Abstract
Introduction
Although the COVID‐19 vaccination is deemed safe, exact incidence and nature if adverse effects, particularly dermatological ones, are still unknown.
Objective
To describe the demographic, clinical, morphological characteristics, outcomes, and timing of development of herpes zoster to the various COVID‐19 vaccines. And to identify on whether COVID‐19 vaccine has temporal relationship between development of herpes zoster (HZ).
Methods
We have performed a systemic review of articles from PubMed and Embase using MeSH and keywords like “Shingles,” “Herpes zoster,” “Varicella zoster,” “COVID‐19,” “Vaccine,” “SARS‐CoV‐2.” No filters including country of publication, language, type of articles were applied. Individual case report references were filtered for any pertinent cases.
Results
A total of 54 cases consisting of 27 male and 27 female patients have been reported. There were cases with known risk factors for herpes zoster, which included age more than 50 years (n = 36), immunological disorders (n = 10), chronic disease (n = 25), metabolic disorder (n = 13), malignancy (n = 4), and psychiatric disorder (n = 2). The mean (SD) period between development of herpes zoster and COVID‐19 vaccination was 7.64 (6.92) days. Majority of the cases were from the high‐income and/or middle‐income countries. 86.27% of the cases of HZ were reported due to mRNA vaccine. Thirty‐six patients 36/45 (80%) developed herpes zoster following the priming dose of COVID‐19 vaccine among those who received mRNA vaccine.
Conclusion
We could not establish definite link but there may be possible association between COVID‐19 vaccine and shingles. Large‐scale studies may help to understand the cause‐effect relationship.
Keywords: COVID‐19, herpes zoster, vaccine, Varicella zoster
1. INTRODUCTION
The World Health Organization (WHO) announced the coronavirus disease 2019 (COVID‐19) outbreak a pandemic on March 11, 2020 and the development of a safe and effective COVID‐19 vaccine quickly became a worldwide priority. As of July 2021, 27.6% of the world population has received at least one dose of COVID‐19 vaccine. 1 In an attempt to prevent severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) viral transmission, non‐replicating viral vector vaccines, DNA‐based or RNA‐based vaccines, and inactivated vaccines, protein subunit recombinant vaccines have been developed recently. Adverse events after vaccination (AEAV) might be a coincidence or a direct result of the vaccine. In order to answer this question, a temporal relationship between vaccination and AEAV is required, as well as a biological mechanism is necessary to explain the link.
The platform for COVID‐19 vaccine leaders is based on gene therapy. Still, it is the matter of research for the dermatologists, to investigate the long‐term consequences of older generation vaccines. As a result, the long‐term consequences of vaccinations based on gene therapy remain essentially unknown. COVID‐19 vaccines have been reported to be associated with reactogenicity with symptoms such as fever, fatigue, and headache being frequently reported. This is due to the vaccines' intrinsic character, even though they have been shown to be safe in clinical trials. 2 , 3 Although the COVID‐19 vaccination is deemed safe, adverse effects, particularly dermatological ones, are still unknown. Clinical studies have reported that the most common cutaneous adverse effects are injection site reaction and pruritus; allergic reactions such as urticaria and widespread erythematous rash. 4 In the literature, various cutaneous conditions have been observed including anecdotal cases of erythema multiforme with morbilliform rash, delayed‐type hypersensitivity reaction, bullous drug eruption, pernio/chilblains (eg, “COVID toes”), erythromelalgia, and pityriasis‐rosea‐like exanthems, and herpes zoster (HZ). 5 , 6 , 7 , 8 Studies have reported development of herpes zoster due to SARS‐CoV‐2 infection either at the time of disease progression or following recovery from the disease. In the context of COVID‐19 pandemic, studies observed that COVID‐19 is associated lymphopenia, particularly CD3+, CD8+ lymphocytes, and functional impairment of CD4+ T cells, might make a patient more vulnerable to development of herpes zoster. 9
While there have been several case reports and case series published in literature on COVID‐19 vaccine‐induced HZ, to our knowledge, there is no comprehensive review on this subject. Given how crucial widespread vaccination is in curbing the COVID‐19 pandemic, limited data on HZ after COVID‐19 vaccine with handful of case reports and case series promoted us to systemically review published cases of HZ to describe the demographic, clinical, morphological characteristics, outcomes, and timing of development of herpes zoster to the various COVID‐19 vaccines.
2. METHOD
2.1. Search strategy
A systemic review was conducted due to the sparse but quickly increasing scientific literature on HZ and COVID‐19. We performed a search in PubMed and Embase using “Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA)” guidelines by using MeSH and keywords like “Shingles,” “Herpes zoster,” “Varicella zoster,” “COVID‐19,” “Vaccine,” “SARS‐CoV‐2.” Boolean operators (“OR”; “AND”) were used to combine the search results. No filters including country of publication, language, type of articles were applied. Individual case report references were filtered for any pertinent cases. The findings were saved in an EndNote library. The search strategy is shown in PRISMA diagram (Figure 1). Articles published until August 14, 2021 were included.
FIGURE 1.
