Abstract
Periodontal disease is a chronic inflammatory, multifactorial condition, that, in the absence of an early and adequate treatment, may lead to a progressive damaging of the alveolar tissues that support the teeth (periodontal ligament, cement and alveolar bone) followed by teeth mobility and, subsequently, their loss. Periodontal disease is one of the most common inflammatory disease affecting adult individuals all over the world, being considered a real worldwide pandemic. This disease may influence the progression of certain systemic diseases: diabetes mellitus, cardiovascular diseases, ischemic cardiomyopathy, myocardial infarction, stroke, neurodegenerative diseases, chronic kidney diseases, cancer, etc. The association between smoking and periodontal disease was described in numerous clinical and epidemiological studies, suggesting that products derived from tobacco burning may change the clinical aspects and the disease progression. The present study analyzed microscopically and immunohistochemically 58 periodontal fragments, from 50 patients, chronic smokers, clinically diagnosed with severe periodontitis. There were highlighted major changes in the gingival epithelium (epithelium thickening, acanthosis, intraepithelial edema, infiltrates of neutrophils or lymphocytes, epithelial necrosis), in the periodontal conjunctive tissue (more or less intense inflammatory infiltrates, microhemorrhages, vascular congestion, intense immunohistochemical expression for some matrix metalloproteinases). The periodontal changes may be the expression of both toxic factors present in tobacco smoke and due to the changes caused by tobacco in the microbial flora of the oral cavity.
Keywords: periodontitis, smoking, inflammation, metalloproteinase, lymphocyte
⧉ Introduction
Periodontal disease is a chronic inflammatory, multifactorial condition, that, in the absence of an early and adequate treatment, may lead to a progressive damaging of the alveolar tissues that support the teeth (periodontal ligament, cement and alveolar bone) followed by teeth mobility and, subsequently, their loss [1,2]. Periodontal disease is one of the most common inflammatory disease affecting adult individuals all over the world. A study published in 2013 showed that in 2010, worldwide, there were approx. 3.9 billion people suffering from periodontal diseases, the prevalence of mild periodontitis being 35%, while moderate and severe periodontitis was 11% [3,4]. Due to the high number of patients, periodontal disease is considered a real worldwide pandemic.
With age, the number of patients with periodontal disease increases, causing problems of mastication, speech or other disabilities and thus affecting the quality of life. Moreover, numerous studies showed that periodontal disease influences the progression of certain systemic diseases: diabetes mellitus, cardiovascular diseases, ischemic cardiomyopathy, myocardial infarction, stroke, neurodegenerative diseases, chronic kidney diseases, cancer, etc. [5,6,7,8,9,10]. Other studies showed that between periodontal disease and other systemic diseases there is a bidirectional association, namely the periodontal disease intensity determine the severity of a systemic disease and vice versa [11].
The association between smoking and periodontal disease was described in numerous clinical and epidemiological studies, suggesting that products derived from tobacco burning may change the clinical aspects and the disease progression [12]. Also, there was shown that cigarettes are the main risk factor for the prevalence and severity of periodontal diseases [13,14,15].
In the US, the prevalence of periodontal disease is about 40%; the number of smokers presenting periodontitis is at least 50% higher than the number of non-smokers [16]. Epidemiological studies showed that smokers, after non-surgical periodontal treatment, present a lower improvement of symptoms than non-smokers [17].
Aim
In the present study, we proposed to analyze the microscopic changes in a group of patients, chronic smokers, diagnosed with periodontal disease in advanced stages.
⧉ Materials and Methods
The biological material under study was represented by 58 periodontal fragments, coming from 50 patients, chronic smokers, clinically diagnosed with severe periodontitis. The biological material was harvested during some surgical treatments specific to this disease. In accordance with medical ethical specifications, the harvesting of periodontal tissue fragments was performed after receiving an informed consent of all the patients included in the study group. The harvesting was performed between 2016–2019 in the dental practice offices of S.C. Smile Gabi Dent S.R.L. in Galaţi and Brăila, Romania. The harvesting of periodontium fragments was performed after applying local anesthesia to the patient, with a maximum of attention, to avoid an extra damaging of the dental-maxillary system structures affected by periodontal disease.
Immediately after harvesting, the biological material was processed by fixing the biological material in 10% neutral buffered formalin solution for 24–48 hours followed by the classic histological technique of paraffin inclusion within the Research Center for Microscopic Morphology and Immunology of the University of Medicine and Pharmacy of Craiova, Romania. After paraffin inclusion, the material was sectioned with the HMB450 rotary microtome (Thermo Scientific), equipped with a Peltier cooling system of the biological material and with a water transfer system of sections. The histological fragments were stained with Hematoxylin–Eosin and Goldner–Szekely (GS) trichrome.
The immunohistochemical (IHC) study was performed on the same biological material. The histological sections were harvested on special histological slides, covered with poly-L-lysine, subsequently introduced in an incubator at 35°C and kept overnight (18 hours) for a good adherence. After that, there was performed the deparaffinization of the sections by their immersion in three xylene baths (five minutes every bath), rehydration, by their immersion in baths of ethanol with a decreasing concentration (100%, 70%, 50%, five minutes every bath) and hydration of histological slides by immersion on distilled water for 10 minutes.
Antigenic recovery and demasking were performed by boiling the histological sections in the microwave oven in a special solution [sodium citrate or Ethylenediaminetetraacetic acid (EDTA)], chosen according to the primary used antibody, according to the protocol of antibody manufacturer. To avoid a rapid evaporation of the fluid from the boiling bowl, this was air-proof covered and then placed in a microwave oven at 650 W. Boiling lasted for 21 minutes (seven cycles of three minutes each). Then, the biological material was washed thoroughly with tap water and distilled water for 15 minutes.
During the next stage, there was performed the endogenous peroxidase blocking by incubating the biological material for 30 minutes in 1% hydrogen peroxide, at room temperature (RT), followed by the wash of histological sections with abundant distilled water and then passed through a phosphate-buffered saline (PBS). Blocking the non-specific sites was performed by passing the biological material and the gingival mucosa fragments, respectively, through a bath of 2% skimmed milk for 30 minutes. The prepared biological material received the primary antibody on a slide, kept in a refrigerator at 4°C for 12–14 hours (overnight) in a moist box (filter paper impregnated with distilled water) to prevent the section drying up.
The following day, the slides were taken out of the refrigerator and left to reach RT for 30 minutes, followed by abundant washings in PBS, for at least 15 minutes each, to remove any excess of primary antibody. After that, there was applied the biotinylated secondary antibody on the biological material, for 30 minutes, at RT, followed by a washing in a 1% PBS (three baths of five minutes each), applying Streptavidin–Horseradish peroxidase (HRP) for 30 minutes at RT, followed by washing the slides in 1% PBS for 15 minutes.
An actual detection of the IHC signal was performed by incubating the sections with a sub-layer for peroxidase, in the presence of a proton source [3,3’-Diaminobenzidine (DAB)] and of 1% hydrogen peroxide. At first, DAB oxidation led to a brown deposit, insoluble in alcohol and other organic solvents. The detection reaction was observed under the microscope, therefore, after the optimal staining, the sections were thoroughly washed in PBS to stop the reaction. For an optimal contrast, the sections were stained with Mayer’s Hematoxylin for nuclear staining. In the end, the sections were dehydrated by passing them through three baths of ethanol in an ascending concentration (70%, 95%, 100% ethanol), clarified in xylene (three baths of five minutes each), covered with a xylene-based setting environment (DPX, Fluka) and protection slide. In this way, we performed permanent samples of immunohistochemistry where the antibodies were strictly localized on the antigens subjected to study, their color being brown and the cell nuclei blue-colored.
