Abstract
The multifidus is an important muscle for the active stabilization of the spine. Unfortunately, clinical procedures such as posterior lumbar fusion (PLF) and radio frequency neurotomy (RFN) cause injury to these muscles affecting their function. However, evaluating multifidus function using traditional biomechanical methods is challenging due to its unique anatomical features. The change in muscle shear modulus during contraction has been corrected to force generation for several skeletal muscles. Therefore, the change in shear modulus can be used to quantify muscle contraction. The objective of this study was to evaluate multifidus dysfunction by comparing changes in shear modulus during muscle contraction in healthy individuals and patients who received RFN and PLF in the lumbar spine. We used our recently developed protocol which consists of measuring changes of multifidus shear modulus at lying prone, sitting up, and sitting up with the arms lifted. In healthy individuals, the median multifidus shear modulus increased progressively from prone, sitting, and sitting with arms raised: 18.55 kPa, 27.14 kPa, and 38.45 kPa, respectively. A moderate increase in shear modulus for these body positions was observed in PLF patients: 9.81 kPa, 17.26 kPa, and 21.85 kPa. In RFN patients, the shear modulus remained relatively constant: 14.44 kPa, 16.57 kPa, and 17.26 kPa. Overall, RFN and PLF caused a reduction in the contraction of multifidus muscles. However, the contraction of multifidus muscle slightly increased during multifidus activation in PLF patients, while it did not change in RFN patients. These preliminary measurements suggest that the proposed protocol using SWE can provide important information about the function of individual spine muscles to guide the design and evaluation of postsurgical rehabilitation protocols.
Keywords: activation forces, multifidus muscle, shear modulus, radiofrequency neurotomy, posterior lumbar fusion
Introduction
Chronic low back pain is one of the most common sources of disability in the middle-aged and elderly population with significant associated medical expenditures [1]. Radio frequency neurotomy (RFN) and posterior lumbar fusion (PLF) are common procedures for the treatment of different spine conditions. The lumbar facet joints are the primary source of pain in approximately 10–40% of chronic low back pain patients [2–4]. Common interventional treatment for the pain caused by lumbar facet joints is medial RFN. Unfortunately, RFN also causes denervation and subsequent atrophy of the multifidus muscle [5]. Since multifidus muscle is considered as one of the primary active stabilizers of the lumbar spine [6], multifidus dysfunction after RFN may have an impact on segmental stability, possibly affecting other structures of the spine like the intervertebral disk [7]. Similarly, muscle dissection and retraction during PLF procedures cause a localized multifidus iatrogenic injury that results in atrophy [8] and reduced trunk strength [9]. Atrophy in paraspinal muscles has been associated with worse clinical outcomes after PLF [10]. Therefore, evaluating multifidus dysfunction is important to understand and improve the long-term outcomes of these treatments.
Evaluating the multifidus function is challenging. Several techniques, including rehabilitative ultrasound imaging (RUSI), strength tests, and electromyography (EMG), have been used to evaluate spine muscles. However, these techniques have some limitations. Multifidus function has been assessed using RUSI in patients with chronic low back pain by quantifying changes of multifidus thickness during volitional contractions [11–13]. However, the linearity of the relationship between muscle force and change in thickness is limited to the range from 18% to 50% of the maximum voluntary contraction [14–16]. Additionally, the bony anatomy of the spine changes after fusion surgery making landmark identification during ultrasound evaluation very difficult. In fact, most of RUSI studies exclude patients with spinal surgeries [17,18]. Isokinetic or isometric strength tests (i.e., dynamometry) cannot quantify localized multifidus contraction independent from neighboring agonist muscles. Additionally, adjacent muscles may compensate for local multifidus dysfunction, which may result in no change of the overall strength of the back. Surface EMG also has some limitations, such as maintaining robust contact of the electrode with skin and crosstalk for evaluating localized multifidus muscle independent of neighboring muscles. Needle EMG is invasive, and many factors affect its amplitude, such as the direction of muscle fibers relative to the location of electrodes [19,20]. The limitation of these techniques has made the evaluation of the role of multifidus function on back pain very challenging.
Ultrasound shear wave elastography (SWE) is a reliable and noninvasive method that can potentially quantify the shear modulus of the tissues [21–23]. Changes in shear modulus are linearly correlated to muscle force [24–26]. Therefore, SWE can be used to evaluate the function of individual muscles, which cannot be performed with other tools [27]. Moreau et al. [28] evaluated the reliability of ultrasound SWE in the assessment of lumbar shear modulus at level L4-5 and L2-3 during rest and sitting on an ergonomic forward-leaning chair. They reported excellent intra- and interobserver reliability. Recently, we evaluated the reliability of ultrasound SWE in the evaluation of the multifidus shear modulus at three postures: lying prone, sitting up, and sitting up with the right arm lifted postures in healthy individuals, reporting excellent reliability [29]. An essential advantage of using SWE for quantifying multifidus muscle contraction is that this is a localized method that can quantify multifidus function in the specific injured lumbar level independent of the neighboring muscles. Additionally, our protocol reported the differences in activation between the multifidus layers, which indicates the importance of developing a technique to quantify localized contraction of spinal muscles. These results suggest that SWE is a reliable method to quantify localized multifidus contraction after RFN and PLF procedures.
