Abstract
Our previous paper reported the structure of ginseng pectic polysaccharides related to the cell migration inhibitory effects, but the underlying mechanisms are poorly understood. In this manuscript, rhamnogalacturonan I (RGI)-rich pectins prepared from ginseng pectin were investigated for their effect on cell migration. The results indicated that the combination of homogalacturonan (HG) and RGI-rich pectins exerted stronger effects than either HG- or RGI-rich pectin alone. Further studies revealed that the effects of HG- and RGI-rich pectins were dependent on pretreatment, which caused alterations in cell morphologies such as cell size and shape, focal adhesion, and the organization of actin filaments, suggesting that HG and RGI pectins exert synergistic effects on cell migration, likely through different ways. Morphological data and quantitative cell adhesion and spreading assays showed that HG- and RGI-rich pectin treatment decreased cell adhesion and cell spreading on the substratum, suggesting that HG- and RGI-rich pectins may exert their effects on cell migration via decreasing cell adhesion and cell spreading. Additionally, we showed that L-929 cells expressed little galectin-3 (Gal-3) and that lactose, an inhibitor of Gal-3 did not block the activities of HG- and RGI-rich pectins, implicating that cell migration inhibited by pectin did not correlate to Gal-3.
Keywords: Cell migration, Ginseng pectin, Galectin-3, Homogalacturonan, Rhamnogalacturonan I
1. Introduction
Cell migration is a highly integrated multistep process in normal physiology as well as disease pathology, including embryonic morphogenesis, tissue repair, immunological surveillance, mental retardation, chronic inflammatory occurrence, and cancer progression. Cell migration is comprised of a cycle of several interdependent physical steps-polarization, protrusion, adhesion, contraction, and de-adhesion and retraction [1–3]. All these steps are coordinated spatially and temporally by a complex signaling network. Integrin, which is a heterodimeric receptor consisting of α and β subunits with a ligand-binding extracellular domain and a cytoplasmic domain, is one of the most studied transmembrane glycoproteins in cell migration signaling pathways, and influences polarization, adhesion formation, cytoskeleton organization and dynamic during cell migration.
Cell migration is initiated by extracellular ligand-membrane receptor binding directly or via extracellular matrix (ECM) components indirectly. Therefore, soluble growth factors and ECM constituents have been studied extensively [4,5]. Hyaluronan and proteoglycans are the main carbohydrate components of the ECM. Their functions in regulating cell migration have been demonstrated by a number of studies in recent years [6–8]. In addition, it has been reported that modified citrus pectin blocked chemotaxis of cancer cells, impaired myeloma cell metastasis and angiogenesis, and inhibited galetin-3-mediated human breast cancer cell invasion.
Ginseng panax C. A. Meyer (ginseng) has been used in Asian countries for a long time as a traditional medicine with a number of pharmacological activities. Pectin is the main active component with many bioactivities [9–11]. Ginseng pectin is mainly composed of arabinogalactan (AG), rhamnogalacturonan I (RGI) and homogalacturonan (HG). In our previous studies, ginseng pectin was fractionated into four homogenous fractions of homogalacturonans (WGPA-1HG, -2HG, -3HG, -4HG), two homogenous fractions of arabinogalactans (WGPA-1RG, -2RG), and two fractions consisting of both rhamnogalacturonans and homogalacturonans (WGPA-3RG, -4RG) [12,13]. Afterwards, our research group has isolated one of RGI-rich pectins (named RGI-4) with the highest RGI content from ginseng pectin by endo-polygalacturonase hydrolysis and a combination of ion exchange and gel permeation chromatography [14].
Although the inhibitory effects of HG-rich ginseng pectins on cell migration have been observed, structural features related to the effects and the underlying mechanisms are not elucidated. In this study, we chose WGPA-3HG and RGI-rich as a representative of ginseng HG and RGI pectins, respectively, and studied their effects and mechanisms on cell migration.
2. Materials and methods
2.1. Materials
Na3VO4 and HEPES were purchased from Sigma. RPMI1640 medium and fetal calf serum were obtained from Gibco. Penicillin/streptomycin antibiotics were from PAA. Protease inhibitor cocktail was purchased from Roche, crystal violet from Genview, precision plus protein standards from Bio-Rad, phenylmethanesulfonyl fluoride and trypsin from Amresco, and Hybond-c extra nitrocellulose membrane and ECL plus western blotting detection reagent from GE Healthcare. The antibodies were purchased as follows: mouse monoclonal vinculin antibody (hVIN-1) and rabbit polyclonal α-actin antibody from Sigma; Rhodamine-conjugated phalloidin (in the actin cytoskeleton/focal adhesion staining kit) from Chemicon (Millipore); mouse monoclonal phosphotyrosine antibody PY20 and mouse monoclonal Galectin-3 antibody (A3A12) from Santa Cruze; FITC-conjugated and HRP-conjugated goat anti-mouse secondary antibodies from Jackson Laboratories. All other reagents were of analytical grade or better.
2.2. RGI-rich pectins preparation
RGI-high, it was prepared from ginseng pectin WGPA according to the method described by Yu et al. [14]. Briefly, 58 g of WGPA dissolved in 580 ml of 25 mM sodium acetate buffer (pH 4.2) was incubated with 2552 units of Endo-PG at 50 °C for two hours and then applied to a Sephadex G-25 column. The materials eluted in the void volume was recovered and separated on a preparative DEAE-Sepharose fast flow column eluted sequentially with H2O, 0.07 M, 0.16 M, 0.22 M and 0.3 M NaCl. The materials eluted by 0.3 M NaCl were further purified on Sepharose CL-6B yielding 1.39 g RGI-high.
RGI-low, it was prepared from ginseng pectin fraction WGPA-3RG. 200 mg of WGPA-3RG was treated with enzyme Endo-PG as described above. The digest was applied onto a Sepharose CL–6 B column (1.5 × 90 cm) and eluted with 0.15 M NaCl at 0.15 ml/min (Supplementary Fig. 1). The materials eluted between 60 and 130 ml were combined yielding 86 mg RGI-low. Its sugar composition was determined and listed in Table 1.
Table 1.
Sugar composition of the RGI pectins.
| pectins | Monosaccharide composition (%) | ||||||
|---|---|---|---|---|---|---|---|
| Gal | Glc | Ara | Rha | Man | GalA | GlcA | |
| RGI-low | 24.9 | 1.5 | 43.0 | 11.1 | 0.3 | 16.9 | 2.0 |
| RGI-high | 19.5 | 3.0 | 9.2 | 21.8 | 0.4 | 33.8 | 2.2 |
2.3. Determination of total sugar, uronic acid and protein contents
Total sugar content was measured by the phenol-sulfuric acid method [15]. The standard was a combination of various monosaccharides prepared according to sugar composition. The content of uronic acid was measured by a modified sulfamate/m-hydroxydiphenyl method [16], using galacturonic acid as the standard. Protein content was determined by Bradford assay, with bovine serum albumin as the standard.
2.4. Determination of sugar composition
Each polysaccharide sample (1 mg) was hydrolyzed first by methanolysis with 1 M MeOH/HCl at 80 °C for 16 h and then by acid hydrolysis with 2 M TFA at 120 °C for 1 h. The resulting monosaccharides were derivatized with 1-phenyl-3-methyl-5-pyrazolone (PMP) and subsequently separated on a pre-calibrated DIKMA Inertsil ODS-3 column (150 × 4.6 mm) assembled on a Shimadzu HPLC system [17]. The column was eluted isocratically at 1 ml/min with 18% (v/v) acetonitrile/72% (v/v) of 0.1 M phosphate buffer, pH 7.0, and monitored by absorbance at 245 nm.
2.5. Cell culture
L-929 cell was obtained from American Type Culture Collection. It was cultured in RPMI 1640 medium. The medium was supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 10 mM HEPES at 37 °C in a humidified atmosphere of 5% CO2.
2.6. Cell migration by scratching assays
Cells were seeded on a 12-well tissue culture plate (Costar) at 2.5 × 105 cells/well and incubated in a CO2 incubator for 24 h. The culture medium was then aspirated and the pretreatment medium (culture medium plus various carbohydrate samples) was added. Cells were maintained in this medium for 24 h and then scratched with a yellow tip. A horizontal and a vertical scratch crossing each other were made for each well routinely to facilitate subsequent measurements. Scraped cells were rinsed off and fresh pretreatment medium was added. Cells were observed using an inverted microscope at 100× magnifications and photographed. The width of the scratch was measured and referred to as Wbefore. Then, the plate was returned to the incubator and left for 3 h for cell migration into the space. The width of the same scratch was measured again and referred to as Wafter. Control cells were pretreated and scratched similarly except that pretreatment medium without carbohydrates was used.
Migrating distance was calculated by subtracting Wafter from Wbefore. The relative migration, defined as the ratio of the migrating distance of carbohydrate-treated cells to that of control cells, was presented for each assay. The migration of the control was set as 100.
2.7. Cell migration by transwell assays
Transwell assays were performed using 24-well Transwell units with 8 μm pore size polycarbonate inserts (BD Biosciences). Cells grown on tissue culture plates were incubated for 24 h in pretreatment medium without (as control) or with 0.5 mg/ml of pectin as described in the scratching assay and then detached from the plates by a 30-min incubation with PBS containing 1 mM EDTA at 37 °C. Detached cells (5 × 104) were re-suspended in 200 μl pretreatment medium lacking serum and added to the upper chamber of a Transwell unit. The lower chamber was filled with complete pretreatment medium. Cells were allowed to migrate at 37 °C in a CO2 incubator for 3 h in the case of L-929. Then the cells on the upper side of the filter were removed using a cotton swab. The cells that migrated to the underside were fixed for 15 min at room temperature in 2.7% paraformaldehyde and stained for 10 min in 0.5% crystal violet in 20% methanol/80% H2O. Subsequently, the filter was washed three times with water to remove excess dye and incubated with 1% SDS for 30 min to solubilize the cells. The resulting cell extract was transferred to a 96 well plate at 150 μl per well and read at 570 nm using a microplate reader.
2.8. Cell adhesion assays
L-929 cells grown on a 12-well tissue culture plate (Costar) were incubated for 24 h in pretreatment medium without (as control) or with 0.5 mg/ml of pectins. Then the medium was changed to PBS and the plate was rotated at 250 rpm for 2 h on an orbital shaker. The cells released into the medium were collected into microcentrifuge tubes and pelleted by centrifugation. Both the released cells and the cells remained on the plate were fixed with paraformaldehyde, stained with crystal violet, and measured at 570 nm as described above in the transwell migration assay. The percentage of cells remained on the plate after rotation, representing relative cell adhesion strength, was calculated in each case.
2.9. Cell spreading assays
L-929 cells grown on tissue culture plates were incubated for 24 h in pretreatment medium without (as control) or with 0.5 mg/ml of pectin and then detached as described in the transwell assay. Detached cells were re-suspended in respective pretreatment medium, plated at 2 × 105 cells/well onto 12 mm diameter glass cover slips and incubated at 37 °C in a CO2 incubator for 1, 2, and 3 h. Afterwards, the plate was gently washed three times with PBS by rotating at 100 rpm for 5 min each time. The adherent cells were fixed in 2.7% paraformaldehyde at room temperature for 15 min and viewed using a phase contrast microscope. Ten randomly chosen fields per condition each with 120–500 cells were photographed at 200× magnifications. All images were analyzed for cell morphologies that were classified as non-spreading and spreading cells in this paper. This experiment was repeated 4–6 times.