For the IHC study, we used the following antibodies: anti-cluster of differentiation (CD) 3 (monoclonal mouse anti-human CD3, clone F7.2.38, Dako) for highlighting T-lymphocytes; anti-CD20 (monoclonal mouse anti-human CD20cy, clone L26, Dako) for highlighting B-lymphocytes; anti-CD68 (monoclonal anti-human CD68, clone KP1, Dako) for highlighting macrophages; anti-CD79a (monoclonal mouse anti-human CD79a, clone JCB117, Dako) for marking plasma cells; anti-tryptase (monoclonal mouse anti-human mast cell tryptase, clone AA1, Dako) for highlighting mast cells; anti-matrix metalloproteinase (MMP)-1 (monoclonal mouse anti-human MMP-1, clone VT7, Dako); anti-MMP-8 (purified monoclonal mouse IgG2A, clone 100608, R&D Systems); anti-MMP-9 (polyclonal rabbit anti-human MMP-9, Dako); anti-MMP-13 (polyclonal rabbit anti-human MMP-13, Novus); anti-MMP-14 (monoclonal rabbit anti-human MMP-14, clone NBP2-29699, Novus).
⧉ Results
The microscopic study performed showed that all the individuals presenting clinical changes of gingivitis or chronic periodontitis presented multiple histopathological (HP) changes, which explains the clinical symptoms of the patients. Also, it was observed that all the structures of the gingival mucosa were affected both in gingivitis and periodontitis. The gingival epithelium presented various changes, thus showing that the physiopathological mechanisms that cause the onset of periodontal disease are complex ones. Therefore, one of the most frequent changes of the surface epithelium was the thickening of the epithelium with a tendency for hyperkeratosis (Figure 1). In other patients, there was observed a significant reduction of the covering epithelium thickness and erosion, as well (Figure 2).
Figure 1.
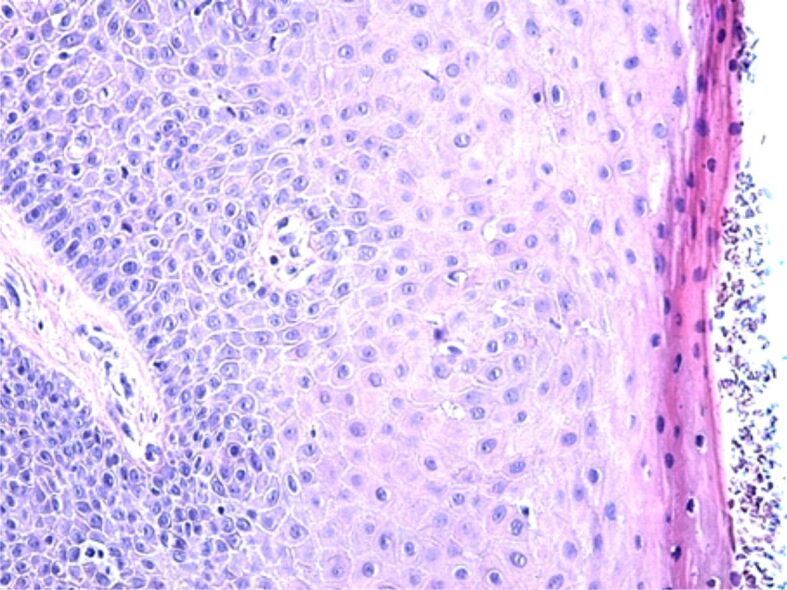
Hypertrophic gingival epithelium with a tendency for hyperkeratosis. Hematoxylin–Eosin (HE) staining, ×200
Figure 2.
Area of gingival epithelium with a reduced thickness and a moderate inflammatory infiltrate in the papillary chorion of the gum. Goldner–Szekely (GS) trichrome staining, ×200
In most patients in our study group, we observed two particular microscopic changes, namely the infiltration of the covering epithelium with leukocytes and intraepithelial edema.
Most often, the surface epithelium was infiltrated with lymphocytes, cells that migrated from the subadjacent connective tissue in a large quantity towards the epithelium surface. These cells are involved both in the inborn immune response and in the adaptive response of the oral cavity mucosa. The presence of these cells in a very high number shows a change in the microbial flora of the oral cavity present in smokers, either as a quantitative increase of some species of bacteria or viruses, or as a selection of bacteria with high aggressive action on the oral mucosa.
The edematous changes were characterized by the increase of the intercellular space, with visualization of the intercellular spikes or even their rupture (Figure 3). Frequently, there were observed areas of intraepithelial cellular necrosis, cellular lesions that could have been generated either by the toxic factors from tobacco smoke, or by the toxins of microbial flora in the oral cavity, or by the interleukins (ILs) and cytokines synthesized by the cells of the immune system stimulated by the aggressive factors in the oral cavity. The edematous changes of the gingival epithelium may be caused by an increase of the permeability of blood vessels in the gingival chorion, a permeability that may be changed by a series of endogenous and exogenous factors present in the oral cavity due to smoking that influence the balance of local bacterial flora, and also the conditions of symbiosis between them and the host organism.
Figure 3.
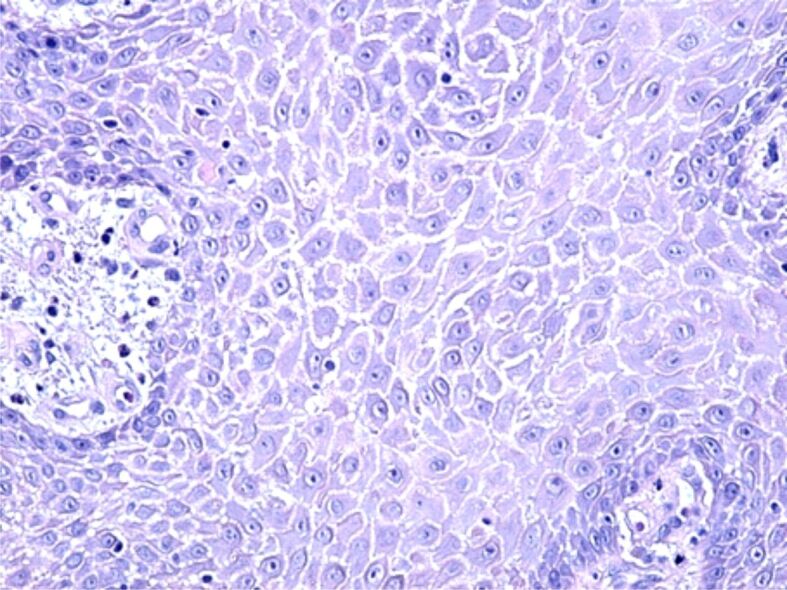
Image of gingivitis with areas of papillary necrosis and intraepithelial edema characterized by the enlargement of intercellular spaces and partial ruptures of the intercellular spikes. HE staining, ×200
The erosions of the gingival epithelium were frequently observed in individuals with periodontitis (Figure 4). They were probably induced by the aggression of the bacteria in the oral cavity and by the decrease of defense ability of the oral mucosa by the immune response reduction caused by chronic smoking. In these cases, due to the disparity of the covering epithelium, the periodontal connective tissue came into direct contact with the microbial flora in the oral cavity, which led to an inflammatory reaction, either an acute one where the periodontal inflammatory infiltrate was mostly made of neutrophils and leukocytes, or a chronic reaction when the inflammatory reaction was mainly formed of lymphocytes, plasma cells and macrophages (Figure 5).
Figure 4.
