Abstract
Purpose:
To evaluate the efficacy and safety of a hinged pupil expansion device (PED) in eyes with small pupils undergoing phacoemulsification.
Methods:
In this prospective, multicenter, interventional case series of 57 eyes with suboptimal pharmacologic pupil dilation (<5 mm diameter), a hinged PED (I-Ring, Beaver-Visitec International, Waltham, MA) was applied to facilitate surgical visualization during cataract surgery. The pupil diameters (PD) were measured at different stages of the procedure and at the 1-month follow-up visit. Rate of successful intraoperative PED deployment, pupil size, and shape were assessed.
Results:
The mean patient age was 70.5 ± 12.1 years. The I-Ring PED was successfully applied in all eyes. The mean PD at various stages were 4.1 ± 1.1 mm (dilation with eye drops only preoperatively), 4.3 ± 1.1 mm (dilation after intracameral epinephrine and ophthalmic viscoelastic device), 6.80 ± 0.00 mm (with PED applied), and 5.7 ± 1.1 mm (end of surgery). A statistically significant difference (P < 0.001) was observed between the mean PD with intracameral medications and with PED application. Postoperative circular pupil was observed in 54 of 57 eyes (94.7%) and the mean eccentricity index (n = 57 eyes) was 0.11 ± 0.22. No significant adverse events were observed.
Conclusion:
The I-Ring PED safely and effectively provided and maintained adequate pupil expansion and surgical visualization in eyes with small pupils undergoing cataract surgery. Postoperatively 95% of eyes attained circular pupils. This hinged PED is an additional instrumentation option for the safe and effective expansion of inadequately sized pupils during cataract surgery.
Keywords: Hinged pupil expansion device, I-ring pupil expander, pupil expansion device, small pupil cataract surgery
Phacoemulsification cataract surgery (Phaco) through a small pupil is technically challenging. Because of limited visualization and manipulation space, Phaco in the presence of a small pupil is prone to adverse intraoperative events such as iris sphincter damage, iris prolapse, bleeding, zonular/capsular damage, incomplete cataract removal, and suboptimal intraocular lens (IOL) placement, as well as postoperative complications such as iritis, intraocular pressure rise, visual disturbances from iris defects, and cosmetic concerns.[1,2,3,4,5,6,7,8,9,10,11,12,13]
Numerous pharmacologic[1,4,14,15,16,17] and mechanical[18,19,20,21,22,23,24,25,26] strategies have been developed to increase pupil size and optimize surgical visualization. Pupil expansion devices (PEDs) such as iris hooks and the Malyugin ring (MST, Seattle, WA) are innovative solutions for controlling intraoperative pupil size. While iris hooks typically lead to postoperative pupil distortion because of the limited points of iris contact, newer PEDs provide increased points of pupillary margin fixation (e.g., Malyugin ring provides 8 points of contact) to provide uniform pupil dilation, resulting in a better intraoperative visualization as well as less postoperative pupil distortion.
The I-Ring Pupil Expander (Beaver-Visitec International, Inc., Waltham, MA) is a novel, single-use, hinged, polypropylene PED which is designed to engage with the iris for 360° while providing a uniform, circular field of view of 6.8 mm in diameter. The device has positioning holes to ensure that instruments (typically Sinskey hook) used during iris engagement and removal do not damage the iris. The present study aims to determine the efficacy and safety of the I-Ring PED when applied during Phaco to eyes with pupils of inadequate size.
Methods
In this prospective, multicenter, interventional, non-comparative, consecutive case series, 59 patients (62 eyes) undergoing cataract surgery and IOL implantation at the Peregrine Eye and Laser Institute were recruited. Primary inclusion criteria included eyes undergoing cataract removal that required application of a PED. Excluded were eyes with previous history of iris trauma, iris surgery, or iris laser treatment (i.e., peripheral iridotomy, laser pupilloplasty), prior use of PED or medications that may influence pupil size (i.e., pilocarpine, amphetamine), zonular weakness, and severely shallow anterior chamber depth (<2.0 mm as measured by optical biometry). In addition, eyes that required concomitant ocular surgical procedures (i.e., pars plana vitrectomy, glaucoma surgery, etc.) in addition to Phaco were likewise excluded.
