solid lesion along the GI tract | |
| EUS-B: EUS with manipulation of a cystic or pseudocystic lesions anywhere in GI tract |
| Context in which antibiotic prophylaxis are recommended | |||
|---|---|---|---|
| Context | Goal of prophylaxis | Organisms to cover | Potential antibiotic choice |
| 1, PEG | Prevent peristomal infection | Cutaneous organisms | Cefazolin |
| 1, ERCP-B | Prevent cholangitis | Enteric gram negatives, enterococcus | Piperacillin/tazobactam |
| 1, ERCP-C | Prevent infection of cyst/pseudocyst | Enteric gram negatives, enterococcus | Piperacillin/tazobactam |
| 1, EUS-B | Prevent infection of cyst | Enteric gram negatives, enterococcus | Piperacillin/tazobactam |
| 4, Regardless of procedural context | Prevent infectious complications, reduce all-cause mortality | Gram positive bacteria, enteric gram negatives | Third-generation cephalosporin or piperacillin/tazobactam |
| Context in which antibiotic prophylaxis should be considered | |||
| Context | Goal of prophylaxis | Organisms to cover | Potential regimen |
| 2, ENDO-B | Prevent endocarditis | Enterococcus | Amoxicillin or ampicillin |
| 3, ENDO-B | Prevent sepsis | Enteric gram negatives, enterococcus | Piperacillin/tazobactam |
| Context in which antibiotic prophylaxis is generally not recommended | |||
| Patient contexts: 5 | |||
| Procedural contexts: ENDO-A, ERCP-A, EUS-A | |||
See Table 2 for high risk cardiac conditions.
ANC = absolute neutrophil count; ERCP = endoscopic retrograde cholangiopancreatography; GI = gastrointestinal; PSC = primary sclerosing cholangitis.
Modified from Banerjee S, Shen B, Baron TH, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc 2008;67:791–798. Khashab MA, Chithadi KV, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc 2015;81:81–89.
In contrast, placement of percutaneous endoscopic gastrostomy tubes is a relatively common procedure in children, and peristomal wound infections are the most common postprocedural complication (96). Wound infections can occur following placement while in the hospital and after discharge (97,98). This is not surprising given the inoculation of the newly formed gastrostomy site with both skin and oral flora that inevitably occurs during the procedure. Rates of predischarge peristomal infections are reported to occur in 4% to 7% of children (97–99). Having a known malignancy, particularly in the setting of neutropenia, is associated with higher rates of peristomal infections, but in most reported cases infections are classified as minor and rarely lead to reversal of the gastrostomy (96,98–101). A systematic review of randomized control trials shows that use of prophylactic antibiotics decreases the incidence of peristomal infections after the procedure in adult patients (odds ratio: 0.36, confidence interval: 0.26–0.5) (102). Based on these data, the ASGE currently recommends the administration of parenteral cefazolin (or equivalent) 30 minutes before undergoing percutaneous endoscopic gastrostomy-tube placement (85).
ERCP is increasingly used in the pediatric population (24). It is important for providers performing this procedure or caring for patients after these procedures to recognize that infectious events may be experienced by patients in the postprocedural period. Although inadequately evaluated in the pediatric population, cholangitis and cholecystitis with or without sepsis are thought to affect 1% adult patients undergoing ERCP (103). A meta-analysis evaluating prophylactic antibiotic administration in all patients undergoing ERCP did not find a significant benefit and is not currently recommended (85,104). It is, however, recommended to give antibiotic prophylaxis in situations in which complete relief of biliary obstruction is not expected to be achieved, such as in the setting of patients with primary sclerosing cholangitis or hilar tumors (85). In addition, antibiotic prophylaxis is recommended in patients with communicating pancreatic cysts or pseudocysts or when drainage of these structures is anticipated to be attempted with ERCP (103). It has also been suggested that antibiotic prophylaxis be considered in patients shown to be at higher risk for developing infectious complications after ERCP, including patients requiring biliary interventions after liver transplantation, stenting in the setting of biliary malignancy, and situations in which a combined percutaneous-endoscopic approach is undertaken (103).
Although diagnostic EUS is believed to carry no more infectious risk than standard endoscopy, EUS-FNA of fluid and for tissue sampling outside the GI tract can increase patient risks for infectious complications (105). Although large prospective studies are lacking, the available literature suggest that infectious complications for EUS-FNA of solid lesions anywhere along the GI tract occurs in <1% (106); hence, prophylactic antibiotics are generally not recommended in this setting (105). For the diagnosis and treatment of cystic lesions anywhere along the GI tract, prophylactic antibiotics are recommended as infections can occur in up to 14% of these patients (107). In adult patients, a fluoroquinolone (or equivalent) is recommended and is generally continued for 3 to 5 days after the procedure (85).
