Abstract
The TATA binding protein (TBP) interacts with two transcription factor complexes, upstream activating factor (UAF) and core factor (CF), to direct transcription by RNA polymerase I (polI) in the yeast Saccharomyces cerevisiae. Previous work indicates that one function of TBP is to serve as a bridge, ennabling UAF to recruit and stabilize the binding of CF (23, 24). In this work we show that, in addition to aiding recruitment, TBP also directly aids CF function. Overexpression of TBP in strains with UAF components deleted will stimulate CF-directed transcription nearly to wild-type levels in vivo. In vitro, increasing the concentration of TBP stimulates CF-directed transcription in the absence of either UAF or its DNA binding site. This dual function of TBP, serving as a critical member of a core promoter complex as well as a contact point for upstream activators, appears similar to the dual roles that TBP also plays in transcription by RNA polII.
The promoter which directs rRNA synthesis in the yeast Saccharomyces cerevisiae consists of two functionally distinct sequence elements (2, 14, 18). One of these elements is the core domain, which overlaps the site of transcription initiation. Both in vitro and in vivo the core domain is capable of directing a low level of accurate initiation by RNA polymerase I (polI). Transcription from the core domain is strongly stimulated by the presence of the upstream domain, a region whose upstream boundary is close to position −150 and whose downstream boundary has been mapped to approximately −100.
Genetic and biochemical studies have shown that there are distinct multiprotein complexes which specifically interact with each of these promoter domains. Core factor (CF) interacts with the core domain and contains Rrn6p, Rrn7p, and Rrn11p, proteins which are essential for polI transcription (12, 15, 17). Upstream activating factor (UAF) interacts with the upstream domain and contains Rrn5p, Rrn9p, and Rrn10p (11), the two histones H3 and H4 (9), and an unidentified protein, p30. UAF cannot independently direct transcription but strongly stimulates polI transcription when CF is also present.
The TATA binding protein (TBP) is clearly required for activated levels of polI transcription (5, 22) and its precise interaction with the UAF and CF complexes has been an ongoing matter of investigation. It was initially reported that CF did not contain TBP (12), but subsequent experiments (17, 23) showed that TBP is associated with this complex. In addition, it has been shown that TBP binds to UAF and that this association is required for UAF to stimulate polI transcription (23). One of the UAF components, Rrn9p, has been shown to be a direct target of TBP binding, and mutations in Rrn9p that impair TBP binding can be suppressed by fusing Rrn9p to TBP (24). It has further been shown that basal levels of in vitro transcription can be obtained with a reconstituted system apparently lacking TBP (10). These experiments raise the possibility that in the yeast polI system the sole function of TBP is to serve as a bridge between UAF and CF and thereby enable UAF to recruit CF and position it over the core domain.
In this paper we report experiments which indicate that TBP plays an important role in the functions of the CF complex in addition to any role it may have in aiding recruitment by UAF. We show that overexpression of TBP can bypass the requirement for UAF to achieve activated levels of polI transcription in vivo. In vitro experiments show that raising the level of TBP stimulates polI transcription in the absence of UAF subunits or the upstream domain of the promoter. Taken together, these results support a model in which TBP has at least two important roles in polI transcription. TBP binding to UAF stimulates CF recruitment and overall stabilization of the initiation complex. In addition, TBP interaction with CF facilitates location of the core domain of the promoter, polI recruitment, and production of high levels of accurate transcription initiation. The last role of TBP appears similar to its function at many other promoters recognized by RNA polII and polIII.
MATERIALS AND METHODS
Yeast strains and plasmids.
Yeast strains and plasmid used in this paper are described in Table 1. All strains are derivatives of RLY01. Strains carrying knockouts of individual RRN genes were constructed by replacing part of the coding region with the HIS3 gene as described previously (11, 12), except with RLY08, in which the entire coding region of the RRN9 gene was replaced by a DNA fragment carrying the TRP1 gene.
TABLE 1.
Yeast strains and plasmids
| Strain or plasmid | Description |
|---|---|
| Strains | |
| RLY01 | MATα ade2-1 ade3::hisG ura3-1 his3-11 trp1-1 leu2-112 can1 |
| RLY02 | MATα ade2-1 ade3::hisG ura3-1 his3-11 trp1-1 leu2-112 can1 Δrrn11::HIS3 pNOY103 |
| RLY05 | MATα ade2-1 ade3::hisG ura3-1 his3-11 trp1-1 leu2-112 can1 Δrrn7::HIS3 pNOY103 |
| RLY06 | MATα ade2-1 ade3::hisG ura3-1 his3-11 trp1-1 leu2-112 can1 Δrrn6::HIS3 pNOY103 |
| RLY07 | MATα ade2-1 ade3::hisG ura3-1 his3-11 trp1-1 leu2-112 can1 Δrrn5::HIS3 pNOY103 |
| RLY08 | MATα ade2-1 ade3::hisG ura3-1 his3-11 trp1-1 leu2-112 can1 Δrrn9::TRP1 pNOY103 |
| RLY09 | MATα ade2-1 ade3::hisG ura3-1 his3-11 trp1-1 leu2-112 can1 Δrrn10::HIS3 pNOY103 |
| RLY4811 | Isogenic to RLY07, which carries the temperature-sensitive allele of the RPA190 gene |
| Plasmids | |
| pNOY103 | 2μm-, URA3-, and ADE3-expressing GAL7 35S rRNA (19) |
| pSII223 | YEp351 expressing TBP under the control of its own promoter (S. Hahn, Fred Hutchinson Cancer Research Center) |
| pRS315 | ARS CEN LEU2 vector (19a) |
| pRS315RRN5 | pRS315 expressing RRN5 under the control of its own promoter |
Strain RLY4811 (carrying a temperature-sensitive mutation in the RPA190 subunit of polI) was found in a genetic screen similar to that described by Nogi et al. (19). Strain RLY01 was transformed with plasmid pNOY103 (which expresses rRNA under the control of the GAL7 promoter) and mutagenized with ethylmethanesulfonate, and colonies that could grow on galactose but were unable to grow on glucose were selected. Among the galactose-dependent colonies, one exhibited a temperature-sensitive phenotype which was completely rescued by transformation with a cloned copy of the RPA190 gene and was not rescued by transformation with genes for several of the other polI subunits or polI transcription factors. This strain was designated RLY4811.