Comprehensive eye examination was performed in the clinic. Eyes with surgical cataracts and non-dilating pupils (<5 mm) were evaluated for preoperative inclusion/exclusion criteria. Eyes that met the study criteria proceeded to surgery. Intraoperatively, the eyes were enrolled into the study after failure to achieve pupil dilation of 5 mm or more with pharmacologic and mechanical maneuvers such as administration of topical and intracameral mydriatic agents, viscodilation, membranectomy, and/or synechiolysis. Of 62 screened eyes (59 patients), 57 eyes (54 patients) subsequently required the application of I-Ring Pupil Expander (Beaver-Visitec International, Inc., Waltham, MA) and were enrolled into the study.
Informed consent was taken from all subjects after explaining the risks and benefits of the surgery. The Institutional Review Board at Peregrine Eye and Laser Institute approved this study and it was conducted in accordance with the tenets of the Declaration of Helsinki.
Surgical technique
All Phaco procedures were performed by two surgeons using the temporal approach. Anesthesia was attained with topical proparacaine HCL 0.5% (Alcaine, Alcon Laboratories, Ft Worth, TX) or retrobulbar injection of 2% xylocaine. Preoperatively, phenylephrine HCl 5% plus tropicamide 0.5% (Sanmyd-P, Santen Inc., Osaka, Japan) was instilled every 5 min for 15 min into the operated eye and if at 30 min following start of dilation, pupil diameter remained at less than 5 mm, additional drops of 10% phenylephrine were applied.
Epinephrine 0.025%, lidocaine 0.75% and sodium chondroitin sulphate/sodium hyaluronate (Viscoat, Alcon Surgical, Ft Worth, TX) were sequentially injected into the anterior chamber (AC) through a paracentesis incision. A 2.4 mm temporal clear corneal incision (CCI) was created. Apparent posterior synechiae were released from the anterior lens capsule using capsulorrhexis forceps and various iris manipulating instruments. The AC was then additionally reformed with OVD and pupil diameter (PD) was measured to the nearest 0.5 mm using Castroviejo surgical calipers. For eyes with PD remaining less than 5.0 mm, the I-Ring was applied.
Surgical technique: I-Ring application and removal
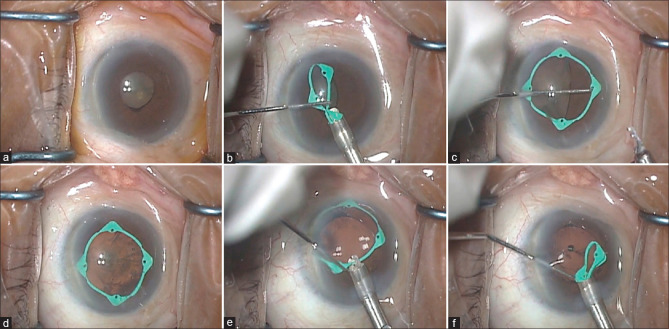
The slider on the inserter device was retracted until the preloaded I-Ring was entirely drawn into the inserter. The tip of the inserter was introduced into the AC via the CCI. The slider was then slowly advanced, delivering the I-Ring completely into the AC. The inserter was then removed and a Sinskey hook was used to manipulate the I-Ring to engage its four channels upon the pupil borders, thereby resulting in iris retraction and pupil expansion [Fig. 1a-d]. Phaco and IOL insertion were then performed, whereupon either the Sinskey hook or the prong of the I-Ring inserter was used to disengage the I-Ring from the iris margin. Once freed, the I-Ring was then withdrawn into the inserter and removed from the AC [Fig. 1e and f]. Removal of residual OVD and wound hydration were followed by intracameral and topical antibiotics. Typical postoperative regimen included topical fluoroquinolone, prednisone acetate 1%, and a non-steroidal anti-inflammatory drug in tapering doses for one month.
Figure 1.