PATIENT ASSESSMENT
Standardized “Preprocedure” Assessment
Implementing a systematic means of “preprocedure assessment” for all patients undergoing pediatric GI procedures is recommended across all institutions, because it may be useful at generally mitigating risk (108) (Table 4). Perhaps best conceived as a “checklist,” preprocedure assessment as a process should be integrated with procedure scheduling and may be essential to planning the following components of endoscopy: optimal location of procedure (ie, main operating room vs dedicated procedure unit); planned type of sedation (ie, anesthesiologist vs. endoscopist administered); patient readiness for goals of procedure (ie, ability to prepare for diagnostic procedures vs fitness to undergo therapeutic procedure).
TABLE 4.
Sample preprocedure checklist for patients undergoing endoscopy
| Consideration | Y/N | Potential impact on procedural planning |
|---|---|---|
| Premature infant <60 wk gestation or term infant <45 days old | □Yes □No |
May affect location of procedure (eg, hospital, not ambulatory surgical center); postprocedural admission for monitoring |
| Difficult airway | □Yes □No |
May affect sedation planning |
| BMI >35 | □Yes □No |
May affect location of procedure and sedation planning |
| Cardiac disease | □Yes □No |
May affect location of procedure, sedation planning, need for prophylactic antibiotics. |
| Guardianship/social services involved | □Yes □No |
May impact planning for informed consent |
| Diabetes/endocrinopathy | □Yes □No |
May affect procedure timing (ie, schedule as first procedure) and/or glucose monitoring |
| Hematologic/oncologic | □Yes □No |
May affect need for prophylactic antibiotics; need for blood products to be available prn |
| Hypo/hypertonia | □Yes □No |
May affect sedation planning |
| Neurologic/seizure disorder/ventilatory status | □Yes □No |
May affect sedation planning |
| Psych issues/anxiety | □Yes □No |
May affect sedation planning; pre-procedural approach; recovery |
| Renal/metabolic | □Yes □No |
May affect procedure timing (ie, schedule as first procedure) and/or glucose monitoring |
| Respiratory/pulmonary/asthma | □Yes □No |
May affect sedation planning |
| Short bowel syndrome | □Yes □No |
May affect procedure planning |
| Other medical issues | □Yes No |
If yes, specify issue and impact on procedural planning: |
Of note, it has been helpful to avoid prepopulating dichotomous answers (ie, avoid prechecking the answer “no”), so as to encourage careful consideration by providers about each item, before scheduling.
The goals of a systematic approach to preprocedural assessment are to identify procedural risk factors before the procedure begins, so that all appropriate steps can be taken to maintain patient safety. To do this well, it is critical that preprocedure assessments establish a patient’s medical history and catalogue their preprocedure medications (109). Knowledge of a patient’s comorbidities and risk factors can help facilitate consultation and communication with other specialty physicians, as necessary. In certain instances, a careful preprocedural assessment can optimize the patient’s physiologic condition before procedure; reduce patient anxiety through education; and allows the personalization of both informed consent for the procedure and a sedation/anesthesia plan. Defining a sedation plan before beginning a procedure is a Joint Commission mandate, and has been demonstrated to diminish risks of sedation during the procedure (108). Ultimately, preprocedure assessment should allow the formulation and communication of sedation/anesthesia plan to everyone involved in the procedure, including nursing staff, technicians, anesthesiologists, and endoscopists.
The American Society of Anesthesiologists (ASA) has devised a classification of patient status that may be of use in helping endoscopists to stratify patients by their risks of experiencing a cardiopulmonary event (110) (Table 5). The ASA patient classification system was not specifically developed to estimate anesthesia risk, but rather to provide relative guidelines for determining which patients are safe for moderate sedation, and which should be considered for general anesthesia. Generally patients who are status 1 and 2 are considered good candidates for procedural (moderate) sedation. Status 3 patients should be evaluated carefully, whereas status 4 and 5 patients likely will require general anesthesia.
TABLE 5.
American Society of Anesthesiologist Physical Status Classification System (110)
| ASA PS classification | Definition | Examples, including, but not limited to: |
|---|---|---|
| ASA I | A normal healthy patient | Healthy, nonsmoking, no or minimal alcohol use |
| ASA II | A patient with mild systemic disease | Mild diseases only without substantive functional limitations. Examples include (but not limited to): controlled inflammatory conditions including IBD, obesity (30 < BMI <40), well-controlled DM/HTN, mild lung disease |
| ASA III | A patient with severe systemic disease | Substantive functional limitations; One or more moderate to severe diseases. Examples include (but not limited to): poorly controlled DM or HTN, COPD, morbid obesity (BMI ≥40), active hepatitis, alcohol dependence or abuse, implanted pacemaker, moderate reduction of ejection fraction, ESRD undergoing regularly scheduled dialysis, premature infant PCA <60 weeks, history (>3 months) of MI, CVA, TIA, or CAD/stents. |
| ASA IV | A patient with severe systemic disease that is a constant threat to life | Examples include (but not limited to): recent (<3 months) MI, CVA, TIA, or CAD/stents, ongoing cardiac ischemia or severe valve dysfunction, severe reduction of ejection fraction, sepsis, DIC, ARD or ESRD not undergoing regularly scheduled dialysis |
| ASA V | A moribund patient who is not expected to survive without the operation | Examples include (but not limited to): ruptured abdominal/thoracic aneurysm, massive trauma, intracranial bleed with mass effect, ischemic bowel in the face of significant cardiac pathology or multiple organ/system dysfunction |
| ASA VI | A declared brain-dead patient whose organs are being removed for donor purposes |
The addition of “E” denotes Emergency surgery: (An emergency is defined as existing when delay in treatment of the patient would lead to a significant increase in the threat to life or body part).
