ABSTRACT
Objectives
First, to compare published Doppler reference charts of the ratios of flow in the fetal middle cerebral and umbilical arteries (i.e. the cerebroplacental ratio (CPR) and umbilicocerebral ratio (UCR)). Second, to assess the association of thresholds of CPR and UCR based on these charts with short‐term composite adverse perinatal outcome in a cohort of pregnancies considered to be at risk of late preterm fetal growth restriction.
Methods
Studies presenting reference charts for CPR or UCR were searched for in PubMed. Formulae for plotting the median and the 10th percentile (for CPR) or the 90th percentile (for UCR) against gestational age were extracted from the publication or calculated from the published tables. Data from a prospective European multicenter observational cohort study of singleton pregnancies at risk of fetal growth restriction at 32 + 0 to 36 + 6 weeks' gestation, in which fetal arterial Doppler measurements were collected longitudinally, were used to compare the different charts. Specifically, the association of UCR and CPR thresholds (CPR < 10th percentile or UCR ≥ 90th percentile and multiples of the median (MoM) values) with composite adverse perinatal outcome was analyzed. The association was also compared between chart‐based thresholds and absolute thresholds. Composite adverse perinatal outcome comprised both abnormal condition at birth and major neonatal morbidity.
Results
Ten studies presenting reference charts for CPR or UCR were retrieved. There were large differences between the charts in the 10th and 90th percentile values of CPR and UCR, respectively, while median values were more similar. In the gestational‐age range of 28–36 weeks, there was no relationship between UCR or CPR and gestational age. From the prospective observational study, 856 pregnancies at risk of late‐onset preterm fetal growth restriction were included in the analysis. The association of abnormal UCR or CPR with composite adverse perinatal outcome was similar for percentile thresholds or MoM values, as calculated from the charts, and for absolute thresholds, both on univariable analysis and after adjustment for gestational age at measurement, estimated fetal weight MoM and pre‐eclampsia. The adjusted odds ratio for composite adverse perinatal outcome was 3.3 (95% CI, 1.7–6.4) for an absolute UCR threshold of ≥ 0.9 or an absolute CPR threshold of < 1.11 (corresponding to ≥ 1.75 MoM), and 1.6 (95% CI, 0.9–2.9) for an absolute UCR threshold of ≥ 0.7 to < 0.9 or an absolute CPR threshold of ≥ 1.11 to < 1.43 (corresponding to ≥ 1.25 to < 1.75 MoM).
Conclusions
In the gestational‐age range of 32 to 36 weeks, adjustment of CPR or UCR for gestational age is not necessary when assessing the risk of adverse outcome in pregnancies at risk of fetal growth restriction. The adoption of absolute CPR or UCR thresholds, independent of reference charts, is feasible and makes clinical assessment simpler than if using percentiles or other gestational‐age normalized units. The high variability in percentile threshold values among the commonly used UCR and CPR reference charts hinders reliable diagnosis and clinical management of late preterm fetal growth restriction. © 2021 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: adverse outcome, brain sparing, cerebroplacental ratio, Doppler, fetal growth restriction, middle cerebral artery, percentile, reference chart, umbilicocerebral ratio
CONTRIBUTION —
What are the novel findings of this work?
Comparison of different cerebroplacental ratio (CPR) or umbilicocerebral ratio (UCR) reference charts demonstrated large differences in threshold values. In pregnancies at risk of late preterm fetal growth restriction (FGR) (32 + 0 to 36 + 6 weeks), absolute thresholds for CPR or UCR had a similar association with adverse short‐term perinatal outcome as did percentile thresholds or thresholds based on other gestational‐age normalized units.
What are the clinical implications of this work?
Absolute UCR or CPR thresholds can be used for clinical management of late preterm FGR. This makes fetal assessment simpler and does not require Doppler reference charts.
INTRODUCTION
Doppler assessment of the fetal umbilical and cerebral circulations has become important in the diagnosis and management of fetal growth restriction (FGR) 1 , 2 . Cerebral blood‐flow redistribution (otherwise known as ‘brain sparing’) indicates preferential fetal cardiac output redistribution toward the brain, heart and adrenal glands in the presence of hypoxemia 3 . It can be evaluated through the assessment of fetal middle cerebral artery (MCA) pulsatility index (PI), systolic/diastolic ratio or resistance index, or the ratio between MCA‐PI and umbilical artery (UA) PI, namely the so‐called cerebroplacental ratio (CPR) 4 and umbilicocerebral ratio (UCR) 5 . Several methods for relating cerebral blood‐flow redistribution to gestational age have been proposed, such as computing percentiles, multiples of the median (MoM), Z‐scores or absolute values 6 . Calculation of the ratio of MCA‐PI to UA‐PI (i.e. CPR) has gained prominence, and CPR < 5th percentile has been proposed to define cerebral blood‐flow redistribution 2 . This requires the use of Doppler reference charts with accurate and reproducible percentile thresholds.
Many studies have reported an association between cerebral blood‐flow redistribution and adverse short‐ and long‐term outcomes in FGR 7 , 8 , 9 , 10 , 11 , 12 . However, the value of cerebral blood‐flow redistribution ratios in determining the timing of delivery is still not known 13 , 14 , 15 . This uncertainty may, at least in part, be due to the heterogeneity of reference charts for fetal arterial Doppler parameters 16 , some of which may be explained by methodological shortcomings. Ruiz‐Martinez et al. 17 performed a simulation analysis in a cohort of small‐for‐gestational‐age fetuses using percentile thresholds from the 10 most frequently cited Doppler reference charts for UA‐PI, MCA‐PI and CPR. They concluded that the variation in percentile thresholds could result in large variation in clinical management. The objectives of the current study were: (1) to compare published Doppler reference charts of the ratios of MCA‐PI and UA‐PI (i.e. CPR and UCR); and (2) to assess the association of thresholds of these reference charts with short‐term adverse perinatal outcomes in a cohort of pregnancies considered to be at risk of late preterm FGR.
METHODS
Doppler reference chart selection and evaluation
Reference charts that described values for UA‐PI and MCA‐PI and their ratio (UCR or CPR), within the gestational‐age range of 20–40 weeks in a normal population, were selected from PubMed using the search string: ““((“1970/01/01”[Date‐Publication] : “2020/07/10”[Date ‐ Publication])) AND ((((reference chart)[Title/Abstract] OR (reference value))[Title/Abstract] AND ((cerebro‐placental)[Title/Abstract] OR (cerebroplacental)[Title/Abstract] OR (umbilical cerebral artery)[Title/Abstract] OR (umbilical‐cerebral)[Title/Abstract] OR (umbilicocerebral))[Title/Abstract] AND (ratio))[Title/Abstract])””. The aim was to identify studies that allowed the extraction of a formula using gestational age for the calculation of median values as well as the 10th percentile values for CPR or the 90th percentile values for UCR. If such formulae were not available, data from published tables specifying the median and 10th or 90th percentile values for each week of gestation were used to determine a gestational‐age‐dependent formula. The data were fitted in polynomial models of increasing order, and the model with the highest R 2 was selected. If only mean and SD were reported, then it was assumed that the data distribution was normal, and these were used for calculation of the 10th or 90th percentiles. If the 10th or 90th percentiles were not available in the publication but the 5th or 95th percentiles were provided, the 10th or 90th percentiles were calculated as (mean ± 1.282 × ((95th percentile – 50th percentile)/1.645)) for each week of gestation. Studies in which a mean or median value for each week of gestation was not available were excluded from further assessment. Data from all 10th percentile (CPR), 90th percentile (UCR) and median formulae were plotted against gestational age.
