Abstract
The circle of Willis is a very important vascular mechanism of protecting against cerebral ischemia, especially when circulation within the main arteries irrigating the brain is somehow impeded. As result of congenital malformation arising early in embryonic development, the fetal-type posterior circle of Willis remains as such during the rest of one’s life. Consequently, the posterior cerebral artery (PCA) becomes a branch of the internal carotid artery (ICA), rather than of the basilar artery (BA). Furthermore, the rest of collateral circulation, between the anterior and the posterior regions of the brain, is also negatively affected (e.g., leptomeningeal vessels). The anatomical variant represented by the artery of Percheron (AOP) has its origin on one of the PCAs, supplying singlehandedly both paramedian areas of the thalamus (right and left) and posterior regions of the midbrain. In the present study, we report a case of bilateral thalamic infarction with midbrain involvement, where the correct diagnosis was made retrospectively using computed tomography (CT) scan, magnetic resonance imaging (MRI), diffusion-weighted imaging (DWI) and three-dimensional time-of-flight magnetic resonance angiography (3D TOF MRA).
Keywords: artery of Percheron, stroke, anatomic variant, posterior cerebral artery, MRI, circle of Willis
⧉ Introduction
The posterior cerebral arteries (PCAs) originate from the basilar artery (BA), close to the pituitary stalk at the pontomesencephalic joint [1,2]. These PCAs are supplying with oxygenated blood: the midbrain, the thalamus, the occipital lobes, the posteromedial aspect of the temporal lobes and the regions of the posterior inferior parietal lobes [3,4].
Studies of human cadaveric brains demonstrated the diversity existent in the morphology of the posterior cerebral circulation. The anomalies found include partial circle, uneven with duplication, triplication, lack or union of components and fenestrations of the vertebrobasilar junction [5,6,7].
According to the autopsy studies on various deviations from normality regarding the circle of Willis, vessels reduced in thickness and absent vessels predominated in posterior cerebral circulation [7,8,9,10]. Most variants described in literature are either string-like vessels or persistent embryonic variants of the PCAs from the internal carotid arteries (ICAs) [4, 11]. 48–58% of the population has an asymmetrical circle of Willis, arising during fetal development [12,13].
Fetal posterior cerebral artery (FPCA) represents an anatomic variant of PCA, deriving directly from the ICA, without having a link with the BA [14,15,16]. The embryonic origin of the PCA was detected by angiographic studies in 11% to 46% of the general population, most commonly being found unilaterally (4–26%), while bilaterally was found in only 2–4% of cases [2, 11,12,13,14,15]. An FPCA is named a full FPCA if the precommunicating arterial segment (P1) of the PCA is absent or hypoplastic, not being detected by imaging or contrast substance, and partial FPCA, when the P1 portion is present, but is smaller than the posterior communicating artery (PCoA) [11,12,13,14].
The leptomeningeal vessels represent secondary collaterals established between the anterior cerebral artery (ACA), middle cerebral artery (MCA) and the PCA, which are used when the primary ones are incapable [12, 17]. Persistence of a FPCA makes impossible the development of collaterals between the anterior and posterior cerebral circulation, since the FPCA is part solely of the anterior cerebral circulation, without a connection to the vertebrobasilar system [11,12, 15].
Four variants of neurovascular supply to the midbrain and thalamus were described by Gérard Percheron in 1973. The artery of Percheron (AOP) represents a congenital malformation of the paramedian arterial supply, in which an artery, emergent from the P1 segment, endows, by itself, through bifurcation both paramedian thalamus and, in some cases, the rostral mesencephalon [18,19,20].
Aim
We discuss the case of a patient with an anatomical variant of the posterior cerebral circulation who was diagnosed after several cerebral imaging investigations. The main purpose of our paper is to highlight the importance in the current practice of corroborating magnetic resonance imaging (MRI) with three-dimensional time-of-flight magnetic resonance angiography (3D TOF MRA) in patients with the anatomical variant of the Willis polygon in the posterior territory, which according to the literature can precipitate the production of a stroke. More than this, it is important to carry out this imaging examination to facilitate the right treatment and to have the possibility of performing some rescue procedures at the right time, such as thrombectomy.