Modified from American Society of Anesthesiology, ASA Physical Status Classification System (Last approved by the ASA House of Delegates on October 15, 2014). https://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system. Accessed August 15, 2018.
ASA scores may also be helpful with assessing overall endoscopic risk in children. Several studies have demonstrated that higher ASA class has been associated with increased risk of AEs (1,111). Nevertheless, there are several caveats of ASA classification. ASA classes represent crude categories and may not be useful for capturing complex pediatric clinical scenarios. Interprovider disagreement between nurses, endoscopists, and anesthesiologists, regarding patient status may be common (112). It is generally considered most helpful to view a child’s ASA score in the context of other risk factors, including airway compromise, cardiac disease, risks of bleeding, or immunocompromised status.
If preprocedural preparation determines that patients are at high risk of cardiopulmonary, bleeding, or infectious complications, it may be appropriate to obtain an anesthesia consult (113). It may also be appropriate to consider performing an unsedated procedure if the risks of sedation are deemed too high (15,114).
CONCLUSIONS
Pediatric endoscopy should be considered an integral and safe component of pediatric gastroenterology. Nevertheless, there are patients who should be recognized to be at higher risk for potential complications and AEs. This document outlines best understanding at this time based on evidence that exists of which patients and procedures may benefit from careful consideration to mitigate risk, and sets groundwork for further study in this area.
Acknowledgments:
The authors would like to thank Dr Robert Kramer for critical review of this manuscript.
J.R.L. has received speaker honoraria from Mead Johnson and has a research grant from Abbvie. D.S.F. is a contributor to UpToDate for a chapter on caustic ingestions; Q.Y.L., B.S., D.M.T., and M.T. have no conflicts of interest related to this manuscript.
Footnotes
Publisher's Disclaimer: Disclaimer: The NASPGHAN practice guidelines are evidence-based decision-making tools for managing health conditions. Practice Guidelines include Clinical Practice Guidelines (CPGs), clinical reports, technical reports, and position statements. They are authorized by the NASPGHAN Executive Council, peer reviewed, and periodically updated.
Publisher's Disclaimer: They are not to be construed as standards of care and should not be construed as establishing a legal standard of care or as encouraging, advocating, requiring, or discouraging any particular treatment. All decisions regarding the care of a patient should be made by the health care team, patient, and family in consideration of all aspects of the individual patient’s specific medical circumstances.
Publisher's Disclaimer: While NASPGHAN makes every effort to present accurate and reliable information, these guidelines are provided “as is” without any warranty of accuracy, reliability, or otherwise, either express or implied. NASPGHAN does not guarantee, warrant, or endorse the products or services of any firm, organization, or person. Neither NASPGHAN nor its officers, directors, members, employees, or agents will be liable for any loss, damage, or claim with respect to any liabilities, including direct, special, indirect, nor consequential damages, incurred in connection with the guidelines or reliance on the information presented.
The authors report no conflicts of interest.