Clinical assessment of Doppler reference charts
Study population
Data were collected during a prospective multicenter observational study conducted between 1st April 2017 and 1st July 2018 in 33 European perinatal centers with fetal medicine and specialized neonatal intensive care services. Observational data from this study have been described previously 12 . In brief, women were eligible if they had a singleton pregnancy at 32 + 0 to 36 + 6 weeks' gestation with a fetus considered to be at risk for growth restriction, defined as estimated fetal weight (EFW) or abdominal circumference (AC) < 10th percentile, abnormal arterial Doppler or a fall in AC growth velocity of at least 40 percentile points from the 20‐week scan. The references for EFW, AC and Doppler parameters were based on local charts. Fetuses with absent end‐diastolic flow in the UA, an abnormal cardiotocogram, an immediate indication for delivery or a structural abnormality were not eligible. Pre‐eclampsia was defined as hypertension and proteinuria, or hypertension and clinical signs of pre‐eclampsia, at any time during pregnancy 18 .
Study endpoint
The primary outcome was a composite of abnormal condition at birth, major neonatal morbidity and mortality. Abnormal condition at birth was defined as at least one of the following: Apgar score < 7 at 5 min, UA pH < 7.0, umbilical vein pH < 7.1, need for resuscitation with intubation, chest compressions or medication, or stillbirth. Major neonatal morbidity was defined as at least one of the following: neurological abnormality (intracerebral hemorrhage Grade 3 or 4, periventricular leukomalacia Grade 2 or 3, encephalopathy or seizures necessitating antiepileptic drug treatment), cardiovascular abnormality (hypotensive treatment, ductus arteriosus treatment or disseminated coagulopathy), respiratory morbidity (respiratory support for more than 1 week, mechanical ventilation, meconium aspiration or persistent pulmonary hypertension) or sepsis (clinical sepsis with positive blood culture, necrotizing enterocolitis (Bell's Stage 2 or greater) or meningitis).
Statistical analysis
Using all selected reference charts, the median and 10th or 90th percentile values were calculated, based on the gestational age at measurement. CPR measurements < 10th percentile or UCR measurements ≥ 90th percentile were coded as abnormal. Median values from reference charts for CPR were transformed to 1/UCR or vice versa. However, percentiles were not transformed because the distributions of UCR and CPR are skewed in an opposite direction and transformation may have caused bias. For each UCR or CPR measurement, MoM was calculated as: observed value/median value for gestational age.
The fetal arterial Doppler measurement obtained at inclusion was selected from each woman for assessment of the association between composite adverse perinatal outcome and the 10th and 90th percentile thresholds of CPR and UCR, respectively, based on the selected reference charts. True‐positive and false‐negative rates and odds ratios (OR) with 95% CIs were calculated. Furthermore, ORs with 95% CIs were calculated for the association of all threshold values with composite adverse perinatal outcome, with adjustment for gestational age at measurement, EFW MoM and pre‐eclampsia, using logistic regression analysis.
The predictive value of all models was estimated by plotting a receiver‐operating‐characteristics (ROC) curve and calculating the area under the curve (AUC). The coordinates of the curves were used to determine the sensitivity and specificity of the models for the prediction of composite adverse outcome. This analysis was repeated for absolute UCR and CPR threshold values to assess if MoM values, which were adjusted for gestational age, had a better predictive value than unadjusted UCR or CPR for the composite adverse perinatal outcome.
From the available reference chart data, we aimed to ascertain if a UCR or CPR threshold value that improves the prediction of composite adverse perinatal outcome could be selected.
Ethical approval
This study was observational, and practice (monitoring, delivery and steroid administration) was based on existing local guidance. Data were recorded and anonymized after delivery outcomes had been ascertained. In six countries (19 centers), ethical approval was required and obtained, and participating women gave informed written consent. In the remaining five countries, this was not required.
RESULTS
Doppler reference chart selection
The PubMed search identified 66 publications. Figure 1 shows a flowchart of the selection process. Ten studies complied with the selection criteria and were included in the study 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 . Table S1 summarizes the characteristics of the selected studies. Two studies recruited women prospectively 21 , 24 ; all other studies selected data from women who visited the antenatal clinic.
Figure 1.
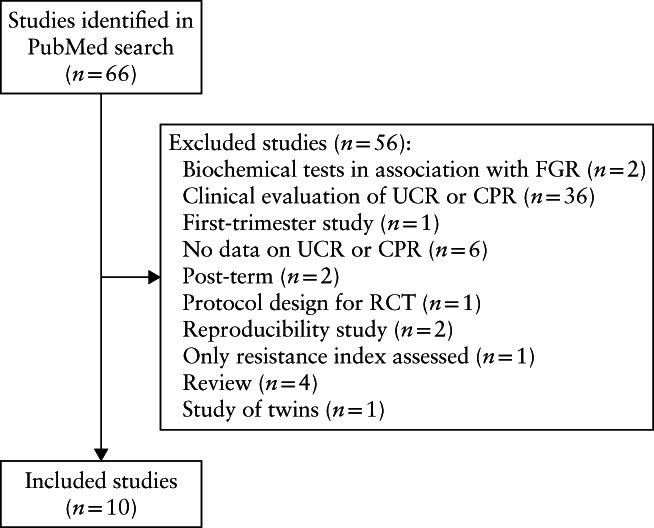
Flowchart showing selection of studies reporting reference charts of ratios of middle cerebral artery and umbilical artery pulsatility index (i.e. cerebroplacental ratio (CPR) or umbilicocerebral ratio (UCR)). FGR, fetal growth restriction; RCT, randomized controlled trial.
Three of the included studies reported reference formulae for the mean values of CPR or UCR 19 , 20 , 21 . Three studies contained a formula for the median 22 , 23 , 24 . For the four other studies, gestational‐age‐dependent formulae for the median were determined from the published table data of the median values 25 , 26 , 27 , 28 .
Two studies reported a formula for percentiles 22 , 24 . For two charts, the percentiles were calculated from a published formula for the mean or median and SD 21 , 23 , while in two other studies, a formula for the mean was available, but the formula for SD had to be determined from the tables 19 , 20 . For the remaining four charts, the SD was derived from table data for the percentile values 25 , 26 , 27 , 28 . Formulae were determined from chart data using a polynomial model with an order (second or third) that achieved the highest R 2 value.
Doppler reference chart evaluation
For all charts, the 10th percentile of CPR was plotted against gestational age (Figure 2a). Because all studies, except one 19 , reported CPR data, CPR was used for the plot, and UCR data from the study of Arduini and Rizzo 19 were transformed to CPR (1/UCR). This was done for ease of presentation, although this transformation might have caused some bias. Variation in the percentile threshold values between the charts was large; in the most clinically relevant gestational‐age period of 28 to 36 weeks, the 10th percentile value of CPR varied between approximately 0.9 and 1.8.
Figure 2.