Surgical microscope view of insertion and removal of hinged pupil expansion device (PED) in right eye of a patient. (a) Non-dilating pupil of approximately 4 mm in diameter. (b) Insertion of hinged PED into anterior chamber using its single-use injector/manipulator. (c) PED with three of four channels already capturing the temporal, superior, and nasal pupil edges. A Sinskey hook is being used to manipulate the final channel to capture the inferior pupil edge. (d) The fully deployed, centrally positioned, PED provides an enhanced intraoperative view of the cataract. (e) The injector prong grasping the proximal hinge portion of the PED in preparation for removal. (f) As the inserter is retracted, the PED separates readily from the iris edge and is withdrawn into the injector
Study outcome measures
The primary outcome measure was the proportion of eyes with successful deployment of the I-Ring. Deployment was considered successful if all four channels were attached to the pupil edges and remained stable and engaged throughout the surgery. Secondary outcome measures included (a) PD in the various stages of the Phaco procedure and at the 1-month follow-up visit, (b) postoperative pupil shape and irregularity, and (c) frequency of adverse events. PD was measured using surgical calipers at 6 pre-defined time-points: (1) preoperative baseline, (2) after maximal preoperative dilation with topical mydriatics, (3) after intracameral dilation with epinephrine and OVD, (4) following application of the I-Ring, (5) at the end of the surgery, following withdrawal of the I-Ring, and (6) at 1 month postoperative visit.
Pupil shape was qualitatively and quantitatively assessed. Quantitative assessment was carried out by measuring the longest and shortest horizontal axes after I-Ring removal and computing for the eccentricity index (ε) [Fig. 2] as determined by:
Figure 2.
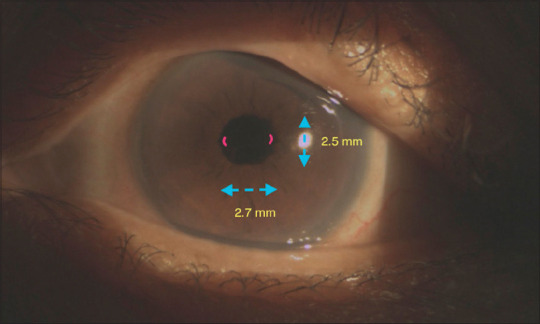
Slit-lamp photograph of postoperative eye with mild ovalization. The horizontal diameter (dashed line) is 2.7 mm while the vertical diameter is 2.5 mm resulting in an eccentricity index of 0.38. The two irregular areas (red solid curved lines) measure 4 degrees of arc translating to 1.1% irregularity
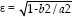
Where a is the radius of the semi-major axis and b is the radius of the semi-minor axis. An e of 0 denotes a perfectly circular pupil, while e equal to 1.0 denotes highest degree of eccentricity. Lastly, degree of pupil irregularity was assessed by estimating the percentage of pupil irregularity using photographs. The formula for irregularity (Irr) was:
% Irr = degrees of pupil irregularity/360 * 100
Statistical analysis
Data was encoded in Microsoft Excel version 14.4.7. Statistical analysis was performed using SPSS version 17.0. Descriptive statistics (mean, median, standard deviation) were used for continuous variables, while proportions and percentages were used to report discrete variables. Normality of data samples was evaluated by means of the Shapiro-Wilk test and Q-Q plots. If the data were normally distributed, the Student’s t-test for paired data was used for comparisons of pupil diameters at different time-points (preoperatively, after topical dilation, intracameral dilation, and postoperatively). If the data were not normally distributed, Wilcoxon rank-sum test was applied. For comparison of pupil diameter before and after I-Ring insertion, one-sample Wilcoxon signed rank test was applied. For all statistical tests, a P value of < 0.05 was considered statistically significant.
Results
Fifty-seven (57) eyes of 54 patients were included in the analysis. Patient demographic data and probable risk factors for the poorly dilating pupil are summarized in Table 1. The pupil diameters at the 5 pre-defined study time-points are illustrated in Table 2. The baseline mean pupil diameter was 3.1 ± 1.1 mm. After the instillation of standard topical mydriatic agents, the mean pupil diameter increased to 4.1 ± 1.1 mm. This average increase in pupil diameter of 1.0 mm was statistically significant (P < 0.001). Following intracameral administration of epinephrine, mean pupil diameter increased to 4.3 ± 1.1 mm. Again, a statistically significant difference was noted between the mean pupil diameters after topical mydriatics and intracameral epinephrine (P < 0.001). With the application of I-Ring, the mean PD further expanded to 6.8 mm which was statistically significant from the previous measurement (P < 0.001). Lastly, mean PD at the end of the surgery and at 1-month postoperative visits were 5.7 ± 1.16 and 3.6 ± 0.78 mm, respectively. Comparing baseline PD from PD at 1-month follow-up, a significant increase of 0.5 mm was noted (P < 0.001).
Table 1.
