ABSTRACT
Superficial fungal infections are prevalent worldwide, with dermatophytes as the most common cause. Various antifungal agents including azoles and allylamines are commonly used to treat dermatophytosis. However, their overuse has yielded drug-resistant strains, calling for the development of novel antimycotic compounds. Olorofim is a newly developed antifungal compound that targets pyrimidine biosynthesis in molds. The purpose of this study was to determine the in vitro and in vivo antifungal effects of olorofim against common dermatophytes. The in vitro activity of olorofim against dermatophytes was assessed by microtiter broth dilution method. Bioinformatic analysis of olorofim binding to dihydroorotate dehydrogenase (DHODH) of dermatophytes was also performed, using Aspergillus fumigatus DHODH as a template. The in vivo efficacy of the drug was investigated, using a guinea pig model, experimentally infected with Microsporum gypseum. Microtiter assays confirmed the high in vitro sensitivity of dermatophytes to olorofim (MIC = 0.015–0.06 mg/liter). Amino acid sequence analysis indicated that DHODH is highly conserved among dermatophytes. The critical residues, in dermatophytes, involved in olorofim binding were similar to their counterparts in A. fumigatus DHODH, which explains their susceptibility to olorofim. Typical skin lesions of dermatophyte infection were observed in the guinea pig model at 7 days postinoculation. Following 1 week of daily topical administration of olorofim, similar to the clotrimazole group, the skin lesions were resolved and normal hair growth patterns appeared. In light of the in vitro and in vivo activity of olorofim against dermatophytes, this novel agent may be considered as a treatment of choice against dermatophytosis.
KEYWORDS: Microsporum gypseum, antifungals, dermatophytes, dihydroorotate dehydrogenase, olorofim
INTRODUCTION
According to a recent estimate of the prevalence of fungal diseases, the burden of superficial fungal infections, including skin, hair and nail, approximates 750 million people worldwide (1). Superficial mycosis is mainly attributed to two divergent fungal groups: dermatophytes, as the most common causative agents, including the genera Trichophyton, Microsporum, and Epidermophyton; and non-dermatophytes yeasts from the genera Candida, Trichosporon, and Malassezia (2). In 2017, an estimated five million people in the US were diagnosed with dermatophytes, imposing a direct cost of ∼$821 million to the US health care system (https://www.cdc.gov/fungal/cdc-and-fungal/burden.html). Dermatophytes spread via direct contact with infected people, animals, or soil, as well as indirectly from fomites. These pathogens can colonize the keratinized structures present in skin, hair, and nails, causing superficial infections, known as dermatophytosis (3). Dermatophytoses are mainly treated by local application and/or systemic administration of azole-based drugs including clotrimazole, econazole, ketoconazole, miconazole, tioconazole, as well as terbinafine, amorolfine, tolnaftate, and griseofulvin (4, 5).
Antifungal susceptibility testing is not routinely undertaken in cases of dermatophytosis, as the infecting organism is rarely identified. Resistance to commonly administered topical azoles has been increasingly reported (6). Moreover, a number of reports indicate that the terbinafine-resistant T. mentagrophytes are emerging and spreading globally (7–11). Although dermatophytosis is non-fatal, it can be disfiguring and contagious, requiring immediate treatment. Long-term treatment is often required, and compliance can be poor, and this can bring about the emergence of drug resistance strains (12, 13).
This fact emphasizes the urgent need for introducing new classes of antifungals, with novel mechanisms of action. A recently developed antifungal compound, olorofim (F2G Ltd, UK), has demonstrated high efficiency against Aspergillus species and some other molds (14, 15). Belonging to a new class of antifungals titled the orotomides, it specifically targets fungal dihydroorotate dehydrogenase (DHODH) (14), an essential enzyme in the de novo pyrimidine biosynthesis pathway (16). Olorofim does not inhibit human DHODH and is currently in a phase IIb open-label study, focusing on rare and resistant, life-threatening, invasive fungal infections (www.F2g.com).