For the analysis of tyrosine phosphorylation during cell spreading, cells were pretreated with various pectins and re-plated essentially the same as described above except that cells were re-plated on tissue culture plates in stead of cover slips and incubated for 0.5 h, 1 h, and 2 h. Following a gentle wash to remove the non-adherent cells, the adherent cells were scrapped off the plates and processed for SDS-PAGE and western blot analysis as described in the following section.
2.10. SDS-PAGE and Western blot analysis
The samples for SDS-PAGE and Western blot analysis were prepared as follows. For the comparison of galectin 3 expression, Hela and L-929 cells were detached from tissue culture plates by EDTA treatment and solubilized directly with SDS-PAGE loading buffer. For the determination of protein tyrosine phosphorylation during cell spreading, cells were scratched off the plate in cold PBS and then lysed in lysis buffer (150 mM NaCl, 1% TX-100, 1 mM EDTA, 1 mM PMSF, 1 mM Na3VO4, protease inhibitor cocktail (Roche), Tris-Cl, pH 7.4). After centrifugation at 14,000 rpm for 5 min, the supernatant was recovered and mixed with SDS-PAGE loading buffer.
SDS-PAGE was performed as described previously. The separated proteins on the gel were transferred to nitrocellulose membrane and blocked in 5% non-fat milk. Then the membrane was incubated with first antibodies (PY20 at 1:100; Galectin-3 at 1:500) followed by HRP conjugated secondary antibodies. Protein bands were revealed by ECL solutions (GE).
2.11. Morphological studies
L-929 cells were grown on 12 mm diameter cover slips contained in 24-well plates. After pretreatment with various pectins, cells were gently washed with PBS and then fixed for 15 min at room temperature in 2.7% paraformaldehyde. For crystal violet staining, fixed cells were stained for 10 min in 0.5% crystal violet in 20% methanol/80% H2O. After three washes with water, the cells were visualized under a microscope (Olympus BX51) using a 20× objective. For fluorescence staining, fixed cells were perme-abilized in 0.1% Triton X-100/PBS for 15 min and then blocked in blocking buffer (2% (w/v) BSA and 5% (v/v) bovine serum in PBS) for 20 min. Afterwards, cells were incubated with vinculin antibody (at 1:100 dilution) for 1 h, washed three times, and incubated with FITC-conjugated goat anti mouse antibody (at 1: 100 dilution) and Rhodamine-conjugated phalloidin (at 1:400 dilution) for 1 h. After five washes, the cover slips were mounted and viewed under an epi-fluorescence microscope (Olympus BX51) with a 100 × objective. Cell images were recorded with a DP71 camera.
2.12. Statistical analysis
The results were expressed as the means ± SD. Statistical analysis of the data was performed using one-way ANOVA (SPSS Statistics 17.0). Differences were considered significant when p < 0.01.
3. Results
3.1. Preparation of RGI-rich pectins
In our previous study, ginseng pectin WGPA was fractionated into five rhamnogalacturonan I-rich pectins by endo-polygalacturonase hydrolysis and a combination of ion-exchange and gel permeation chromatography [14]. According to this procedure, we prepared two RGI-rich samples with little HG from ginseng. Sample RGI-high (with higher Rha content) was a relatively homogenous RGI-rich fragment prepared from ginseng pectin WGPA. As shown in Table 1, RGI-high contained 21.8% Rha and 33.8% GalA, with a GalA/Rha ratio of 1.55. The other sample, RGI-low (with lower Rha content) was prepared by treatment of WGPA-3RG [12] with the enzyme Endo-PG followed by gelfiltration chromatography to remove the resulting GalA enriched (mainly HG) oligosaccharides (Supplementary Fig. 1). It contained 11.1% Rha and 16.9% GalA (Table 1), with a GalA/Rha ratio of 1.52.
3.2. RGI-rich pectins inhibit cell migration
Rha-containing structures have been proposed to contribute to the inhibitory effects on cell migration [12]. To test this hypothesis, we performed cell migration assays in L-929 cells. We found that RGI-high was indeed a potent inhibitor of cell migration (Fig. 1A, Table 2). Migration was reduced by about 70% at maximum. In addition, RGI-high also displayed dose-dependent effects, with detectable effects starting from 0.015 mg/ml to 50% effect at 0.125–0.25 mg/ml. Sample RGI-low also inhibited cell migration albeit to a less extent. Migration was reduced by 40% at maximum (Fig. 1A, Table 2). The results of these RGI fragments provided further evidence that RGI domain was a functional element.
Fig. 1.
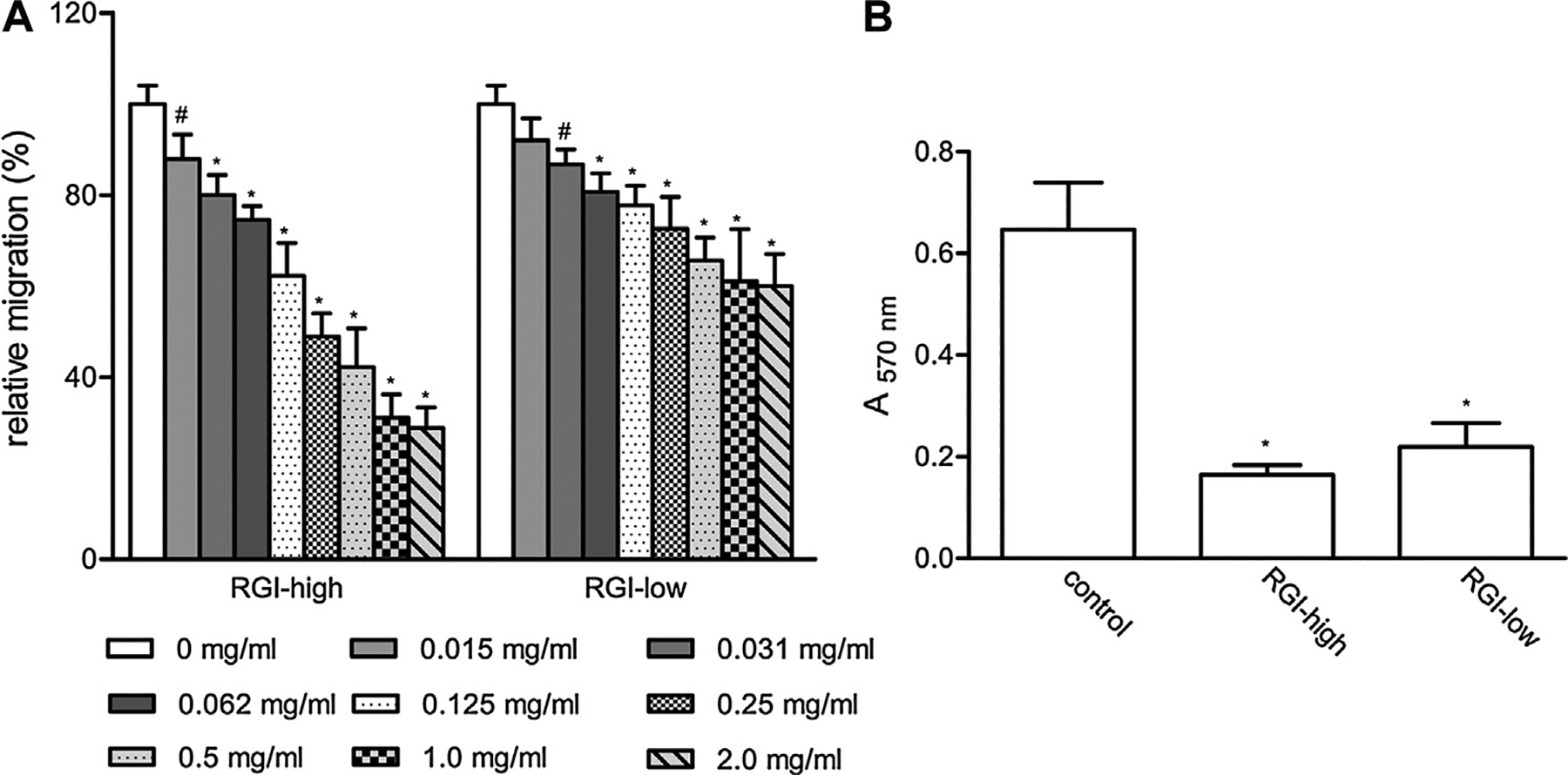
The effects of RGI pectins on L-929 cell migration. (A) The effects of RGI samples on cell migration as measured by scratching assay; (B) the effects of RGI samples on cell migration as measured by transwell assay. The values are means ± SD, * p < 0.001, # 0.001 < p < 0.01.
Table 2.
Comparison of the effects of different pectins on cell migration.
| Pectins | Maximum inhibition | Minimum dose for significant inhibition (mg/ml) | Does for 50% inhibition (mg/ml) |
|---|---|---|---|
| Scratching migration assay | |||
| WGPA-3HG | ~46% | <0.015 | NA |
| RGI-high | ~70% | <0.015 | 0.125–0.25 |
| RGI-low | ~40% | 0.031 | NA |
| WGPA-3HG+ RGI-high | ~80%a | ND | ND |
| WGPA-3HG+ RGI-low | ~65%a | ND | ND |
| Transwell migration assay | |||
| WGPA-3HG | ~50% | <0.031 | 0.25 |
| RGI-high | ~75% | <0.031 | 0.125 |
| WGPA-3-HG + RGI-high | ~85% | <0.031 | 0.062–0.125 |
NA not applicable; ND not determined.
The inhibition at 1.0 mg/ml.
The inhibitory effects of RGI-rich pectins on cell migration were also observed in transwell migration assays. As shown in Fig. 1B, the absorbance of L-929 cells treated with 0.5 mg/ml RGI-high was 34% of that in control cells, indicating that RGI-high inhibited cell migration through the filter. When increasing concentrations of RGI-high were tested, cell migration was reduced accordingly, indicating a dose-dependent response of the compounds (Fig. 2B). The maximal inhibition of RGI-high was approximately 75%, higher than that in the scratching assay (Table 2).
Fig. 2.
The effects of ginseng pectins on L-929 cell migration. Cells were treated with 0.5 mg/ml of ginseng pectins in scratching assay. The values are means ± SD, * p < 0.001.