The objective of this study was to evaluate multifidus dysfunction by comparing changes in shear modulus during muscle contraction in healthy individuals and patients who received RFN and PLF in the lumbar spine. We hypothesized that the shear modulus in multifidus muscle after PLF and RFN procedures would be lower compared to healthy participants. The results of this preliminary study will assist in the design of appropriate rehabilitative recovery procedures for improving multifidus muscle function in RFN and PLF patients.
Methods
Overview.
The Institutional Review Board (IRB) of the Pennsylvania State University approved the study (STUDY00010509), and human participants gave informed consent before the data collection. This study quantified multifidus shear modulus in three groups: (1) patients who have received RFN within the past 2 years, (2) patients who have received PLF within the past 5 years, and (3) age- and gender-matched healthy participants. RFN and PLF participants were excluded if they had previous spinal surgeries other than RFN or PLF; had a history of scoliosis; were pregnant, or had any neurological disease. Healthy participants were excluded if they ever had low back surgery; had a history of chronic back pain; had received services for low back pain within the past 6 months; had difficulty performing the requested tasks; had experienced a recent traumatic event such as a motor vehicle collision; had any neurological disease, or terminal illness.
A customized supersonic SWE method [30] was implemented to quantify the shear modulus of multifidus muscle using a Verasonic ultrasound system (Verasonic, Inc., Redmond, WA) with an ATL C5-2 transducer (128 elements, beamwidth = 2–5 MHz, center frequency = 3.1025 MHz) [29]. SWE is one of the ultrasound elastography techniques that uses shear waves induced by push pulses to measure tissue stiffness quantitatively. Sixty-four elements of the transducer emitted focused ultrasound “push” pulses (500 cycles) successively at seven increasing, equally spaced focal depths to create quasi-plane shear waves propagating through the tissue. This sequence of push pulses is called “supersonic” since the shear wave source (the focal points), moves faster than the shear wave propagation speed. The propagation speed was calculated within the region of interest using high frame rate plane wave imaging (at 10,000 frames/s). This method was validated on a homogeneous elasticity phantom made of Zerdine hydrogel polymer (Model 040GSE, CIRS, Norfolk, VA) with the dimensions of 17.8 cm × 12.7 cm × 20.3 cm [31]. We successfully applied this method in several previous studies [32–34].
Evaluation of Multifidus Muscle Function.
The RFN and PLF patients were recruited from the Hershey Medical Center (Hershey, PA). Thirteen patients (six men and seven women) who have received RFN within 2 years before ultrasound evaluation (mean elapsed time after surgery ± SD, 11.42 ± 6.57 months) were recruited (mean age ± SD, 61.15 ± 11.09 yr) (Table 1). Thirteen healthy age- and gender-matched controls (six men and seven women) who met the study's inclusion criteria were recruited to match the RFN group (mean age ± SD, 60.31 ± 9.00 yr). Ten patients (six men and four women) who have received PLF within the past five years (mean elapsed time after surgery ± SD, 33.88 ± 15.62 months) were recruited (mean age ± SD, 60.90 ± 11.08 yr). Ten healthy age- and gender-matched controls (six men and four women) that met the study's inclusion criteria were recruited to match the PLF group (mean age ± SD, 60.80 ± 9.59 yr). Participants were asked to fill out the pain catastrophizing scale (PCS) [35], modified Oswestry low back pain disability questionnaire (ODQ) [36], and visual analog scale (VAS) pain intensity rating questionnaire [37] to assess their pain intensity and perceived disability. The changes in multifidus shear modulus were quantified in three postures (prone, sitting up, and sitting up with the arms lifted). Participants laid prone with the spine muscles in a fully relaxed and neutral position (Fig. 1(a)). The shear modulus measurements of multifidus in the group of patients were performed bilaterally on the surgery lumbar level and the average was considered. For consistency in measuring shear modulus at one lumbar level, in the case of patients with multiple surgery levels, the multifidus shear modulus was only measured at the middle level. The transducer was placed longitudinally lateral to the spinous processes and angled medially (10–15 deg), similar to that used in previous multifidus studies [14,15,38,39]. To minimize contact pressure of the transducer and the skin, an adjustable fixture (goose neck holder) was used to securely hold the probe and maintain minimal contact pressure. This is desirable to avoid changes in mechanical properties of the muscle due to compression and to minimize user dependency (Fig. 1(a)). For the measurement of the shear modulus of multifidus during muscle contraction in sitting posture, each participant was first asked to sit up (Fig. 1(b)). They lifted their sternum straight up and sat upright in a resting posture, and the transducer was placed back on the skin, while their feet were not on the floor. The participants were asked to sit up with their arms horizontal to measure the shear modulus of the multifidus (Fig. 1(c)).
Table 1.
Demographic low back pain patient data
| Procedure | Number | Age (mean ± SD) | Gender | Time elapsed after surgery (mean±SD) |
|---|---|---|---|---|
| RFN | 13 | 61.15 ± 11.09 | Six men and seven women | 11.42 ± 6.57 months |
| PLF | 10 | 60.90 ± 11.08 yr | Six men and four women | 33.88 ± 15.62 months |
Fig. 1.