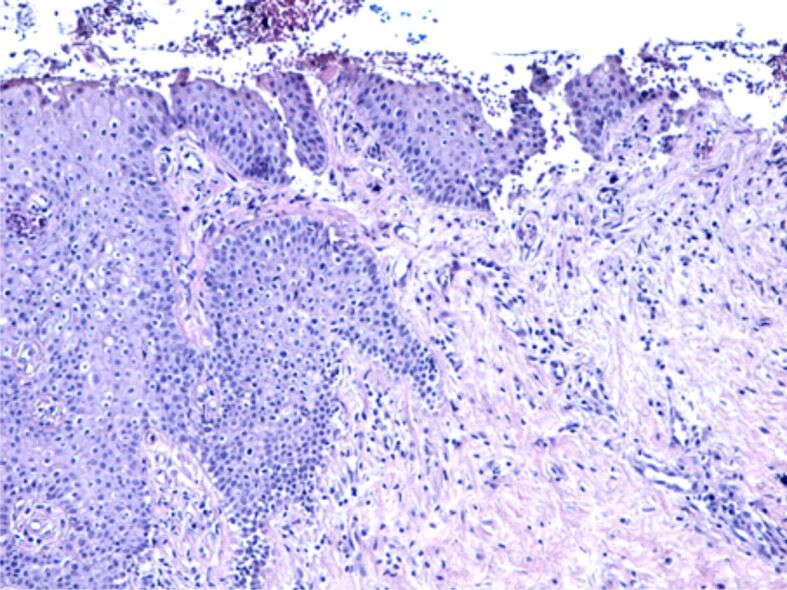
Image of chronic periodontitis with extended epithelial erosions, partial thickness of the gingival epithelium, moderate inflammatory infiltrate and fibrosis in the gingival chorion. HE staining, ×100
Figure 5.
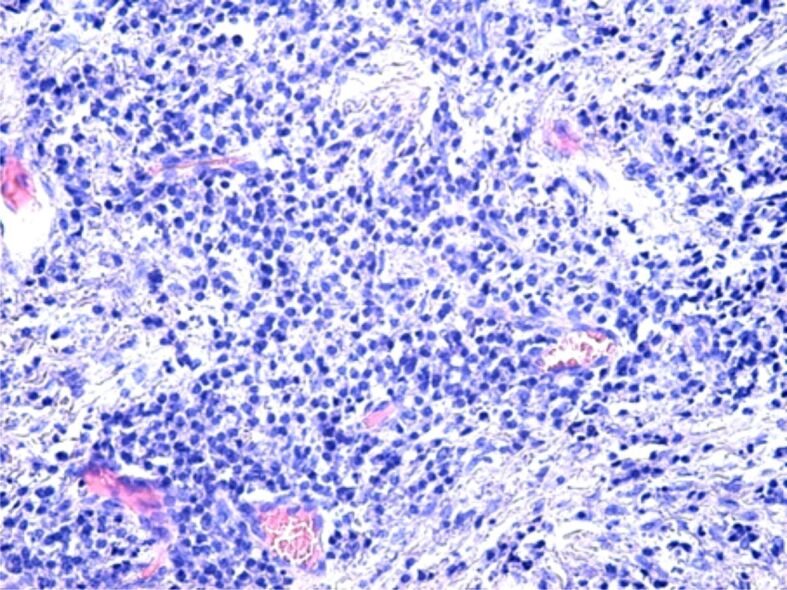
Image of chronic periodontitis with a chronic inflammatory infiltrate and moderate cellular necrosis in the periodontal connective tissue. HE staining, ×200
Chronic periodontal lesions resulted into the damaging of soft tissues in this area and, especially of periodontal ligaments, with the presence of a local reparatory reaction. Thus, in chronic periodontitis, the periodontal connective tissue suffered deep changes, characterized by the onset of a chronic inflammatory infiltrate, remodeling of the periodontal area, with frequent regenerative, desmoplastic reactions, characterized by the proliferation of fibroblasts and formation of a heterogenous arrangement of the collagen fibers with that constituted the periodontal ligaments. Proliferation of local fibroblasts represents a normal reparatory reaction of the connective tissue lesions, yet this connective tissue will progress into a densely heterogeneous arranged, non-functional tissue.
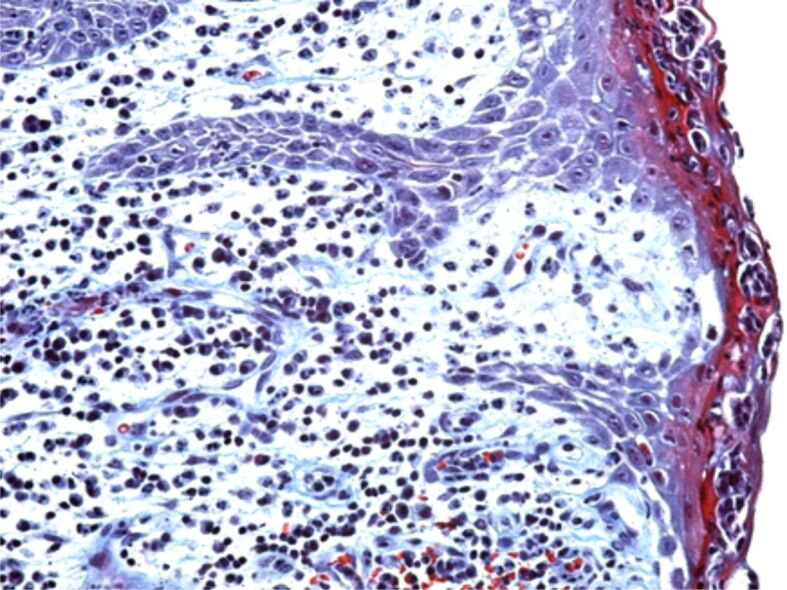
The intensity of the inflammatory infiltrate from the periodontium was variable from one patient to another, even from one area to another of the periodontium, thus showing a diverse reaction of every patient to the aggressive factors of the periodontium. Sometimes, the presence of the inflammatory infiltrate was associated to vascular congestion phenomena or even to periodontal microhemorrhages (Figure 6).
Figure 6.
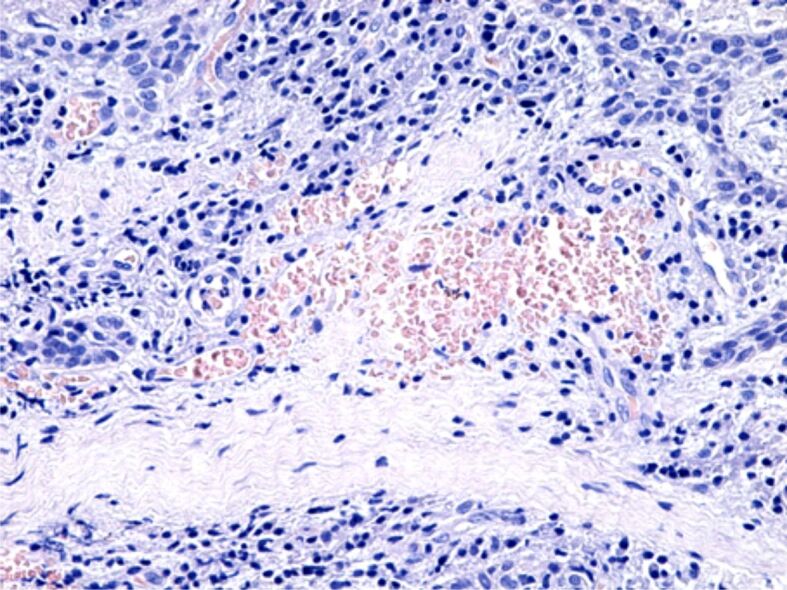
Area of chronic periodontitis with a moderate inflammatory infiltrate, connective fibrosis, vascular congestion and microhemorrhages. HE staining, ×200
The IHC studies were intended to make qualitative observations regarding the type of cells participating in the inflammatory reaction present both in the gingivitis patients and in the ones with periodontitis, being well-known the fact that the periodontal disease is an inflammatory response to the accumulation of bacterial biofilms around the teeth and oral mucosa. Also, we wanted to identify the main MMPs that interfere with the destruction and remodeling of the periodontal connective tissue.
In the structure of periodontal inflammatory infiltrates there were identified both T- and B-lymphocytes (Figures 7 and 8). The two types of cells had a completely heterogeneous arrangement, being identified intensely infiltrated areas with lymphocytes and areas with rare lymphocytes, with no other microscopic changes. The largest number of lymphocytes was identified in periodontitis, while the smallest number was identified in early gingivitis. Of the two categories of lymphocytes, the population of T-lymphocytes was the most numerous one, which shows that in some cases of periodontal disease, the immune response is mainly of the cellular type. Also, in gingivitis there were frequently identified T-cells in the covering epithelium structure.