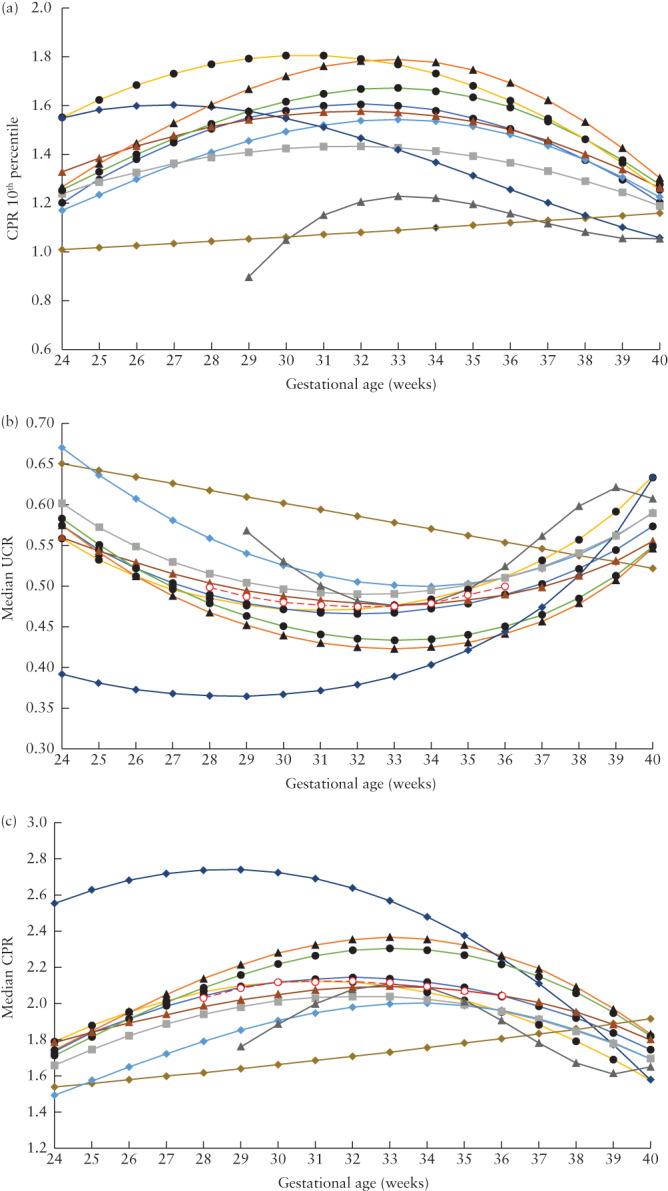
Cerebroplacental ratio (CPR) 10th percentile values (a) and median umbilicocerebral ratio (UCR) (b) and CPR (c) values for different reference charts, according to gestational age. The UCR charts of Arduini and Rizzo
19
were transformed to CPR (1/UCR) in (a) and (c); all other charts were for CPR and were transformed to UCR (1/CPR) in (b). Only first author of study is given:  Arduini (1990)
19
;
Arduini (1990)
19
;  , Baschat (2003)
20
;
, Baschat (2003)
20
;  , Ebbing (2007)
21
;
, Ebbing (2007)
21
;  , Morales‐Rosello (2015)
22
;
, Morales‐Rosello (2015)
22
;  , Srikumar (2017)
26
;
, Srikumar (2017)
26
;  , Flatley (2019)
25
;
, Flatley (2019)
25
;  , Ciobanu (2019)
23
;
, Ciobanu (2019)
23
;  , Dias (2019)
27
;
, Dias (2019)
27
;  , Zohav (2019)
28
;
, Zohav (2019)
28
;  , Acharya (2020)
24
;
, Acharya (2020)
24
;  , Median of all medians in (b) and (c).
, Median of all medians in (b) and (c).
Figure 2b shows the median UCR values for all reference charts. There were two outliers: the charts of Dias et al. 27 and of Arduini and Rizzo 19 . The chart of Dias et al. 27 had far higher CPR values (or lower UCR values) in the earlier gestational‐age range of 24–30 weeks, as compared with the other charts. All charts had a polynomial profile, except for the chart of Arduini and Rizzo 19 , which had a linear profile and differed from the other charts in the gestational‐age window of 28–35 weeks. When the charts of Dias et al. 27 and Arduini and Rizzo 19 were excluded, the range between the median values of UCR in the remaining eight charts was less than 0.1. Figure 2c shows a plot of the median values of CPR. When the charts of Dias et al. 27 and Arduini and Rizzo 19 were excluded, the range between the median values of CPR was approximately 0.4, which was proportionally similar to the variation observed for UCR.
Assessment of the graph of UCR median values for the different charts (Figure 2b) showed that, in the gestational‐age range of 28 to 36 weeks, the profile of the median values was nearly linear and not related to gestational age. The pooled median of all reference charts in the range of 28 to 36 weeks had an ellipsoid pattern (Figure 2b). However, the difference between the highest UCR values, at 28 and 36 weeks, and the nadir at 33 weeks was only 0.025. For CPR, plotting the pooled median of all charts (Figure 2c) showed that the difference between the lowest values, at 28 and 36 weeks, and the peak at 31 weeks was 0.09. These differences were both roughly 5% of the average value of the respective parameter.
Clinical assessment of Doppler reference charts
In the prospective study, complete delivery and outcome data were recorded for 873 patients at risk of late‐onset preterm FGR in the study database. Seventeen cases were excluded because of the presence of a major congenital abnormality, leaving 856 pregnancies for the final cohort analysis. Demographic, obstetric and fetal Doppler velocimetry characteristics of the included pregnancies are shown in Table 1 and neonatal outcome is given in Table 2.
Table 1.
Demographic and obstetric characteristics of study population of 856 singleton pregnancies at risk for late‐onset preterm fetal growth restriction
| Variable | Value |
|---|---|
| Maternal age (years) | 31 (28–35) |
| Nulliparous | 524 (61) |
| Body mass index (kg/m2) | 22.5 (20.3–26.0) |
| Smoker | 68 (8) |
| Diabetes (Type 1, 2 or gestational) | 70 (8) |
| Chronic hypertension | 19 (2) |
| At inclusion | |
| Gestational age (weeks) | 34 (33–35) |
| Indication for inclusion* | |
| EFW or AC < 10th percentile | 792 (93) |
| AC growth velocity drop ≥ 40 percentile points | 50 (6) |
| Doppler abnormality | 98 (11) |
| EFW (g) | 1894 (1624–2145) |
| EFW Z‐score | –1.52 (–2.0 to –1.1) |
| Umbilical artery PI | 1.00 (0.86–1.14) |
| Middle cerebral artery PI | 1.75 (1.51–2.01) |
| UCR | 0.56 (0.47–0.69) |
| CPR | 1.79 (1.45–2.14) |
| Before delivery | |
| Pre‐eclampsia or HELLP | 79 (9) |
| Any hypertensive disorder of pregnancy | 119 (14) |
| Corticosteroids for fetal lung maturation† | 98 (11) |
| Total number of arterial Doppler measurements | 2770 |
| Number of arterial Doppler measurements per woman | 3 (2–4) |
| Inclusion–delivery interval (days) | 27 (14–38) |
| Delivery | |
| Planned CS for: | 219 (26) |
| Fetal condition (CTG or Doppler) | 155/219 (71) |
| Fetal growth/EFW | 25/219 (11) |
| Maternal condition | 39/219 (18) |
| Induction of labor for: | 369 (43) |
| Fetal condition (CTG or Doppler) | 112/369 (30) |
| Fetal growth/EFW | 213/369 (58) |
| Maternal condition | 44/369 (12) |
| Spontaneous onset of labor | 268 (31) |
| CS after onset of labor | 117/637 (18) |
Data are presented as median (interquartile range), n (%) or n/N (%).
Multiple indications possible.
> 24 h before delivery.
AC, abdominal circumference; CPR, cerebroplacental ratio; CS, Cesarean section; CTG, cardiotocography; EFW, estimated fetal weight; PI, pulsatility index; UCR, umbilicocerebral ratio.
Table 2.