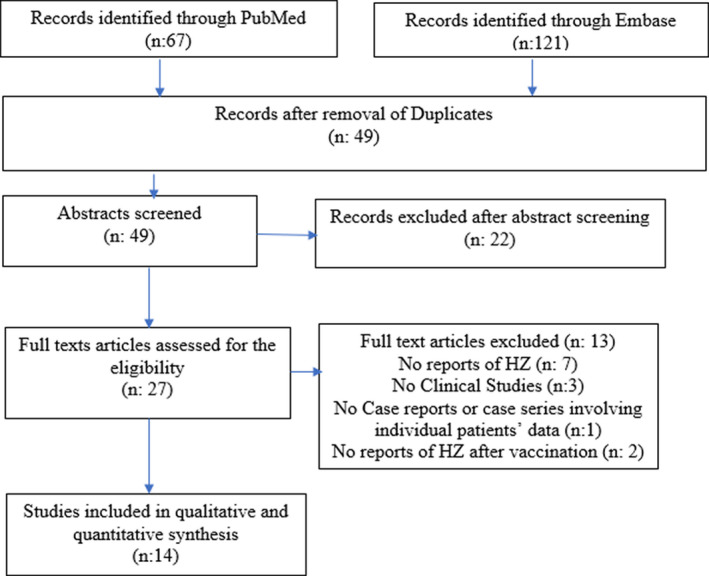
PRISMA flowchart of study selection
2.2. Study selection
We selected all the available individual case reports and case series. During phase 1, two authors (H.D. and K.S.) independently assessed the abstracts, titles, and categories of research that met the requirements. The disagreements were resolved by consensus with another two authors (J.P and M.G). Based on the inclusion criteria, the second phase of the search involved assessing full‐text articles in order to identify items for data extraction. Data from the article were curated and summarized in the form of age, gender, country of origin, symptoms and clinical characteristic of the lesion, vaccine type, and dose, days until symptom onset of post‐vaccination, location of the lesion, past medical history/comorbidities, medical intervention during hospitalization and confirmatory test. Descriptive analysis and data collection were performed using Microsoft Excel software.
3. RESULTS
The search yielded a total of 121 and 67 hits from Embase and PubMed, respectively. We identified 14 articles that met as per the study requirement.
3.1. Description of Herpes zoster cases in patients with COVID‐19 vaccination
3.1.1. Demographic and comorbid conditions
The search yielded a total of 54 patients consisting of 27 male and 27 females. Cases were reported worldwide including USA, 10 , 11 Lebanon, 12 Turkey, 13 , 14 , 15 Greece, 16 Italy, 17 India, 18 Israel, 19 Finland, 20 Taiwan, 21 Spain, 22 and Portugal. 23 There were cases with known risk factors for herpes zoster, which included age more than 50 years (n = 36), immunological disorders (n = 10), chronic disease (n = 25), metabolic disorder (n = 13), malignancy (n = 4), and psychiatric disorder (n = 2). Table 1 shows the details of cases.
TABLE 1.
Conducted studies on COVID‐19 vaccine and Varicella zoster
| Author/Country | Age/Sex | Comorbidities | Symptoms and clinical characteristics of lesion | Vaccine type and dose | Days until symptom onset post‐vaccination (PV) | Location | Treatment | Confirmatory test |
|---|---|---|---|---|---|---|---|---|
| Eid E, et al./Lebanon 12 | 79/M | Hypertension, coronary artery disease, and antineutrophilic cytoplasmic antibody‐related glomerulonephritis | Vesicles, some excoriated and overlying an erythematous base | mRNA COVID vaccine/NR | 5 | Right thigh | Systemic antiviral | Clinically diagnosed |
| Lee C, et al./USA 10 | 77/M | Crohn's Disease, h/o treated Aspergilloma, Psoriasis | Painful, unilateral dermatomal herpetiform eruption |
mRNA/1st (Moderna) |
4 | T11 distribution from the right mid‐abdomen, right flank, and right mid‐back | Valacyclovir, Gabapentin, LMX | Clinically diagnosed |
| 56/M | Atopic Eczema | Unilateral dermatomal herpetiform eruption |
mRNA/1st (Moderna) |
4 | Right chest | Valacyclovir, Gabapentin, LMX | VZV IgG (+), IgM (−) | |
| 54/F | Thyroidectomy, Hysterectomy, Cholecystectomy, Melanoma | Itchiness and deep pain, unilateral dermatomal herpetiform eruption |
mRNA/1st (Moderna) |
5 | Left back, Left shoulder, Left triceps | Valacyclovir, Gabapentin, LMX | VZV PCR (+) | |
| 65/M | Hypertension | Itchiness and pain, unilateral dermatomal herpetiform eruption |
mRNA/2nd (Pfizer) |
38 | Left axilla, Left shoulder, Left triceps | Valacyclovir, Gabapentin, LMX | VZV PCR (+) | |
| 43/F | Hypertension | Severe pain at injection site and body aches, unilateral dermatomal herpetiform eruption |
mRNA/1st (Moderna) |
4,8 | "Bump" on R neck, R collarbone & R lower jaw | Valacyclovir & Terrasil Shingles cream | Clinically diagnosed | |
| 69/F | Polycythemia Vera, Hypertension, Dyslipidemia, Osteopenia | Severe pain in upper Left back, unilateral dermatomal herpetiform eruption |
mRNA/1st (Moderna) |
4,7 | Worsening blistering of L axilla and L upper breast | Antiviral | Clinically diagnosed | |
| 42/M | — | 14 days PV: tingling, mild “skin irritation sensation” radiating from R lower chest to mid‐ABD. 21 days: burning and itchiness, unilateral dermatomal herpetiform eruption |