Figure 7.
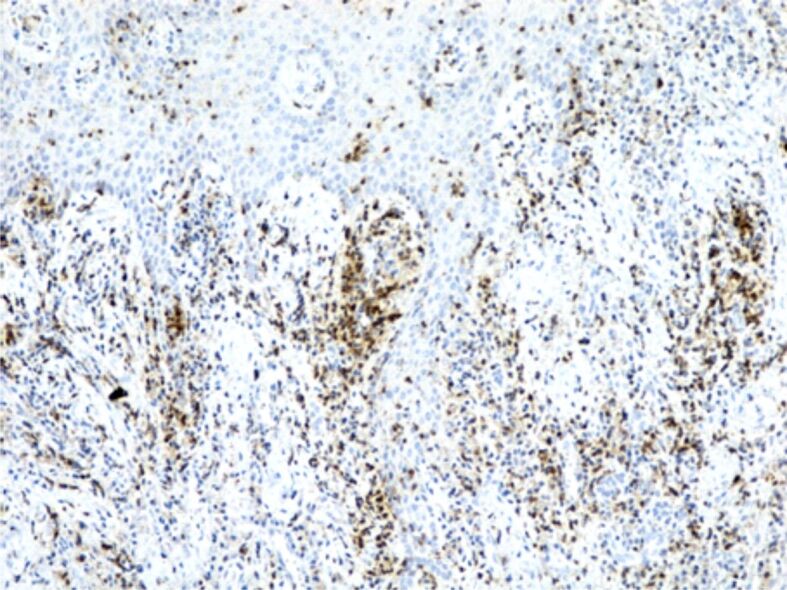
Image of gingivitis with a chronic inflammatory infiltrate in the chorion, rich in CD3+ T-lymphocytes. It is also observed the presence of T-lymphocytes in the structure of the gingival epithelium, as well. Immunostaining with anti-CD3 antibody, ×100. CD3: Cluster of differentiation 3
Figure 8.
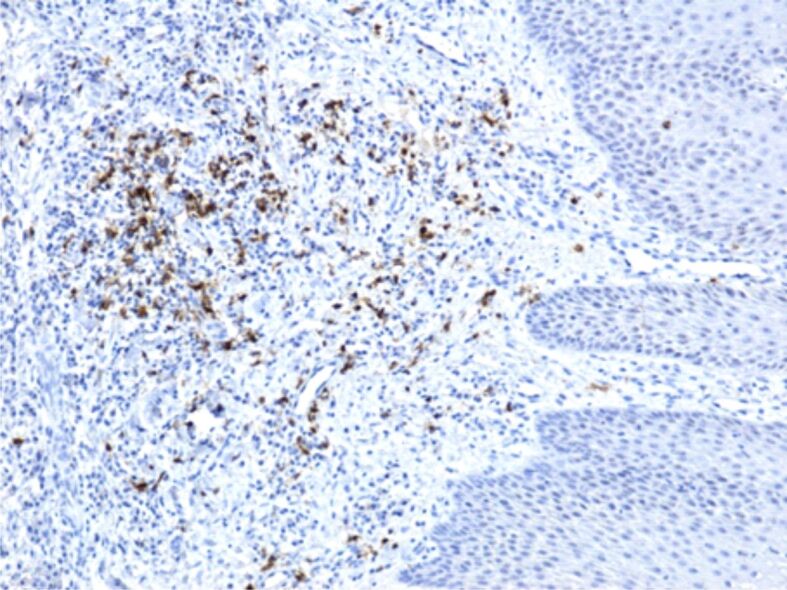
Chronic gingivitis, with a moderate inflammatory infiltrate in the chorion, with a relatively low number of CD20+ B-lymphocytes, with a heterogenous arrangement. Immunostaining with anti-CD20 antibody, ×100. CD20: Cluster of differentiation 20
Regarding the reaction of the macrophage system cells, in our study, most macrophages were identified in periodontitis, where the inflammatory processes and cellular necrosis were more intense (Figure 9). This microscopic aspect shows that, both bacterial aggression and cellular and tissue debris resulted from necrosis, represent important stimuli for the migration and local accumulation of the monocyte/macrophage cells, the main role played by macrophages being that of removing all the invading bacteria from the tissues and to phagocyte cellular and tissue debris. Also, there were identified macrophage cells in the structure of the gingival epithelium, probably as a response to the presence of an aggressive subgingival bacterial plaque. Similarly to T- and B-lymphocytes, most macrophages have a blood origin, being under the influence of some stimuli released through the capillary wall directly into the inflamed connective tissue.
Figure 9.
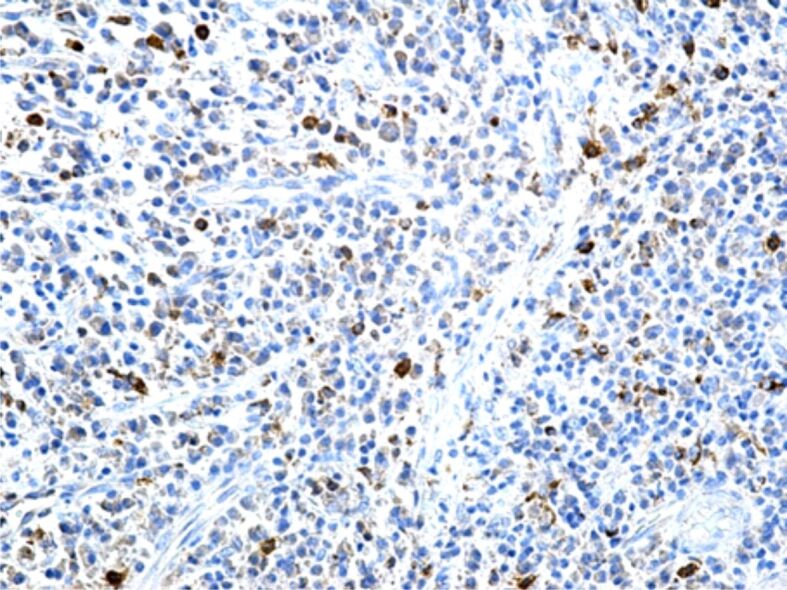
Periodontitis with a relatively high number of CD68+ macrophages. Immunostaining with anti-CD68 antibody, ×200. CD68: Cluster of differentiation 68
Other cells of the immune system found in the periodontal infiltrate were plasma cells, cells participating in the humoral immunity through the synthesis and the secretion of antibodies. In our study, both in gingivitis and periodontitis, it was identified a high number of plasma cells, which shows that humoral immunity also represents, in some patients, an intense immune response to the aggression of bacterial species from the oral cavity (Figure 10).
Figure 10.
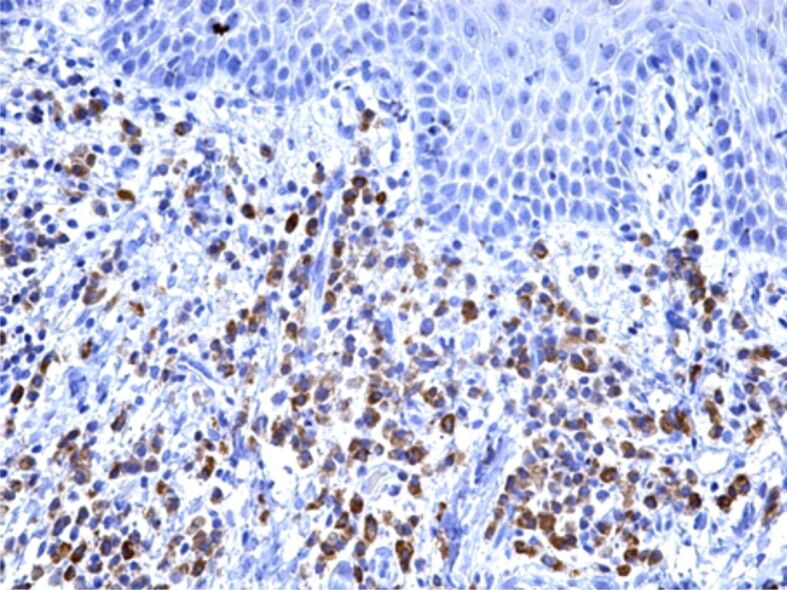
Image of chronic gingivitis with an abundant inflammatory infiltrate in the chorion, mainly formed of CD79a+ plasma cells. Immunostaining with anti-CD79a antibody, ×200. CD79a: Cluster of differentiation 79a
Regarding the mast cell reaction, cells with an essential role in various physiological mechanisms of local defense, protection against pathogenic agents and triggering of the inflammatory process, in our study we observed a high number of inflammatory infiltrates during the periodontal inflammatory processes. If in gingivitis, the number of these cells was moderately high, with a perivascular or subepithelial arrangement, in some periodontitis cases, the number of mast cells was much higher, with a completely heterogenous arrangement (Figures 11 and 12).