Patient demographics and probable risk factors for poorly dilating pupil
| Patient Characteristics (N=54) | Frequency |
|---|---|
| Age (years) (Mean±SD) | 70.5±12.1 |
| Gender, n (%) | |
| Male | 34 (60%) |
| Female | 23 (40%) |
| Race, n (%) | |
| Asian | 27 (47%) |
| Caucasian | 25 (44%) |
| African-American | 5 (9%) |
| Concomitant risk factors for poorly dilating pupil n (%) | |
| Intraoperative floppy iris syndrome | 17 (30%) |
| Diabetes mellitus | 9 (16%) |
| Uveitis | 9 (16%) |
| Pseudoexfoliation syndrome | 7 (12%) |
| Previous ocular surgery | 7 (12%) |
| Idiopathic or age-related | 6 (11%) |
| Previous ocular trauma | 2 (3%) |
Table 2.
Pupil diameters at predefined study time points
| Time Points | Baseline | After topical mydriatics | After intracameral mydriatics | With I-Ring application | At the end of surgery | Postoperative 1-month |
|---|---|---|---|---|---|---|
| Mean (SD) | 3.1 (1.1) | 4.1 (1.2) | 4.3 (1.1) | 6.8 (0.0) | 5.7 (1.2) | 3.6 (0.8) |
| Median (IQR) | 3.0 (2.1-4) | 4.5 (3.5-5.0) | 4.5 (3.5-5.0) | 6.8 (6.8-6.8) | 5.7 (4.5-7.0) | 3.5 (3.0-4.0) |
SD: Standard deviation; IQR: Interquartile range
Application of the I-Ring was successful in all eyes (57/57, 100%). The I-Ring remained engaged and stable throughout the surgery. Pupil shape and irregularity were also assessed at the conclusion of surgery and the mean eccentricity index was determined to be 0.11 ± 0.22. At least some pupil irregularity was observed in 15 of 57 eyes (26%). Overall (n = 57), the mean irregularity of all eyes was 4.3 ± 9.0%.
With respect to safety, the only significant adverse event encountered was a single case of zonular dehiscence which was unrelated to I-Ring application.
Discussion
The success of cataract surgery, the most frequently performed intraocular surgical procedure, depends on several factors including surgeon skill, adequacy of surgical instrumentation, cataract density, and degree of surgical visualization. By limiting the surgeon’s field of view of the lens, small pupils increase the likelihood of intraoperative and postoperative complications. There is no standard definition in the literature for what constitutes a small pupil. Most commonly pupil diameter less than 5 mm is considered as small[22,27,28]; however, there is a study that considered pupil diameter of 4 mm or less as small pupil.[6]
Numerous risk factors for small pupils have been reported in literature including: pseudoexfoliation syndrome, uveitis, diabetes mellitus, ocular trauma, prior ocular surgery, prior femtosecond laser treatment, and use of certain pharmacologic agents (e.g. systemic alpha-adrenergic antagonists, pilocarpine, or carbachol).[29] The patient population in the present study includes a wide sampling of these risk factors.
Efforts to mechanically enlarge pupil size among eyes with insufficient response to pharmacologic agents and mechanical stretching have demonstrated improved surgical visualization and reduced intraoperative complications.[21,30] In our practice, we have devised an algorithm to systematically approach the small pupil problem as follows [Fig. 3]: First, standard topical and intracameral pharmacologic dilation and viscomydriasis are used. If these are inadequate to achieve adequate pupillary dilation, the iris is carefully examined to look for any irido-lenticular adhesions or membranes, and if needed, synechiolysis or membranectomy is performed. If pupil still fails to dilate after a second attempt of intracameral pharmacologic dilation, then a PED is applied and surgery is performed.
Figure 3.