3.3. HG- and RGI-rich pectins display synergistic effects
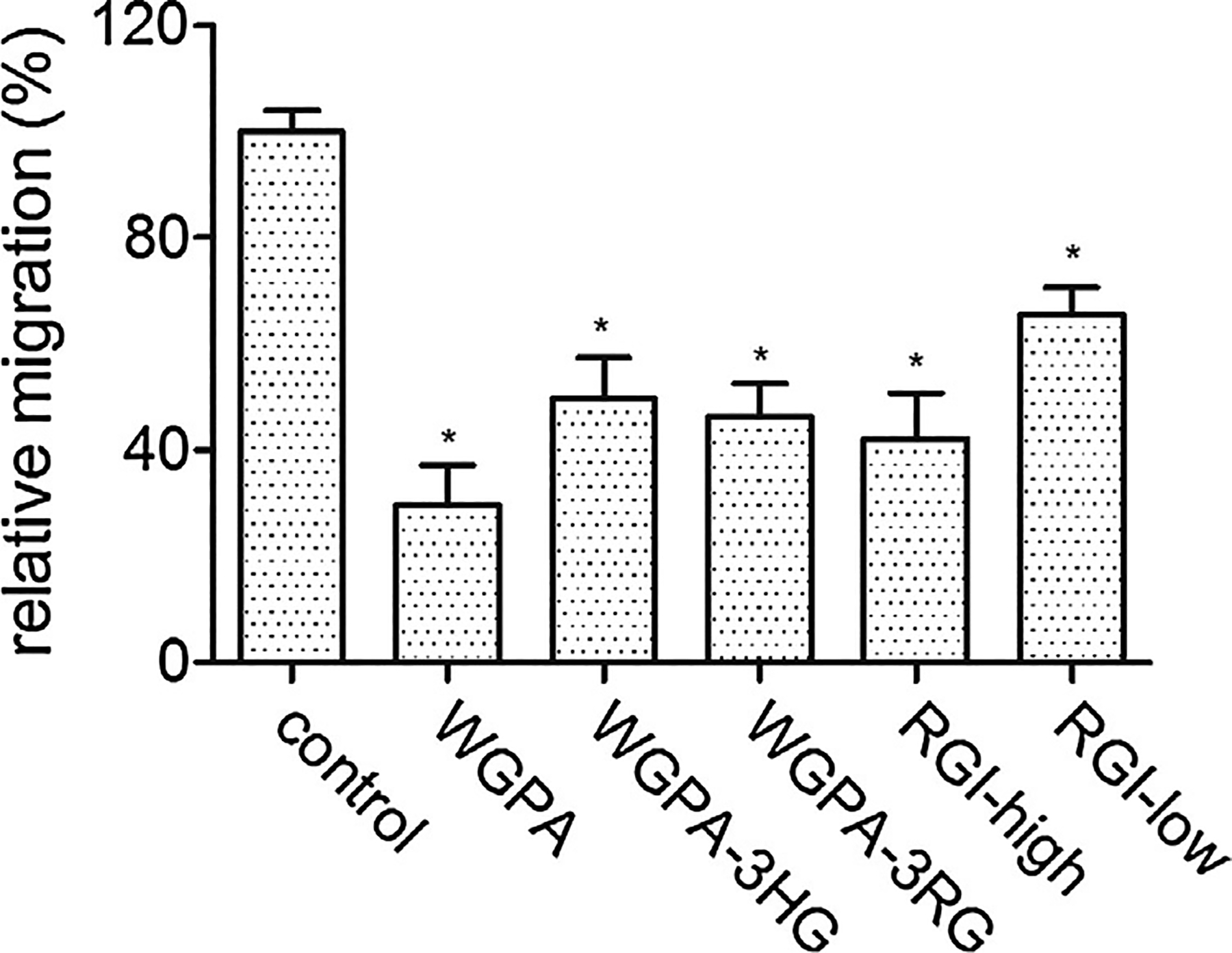
The effects of ginseng pectin WGPA and its fractions on cell migration were analyzed and compared (Fig. 2). Based on our previous reported results, WGPA-3HG is the pectin with high content of GalA, it is composed of 90.9% GalA, 1.5% Rha, 2.2% Ara, 3.5% Gal and 1.3% Glc [12]. Here, we chose WGPA-3HG as HG-rich pectin. As shown in Fig. 2, both HG- and RGI-rich pectins inhibited cell migration, although their effects were lower than that of the parental materials that contain both HG and RGI structures. For example, the individual fractions of ginseng pectin WGPA-3HG and RGI-high had lower effects than the un-fractionated ginseng pectin WGPA. Likewise, RGI-low fractionated from WGPA-3RG had lower effects than WGPA-3RG. The results suggested that HG and RGI structures may exert synergistic effects on inhibiting cell migration.
To test this hypothesis, we mixed RGI-rich pectin (RGI-high or RGI-low) and HG-rich pectin (WGPA-3HG) [12] at 1:1 ratio and then assayed the effects of the mixtures on cell migration. Fig. 3A showed that 1.0 mg/ml mixture comprised of 0.5 mg/ml RGI-high and 0.5 mg/ml WGPA-3HG reduced cell migration to 19% of control, which is significantly lower than that with either 1.0 mg/ml RGI-high (31%) or WGPA-3HG (50%) alone. Similarly, 1.0 mg/ml mixture comprised of 0.5 mg/ml RGI-low and 0.5 mg/ml WGPA-3HG also reduced cell migration to lower extent (35% of control) than either 1.0 mg/ml RGI-low (65%) or WGPA-3HG (50%) alone (Fig. 3A). These data indicated that although individual HG or RGI containing pectins could inhibit cell migration, the combination of both exerted stronger effects.
Fig. 3.
HG- and RGI-rich pectins exhibited synergistic effect on cell migration. (A) The synergistic effect of WGPA-3HG and RGI-high or RGI-low on L-929 cell migration in scratching assay. Cells treated with either 1.0 mg/ml of RGI-high, RGI-low, WGPA-3HG, or 1.0 mg/ml of mixture comprising equal amount of RGI-high (or RGI-low) and WGPA-3HG were tested. (B) The synergistic effect of WGPA-3HG and RGI-high on L-929 cell migration in transwell assay. Cells treated with increasing concentrations of either RGI-high, or WGPA-3HG, or the mixture comprising equal proportion of RGI-high and WGPA-3HG were tested. Values were means ± SD from three experiments in A and two experiments in B, * p < 0.001, # 0.001 < p < 0.01.
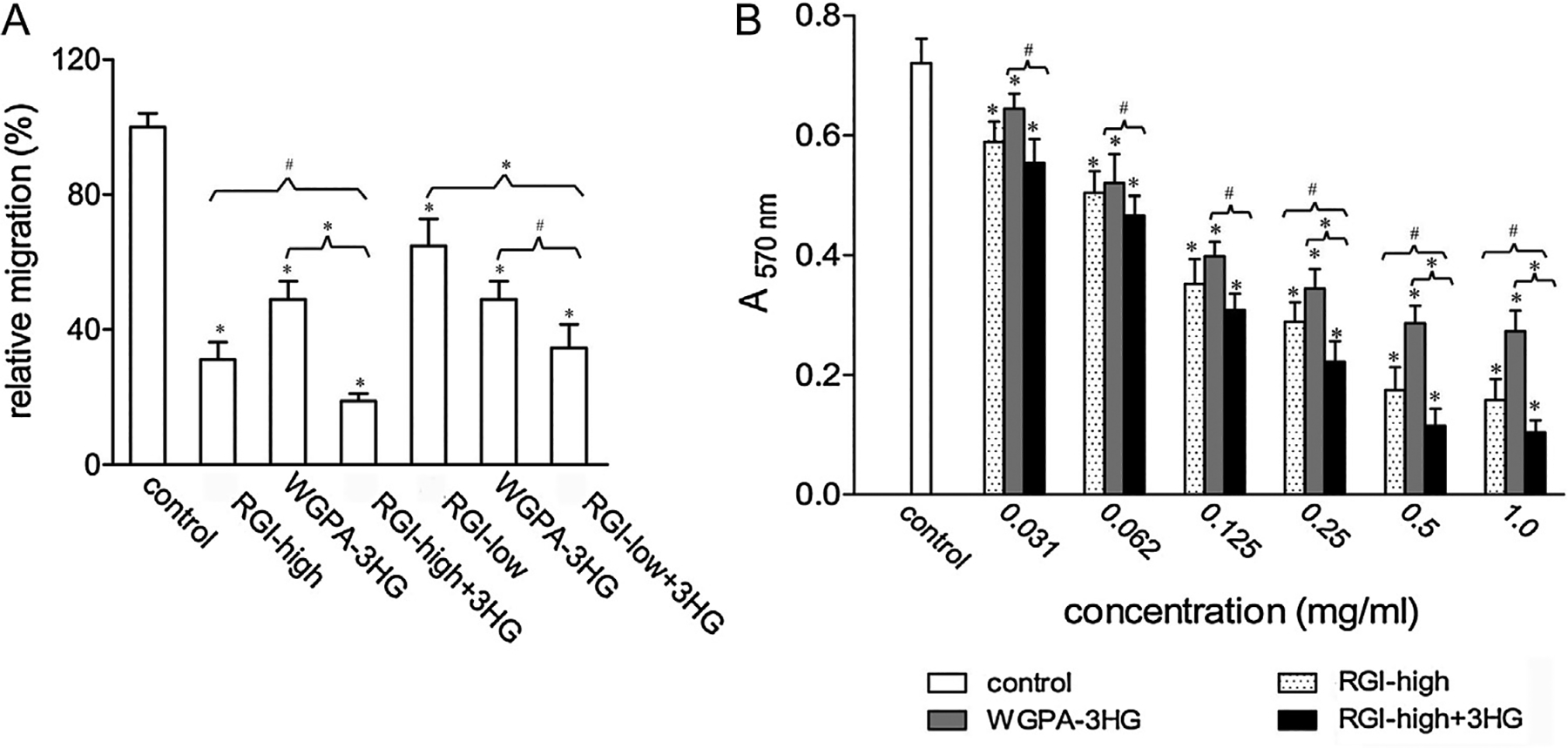
The synergistic effect of HG- and RGI-rich pectins was also demonstrated by transwell migration assays. Fig. 3B showed that the mixture consisted of RGI-high and WGPA-3HG at 1:1 ratio exerted stronger effects than either RGI-high or WGPA-3HG alone at all concentrations tested. For example, at the concentration of 0.125 mg/ml, cell migration was 49% of control for RGI-high and 53% for WGPA-3HG, but was 40% for the mixture; at 0.5 mg/ml, the value was 26% for RGI-high and 40% for WGPA-3HG, but was 16% for the mixture. The mixture exhibited 50% inhibition at 0.062–0.125 mg/ml, while RGI-high showed 50% inhibition at approximately 0.125 mg/ml and WGPA-3HG at approximately 0.125–0.25 mg/ml. Besides, the maximal effect of the mixture (~85% inhibition) was also greater than that of either RGI-high (~75% inhibition) or WGPA-3HG (~55% inhibition).
3.4. Pretreatment is required for both HG- and RGI-rich pectins to exert maximal effects
In our standard cell migration assays, cells were pretreated for 24 h in carbohydrate-containing pretreatment medium and then subjected to migration assays in the same pretreatment medium. To get better understanding of the actions of HG-rich and RGI-rich pectins, we investigated whether pectin treatment before and during migration stage was necessary, using WGPA-3HG and RGI-high as representatives of HG-rich pectin and RGI-rich pectin, respectively (Fig. 4). Without pretreatment, cells migrated at 82% of control in the case of WGPA-3HG and 77% of control in the case of RGI-high. In contrast, pretreated cells migrated at 60% and 34% of control under standard conditions. These data clearly indicated that the effects of these pectins on cell migration were dependent on pretreatment. We next asked if continuous treatment during migration was required. To this end, we pretreated cells as aforementioned but carried out cell migration in the absence of pectins. Under these conditions, cell migration was 89% and 36% of control for WPGA-3HG and RGI-high, respectively. Compared with the migration under standard conditions, the inhibitory effect of WPGA-3HG was almost abolished, whereas the effect of RGI-high was only slightly changed. These data indicated that the presence of WPGA-3HG, but not RGI-high, during the migration stage was necessary.