mRNA/1st (Moderna) |
21 | Scattered blistering | NR | Clinically diagnosed | |
| 47/F | Vascular issues to extremities | 12 days PV: burning pain to Right axilla and Right chest, unilateral dermatomal herpetiform eruption |
mRNA/1st (Moderna) |
15 | T1 dermatome | Valacyclovir, Gabapentin, Prednisone | Clinically diagnosed | |
| 39/F | DM, HTN | Itchiness |
mRNA/1st (Moderna) |
5 | Right back and Right flank | Valacyclovir | Clinically diagnosed | |
| 74/M | Kidney failure, Anemia, HTN, Gout, Heart failure, A‐Fib | None |
mRNA/2nd (Pfizer) |
3 | Mid‐chest, Right arm | Valacyclovir & Mupirocin | Clinically diagnosed | |
| 48/M | None | Arm soreness from vaccine |
mRNA/1st (Pfizer) |
12 | Unilateral dermatomal Left flank and altered skin sensation | Valacyclovir, Gabapentin, LMX | VZV PCR (+) | |
| 68/F | Hypothyroidism, HTN, adrenal fatigue | Red blotches erupted |
mRNA/1st (Moderna) |
12 | Left axilla, Left triceps, Left scapula | Valacyclovir, Tramadol | Clinically diagnosed | |
| 46/M | None | Pain at lesion side |
mRNA/1st (Pfizer) |
9 | Right flank | Valacyclovir, Gabapentin, Hydrocodone/acetaminophen | Clinically diagnosed | |
| 60/F | Treated thyroid cancer, Gout | Itching and burning at site of future skin eruption |
mRNA/2nd (Moderna) |
26,28 | Right forehead along hairline | Valacyclovir | Clinically diagnosed | |
| 43/M | None | Hotness and altered skin sensation at site of future skin eruption |
mRNA/1st (Moderna) |
3 | Unilateral dermatomal right flank and back | Valacyclovir and Gabapentin | Clinically diagnosed | |
| 65/F | None | Tingling, itching, burning, and eruption around and under right eye |
mRNA/1st (Moderna) |
11 | Upper right eye | Valacyclovir | Clinically diagnosed | |
| 44/M | None | Itchiness |
mRNA/1st (Pfizer) |
5 | Left eyebrow | Acyclovir, Desonide cream | Clinically diagnosed | |
| 37/F | None | Nerve pain |
mRNA/1st (Moderna) |
4,5 | Left back and left ABD | Valacyclovir | Clinically diagnosed | |
| 69/M | Hypercholesterolemia | None |
mRNA/1st (Moderna) |
14 | Unilateral right back and right arm | Valacyclovir | Clinically diagnosed | |
| 72/F | Hypertension, Treated with breast cancer | Raised, red spot at injection site with accompanying pain; 4–5 days PV: itching and burning at site of future skin eruption |
mRNA/2nd (Moderna) |
5,6 | Left arm, left upper back, left breast | Valacyclovir | Clinically diagnosed | |
| Bostan E, et al./Turkey 14 | 78/M | Coronary artery disease, cerebrovascular accident, hypertension, chronic pulmonary obstructive disease, radical cystectomy, and prostatectomy performed seven years ago for bladder cancer | Erythematous, painful, and pruritic lesions on his chest | Inactivated vaccine | 5 | Crusted, hemorrhagic vesicles upon an erythematous base occupying an area corresponding to left T3‐T4 dermatomes | Valacyclovir | Clinically diagnosed |
| Arora P, et al./India 18 | 60/M | Type II diabetes mellitus and hypertension | Multiple grouped vesicles on an erythematous base | Inactivated Vaccine/1st Dose | 5 | Over the knee, and anterior aspect of right thigh, L2 and L3 dermatome | Valacyclovir and topical fusidic acid | Skin Biopsy and clinically diagnosed |
| Furer V, et al./Israel 19 | 44/F | Sjogren's syndrome | Skin rash, pruritus, pain, inguinal lymphadenopathy |
mRNA/1st (Pfizer) |
3 | L5 dermatome | None | Clinically diagnosed |
| 56/F | Rheumatoid arthritis | Headache, tingling and burning, facial skin rash, eyelids swelling, conjunctivitis |
mRNA/1st (Pfizer) |
4 | Left eye and forehead, V1 of V cranial nerve | Acyclovir | Clinically diagnosed | |
| 59/F | Rheumatoid arthritis | Skin rash, pain, inguinal lymphadenopathy, slow healing of skin lesions |
mRNA/2nd (Pfizer) |
2 | Low abdomen, inguinal area, upper thigh, and buttock, L1‐L2 Dermatome | Valacyclovir | Clinically diagnosed | |
| 36/F | Rheumatoid arthritis, Interstitial Lung Disease | Skin rash, pruritus, pain |
mRNA/1st (Pfizer) |
10 | The abdomen and back, T10 Dermatome | Acyclovir | Clinically diagnosed | |
