Dear Editor,
The “Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)” disease has caused worldwide colossal challenges and disruptions of social and economic life. The vaccination undoubtedly is the most critical step to restore normalcy and to bounce back from the pandemic.
It has been reported that the SARS-CoV-2 may trigger or exacerbate inflammatory or demyelinating disease and the same effects can be induced by vaccination. The US Vaccine Adverse Events Reporting System (VAERS) has reported ophthalmic adverse effects like optic neuritis, gaze palsies, cranial nerve palsies including third, fourth, and sixth nerve palsies after various live attenuated and mixed vaccines.[1,2,3] VAERS might represent true vaccine reactions, and others might be coincidental adverse health events not related to the vaccination at all.[1,2,3,4]
Unfortunately in India and other developing countries, there is no such VAERS.
Here, we report four cases in young adults who were suspected to have Coronavirus Disease 19(COVID-19)-vaccine-related ophthalmic adverse events. All of the patients were systemic, healthy with no comorbidities, no prior history of COVID-19, and had negative RT-PCR at the time of presentation.
The first case was of a 28 year healthy female who presented with a sudden decrease of vision in the left eye (LE). The right eye had 6/6 while the LE had the best-corrected visual acuity (BCVA) of 6/36. The fundus examination was unremarkable in RE and LE had mild blurring of disk margin. The Resonance Imaging(MRI) brain was normal with LE optic nerve findings consistent with optic neuritis. Anti-nuclear antibody (ANA), angiotensin-converting enzyme (ACE), and anti-AQP4-Immunoglobulin (IgG) levels, and routine blood investigations were normal. She had received the first dose of the COVID-19 vaccine 3 weeks prior to this presentation. She regained 6/6 in the LE after a course of intravenous methylprednisolone followed by a tapering steroid dose.
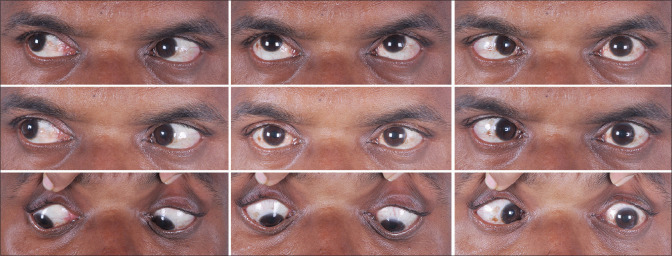
The second female, 24 years old, presented with complaints of diplopia and squinting for 2 days. On examination, she had restricted elevation (−2 limitation) of both eyes. Her MRI and neurological examination were normal. She was diagnosed as Both Eye(BE) sudden-onset vertical gaze palsy. She had no diplopia with complete resolution of the elevation restriction within 10 days of systemic steroids. She had her first dose of the COVID-19 vaccine 3 weeks back. The third case was of a 44-year-old male who developed acute sixth nerve palsy in the LE and diplopia after 1 month of COVID-19 vaccination along with a new onset of diabetes mellitus [Fig. 1]. He had polio during his childhood but was otherwise healthy. He was given LE botox injection to the medial rectus which resolved his diplopia with minimal residual esotropia. The fourth case was of a young adult with a history of recurrent sixth nerve palsy LE following chickenpox. He developed acute onset Esotropia in the LE 6 days after vaccination along with a headache which resolved after 4 weeks. Both cases (3,4) had unremarkable MRI, normal fundus examination, and no other neurological deficit.
Figure 1.
Nine cardinal gaze showing the LE esotropia following 3 weeks after the COVID-19 vaccination in case 3
All the patients received recombinant, adenovirus vector encoding the SARS-CoV-2 spike (S) glycoprotein vaccine. Following administration, the genetic material of the part of coronavirus is expressed, which stimulates an immune response which is almost similar to post-viral infections.
Although it is important to highlight these incidental complications, this should not outweigh the benefits of the COVID-19 vaccine. The ophthalmologist should be aware of the possibilities of these immune-related and neurological complications of the COVID-19 vaccine. The possibilities of the need for booster doses, rise in trajectory and considering the ongoing pandemic, various ophthalmic communities should observe and build a national reporting system.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Woo EJ, Winiecki SK, Ou AC. Motor palsies of cranial nerves (excluding VII) after vaccination:reports to the US Vaccine Adverse Event Reporting System. Hum Vaccin Immunother. 2014;10:301–5. doi: 10.4161/hv.27032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varricchio F, Iskander J, Destefano F, Ball R, Pless R, Braun MM, et al. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J. 2004;23:287–94. doi: 10.1097/00006454-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines:Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci. 2021;25:1663–9. doi: 10.26355/eurrev_202102_24877. [DOI] [PubMed] [Google Scholar]
- 4.Reyes-Capo DP, Stevens SM, Cavuoto KM. Acute abducens nerve palsy following COVID-19 vaccination. J AAPOS. 2021 doi: 10.1016/j.jaapos.2021.05.003. S1091-8531(21)00109-9. https://doi.org/10.1016/j.jaapos.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]