Neonatal outcome of study population of 856 singleton pregnancies at risk for late‐onset preterm fetal growth restriction
| Variable | Value |
|---|---|
| Gestational age at delivery (weeks) | 38 (37–39) |
| Birth weight (g) | 2478 (2140–2790) |
| Birth‐weight Z‐score | –1.7 (–2.3 to –1.1) |
| Birth weight < 10th percentile | 596 (70) |
| Male sex | 372 (43) |
| Composite adverse outcome* | 93 (11) |
| Abnormal condition at birth† | 27 (3) |
| Fetal death | 2 (0) |
| Umbilical artery pH < 7.0 or umbilical vein pH < 7.1‡ | 7 (1) |
| 5‐min Apgar score < 7 | 15 (2) |
| Resuscitation with intubation or medication | 10 (1) |
| Major neonatal morbidity† | 77 (9) |
| Cerebral | 7 (1) |
| Cardiovascular | 7 (1) |
| Respiratory§ | 53 (6) |
| Infection | 17 (2) |
| Neonatal death | 0 (0) |
Data are presented as median (interquartile range) or n (%).
Defined as abnormal condition at birth or major neonatal morbidity or mortality.
Multiple conditions possible.
Data were missing for 17% of cases.
Of the 53 cases of respiratory morbidity, 39 (74%) had only some respiratory support of short duration in the first week postpartum.
Although UCR ≥ 90th percentile or CPR < 10th percentile was associated significantly with composite adverse outcome for all reference charts, the false‐negative rate was high (Table 3). The differences between the charts in the 90th and 10th percentiles caused variation in sensitivity (ranging from 15% in the study of Srikumar et al. 26 to 28% in the study of Arduini and Rizzo 19 ) and in specificity in the opposite direction (93% and 90%, respectively).
Table 3.
Performance of umbilicocerebral ratio (UCR) ≥ 90th percentile or cerebroplacental ratio (CPR) < 10th percentile at inclusion, in the prediction of composite adverse perinatal outcome in 856 pregnancies at risk of late‐onset preterm fetal growth restriction, using different published reference charts
| Study | Ratio assessed | TPR* | FPR | Specificity (%)† | Crude OR (95% CI) |
|---|---|---|---|---|---|
| Arduini (1990) 19 | UCR | 18/64 (28) | 75/792 (9) | 90 | 3.7 (2.1–6.8) |
| Baschat (2003) 20 | CPR | 51/270 (19) | 42/586 (7) | 93 | 3.0 (1.9–4.7) |
| Ebbing (2007) 21 | CPR | 60/404 (15) | 33/452 (7) | 93 | 2.2 (1.4–3.5) |
| Morales‐Rosello (2015) 22 | CPR | 39/184 (21) | 54/672 (8) | 92 | 3.1 (2.0–4.8) |
| Srikumar (2017) 26 | CPR | 58/377 (15) | 35/479 (7) | 93 | 2.3 (1.5–3.6) |
| Ciobanu (2019) 23 | CPR | 49/252 (19) | 44/604 (7) | 93 | 3.1 (2.0–4.8) |
| Dias (2019) 27 | CPR | 39/161 (24) | 54/695 (8) | 92 | 3.8 (2.4–6.0) |
| Flatley (2019) 25 | CPR | 50/263 (19) | 43/593 (7) | 93 | 3.0 (1.9–4.6) |
| Zohav (2019) 28 | CPR | 22/84 (26) | 71/772 (9) | 91 | 3.5 (2.0–6.0) |
| Acharya (2020) 24 | CPR | 53/339 (16) | 40/517 (8) | 92 | 2.2 (1.4–3.4) |
Only first author's name is given for each study.
Data are given as n/N (%), unless indicated otherwise.
True‐positive rate (TPR) is equal to sensitivity.
Specificity is equal to 100 − false‐positive rate (FPR).
OR, odds ratio.
ORs for the association between composite adverse perinatal outcome and the reference chart thresholds (UCR ≥ 90th percentile or CPR < 10th percentile) were calculated using logistic regression analysis with adjustment for gestational age at measurement, EFW MoM and pre‐eclampsia (Table 4). The adjusted OR values varied from 1.7 to 2.7. However, the AUC of the ROC curve of the predicted probability for composite adverse perinatal outcome was similar for all charts (range, 0.71–0.73) 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 . ORs for composite adverse perinatal outcome were also calculated for each reference chart using UCR MoM at inclusion, with adjustment for gestational age at measurement, EFW MoM at inclusion and pre‐eclampsia (Table 5). The adjusted ORs were similar for all charts (range, 1.5–1.7) and the AUCs of the ROC curve of the calculated probability of composite adverse perinatal outcome were the same (0.71 (95% CI, 0.66–0.77)). Repeating these calculations for CPR MoM produced similar results, with identical AUC values (Table 5).
Table 4.
Association between composite adverse perinatal outcome and umbilicocerebral ratio (UCR) ≥ 90th percentile or cerebroplacental ratio (CPR) < 10th percentile at inclusion, adjusted for gestational age at measurement (GA), estimated fetal weight multiples of the median (EFW MoM) and pre‐eclampsia, in 856 pregnancies at risk for late‐onset preterm fetal growth restriction, using different published reference charts
| Study | Ratio assessed | aOR (95% CI) | AUC (95% CI) |
|---|---|---|---|
| Arduini (1990) 19 | UCR | 2.7 (1.4–5.1) | 0.73 (0.67–0.78) |
| Baschat (2003) 20 | CPR | 2.2 (1.4–3.5) | 0.73 (0.67–0.78) |
| Ebbing (2007) 21 | CPR | 1.7 (1.1–2.7) | 0.71 (0.65–0.77) |
| Morales‐Rosello (2015) 22 | CPR | 2.3 (1.4–3.8) | 0.72 (0.66–0.78) |
| Srikumar (2017) 26 | CPR | 1.7 (1.0–2.7) | 0.71 (0.66–0.77) |
| Ciobanu (2019) 23 | CPR | 2.2 (1.4–3.6) | 0.72 (0.66–0.78) |
| Dias (2019) 27 | CPR | 2.6 (1.6–4.3) | 0.73 (0.67–0.78) |
| Flatley (2019) 25 | CPR | 2.2 (1.4–3.5) | 0.72 (0.66–0.78) |
| Zohav (2019) 28 | CPR | 2.4 (1.3–4.4) | 0.73 (0.67–0.78) |
| Acharya (2020) 24 | CPR | 1.7 (1.0–2.6) | 0.71 (0.65–0.77) |
| Adjustment parameters | |||
| GA (in weeks) | — | 0.8 (0.7–1.0) | — |
| EFW MoM | — | 0.6 (0.4–0.7) | — |
| Pre‐eclampsia | — | 2.1 (1.2–3.7) | — |
Only first author's name is given for each study.
At a sensitivity of 70%, specificity was approximately 60% for all models.
aOR, adjusted odds ratio; AUC, area under receiver‐operating‐characteristics curve.
Table 5.