| 38/F | Undifferentiated Connective tissue disease, Antiphospholipid antibody syndrome | Skin rash, pruritus, pain |
mRNA/1st (Pfizer) |
14 | The right breast, T4 Dermatome | Acyclovir | Clinically diagnosed | |
| 61/F | Rheumatoid arthritis | Skin rash |
mRNA/1st (Pfizer) |
14 | T6 Dermatome | Valacyclovir | Clinically diagnosed | |
| Tessas I, et al./Finland 20 | 44/M | dyslipidemia and active smoking | Neuropathic pain, Rash |
mRNA/1st (Pfizer) |
7 | Left upper back, lateral side, and inner side of the left arm, C5–C6 dermatomes | Valacyclovir | Clinically diagnosed |
| Psichogiou M, et al./Greece 16 | 51/F | None | NR |
mRNA/1st (Pfizer) |
9 | Lumbar Dermatome | Valacyclovir | Clinically diagnosed |
| 56/F | Osteoporosis, Dyslipidemia | NR |
mRNA/1st (Pfizer) |
14 | Thoracic Dermatome | Valacyclovir | Clinically diagnosed | |
| 69/F | Mitral valve surgery | Postherpetic Neuralgia |
mRNA/1st (Pfizer) |
8 | 5th cranial nerve | Valacyclovir | Clinically diagnosed | |
| 86/M | Prostate cancer, Hypertension | NR |
mRNA/2nd (Pfizer) |
7 | Thoracic Dermatome | Valacyclovir | Clinically diagnosed | |
| 90/M | Hypertension, COPD, hyperuricemia | NR |
mRNA/2nd (Pfizer) |
9 | Thoracic Dermatome | Valacyclovir | Clinically diagnosed | |
| 91/M | Dyslipidemia, hypertension | Postherpetic Neuralgia |
mRNA/1st (Pfizer) |
7 | Fifth cranial nerve, Herpes zoster ophthalmicus | Acyclovir IV and then valacyclovir | Clinically diagnosed | |
| 94/M | Heart Failure, Chromic kidney disease | Postherpetic Neuralgia |
mRNA/1st (Pfizer) |
20 | Thoracic Dermatome | Valacyclovir | Clinically diagnosed | |
| Aksu SB, et al./Turkey 15 | 68/M | hypertension, dysrhythmia, and anxiety | stinging sensation and pain, multiple pinheaded vesicular lesions upon an erythematous base |
Inactivated Vaccine, 2nd Dose |
5 | Right mammary region and back corresponding to T3–T5 dermatomes | Valacyclovir | Clinically diagnosed |
| Rodríguez‐Jiménez P, et al./Spain 22 | 58/M | Hypertension | Asymptomatic herpetiform umbilicated vesicles, Fever, Cervical Lymphadenopathy |
mRNA/1st (Pfizer) |
1 | C6 Dermatome | VZV PCR (+) | |
| 47/F | None | Herpetiform umbilicated vesicles, Fever, Dysesthesia |
mRNA/1st (Pfizer) |
5 | Dorsal 2 to Dorsal 4 Dermatome | VZV PCR (+) | ||
| 39/M | None | Painful herpetiform umbilicated vesicles |
mRNA/1st (Pfizer) |
3 | Dorsal 4 Dermatome | Clinically diagnosed | ||
| 56/F | None | Herpetiform umbilicated vesicles, Dysesthesia |
mRNA/2nd (Pfizer) |
2 | Cranial Nerve 1 | VZV PCR (+) | ||
| 41/F | None | Herpetiform umbilicated vesicles, Dysesthesia |
mRNA/2nd (Pfizer) |
16 | Dorsal 5 Dermatome | Clinically diagnosed | ||
| Chiu HH, et al./Taiwan 21 | 42/M |
None replicating viral vector 1st (Astrazeneca) |
7 | T10 Dermatome | ||||
| 71/M | None | Erythematous papules and vesicles, with itching and pain |
mRNA/1st (Moderna) |
2 | Left flank T8 dermatome | Acyclovir | Clinically diagnosed | |
| 46/M | NR | Grouped vesicles |
None replicating viral vector 1st (Astrazeneca) |
2 | T11 Dermatome | Acyclovir | clinically Diagnosed | |
| Alpalhao M, et al./Portugal 23 | 70/F | Hallux Valgus |
None replicating viral vector 1st (Astrazeneca) |
3 | Left V2 Territory | Valacyclovir | VZV PCR (+) | |
| 73/F | Mechanical Mitral Valve Prosthesis |
None replicating viral vector 1st (Astrazeneca) |
4 | Right V3 Territory | Valacyclovir | VZV PCR (+) | ||
| 63/F | NR |
mRNA/1st (Pfizer) |
6 | Left C8 Territory | Valacyclovir | VZV PCR (+) | ||
| 69/M |
Systemic Lupus Erythematous Hypertension Plaque type psoriasis Hemophilia A Psoriatic Arthritis Antiphospholipid antibody syndrome |
mRNA/1st (Pfizer) |
3 | Left V2/V3 Territory | Valacyclovir | VZV PCR (+) | ||
| Nastro F, et al./Italy 17 | 84/F | Chronic kidney disease and Depressive disorder | Burning pain over distal part of right leg and foot, followed by multiple nonconfluent purpuric papules and vesicles |
mRNA/1st (Pfizer) |
4 (hours) | Right leg and foot | Famcyclovir | VZV PCR (+), Skin biopsy |