RNA pulse-labeling in vivo.
Yeast cells were grown at 30°C in minimal yeast nitrogen base (YNB) medium supplemented with galactose and required amino acids. At an A600 of 0.5, cells were harvested by centrifugation, washed with water, and resuspended in minimal YNB medium supplemented with glucose, amino acids, and uracil decreased to 1/100 of the normal amount (0.2 mg/liter). After 30 min at 30°C, [5,6-3H]uracil (50 Ci/mmol) was added at 100 μCi/ml, and cells were harvested by centrifugation 15 min later. Total RNA was extracted using TRIzol (Gibco BRL) according to the manufacturer's instructions. RNA samples containing the same amount of incorporated radioactive label were separated on 1.2% agarose–formaldehyde gels. Gels were treated with Amplify (Amersham) according to the manufacturer's instructions, dried, and autoradiographed using Kodak Biomax-MR film.
Western blotting of TBP.
Cells were grown as described above for the pulse-labeling experiment in 10 ml of medium. Equal numbers of cells were harvested by centrifugation and resuspended in 100 μl of loading buffer (0.06 M Tris-HCl [pH 6.8], 10% glycerol, 2% sodium dodecyl sulfate, 5% 2-β-mercaptoethanol, 0.001% bromophenol blue). After incubation at 100°C for 5 min, samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and reacted with anti-TBP antibodies. Signals were detected as described previously (17).
In vitro transcription assay.
Whole-cell extracts were prepared from cultures grown to an A600 of 1.0 essentially as described previously (21) except that ground cells were thawed into 1/10 volume of extraction buffer containing 10% glycerol. To normalize extracts to each other, each extract was titrated to determine the amount yielding the highest level of specific polI transcription activity when it was assayed with 10 μg of a wild-type promoter template per ml. All transcription experiments shown in this paper were performed using one-third of the amount of extract which gives maximal activity. Each experiment has been repeated with at least two independently prepared whole-cell extracts.
Templates for in vitro transcription contained either a wild-type polI promoter, a promoter bearing a linker scanner mutation in the upstream domain (LS −142/−122), or a promoter bearing a linker scanner mutation in the core domain (LS −29/−2). In some experiments (see Fig. 5B and 6) upstream regions of the promoter were deleted down to −122, −42, or −2 and replaced by vector sequence. The construction of these linker scanners or deletions has been described previously (2). To reduce background from nonspecific transcription, each promoter was excised as an EcoRI-XhoI fragment and inserted into the polylinker of pUC19. Transcription was assayed by primer extension as described previously (21) and quantitated by PhosphorImager analysis.
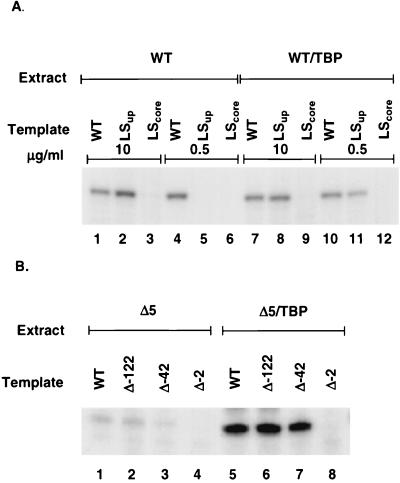
FIG. 5.