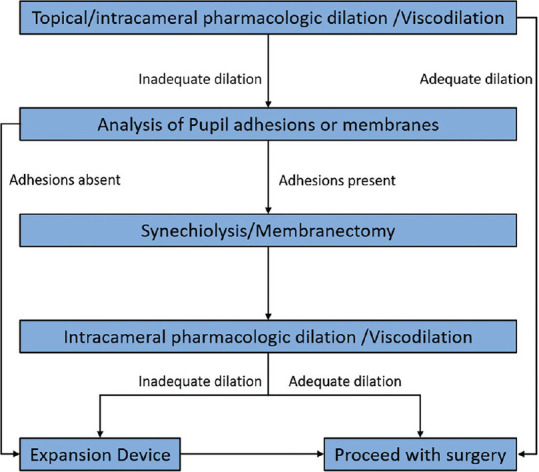
Peregrine Eye and Laser Institute Institute Small Pupil Algorithm
For the past 2 decades, PEDs have become important components of the surgical tool kit for small pupil cataract surgery, especially as alpha-adrenergic antagonist pharmaceuticals (e.g. tamsulosin for urinary retention) have become more prevalent and cause not only inadequate pupillary dilation but also increased iris flaccidity, termed the intraoperative floppy iris syndrome (IFIS). Consequently, multiple PEDs are currently available including: 5S Iris Ring (Morcher, GmBH, Stuttgart, Germany), Perfect Pupil (Milvella Inc. Eden Prairie, MN), Graether Expander (Eagle Vision Inc., Memphis, TN), Malyugin Ring (MicroSurgical Technology, Redmond, WA), APX 200 (APX Ophthalmology), Canabrava Ring (CR; AJL Ophthalmic SA, Spain), Bhattacharjee B-HEX Pupil Expander (Med-Invent Devices, Kolkata, India), as well as the I-Ring (Beaver-Visitec International, Waltham, MA, USA).
Relative to the use of multiple solitary iris hook retractors, PEDs have the advantage of achieving larger and more consistent PD, broader support of flaccid iris tissue (as in IFIS), sustained pupil dilatation, faster application, ease of use, insertion through the primary limbal incision rather than multiple additional paracenteses, and protection of iris sphincter from surgical trauma.
Specifically concerning the I-Ring, the learning curve for its insertion, deployment, and removal is brief, as these steps are based on standard anterior segment surgical manipulations. Beyond the scope of the present study, we have utilized the I-Ring on multiple occasions with resident surgeons having neither prior experience nor even exposure to the device, and they have invariably adapted to its use without difficulty. Unlike iris hooks and some other PEDs, the I-Ring can be inserted and removed through the same CCI without creation of any additional incisions. With minimal experience, deployment of the I-Ring is usually completed within 1 minute, and removal is also expedient as only a single channel needs to be disengaged from the iris in order to allow the inserter prong to engage the I-Ring for withdrawal into the inserter cartridge. As such, the total increase in surgical time is minimal.
In this consecutive case series involving 57 eyes with small pupils of various etiologies, successful insertion and positioning of the I-Ring was achieved in all eyes and was accomplished without complication, such as iris sphincter tears. The relatively flexible polyurethane material of the I-Ring seems less likely to damage the iris tissue during ring engagement or removal. Following deployment, sufficient visualization through a pupil diameter of 6.8 mm was maintained throughout surgery, and no additional maneuvers were required to obtain an adequate surgical field of view.
Postoperatively, the I-Ring was not observed to have caused significant iris distortion, as pupil shape after surgery remained round in nearly all cases, with mean eccentric index approaching that of the perfectly circular pupil. In a case report by Tian et al.,[25] small-pupil cataract surgery with the I-Ring resulted in less pupil distortion than when a Malyugin ring was used in the fellow eye of the same patient. Pupil size after surgery was also close to preoperative baseline pupil size, averaging only a 0.5 mm enlargement. Inability of the pupil to return to its preoperative size has been observed after use of PEDs, as other studies utilizing various other PEDs documented postoperative pupil size increases of 0.64 to 1.1 mm. Excessive postoperative pupil size is undesirable, leading to glare and negative dysphotopsias.[10,19]
An innovative PED is the B-HEX Pupil Expansion Ring (Med-Invent Devices, Kolkata, India) developed by Dr. Suven Bhattacharjee.[31] Made of 5-0 monofilament polyamide (Nylon), the B-Hex has a thin planar (0.075 mm) profile allowing insertion through a 1.0 mm or wider incision and contains notches and flanges that are used to fixate the pupillary margin to create a 5.5 mm expanded pupil. The B-HEX is preloaded onto a carrier platform that is situated at the main wound entrance. A Sinskey hook manipulator or 23-gauge DSEK forceps is then used to maneuver the device into the anterior chamber whereby the flanges are tucked onto the iris. Both I-Ring and B-Hex provide adequate and stable intraoperative pupil dilation and are intended for single use. The I-Ring produces a slightly larger pupil diameter, is more widely available worldwide, and includes an injector device that can aid both PED deployment and removal. Compared to the I-Ring, the B-hex requires a smaller entry wound.