REFERENCES
- 1.Thakkar K, El-Serag HB, Mattek N, et al. Complications of pediatric EGD: a 4-year experience in PEDS-CORI. Gastrointest Endosc 2007;65:213–21. [DOI] [PubMed] [Google Scholar]
- 2.Thakkar K, El-Serag HB, Mattek N, et al. Complications of pediatric colonoscopy: a five-year multicenter experience. Clin Gastroenterol Hepatol 2008;6:515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 2010;71:446–54. [DOI] [PubMed] [Google Scholar]
- 4.Kramer RE, Narkewicz MR. Adverse events following gastrointestinal endoscopy in children: classifications, characterizations, and implications. J Pediatr Gastroenterol Nutr 2016;62:828–33. [DOI] [PubMed] [Google Scholar]
- 5.ASGE Standards of Practice Committee Lightdale JR, Acosta R, Shergill AK, et al. Modifications in endoscopic practice for pediatric patients. Gastrointest Endosc 2014;79:699–710. [DOI] [PubMed] [Google Scholar]
- 6.Gilger MA, Gold BD. Pediatric endoscopy: new information from the PEDS-CORI project. Curr Gastroenterol Rep 2005;7:234–9. [DOI] [PubMed] [Google Scholar]
- 7.Cravero JP. Risk and safety of pediatric sedation/anesthesia for procedures outside the operating room. Curr Opin Anaesthesiol 2009;22:509–13. [DOI] [PubMed] [Google Scholar]
- 8.Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J 1985;32:429–34. [DOI] [PubMed] [Google Scholar]
- 9.Mallampati Classification. Samsoon GL, Young Jr. Difficult tracheal intubation: a retrospective study. Anaesthesia 1987;42:487–90. [DOI] [PubMed] [Google Scholar]
- 10.Wani S, Azar R, Hovis CE, et al. Obesity as a risk factor for sedation-related complications during propofol-mediated sedation for advanced endoscopic procedures. Gastrointest Endosc 2011;74:1238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelhardt T, Weiss M. A child with a difficult airway: what do I do next? Curr Opin Anaesthesiol 2012;25:326–32. [DOI] [PubMed] [Google Scholar]
- 12.Sharma VK, Nguyen CC, Crowell MD, et al. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc 2007;66:27–34. [DOI] [PubMed] [Google Scholar]
- 13.Nusrat S, Mahmood S, Bitar H, et al. The impact of chronic opioid use on colonoscopy outcomes. Dig Dis Sci 2015;60:1016–23. [DOI] [PubMed] [Google Scholar]
- 14.Andropoulos DB, Greene MF. Anesthesia and developing brains—implications of the FDA warning. N Engl J Med 2017;376:905–7. [DOI] [PubMed] [Google Scholar]
- 15.Friedlander JA, DeBoer EM, Soden JS, et al. Unsedated transnasal esophagoscopy for monitoring therapy in pediatric eosinophilic esophagitis. Gastrointest Endosc 2016;83:299.e1–306.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenbit A, Flume PA. Pulmonary complications in adult patients with cystic fibrosis. Am J Med Sci 2008;335:55–9. [DOI] [PubMed] [Google Scholar]
- 17.Wiehe M Massive hemoptysis during monitored anesthesia care for esophagogastroduodenoscopy with percutaneous endoscopic gastrostomy tube placement: a case report. AANA J 2010;78:43–6. [PubMed] [Google Scholar]
- 18.Yao MD, Von Rosenvinge EC, Groden C, et al. Multiple endoscopic biopsies in research subjects: safety results from a National Institutes of Health series. Gastrointest Endosc 2009;69:906–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amornyotin S, Aanpreung P. Clinical effectiveness of an anesthesiologist-administered intravenous sedation outside of the main operating room for pediatric upper gastrointestinal endoscopy in Thailand. Int J Pediatr 2010;2010:pii: 748564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ASGE Standards of Practice Committee Fisher DA, Maple JT, Ben-Menachem T, et al. Complications of colonoscopy. Gastrointest Endosc 2011;74:745–52. [DOI] [PubMed] [Google Scholar]
- 21.Cerezo Ruiz A, Parras Mejias E, Martos Becerra JM. A complication following a biopsy sample in eosinophilic esophagitis. Rev Esp Enferm Dig 2017;109:537. [DOI] [PubMed] [Google Scholar]
- 22.Katsurahara M, Horiki N, Kitade T, et al. Acute colonic intramural hematoma: a rare complication of colonoscopy. Endoscopy 2014;46(suppl 1 UCTN):E180–1. [DOI] [PubMed] [Google Scholar]
- 23.Yankov IV, Spasova MI, Andonov VN, et al. Endoscopic diagnosis of intramural hematoma in the colon sigmoideum in a child with high titer inhibitory hemophilia A. Folia Med (Plovdiv) 2014;56:126–8. [DOI] [PubMed] [Google Scholar]