Requirement for either the upstream domain of the polI promoter or the UAF complex can be bypassed in vitro by overexpression of TBP. (A) Transcription extracts were made from either wild-type (WT) (RLY01) cells (lanes 1 through 6) or from wild-type cells overexpressing TBP (lanes 7 through 12). Each extract was used to transcribe templates bearing either a wild-type polI promoter (lanes 1, 4, 7, and 10), a promoter with an inactivated upstream domain (LSup; lanes 2, 5, 8, and 11), or a promoter with an inactivated core domain (LScore; lanes 3, 6, 9, and 12). Assays were performed at either a high (10-μg/ml) or a low (0.5-μg/ml) template concentration. Each extract was titrated to determine the amount which gave the maximum transcription with a wild-type template at 10 μg of DNA per ml. Thirty percent of the maximal amount of extract was used for each of the experiments whose results are shown. In wild-type extracts, the negative effect of inactivating the upstream domain (LSup) can be overcome by increasing the template concentration (compare lanes 2 and 5). Inactivation of the core domain (LScore) eliminates transcription at all template concentrations (lanes 3 and 6). Overexpression of TBP strongly stimulates transcription from a template lacking an upstream domain at a low template concentration (compare lanes 5 and 11). (B) Extracts were made from a ΔRRN5 strain (RLY07) or from a ΔRRN5 strain overexpressing TBP. Each extract was used to transcribe either a wild-type template (lanes 1 and 5), a template with a 5′ deletion to position −122 of the promoter (lanes 2 and 6), a template deleted to −42 (lanes 3 and 7), or a template deleted to −2 (lanes 4 and 8) at a 10-μg/ml template concentration. Extracts were used at 30% of the amount which gave maximal activity on a wild-type template. Overexpressing TBP stimulates transcription from promoters bearing deletions of the UAF binding region (lanes 6 and 7) to the same level seen with an intact promoter (lane 5).
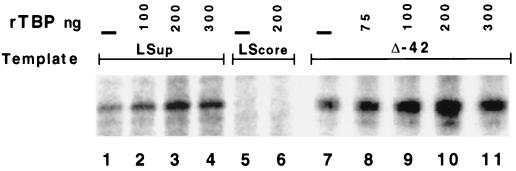
FIG. 6.
Recombinant TBP stimulates transcription from the core domain in an extract defective for UAF. Extract from strain RLY07 was used to transcribe templates either mutated in the upstream domain (LSup, lanes 1 through 4), mutated in the core domain (LScore, lanes 5 and 6), or having the upstream domain deleted (Δ−42, lanes 7 through 11). Template concentrations were 10 μg/ml, and recombinant TBP (rTBP) was added to the reaction mixtures as indicated. PhosphorImager quantitation indicates about a threefold stimulation of transcription between lanes 1 and 3 and about a fourfold stimulation between lanes 7 and 10.
RESULTS
TBP overexpression rescues deletions of UAF components but not CF components.
Genes for CF components, RRN6, RRN7, and RRN11, were individually disrupted in strain RLY01 carrying plasmid pNOY103 (20). As previously shown, these disruption strains are completely deficient in rRNA transcription as shown by their inability to grow on glucose-containing media (12, 15, 17) (see Fig. 1A). In contrast, these strains all grow on galactose due to transcription of rRNA from the GAL7 promoter on pNOY103. Genes for the UAF components, RRN5, RRN9, and RRN10, were also individually disrupted in the same background. As previously reported (11), disruption of these genes impairs but does not abolish rRNA production since the strains are capable of very slow growth on glucose (see Fig. 1B). Results similar to these have previously been interpreted as evidence that CF is absolutely required for rRNA transcription. In contrast, a low level of rRNA synthesis, sufficient to support cell growth at reduced rates, continues in the absence of UAF.
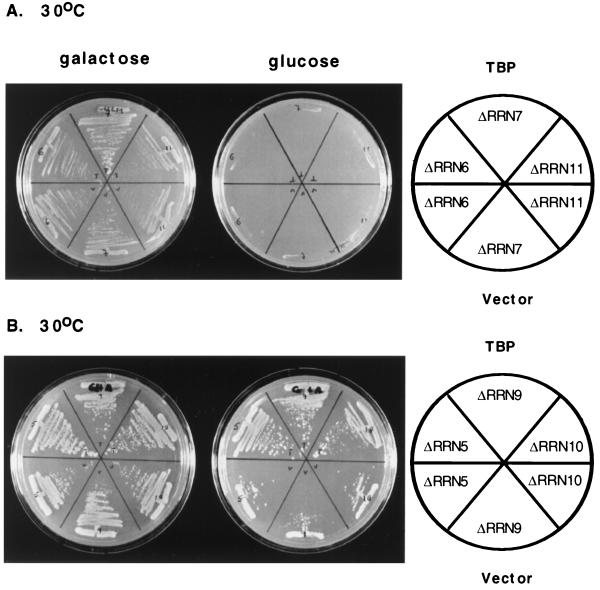
FIG. 1.
Overexpression of TBP bypasses the requirement for UAF components in vivo. (A) Strains carrying disruptions of any of the CF subunits (ΔRRN6, ΔRRN7, and ΔRRN11) are able to grow on galactose (left plate) due to rRNA transcribed from the GAL7 promoter on pNOY103 but are unable to grow on glucose (right plate). Overexpression of TBP (top sectors) does not alter this phenotype (compare to bottom sectors). (B) Strains carrying disruptions of UAF subunits (ΔRRN5, ΔRRN9, and ΔRRN10) also grow on galactose due to rRNA transcribed from pNOY103 (left plate). On glucose the UAF disruption strains grow very slowly (right plate, bottom sectors). However, overexpression of TBP allows the UAF disruption strains to grow at near wild-type rates (right plate, top sectors). This result indicates that TBP overexpression bypasses the requirement for the UAF complex.