| Channa L, et al./USA 11 | 81/M | Diabetes, Hypertension, Coronary Artery Disease | Dermatomal rash |
mRNA/2nd (Moderna) |
3 | Not reported | No intervention | Clinically diagnosed |
| Özdemir AK, et al./Turkey 13 | 23/F | None | Itchy and Painful rash, Grouped vesicles on erythematous base | Inactivated SARS‐CoV−2 Vaccine | 1 | Lower Back | Brivudine | Clinically diagnosed |
| 21/M | None | Painful blister, Grouped vesicles on erythematous base | Inactivated SARS‐CoV−2 Vaccine | 2 | Abdomen | Valacyclovir | Clinically diagnosed |
3.2. Period between diagnosis of herpes zoster (HZ) and COVID‐19 vaccination, vaccine component, and its number of dose
The mean (SD) period between herpes zoster and COVID‐19 vaccination was 7.64 (6.92) days (Figure 2). In most of the cases, 52 (96.07%) HZ was developed within 1–3 weeks following COVID‐19 vaccine. Among, 29 (50.98%) of the cases it developed within 1st week of vaccination irrespective of the number of vaccine dose. Only 1 case reported after the one month of COVID‐19 vaccine. Majority of the patients 45/54 (86.27%) received mRNA vaccine followed by 5/54 (5.88%) inactivated COVID‐19 vaccine, 4/51 (7.84%) non‐replicating viral vector. Thirty‐six patients 36/45 (80%) developed herpes zoster following the priming dose of COVID‐19 vaccine among those who received mRNA vaccine. All the patients who received non‐replicating viral vector vaccine developed herpes zoster after the 1st dose. Eleven patients (11/54) were diagnosed through VZV PCR followed by clinical diagnosis (40/54).
FIGURE 2.
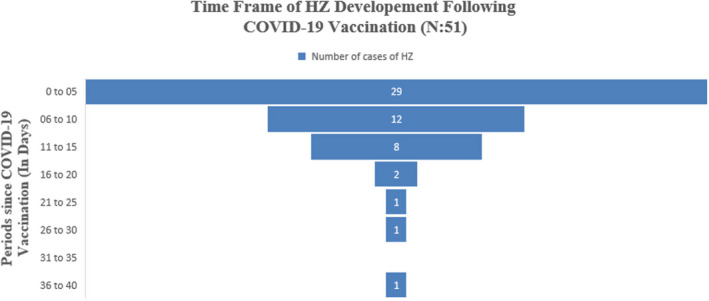
Period of herpes zoster development following COVID‐19 vaccination (n = 51)
3.3. Clinical presentation and past history of varicella zoster (VZV) infection or vaccine
Majority of the herpes zoster cases diagnosed clinically (40/54), followed by VZV PCR (11/54), VZV IgG (1/54), 10 and skin biopsy (2/54). 17 , 18 Various dermatomal lesion has been observed including trunk, 10 hips/buttocks or inguinal region, 19 herpes zoster ophthalmicus (HZO), 10 , 19 upper limb, 10 thigh, 12 and abdomen and flank. 10 There were 3 cases who developed lymphadenopathy including cervical and inguinal lymphadenopathy. 19 , 22 Postherpetic neuralgia was reported in 3 cases. 16 Prior VZV infection was reported in 13 cases, 10 , 19 , 20 and 5 cases 10 , 11 , 19 reported prior zoster vaccination.
4. DISCUSSION
Vaccinations have been found to have a high level of reactogenicity, with headache, fever, and tiredness being more prevalent than with other vaccines. The distinctive inflammatory nature of these vaccinations may explain the higher‐than‐usual reported adverse effects. These vaccinations have been proven to strengthen the cellular immune system and generate a Th1‐type response with high IFNg, TNFa, and IL2 levels. As a result, they may hypothetically play a role in the flare‐up of dermatological disorders including psoriasis, lichen planus, vitiligo, and other diseases with a known Th1 function in pathogenesis. 24
Herpes zoster following vaccination is rarely reported in the literature. Studies have reported herpes zoster following vaccination of inactivated vaccines for influenza, hepatitis A, and rabies and Japanese encephalitis, and yellow fever. In this review, we studied baseline clinical and demographic characteristics, and timing of development of herpes zoster following COVID‐19 vaccine. HZ has been reported due to almost all types of COVID‐19 vaccine. Among, majority of the cases were reported due to mRNA vaccine. However, underreporting, fluctuating data quality, the absence of specified diagnostic criteria, missing denominator information, and reporter bias all restrict the reporting of AEAV occurrences in major databases like the Vaccine Adverse Event Reporting System (VAERS). 25 Majority of the HZ cases reported from Turkey, USA, and there were no reports of herpes zoster from patients in low‐ and middle‐income countries, raising attention to global inequities in COVID‐19 vaccine access or lack of reporting bias.