- 24.Enestvedt BK, Tofani C, Lee DY, et al. Endoscopic retrograde cholangiopancreatography in the pediatric population is safe and efficacious. J Pediatr Gastroenterol Nutr 2013;57:649–54. [DOI] [PubMed] [Google Scholar]
- 25.Sahn B, Anupindi SA, Dadhania NJ, et al. Duodenal hematoma following EGD: comparison with blunt abdominal trauma-induced duodenal hematoma. J Pediatr Gastroenterol Nutr 2015;60:69–74. [DOI] [PubMed] [Google Scholar]
- 26.Zinelis SA, Hershenson LM, Ennis MF, et al. Intramural duodenal hematoma following upper gastrointestinal endoscopic biopsy. Dig Dis Sci 1989;34:289–91. [DOI] [PubMed] [Google Scholar]
- 27.Guzman C, Bousvaros A, Buonomo C, et al. Intraduodenal hematoma complicating intestinal biopsy: case reports and review of the literature. Am J Gastroenterol 1998;93:2547–50. [DOI] [PubMed] [Google Scholar]
- 28.Bechtel K, Moss RL, Leventhal JM, et al. Duodenal hematoma after upper endoscopy and biopsy in a 4-year-old girl. Pediatr Emerg Care 2006;22:653–4. [DOI] [PubMed] [Google Scholar]
- 29.Ramakrishna J, Treem WR. Duodenal hematoma as a complication of endoscopic biopsy in pediatric bone marrow transplant recipients. J Pediatr Gastroenterol Nutr 1997;25:426–9. [DOI] [PubMed] [Google Scholar]
- 30.Lipson SA, Perr HA, Koerper MA, et al. Intramural duodenal hematoma after endoscopic biopsy in leukemic patients. Gastrointest Endosc 1996;44:620–3. [DOI] [PubMed] [Google Scholar]
- 31.Grasshof C, Wolf A, Neuwirth F, et al. Intramural duodenal haematoma after endoscopic biopsy: case report and review of the literature. Case Rep Gastroenterol 2012;6:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hameed S, McHugh K, Shah N, et al. Duodenal haematoma following endoscopy as a marker of coagulopathy. Pediatr Radiol 2014;44:392–7. [DOI] [PubMed] [Google Scholar]
- 33.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013; 368:11–21. [DOI] [PubMed] [Google Scholar]
- 34.De Franchis R, Baveno VIF. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63:743–52. [DOI] [PubMed] [Google Scholar]
- 35.Khan K, Schwarzenberg SJ, Sharp H, et al. Diagnostic endoscopy in children after hematopoietic stem cell transplantation. Gastrointest Endosc 2006;64:379–85quiz 389–392. [DOI] [PubMed] [Google Scholar]
- 36.Chu DZ, Shivshanker K, Stroehlein JR, et al. Thrombocytopenia and gastrointestinal hemorrhage in the cancer patient: prevalence of unmasked lesions. Gastrointest Endosc 1983;29:269–72. [DOI] [PubMed] [Google Scholar]
- 37.Ben-Menachem T, Decker GA, Early DS, et al. Adverse events of upper GI endoscopy. Gastrointest Endosc 2012;76:707–18. [DOI] [PubMed] [Google Scholar]
- 38.Krishna SG, Rao BB, Thirumurthi S, et al. Safety of endoscopic interventions in patients with thrombocytopenia. Gastrointest Endosc 2014;80:425–34. [DOI] [PubMed] [Google Scholar]
- 39.Friedmann AM, Sengul H, Lehmann H, et al. Do basic laboratory tests or clinical observations predict bleeding in thrombocytopenic oncology patients? A reevaluation of prophylactic platelet transfusions. Transfus Med Rev 2002;16:34–45. [DOI] [PubMed] [Google Scholar]
- 40.Park YB, Lee JW, Cho BS, et al. Incidence and etiology of overt gastrointestinal bleeding in adult patients with aplastic anemia. Dig Dis Sci 2010;55:73–81. [DOI] [PubMed] [Google Scholar]
- 41.Li M, Wang Z, Ma T, et al. Enhanced platelet apoptosis in chronic uremic patients. Ren Fail 2014;36:847–53. [DOI] [PubMed] [Google Scholar]
- 42.ASGE Standards of Practice Committee Anderson MA, Ben-Menachem T, Gan SI, et al. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc 2009;70:1060–70. [DOI] [PubMed] [Google Scholar]
- 43.ASGE Standards of Practice Committee Pasha SF, Acosta R, Chandrasekhara V, et al. Routine laboratory testing before endoscopic procedures. Gastrointest Endosc 2014;80:28–33. [DOI] [PubMed] [Google Scholar]
- 44.Friedt M, Welsch S. An update on pediatric endoscopy. Eur J Med Res 2013;18:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu EK, Chugh P, Kronman MP, et al. Incidence of perforation in pediatric GI endoscopy and colonoscopy: an 11-year experience. Gastrointest Endosc 2013;77:960–6. [DOI] [PubMed] [Google Scholar]
- 46.Wilde PH, Mullany CJ. Oesophageal perforation—a review of 37 cases. Aust N Z J Surg 1987;57:743–7. [DOI] [PubMed] [Google Scholar]
- 47.Church JM. Ancillary colonoscope insertion techniques. An evaluation. Surg Endosc 1993;7:191–3. [DOI] [PubMed] [Google Scholar]