Fig. 4.
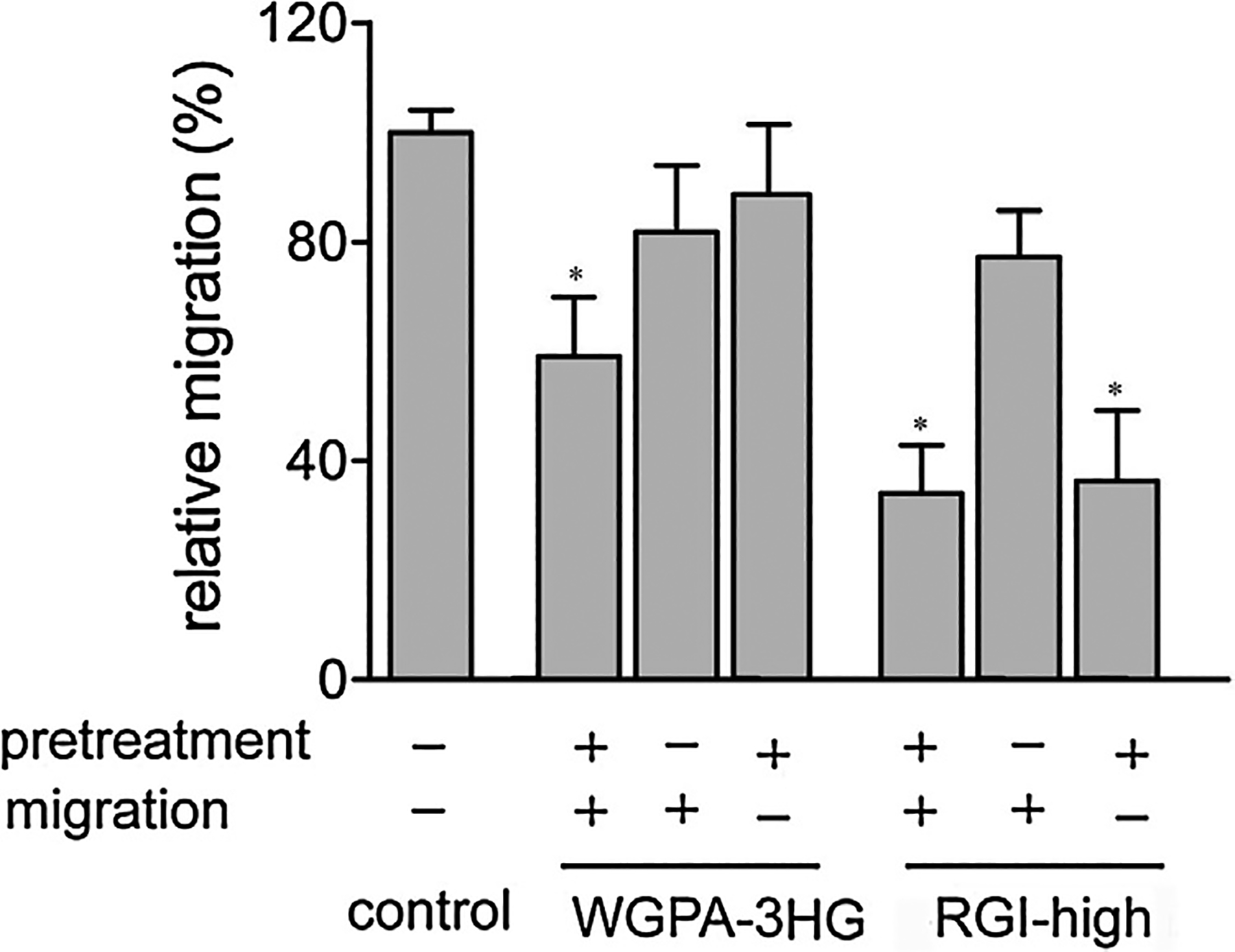
Pretreatment of cells with pectins was required for HG- and RGI-rich pectins to exert maximum effects. Cells pretreated (+) or not pretreated (−) with 0.5 mg/ml WGPA-3HG or RGI-high were subjected to scratching assay in the presence (+) or absence (−) of the same pectins. Values were means ± SD from more than three experiments, * p < 0.001.
In summary, while pretreatment of cells were absolutely required for both HG-rich and RGI-rich pectins to exert maximal inhibitory effects, the continuous pectin treatment during the migration stage was only required for HG-rich pectins but not RGI-rich pectins. The different requirement implied that HG-rich and RGI-rich pectins may inhibit cell migration by different mechanisms, which supports our findings that HG-rich and RGI-rich pectins exerted synergistic effects.
3.5. Pretreatment of HG- and RGI-rich pectins alters cell morphology but not cell viability
In order to elucidate the mechanisms by which HG-rich and RGI-rich pectins inhibit cell migration, we investigated whether pretreatment resulted in any alterations in cell morphology, cell adhesion and cell spreading in this and the following sections.
Cells were pretreated with HG-rich pectins (WGPA-3-HG), RGI-rich pectins (RGI-high), or pectic arabinogalactan (WGPA-1RG) as described in cell migration assays and visualized under a microscope following crystal violet staining. As shown in Fig. 5, the overall images of control L-929 cells were similar to that shown in the data sheet of ATCC, i.e. the majority of cells displayed polygonal morphologies, while a small number of cells displayed elongated fibroblastoid morphologies. Control cells were large and well spread. Their staining intensity was high in the central areas but low in the peripheral areas. Notably, cells pretreated with RGI-high, appeared smaller and poorly spread. The majority of cells displayed oval and spindle-like shapes with heavy and uniform staining. Cells pretreated with WGPA-3HG appeared more slender than control cells. About 40% cells shaped like elongated spindles and about 30% shaped like oval and short spindles. Thus, both HG-rich and RGI-rich pectins altered cell morphologies. In contrast, cells pretreated with WGPA-1RG, which did not significantly inhibit cell migration [12], showed similar morphologies to the control.
Fig. 5.
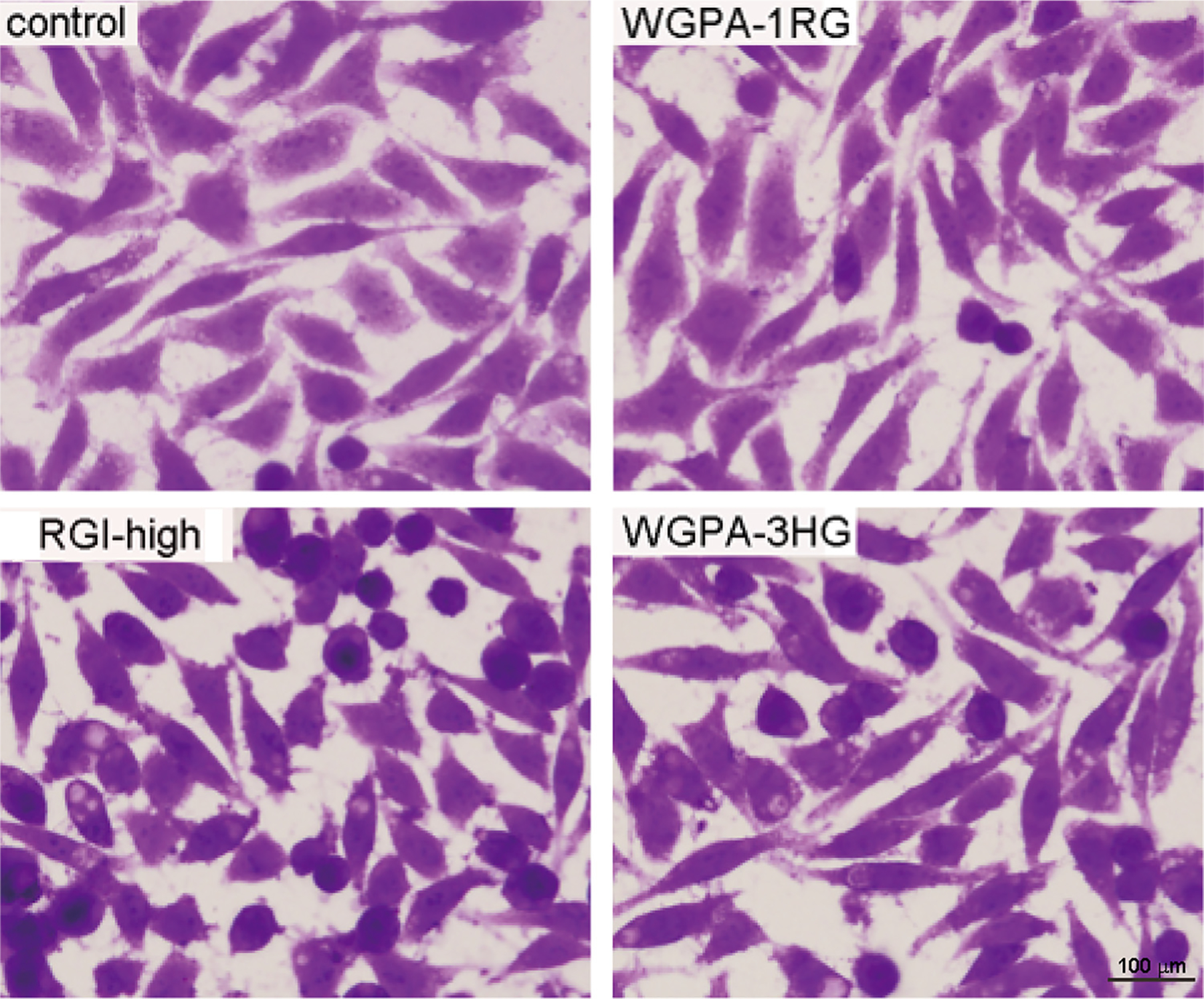
Pretreatment of cells by HG- and RGI-rich pectins altered cell morphology. L-929 cells treated without (control) or with 0.5 mg/ml WGPA-1RG, RGI-high, WGPA-3HG for 24 h were fixed, stained with crystal violet and visualized under microscope at 200× magnifications. This experiment was repeated twice. The representative images from total 32 fields under each condition were shown.