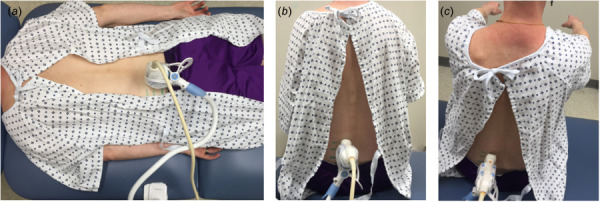
Experimental setup for the shear modulus measurement of multifidus muscle with the transducer located lateral to the spinous processes and angled medially at prone (a), sitting up (b), and sitting up with the arms lifted in a horizontal position (c)
The investigator monitored the brightness-mode (B-mode) ultrasound images during measurements to ensure that there was no significant body motion. After identifying the multifidus muscle in the B-mode image (Fig. 2), the elastography mode was activated to measure the multifidus shear modulus. An ROI with variable height to cover the entire thickness of the multifidus (from the fascial line of the muscle to 2 mm above the facet joint) and the 20 mm width was considered [29]. The first dark pixel before the fascial line of the muscle was selected as the upper limit of the ROI. Figure 2 shows the representative B-mode image of the multifidus in an RFN, PLF, and matched healthy participant. In each round of measurements, the shear wave speed was calculated five times, and the median value was reported.
Fig. 2.
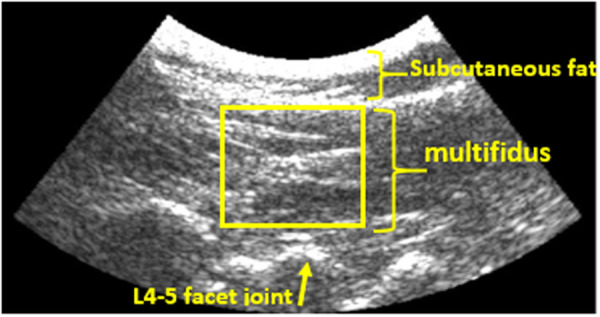
Representative B-mode ultrasound image of the multifidus in the prone position
Statistical Analysis.
Shapiro–Wilks test was used to analyze the normality of data distribution. A linear mixed effects model followed by Sidak's multiple comparison tests was used to find significant differences between multifidus shear modulus between patients (RFN or PLF) and matched controls for each of the body positions (lying down, sitting up, and sitting up with the arms lifted). The Spearman's rank correlation coefficient was used to evaluate the correlation between the multifidus shear modulus and PCS score in the RFN and PLF group, between the multifidus shear modulus and ODQ score in the RFN and PLF group. In all analyses, the p < 0.05 was considered as the level of statistical significance. SPSS statistics software (v24, IBM) was used for all statistical analyses.
Results
The representative shear modulus maps of the multifidus muscle in healthy controls, RFN, and PLF patients in the sitting up with lifted arms position are shown in Fig. 3. From the normality test, it was determined that the shear modulus data from the prone position measurements in the RFN group as well as data from the sitting position in the control groups were not normally distributed (data from the other positions passed the normality test). Therefore, data is presented as median (interquartile range). The shear modulus for the affected multifidus in the RFN patients for the prone, sitting up and lifted arms states was 14.44 (6.62) kPa, 16.57 (9.59) kPa, and 20.07 (10.96) kPa, respectively; for the healthy controls, values were 18.55 (5.59) kPa, 27.14 (5.08) kPa, and 38.45 (16.71) kPa, respectively (Fig. 4(a)). Similarly, the shear modulus for the affected multifidus in PLF patients for the prone, sitting up and lifted arms states was 9.81 (3.75) kPa, 17.26 (8.68) kPa, and 21.85 (13.83) kPa, respectively; for the healthy controls, values were 16.08 (11.85) kPa, 28.11 (3.87) kPa, and 37.26 (11.87) kPa, respectively (Fig. 4(b)). The shear modulus of the multifidus muscle in the RFN and PLF patients was significantly lower than that of in healthy controls (p < 0.01) in the sitting and sitting with arms raised positions, indicating lower levels of muscle contraction. There was a significant increase in shear modulus of the multifidus muscle in the PLF patients from prone to sitting up and from sitting up to the lifted arms posture (p < 0.01), while there was no significant difference in shear modulus of the multifidus muscle between different postures in the RFN patients (p = 0.13).
Fig. 3.
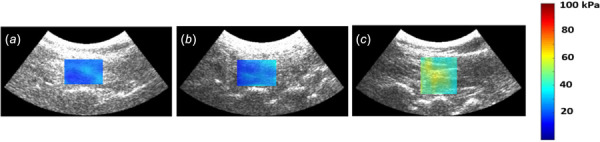
Representative shear modulus maps of the L4-5 multifidus muscle in the sitting up with the lifted arms position: PLF patient (a), RFN patient (b), and age- and gender-matched healthy participant (c). The lower shear modulus in the RFN and PLF patient compared to the healthy individual indicates multifidus dysfunction.
Fig. 4.