- 48.Anderson ML, Pasha TM, Leighton JA. Endoscopic perforation of the colon: lessons from a 10-year study. Am J Gastroenterol 2000;95:3418–22. [DOI] [PubMed] [Google Scholar]
- 49.Teshima CW, Aktas H, Haringsma J, et al. Single-balloon-assisted colonoscopy in patients with previously failed colonoscopy. Gastrointest Endosc 2010;71:1319–23. [DOI] [PubMed] [Google Scholar]
- 50.Kaltenbach T, Soetikno R, Friedland S. Use of a double balloon enteroscope facilitates caecal intubation after incomplete colonoscopy with a standard colonoscope. Dig Liver Dis 2006;38:921–5. [DOI] [PubMed] [Google Scholar]
- 51.Keswani RN. Single-balloon colonoscopy versus repeat standard colonoscopy for previous incomplete colonoscopy: a randomized, controlled trial. Gastrointest Endosc 2011;73:507–12. [DOI] [PubMed] [Google Scholar]
- 52.Dib J Jr. Post-polypectomy syndrome. Am J Gastroenterol 2017; 112:390. [DOI] [PubMed] [Google Scholar]
- 53.Shin YJ, Kim YH, Lee KH, et al. CT findings of post-polypectomy coagulation syndrome and colonic perforation in patients who underwent colonoscopic polypectomy. Clin Radiol 2016;71:1030–6. [DOI] [PubMed] [Google Scholar]
- 54.Farley DR, Bannon MP, Zietlow SP, et al. Management of colonoscopic perforations. Mayo Clin Proc 1997;72:729–33. [DOI] [PubMed] [Google Scholar]
- 55.Sajid MS, Caswell J, Bhatti MI, et al. Carbon dioxide insufflation vs conventional air insufflation for colonoscopy: a systematic review and meta-analysis of published randomized controlled trials. Colorectal Dis 2015;17:111–23. [DOI] [PubMed] [Google Scholar]
- 56.Bassan MS, Holt B, Moss A, et al. Carbon dioxide insufflation reduces number of postprocedure admissions after endoscopic resection of large colonic lesions: a prospective cohort study. Gastrointest Endosc 2013;77:90–5. [DOI] [PubMed] [Google Scholar]
- 57.Saunders MD. Acute colonic pseudo-obstruction. Best Pract Res Clin Gastroenterol 2007;21:671–87. [DOI] [PubMed] [Google Scholar]
- 58.Prost B, Poncet G, Scoazec JY, et al. Unusual complications of argon plasma coagulation. Gastrointest Endosc 2004;59:929–32. [DOI] [PubMed] [Google Scholar]
- 59.Barth BA, Banerjee S, Bhat YM, et al. Equipment for pediatric endoscopy. Gastrointest Endosc 2012;76:8–17. [DOI] [PubMed] [Google Scholar]
- 60.Lightdale JR. Patient preparation and sedation for endoscopy. In: Classen M, Tytgat GNJ, Lightdale CJ, eds. Gastroenterological Endoscopy. 2nd ed New York, NY: Thieme; 2010:57–67. [Google Scholar]
- 61.Hunter A, Mamula P. Bowel preparation for pediatric colonoscopy procedures. J Pediatr Gastroenterol Nutr 2010;51:254–61. [DOI] [PubMed] [Google Scholar]
- 62.Church J, Kao J. Bedside colonoscopy in intensive care units: indications, techniques, and outcomes. Surg Endosc 2014;28:2679–82. [DOI] [PubMed] [Google Scholar]
- 63.Wai CT, Yeoh KG, Ho KY. Esophageal intubation with duodenoscope in the presence of pharyngeal pouch by a guidewire and catheter-guided technique. Surg Laparosc Endosc Percutan Tech 2002;12:362–3. [DOI] [PubMed] [Google Scholar]
- 64.Thakkar K, Lucia CJ, Ferry GD, et al. Repeat endoscopy affects patient management in pediatric inflammatory bowel disease. Am J Gastroenterol 2009;104:722–7. [DOI] [PubMed] [Google Scholar]
- 65.Navaneethan U, Kochhar G, Phull H, et al. Severe disease on endoscopy and steroid use increase the risk for bowel perforation during colonoscopy in inflammatory bowel disease patients. J Crohns Colitis 2012;6:470–5. [DOI] [PubMed] [Google Scholar]
- 66.ReMine SG, McIlrath DC. Bowel perforation in steroid-treated patients. Ann Surg 1980;192:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stillman AE, Painter R, Hollister DW. Ehlers-Danlos syndrome type IV: diagnosis and therapy of associated bowel perforation. Am J Gastroenterol 1991;86:360–2. [PubMed] [Google Scholar]
- 68.Okada T, Sasaki F, Shimizu H, et al. Effective esophageal balloon dilation for esophageal stenosis in recessive dystrophic epidermolysis bullosa. Eur J Pediatr Surg 2006;16:115–9. [DOI] [PubMed] [Google Scholar]
- 69.Gottschalk A, Venherm S, Vowinkel T, et al. Anesthesia for balloon dilatation of esophageal strictures in children with epidermolysis bullosa dystrophica: from intubation to sedation. Curr Opin Anaesthesiol 2010;23:518–22. [DOI] [PubMed] [Google Scholar]