To gain more insight into the morphological changes, we examined actin filaments, as the organization of actin filaments was important to cell migration. Cells pretreated as described above were stained with Rhodamine-conjugated phalloidin and observed under an epi-fluorescence microscope. It should be noted that pectin treated cells were not easy to focus under high magnifications such as 1000× as shown in Fig. 6, which may result from the increased cell thickness due to poor spreading or from the lack of distinct focal section of actin filaments. Cell shapes outlined by actin filament staining were similar to those revealed by crystal violet staining. For example, control cells were large and well spread, and the majority of cells had polygonal appearances; RGI-rich pectin treated cells were smaller and poorly spread, and the majority of cells shaped like oval and short spindles; HG-rich pectin treated cells appeared slender, and many cells shaped like elongated spindles. However, there were not so many oval shaped cells in the images derived from actin filament staining as in the images derived from crystal violet staining. The possible reason was that oval shaped cells did not adhere firmly on the substratum and some of those were lost during the extensive washing steps that were required for fluorescence imaging studies.
Fig. 6.
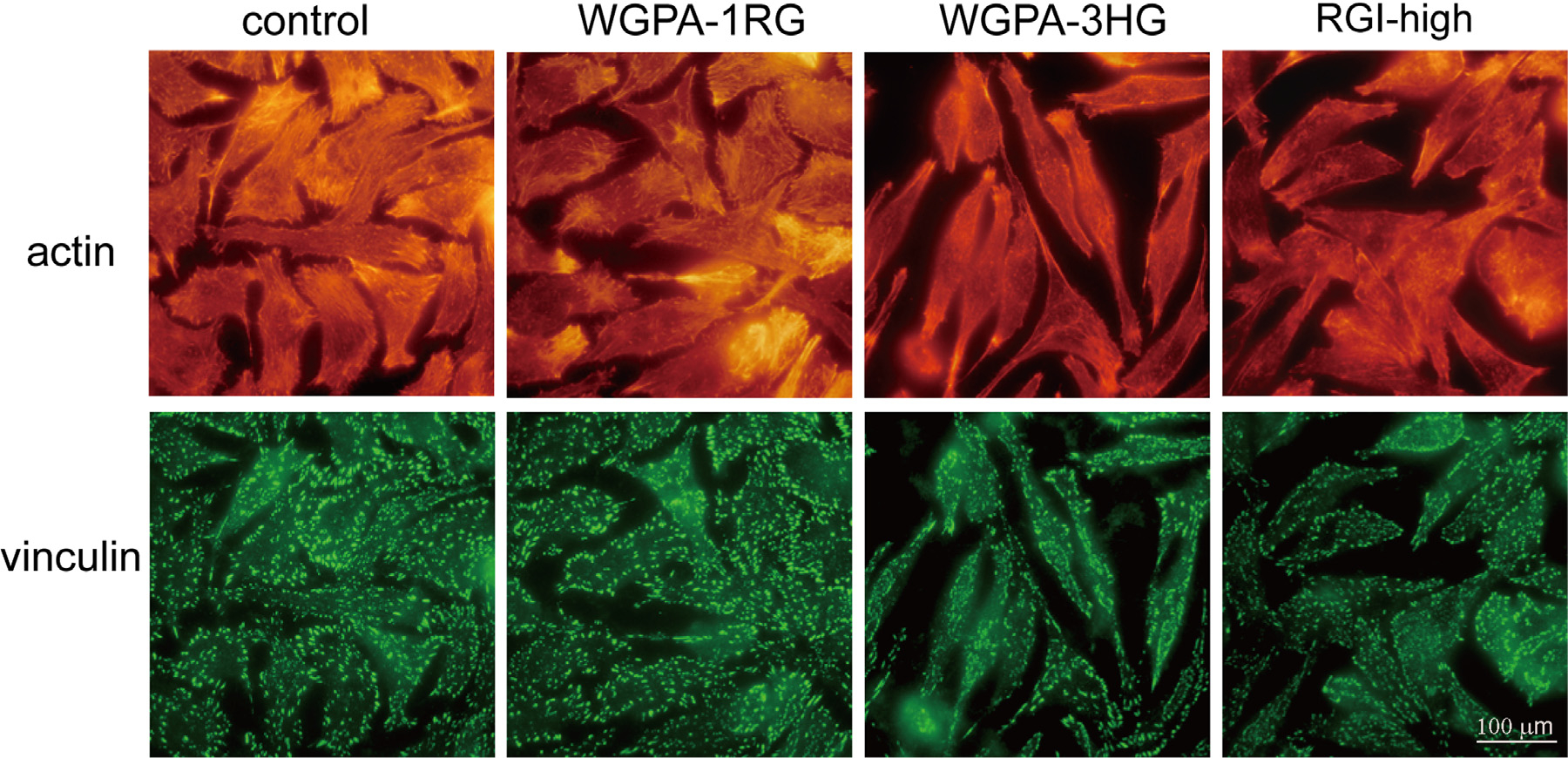
HG- and RGI-rich pectins altered actin filaments and focal adhesions. L-929 cells pretreated without (control) or with 0.5 mg/ml of WGPA-1RG, RGI-high, or WGPA-3HG for 24 h were processed for immunofluorescent imaging using vinculin antibody for focal adhesions (green) and Rhodamine-phalloidin for actin filaments (red). The representative images were from three experiments of 40 different fields under each condition.
Apart from the overall cell shapes, the organization of actin filament within the cells was altered by RGI-rich and HG-rich pectins as well (Fig. 6). Control cells had numerous thick and long stress fibers distributed in the entire cells and numerous membrane protrusions such as filopodia and lamellipodia at cell edges. In contrast, there were less stress fibers and filopodia and lamellipodia in the cells pretreated with RGI-rich pectins (RGI-high) and HG-rich pectins (WGPA-3HG). The edges of these cells were relatively smooth. Stress fibers and lamellipodia were only found near the poles of the spindles. Taken together, both RGI-rich and HG-rich pectins altered cell shapes and the organization of actin filaments. These alterations may relate to the inhibition of cell migration, as reorganization of actin filaments is involved in all four steps that accomplish cell migration.
As pretreatment severely altered cell morphologies, it is necessary to check whether these pectins have cytotoxic effects on L-929 cells. Thus, we determined cell viability using an MTT assay. Live cells can utilize MTT and produce formazan that gives rise to high absorbance at 570 nm after being solubilized. The assay showed that cells pretreated with 0.5 mg/ml of pectin for 24 h had similar absorbance to that of control cells. The absorbance was 1.93, 1.95 and 1.97 for the control, RGI-high and WGPA-3HG treated cells, respectively. In addition, pretreated cells maintained the ability of proliferation. Even when the pretreatment lasted for 48 h, the absorbance of these cells was still similar to that of control (data not shown). These data indicated that neither RGI-rich nor HG-rich pectins altered cell viability, at least under the conditions used in cell migration assays.
3.6. Pretreatment of RGI-rich pectins decreases cell adhesion to substratum
Cell adhesion plays a pivotal role in cell migration. It has been shown that medium adhesion gives rise to fast migration, while both weak and strong adhesion lead to retarded migration [18,19]. In our previous study, we deduced that HG-rich pectins inhibited cell migration via decreasing cell adhesion. Thus, we investigated whether the retardation of cell migration induced by RGI-rich pectins was related to cell adhesion impairment or enhancement. Cells pretreated with RGI-rich pectins (RGI-high) were subjected to vigorous rotation on an orbital shaker [12]. The speed of rotation was adjusted such that weakly adherent cells were released into the medium while firmly adherent cells remained on the plate. At the end of the rotation, both the cells in the medium and the cells on the plate were measured. The result obtained under one of such conditions, e.g., rotation at 250 rpm for 2 h at room temperature was presented in Fig. 7. The percentage of cells that remained on the plate was 40% for the control and 25% for RGI-high treated cells. These data implied that control cells adhered more strongly on the substratum than pectin-treated cells, indicating that pretreatment with RGI-rich pectins decreased cell adhesion. The decrease of cell adhesion by pectin treatment was also observed in morphological studies (Fig. 6). In control cells, there were numerous large and long focal adhesions oriented and associated with the thick and long stress fibers, features typical for firm adhesion to the substratum. Compared with the control, cells pretreated with HG-rich and RGI-rich pectins had fewer and smaller focal adhesions generally. Large focal adhesions were only sparsely observed near the poles of the spindles and along cell edges. These morphological data provided further evidence that HG- and RGI-rich pectins decreased cell adhesion. We propose that the decrease of cell adhesion contributes to their inhibition on cell migration.
Fig. 7.
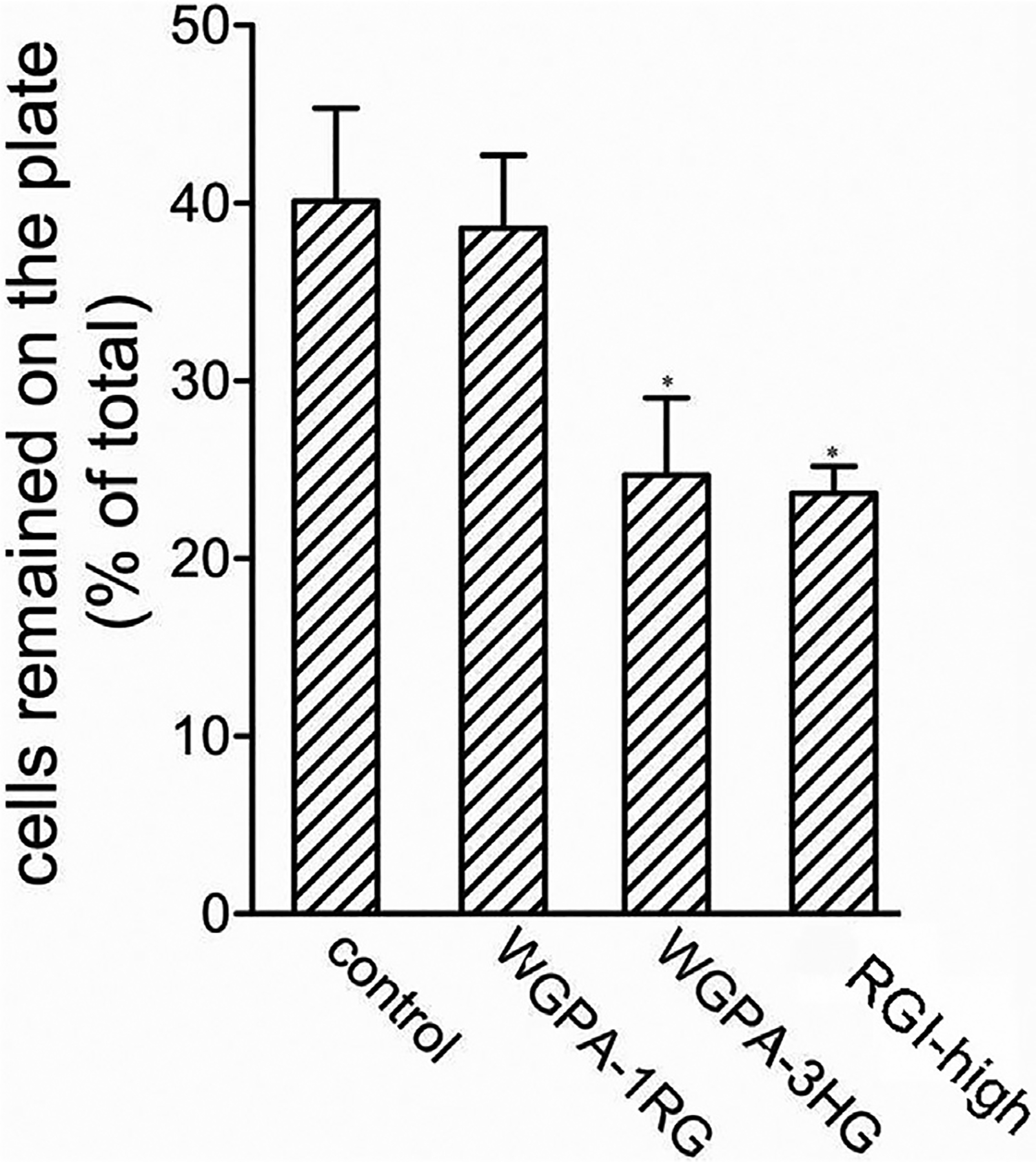
HG- and RGI-rich pectins decreased cell adhesion to substratum. Cells pretreated with 0.5 mg/ml of WGPA-1RG, WGPA-3HG or RGI-high for 24 h were washed with PBS and rotated at 250 rpm for 2 h. Both the released cells and the cells remained on the plate were determined. The percentage of cells remained on the plate under each condition was plotted. This experiment was repeated five times. Values were means ± SD, * p < 0.001.