Considering the strong antifungal activity of olorofim against different pathogenic molds (9, 14, 17), here we have investigated the in vitro and in vivo effects of this compound against dermatophytes, using an animal model of dermatophytosis.
RESULTS
In vitro sensitivity of dermatophytes to olorofim.
MICs of olorofim were determined for different dermatophyte and Aspergillus strains, in comparison to posaconazole and voriconazole. As demonstrated by the MICs listed in Table 1, both groups (dermatophyte and Aspergillus strains) were far more sensitive to olorofim, compared to posaconazole and voriconazole. A. fumigatus and A. flavus showed identical MIC value of 0.01 mg/liter; however, the susceptibility range for the dermatophyte isolates was 0.01–0.06 mg/liter. The highest MIC was observed in T. tonsurans.
TABLE 1.
In vitro susceptibility of aspergilli and dermatophytes to olorofim, posaconazole, voriconazole, and clotrimazole
| Antifungal compound | MIC (mg/L) |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Aspergillus
|
Dermatophytes |
|||||||
|
Aspergillus fumigatus PTCC5009 |
Aspergillus flavus PTCC5004 |
Trichophyton mentagrophytes NBRC5809 |
Trichophyton tonsurans CBS 130814 |
Trichophyton rubrum IR613 |
Epidermophyton floccosum CBS 130793 |
Microsporum canis PTCC5069 |
Microsporum gypseum PTCC5070 |
|
| Olorofim | 0.01 | 0.01 | 0.01 | 0.06 | 0.01 | 0.03 | 0.03 | 0.03 |
| Posaconazole | 0.15 | 0.3 | 0.04 | 0.6 | 0.08 | 0.12 | 0.3 | 0.6 |
| Voriconazole | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.12 | 0.6 | 0.6 |
| Clotrimazole | 2 | 4 | 0.25 | 1 | 16 | 2 | 4 | 1 |
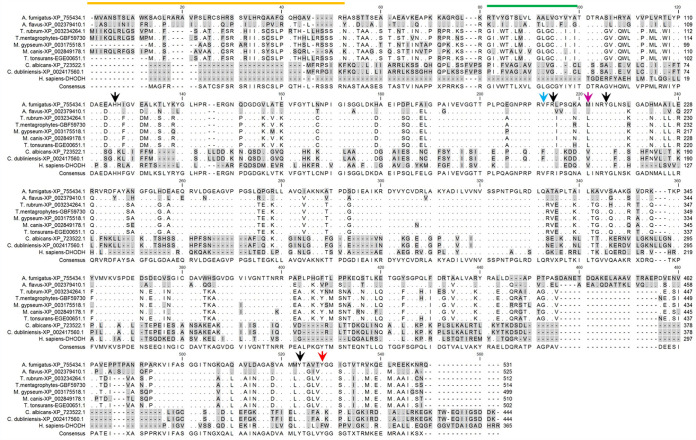
DHODH is highly conserved among dermatophytes.
The above MIC results led us to analyze the DHODH sequences of the dermatophytes tested, except Epidermophyton flocossum, for which no genome was available, and compare them to other fungal and human DHODH. The amino acid sequences revealed a significant conservation, among the aligned sequences (Fig. 1). The DHODH sequence similarity and identity among different strains of dermatophytes ranged from 74.3 to 95.5% and 73 to 90%, respectively. The overall similarity between DHODH of the investigated dermatophytes and A. fumigatus was about 63%. In previous reports, the key residues for olorofim binding were identified as His116, Val200, Arg202, Met209, Tyr213, Tyr507, and Tyr512 (14, 22). Our results indicated that dermatophytes share six out of these seven critical residues with A. fumigatus. The only differing residue was Met209, which was replaced by Leu in Trichophyton and Microsporum, and Val in Candida strains.
FIG 1.