Antiviral medicines and analgesics are currently mainstay of the treatment of herpes zoster, and the initial discomfort and skin rash are well controlled. All the patients were treated with antivirals either valacyclovir or acyclovir or famciclovir and no any causality was observed.
4.1. Is there a medical or biological basis for an increased risk of COVID‐19 vaccine‐induced HZ?
Several theories can be postulated to explain the relationship between development of herpes zoster and COVID‐19 vaccines. Age was found to be the major risk factor for the development of HZ partly due to age‐related decline in cell‐mediated immune responses to VZV, whereas disease‐related immunocompromise is another risk factor including such as HIV infection, iatrogenic immunocompromission, physical trauma, or comorbid conditions such as malignancy or chronic kidney or liver disease. Studies have reported cross‐reactivity between spike protein and self‐antigen may lead to development of immune‐mediated disorders in COVID‐19 patients in the long run. The authors hypothesized that similar response can happen following COVID‐19 vaccine. 9 , 10 Toll‐like receptors (TLR) stimulation of innate immunity might be the connection between COVID‐19 vaccine and HZ development. 15 The stimulation of these receptors has been related to the reactivation of VZV, allowing the latent virus to remain dormant in the afflicted people. 14 The COVID‐19 immunization may lead to the production of type I IFNs and other inflammatory cytokines, activating T‐ and B‐cell immunity and negatively affecting antigen expression, resulting in herpes zoster reactivation. 16 , 17 , 18 The peak of antigen expression is determined by the administration method and vaccine composition, which is another approach to modulate the immune response. 11 , 12 , 13
Furthermore, herpes zoster is more common in HIV patients with lower CD4 cell counts, underlining the significance of T‐cell immunity in sustaining VZV latency. 26 , 27 According to Sahin U et al., 28 vaccination with BNT162b2 produces coordinated humoral and cellular adaptive immunity in healthy individuals. A robust cellular response with spike‐specific CD8+ T cells and T helper type 1 (Th1) CD4+ T cells is growing seven days after the booster dosage, with a high proportion of them generating interferon (IFN), a cytokine involved in numerous antiviral responses. S1‐binding IgG associated positively with the S‐specific CD4+ T‐cell responses, as did the intensity of S‐specific CD8+ T‐cell responses, which correlated positively with S1‐binding IgG. Furthermore, among participants over 55 years old, the SARS‐CoV‐2 mRNA‐1273 vaccination generated a robust CD4 cytokine response involving type 1 helper T cells. 29 After a large shift of naive CD8+ cells to create CD8+ cells specific to control HIV or VZV, we hypothesize that VZV‐specific CD8+ cells are momentarily incapable of regulating VZV.
4.2. Is there temporal association of development of HZ and COVID‐19 vaccine?
WHO and CDC have established standard methods for conducting causation evaluations of individual instances of AEAV. When an incident occurs during the time frame defined for increased risk, it is said to be "consistent with" a causal association. As per WHO updated guidelines on causality definitions, a "probable" association suggests a temporal relationship and the existence of a biologic mechanism for causal association between the vaccination and the occurrence. 25 , 30 , 31 Of 54 herpes zoster reported cases, 52 (96.29%) of the patients developed it at the defined higher risk timeframe (1–21 days after the initial dose). 23 As a result, the AEAV may be categorized as "compatible with," and a "likely" causal relationship based on the World Health Organization Working Group can be presented, indicating the existence of a temporal link and a credible physiological mechanism. 25 , 30 , 31 , 32
4.3. Interpretation of the most up‐to‐date clinical evidence
Despite the fact that the case fatality rate (CFR) for HZ is exceedingly low in COVID‐19 patients. 9 Herpes zoster is often associated with disability, especially among aged individuals, and available management only decreases viral shedding reducing the risk of transmission and prevents post‐herpetic neuralgia and the severity and the duration of pain. In immunocompetent people over the age of 50, the recombinant zoster vaccination is indicated. 33 Clinicians may not be aware of the link between HZ and COVID‐19 immunization. The awareness of this clinical condition encourages additional reporting and communication of HZ after vaccination. Now the question raised whether to use antiviral or not in highly suspected old aged immunocompromised people as to prevent the risk of development of HZ. Therefore, clinical review committees need to decide whether to recommend antiviral treatment or not before initiating SARS‐CoV‐2 immunization.
4.4. What kind of clinical/epidemiological evidence is required to determine whether HZ cases have increased due to COVID‐19 vaccine?