- 70.Van Den Heuvel I, Boschin M, Langer M, et al. Anesthetic management in pediatric patients with epidermolysis bullosa: a single center experience. Minerva Anestesiol 2013;79:727–32. [PubMed] [Google Scholar]
- 71.Vowinkel T, Laukoetter M, Mennigen R, et al. A two-step multidisciplinary approach to treat recurrent esophageal strictures in children with epidermolysis bullosa dystrophica. Endoscopy 2015;47:541–4. [DOI] [PubMed] [Google Scholar]
- 72.Rosseneu S, Afzal N, Yerushalmi B, et al. Topical application of mitomycin-C in oesophageal strictures. J Pediatr Gastroenterol Nutr 2007;44:336–41. [DOI] [PubMed] [Google Scholar]
- 73.Allaparthi S, Verma H, Burns DL, et al. Conservative management of small bowel perforation in Ehlers-Danlos syndrome type IV. World J Gastrointest Endosc 2013;5:398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burcharth J, Rosenberg J. Gastrointestinal surgery and related complications in patients with Ehlers-Danlos syndrome: a systematic review. Dig Surg 2012;29:349–57. [DOI] [PubMed] [Google Scholar]
- 75.Yoneda A, Okada K, Okubo H, et al. Spontaneous colon perforations associated with a vascular type of Ehlers-Danlos syndrome. Case Rep Gastroenterol 2014;8:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jain P, Debnath PR, Jain V, et al. Multiple anastomotic complications following repair of oesophageal atresia with tracheoesophageal fistula: a report of two cases. Afr J Paediatr Surg 2011;8:244–8. [DOI] [PubMed] [Google Scholar]
- 77.Soreide JA, Viste A. Esophageal perforation: diagnostic work-up and clinical decision-making in the first 24 hours. Scand J Trauma Resusc Emerg Med 2011;19:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reprocessing Guideline Task Force Petersen BT, Cohen J, Hambrick RD 3rd et al. Multisociety guideline on reprocessing flexible GI endoscopes: 2016 update. Gastrointest Endosc 2017;85:282.e1–94.e1. [DOI] [PubMed] [Google Scholar]
- 79.Visrodia K, Petersen BT. Echoing concerns related to endoscope reprocessing. Gastrointest Endosc 2017;85:398–400. [DOI] [PubMed] [Google Scholar]
- 80.Spach DH, Silverstein FE, Stamm WE. Transmission of infection by gastrointestinal endoscopy and bronchoscopy. Ann Intern Med 1993;118:117–28. [DOI] [PubMed] [Google Scholar]
- 81.Petersen BT, Chennat J, Cohen J, et al. Multisociety guideline on reprocessing flexible GI endoscopes: 2011. Infect Control Hosp Epidemiol 2011;32:527–37. [DOI] [PubMed] [Google Scholar]
- 82.Epstein L, Hunter JC, Arwady MA, et al. New Delhi metallo-beta-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA 2014;312:1447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Administration FaD. U.S. Food and Drug Administration. Infections associated with reprocessed duodenoscopes. https://www.fda.gov/medicaldevices/productsandmedicalprocedures/reprocessingfreusabledevices/ucm454630.htm. Last accessed January 15, 2019.
- 84.Snyder J, Bratton B. Antimicrobial prophylaxis for gastrointestinal procedures: current practices in North American academic pediatric programs. J Pediatr Gastroenterol Nutr 2002;35:564–9. [DOI] [PubMed] [Google Scholar]
- 85.ASGE Standards of Practice Committee Khashab MA, Chithadi KV, Acosta RD, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc 2015;81:81–9. [DOI] [PubMed] [Google Scholar]
- 86.El-Baba M, Tolia V, Lin CH, et al. Absence of bacteremia after gastrointestinal procedures in children. Gastrointest Endosc 1996;44:378–81. [DOI] [PubMed] [Google Scholar]
- 87.Byrne WJ, Euler AR, Campbell M, et al. Bacteremia in children following upper gastrointestinal endoscopy or colonoscopy. J Pediatr Gastroenterol Nutr 1982;1:551–3. [DOI] [PubMed] [Google Scholar]
- 88.Hirota WK, Petersen K, Baron TH, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc 2003;58:475–82. [DOI] [PubMed] [Google Scholar]
- 89.Banerjee S, Shen B, Baron TH, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc 2008;67:791–8. [DOI] [PubMed] [Google Scholar]
- 90.Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007;116:1736–54. [DOI] [PubMed] [Google Scholar]
- 91.Buderus S, Sonderkotter H, Fleischhack G, et al. Diagnostic and therapeutic endoscopy in children and adolescents with cancer. Pediatr Hematol Oncol 2012;29:450–60. [DOI] [PubMed] [Google Scholar]
- 92.Bianco JA, Pepe MS, Higano C, et al. Prevalence of clinically relevant bacteremia after upper gastrointestinal endoscopy in bone marrow transplant recipients. Am J Med 1990;89:134–6. [DOI] [PubMed] [Google Scholar]