Figure 11.
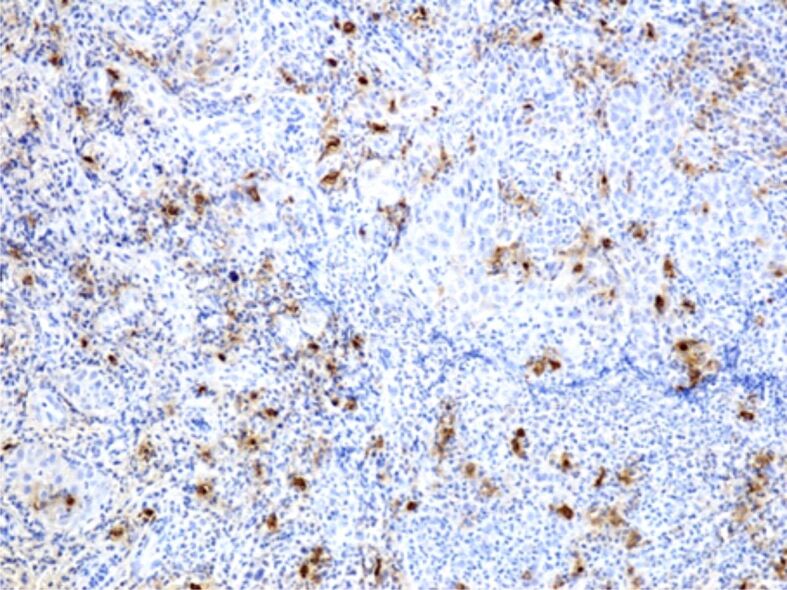
Intense reaction of mast cells in a case of periodontitis. Immunostaining with anti-tryptase antibody, ×100
Figure 12.
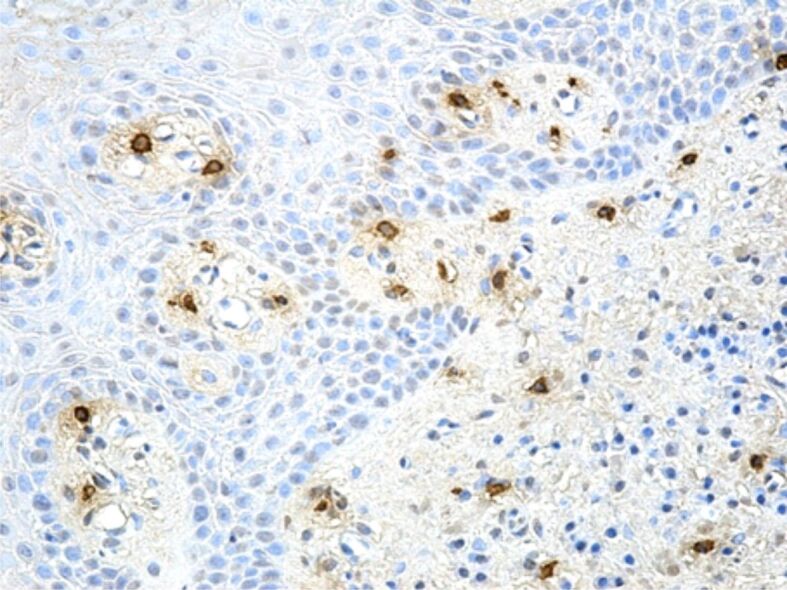
Moderate reaction of mast cells in a case of gingivitis. Immunostaining with anti-tryptase antibody, ×200
Other studied IHC markers were MMPs. These represent a family of 25 zinc-dependent proteolytic enzymes (metallo-endopeptidases), which may damage or split-up many components of the extracellular matrix, as well as most extracellular proteins, both during normal physiological processes and during the progression of some diseases. In our study, the intensity of the IHC reaction of MMPs was quite varied, which shows a variable quantity of enzymes synthesized by various cells in the gingival mucosa and the periodontium. In almost all cases, MMP-1 had a relatively intense expression, similar to the MMP-9 expression (Figures 13,14,15). The most intense IHC reaction was observed in MMP-8; in contrast, MMP-13 had a low reaction, while MMP-14 had a very low reaction (Figures 16 and 17).
Figure 13.
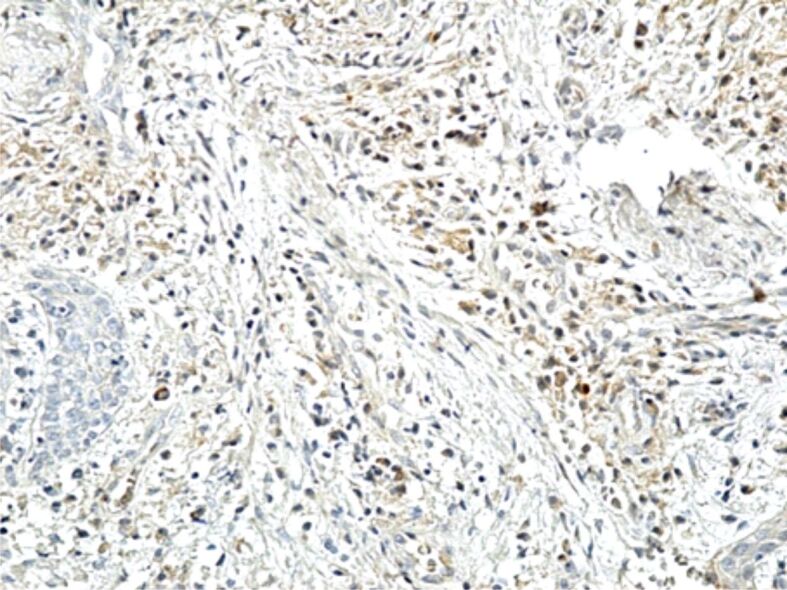
Image of chronic gingivitis with a moderate reaction to MMP-1. Immunostaining with anti-MMP-1 antibody, ×200. MMP-1: Matrix metalloproteinase-1
Figure 14.
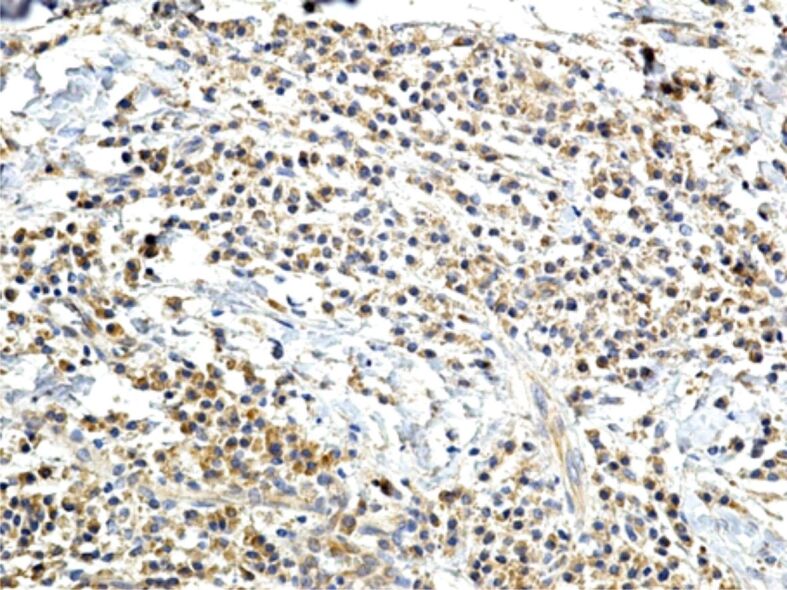
Intense chronic inflammatory infiltrate in a case of periodontitis, mainly formed of lymphocytes, plasma cells and macrophages, with an intense reaction to MMP-8. Immunostaining with anti-MMP-8 antibody, ×200. MMP-8: Matrix metalloproteinase-8
Figure 15.