⧉ Case presentation
A 70-year-old male patient arrived at our Emergency Department of the Emergency County Hospital, Sibiu, Romania, in August 2020 (with reduced level of consciousness, psychomotor agitation, motor deficit in the left limbs, dysarthric speech disorders, and ataxia. His past medical history includes hypertension, hypertensive heart disease, ischemic heart disease and type II diabetes mellitus. His modified Rankin Scale (mRS) score prior to admission was zero. He had no past history of hypoglycemia, transient ischemic attack, substance abuse, head injury, trauma or seizure activity. Blood pressure was 180/90 mmHg with rest of the vitals being normal. The neurological examination revealed a Glasgow Coma Scale (GCS) of 12/15 (eye opening response – 3; best verbal response – 4; best motor response – 5) with left hemiparesis [3/5 Medical Research Council (MRC) superior limb and 2/5 MRC inferior limb], bilateral extensor plantars and absent neck rigidity. On examination, pupils were unequal, with sluggish light reaction, divergent strabismus on the right eye, horizontally nystagmus to the right. The National Institutes of Health Stroke Scale (NIHSS) score was 13 points.
Relevant physiological markers, including blood glucose, complete blood count, serum electrolytes, liver and renal function tests and arterial blood gas were unremarkable. Electrocardiogram showed normal sinus rhythm. The ultrasound examination of the extracranial vessels revealed multiple atherosclerotic plaques situated on the left common carotid artery, left ICA, left external carotid artery, and right vertebral artery (which is completely obstructed).
The initial computed tomography (CT) showed no obvious brain lesion. MRI done three days later showed features of a subacute infarction in paramedian thalami and rostral midbrain bilaterally (right>left) (Figure 1).
Figure 1.
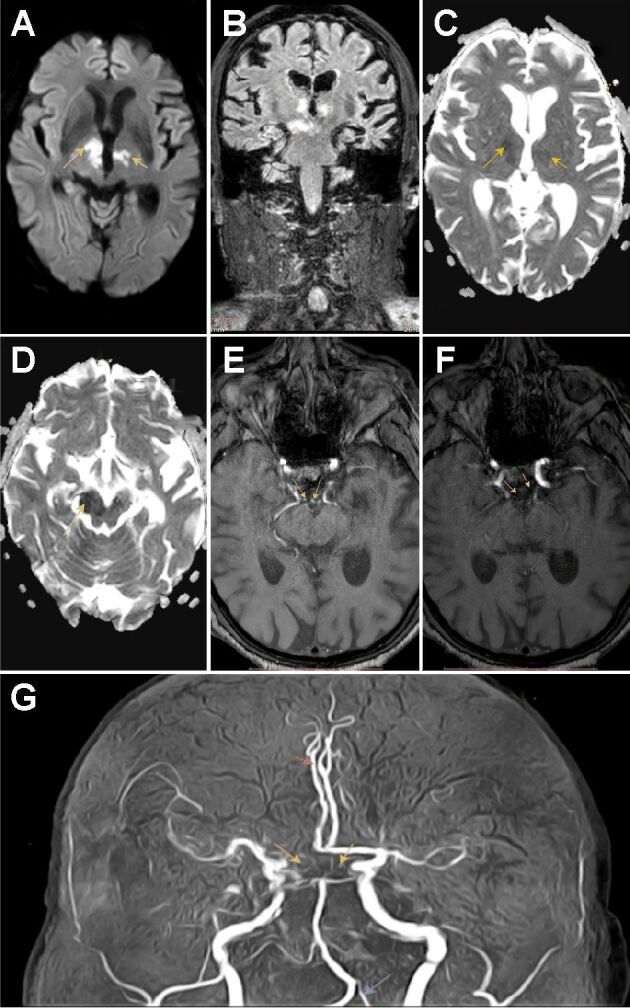
(A) Coronal section with bilateral para-median thalamic hyperintensities with extension to the midbrain; (B and D) TOF MRA revealed a bilateral fetal-type posterior circle of Willis, with arrows pointing where the two P1 segments of PCAs should have been; (C and E) ADC axial images illustrates bilateral para-median thalamic and midbrain diffusion restriction; (F) DWI axial image showing focal areas of restricted diffusion in bilateral paramedian thalami; (G) MRA shows a full fetal variant of the PCA (yellow arrows), left hypoplastic vertebral artery (blue arrows) and anatomic variant of right ACA (red arrows). ACA: Anterior cerebral artery; ADC: Apparent diffusion coefficient; DWI: Diffusion-weighted imaging; MRA: Magnetic resonance angiography; PCA: Posterior cerebral artery; TOF; Time-of-flight
Based on our initial findings (neurological examination and cerebral imaging), the initial diagnosis presumed was acute ischemic stroke in the territory of AOP. For a final diagnosis, a 3D TOF MRA was performed. The examination revealed a bilateral fetal-type posterior circle of Willis, demonstrated by the lack of visualization of the P1 segment of the PCA, with the P2 segment continuing with the PCoA, which shows an increased caliber (Figure 1).