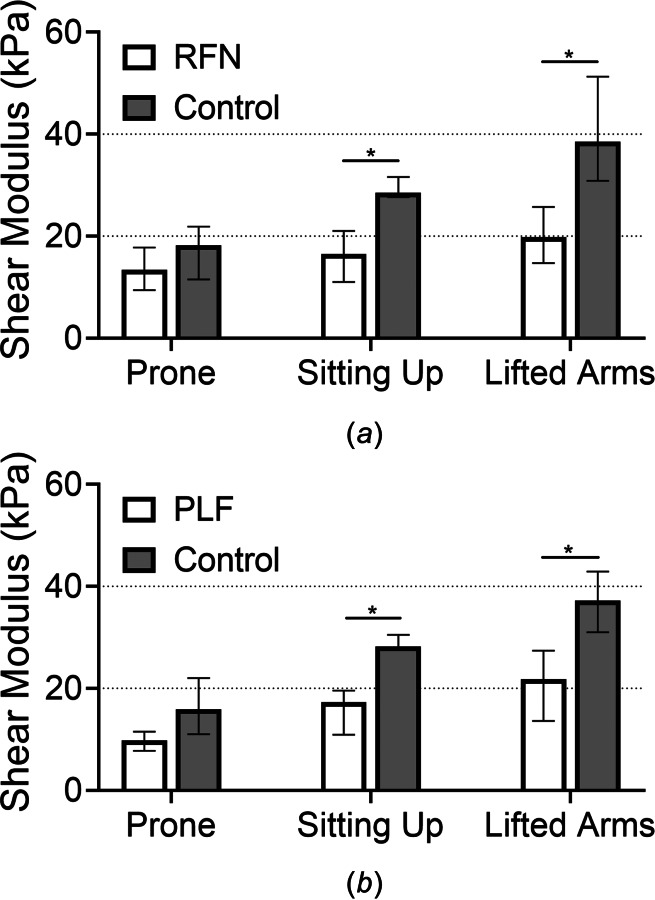
The shear modulus of the affected multifidus (median, interquartile range) was lower in the sitting up and sitting up with lifted arms positions in the RFN (a) and PLF (b) groups compared to age- and gender-matched healthy controls
The PCS score for the RFN and PLF group was 15.0 (13.0) and 13.5 (16.75), respectively; the VAS score for the RFN and PLF group was 4.0 (2.85) and 5.55 (5.66), respectively; and the ODQ score for the RFN and PLF group was 42.0% (19%) and 28.0% (22.5%), respectively. Although there was no statistical difference in PCS, VAS score, and ODQ score between RFN and PLF, the median ODQ score of the RFN group was in the severe disability range (41–60%), while the PLF group was in the moderate disability range (21–40%). There was not enough power to determine the possible correlations between multifidus function and patient-reported outcomes.
Discussion
This study evaluated localized lumbar multifidus muscle contraction after RFN and PLF procedures using ultrasound SWE. Changes in muscle shear modulus during muscle contraction correlate to force. Therefore, shear modulus can be used as a marker of muscle function. The change in median shear modulus from lying prone to sitting up and from sitting up to sitting up with the lifted arms was more significant in the healthy participants compared to changes in the other groups. During lifting arms in healthy controls, the higher multifidus shear modulus indicates increased contraction in multifidus muscle to keep the balance of the torso. The multifidus muscle showed lower shear modulus in RFN and PLF group in all postures, indicating a reduced muscle contraction after RFN and PLF procedures. The preliminary results in this study demonstrate that the new protocol is useful for evaluating multifidus contraction and lumbar multifidus dysfunction after RFN and PLF procedures.
The multifidus shear modulus has been evaluated in healthy individuals in a few previous studies. Sadeghi et al. [29] assessed the reliability of SWE for quantifying L4-5 multifidus shear modulus in 18 healthy individuals at prone, sitting up and sitting up with an arm lifted postures, reporting shear modulus of 16.15 (6.69), 27.28 (15.72), and 45.02 (25.27), respectively. These numbers are very similar to the multifidus shear modulus in matched healthy controls in this study. The similarity of the shear modulus between these two groups with different age groups (average age: 35 versus 60 yr old) suggests that age may not have a significant effect in the multifidus muscle shear modulus. The similarity of muscle shear modulus between different age groups has been reported in other studies as well. For example, Akagi et al. [40] investigated the age-related differences between the shear moduli in the lower extremity of younger and elderly individuals, reporting no age-related differences between the soleus shear modulus in two groups. Dieterich et al. [41] evaluated the shear modulus of cervical multifidus in a group of healthy individuals, reporting a shear modulus of 14.9 kPa during resting in prone posture. This result was similar to our findings in healthy individuals for the prone posture. They showed that shear modulus increased with increasing the loading of the neck. The results of this study also show increased shear modulus of the multifidus at different body postures, which were expected because different levels of contraction are required in those positions to keep balance of the torso [29]. Alis et al. [42] evaluated the shear modulus of the lumbar multifidus in a prone posture in patients diagnosed with disk herniation, reporting a significantly lower average shear modulus in the affected side (14.08 ± 3.57 kPa) compared to the contralateral side (18.81 ± 3.95 kPa). These values are higher than our values for patients with RFN and PLF. The differences may be because of the severity of nerve compression after disk herniation. The presence of lumbar disk herniation compresses the adjacent nerve root, which may cause a change in multifidus function depending on the compression severity. Alis et al. [42] reported a negative correlation between multifidus shear modulus and the severity of the nerve compression. Therefore, multifidus shear modulus may change differently after different spinal pathologies.