Association between composite adverse perinatal outcome and umbilicocerebral ratio (UCR) or cerebroplacental ratio (CPR) multiples of the median (MoM) at inclusion (as a continuous variable), adjusted for gestational age at measurement (GA), estimated fetal weight (EFW) MoM and pre‐eclampsia, in 856 pregnancies at risk of late‐onset preterm fetal growth restriction, using different published reference charts
| Study | Adjusted OR (95% CI) | AUC (95% CI) | |
|---|---|---|---|
| UCR MoM | CPR MoM | ||
| Arduini (1990) 19 | 1.74 (1.04–2.90) | 0.45 (0.21–0.98) | 0.71 (0.66–0.77) |
| Baschat (2003) 20 | 1.58 (1.04–2.41) | 0.38 (0.15–0.97) | 0.71 (0.66–0.77) |
| Ebbing (2007) 21 | 1.51 (1.03–2.22) | 0.34 (0.12–0.96) | 0.71 (0.66–0.77) |
| Morales‐Rosello (2015) 22 | 1.62 (1.04–2.52) | 0.39 (0.16–0.97) | 0.71 (0.66–0.77) |
| Srikumar (2017) 26 | 1.60 (1.04–2.47) | 0.38 (0.15–0.97) | 0.71 (0.66–0.77) |
| Ciobanu (2019) 23 | 1.63 (1.04–2.56) | 0.40 (0.16–0.97) | 0.71 (0.66–0.77) |
| Dias (2019) 27 | 1.46 (1.02–2.09) | 0.33 (0.11–0.90) | 0.71 (0.66–0.77) |
| Flatley (2019) 25 | 1.59 (1.04–2.45) | 0.38 (0.15–0.97) | 0.71 (0.66–0.77) |
| Zohav (2019) 28 | 1.60 (1.04–2.47) | 0.38 (0.15–0.95) | 0.71 (0.66–0.77) |
| Acharya (2020) 24 | 1.53 (1.03–2.26) | 0.35 (0.13–0.96) | 0.71 (0.66–0.77) |
| Adjustment parameters | |||
| GA (in weeks) | 0.86 (0.73–0.99) | — | |
| EFW MoM | 0.54 (0.41–0.71) | — | |
| Pre‐eclampsia | 2.27 (1.33–3.86) | — | |
Only first author's name is given for each study.
At a sensitivity of 70%, specificity was approximately 60% for all models.
For assessment of CPR, the UCR charts of Arduini and Rizzo 19 were transformed (1/UCR); all other charts were for CPR and were transformed (1/CPR) for assessment of UCR.
AUC, area under receiver‐operating‐characteristics curve; OR, odds ratio.
For the classification of UCR values, we used the pooled median of all charts for all gestational ages. UCR was categorized as < 1.25 MoM, ≥ 1.25 to < 1.75 MoM and ≥ 1.75 MoM, which corresponded to absolute UCR values of < 0.7, ≥ 0.7 to < 0.9 and ≥ 0.9, respectively, or absolute CPR values of ≥ 1.43, ≥ 1.11 to < 1.43 and < 1.11, respectively. These values were close to the average 75th and 90th percentiles calculated for UCR from the selected charts. Specifying these absolute UCR categories for gestational age at inclusion at 32–33 weeks, 34–35 weeks and 36 weeks showed that abnormal UCR ≥ 0.9 or CPR < 1.11 was associated significantly with composite adverse perinatal outcome in each gestational‐age category (Table 6). The absolute UCR/CPR thresholds, gestational age at measurement, EFW MoM and pre‐eclampsia were entered into a logistic regression model with composite adverse perinatal outcome as the dependent variable (Table 7). UCR ≥ 0.9 or CPR < 1.11 had an adjusted OR for composite adverse outcome of 3.3 (95% CI, 1.7–6.4). The AUC of the model was 0.73 (95% CI, 0.67–0.78), with a sensitivity of 70% and specificity of 64%.
Table 6.
Rate of composite adverse perinatal outcome in 856 pregnancies at risk of late‐onset preterm fetal growth restriction, overall and according to umbilicocerebral ratio (UCR) and cerebroplacental ratio (CPR) absolute thresholds at inclusion and gestational age at measurement (GA)
| GA | UCR/CPR value threshold | All | ||
|---|---|---|---|---|
| < 0.7/≥ 1.43 | ≥ 0.7 to < 0.9/≥ 1.11 to < 1.43 | ≥ 0.9/< 1.11 | ||
| 32–33 weeks | 28/311 (9) | 14/61 (23)* | 12/29 (41)* | 54/401 (13) |
| 34–35 weeks | 21/242 (9) | 4/52 (8) | 6/28 (21)* | 31/322 (10) |
| 36 weeks | 5/105 (5) | 2/22 (9) | 1/6 (17)* | 8/133 (6) |
| All (32–36 weeks) | 54/658 (8) | 20/135 (15) | 19/63 (30)* | 93/856 (11) |
Data are given as n/N (%).
Pearson χ‐square P < 0.05, compared with UCR < 0.7 or CPR ≥ 1.43.
Table 7.
Association between composite adverse perinatal outcome and absolute thresholds of umbilicocerebral ratio (UCR) or cerebroplacental ratio (CPR) assessed at inclusion, gestational age at measurement (GA), estimated fetal weight multiples of the median (EFW MoM) and pre‐eclampsia, in 856 pregnancies at risk of late‐onset preterm fetal growth restriction
| Parameter | aOR (95% CI) | P |
|---|---|---|
| UCR < 0.7/CPR ≥ 1.43 | — | < 0.001 |
| UCR ≥ 0.7 to < 0.9/CPR ≥ 1.11 to < 1.43 | 1.6 (0.9–2.9) | 0.10 |
| UCR ≥ 0.9/CPR < 1.11 | 3.3 (1.7–6.4) | < 0.001 |
| GA (in weeks) | 0.8 (0.7–0.9) | 0.03 |
| EFW MoM | 0.6 (0.4–0.7) | < 0.001 |
| Pre‐eclampsia | 2.0 (1.2–3.5) | 0.01 |
Area under receiver‐operating‐characteristics curve of the model was 0.73 (95% CI, 0.67–0.78), with sensitivity of 70% and specificity of 64%.
aOR, adjusted odds ratio.
DISCUSSION
Principal findings
Different reference charts for UCR and CPR are not directly comparable, but all demonstrate that the indices are not related to gestational age between 28 and 36 weeks. In prospectively collected observational data of women at risk for FGR at a gestational age of 32 + 0 to 36 + 6 weeks, percentiles, MoM values and absolute thresholds of UCR and CPR had a similar association with short‐term adverse perinatal outcome. There is, in our view, no reason to use percentile values of UCR or CPR for the clinical management of fetuses at risk of FGR, as the same information can be obtained more simply from absolute thresholds (at least in the gestational‐age window of 32 + 0 to 36 + 6 weeks, as assessed in our study), reducing the risk of error and variability.
We found large differences in reported percentile threshold values for CPR and UCR between different Doppler reference charts, which was similarly demonstrated in two previous studies 16 , 17 . The most probable reason for these differences is an insufficient number of included cases for reliable determination of the limits of normality for each week of gestation, in addition to other methodological aspects 16 . As values close to normal are far more common than abnormal values, the variation in median values is much smaller than that for the percentile threshold values at the limits of normality, thus MoM values might be preferred over percentiles. However, because the median reference chart values have a nearly linear profile at 28 to 36 weeks, without a relationship with gestational age, adjustment for gestational age seems unnecessary, at least in fetuses in the gestational‐age window of 32 + 0 to 36 + 6 weeks, as assessed in the current study.
Based on the assessment of our observational data in pregnancies at 32 + 0 to 36 + 6 weeks, in relation to composite adverse perinatal outcome, an absolute UCR threshold of < 0.7 might be considered normal, ≥ 0.7 to < 0.9 as moderately abnormal and ≥ 0.9 as abnormal (with corresponding CPR thresholds of ≥ 1.43, ≥ 1.11 to < 1.43 and < 1.11, respectively). Two studies compared percentile CPR thresholds with absolute CPR thresholds for predicting adverse perinatal outcome in a cohort of pregnancies with FGR, and concluded that CPR < 1.08 (or UCR > 0.93) was as effective as the 5th percentile of CPR 29 , 30 . Indeed, a consensus meeting of experts in fetal medicine revealed that they would be willing to randomize pregnancies to delivery based on a UCR threshold of ≥ 0.8 from 34 weeks onwards, while for earlier gestational ages (< 34 weeks), a higher threshold of 1.0 was preferred 31 . This reflects the natural caution of clinicians, with the aim of delivering very preterm fetuses only when their condition is thought to be critical.