Our patient could not benefit from recombinant tissue plasminogen activator (rtPA) therapy because we did not know exactly the onset timing of symptoms. He received antithrombotic therapy and followed neuromotor recovery. He was discharged with a Rankin score of 3 points, which means that our patient was able to walk without support and his disability was moderate.
⧉ Discussions
Our patient presented neurological signs and symptoms compatible with thalamic infarction. We used cerebral CT scan as first-line procedure when it comes to imaging. MRI was postponed, not being performed at the symptom’s onset, because the state of the patient was severe. Three days later, the diffusion-weighted imaging (DWI) was obtained, showing high-signal intensity in the paramedian thalami and in the rostral midbrain (right>left).
The midbrain and the thalamus receive blood supply from perforating branches arising from the P1 and P2 segments of the PCA and the PCoA. In one of Percheron’s variations, all these perforators arise from a single branch of the PCA – the AOP, also named the internal optic artery of Duret or the thalamoperforating artery of Foix and Hillerman (Figure 2) [21,22,23,24].
Figure 2.
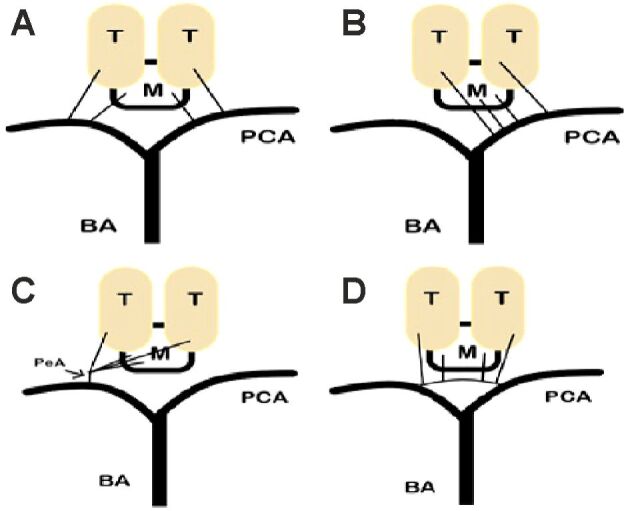
Four variants of the paramedian thalamic–mesencephalic arterial supply: (A) Variant 1 – normal anatomy; (B) Variant 2a – the left P1 segment is the wellhead of both paramedian arteries; (C) Variant 2b – illustrates the PeA; (D) Variant 3 – in this anatomical variant, an arcade gives rise to the paramedian arteries connecting the left and right P1 segments. BA: Basilar artery; M: Midbrain; PCA: Posterior cerebral artery; PeA: Percheron artery; T: Thalamus
Following the enclosement of the neural tube, a series of primitive endothelial vascular cells form a certain number of channels. The development of the entire brain vascularization originates in those particular cells.
The development of the posterior brain circulation is linked to the development of the occipital lobes. The occipital lobe and the brainstem growth in size provide the initial stimulus for the first stages of development of the posterior cerebral circulation. The first posterior arteries to develop are the BA followed closely by the vertebral artery. As the embryo is smaller than 5 mm, the developing posterior fossa is supplied by several endothelial channels called neural arteries that are connected to the carotid system via several collaterals such as the trigeminal or hypoglossal artery. The vertebral artery is linked to the ICA via the proatlantal artery, which regresses at a later stage. As the neural arteries grow, they develop into the BAs and vertebral arteries respectively. Following the growth of the PCoA, those anastomoses regress with occasional persistence in adult life [25,26]. As the embryonic development reaches day 24 (3 mm), the primitive ICA forms. Following this event, the primitive ICA gives rise to two divisions: the anterior division, which will for the anterior brain circulation, and the posterior division, which forms the FPCA and the primitive posterior choroidal artery. The FPCA will develop and eventually form the PCoA. As the BA grows, it will eventually branch and give rise to the P1 segment of the adult PCA. The two segments will eventually fuse resulting in the adult PCA, which will be linked to the internal carotid system by the PCoA.
The most common posterior circulation anomaly, also present in our patient, is linked to the formation of the primitive or FPCA. These anomalies can present either as a complete absence of one PCA or the absence of both. Another anomaly is the absence of one segment of the PCA, especially the P1 [27].