The changes in multifidus shear modulus in the PLF patients were different from those observed in RFN patients reflecting differences in muscle functionality. There was a moderate increase in shear modulus from lying prone to sitting up and from sitting up to lifted arms posture in the PLF group, while the shear modulus remained relatively constant at all three positions in the RFN group. These results suggest that multifidus has moderate levels of contraction in PLF patients, but a more severe dysfunction in RFN patients. The difference may be explained by the different mechanisms involved in the RFN and PLF procedures affecting multifidus function. In the PLF, a direct injury to the multifidus due to the incision during surgery and retraction with long periods of excessive pressure [43], while the nerve damage caused by RFN seems to completely inhibit muscle contraction. These results suggest that our SWE protocol can discriminate between different patterns and severity of multifidus dysfunction after injury or disease.
Quantifying multifidus contraction may clarify possible relationships between multifidus dysfunction and spinal pathologies. Our protocol can potentially determine the extent of muscle dysfunction after RFN or PLF. For instance, it has been shown that there is an increased load on adjacent segments of an injured level after the PLF procedure, which may contribute to the onset of adjacent segment disease [44]. SWE can be used to evaluate how decreased multifidus muscle contraction affects the loading of adjacent intervertebral disks or facet joints. The ability of SWE to evaluate function from individual muscles can also be exploited to understand interactions between back muscles. Kong et al. [45] suggested that multifidus muscle dysfunction affects the normal functioning of other paraspinal muscles, causing spinal disorder. Our proposed protocol may also help clinicians evaluate the health status and monitor the recovery process of the muscle after interventions are applied to mitigate the severity of its dysfunction.
Evaluating the function of the multifidus muscle is difficult even when using shear wave elastography. A linear relationship between force and shear modulus during contraction has been measured for several skeletal muscles [46]. However, unlike other muscles, the multifidus muscle does not have a single insertion point. Fascicles of the multifidus originate in the mammillary process of lumbar vertebrae and insert in the spinous process of the following three vertebrae above its origin [47]. This fascicle arrangement is repeated at most levels of the spine. Since there is not a single insertion point or a single moment arm, measuring force production of the multifidus through changes in shear modulus is nearly impossible. Instead, changes in shear modulus during different body positions can be used as a marker to evaluate local muscle contraction rather than quantifying force. Future studies will focus on evaluating relationships between shear modulus and specific tension (tensile stress) rather than total force produced by the multifidus.
This study has several limitations. The sample size in this study was relatively small, which might have contributed to the lack of a significant correlation between the shear modulus results and pain symptom scores. However, the sample size did not compromise the linear mixed effects model results showing differences between patients and healthy controls (p < 0.01). Patients in this cross-sectional study were recruited at different time points after underwent RFN or PLF, which may contribute to the variability in the shear modulus. Future studies will focus on longitudinal studies evaluating multifidus function before and after the RFN/PLF procedure.
In conclusion, this study showed the quantification of localized lumbar multifidus muscle dysfunction after RFN and PLF procedures using ultrasound SWE. The patterns of multifidus dysfunction were different between these procedures. A moderate increase in multifidus shear modulus from lying prone to sitting up and from sitting up to sitting up with lifted arms posture was observed in PLF patients, while the shear modulus remained relatively constant in RFN patients. The results in this study provided a preliminary data into abnormal patterns of contraction in injured multifidus muscles. The proposed protocol has the potential to assess individual spine muscles in response to postsurgical rehabilitation protocols.
Acknowledgment
The content is solely the responsibility of the authors and does not necessarily represent the official vies of the NIH.