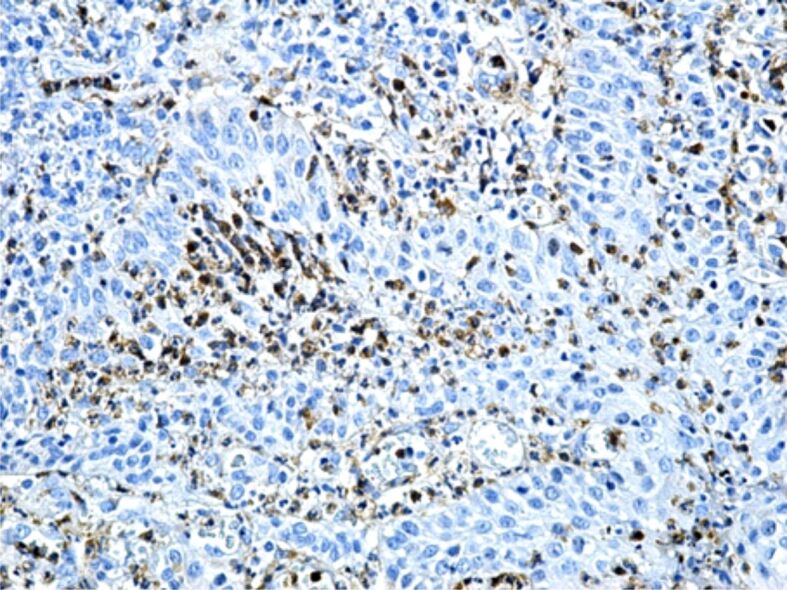
Chronic gingivitis with an intensely moderate reaction of some inflammatory cells in the gingival chorion to MMP-9. Immunostaining with anti-MMP-9 antibody, ×200. MMP-9: Matrix metalloproteinase-9
Figure 16.
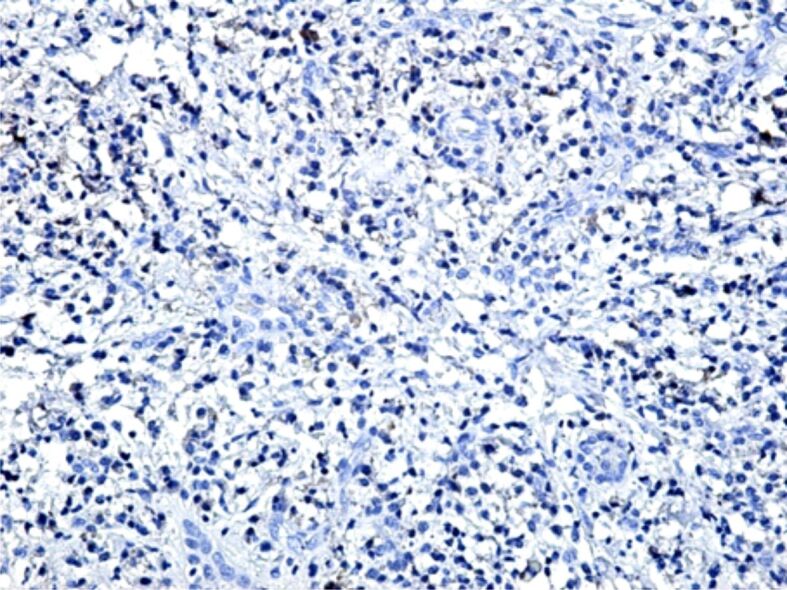
Microscopic image in the periodontium where there is observed the presence of a chronic inflammatory infiltrate, with a low immunohistochemical expression of MMP-13. Immunostaining with anti-MMP-13 antibody, ×100. MMP-13: Matrix metalloproteinase-13
Figure 17.
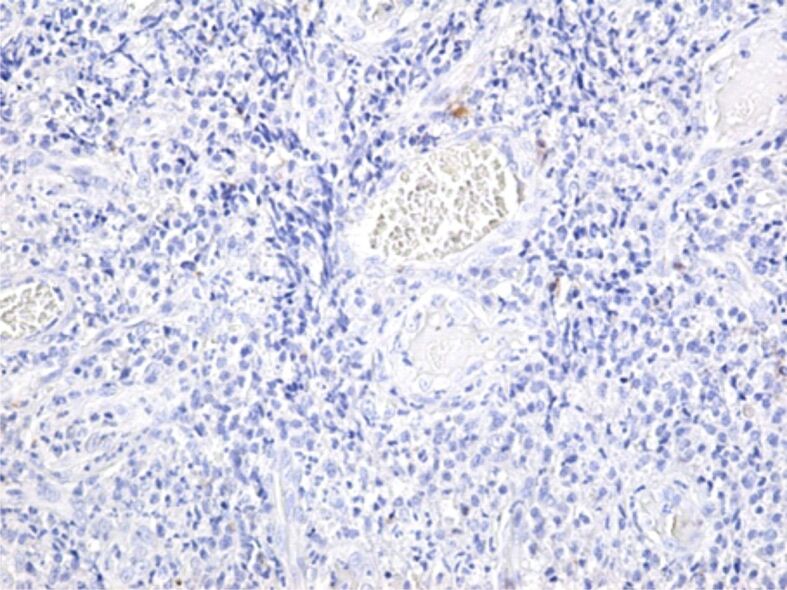
Microscopic image of periodontitis with an intense inflammatory reaction, with a very low expression of MMP-14. Immunostaining with anti-MMP-14 antibody, ×100. MMP-14: Matrix metalloproteinase-14
⧉ Discussions
Our study highlighted the HP changes in the gingival mucosa and the periodontium, in chronic smokers, clinically diagnosed with periodontitis, for the assessment of the particularities of this disease caused by smoking. At present, it is well-established the fact that smoking is the major risk factor for the onset and progression of periodontal disease [18,19]. Clinical studies showed that smokers have two up to seven times more chances to develop periodontitis, compared to non-smokers, as smoking was highly associated with tooth loss, because of deep changes of the periodontium, especially after the damaging of periodontal ligaments [20]. Moreover, there was established that there is a direct connection between the quantity of tobacco intake and periodontal lesions, including loss of periodontal attachment and tooth loss [21].
If gingivitis is characterized only by clinical signs like inflammation and gum bleeding, spontaneous or on touch, periodontitis progresses in time with the accumulation of dental plaque, bacterial dysbiosis, formation of periodontal pockets, gum retraction, damaging of the periodontal tissue and alveolar bone loss, eventually leading to tooth loss. As such, gingivitis is considered an early form of periodontal disease, while periodontitis is a chronic disease, more severe than gingivitis, which left untreated may have a slow progression leading to onset of complications caused by systemic diseases and tooth loss [22,23,24,25]. Periodontal treatment may slow down the periodontal disease progression by removing dental plaque and reducing inflammation, yet once the periodontal tissue and alveolar bone damaging occurs, the disease becomes permanent [21, 26,27,28].