With the complete absence of both P1 segments of the PCAs, the circulation of both occipital lobes is considered fetal as the PCoA will provide full blood supply to both occipital lobes as the carotid arteries will eventually provide full blood supply to most of the occipital lobes. This type of vascular malformation is considered extremely rare and is potentially linked with an increased severity of ischemic cerebrovascular events [27].
For an assured diagnosis, it is important to examine the patient’s potential morphological and anatomical variations of the circle of Willis. Thus, we performed a 3D TOF MRA, which is a well-recognized method for such a goal [28]. This highlighted a bilateral FPCA circle of Willis, which is characterized by the abnormal fetal development of basilar circulation, resulting in the absence of the P1 arterial segment (Figure 3). At the same time, on our patient’s MRA, the BA was significantly smaller in size than what is considered to be normal, this aspect being supported by the current literature [28].
Figure 3.
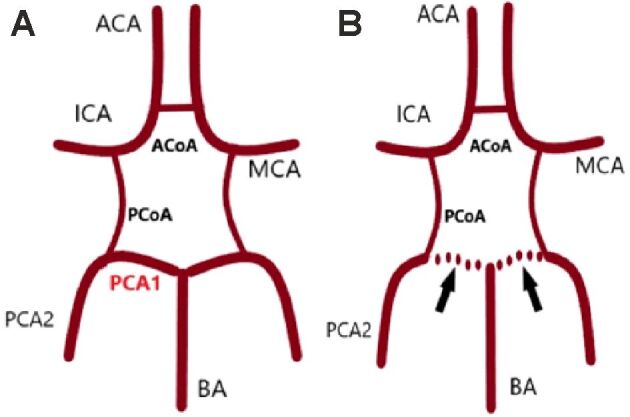
Circle of Willis: (A) Complete circle of Willis; (B) Full bilateral fetal PCAs. ACA: Anterior cerebral artery; ACoA: Anterior communicating artery; BA: Basilar artery; ICA: Internal carotid artery; MCA: Middle cerebral artery; PCA1: P1 segment of posterior cerebral artery; PCA2: P2 segment of posterior cerebral artery; PCoA: Posterior communicating artery
We have reviewed the literature available regarding the incidence of FPCA arising from the ICA. Iqbal (2013) studied 50 adult brains during autopsy to have an inner-most understanding of the variations in the circle of Willis [4]. He mentioned thar 6% of the specimens had an incomplete circle owing to the non-existence of the P1 segment of the PCA. The complete agenesis of P1 segment may be encountered bilaterally in 2% to 4% of cases [14].
Collateral cerebral circulation is complex, connecting all three main arteries: anterior, MCA and PCA. The leptomeningeal collaterals represent communications of the terminal cortical branches of the important cerebral arteries that are joined at the boundaries of their distribution areas. Collateral flow through anastomotic systems might be “non-functional” in various pathological conditions.
In the full FPCAs, collateral circulation is lacking between the anterior and the posterior part of the cerebrum, because a unilateral ICA supplies both the MCA and the PCA [2, 16, 29].
In patients suffering from such syndrome, like our patient, there is an increased risk of ischemic maladies, especially to the posterior cerebral area, and a higher incidence of strokes [30], with the latter being confirmed by post-mortem reports [12, 30].
The communicating arteries of the cerebral polygon are the first collateral enlisted. In case of FPCAs, the ICAs must deliver a higher debit of blood than in the normal arrangement of the polygon. Patients with ICA obstruction associated with full FPCAs are exposed to a higher risk for ischemic issues, compared to patients with a normal configuration of the circle of Willis, in which the collaterals can expand between the anterior and posterior cerebral circulation [29,30,31]. Furthermore, the lack of contralateral A1 segment exposes patients to an even greater risk, considering that a single ICA must feed with blood the areas of anterior, MCA and PCA.
As in our case, two subsequent studies [23,24] have reached the conclusion that FPCAs represents a major risk factor for vascular events, since such variants were confirmed in autopsy studies of people who died from infarcts. One of these studies found that the configuration of the anterior system of the circle of Willis is also valuable in the risk estimation. The biggest infarct incidence in brains have been described especially when one ICA preferably fed both ACAs, an MCA and a PCA.