Funding Data
National Center for Advancing Translational Sciences, National Institutes of Health (Grant No. UL1 TR002014; Funder ID: 10.13039/100000002).
References
- [1]. Pangarkar, S. , and Miedema, M. L. , 2014, “ Pulsed Versus Conventional Radio Frequency Ablation for Lumbar Facet Joint Dysfunction,” Curr. Phys. Med. Rehabil. Rep., 2(1), pp. 61–65. 10.1007/s40141-013-0040-z [DOI] [Google Scholar]
- [2]. Streitberger, K. , Müller, T. , Eichenberger, U. , Trelle, S. , and Curatolo, M. , 2011, “ Factors Determining the Success of Radiofrequency Denervation in Lumbar Facet Joint Pain: A Prospective Study,” Eur. Spine J., 20(12), pp. 2160–2165. 10.1007/s00586-011-1891-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Cohen, S. P. , and Raja, S. N. , 2007, “ Pathogenesis, Diagnosis, and Treatment of Lumbar Zygapophysial (Facet) Joint Pain,” Anesthesiol.: J. Am. Soc. Anesthesiol., 106(3), pp. 591–614. 10.1097/00000542-200703000-00024 [DOI] [PubMed] [Google Scholar]
- [4]. Suseki, K. , Takahashi, Y. , Takahashi, K. , Chiba, T. , Tanaka, K. , Morinaga, T. , Nakamura, S.-I. , and Moriya, H. , 1997, “ Innervation of the Lumbar Facet Joints: Origins and Functions,” Spine, 22(5), pp. 477–485. 10.1097/00007632-199703010-00003 [DOI] [PubMed] [Google Scholar]
- [5]. Dreyfuss, P. , Stout, A. , Aprill, C. , Pollei, S. , Johnson, B. , and Bogduk, N. , 2009, “ The Significance of Multifidus Atrophy After Successful Radiofrequency Neurotomy for Low Back Pain,” PMR, 1(8), pp. 719–722. 10.1016/j.pmrj.2009.05.014 [DOI] [PubMed] [Google Scholar]
- [6]. Ward, S. R. , Kim, C. W. , Eng, C. M. , Gottschalk, L. J., IV , Tomiya, A. , Garfin, S. R. , and Lieber, R. L. , 2009, “ Architectural Analysis and Intraoperative Measurements Demonstrate the Unique Design of the Multifidus Muscle for Lumbar Spine Stability,” J. Bone Jt. Surg. Am. Vol., 91(1), pp. 176–185. 10.2106/JBJS.G.01311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Smuck, M. , Crisostomo, R. A. , Demirjian, R. , Fitch, D. S. , Kennedy, D. J. , and Geisser, M. E. , 2015, “ Morphologic Changes in the Lumbar Spine After Lumbar Medial Branch Radiofrequency Neurotomy: A Quantitative Radiological Study,” Spine J., 15(6), pp. 1415–1421. 10.1016/j.spinee.2013.06.096 [DOI] [PubMed] [Google Scholar]
- [8]. Goubert, D. , De Pauw, R. , Meeus, M. , Willems, T. , Cagnie, B. , Schouppe, S. , Van Oosterwijck, J. , Dhondt, E. , and Danneels, L. , 2017, “ Lumbar Muscle Structure and Function in Chronic Versus Recurrent Low Back Pain: A Cross-Sectional Study,” Spine J., 17(9), pp. 1285–1296. 10.1016/j.spinee.2017.04.025 [DOI] [PubMed] [Google Scholar]
- [9]. Rahmani, N. , Kiani, A. , Mohseni-Bandpei, M. A. , and Abdollahi, I. , 2018, “ Multifidus Muscle Size in Adolescents With and Without Back Pain Using Ultrasonography,” J. Bodywork Mov. Ther., 22(1), pp. 147–151. 10.1016/j.jbmt.2017.05.016 [DOI] [PubMed] [Google Scholar]
- [10]. Addison, O. , Marcus, R. L. , LaStayo, P. C. , and Ryan, A. S. , 2014, “ Intermuscular Fat: A Review of the Consequences and Causes,” Int. J. Endocrinol., 2014, pp. 1–11. 10.1155/2014/309570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Wallwork, T. L. , Hides, J. A. , and Stanton, W. R. , 2007, “ Intrarater and Interrater Reliability of Assessment of Lumbar Multifidus Muscle Thickness Using Rehabilitative Ultrasound Imaging,” J. Orthop. Sports Phys. Ther., 37(10), pp. 608–612. 10.2519/jospt.2007.2418 [DOI] [PubMed] [Google Scholar]
- [12]. Stokes, M. , Hides, J. , Elliott, J. , Kiesel, K. , and Hodges, P. , 2007, “ Rehabilitative Ultrasound Imaging of the Posterior Paraspinal Muscles,” J. Orthop. Sports Phys. Ther., 37(10), pp. 581–595. 10.2519/jospt.2007.2599 [DOI] [PubMed] [Google Scholar]
- [13]. Kiesel, K. B. , Uhl, T. L. , Underwood, F. B. , Rodd, D. W. , and Nitz, A. J. , 2007, “ Measurement of Lumbar Multifidus Muscle Contraction With Rehabilitative Ultrasound Imaging,” Manual Ther., 12(2), pp. 161–166. 10.1016/j.math.2006.06.011 [DOI] [PubMed] [Google Scholar]
- [14]. Sions, J. M. , Velasco, T. O. , Teyhen, D. S. , and Hicks, G. E. , 2015, “ Reliability of Ultrasound Imaging for the Assessment of Lumbar Multifidi Thickness in Older Adults With Chronic Low Back Pain,” J. Geriatr. Phys. Ther. (2001), 38(1), p. 33. 10.1519/JPT.0000000000000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Sions, J. M. , Smith, A. C. , Hicks, G. E. , and Elliott, J. M. , 2016, “ Trunk Muscle Size and Composition Assessment in Older Adults With Chronic Low Back Pain: An Intra-Examiner and Inter-Examiner Reliability Study,” Pain Med., 17(8), pp. 1436–1446. 10.1093/pm/pnv023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Koppenhaver, S. L. , Hebert, J. J. , Fritz, J. M. , Parent, E. C. , Teyhen, D. S. , and Magel, J. S. , 2009, “ Reliability of Rehabilitative Ultrasound Imaging of the Transversus Abdominis and Lumbar Multifidus Muscles,” Arch. Phys. Med. Rehabil., 90(1), pp. 87–94. 10.1016/j.apmr.2008.06.022 [DOI] [PubMed] [Google Scholar]