The current series also demonstrates the excellent overall intraoperative safety profile of I-Ring use, as only 1 unrelated case of zonular dehiscence occurred and no cases of spontaneous PED disengagement, iris bleeding, iris damage, or capsulorhexis tears were encountered.
Although this study does not compare I-Ring with other PEDs, our collective experience with various alternative devices does suggest several design advantages. The I-Ring contacts and expands the pupillary margin for its entire 360° circumference, protecting it from inadvertent surgical trauma, and allows for distribution of the centrifugal stretching force along the entire pupillary margin versus the four or more discreet contact points of other devices. The I-Ring’s distinctive color increases its visibility and facilitates intraoperative manipulation. Its softer polyurethane material also causes less trauma to the iris.[25] These several factors may contribute to restoration of a more uniform and esthetically consistent postoperative pupil configuration.
Small pupil Phaco can also be performed without the use of PED. A recent retrospective study of 114 eyes with small pupils that underwent pupillary sphincterotomy reported successful surgery in all cases. However, postoperative complications included transient ocular hypertension (4%), sustained ocular hypertension (1%), persistent uveitis of more than 1 month (4%), and cystoid macular edema (5%).[32] In our series, no such significant adverse events were encountered, apart from the single unrelated case of zonular dialysis. This relative lack of complications suggests that the use of a PED such as the I-Ring may be less traumatic and less inflammatory than pupil enlargement by cutting the iris.
The main disadvantage of I-Ring usage is cost which while comparable to other PEDs is substantially greater than iris hooks. Considering the cost and morbidity of the surgical complications which I-Ring and other PEDs greatly reduce, their relative expense seems more than justified.
Limitations
One of the limitations of this study was the use of Castroviejo caliper to measure pupil diameter. The Castroviejo caliper has limited accuracy of 1 mm so values smaller than 1 mm will merely be estimated. In this study, the Castroviejo caliper was utilized because of its low cost, universal availability, and ability to be used both in the clinic and operating theater. Other devices that measure pupil size with greater accuracy include infrared pupilometers, wavefront aberrometers, optical biometers, and Scheimpflug camera systems. Measurement of pupil sizes using a surgical microscope or slit-lamp biomicroscope results in larger-than-actual measurements because of corneal magnification. This explains why our clinically measured I-Ring diameter was 6.8 mm while the manufacturing specifications report a 6.3 mm diameter. Because it is technically difficult to measure the actual pupil size in vivo, we used the clinically measured diameters for consistency and to show the relative effect of using the I-Ring PED. A recently approved irrigant-additive mydriatic plus non-steroidal anti-inflammatory (phenylephrine 1% + ketorolac 0.3%, Omidria, Omeros) was not available to either surgeon; hence, its presumed comparable effects could not be assessed. It is also important to reinforce that although I-Ring and other PEDs can be used in most of the small pupil Phaco cases, they are not appropriate for extremely small pupils (<3 mm maximal post-dilation diameter), for extremely shallow anterior chambers, or for irises which are atrophic or torn. In such situations, iris hooks may afford more individualized control and hence increased safety. Other limitations of this study include diversity of cases, although the similar surgical experience and technique of the 2 surgeons presumably conferred uniformity. A priori sample size calculations were not performed; all eligible patients during the study period (Jan 1, 2018 to June 30, 2018) were recruited. Nevertheless, all the statistical comparisons were statistically significant (P < 0.001). Future studies with larger dataset may evaluate the outcomes of I-Ring in comparison with other PEDs.
Conclusion
In conclusion, the I-Ring is an effective and safe PED for small pupil cataract surgery. It can intraoperatively expand and maintain the pupil to 6.8 mm and does so with great stability and negligible iris tissue stress. Excellent functional and esthetic postoperative pupillary outcomes confirm its useful addition to the small pupil tool kit.
Financial support and sponsorship
Nil.
Conflicts of interest
HSU is a consultant to Beaver Visitec International.
Acknowledgements
IrisARC - Analytics, Research, and Consulting provided editorial and statistical assistance in the preparation of the manuscript.