Clinical implications
In the most clinically relevant gestational‐age period at which abnormalities of UA and MCA Doppler waveforms and their ratios might guide fetal management in FGR (28–36 weeks' gestation), the 10th percentile value of the different charts for CPR varied from 0.9 to 1.8. These differences resulted in different sensitivities and ORs for composite adverse perinatal outcome and would also affect the proportion of FGR cases that are diagnosed, given that CPR is a criterion used in the diagnosis of this condition 2 . Our findings suggest that absolute UCR or CPR threshold values, rather than percentile thresholds or thresholds based on other gestational‐age normalized units, might be more practical for clinical use, with the added advantage that, as a simple ratio that is independent of gestational age, Doppler reference charts are not required.
Research implications
The overall performance of ratios of UA‐PI and MCA‐PI in predicting composite adverse perinatal outcome in late preterm FGR from 32 + 0 to 36 + 6 weeks was poor, with a specificity of approximately 60% at a sensitivity of 70%, which does not make a strong case for the utility of these ratios for triggering delivery in a clinical setting. This inference must be placed in context; as in many similar studies, the results that we describe are influenced by clinical management. The major confounder for the interpretation of these results is obstetric intervention, namely delivery of the baby, which was aimed at diminishing the risk of adverse infant outcome. It is not possible to control for the effect of this intervention. It is also not possible to determine whether and how knowledge of the ratios of UA‐PI and MCA‐PI was used in making the decision for delivery. Furthermore, longer‐term outcome data in infancy were not available for this and other studies. Only a randomized controlled interventional trial based on an index of cerebral blood‐flow redistribution and with long‐term follow‐up may provide a definitive answer to the question of whether delivery based on cerebral blood‐flow redistribution in late preterm FGR is beneficial.
Strengths and limitations
The main strength of this study is that UCR or CPR Doppler reference charts and absolute thresholds of the ratios were tested in a large, prospectively recruited cohort of fetuses at risk of late preterm FGR. However, it should be acknowledged that an inclusion criterion of the present cohort, derived from the TRUFFLE‐2 observational study 12 , was gestational age between 32 + 0 and 36 + 6 weeks, thus the results of this analysis can be applied only to this gestational‐age window. Furthermore, it should be taken into account that our study population had a high risk for adverse perinatal outcome. Application of Doppler thresholds for the prediction of adverse perinatal outcome in a low‐risk population will result in lower sensitivity and specificity. The findings of two Cochrane reviews support this; one showed an improvement in perinatal outcome associated with routine fetal Doppler assessment in a high‐risk population, while the other did not observe a benefit in low‐risk pregnant women 32 , 33 . Lastly, as is common in all observational studies, the association between diagnostic criteria and outcome is influenced by clinical management.
There has been some debate in relation to whether CPR 34 , 35 or UCR 36 , 37 is preferred for describing the degree of cerebral redistribution. Our preference for UCR derives from the analysis of the early FGR TRUFFLE cohort 38 , in which UCR and MCA Z‐score, but not CPR, were associated with long‐term outcome. Moreover, most ratios used in medicine show progressively greater differentiation of values in the abnormal (not normal) range, which is the case for UCR but not CPR; this is true for the soluble fms‐like tyrosine kinase‐1/placental growth factor ratio for risk assessment of pre‐eclampsia, protein/creatinine ratio in the diagnosis of pre‐eclampsia and ventilation/perfusion ratio for ventilation–perfusion mismatch.
Conclusions
The findings of this study confirm the presence of large differences between fetal Doppler reference charts for indices describing the relationship between UA and MCA impedance, particularly in the extreme ranges (< 10th percentile or ≥ 90th percentile), which might have a major clinical impact, particularly when used for the diagnosis and management of FGR. However, we observed that, in the gestational‐age window of 32 + 0 to 36 + 6 weeks, adjustment of UCR or CPR for gestational age had no advantage in the prediction of adverse outcome, as compared with absolute threshold values. This means that adoption of absolute UCR or CPR thresholds independent of Doppler reference charts is feasible, with these thresholds being simpler to use in clinical practice.
TRUFFLE‐2 GROUP AND CONTRIBUTING AUTHORS
B. Arabin, Department of Obstetrics Charite, HumboldtUniversity Berlin and Clara Angela Foundation, Berlin,Germany
A. Berger, Department of Obstetrics and Gynecology,Medical University of Innsbruck, Innsbruck, Austria
E. Bergman, Department of Women's and Children'sHealth, Uppsala University, Uppsala, Sweden
A. Bhide, Fetal Medicine Unit, St George's UniversityHospitals NHS Foundation Trust, London, UK; Molecular & Clinical Sciences Research Institute, St George'sUniversity of London, London, UK
C. M. Bilardo, Department of Obstetrics and Gynecology, Amsterdam University Medical Centers, University of Amsterdam, Location VUMC, Amsterdam,The Netherlands
A. C. Breeze, Fetal Medicine Unit, Leeds GeneralInfirmary, Leeds Teaching Hospitals NHS Trust, Leeds,UK
J. Brodszki, Department of Pediatric Surgery and Neonatology, Lund University, Skane University Hospital,Lund, Sweden
P. Calda, Department of Obstetrics and Gynaecology,General University Hospital and First Faculty ofMedicine, Charles University, Prague, Czech Republic
E. Cesari, Department of Obstetrics and Gynecology,Vittore Buzzi Children's Hospital, University of Milan,Milan, Italy
I. Cetin, Department of Obstetrics and Gynecology,Vittore Buzzi Children's Hospital, University of Milan,Milan, Italy
J. Derks, Department of Perinatal Medicine, Universityof Utrecht, Utrecht, The Netherlands
C. Ebbing, Department of Obstetrics and Gynecology,Haukeland University Hospital, Bergen, Norway
E. Ferrazzi, Department of Obstetrics and Gynecology,Fondazione IRCCS Ca' Granda Ospedale MaggiorePoliclinico and Department of Clinical Sciences andCommunity Health, Università degli Studi di Milano,Milan, Italy
T. Frusca, Department of Obstetrics and Gynecology,University of Parma, Parma, Italy
W. Ganzevoort, Department of Obstetrics and Gynecology, Amsterdam University Medical Centers, location AMC, Amsterdam, The Netherlands
S. J. Gordijn, Department of Obstetrics and Gynaecology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands
W. Gyselaers, Faculty of Medicine and Life Sciences,Hasselt University, Agoralaan, Diepenbeek, Belgium;Department of Obstetrics & Gynaecology, ZiekenhuisOost‐Limburg, Genk and Department Physiology,Hasselt University, Diepenbeek, Belgium
K. Hecher, Department of Obstetrics and FetalMedicine, University Medical Centre Hamburg‐Eppendorf, Hamburg, Germany
P. Klaritsch, Department of Obstetrics and Gynecology,Medical University of Graz, Graz, Austria
L. Krofta, Institute for the Care of Mother and Child,Prague, Czech Republic; Third Medical Faculty, CharlesUniversity, Prague, Czech Republic
P. Lindgren, Center for Fetal Medicine, KarolinskaUniversity Hospital, Stockholm, Sweden
S. M. Lobmaier, Department of Obstetrics and Gynecology, Klinikum Rechts Der Isar, Technical Universityof Munich, Munich, Germany
N. Marlow, UCL Elizabeth Garrett Anderson Institutefor Women's Health, University College London,London, UK
G. M. Maruotti, Department of Neurosciences,Reproductive and Dentistry Sciences, University ofNaples ‘Federico II’, Naples, Italy
F. Mecacci, Department of Health Sciences, Universityof Florence, Obstetrics and Gynecology, CareggiUniversity Hospital, Florence, Italy
K. Myklestad, St Olav's Hospital, Trondheim, Norway
R. Napolitano, UCL Elizabeth Garrett AndersonInstitute for Women's Health, University CollegeLondon, London, UK; Fetal Medicine Unit, UniversityCollege London Hospitals NHS Foundation Trust,London, UK
F. Prefumo, Department of Obstetrics and Gynecology,ASST Spedali Civili di Brescia and University of Brescia,Brescia, Italy
L. Raio, Department of Obstetrics & Gynecology,University Hospital of Bern, Bern, Switzerland
J. Richter, Department of Gynecology and Obstetrics,UZ Leuven and Department of Regeneration andDevelopment, KU Leuven, Leuven, Belgium
R. K. Sande, Department of Obstetrics and Gynecology,Stavanger University Hospital, Stavanger and Department of Clinical Science, University of Bergen, Bergen,Norway
J. Thornton, School of Clinical Sciences, University ofNottingham, Division of Obstetrics and Gynaecology,Maternity Department, City Hospital, Nottingham, UK
H. Valensise, Department of Surgery, Division ofObstetrics and Gynecology, Tor Vergata, University,Policlinico Casilino Hospital, Rome, Italy
G. H. A. Visser, Department of Perinatal Medicine,University of Utrecht, Utrecht, The Netherlands
L. Wee, The Princess Alexandra Hospital NHS Trust,Harlow, UK
Supporting information
Appendix S1 TRUFFLE‐2 collaborating authors
Table S1 Characteristics of included studies presenting Doppler reference charts
ACKNOWLEDGMENTS
We acknowledge the TRUFFLE‐2 collaborating authors listed in Appendix S1.