⧉ Conclusions
The anatomical variations of the posterior cerebral circulation were probably detailed during fetal development and persist in postpartum period. Anomalies in the circle of Willis like hypoplasia and absent vessels were more common in posterior cerebral arterial system. FPCA can increase the risk of cerebral ischemia, the neurologists should therefore eagerly tackle the stroke risk factors of patients with these variants, such as ICA stenosis and atrial fibrillation. This article brings into discussion the importance in the current practice of corroborating MRI with 3D TOF MRA (which demonstrated high sensitivity in evaluating component vessels in the circle of Willis), for patients with the anatomical variant of the Willis polygon in the posterior territory. A complete knowledge of the various possible arrangements cerebral vessels can exhibit, is very important for neurosurgeons, neurologists and neuroradiologists when putting the correct diagnosis and selecting the appropriate therapeutic strategy.
Conflict of interests
Conflict of interests
The authors declare that they have no conflict of interests.
Patient consent
Written informed consent from the patient for the publication of this report and accompanying images was obtained. The report was conducted in accordance with the ethical standards, being approved by the Ethics Committee of the Emergency County Hospital, Sibiu, Romania.
References
- 1.Kuybu O, Tadi P, Dossani RH. In: StatPearls, editor. Treasure Island (FL): StatPearls Publishing; 2021. Posterior cerebral artery stroke. 2021 Jan 31. [PubMed] [Google Scholar]
- 2.Veras TWR, Elhert GW. Variation of the posterior cerebral artery and its embryological explanation: a cadaveric study. Bol Asoc Med P R. 2010;102(3):55–58. [PubMed] [Google Scholar]
- 3.Caplan LR, van Gijn J, editors. Stroke syndromes. 3. Cambridge, UK: Cambridge University Press; 2012. [Google Scholar]
- 4.Iqbal S. A comprehensive study of the anatomical variations of the circle of Willis in adult human brains. J Clin Diagn Res. 2013;7(11):2423–2427. doi: 10.7860/JCDR/2013/6580.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunnal SA, Farooqui MS, Wabale RN. Study of posterior cerebral artery in human cadaveric brain. Anat Res Int. 2015;2015:681903–681903. doi: 10.1155/2015/681903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zampakis P, Panagiotopoulos V, Petsas T, Kalogeropoulou C. Common and uncommon intracranial arterial anatomic variations in multi-detector computed tomography angiography (MDCTA). What radiologists should be aware of. Insights Imaging. 2015;6(1):33–42. doi: 10.1007/s13244-014-0381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alpers BJ, Berry RG, Paddison RM. Anatomical studies of the circle of Willis in normal brain. AMA Arch Neurol Psychiatry. 1959;81(4):409–418. doi: 10.1001/archneurpsyc.1959.02340160007002. [DOI] [PubMed] [Google Scholar]
- 8.Riggs HE, Rupp C. Variation in form of circle of Willis. The relation of the variations to collateral circulation: anatomic analysis. Arch Neurol. 1963;8(1):8–14. doi: 10.1001/archneur.1963.00460010024002. [DOI] [PubMed] [Google Scholar]
- 9.Kamath S. Observations on the length and diameter of vessels forming the circle of Willis. J Anat. 1981;133(Pt 3):419–423. [PMC free article] [PubMed] [Google Scholar]
- 10.Blackburn IW. On the median anterior cerebral artery as found among the insane. J Comp Neurol Psychol. 1910;20(3):185–194. [Google Scholar]
- 11.Nouh A, Remke J, Ruland S. Ischemic posterior circulation stroke: a review of anatomy, clinical presentations, diagnosis, and current management. Front Neurol. 2014;5:30–30. doi: 10.3389/fneur.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Raamt AF, Mali WPTM, van Laar PJ, van der Graaf Y. The fetal variant of the circle of Willis and its influence on the cerebral collateral circulation. Cerebrovasc Dis. 2006;22(4):217–224. doi: 10.1159/000094007. [DOI] [PubMed] [Google Scholar]