References
- 1.Bonnell LN, SooHoo JR, Seibold LK, Lynch AM, Wagner BD, Davidson RS, et al. One-day postoperative intraocular pressure spikes after phacoemulsification cataract surgery in patients taking tamsulosin. J Cataract Refract Surg. 2016;42:1753–8. doi: 10.1016/j.jcrs.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Chang DF, Braga-Mele R, Mamalis N, Masket S, Miller KM, Nichamin LD, et al. ASCRS white paper:Clinical review of intraoperative floppy-iris syndrome. J Cataract Refract Surg. 2008;34:2153–62. doi: 10.1016/j.jcrs.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 3.Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg. 2005;31:664–73. doi: 10.1016/j.jcrs.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Eyeson-Annan ML, Hirst LW, Battistutta D, Green A. Comparative pupil dilation using phenylephrine alone or in combination with tropicamide. Ophthalmology. 1998;105:726–32. doi: 10.1016/S0161-6420(98)94030-1. [DOI] [PubMed] [Google Scholar]
- 5.Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc. 1998;96:557–634. [PMC free article] [PubMed] [Google Scholar]
- 6.Gimbel HV. Nucleofractis phacoemulsification through a small pupil. Can J Ophthalmol. 1992;27:115–9. [PubMed] [Google Scholar]
- 7.Greenberg PB, Tseng VL, Wu WC, Liu J, Jiang L, Chen CK, et al. Prevalence and predictors of ocular complications associated with cataract surgery in United States veterans. Ophthalmology. 2011;118:507–14. doi: 10.1016/j.ophtha.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Hashemi H, Seyedian MA, Mohammadpour M. Small pupil and cataract surgery. Curr Opin Ophthalmol. 2015;26:3–9. doi: 10.1097/ICU.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 9.Katsimpris JM, Petropoulos IK, Apostolakis K, Feretis D. Comparing phacoemulsification and extracapsular cataract extraction in eyes with pseudoexfoliation syndrome, small pupil, and phacodonesis. Klin Monbl Augenheilkd. 2004;221:328–33. doi: 10.1055/s-2004-812863. [DOI] [PubMed] [Google Scholar]
- 10.Kershner RM. Management of the small pupil for clear corneal cataract surgery. J Cataract Refract Surg. 2002;28:1826–31. doi: 10.1016/s0886-3350(02)01206-3. [DOI] [PubMed] [Google Scholar]
- 11.Muhtaseb M, Kalhoro A, Ionides A. A system for preoperative stratification of cataract patients according to risk of intraoperative complications:A prospective analysis of 1441 cases. Br J Ophthalmol. 2004;88:1242–6. doi: 10.1136/bjo.2004.046003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasavada A, Singh R. Phacoemulsification in eyes with a small pupil. J Cataract Refract Surg. 2000;26:1210–8. doi: 10.1016/s0886-3350(00)00361-8. [DOI] [PubMed] [Google Scholar]
- 13.Vollman DE, Gonzalez-Gonzalez LA, Chomsky A, Daly MK, Baze E, Lawrence M. Intraoperative floppy iris and prevalence of intraoperative complications:Results from ophthalmic surgery outcomes database. Am J Ophthalmol. 2014;157:1130–5.e1. doi: 10.1016/j.ajo.2014.02.052. [DOI] [PubMed] [Google Scholar]
- 14.Akman A, Yilmaz G, Oto S, Akova YA. Comparison of various pupil dilatation methods for phacoemulsification in eyes with a small pupil secondary to pseudoexfoliation. Ophthalmology. 2004;111:1693–8. doi: 10.1016/j.ophtha.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Chiambaretta F, Pleyer U, Behndig A, Pisella PJ, Mertens E, Limao A, et al. Pupil dilation dynamics with an intracameral fixed combination of mydriatics and anesthetic during cataract surgery. J Cataract Refract Surg. 2018;44:341–7. doi: 10.1016/j.jcrs.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Donnenfeld ED, Whitaker JS, Jackson MA, Wittpenn J. Intracameral ketorolac and phenylephrine effect on intraoperative pupil diameter and postoperative pain in cataract surgery. J Cataract Refract Surg. 2017;43:597–605. doi: 10.1016/j.jcrs.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 17.Hovanesian JA, Sheppard JD, Trattler WB, Gayton JL, Malhotra RP, Schaaf DT, et al. Intracameral phenylephrine and ketorolac during cataract surgery to maintain intraoperative mydriasis and reduce postoperative ocular pain:Integrated results from 2 pivotal phase 3 studies. J Cataract Refract Surg. 2015;41:2060–8. doi: 10.1016/j.jcrs.