C.C.L. is supported by the UK National Institute for Health Research Biomedical Research Centre (BRC) based at Imperial College Healthcare National Health Service Trust and Imperial College.
Contributor Information
H. Wolf, Email: h.wolf@amsterdamumc.nl.
C. C. Lees, Email: christoph.lees@nhs.net.
the TRUFFLE Study Group:
B. Arabin, A. Berger, E. Bergman, A. Bhide, C. M. Bilardo, A. C. Breeze, J. Brodszki, P. Calda, E. Cesari, I. Cetin, J. Derks, C. Ebbing, E. Ferrazzi, T. Frusca, W. Ganzevoort, S. J. Gordijn, W. Gyselaers, K. Hecher, P. Klaritsch, L. Krofta, P. Lindgren, S. M. Lobmaier, N. Marlow, G. M. Maruotti, F. Mecacci, K. Myklestad, R. Napolitano, F. Prefumo, L. Raio, J. Richter, R. K. Sande, J. Thornton, H. Valensise, G. H. A. Visser, and L. Wee
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Lees CC, Stampalija T, Baschat A, da Silva Costa F, Ferrazzi E, Figueras F, Hecher K, Kingdom J, Poon LC, Salomon LJ, Unterscheider J. ISUOG Practice Guidelines: diagnosis and management of small‐for‐gestational‐age fetus and fetal growth restriction. Ultrasound Obstet Gynecol 2020; 56: 298–312. [DOI] [PubMed] [Google Scholar]
- 2. Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN, Silver RM, Wynia K, Ganzevoort W. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 2016; 48: 333–339. [DOI] [PubMed] [Google Scholar]
- 3. Richardson BS, Bocking AD. Metabolic and circulatory adaptations to chronic hypoxia in the fetus. Comp Biochem Physiol A Mol Integr Physiol 1998; 119: 717–723. [DOI] [PubMed] [Google Scholar]
- 4. Arbeille P, Patat F, Tranquart F, Body G, Berson M, Roncin A, Saliba E, Magnin G, Berger C, Pourcelot L. Doppler examination of the umbilical and cerebral arterial circulation of the fetus. J Gynecol Obstet Biol Reprod (Paris) 1987; 16: 45–51. [PubMed] [Google Scholar]
- 5. Arduini D, Rizzo G, Romanini C, Mancuso S. Fetal blood flow velocity waveforms as predictors of growth retardation. Obstet Gynecol 1987; 70: 7–10. [PubMed] [Google Scholar]
- 6. DeVore GR. The importance of the cerebroplacental ratio in the evaluation of fetal well‐being in SGA and AGA fetuses. Am J Obstet Gynecol 2015; 213: 5–15. [DOI] [PubMed] [Google Scholar]
- 7. Hecher K, Spernol R, Stettner H, Szalay S. Potential for diagnosing imminent risk to appropriate‐ and small‐for‐gestational‐age fetuses by Doppler sonographic examination of umbilical and cerebral arterial blood flow. Ultrasound Obstet Gynecol 1992; 2: 266–271. [DOI] [PubMed] [Google Scholar]
- 8. Hernandez‐Andrade E, Stampalija T, Figueras F. Cerebral blood flow studies in the diagnosis and management of intrauterine growth restriction. Curr Opin Obstet Gynecol 2013; 25: 138–144. [DOI] [PubMed] [Google Scholar]
- 9. Meher S, Hernandez‐Andrade E, Basheer SN, Lees C. Impact of cerebral redistribution on neurodevelopmental outcome in small‐for‐gestational‐age or growth‐restricted babies: a systematic review. Ultrasound Obstet Gynecol 2015; 46: 398–404. [DOI] [PubMed] [Google Scholar]
- 10. Monteith C, Flood K, Pinnamaneni R, Levine TA, Alderdice FA, Unterscheider J, McAuliffe FM, Dicker P, Tully EC, Malone FD, Foran A. An abnormal cerebroplacental ratio (CPR) is predictive of early childhood delayed neurodevelopment in the setting of fetal growth restriction. Am J Obstet Gynecol 2019; 221: 273.e1–9. [DOI] [PubMed] [Google Scholar]
- 11. Conde‐Agudelo A, Villar J, Kennedy SH, Papageorghiou AT. Predictive accuracy of cerebroplacental ratio for adverse perinatal and neurodevelopmental outcomes in suspected fetal growth restriction: systematic review and meta‐analysis. Ultrasound Obstet Gynecol 2018; 52: 430–441. [DOI] [PubMed] [Google Scholar]
- 12. Stampalija T, Thornton J, Marlow N, Napolitano R, Bhide A, Pickles T, Bilardo CM, Gordijn SJ, Gyselaers W, Valensise H, Hecher K, Sande RK, Lindgren P, Bergman E, Arabin B, Breeze AC, Wee L, Ganzevoort W, Richter J, Berger A, Brodszki J, Derks J, Mecacci F, Maruotti GM, Myklestad K, Lobmaier SM, Prefumo F, Klaritsch P, Calda P, Ebbing C, Frusca T, Raio L, GHA Visser, Krofta L, Cetin I, Ferrazzi E, Cesari E, Wolf H, Lees CC, TRUFFLE‐2 Group . Fetal cerebral Doppler changes and outcome in late preterm fetal growth restriction: prospective cohort study. Ultrasound Obstet Gynecol 2020; 56: 173–181. [DOI] [PubMed] [Google Scholar]
- 13. Vollgraff Heidweiller‐Schreurs CA, De Boer MA, Heymans MW, Schoonmade LJ, Bossuyt PMM, Mol BWJ, De Groot CJM, Bax CJ. Prognostic accuracy of cerebroplacental ratio and middle cerebral artery Doppler for adverse perinatal outcome: systematic review and meta‐analysis. Ultrasound Obstet Gynecol 2018; 51: 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vollgraff Heidweiller‐Schreurs CA, van Osch IR, Heymans MW, Ganzevoort W, Schoonmade LJ, Bax CJ, Mol B, de Groot C, Bossuyt P, de Boer MA; CPR IPD Study Group . Cerebroplacental ratio in predicting adverse perinatal outcome: a meta‐analysis of individual participant data. BJOG 2021; 128: 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stampalija T, Arabin B, Wolf H, Bilardo CM, Lees C. An abnormal cerebroplacental ratio (CPR) is predictive of early childhood delayed neurodevelopment in the setting of fetal growth restriction. Am J Obstet Gynecol 2020; 222: 391–392. [DOI] [PubMed] [Google Scholar]
- 16. Oros D, Ruiz‐Martinez S, Staines‐Urias E, Conde‐Agudelo A, Villar J, Fabre E, Papageorghiou AT. Reference ranges for Doppler indices of umbilical and fetal middle cerebral arteries and cerebroplacental ratio: systematic review. Ultrasound Obstet Gynecol 2019; 53: 454–464. [DOI] [PubMed] [Google Scholar]