Despite the rarity of the articles, HZ is a common occurrence. In the VAERS, there were 232 HZ‐related adverse events recorded for the COVID‐19 vaccinations. 34 As of March 21, 2021, there were 331 cases of HZ after the Pfizer/BioNTech vaccination and 297 after the Astra‐Zeneca vaccine reported in the Medicines and Healthcare Products Regulatory Agency of the United Kingdom (MHRA) Yellow Card adverse reaction reporting program. 35 , 36 , 37 However, because HZ is underreported as an adverse event following vaccination for other infectious agents, these findings may underestimate the prevalence of herpes zoster. So, ongoing studies are required to understand the immunological mechanism that control SARS‐CoV‐2 and VZV long‐term protection. Studies could assess either the risk or trend of development of HZ due to COVID‐19 vaccine. Studies designed to assess the risk of HZ in patients with and without COVID‐19 immunization could assess whether there has been an increased risk of HZ in COVID‐19 vaccinated individual. Both retrospective cohort and case‐control studies can be considered. Cases and controls may require to be matched. Studies can focus to determine the prevalence of herpes zoster in the general population or in specialized populations (eg, administrative or hospital databases). Interestingly, a study was done by Pedro et al. observed that incidence of HZ development after 1 month of follow‐up of all vaccinated patients was 5–6 times higher than the usual annual HZ incidence in their geographical area. Future studies need to focus on cytokine function, T cells, and absolute lymphocytes in patients who present with reactivation of VZV following COVID‐19 immunization and its effects on cellular immunity with regard to its role in etiology, prognosis, and manifestation. 22
Our study was limited by publication bias, small sample size, missing data, and lack of generalizability in demographics of the series analyzed. In the majority of the cases, diagnosis of HZ was pertinent to the HZ clinical findings.
5. CONCLUSION
Our study does not establish any causality or definite link but draws the attention to a possible association between COVID‐19 vaccine and shingles. Large‐scale immunological, epidemiological, and clinical studies may help to understand the cause‐effect relationship. Based on the criteria of temporal connection with vaccination and a plausible biological link, HZ appears to be a "possible" but uncommon AEAV. Furthermore, these findings may be therapeutically relevant in deciding whether to use antiviral as a temporary prophylactic prior to immunization for individuals who are at a greater risk of VZV reactivation following SARS‐CoV‐2 vaccination.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Hardik D Desai: Writing and revising the manuscript. Kamal Sharma, Anchal Shah, Jaimini Patoliya, Anant Patil, Zahra Hooshanginezhad, and Stephan Grabbe: Review and revising the manuscript. Mohamad Goldust: Conception, writing, review and revising the manuscript.
DISCLAIMER
We confirm that the manuscript has been read and approved by all the authors, that the requirements for authorship as stated earlier in this document have been met and that each author believes that the manuscript represents honest work.
Ethical Approval
This study was conducted using published online material, hence the ethical approval is waived.
ACKNOWLEDGEMENTS
Open access funding enabled and organized by Projekt DEAL.
Desai HD, Sharma K, Shah A, et al. Can SARS‐CoV‐2 vaccine increase the risk of reactivation of Varicella zoster? A systematic review. J Cosmet Dermatol. 2021;20:3350–3361. 10.1111/jocd.14521
Contributor Information
Hardik D. Desai, Email: hardikdesai198@yahoo.com.
Mohamad Goldust, Email: mgoldust@uni-mainz.de.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Coronavirus (COVID‐19) Vaccination. Available at https://ourworldindata.org/covid‐vaccinations. Accessed October 1, 2021.
- 2. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2020;397:99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85:46‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnston MS, Galan A, Watsky KL, Little AJ. Delayed localized hypersensitivity reactions to the moderna COVID‐19 vaccine: a case series. JAMA Dermatol. 2021;157:716‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kong J, Cuevas‐Castillo F, Nassar M, et al. Bullous drug eruption after second dose of mRNA‐1273 (Moderna) COVID‐19 vaccine: Case report. J Infect Public Health. 2021. 10.1016/j.jiph.2021.06.021. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gambichler T, Scholl L, Dickel H, Ocker L, Stranzenbach R. Prompt onset of Rowell's syndrome following the first BNT162b2 SARS‐CoV‐2 vaccination. J Eur Acad Dermatol Venereol. 2021;35(7):e415‐e416. 10.1111/jdv.17225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ackerman M, Henry D, Finon A, Binois R, Esteve E. Persistent maculopapular rash after the first dose of Pfizer‐BioNTech COVID‐19 vaccine. J Eur Acad Dermatol Venereol. 2021;35(7):e423‐e425. 10.1111/jdv.17248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diez‐Domingo J, Parikh R, Bhavsar AB, Cisneros E, McCormick N, Lecrenier N. Can COVID‐19 increase the risk of herpes zoster? A narrative review. Dermatol Ther. 2021;11:1119‐1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee C, Cotter D, Basa J, Greenberg HL. 20 Post‐COVID‐19 vaccine‐related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960‐1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Channa L, Torre K, Rothe M. Herpes zoster reactivation after mRNA‐1273 (Moderna) SARS‐CoV‐2 vaccination. JAAD Case Rep. 2021;15:60‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eid E, Abdullah L, Kurban M, Abbas O. Herpes zoster emergence following mRNA COVID‐19 vaccine. J Med Virol. 2021;93:5231‐5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Özdemir AK, Kayhan S, Çakmak SK. Herpes zoster after inactivated SARS‐CoV‐2 vaccine in two healthy young adults. J Eur Acad Dermatol Venereol. 2021. 10.1111/jdv.17577. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bostan E, Yalici‐Armagan B. Herpes zoster following inactivated COVID‐19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566‐1567. [DOI] [PubMed] [Google Scholar]