- [17]. Whitehead, N. , Weerakkody, N. , Gregory, J. , Morgan, D. , and Proske, U. , 2001, “ Changes in Passive Tension of Muscle in Humans and Animals After Eccentric Exercise,” J. Physiol., 533(2), pp. 593–604. 10.1111/j.1469-7793.2001.0593a.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Todorov, P. T. , Nestorova, R. , and Batalov, A. , 2018, “ Diagnostic Value of Musculoskeletal Ultrasound in Patients With Low Back Pain—A Review of the Literature,” Med. Ultrasonography, 1(1), p. 80. 10.11152/mu-1245 [DOI] [PubMed] [Google Scholar]
- [19]. Shair, E. , Ahmad, S. , Marhaban, M. , Mohd Tamrin, S. , and Abdullah, A. , 2017, “ EMG Processing Based Measures of Fatigue Assessment During Manual Lifting,” BioMed Res. Int., 2017, pp. 1–12. 10.1155/2017/3937254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Smith, L. H. , and Hargrove, L. J. , 2013, “ Comparison of Surface and Intramuscular EMG Pattern Recognition for Simultaneous Wrist/Hand Motion Classification,” 35th Annual International Conference of the IEEE, Proceedings Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, July 3–7, pp. 4223–4226. 10.1109/EMBC.2013.6610477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Palmeri, M. L. , and Nightingale, K. R. , 2011, “ Acoustic Radiation Force-Based Elasticity Imaging Methods,” Interface Focus, 1(4), pp. 553–564. 10.1098/rsfs.2011.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Cortes, D. H. , Suydam, S. M. , Silbernagel, K. G. , Buchanan, T. S. , and Elliott, D. M. , 2015, “ Continuous Shear Wave Elastography: A New Method to Measure Viscoelastic Properties of Tendons In Vivo,” Ultrasound Med. Biol., 41(6), pp. 1518–1529. 10.1016/j.ultrasmedbio.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Song, P. , Urban, M. W. , Manduca, A. , Zhao, H. , Greenleaf, J. F. , and Chen, S. , 2013, “ Comb-Push Ultrasound Shear Elastography (CUSE) With Various Ultrasound Push Beams,” IEEE Trans. Med. Imaging, 32(8), pp. 1435–1447. 10.1109/TMI.2013.2257831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Nordez, A. , and Hug, F. , 2010, “ Muscle Shear Elastic Modulus Measured Using Supersonic Shear Imaging is Highly Related to Muscle Activity Level,” J. Appl. Physiol., 108(5), pp. 1389–1394. 10.1152/japplphysiol.01323.2009 [DOI] [PubMed] [Google Scholar]
- [25]. Koo, T. K. , Guo, J.-Y. , Cohen, J. H. , and Parker, K. J. , 2013, “ Relationship Between Shear Elastic Modulus and Passive Muscle Force: An Ex-Vivo Study,” J. Biomech., 46(12), pp. 2053–2059. 10.1016/j.jbiomech.2013.05.016 [DOI] [PubMed] [Google Scholar]
- [26]. Bouillard, K. , Hug, F. , Guével, A. , and Nordez, A. , 2012, “ Shear Elastic Modulus Can Be Used to Estimate an Index of Individual Muscle Force During a Submaximal Isometric Fatiguing Contraction,” J. Appl. Physiol., 113(9), pp. 1353–1361. 10.1152/japplphysiol.00858.2012 [DOI] [PubMed] [Google Scholar]
- [27]. Hug, F. , Tucker, K. , Gennisson, J.-L. , Tanter, M. , and Nordez, A. , 2015, “ Elastography for Muscle Biomechanics: Toward the Estimation of Individual Muscle Force,” Exercise Sport Sci. Rev., 43(3), pp. 125–133. 10.1249/JES.0000000000000049 [DOI] [PubMed] [Google Scholar]
- [28]. Moreau, B. , Vergari, C. , Gad, H. , Sandoz, B. , Skalli, W. , and Laporte, S. , 2016, “ Non-Invasive Assessment of Human Multifidus Muscle Stiffness Using Ultrasound Shear Wave Elastography: A Feasibility Study,” Proc. Inst. Mech. Eng., Part H: J. Eng. Med., 230(8), pp. 809–814. 10.1177/0954411916656022 [DOI] [PubMed] [Google Scholar]
- [29]. Sadeghi, S. , Quinlan, K. , Eilertson, K. E. , Billy, G. G. , Bible, J. , Sions, J. M. , and Cortes, D. H. , 2019, “ Changes in Shear Modulus of the Lumbar Multifidus Muscle During Different Body Positions,” ASME J. Biomech. Eng., 141(8), p. 081003. 10.1115/1.4043443 [DOI] [PubMed] [Google Scholar]
- [30]. Bercoff, J. , Tanter, M. , and Fink, M. , 2004, “ Supersonic Shear Imaging: A New Technique for Soft Tissue Elasticity Mapping,” IEEE Trans. Ultrasonics, Ferroelectr., Frequency Control, 51(4), pp. 396–409. 10.1109/TUFFC.2004.1295425 [DOI] [PubMed] [Google Scholar]
- [31]. Lin, C.-Y. , Sadeghi, S. , Bader, D. A. , and Cortes, D. H. , 2018, “ Ultrasound Shear Wave Elastography of the Elbow Ulnar Collateral Ligament: Reliability Test and a Preliminary Case Study in a Baseball Pitcher,” ASME J. Eng. Sci. Med. Diagn. Ther., 1(1), p. 011004. 10.1115/1.4038259 [DOI] [Google Scholar]