Each of the RRN gene disruption strains was secondarily transformed with the 2μm-based expression vector pSH223, which carries the gene for TBP under the control of its own promoter, and plated on either glucose- or galactose-containing medium. As we expected, the TBP expression vector did not rescue any of the strains containing disruptions of CF components as these strains remained galactose dependent and showed no growth on glucose (Fig. 1A). In contrast, strains disrupted in any of the UAF components (RRN5, RRN9, or RRN10) showed nearly wild-type levels of growth on glucose when they were transformed with the TBP expression construct (Fig. 1B). We also verified that the transformed UAF disruption strains were capable of growth in the presence of 5-fluoro-orotic acid, demonstrating that growth on glucose-containing media is completely independent of pNOY103 (data not shown).
These results suggest that increasing TBP levels within the cell can bypass the requirement for the UAF complex and strongly stimulate rRNA transcription. To examine this hypothesis, we monitored both rRNA synthesis and TBP expression in these strains directly.
TBP levels are elevated in cells transformed with a TBP expression vector.
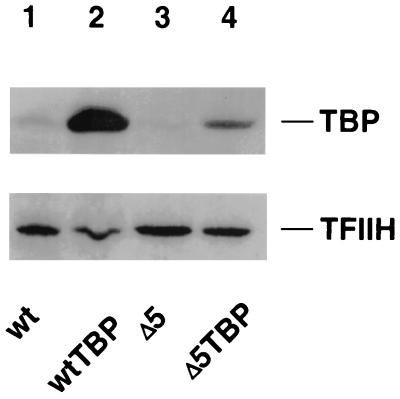
To verify that cells transformed with pSH223 overexpress TBP, we examined TBP levels in extracts from both wild-type and UAF disruption strains by Western blot analysis. Figure 2 shows that introduction of the 2μm TBP expression vector elevates TBP levels in both a wild-type strain (compare lanes 1 and 2) and in a strain where the UAF component RRN5 has been disrupted (compare lanes 3 and 4). We observed that TBP levels were reproducibly lower in the RRN5 disruption strain than in the wild-type strain (compare lanes 1 and 3), possibly reflecting the fact that the ΔRRN5 strain grows more slowly on galactose than does the wild type. We also observed that introduction of the TBP expression vector into both the wild-type and the RRN5 disruption strains reproducibly led to a substantial increase in TBP levels.
FIG. 2.
Transformation with a TBP expression vector (pSH223) results in overexpression of the protein. Protein extracts from various strains were blotted onto nitrocellulose and probed with a polyclonal antibody against yeast TBP. As a loading control the same blots were also probed with an antibody against the polII transcription factor TFIIH. Lane 1, extract from wild-type (wt) cells (strain RLY01); lane 2, wild type transformed with pSH223; lane 3, strain disrupted in RRN5 (RLY07); lane 4, ΔRRN5 strain transformed with pSH223. Transformation with pSH223 increases TBP levels significantly in either wild-type or ΔRRN5 cells (lanes 3 and 4) without affecting the level of TFIIH.
TBP overexpression stimulates rRNA transcription in UAF disruption strains.
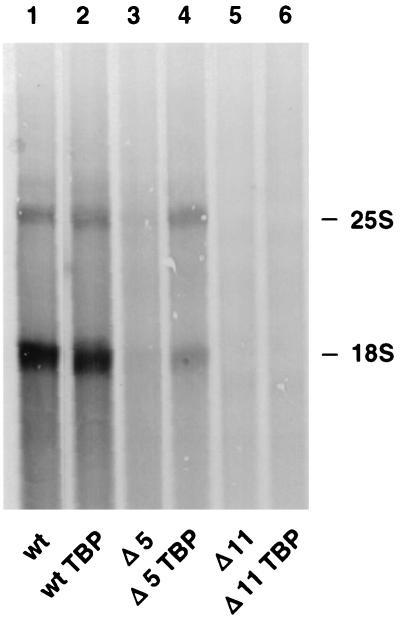
We next compared levels of rRNA synthesized in wild-type and disruption strains transformed with the TBP expression vector. Cells were pulse-labeled with [5,6-3H]uracil for 15 min, and relative levels of isotope incorporated into rRNA were measured by autofluorography. As expected, TBP overexpression in a CF disruption strain (ΔRRN11) did not stimulate rRNA transcription. rRNA transcripts remained undetectable in the presence or absence of pSH223 (Fig. 3, compare lanes 5 and 6), consistent with the observation that the TBP expression vector does not rescue CF disruption strains. In contrast, TBP overexpression in a UAF disruption strain (ΔRRN5) stimulated polI transcription to nearly wild-type levels (Fig. 3, lanes 3 and 4). Again, the results of the pulse-labeling experiment were consistent with the observation that transformation of UAF disruption strains with pSH223 allows growth on glucose. As a control we compared the wild-type strain RLY01 (lane 1) with a RLY01 strain transformed with the TBP expression vector. The intensities of the bands corresponding to 18S and 25S rRNAs showed that TBP overexpression in the wild-type strain had no effect on rRNA synthesis. We conclude that TBP overexpression stimulates rRNA transcription in the absence of a functional UAF complex.