The complexity of the HP changes, highlighted in smokers with chronic periodontitis, shows the multitude of the etiopathological mechanisms involved. Therefore, we consider that, in the progression of periodontal disease, besides the toxic factors in the tobacco smoke, there also interfere other factors, especially the change of the microbial flora of the oral cavity and the decrease of the immune system defense capacity. Various microbiological studies showed that smokers have a higher prevalence of bacterial species connected to periodontal disease in comparison to non-smokers, including Porphyromonas gingivalis, Bacteroides forsythus, Prevotella intermedia, Fusobacterium nucleatum, and relatively recent studies, using the polymerase chain reaction (PCR) technique, showed a positive reaction between the quantity of tobacco intake and the quantity of bacteria in dental plaque [29,30].
Even though the epidemiological studies showed a close connection between smoking and periodontal disease, the cellular and molecular mechanisms of this pathological association are still unknown. Nicotine is one of the toxic compounds that negatively affect cellular proliferation, induce the synthesis and release of proinflammatory cytokines by the gingival fibroblasts and alter the alveolar bone structure [31].
Other studies showed that cigarette smoke may interfere with the oxidative stress mechanisms of periodontal tissues, it may inhibit the defense against plaque bacteria and may lead to vasoconstriction and slow healing of the wounds [32]. It is well-known the fact that reactive oxygen species (ROS) are caused by inflammatory cells, like polymorphonuclear leukocyte neutrophils, and they represent an important defense factor in periodontal disease. Nevertheless, an excessive production of ROS will alter the oxidative balance homeostasis in the tissues, thus becoming a progression factor for periodontal disease [33,34].
The complex, heterogeneous aspect of the inflammatory infiltrate observed in smokers, with chronic periodontitis, the damaging of the covering epithelium and remodeling of the periodontal connective tissue, show a different answer of the local defense mechanisms from one patient to another. The immune system cells, in a first stage, are opposing to the altering of local homeostasis, yet subsequently, by an excess secretion of some cellular signaling, they favor the progression of periodontal disease. Inflammatory cytokines, associated with periodontal disease, secreted by fibroblasts and the immune system cells, especially IL-1, IL-6 and IL-8 and the tumor necrosis factor-alpha (TNF-α), increase vascular permeability that may increase bacteriemia and stimulate fibroblasts and inflammatory cells, which, in their turn, induce the synthesis and release of other cytokines [10]. Moreover, inflammatory cytokines favor bone resorption and inhibit bone formation, lesions that are associated with local expansion of periodontal lesions, with alveolar bone loss, increase of dental mobility and, ultimately, tooth loss [35].
The IHC examinations performed highlighted the complexity of the inflammatory reaction in the periodontal connective tissue. The immunohistochemistry studies bring new data, which are essential in the presentation of various pathological lesions [36,37,38]. Using specific markers, T- and B-lymphocytes, plasma cells, macrophages and even mast cells were identified in variable quantities from one patient to another, and from one area to another in the periodontium. Also, we showed that the inflammatory reaction in periodontitis may be an intense one, involving all the cells of both the inborn and the adaptive immune systems. We believe that the variation of inflammatory reaction may be due to the toxic products in tobacco smoke, and also to the presence of bacterial flora, due to the fact that various pathogenic bacterial species are capable of invading the gingival and periodontal tissues [39,40].
Starting from the findings that in the inflamed tissues there are released a multitude of enzymes, in our study we investigated the IHC expression of five MMPs, namely: MMP-1, MMP-8, MMP-9, MMP-13, and MMP-14. The most intense IHC reactions in the smokers diagnosed with periodontal disease were observed to MMP-1, MMP-8 and MMP-9, enzymes that have fibrillary proteins as their main substrate (various types of collagen, elastin), and also non-fibrillary biochemical structures like: gelatin, aggrecan, elastin, fibronectin, laminin. We consider that a prolonged, intense activity of these enzymes may cause the damaging of dental ligaments and tooth movement in their alveolar socket.
It is well-known the fact that MMPs are enzymes with a crucial role played in various physiological and pathological processes, including the remodeling of tissues and organ development, in the regulation of inflammatory processes, in systemic diseases and even cancer [41,42,43]. In physiological conditions, they are synthesized, in various quantities, by different cells (epithelial, connective, endothelial or even thrombocyte cells), being present in most connective tissues, still with a lower IHC expression. In pathological conditions, the IHC expression of MMPs changes a lot according to the pathological process.
⧉ Conclusions
The microscopic aspects found in periodontal disease in chronic smokers are extremely complex and variable. In the covering epithelium, there were observed: epithelial thickening with a tendency to hyperkeratosis, intraepithelial edema, intraepithelial cellular necrosis, granulocyte or lymphocyte cell infiltrate, reduction of the epithelium thickness and epithelial necrosis. The periodontal connective tissue was the location of chronic inflammatory infiltrates, mainly with lymphocyte, plasma cell and macrophage round cells, associated with vascular congestion, periodontal microhemorrhages and more or less intense remodeling of the extracellular connective matrix. The IHC examinations highlighted the presence of T- and B-lymphocytes, of plasma cells, macrophages and mast cells in the inflammatory infiltrates. Of the two categories of lymphocytes, the population of T-lymphocytes was the most numerous one, thus showing that, in some cases of periodontal disease, the main immunity is the cellular one. The macrophages were mainly identified in periodontitis, where there were more intense inflammatory processes and more extended cellular necrosis. The most intense IHC reactions observed were to MMP-1, MMP-8 and MMP-9, enzymes that have the main substrate made of fibrillary proteins (collagen, elastin), and also other non-fibrillary biochemical structures, like gelatin, aggrecan, elastin, fibronectin, laminin.
Conflict of interest
The authors declare no conflict of interests.
References
- 1.Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim) 2017;11(2):72–80. [PMC free article] [PubMed] [Google Scholar]
- 2.Liccardo D, Cannavo A, Spagnuolo G, Ferrara N, Cittadini A, Rengo C, Rengo G. Periodontal disease: a risk factor for diabetes and cardiovascular disease. Int J Mol Sci. 2019;20(6):1414–1414. doi: 10.3390/ijms20061414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards D. Oral diseases affect some 3.9 billion people. Evid Based Dent. 2013;14(2):35–35. doi: 10.1038/sj.ebd.6400925. [DOI] [PubMed] [Google Scholar]
- 4.Bui FQ, Almeida-da-Silva CLC, Huynh B, Trinh A, Liu J, Woodward J, Asadi H, Ojcius DM. Association between periodontal pathogens and systemic disease. Biomed J. 2019;42(1):27–35. doi: 10.1016/j.bj.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeo BK, Lim LP, Paquette DW, Williams RC. Periodontal disease - the emergence of a risk for systemic conditions: pre-term low birth weight. Ann Acad Med Singap. 2005;34(1):111–116. [PubMed] [Google Scholar]