3.7. Pretreatment RGI-rich pectins inhibits cell spreading
In the morphological studies, we observed that cells pretreated with HG- and RGI-rich pectins did not spread well. For example, they were not as large and flat as control cells; RGI pectin treated cells even appeared shrunk; they exhibited relatively smooth cell edges with fewer filopodia and lamellipodia. As cell spreading is an important process during cell migration, we investigated the effects of pectins on cell spreading in more detail. Cells were pretreated as described in cell migration assays with HG-rich pectins (WGPA-3HG), RGI-rich pectins (RGI-high), or WGPA-1RG as negative control. They were then detached and re-plated on glass cover slips and incubated for 1, 2, and 3 h for cell adhesion and spreading. After a gentle wash to remove non-adherent cells, the adherent cells were fixed and examined using a phase contrast microscope. The images representing the morphologies of the control and pectin-treated cells were showed in Fig. 8A. There were basically three morphologies under the microscope – non-spreading cells that were round with smooth rims, partially spread cells that were enlarged and flattened with small protrusions, and well-spread cells that exhibited polygon or fibroblastoid morphologies. In this study, both partially spread and well-spread cells were categorized and counted as spreading cells. The percentage of spreading cells under each condition was calculated and plotted (Fig. 8B). As shown in Fig. 7A and B, control cells spread quickly. Spreading cells accounted for 37%, 59%, and 68% of total adherent cells after 1, 2, and 3 h of incubation, respectively. HG- and RGI-rich pectin treatment significantly impaired cell spreading at these time points. Spreading cells accounted for only 2.4%, 28%, and 39% in the case of WGPA-3HG. The spreading of RGI-rich pectin treated cells was even slower, only 3.5%, 15% and 34%, respectively. In contrast, treatment with WGPA-1RG, which did not significantly inhibit cell migration [12], had no significant effect on cell spreading. These data indicated that HG-rich and RGI-rich pectins specifically reduced cell spreading on the substratum. Thus, the reduction of cell spreading may contribute to their inhibition in cell migration.
Fig. 8.
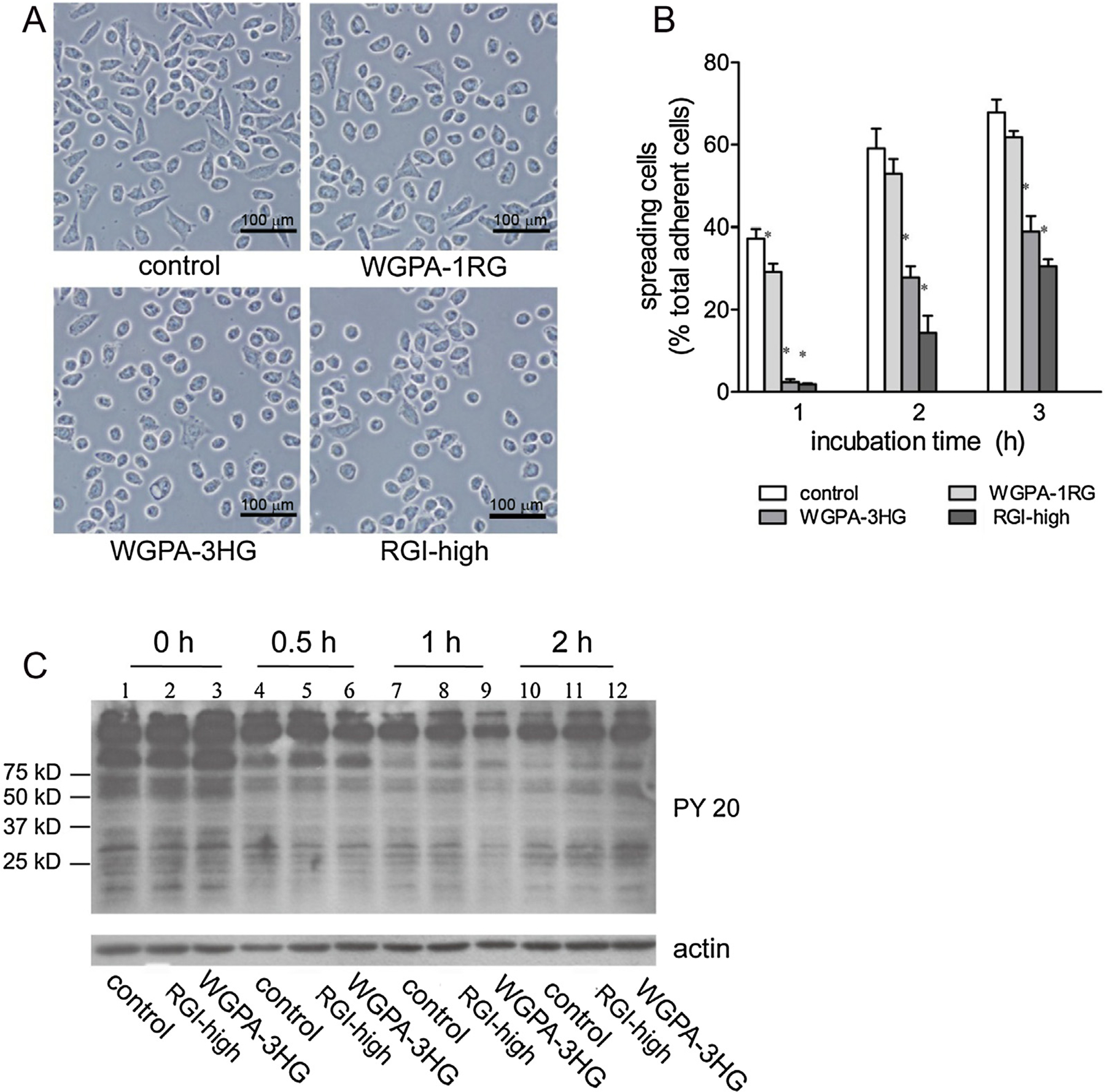
HG- and RGI-rich pectins inhibited cell spreading. (A) and (B) Cells pretreated without (control) or with 0.5 mg/ml of WGPA-1RG, WGPA-3HG or RGI-high were plated on glass coverslips and incubated for 1, 2 or 3 h as indicated. The adherent cells were measured by a phase contrast microscope. (A) Representative images of the control and pectin-treated cells after 2 h incubation. Bar, 100 μm. (B) Quantification of cell spreading. Values were means ± SD from three to four experiments in which 3000 (for 1 h) –10,000 (for 2 and 3 h) cells were scored under each condition. (C) The effects of HG- and RGI-rich pectins on protein tyrosine phosphorylation during cell spreading. Cells pretreated without (control) or with 0.5 mg/ml of WGPA-3HG or RGI-high for 24 h were plated on tissue culture plate and incubated for 0, 0.5, 1 or 2 h. The adherent cells were subjected to Western blotting analysis using phosphotyrosine antibody (PY20) and actin antibody (as loading control).
The effects of WGPA-3HG and RGI-high on cell spreading were also demonstrated by monitoring the changes of protein tyrosine phosphorylation during the course of cell spreading. Cells pretreated with WGPA-3HG or RGI-high were re-plated on tissue culture plates and incubated for 0 h, 0.5 h, 1 h or 2 h. The adherent cells were scrapped, lysed, and subjected to western blot analysis using PY20 antibody. As shown in Fig. 8C, the phosphorylation state of a number of proteins had changed upon cell attachment and spreading. Notably, among them was a protein at about 100 kDa, referred to as band 100. Because only adherent cells were examined at 0.5 h, 1 h, and 2 h time points, the different intensities of band 100 at these time points were related to different stages of cell spreading rather than cell attachment. As spreading proceeded, the intensity of band 100 declined quickly in the control (lane 4, 7, and 10), which implied that lower intensity corresponded to better spreading. Different from the control, the intensity of band 100 declined slowly in RGI-high (lane 5, 8, and 11) or WGPA-3HG (Fig. 7C, lane 6, 9, and 12) treated cells and remained higher than that of control for at least 2 h, which reflected the slower progression in cell spreading. These data supported our postulation that HG-rich pectin and RGI-rich pectin inhibited cell spreading.
3.8. The effects of HG- and RGI-rich pectins are not mediated by Gal-3
As some pectic polysaccharides or fragments interact with Gal-3 (Galectin-3) [20], pectins have been proposed to bind to and interfere with the functions of Gal-3 in various cellular processes [21]. Thus, we set out to determine whether HG-rich and RGI-rich pectins inhibited cell migration by interfering with the cellular functions of Gal-3. Firstly, we examined the expression of Gal-3 in L-929 cells by Western blotting assays using a monoclonal antibody (A3A12) that recognizes both human and mouse Gal-3. As shown in Fig. 9A, an intensive band corresponding to Gal-3 was observed in HeLa cells, while such band was not observed in L-929 cells. These data implied that L-929 cells express little, if any, Gal-3. Thus, it is unlikely that Gal-3 plays a major role in L-929 cell migration. To directly test the roles of Gal-3 in cell migration, we performed a lactose blocking assay, which has been widely used in functional studies on galectins including Gal-3. We showed that lactose at 5, 10, 20, and 40 mM (i.e. 1.8, 3.6, 5.4, 10.8 mg/ml, respectively) did not block the inhibition exerted by WGPA-3HG and RGI-high (Fig. 9B), which confirmed that Gal-3, or more precisely, the carbohydrate recognition domain of Gal-3, was not involved in the actions of HG- and RGI-rich pectins on cell migration.
Fig. 9.