FIG. 3.
Overexpression of TBP stimulates rRNA transcription in a strain with an inactive UAF (ΔRRN5). Newly made rRNAs in various strains were pulse-labeled and separated by gel electrophoresis, and the radioactive bands were detected by fluorography. The positions of mature 18S and 25S rRNAs are indicated. TBP overexpression has no effect on rRNA synthesis in a wild-type (wt) strain (RLY01, compare lanes 1 and 2). In contrast, TBP overexpression in a ΔRRN5 strain stimulates rRNA synthesis (RLY07) (lanes 3 and 4). As expected, no rRNA synthesis is detected in a ΔRRN11 strain, with or without TBP overexpression (RLY02) (lanes 5 and 6).
TBP overexpression stimulates rRNA synthesis by direct interaction with the polI machinery.
Yeast strains defective for UAF can, with some frequency, undergo an epigenetic alteration that results in transcription of the chromosomal ribosomal DNA by polII rather than by polI (25). UAF-deficient strains which have undergone this polymerase switch can now grow on glucose in the absence of pNOY103. In our own hands we have observed that colonies with the polymerase switch phenotype will arise from strain RLY07 (ΔRRN5) with a frequency of about 10−7 (data not shown). These observations raise the possibility that TBP overexpression might stimulate the frequency with which polymerase switch variants arise and thus account for the ability of UAF-deficient strains to grow on glucose upon TBP overexpression.
To address this possibility we constructed strain RLY4811 (isogenic to RLY07), which expresses a temperature-sensitive allele of the largest subunit of polI (rpa190-1ts) in the background of an RRN5 disruption. Strains RLY07 and RLY4811 were transformed with either pSH223 (TBP expression), pRS425RRN5 (Rrn5p expression), or pRS425 (an empty vector [Table 1]). We compared the ability of the transformants to grow on either glucose or galactose medium at both permissive and nonpermissive temperatures. As expected from previous results, RLY07 (ΔRRN5) transformed with either pSH223 or pRS425RRN5 can grow on either glucose or galactose at either the permissive or nonpermissive temperature. Also as expected, RLY07 transformed with the empty vector can grow only on galactose. These results essentially reproduce what is shown in Fig. 1.
The critical result in Fig. 4 was obtained with strain RLY4811 (which carries a temperature-sensitive allele of the RPA190 gene as well as a disrupted RRN5 gene) transformed with the same set of plasmids. RLY4811 behaves identically to RLY07 at the permissive temperature in that TBP overexpression restores growth on glucose (Fig. 4A). However, strain RLY4811 cannot grow on glucose at the nonpermissive temperature where polI is inactivated (Fig. 4B). This result demonstrates that the rescue of UAF disruption strains by TBP overexpression is due to a stimulation of polI transcription and is not due to transcription of the ribosomal DNA by polII.
FIG. 4.
TBP overexpression stimulates rRNA production by polI. Strain RLY07 (ΔRRN5) was secondarily transformed with either an empty vector (pRS315), a vector expressing RRN5 (pRS315RRN5), or the TBP overexpression vector (pSH223). In parallel, strain RLY4811 (isogenic to RLY07 but also carrying a temperature-sensitive [ts] allele of RPA190) was transformed with the same three vectors. (A) At the permissive temperature (30°C) strains RLY07 and RLY4811 behave the same. They grow on galactose due to the presence of pNOY103 and fail to grow on glucose. Transformation with an expression vector for either RRN5 or TBP allows both strains to grow on glucose. (B) At the nonpermissive temperature (35.5°C) strain RLY4811 cannot grow on glucose even when RRN5 or TBP is expressed (right plate, bottom sectors). This result indicates that TBP overexpression stimulates rRNA production via polI and not via some other RNA polymerase.
TBP overexpression stimulates in vitro transcription from the core domain of the promoter.
UAF requires the upstream domain of the polI promoter in order to stimulate transcription (24). Therefore, if TBP overexpression can stimulate transcription independently of the UAF complex, it should stimulate transcription in the absence of the upstream domain. In Fig. 5 we examine this possibility using an in vitro transcription assay.
In Fig. 5A, whole-cell extracts prepared from either wild-type cells or wild-type cells overexpressing TBP were used to transcribe three different promoter templates: a wild-type promoter, a promoter with the upstream domain inactivated by linker insertion (LS −142/−122), and a promoter with a linker insertion in the core domain (LS −29/−2). Figure 5A, lanes 1 to 6, shows the transcription signal obtained using wild-type extract to transcribe each of the three promoter variants. As previously described (2), polI transcription is unaffected by mutations in the upstream domain at a high template concentration (10 μg/ml; compare lanes 1 and 2) but is abolished by mutations in the core domain (compare lanes 1 and 3). At a low template concentration (0.5 μg/ml), however, transcription is strongly dependent upon the presence of the upstream domain (compare lanes 4 and 5). Figure 5A, lanes 7 to 12, repeats the same transcription shown in lanes 1 to 6 except that the extract was made from wild-type cells overexpressing TBP. At low template levels, the presence of excess TBP in the extract boosted transcription from the LS −142/−122 mutant template to nearly wild-type levels (compare lanes 10 and 11). As expected, overexpression of TBP did not rescue transcription of templates carrying mutations in the core domain under any conditions (lanes 9 and 12). These results indicate that increasing TBP levels can stimulate transcription from the core domain in the absence of the upstream domain.