- 6.Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76(11 Suppl):2075–2084. doi: 10.1902/jop.2005.76.11-S.2075. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006;94(1):10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitmore SE, Lamont RJ. Oral bacteria and cancer. PLoS Pathog. 2014;10(3):e1003933–e1003933. doi: 10.1371/journal.ppat.1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrizales-Sepúlveda EF, Ordaz-Farías A, Vera-Pineda R, Flores-Ramírez R. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart Lung Circ. 2018;27(11):1327–1334. doi: 10.1016/j.hlc.2018.05.102. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura M, Mochizuki Y, Miyata Y, Obata Y, Mitsunari K, Matsuo T, Ohba K, Mukae H, Yoshimura A, Nishino T, Sakai H. Pathological characteristics of periodontal disease in patients with chronic kidney disease and kidney transplantation. Int J Mol Sci. 2019;20(14):3413–3413. doi: 10.3390/ijms20143413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santos CMML, Lira-Junior R, Fischer RG, Santos APP, Oliveira BH. Systemic antibiotics in periodontal treatment of diabetic patients: a systematic review. PLoS One. 2015;10(12):e0145262–e0145262. doi: 10.1371/journal.pone.0145262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergström J. Periodontitis and smoking: an evidence-based appraisal. J Evid Based Dent Pract. 2006;6(1):33–41. doi: 10.1016/j.jebdp.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol 2000. 2012;60(1):15–39. doi: 10.1111/j.1600-0757.2011.00425.x. [DOI] [PubMed] [Google Scholar]
- 14.Johannsen A, Susin C, Gustafsson A. Smoking and inflammation: evidence for a synergistic role in chronic disease. Periodontol 2000. 2014;64(1):111–126. doi: 10.1111/j.1600-0757.2012.00456.x. [DOI] [PubMed] [Google Scholar]
- 15.Assem NZ, Alves MLF, Lopes AB, Gualberto Junior EC, Garcia VG, Theodoro LH. Antibiotic therapy as an adjunct to scaling and root planing in smokers: a systematic review and meta-analysis. Braz Oral Res. 2017;31:e67–e67. doi: 10.1590/1807-3107BOR-2017.vol31.0067. [DOI] [PubMed] [Google Scholar]
- 16.Eke PI, Wei L, Thornton-Evans GO, Borrell LN, Borgnakke WS, Dye B, Genco RJ. Risk indicators for periodontitis in US adults: NHANES 2009 to 2012. J Periodontol. 2016;87(10):1174–1185. doi: 10.1902/jop.2016.160013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heasman L, Stacey F, Preshaw PM, McCracken GI, Hepburn S, Heasman PA. The effect of smoking on periodontal treatment response: a review of clinical evidence. J Clin Periodontol. 2006;33(4):241–253. doi: 10.1111/j.1600-051X.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- 18.Luzzi LIT, Greghi SLA, Passanezi E, Passanezi Sant’ana AC, Lauris JRP, Cestari TM. Evaluation of clinical periodontal conditions in smokers and non-smokers. J Appl Oral Sci. 2007;15(6):512–517. doi: 10.1590/S1678-77572007000600011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oppermann RV, Haas AN, Rösing CK, Susin C. Epidemiology of periodontal diseases in adults from Latin America. Periodontol 2000. 2015;67(1):13–33. doi: 10.1111/prd.12061. [DOI] [PubMed] [Google Scholar]
- 20.Chambrone L, Chambrone D, Lima LA, Chambrone LA. Predictors of tooth loss during long-term periodontal maintenance: a systematic review of observational studies. J Clin Periodontol. 2010;37(7):675–684. doi: 10.1111/j.1600-051X.2010.01587.x. [DOI] [PubMed] [Google Scholar]
- 21.Calsina G, Ramón JM, Echeverría JJ. Effects of smoking on periodontal tissues. J Clin Periodontol. 2002;29(8):771–776. doi: 10.1034/j.1600-051x.2002.290815.x. [DOI] [PubMed] [Google Scholar]
- 22.Grønkjær LL, Holmstrup P, Schou S, Kongstad J, Jepsen P, Vilstrup H. Periodontitis in patients with cirrhosis: a cross-sectional study. BMC Oral Health. 2018;18(1):22–22. doi: 10.1186/s12903-018-0487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanz M, Marco Del Castillo A, Jepsen S, Gonzalez-Juanatey JR, D’Aiuto F, Bouchard P, Chapple I, Dietrich T, Gotsman I, Graziani F, Herrera D, Loos B, Madianos P, Michel JB, Perel P, Pieske B, Shapira L, Shechter M, Tonetti M, Vlachopoulos C, Wimmer G. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol. 2020;47(3):268–288. doi: 10.1111/jcpe.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baeza M, Morales A, Cisterna C, Cavalla F, Jara G, Isamitt Y, Pino P, Gamonal J. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. J Appl Oral Sci. 2020;28:e20190248–e20190248. doi: 10.1590/1678-7757-2019-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nwizu N, Wactawski-Wende J, Genco RJ. Periodontal disease and cancer: epidemiologic studies and possible mechanisms. Periodontol 2000. 2020;83(1):213–233. doi: 10.1111/prd.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michaud DS, Fu Z, Shi J, Chung M. Periodontal disease, tooth loss, and cancer risk. Epidemiol Rev. 2017;39(1):49–58. doi: 10.1093/epirev/mxx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergström J, Eliasson S, Dock J. Exposure to tobacco smoking and periodontal health. J Clin Periodontol. 2000;27(1):61–68. doi: 10.1034/j.1600-051x.2000.027001061.x. [DOI] [PubMed] [Google Scholar]
- 28.Bergström J, Eliasson S, Dock J. A 10-year prospective study of tobacco smoking and periodontal health. J Periodontol. 2000;71(8):1338–1347. doi: 10.1902/jop.2000.71.8.1338. [DOI] [PubMed] [Google Scholar]
- 29.Gomes SC, Piccinin FB, Oppermann RV, Susin C, Nonnenmacher CI, Mutters R, Marcantonio RAC. Periodontal status in smokers and never-smokers: clinical findings and real-time polymerase chain reaction quantification of putative periodontal pathogens. J Periodontol. 2006;77(9):1483–1490. doi: 10.1902/jop.2006.060026. [DOI] [PubMed] [Google Scholar]
- 30.Teixeira SRL, Mattarazo F, Feres M, Figueiredo LC, de Faveri M, Simionato MRL, Mayer MPA. Quantification of Porphyromonas gingivalis and fimA genotypes in smoker chronic periodontitis. J Clin Periodontol. 2009;36(6):482–487. doi: 10.1111/j.1600-051X.2009.01411.x. [DOI] [PubMed] [Google Scholar]
- 31.César Neto JB, Rosa EF, Pannuti CM, Romito GA. Smoking and periodontal tissues: a review. Braz Oral Res. 2012;26(Suppl 1):25–31. doi: 10.1590/s1806-83242012000700005. [DOI] [PubMed] [Google Scholar]
- 32.Chang CH, Han ML, Teng NC, Lee CY, Huang WT, Lin CT, Huang YK. Cigarette smoking aggravates the activity of periodontal disease by disrupting redox homeostasis – an observational study. Sci Rep. 2018;8(1):11055–11055. doi: 10.1038/s41598-018-29163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomofuji T, Ekuni D, Irie K, Azuma T, Tamaki N, Maruyama T, Yamamoto T, Watanabe T, Morita M. Relationships between periodontal inflammation, lipid peroxide and oxidative damage of multiple organs in rats. Biomed Res. 2011;32(5):343–349. doi: 10.2220/biomedres.32.343. [DOI] [PubMed] [Google Scholar]
- 34.Kanzaki H, Wada S, Narimiya T, Yamaguchi Y, Katsumata Y, Itohiya K, Fukaya S, Miyamoto Y, Nakamura Y. Pathways that regulate ROS scavenging enzymes, and their role in defense against tissue destruction in periodontitis. Front Physiol. 2017;8:351–351. doi: 10.3389/fphys.2017.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada H, Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998;9(3):248–266. doi: 10.1177/10454411980090030101. [DOI] [PubMed] [Google Scholar]
- 36.Duerr JS. Immunohistochemistry. WormBook. 2006;19:1–61. doi: 10.1895/wormbook.1.105.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rotaru M, Iancu GM, Gheucă Solovăstru L, Glaja RF, Grosu F, Bold A, Costache A. A rare case of multiple clear cell acanthoma with a relatively rapid development of the lower legs. Rom J Morphol Embryol. 2014;55(3 Suppl):1171–1179. [PubMed] [Google Scholar]
- 38.Ferringer T. Immunohistochemistry in dermatopathology. Arch Pathol Lab Med. 2015;139(1):83–105. doi: 10.5858/arpa.2014-0075-RA. [DOI] [PubMed] [Google Scholar]
- 39.Tribble GD, Lamont RJ. Bacterial invasion of epithelial cells and spreading in periodontal tissue. Periodontol 2000. 2010;52(1):68–83. doi: 10.1111/j.1600-0757.2009.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva N, Abusleme L, Bravo D, Dutzan N, Garcia-Sesnich J, Vernal R, Hernández M, Gamonal J. Host response mechanisms in periodontal diseases. J Appl Oral Sci. 2015;23(3):329–355. doi: 10.1590/1678-775720140259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4(8):617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 42.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