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 18.Bucci FA, Jr, Michalek B, Fluet AT. Comparison of the frequency of use of a pupil expansion device with and without an intracameral phenylephrine and ketorolac injection 1%/0.3% at the time of routine cataract surgery. Clin Ophthalmol. 2017;11:1039–43. doi: 10.2147/OPTH.S132552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canabrava S, Rezende PH, Eliazar GC, Figueiredo SB, Resende AF, Batista WD, et al. Efficacy of the canabrava ring (pupil expansion device) in cataract surgery for eyes with small pupils:The first 30 cases. Arq Bras Oftalmol. 2018;81:202–11. doi: 10.5935/0004-2749.20180042. [DOI] [PubMed] [Google Scholar]
- 20.Chang DF. Use of Malyugin pupil expansion device for intraoperative floppy-iris syndrome:Results in 30 consecutive cases. J Cataract Refract Surg. 2008;34:835–41. doi: 10.1016/j.jcrs.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Goldman JM, Karp CL. Adjunct devices for managing challenging cases in cataract surgery:Pupil expansion and stabilization of the capsular bag. Curr Opin Ophthalmol. 2007;18:44–51. doi: 10.1097/ICU.0b013e3280121b09. [DOI] [PubMed] [Google Scholar]
- 22.Halkiadakis I, Chatziralli I, Drakos E, Katzakis M, Skouriotis S, Patsea E, et al. Causes and management of small pupil in patients with cataract. Oman J Ophthalmol. 2017;10:220–4. doi: 10.4103/ojo.OJO_102_2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malyugin B. Small pupil Phacoemulsification surgery:A new technique. Ann Ophthalmol (Skokie) 2007;39:185–93. doi: 10.1007/s12009-007-0023-8. [DOI] [PubMed] [Google Scholar]
- 24.Oetting TA, Omphroy LC. Modified technique using flexible iris retractors in clear corneal cataract surgery. J Cataract Refract Surg. 2002;28:596–8. doi: 10.1016/s0886-3350(01)01100-2. [DOI] [PubMed] [Google Scholar]
- 25.Tian JJ, Garcia GA, Karanjia R, Lu KL. Comparison of 2 pupil expansion devices for small-pupil cataract surgery. J Cataract Refract Surg. 2016;42:1235–7. doi: 10.1016/j.jcrs.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Visco D. Effect of phenylephrine/ketorolac on iris fixation ring use and surgical times in patients at risk of intraoperative miosis. Clin Ophthalmol. 2018;12:301–5. doi: 10.2147/OPTH.S149522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anisimova NS, Arbisser LB, Petrovski G, Petrichuk SV, Sobolev NP, Petrovski B, et al. Effect of NSAIDs on pupil diameter and expression of aqueous humor cytokines in FLACS Versus conventional phacoemulsification. J Refract Surg. 2018;34:646–52. doi: 10.3928/1081597X-20180814-02. [DOI] [PubMed] [Google Scholar]
- 28.Ventura BV, Rabello LP, Silvestre F, Ventura MC. Efficacy of preoperative nonsteroidal anti-inflammatory drug and the re-dilation technique in minimizing miosis after femtosecond laser in cataract surgery. Arq Bras Oftalmol. 2019;82:111–8. doi: 10.5935/0004-2749.20190025. [DOI] [PubMed] [Google Scholar]
- 29.Al-Hashimi S, Donaldson K, Davidson R, Dhaliwal D, Jackson M, Kieval JZ, et al. Medical and surgical management of the small pupil during cataract surgery. J Cataract Refract Surg. 2018;44:1032–41. doi: 10.1016/j.jcrs.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 30.Wilczynski M, Wierzchowski T, Synder A, Omulecki W. Results of phacoemulsification with Malyugin Ring in comparison with manual iris stretching with hooks in eyes with narrow pupil. Eur J Ophthalmol. 2013;23:196–201. doi: 10.5301/ejo.5000204. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharjee S. B-HEX pupil expander:Pupil expansion redefined. Indian J Ophthalmol. 2017;65:1407–10. doi: 10.4103/ijo.IJO_673_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goh JWY, Harrison R, Tavassoli S, Tole DM. Outcomes of sphincterotomy for small pupil phacoemulsification. Eye (Lond) 2018;32:1334–7. doi: 10.1038/s41433-018-0079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]