We have also examined transcription of mutant polI promoters using extracts made from cells lacking Rrn5p (Fig. 5B). In this experiment promoter templates were either wild type or contained deletions that extended from upstream of the promoter down to 5′ −122, 5′ −42, or 5′ −2. The −122 and −42 deletions are expected to remove part or all of the upstream promoter domain, while the −2 deletion removes the core domain as well (2, 10, 14). When the ΔRRN5 extract was assayed at 30% of maximal activity, even the wild-type promoter gave only a weak signal (lane 1), indicating that UAF activity was absent in this extract. Overexpression of TBP strongly stimulated transcription from the wild-type promoter (lane 5) and stimulated transcription from both of the deletion mutants to the same level as that of the wild type (lanes 6 and 7). Thus, increasing TBP levels can stimulate transcription to wild-type levels in the absence of both the upstream domain and the UAF complex.
Transcription from the core domain is stimulated by addition of recombinant TBP.
Extract from cells lacking Rrn5p produced a weak polI transcription signal on either a wild-type, a Δ−122, or a Δ−42 template (Fig. 5B, lanes 1 through 3). Figure 6 shows the results of a further experiment in which either an LS −142/−122 template or a template that has the upstream domain deleted (Δ−42) was transcribed but in the presence of increasing amounts of recombinant TBP. Recombinant TBP stimulated polI transcription about threefold (compare lane 1 with lane 3) or fourfold (compare lane 7 with lane 10) in the absence of either Rrn5p or the upstream domain. As expected, addition of TBP did not stimulate transcription from a template lacking the core domain (lanes 5 and 6).
DISCUSSION
Elevating TBP concentration stimulates core-dependent polI transcription.
In this paper we show that elevating the intracellular level of TBP can stimulate transcription from the rRNA promoter and that this stimulation is independent of either UAF components in vivo or the upstream domain of the promoter in vitro. These observations support two important conclusions. First, they demonstrate that TBP operates through the core domain of the promoter and is therefore functionally a member of the CF complex. Second, they suggest that the primary role of the UAF complex is to recruit TBP or to stabilize its interactions within the CF complex.
It has been clearly demonstrated that binding of the UAF complex to the upstream domain of the promoter strongly stimulates rRNA transcription and is required for preinitiation complex stability in vitro (11). At issue has been the role of TBP in this process and the mechanism by which UAF stimulates transcription. Although TBP was shown to bind to the CF complex by coimmunoprecipitation (17) and affinity chromatography (23) assays, its requirement for transcription factor activity has not been established. In a previous study, we demonstrated that TBP was specifically coimmunoprecipitated with the CF subunit Rrn11p and that all three CF components (Rrn6p, Rrn7p, and Rrn11p) were jointly required to restore transcription activity to an Rrn11p-depleted extract (17). However, we were unable to address the requirement of TBP for CF activity since the CF-depleted extract still contained substantial amounts of TBP, presumably sequestered in TFIID and TFIIIB complexes utilized for polII and polIII transcription. Steffan and coworkers circumvented this problem by preparing TBP-responsive extracts from a yeast strain carrying the TBP point mutation I143N (23). They showed that I143N extracts supported low levels of transcription from the core promoter but did not support activated transcription from promoters with an upstream domain. They also demonstrated that addition of recombinant TBP stimulated transcription from a wild-type promoter but did not increase basal transcription from a core promoter template. These results demonstrate that TBP is required for UAF-mediated transcriptional activation but do not address its possible role in CF activity since the I143N point mutation may impair interactions with UAF subunits without altering interactions with CF proteins. If this is the case, TBP addition in vitro would not be expected to stimulate transcription from the core promoter alone since the I143N mutant would be functionally wild type for basal transcription. In support of this scenario, we note that several TBP point mutations which specifically impair activated transcription from polII promoters but support wild-type levels of basal transcription in vitro have been reported (13).
In this work we have shown that increasing the concentration of TBP in an in vitro reaction stimulates high levels of polI transcription from templates carrying either a block mutation in the upstream domain (Fig. 5A and 6) or a complete deletion of the upstream domain (Fig. 5B). Likewise, increasing TBP concentration stimulates core-dependent transcription when TBP is overexpressed in a wild-type extract (Fig. 5A), when it is overexpressed in an RRN5 knockout extract (Fig. 5B), and when it is added as recombinant protein to an RRN5 knockout extract (Fig. 6). This ability of TBP to stimulate core-dependent transcription is seemingly in conflict with a previous report by Keener et al. (10) in which polI transcription was reconstituted in vitro from highly purified components. A low level of core-dependent transcription was observed in the apparent absence of TBP, and those authors were unable to stimulate core-dependent transcription by addition of recombinant TBP. It is possible that this discrepancy is due to the use of whole-cell extracts for transcription versus the use of highly purified components. For example, in the polII system, the basal factor TFIIA is required for transcription in vivo and in whole-cell extracts but is dispensable when transcription is reconstituted from purified factors (6).