- 15. Aksu SB, Öztürk GZ. A rare case of shingles after COVID‐19 vaccine: is it a possible adverse effect? Clin Exp Vaccine Res. 2021;10:198‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Psichogiou M, Samarkos M, Mikos N, Hatzakis A. Reactivation of Varicella zoster virus after vaccination for SARS‐CoV‐2. Vaccines. 2021;9:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nastro F, Fabbrocini G, di Vico F, Marasca C. Small vessel vasculitis related to varicella‐zoster virus after Pfizer‐BioNTech COVID‐19 vaccine. J Eur Acad Dermatol Venereol. 2021. 10.1111/jdv.17550. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arora P, Sardana K, Mathachan SR, Malhotra P. Herpes zoster after inactivated COVID‐19 vaccine: a cutaneous adverse effect of the vaccine. J Cosmet Dermatol. 2021. 10.1111/jocd.14268. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Furer V, Zisman D, Kibari A, Rimar D, Paran Y, Elkayam O. Herpes zoster following BNT162b2 mRNA COVID‐19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology. 2021:keab345. 10.1093/rheumatology/keab345. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tessas I, Kluger N. Ipsilateral herpes zoster after the first dose of BNT162b2 mRNA COVID‐19 vaccine. J Eur Acad Dermatol Venereol. 2021;35(10):e620‐e622. 10.1111/jdv.17422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiu HH, Wei KC, Chen A, Wang WH. Herpes zoster following COVID‐19 vaccine: report of 3 cases. QJM. 2021:hcab208. 10.1093/qjmed/hcab208. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodríguez‐Jiménez P, Chicharro P, Cabrera LM, et al. Varicella‐zoster virus reactivation after SARS‐CoV‐2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58‐59. 10.1016/j.jdcr.2021.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alpalhão M, Filipe P. Herpes zoster following SARS‐CoV‐2 vaccination ‐ a series of four cases. J Eur Acad Dermatol Venereol. 2021. 10.1111/jdv.17555. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sinha A, Kumar R, Singh AR. Implication of mass COVID‐19 vaccination on dermatology practice in 2021. Dermatol Ther. 2021;34(2):e14765. 10.1111/dth.14765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loughlin AM, Marchant CD, Adams W, et al. Causality assessment of adverse events reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2012;30:7253‐7259. [DOI] [PubMed] [Google Scholar]
- 26. Veenstra J, Krol A, van Praag RM, et al. Herpes zoster, immunological deterioration and disease progression in HIV‐1 infection. AIDS. 1995;9:1153‐1158. [DOI] [PubMed] [Google Scholar]
- 27. Martínez E, Gatell J, Morán Y, et al. High incidence of herpes zoster in patients with AIDS soon after therapy with protease inhibitors. Clin Infect Dis. 1998;27:1510. [DOI] [PubMed] [Google Scholar]
- 28. Sahin U, Muik A, Vogler I, et al. BNT162b2 induces SARS‐CoV‐2 neutralizing antibodies and T cells in humans. medRxiv. 2020. 10.1101/2020.12.09.20245175. [Epub ahead of print]. [DOI] [Google Scholar]
- 29. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS‐CoV‐2 mRNA‐1273, vaccine in older individuals. N Engl J Med. 2020;383:2427‐2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Halsey NA, Edwards KM, Dekker CL, et al. Causality Working Group of the Clinical Immunization Safety Assessment network. Algorithm to assess causality after individual adverse events following immunizations. Vaccine. 2012;30:5791‐5798. [DOI] [PubMed] [Google Scholar]
- 31. Tozzi AE, Asturias EJ, Balakrishnan MR, Halsey NA, Law B, Zuber PL. Assessment of causality of individual adverse events following immunization (AEFI): a WHO tool for global use. Vaccine. 2013;31:5041‐5046. [DOI] [PubMed] [Google Scholar]
- 32. Shimabukuro T. COVID‐19 Vaccine Safety Update; Proceedings of the Advisory Committee on Immunization Practices (ACIP) Meeting; Atlanta, GA, USA. 27 January 2021.
- 33. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. VAERS Searches: United States Department of Health and Human Services (DHHS) , Public Health Service (PHS) , Centers for Disease Control (CDC)/Food and Drug Administration (FDA) , Vaccine Adverse Event Reporting System (VAERS) . 1990—3/26/2021. CDC WONDER On‐line Database. Available online: http://wonder.cdc.gov/vaers.html. Accessed on April 4, 2021.
- 35. Coronavirus Vaccine—Weekly Summary of Yellow Card Reporting. Updated 25 March 2021. Available online: https://www.gov.uk/government/publications/coronavirus‐covid‐19‐vaccine‐adverse‐reactions/coronavirus‐vaccine‐summary‐of‐yellow‐card‐reporting. Accessed on April 4, 2021.
- 36. COVID‐19 Vaccine AstraZeneca Analysis Print. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/975786/COVID‐19_AstraZeneca_Vaccine_Analysis_Print.pdf. Accessed on April 4, 2021.
- 37. COVID‐19 mRNA Pfizer‐ BioNTech Vaccine Analysis Print. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/975808/COVID‐19_mRNA_Pfizer‐BioNTech_Vaccine_Analysis_Print.pdf. Accessed on April 4, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