- 13.Lambert SL, Williams FJ, Oganisyan ZZ, Branch LA, Mader EC. Fetal-type variants of the posterior cerebral artery and concurrent infarction in the major arterial territories of the cerebral hemisphere. J Investig Med High Impact Case Rep. 2016;4(3):2324709616665409–2324709616665409. doi: 10.1177/2324709616665409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anghelescu A. Uncommon association of two anatomical variants of cerebral circulation: a fetal-type posterior cerebral artery and inferred artery of Percheron, complicated with paramedian thalamomesencephalic stroke - case presentation and literature review. Case Rep Neurol Med. 2018;2018:4567206–4567206. doi: 10.1155/2018/4567206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Javed K, Reddy V, Das JM. In: StatPearls, editor. Treasure Island (FL): StatPearls Publishing; 2021. Neuroanatomy, posterior cerebral arteries. 2020 Jul 31. [PubMed] [Google Scholar]
- 16.Coulier B. Duplication of the posterior cerebral artery (PCA) or "true fetal PCA": an extremely rare variant. J Belg Soc Radiol. 2018;102(1):29–29. doi: 10.5334/jbsr.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liebeskind DS. Collateral circulation. Stroke. 2003;34(9):2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 18.Ranasinghe KMIU, Herath HMMTB, Dissanayake D, Seneviratne M. Artery of Percheron infarction presenting as nuclear third nerve palsy and transient loss of consciousness: a case report. BMC Neurol. 2020;20(1):320–320. doi: 10.1186/s12883-020-01889-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stamm BJ, Lineback CM, Skolarus LE, Morgenstern LB, Shah GV. Artery of Percheron infarct: 12 cases and their complex clinical courses. Neurohospitalist. 2018;8(3):141–145. doi: 10.1177/1941874417748543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García-Casares N, Garzón-Maldonado FJ, de la Cruz-Cosme C. Demencia talámica secundaria a infarto agudo paramediano talámico bilateral por oclusión de la arteria de Percheron [Thalamic dementia secondary to acute bilateral paramedian thalamic infarcts after occlusion of the artery of Percheron] Rev Neurol. 2008;46(4):210–212. [PubMed] [Google Scholar]
- 21.Lochner P, Golaszewski S, Caleri F, Ladurner G, Tezzon F, Zuccoli G, Nardone R. Posterior circulation ischemia in patients with fetal-type circle of Willis and hypoplastic vertebrobasilar system. Neurol Sci. 2011;32(6):1143–1146. doi: 10.1007/s10072-011-0763-5. [DOI] [PubMed] [Google Scholar]
- 22.Tahir RA, Haider S, Kole M, Griffith B, Marin H. Anterior cerebral artery: variant anatomy and pathology. J Vasc Interv Neurol. 2019;10(3):16–22. [PMC free article] [PubMed] [Google Scholar]
- 23.Battacharji SK, Hutchinson EC, McCall AJ. The circle of Willis – the incidence of developmental abnormalities in normal and infarcted brains. Brain. 1967;90(4):747–758. doi: 10.1093/brain/90.4.747. [DOI] [PubMed] [Google Scholar]
- 24.Kameyama M, Okinaka SH. Collateral circulation of the brain with special reference to atherosclerosis of the major cervical and cerebral arteries. Neurology. 1963;13:279–286. doi: 10.1212/wnl.13.4.279. [DOI] [PubMed] [Google Scholar]
- 25.Luh GY, Dean BL, Tomsick TA, Wallace RC. The persistent fetal carotid-vertebrobasilar anastomoses. AJR Am J Roentgenol. 1999;172(5):1427–1432. doi: 10.2214/ajr.172.5.10227532. [DOI] [PubMed] [Google Scholar]
- 26.Burger IM, Siclari F, Gregg L, Gailloud P. Bilateral segmental agenesis of the vertebrobasilar junction: developmental and angiographic anatomy. AJNR Am J Neuroradiol. 2007;28(10):2017–2022. doi: 10.3174/ajnr.A0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menshawi K, Mohr JP, Gutierrez J. A functional perspective on the embryology and anatomy of the cerebral blood supply. J Stroke. 2015;17(2):144–158. doi: 10.5853/jos.2015.17.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He J, Liu H, Huang B, Chi C. Investigation of morphology and anatomic variations of circle of Willis and measurement of diameter of cerebral arteries by 3D-TOF angiography] Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2007;24(1):39–44. [PubMed] [Google Scholar]
- 29.Wu HM, Chuang YM. The clinical relevance of fetal variant of the circle of Willis and its influence on the cerebral collateral circulation. Acta Neurol Taiwan. 2011;20(4):232–242. [PubMed] [Google Scholar]
- 30.Arjal RK, Zhu T, Zhou Y. The study of fetal-type posterior cerebral circulation on multislice CT angiography and its influence on cerebral ischemic strokes. Clin Imaging. 2014;38(3):221–225. doi: 10.1016/j.clinimag.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Maier S, Motataianu A, Bajko Z, Romaniuc A, Balasa A. Pontine cavernoma haemorrhage at 24 weeks of pregnancy that resulted in eight-and-a-half syndrome. Acta Neurol Belg. 2019;119(3):471–474. doi: 10.1007/s13760-019-01147-x. [DOI] [PubMed] [Google Scholar]