Our in vitro results with whole-cell extracts are strongly supported by in vivo data. Increasing the TBP level in a UAF disruption strain leads to increased rRNA synthesis (ΔRRN5) (Fig. 3) and allows galactose-dependent strains carrying disruptions of each of the UAF components (ΔRRN5, ΔRRN9, or ΔRRN10) to grow on glucose (Fig. 1). Thus, increasing the concentration of TBP allows the requirement for UAF activator to be bypassed in vivo as well as in vitro. It further implies that TBP plays an important role in CF function, in addition to its role in UAF-dependent activation.
A simple model for how TBP stimulates core-directed transcription is that it increases the number of promoters with CF bound to them. This may be accomplished by altering the CF complex, by stabilizing its association with DNA, or both. It is also possible that TBP provides DNA sequence specificity to the binding of CF in a manner similar to what has been proposed for the role of TBP in the binding of the polIII core complex, TFIIIB (7, 8). It will require further research to decide among these possibilities.
Yeast CF is functionally homologous with vertebrate SL1.
The vertebrate SL1 complex was first identified by its ability to direct accurate initiation in transcription systems primarily dependent on the core domain of the human polI promoter (16). Subsequently it was shown that SL1 consists of three polypeptides tightly associated with TBP (4) and that TBP is essential for SL1 activity (3). Thus, vertebrate SL1 shares significant functional homologies with yeast CF. Both complexes recruit polI, help it to select the start site, and utilize TBP. It is interesting that SL1 and CF perform similar transcriptional functions despite the fact that the three TBP-associated factors present in SL1 (TAF110, TAF63, and TAF48) share no obvious amino acid sequence similarity with those in CF (Rrn6p, Rrn7p, and Rrn11p). Activation mechanisms seem to have diverged even more strongly from yeast to vertebrates. Even though vertebrate polI promoters have both core and upstream domains, analogous to those of yeast, nothing resembling the yeast UAF activation complex has been found in vertebrates. Instead, SL1 interacts with upstream binding factor (1), a high-mobility-group box protein which stabilizes and stimulates SL1-directed transcription. Nothing related to UBF has been observed in the yeast polI system. At this point it appears that polI transcription systems have retained a TBP-containing core complex from fungi to humans which performs similar functions. In contrast, the activation mechanism seems to have diverged considerably.
ACKNOWLEDGMENTS
We thank Judy Roan for expert technical assistance.
This work was partially supported by NIH grant GM26624 awarded to R.H.R. B.M. was supported by NIH training grant CA09657.
REFERENCES
- 1.Bell S P, Learned R M, Jantzen H-M, Tjian R. Functional cooperativity between transcription factors UBF1 and SL1 mediates human ribosomal RNA synthesis. Science. 1988;241:1192–1197. doi: 10.1126/science.3413483. [DOI] [PubMed] [Google Scholar]
- 2.Choe S Y, Schultz M C, Reeder R H. In vitro definition of the yeast RNA polymerase I promoter. Nucleic Acids Res. 1992;20:279–285. doi: 10.1093/nar/20.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Comai L, Tanese N, Tjian R. The TATA-binding protein and associated factors are integral components of the RNA polymerase I transcription factor, SL1. Cell. 1992;68:965–976. doi: 10.1016/0092-8674(92)90039-f. [DOI] [PubMed] [Google Scholar]
- 4.Comai L, Zomerdijk J C B M, Beckman H, Zhou S, Admon A, Tjian R. Reconstitution of transcription factor SL1: exclusive binding of TBP by SL1 or TFIID subunits. Science. 1994;266:1966–1972. doi: 10.1126/science.7801123. [DOI] [PubMed] [Google Scholar]
- 5.Cormack B P, Struhl K. The TATA-binding protein is required for transcription by all three nuclear RNA polymerases in yeast cells. Cell. 1992;69:685–696. doi: 10.1016/0092-8674(92)90232-2. [DOI] [PubMed] [Google Scholar]
- 6.Hampsey M. Molecular genetics of the RNA polymerase II general transcriptional machinery. Microbiol Mol Biol Rev. 1998;62:465–503. doi: 10.1128/mmbr.62.2.465-503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joazeiro C A P, Kassavetis G A, Geiduschek E P. Alternative outcomes in assembly of promoter complexes: the roles of TBP and a flexible linker in placing TFIIIB on tRNA genes. Genes Dev. 1996;10:725–739. doi: 10.1101/gad.10.6.725. [DOI] [PubMed] [Google Scholar]
- 8.Kassavetis G A, Joazeiro C A, Pisano M, Geiduschek E P, Colbert T, Hahn S, Blanco J A. The role of the TATA-binding protein in the assembly and function of the multisubunit yeast RNA polymerase III transcription factor, TFIIIB. Cell. 1992;71:1055–1064. doi: 10.1016/0092-8674(92)90399-w. [DOI] [PubMed] [Google Scholar]