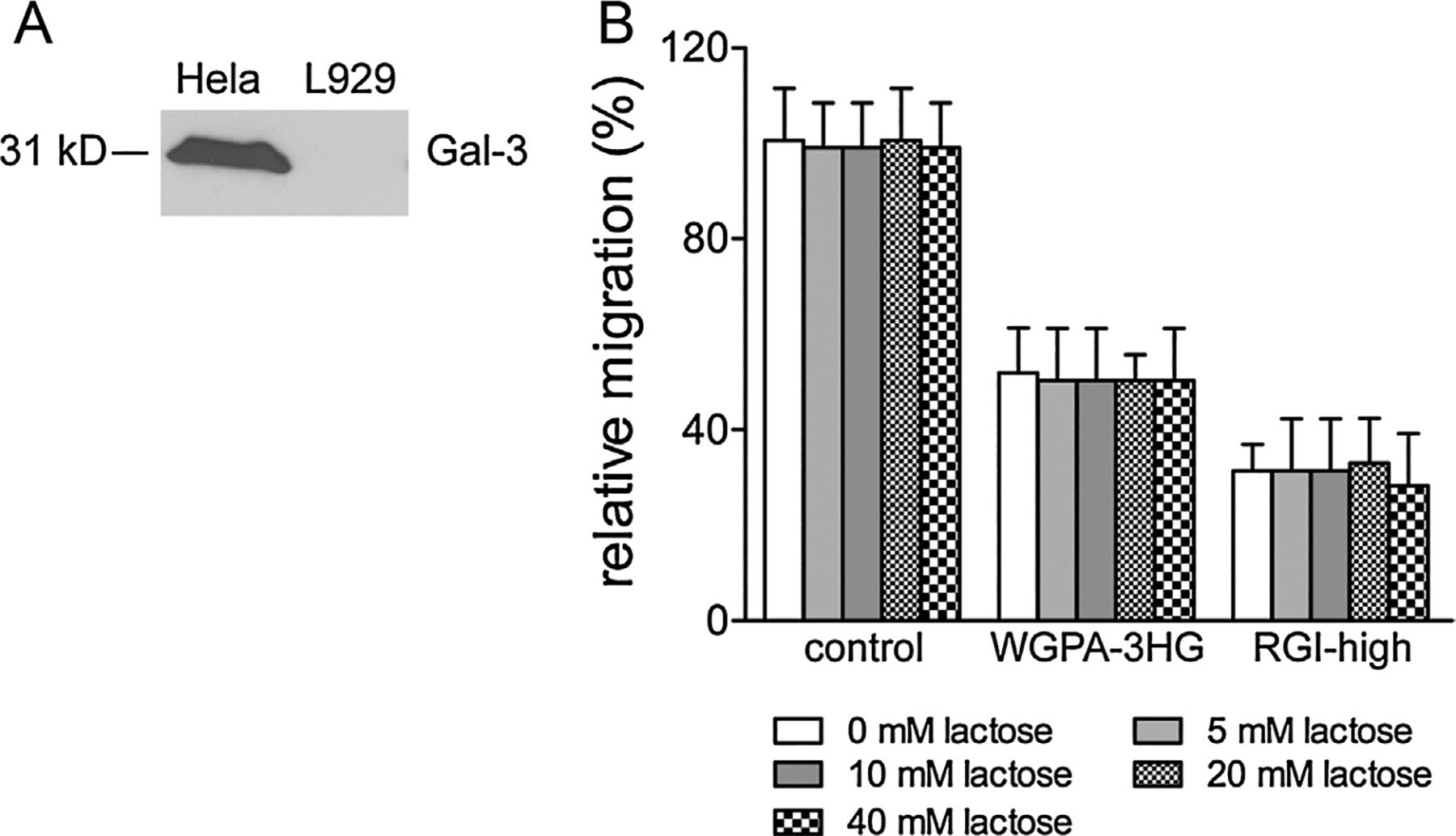
The effects of HG- and RGI-rich pectins were not mediated by Gal-3. (A) Gal-3 expression in L-929 cells. Total cell lysate derived from Hela (as positive control) and L-929 cells (1.25 × 106 cells per lane) were subjected to Western blotting analysis using Gal-3 antibody (A3A12). (B) Lactose did not interfere with the actions of HG- and RGI-rich pectins. Cells treated without (control) or with 0.5 mg/ml of WGPA-3HG or RGI-high in the presence or absence of increasing concentrations of lactose were tested by scratching assay. Values were means ± SD from three experiments.
4. Discussion
We started with ginseng pectin because its fractionation procedure has been well established which could provide structurally divergent pectin fractions for the study of structure and activity relationship [13]. We firstly examined total ginseng pectin on cell migration and found that ginseng pectin selectively and significantly impaired cell migration of some cell lines [12]. Then we examined all individual fractions derived from ginseng pectin to identify the active constituents [12]. After detailed structural correlation studies we demonstrated that both HG- and RGI-rich pectins could inhibit cell migration. Finally we investigated the mechanisms and proposed that both HG- and RGI-rich pectins might exert their effects by decreasing cell adhesion and cell spreading. We also provide evidence that Gal-3 was not involved in the targeting of HG- and RGI-rich pectins leading to cell migration inhibition.
4.1. RGI-rich pectins inhibited cell migration
To study the effects of RGI domains, we prepared two RGI samples with little HG regions from ginseng-sample RGI-high with relatively higher Rha content and sample RGI-low with relatively lower Rha content. Both RGI-high and RGI-low significantly inhibited cell migration, which confirmed that the RGI domains were structural elements that possessed anti-cell migration activities. In scratching assay, RGI-high showed significant effect from less than 0.031 mg/ml and 50% effect at 0.125 mg/ml (Fig. 2B and Table 2). Its maximum inhibition was about 70% in scratching assay and 75% in transwell assay (Table 2). In transwell assay, RGI-high was more effective than RGI-low, which was consistent with that in scratching assay. RGI domains are composed of repeating GalA-Rha disaccharide backbone which are frequently substituted by galactan and arabinogalactan branches. The lack of inhibition by WGPA-1RG that contained significant proportion of arabinogalactan suggested that the disaccharide backbone probably contributed to the effects [12]. Sample RGI-high and RGI-low had similar GalA/Rha ratio, but different contents of Rha, i.e. different contents of RGI backbone. The fact that RGI-high exerted stronger effects than RGI-low was in line with the speculation that RGI backbone might exert the inhibitory effect on cell migration. RGI-low had higher content of AG than RGI-high (Table 1), but RGI-low showed weaker inhibitory effect than RGI-high (Fig. 2), we speculated that AG-domain probably did not affect RGI pectin-induced inhibition of cell migration. This is consistent to our previous observation that WGPA-1RG (34.0% Ara and 56.2% Gal) and WGPA-2RG (40.9% Ara and 44.4% Gal) contained high content of AG, but they showed little effect on L-929 cell migration [12]. Therefore, we concluded that AG-domain had no obvious contribution to the RGI pectin-induced inhibition of cell migration.
Although HG- and RGI-rich pectins could inhibit cell migration, the mixture of them exerted much stronger effects than either HG- or RGI-rich pectin alone, suggesting that HG- and RGI-rich pectins act synergistically. The synergistic effect might explain the strong effect exerted by the un-fractionated ginseng pectin WGPA which contained both HG and RGI structures [12]. In transwell assay, the mixture consisted of RGI-high and WGPA-3HG at 1:1 ratio exerted 50% inhibition at 0.062–0.125 mg/ml, lower than either RGI-high or WGPA-3HG. The maximum inhibition of this mixture was 85%, again higher than either RGI-high or WGPA-3HG (Table 2).
4.2. The similarities and the differences between the actions of HG- and RGI-rich pectins
There were some similarities between the actions of HG- and RGI-rich pectins. For example, both types of pectins required pretreatment for maximum effects; both types inhibited cell adhesion and spreading; the actions of both types were irrelevant to Gal-3. However, there existed some differences. Firstly, their effective doses and maximum effects were different (Table 2). In general, both HG- and RGI-rich pectins displayed dose dependency (Fig. 1) [12]. The effect of RGI-rich pectin was only slightly higher than that of HG-rich pectin (Fig. 2). Secondly, HG- and RGI-rich pectins showed synergistic effects, suggesting that they acted on different molecules or by different pathways. Thirdly, pretreatment by HG- and RGI-rich pectins resulted in different cell morphologies. Cells treated by HG-rich pectin appeared slender, while cells treated by RGI-rich pectin appeared shrink. Fourthly, RGI-rich pectins exerted stronger effects on cell spreading than HG-rich pectins. Lastly, pretreatment is not sufficient for HG-rich pectins to exert their effects. They must exist during the period of migration, otherwise their effects were minimal. In the case of RGI-rich pectins, however, pretreatment was sufficient, their existence during the period of migration was not required. This phenomenon suggested that the modification resulted from HG-rich pectin pretreatment might be short-lived and needed to be sustained by continued supply of the same pectins, while the changes resulted from RGI-rich pectin treatment might be long lasting.
4.3. The possible mechanisms by which HG- and RGI-rich pectins inhibited cell migration HG- and RGI-rich pectins did not act simply as blocking agents during cell migration
Although inhibition of cell migration by other pectins has been reported previously [22–24], there is no clear indication of the importance of pretreatment. Our experiments unambiguously demonstrated that pretreatment is necessary for both HG- and RGI-rich pectins to exert their inhibitory effects. Therefore, the actions of HG- and RGI-rich pectins were different from those of growth factors and hyaluronan to which cells respond rapidly [25,26]. They were also different from the action mode of modified citrus pectin in Gal-3-induced chemotaxis in which endothelial cell migration was inhibited without pretreatment [22]. This discrepancy might result from different cell lines (endothelial cell versus fibroblast cell), different structure of pectins (pH-modified citrus pectin enriched in galactose residues versus purified native HG- and RGI-rich pectins), or different migration methods. Pretreatment of cells by pectins prior to migration assay was also reported by [23], who showed that multiple myeloma cells pretreated for 4 h were inhibited by a type of citrus pectin GCS-100 in vascular endothelial growth-factor-induced chemotaxis. Therefore, we investigated the role of pretreatment with HG- and RGI-rich pectins in cell migration. The prerequisite for pretreatment suggested that HG- and RGI-rich pectins did not act simply as a blocking agent during cell migration. They may, via interacting with lectin-like molecules on cell surface or in the ECM, regulate the expression, modification, localization, or turnover rate of some molecules relevant for cell migration. Indeed, pretreatment resulted in alterations in cell shapes, the organization of actin filaments, cell adhesion and spreading. These alterations might directly or indirectly interfere with cell migration.
4.4. HG- and RGI-rich pectins inhibited cell migration possibly via decreasing cell adhesion and cell spreading
Cell adhesion and cell spreading are important processes in cell migration. Many factors act on these processes and ultimately lead to the inhibition of cell migration. So we investigated if HG- and RGI-rich pectins changed cell adhesion and spreading. Morphological studies showed that the number and length of stress fibers, and the number and size of focal adhesions were both reduced. These changes correlated to reduced cell adhesions. Quantitative analysis showed that when subjected to certain rotating forces, more pectin treated cells than control cells were detached from the substratum, confirming the decrease of cell adhesion by pectin treatment. As optimum adhesion correlated to fast migration, the weaker adhesion caused by HG- and RGI-rich pectins might correlate to slow migration. However, the reduction of cell adhesion alone could not explain the reduction of cell migration for two reasons. One reason was that cell adhesion was reduced by about 15% while migration was reduced by 50–70%. The other reason was that cell adhesion was reduced to similar extent by HG- and RGI-rich pectins, but migration was reduced to higher extent by RGI- than HG-rich pectins. Thus, in addition to cell adhesion, other process such as cell spreading might also be involved. Morphological studies showed that pretreated cells were not as large and flat as control cells, especially RGI-rich pectin treated cells which appeared shrink. Besides, pretreated cells exhibited relatively smooth cell edges with less filopodia and lamellipodia than control cells. All these phenomena corresponded to bad spreading. Quantitative analysis showed that, when cells were re-plated on substratum, HG-rich pectin treated cells spread much more slowly than control cells, and RGI-rich pectin treated cells spread even more slowly than HG-rich pectin treated cells. Therefore, we concluded that the inhibition of cell spreading was a major cause of the inhibition of cell migration.