- [32]. Sadeghi, S. , Lin, C.-Y. , Bader, D. A. , and Cortes, D. H. , 2018, “ Evaluating Changes in Shear Modulus of Elbow Ulnar Collateral Ligament in Overhead Throwing Athletes Over the Course of a Competitive Season,” ASME J. Eng. Sci. Med. Diagn. Ther., 1(4), p. 041008. 10.1115/1.4041503 [DOI] [Google Scholar]
- [33]. Sadeghi, S. , Johnson, M. , Bader, D. A. , and Cortes, D. H. , 2019, “ The Shear Modulus of Lower-Leg Muscles Correlates to Intramuscular Pressure,” J. Biomech., 83, pp. 190–196. 10.1016/j.jbiomech.2018.11.045 [DOI] [PubMed] [Google Scholar]
- [34]. Sadeghi, S. , Lin, C.-Y. , and Cortes, D. H. , 2019, “ Narrowband Shear Wave Generation Using Sinusoidally Modulated Acoustic Radiation Force,” IEEE Trans. Ultrasonics, Ferroelectr., Frequency Control, 66(2), pp. 264–272. 10.1109/TUFFC.2018.2884847 [DOI] [PubMed] [Google Scholar]
- [35]. Sullivan, M. J. , Bishop, S. R. , and Pivik, J. , 1995, “ The Pain Catastrophizing Scale: Development and Validation,” Psychol. Assessment, 7(4), pp. 524–532. 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- [36]. Fritz, J. M. , and Irrgang, J. J. , 2001, “ A Comparison of a Modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale,” Phys. Ther., 81(2), pp. 776–788. 10.1093/ptj/81.2.776 [DOI] [PubMed] [Google Scholar]
- [37]. Langley, G. , and Sheppeard, H. , 1985, “ The Visual Analogue Scale: Its Use in Pain Measurement,” Rheumatol. Int., 5(4), pp. 145–148. 10.1007/BF00541514 [DOI] [PubMed] [Google Scholar]
- [38]. Sions, J. M. , Velasco, T. O. , Teyhen, D. S. , and Hicks, G. E. , 2014, “ Ultrasound Imaging: Intraexaminer and Interexaminer Reliability for Multifidus Muscle Thickness Assessment in Adults Aged 60 to 85 Years Versus Younger Adults,” J. Orthop. Sports Phys. Ther., 44(6), pp. 425–434. 10.2519/jospt.2014.4584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Sions, J. M. , Teyhen, D. S. , and Hicks, G. E. , 2017, “ Criterion Validity of Ultrasound Imaging: Assessment of Multifidi Cross-Sectional Area in Older Adults With and Without Chronic Low Back Pain,” J. Geriatric Phys. Ther., 40(2), pp. 74–79. 10.1519/JPT.0000000000000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Akagi, R. , Yamashita, Y. , and Ueyasu, Y. , 2015, “ Age-Related Differences in Muscle Shear Moduli in the Lower Extremity,” Ultrasound Med. Biol., 41(11), pp. 2906–2912. 10.1016/j.ultrasmedbio.2015.07.011 [DOI] [PubMed] [Google Scholar]
- [41]. Dieterich, A. V. , Andrade, R. J. , Le Sant, G. , Falla, D. , Petzke, F. , Hug, F. , and Nordez, A. , 2017, “ Shear Wave Elastography Reveals Different Degrees of Passive and Active Stiffness of the Neck Extensor Muscles,” Eur. J. Appl. Physiol., 117(1), pp. 171–178. 10.1007/s00421-016-3509-5 [DOI] [PubMed] [Google Scholar]
- [42]. Alis, D. , Durmaz, E. S. M. , Alis, C. , Erol, B. C. , Okur, B. , Kizilkilic, O. , and Mihmanli, I. , 2019, “ Shear Wave Elastography of the Lumbar Multifidus Muscle in Patients With Unilateral Lumbar Disk Herniation,” J. Ultrasound Med., 38(7), pp. 1695–1703. 10.1002/jum.14854 [DOI] [PubMed] [Google Scholar]
- [43]. Min, S.-H. , Kim, M.-H. , Seo, J.-B. , Lee, J.-Y. , and Lee, D.-H. , 2009, “ The Quantitative Analysis of Back Muscle Degeneration After Posterior Lumbar Fusion: Comparison of Minimally Invasive and Conventional Open Surgery,” Asian Spine J., 3(2), p. 89. 10.4184/asj.2009.3.2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Virk, S. S. , Niedermeier, S. , Yu, E. , and Khan, S. N. , 2014, “ Adjacent Segment Disease,” Orthopedics, 37(8), pp. 547–555. 10.3928/01477447-20140728-08 [DOI] [PubMed] [Google Scholar]
- [45]. Kong, W. Z. , Goel, V. K. , Gilbertson, L. G. , and Weinstein, J. N. , 1996, “ Effects of Muscle Dysfunction on Lumbar Spine Mechanics: A Finite Element Study Based on a Two Motion Segments Model,” Spine, 21(19), pp. 2197–2206. 10.1097/00007632-199610010-00004 [DOI] [PubMed] [Google Scholar]
- [46]. Ateş, F. , Hug, F. , Bouillard, K. , Jubeau, M. , Frappart, T. , Couade, M. , Bercoff, J. , and Nordez, A. , 2015, “ Muscle Shear Elastic Modulus is Linearly Related to Muscle Torque Over the Entire Range of Isometric Contraction Intensity,” J. Electromyogr. Kinesiol., 25(4), pp. 703–708. 10.1016/j.jelekin.2015.02.005 [DOI] [PubMed] [Google Scholar]
- [47]. Adnan, R. , Van Oosterwijck, J. , Danneels, L. , Willems, T. , Meeus, M. , Crombez, G. , and Goubert, D. , 2020, “ Structural Changes of Lumbar Muscles in Non-Specific Low Back Pain,” Pain Phys., 19(7), pp. 1–E999. 10.3233/BMR-191548 [DOI] [PubMed] [Google Scholar]