- 9.Keener J, Dodd J A, Lalo D, Nomura M. Histones H3 and H4 are components of upstream activation factor required for the high-level transcription of yeast rDNA by RNA polymerase I. Proc Natl Acad Sci USA. 1997;94:13458–13462. doi: 10.1073/pnas.94.25.13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keener J, Josaitis C A, Dodd J A, Nomura M. Reconstitution of yeast RNA polymerase I transcription in vitro from purified components. J Biol Chem. 1998;173:33795–33802. doi: 10.1074/jbc.273.50.33795. [DOI] [PubMed] [Google Scholar]
- 11.Keys D A, Lee B-S, Dodd J A, Nguyen T T, Vu L, Fantino E, Burson L M, Nogi Y, Nomura M. Multiprotein transcription factor UAF interacts with the upstream element of the yeast RNA polymerase I promoter and forms a stable preinitiation complex. Genes Dev. 1996;10:887–903. doi: 10.1101/gad.10.7.887. [DOI] [PubMed] [Google Scholar]
- 12.Keys D A, Steffan J S, Dodd J A, Yamamoto R T, Nogi Y, Nomura M. RRN6 and RRN7 encode subunits of a multiprotein complex essential for the initiation of rDNA transcription by RNA polymerase I in Saccharomyces cerevisiae. Genes Dev. 1994;8:2349–2362. doi: 10.1101/gad.8.19.2349. [DOI] [PubMed] [Google Scholar]
- 13.Kim T K, Hashimoto S, Kelleher R J, Flanagan P M, Kornberg R D, Horikoshi M, Roeder R G. Effects of activation-defective TBP mutations on transcription initiation in yeast. Nature. 1994;369:252–255. doi: 10.1038/369252a0. [DOI] [PubMed] [Google Scholar]
- 14.Kulkens T, Riggs D L, Heck J D, Planta R J, Nomura M. The yeast RNA polymerase I promoter: ribosomal DNA sequences involved in transcription initiation and complex formation in vivo. Nucleic Acids Res. 1991;19:5363–5370. doi: 10.1093/nar/19.19.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lalo D, Steffan J S, Dodd J A, Nomura M. RRN11 encodes the third subunit of the complex containing Rrn6p and Rrn7p that is essential for the initiation of rDNA transcription by yeast RNA polymerase I. J Biol Chem. 1996;271:21062–21067. doi: 10.1074/jbc.271.35.21062. [DOI] [PubMed] [Google Scholar]
- 16.Learned R M, Cordes S, Tjian R. Purification and characterization of a transcription factor that confers promoter specificity to human RNA polymerase I. Mol Cell Biol. 1985;5:1358–1369. doi: 10.1128/mcb.5.6.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin C-W, Moorefield B, Payne J, Aprikian P, Mitomo K, Reeder R H. A novel 66-kilodalton protein complexes with Rrn6, Rrn7, and TATA-binding protein to promote polymerase I transcription initiation in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:6436–6443. doi: 10.1128/mcb.16.11.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musters W, Knol J, Maas P, Dekker A F, van Heerikhuizen H, Planta R J. Linker scanning of the yeast RNA polymerase I promoter. Nucleic Acids Res. 1989;17:9661–9678. doi: 10.1093/nar/17.23.9661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nogi Y, Vu L, Nomura M. An approach for isolation of mutants defective in 35S ribosomal RNA synthesis in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1991;88:7026–7030. doi: 10.1073/pnas.88.16.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nogi Y, Yano R, Nomura M. Synthesis of large rRNAs by RNA polymerase II in mutants of Saccharomyces cerevisiae defective in RNA polymerase I. Proc Natl Acad Sci USA. 1991;88:3962–3966. doi: 10.1073/pnas.88.9.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schultz M C, Choe S Y, Reeder R H. Specific initiation by RNA polymerase I in a whole-cell extract from yeast. Proc Natl Acad Sci USA. 1991;88:1004–1008. doi: 10.1073/pnas.88.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultz M C, Reeder R H, Hahn S. Variants of the TATA binding protein can distinguish subsets of RNA polymerase I, II, and III promoters. Cell. 1992;69:697–702. doi: 10.1016/0092-8674(92)90233-3. [DOI] [PubMed] [Google Scholar]
- 22a.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in S. cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steffan J S, Keys D A, Dodd J A, Nomura M. The role of TBP in rDNA transcription by RNA polymerase I in Saccharomyces cerevisiae: TBP is required for upstream activation factor-dependent recruitment of core factor. Genes Dev. 1996;10:2551–2563. doi: 10.1101/gad.10.20.2551. [DOI] [PubMed] [Google Scholar]
- 24.Steffan J S, Keys D A, Vu L, Nomura M. Interaction of TATA-binding protein with upstream activation factor is required for activated transcription of ribosomal DNA by RNA polymerase I in Saccharomyces cerevisiae in vivo. Mol Cell Biol. 1998;18:3752–3761. doi: 10.1128/mcb.18.7.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vu L, Siddiqui I, Lee B-S, Josaitis C A, Nomura M. RNA polymerase switch in transcription of yeast rDNA: role of transcription factor UAF (upstream activation factor) in silencing rDNA transcription by RNA polymerase II. Proc Natl Acad Sci USA. 1999;96:4390–4395. doi: 10.1073/pnas.96.8.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]