4.5. The targets of HG- and RGI-rich pectins were not Gal-3 molecules
What are the target molecules of HG- and RGI-rich pectins? This is a central question to the elucidation of glycan and cell interaction. One candidate for such targets was Gal-3, a type of galactose binding lectins. Due to its ability to bind some pectins, Gal-3 has been postulated to mediate the actions of pectins in various cellular processes [27]. We investigated if Gal-3 participated in HG- and RGI-rich pectin mediated migration inhibition. Our results excluded this possibility. Firstly, L-929 cells, the cells used in the migration assay, expressed little Gal-3, therefore it was unlikely that Gal-3 played a major part in these cells. Secondly, pectic galactan, which specifically bound Gal-3 [20], did not significantly inhibit cell migration [12]. Thirdly, lactose, a widely used inhibitor of Gal-3, could not block the action of HG- and RGI-rich pectins, indicating that these pectins worked on different molecules (or domains) from lactose. These evidences, together with our notion that ginseng pectin WGPA had no effect on Hela cells that expressed significant amount of Gal-3, supported the conclusion that Gal-3 or at least the carbohydrate recognition domain of Gal-3 was not involved in the targeting of HG- and RGI-rich pectins for their effects on cell migration. The finding that some activities of pectins were not related to Gal-3 was not unprecedented. Pectin induced apoptosis in prostate cancer cells was also found irrelevant to Gal-3 [28].
Apart from lectins/galectins that specifically recognize carbohydrates, other molecules that exhibit positively charged patches on cell surface or in the ECM may also be the targets, as both HG- and RGI-rich pectins are negatively charged molecules. However, we don’t think it is nonspecific ionic interactions that lead to cell migration inhibition, as the negatively charged monosaccharide GalA, which constituted the HG domain, did not inhibit cell migration. In the case of RGI-rich pectins, RGI-high and RGI-low which contained 33.8% and 16.9% of GalA, respectively, exhibited similar or stronger effects than WGPA-3HG which contained over 90% GalA and low extent of methyl-esterification [13]. Therefore, the interactions between these pectins, especially the RGI-rich pectins, and the cells (or the ECM) were specific. The primary structure or conformation of these pectins should be related to their specific interaction.
To what molecules do HG- and RGI-pectins bind or associate? How does the binding/association influence the organization of cytoskeleton and cell adhesion and spreading? These issues are fundamental to understanding pectin-relevant migration and to understanding cell and glycan interaction in general. Ongoing studies in our laboratory are focused on searching for the target molecule(s) and on detailed study of the structure-activity relationship of these pectins in order to elucidate the molecular mechanisms underlying the effects of HG- and RGI-rich pectins on cell migration. Pectins have been implicated in various bioactivities. Studies on their structure and activity relationship will certainly facilitate their better application.
Supplementary Material
Funding
This work was supported by National Natural Science Foundation of China (Grant Nos. 31300287 and 31470798) and Fundamental Research Funds for the Central Universities (Grant Nos. 2412016KJ044 and 2412017FZ018).
Abbreviations:
- ECM
extracellular matrix
- PMP
1-phenyl-3-methyl-5-pyrazolone
- pretreatment medium
culture medium plus various carbohydrate samples
- Gal-3
galectin-3
- HG
homogalacturonan
- RGI
rhamnogalacturonan I
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijbiomac.2017.08.004.
References
- [1].Friedl P, Gilmour D, Collective cell migration in morphogenesis, regeneration and cancer, Nat. Rev. Mol. Cell. Biol 10 (2009) 445–457. [DOI] [PubMed] [Google Scholar]
- [2].Evje S, An integrative multiphase model for cancer cell migration under influence of physical cues from the microenvironment, Chem. Eng. Sci 165 (2017) 240–259. [Google Scholar]
- [3].Vicente-Manzanares M, Webb DJ, Horwitz AR, Cell migration at a glance, J. Cell Sci 118 (2005) 4917–4919. [DOI] [PubMed] [Google Scholar]
- [4].Crotti S, Piccolo M, Rizzolio F, Giordano A, Nitti D, Agostini M, Extracellular matrix and colorectal cancer how surrounding microenvironment affects cancer cell behavior? J. Cell. Physiol 232 (2017) 967–975. [DOI] [PubMed] [Google Scholar]
- [5].Frangogiannis NG, The extracellular matrix in myocardial injury repair, and remodeling, J. Clin. Invest 127 (2017) 1600–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Toole BP, Hyaluronan promotes the malignant phenotype, Glycobiology 12 (2002) 37R–42R. [DOI] [PubMed] [Google Scholar]
- [7].Evanko SP, Tammi MI, Tammi RH, Wight TN, Hyaluronan-dependent pericellular matrix, Adv. Drug Deliv. Rev 59 (2007) 1351–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Steinar E, An integrative multiphase model for cancer cell migration under influence of physical cues from the microenvironment, Chem. Eng. Sci 165 (2017) 240–259. [Google Scholar]
- [9].Noreen A, Nazli ZIH, Akram J, Rasul I, Mansha A, Yaqoob N, Iqbal R, Tabasum S, Zuber M, Zia KM, Pectins functionalized biomaterials; a new viable approach for biomedical applications: a review, Int. J. Biol. Macromol 101 (2017) 254–272. [DOI] [PubMed] [Google Scholar]
- [10].Inngjerdingen KT, Patel TR, Chen X, Kenne L, Allen S, Morris GA, Harding SE, Matsumoto T, Diallo D, Yamada H, Michaelsen TE, Inngjerdingen M, Paulsen BS, Immunological and structural properties of a pectic polymer from Glinus oppositifolius, Glycobiology 17 (2007) 1299–1310. [DOI] [PubMed] [Google Scholar]
- [11].Inngjerdingen KT, Debes SC, Inngjerdingen M, Hokputsa S, Harding SE, Rolstad B, Michaelsen TE, Diallo D, Paulsen BS, Bioactive pectic polysaccharides from Glinus oppositifolius (L) Aug. DC. a Malian medicinal plant, isolation and partial characterization, J. Ethnopharmacol 101 (2005) 204–214. [DOI] [PubMed] [Google Scholar]
- [12].Fan Y, Cheng H, Li S, Zhang X, Wang J, Liu D, Hao M, Gao X, Fan E, Tai G, Zhou Y, Relationship of the inhibition of cell migration with the structure of ginseng pectic polysaccharides, Carbohyd. Polym 81 (2010) 340–347. [Google Scholar]
- [13].Zhang Xu, Yu Li, Bi Hongtao, Li Xianhua, Ni Weihua, Han Han, Li Nan, Wang Bingqing, Zhou Yifa, Tai Guihua, Total fractionation and characterization of the water-soluble polysaccharides isolated from Panax ginseng C.A. Meyer, Carbohyd. Polym 77 (2009) 544–552. [Google Scholar]
- [14].Yu L, Zhang Y, Liu A, Liu X, Sin L, Liu H, Iteku J, Zhou Y, Tai G, Rhamnogalacturonan I domains from ginseng pectin, Carbohyd. Polym 79 (2010) 811–817. [Google Scholar]
- [15].Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F, Colorimetric method for determination of sugars and related substances, Anal. Chem 28 (1956) 350–356. [Google Scholar]
- [16].Filisetti-Cozzi TMCC, Carpita NC, Measurement of uronic acid without interference from neutral sugars, Anal. Biochem 197 (1991) 157–162. [DOI] [PubMed] [Google Scholar]
- [17].Honda S, Akao E, Suzuki S, Okuda M, Kakehi K, Nakamura J, High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-Phenyl-3-methyl-5-pyrazolone derivatives, Anal. Biochem 180 (1989) 351–357. [DOI] [PubMed] [Google Scholar]
- [18].Gupton S, Waterman-storer CM, Spatiotemoral feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration, Cell 125 (2006) 1361–1374. [DOI] [PubMed] [Google Scholar]
- [19].Hang Q, Isaji T, Hou S, Wang Y, Fukuda T, Gu J, A key regulator of cell adhesion: identification and characterization of important N-glycosylation sites on integrin α5 for cell migration, Mol. Cell. Biol 37 (2017) e00558–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gunning AP, Bongaerts RJM, Morries VJ, Recognition of galactan components of pectin by galectin-3, FASEB J 23 (2008) 415–424. [DOI] [PubMed] [Google Scholar]
- [21].Zhao J, Zhang F, Liu X, Ange KS, Zhang A, Li Q, Linhardt RJ, Isolation of a lectin binding rhamnogalacturonan-I containing pectic polysaccharide from pumpkin, Carbohyd. Polym 163 (2017) 330–336. [DOI] [PubMed] [Google Scholar]
- [22].Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, Raz A, Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin, J. Natl. Cancer Inst 94 (2002) 1854–1862. [DOI] [PubMed] [Google Scholar]
- [23].Chauhan D, Li G, Podar K, Hideshima T, Neri P, He D, Mitsiades N, Richardson P, Chang Y, Schindler J, Carver B, Anderson KC, A novel carbohydrate-based therapeutic GCS-100 overcomes bortezomib resistance and enhances dexamethasone-induced apoptosis in multiple myeloma cells, Cancer Res 65 (2005) 8350–8358. [DOI] [PubMed] [Google Scholar]
- [24].Sathisha UV, Jayaram S, Nayaka MAH, Dharmesh SM, Inhibition of galectin-3 mediated cellular interactions by polysaccharides from dietary sources, Glycoconj. J 24 (2007) 497–507. [DOI] [PubMed] [Google Scholar]
- [25].Ridley AJ, Hall A, The small Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors, Cell 70 (1992) 389–399. [DOI] [PubMed] [Google Scholar]
- [26].Oliferenko S, Kaverina I, Small JV, Huber LA, Hyaluronic acid (HA) binding to CD44 activates Rac1 and induces lamellipodia outgrowth, J. Cell Biol 148 (2000) 1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang T, Zheng Y, Zhao D, Yan J, Sun C, Zhou Y, Tai G, Multiple approaches to assess pectin binding to galectin-3, Int. J. Biol. Macromol 91 (2016) 994–1001. [DOI] [PubMed] [Google Scholar]
- [28].Jackson CL, Dreaden TM, Theobald LK, Tran NM, Beal TL, Eid M, Gao MY, Shirley RB, Stoffel MT, Kumar MV, Mohnen D, Pectin induces apoptosis in human prostate cancer cells: correlation of apoptotic function with pectin structure, Glycobiology 17 (2007) 805–819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