- 93.Allison MC, Sandoe JA, Tighe R, et al. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut 2009;58:869–80. [DOI] [PubMed] [Google Scholar]
- 94.Shneider BL, Bosch J, De Franchis R, et al. Portal hypertension in children: expert pediatric opinion on the report of the Baveno v Consensus Workshop on Methodology of Diagnosis and Therapy in Portal Hypertension. Pediatr Transplant 2012;16:426–37. [DOI] [PubMed] [Google Scholar]
- 95.Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila F, et al. Meta-analysis: antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding—an updated Cochrane review. Aliment Pharmacol Ther 2011;34:509–18. [DOI] [PubMed] [Google Scholar]
- 96.McSweeney ME, Kerr J, Jiang H, et al. Risk factors for complications in infants and children with percutaneous endoscopic gastrostomy tubes. J Pediatr 2015;166:1514.e1–9e. [DOI] [PubMed] [Google Scholar]
- 97.Fortunato JE, Troy AL, Cuffari C, et al. Outcome after percutaneous endoscopic gastrostomy in children and young adults. J Pediatr Gastroenterol Nutr 2010;50:390–3. [DOI] [PubMed] [Google Scholar]
- 98.McSweeney ME, Jiang H, Deutsch AJ, et al. Long-term outcomes of infants and children undergoing percutaneous endoscopy gastrostomy tube placement. J Pediatr Gastroenterol Nutr 2013;57:663–7. [DOI] [PubMed] [Google Scholar]
- 99.Fox VL, Abel SD, Malas S, et al. Complications following percutaneous endoscopic gastrostomy and subsequent catheter replacement in children and young adults. Gastrointest Endosc 1997;45:64–71. [DOI] [PubMed] [Google Scholar]
- 100.Parbhoo DM, Tiedemann K, Catto-Smith AG. Clinical outcome after percutaneous endoscopic gastrostomy in children with malignancies. Pediatr Blood Cancer 2011;56:1146–8. [DOI] [PubMed] [Google Scholar]
- 101.Pedersen AM, Kok K, Petersen G, et al. Percutaneous endoscopic gastrostomy in children with cancer. Acta Paediatr 1999;88:849–52. [DOI] [PubMed] [Google Scholar]
- 102.Lipp A, Lusardi G. Systemic antimicrobial prophylaxis for percutaneous endoscopic gastrostomy. Cochrane Database Syst Rev 2006:CD005571. [DOI] [PubMed] [Google Scholar]
- 103.ASGE Standards of Practice Committee Anderson MA, Fisher L, Jain R, et al. Complications of ERCP. Gastrointest Endosc 2012;75:467–73. [DOI] [PubMed] [Google Scholar]
- 104.Harris A, Chan AC, Torres-Viera C, et al. Meta-analysis of antibiotic prophylaxis in endoscopic retrograde cholangiopancreatography (ERCP). Endoscopy 1999;31:718–24. [DOI] [PubMed] [Google Scholar]
- 105.Early DS, Acosta RD, Chandrasekhara V, et al. Adverse events associated with EUS and EUS with FNA. Gastrointest Endosc 2013;77:839–43. [DOI] [PubMed] [Google Scholar]
- 106.Eloubeidi MA, Tamhane A, Varadarajulu S, et al. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc 2006;63:622–9. [DOI] [PubMed] [Google Scholar]
- 107.Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 1997;112:1087–95. [DOI] [PubMed] [Google Scholar]
- 108.Ragsdale JA. Validating patient safety in the endoscopy unit using the joint commission standards. Gastroenterol Nurs 2011;34:218–23. [DOI] [PubMed] [Google Scholar]
- 109.Campbell EJ, Krishnaraj A, Harris M, et al. Automated before-procedure electronic health record screening to assess appropriateness for GI endoscopy and sedation. Gastrointest Endosc 2012;76:786–92. [DOI] [PubMed] [Google Scholar]
- 110.American Society of Anesthesiology, ASA Physical Status Classification System (Last approved by the ASA House of Delegates on October 15, 2014). https://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system. Accessed August 15, 2018.
- 111.Lightdale JR, Valim C, Mahoney LB, et al. Agitation during procedural sedation and analgesia in children. Clin Pediatr (Phila) 2009;49: 35–42. [DOI] [PubMed] [Google Scholar]
- 112.Haynes SR, Lawler PG. An assessment of the consistency of ASA physical status classification allocation. Anaesthesia 1995;50:195–9. [DOI] [PubMed] [Google Scholar]
- 113.Chung HK, Lightdale JR. Sedation and monitoring in the pediatric patient during gastrointestinal endoscopy. Gastrointest Endosc Clin N Am 2016;26:507–25. [DOI] [PubMed] [Google Scholar]
- 114.Sultan M, Ramprasad J, Jensen MK, et al. Endoscopic diagnosis of pediatric acute gastrointestinal graft-versus-host disease. J Pediatr Gastroenterol Nutr 2012;55:417–20. [DOI] [PubMed] [Google Scholar]