- 17. Ruiz‐Martinez S, Papageorghiou AT, Staines‐Urias E, Villar J, Gonzalez De Aguero R, Oros D. Clinical impact of Doppler reference charts on management of small‐for‐gestational‐age fetuses: need for standardization. Ultrasound Obstet Gynecol 2020; 56: 166–172. [DOI] [PubMed] [Google Scholar]
- 18. Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, Zeeman GG, Brown MA. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens 2014; 4: 97–104. [DOI] [PubMed] [Google Scholar]
- 19. Arduini D, Rizzo G. Normal values of Pulsatility Index from fetal vessels: a cross‐sectional study on 1556 healthy fetuses. J Perinat Med 1990; 18: 165–172. [DOI] [PubMed] [Google Scholar]
- 20. Baschat AA, Gembruch U. The cerebroplacental Doppler ratio revisited. Ultrasound Obstet Gynecol 2003; 21: 124–127. [DOI] [PubMed] [Google Scholar]
- 21. Ebbing C, Rasmussen S, Kiserud T. Middle cerebral artery blood flow velocities and pulsatility index and the cerebroplacental pulsatility ratio: longitudinal reference ranges and terms for serial measurements. Ultrasound Obstet Gynecol 2007; 30: 287–296. [DOI] [PubMed] [Google Scholar]
- 22. Morales‐Rosello J, Khalil A, Morlando M, Hervas‐Marin D, Perales‐Marin A. Doppler reference values of the fetal vertebral and middle cerebral arteries, at 19–41 weeks gestation. J Matern Fetal Neonatal Med 2015; 28: 338–343. [DOI] [PubMed] [Google Scholar]
- 23. Ciobanu A, Wright A, Syngelaki A, Wright D, Akolekar R, Nicolaides KH. Fetal Medicine Foundation reference ranges for umbilical artery and middle cerebral artery pulsatility index and cerebroplacental ratio. Ultrasound Obstet Gynecol 2019; 53: 465–472. [DOI] [PubMed] [Google Scholar]
- 24. Acharya G, Ebbing C, Karlsen HO, Kiserud T, Rasmussen S. Sex‐specific reference ranges of cerebroplacental and umbilicocerebral ratios: longitudinal study. Ultrasound Obstet Gynecol 2020; 56: 187–195. [DOI] [PubMed] [Google Scholar]
- 25. Flatley C, Kumar S, Greer RM. Reference centiles for the middle cerebral artery and umbilical artery pulsatility index and cerebro‐placental ratio from a low‐risk population – a Generalised Additive Model for Location, Shape and Scale (GAMLSS) approach. J Matern Fetal Neonatal Med 2019; 32: 2338–2345. [DOI] [PubMed] [Google Scholar]
- 26. Srikumar S, Debnath J, Ravikumar R, Bandhu HC, Maurya VK. Doppler indices of the umbilical and fetal middle cerebral artery at 18–40 weeks of normal gestation: A pilot study. Med J Armed Forces India 2017; 73: 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dias T, Abeykoon S, Mendis P, Gunawardena C, Pragasan G, Padeniya T, Pathmeswaran A, Kumarasiri S. Fetal Doppler reference values in women with a normal body mass index. Ceylon Med J 2019; 64: 59–65. [DOI] [PubMed] [Google Scholar]
- 28. Zohav E, Zohav E, Rabinovich M, Alasbah A, Shenhav S, Sofer H, Ovadia YS, Anteby EY, Grin L. Third‐trimester Reference Ranges for Cerebroplacental Ratio and Pulsatility Index for Middle Cerebral Artery and Umbilical Artery in Normal‐growth Singleton Fetuses in the Israeli Population. Rambam Maimonides Med J 2019; 10: e0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gramellini D, Folli MC, Raboni S, Vadora E, Merialdi A. Cerebral‐umbilical Doppler ratio as a predictor of adverse perinatal outcome. Obstet Gynecol 1992; 79: 416–420. [DOI] [PubMed] [Google Scholar]
- 30. Odibo AO, Riddick C, Pare E, Stamilio DM, Macones GA. Cerebroplacental Doppler ratio and adverse perinatal outcomes in intrauterine growth restriction: evaluating the impact of using gestational age‐specific reference values. J Ultrasound Med 2005; 24: 1223–1228. [DOI] [PubMed] [Google Scholar]
- 31. Mylrea‐Foley B, Bhide A, Mullins E, Thornton J, Marlow N, Stampalija T, Napolitano R, Lees CC. Building consensus: thresholds for delivery in TRUFFLE‐2 randomized intervention study. Ultrasound Obstet Gynecol 2020; 56: 285–287. [DOI] [PubMed] [Google Scholar]
- 32. Alfirevic Z, Stampalija T, Dowswell T. Fetal and umbilical Doppler ultrasound in high‐risk pregnancies. Cochrane Database Syst Rev 2017; 6: CD007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alfirevic Z, Stampalija T, Medley N. Fetal and umbilical Doppler ultrasound in normal pregnancy. Cochrane Database Syst Rev 2015; 2015: CD001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalafat E, Khalil A. Umbilicocerebral ratio: potential implications of inversing the cerebroplacental ratio. Ultrasound Obstet Gynecol 2020; 56: 159–162. [DOI] [PubMed] [Google Scholar]
- 35. Kalafat E, Ozturk E, Kalaylioglu Z, Akkaya AD, Khalil A. Re: Ratio of umbilical and cerebral artery pulsatility indices in assessment of fetal risk: numerator and denominator matter. Ultrasound Obstet Gynecol 2020; 56: 290–292. [DOI] [PubMed] [Google Scholar]
- 36. Wolf H, Stampalija T, Monasta L, Lees CC. Ratio of umbilical and cerebral artery pulsatility indices in assessment of fetal risk: numerator and denominator matter. Ultrasound Obstet Gynecol 2020; 56: 163–165. [DOI] [PubMed] [Google Scholar]
- 37. Wolf H, Stampalija T, Monasta L, Lees CC. Reply. Ultrasound Obstet Gynecol 2020; 56: 292–293. [DOI] [PubMed] [Google Scholar]
- 38. Stampalija T, Arabin B, Wolf H, Bilardo CM, Lees C; TRUFFLE investigators . Is middle cerebral artery Doppler related to neonatal and 2‐year infant outcome in early fetal growth restriction? Am J Obstet Gynecol 2017; 216: 521.e1–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 TRUFFLE‐2 collaborating authors
Table S1 Characteristics of included studies presenting Doppler reference charts
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