Alignment of different dermatophytes, A. fumigatus, C. albicans, and human (H. sapiens) DHODH amino acid sequences. Identical residues are distinguished by dots, and similar residues are highlighted in gray. The predicted mitochondrial targeting sequences are indicated by the orange line, and the predicted transmembrane domains are shown by the green line. The seven residues predicted to be important for olorofim binding in A. fumigatus DHODH are depicted by arrows, whose colors illustrate conservation status—black, purple, and blue for conserved residues unique to A. fumigatus, Candida, and humans, respectively.
In vivo sensitivity of dermatophytes to olorofim.
All infected guinea pigs showed multiple signs of superficial fungal infection, including erythema, ulceration, mild shedding, and scaly skin, in the infected area, 7 days postinfection (Fig. 2). Fungal infection was validated by the presence of fungal elements in microscopic examination of scrapings (data not shown).
FIG 2.
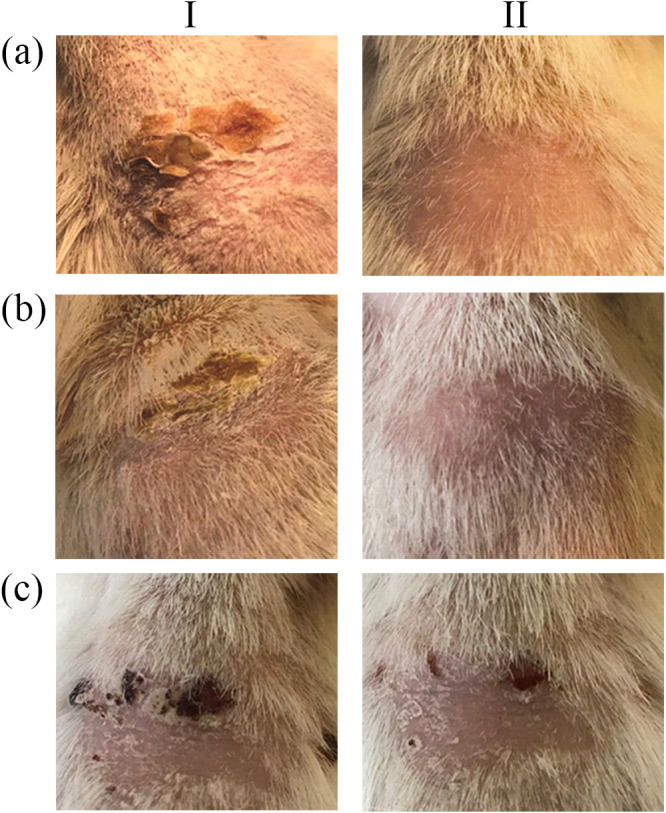
The effect of different treatments on skin lesions in guinea pigs Infected with M. gypseum. aI, before treatment with olorofim; aII, After 1 week treatment with olorofim (10 μg/lesion); bI, before treatment by clotrimazole; bII, after 1 week treatment with clotrimazole (1%); cI, before treatment by PEG 300; cII, after 1 week treatment with PEG 300.
Following topical antifungal treatment, the skin samples were collected by scraping the edge of the healed lesions and examined by direct microscopy. In the control group (c: PEG-treated), the patches of hair loss and the readily visible ulcerated or scaly skin were still present, 1 week after PEG administration (Fig. 2 cI & cII). In this group, the lesions self-healed after 3–4 weeks of infection. However, in the olorofim-treated group (Group a), similar to the clotrimazole-treated group (Group b), no fungal elements were observed, at 7 days post treatment. Furthermore, olorofim treatment significantly reduced the redness and the skin lesions, followed by the appearance of normal pattern of hair growth and no sign of scaly skin after 1 week (Fig. 2 aII and bII).
DISCUSSION
Superficial mycosis, as the most prevalent fungal infection, affects 20–25% of the world population (23, 24). Dermatophytes are known as the main causative agents of the disease and are often treated by antifungal drugs, targeting the ergosterol biosynthetic pathway, including azoles and allylamines. Unfortunately, the widespread and unattended use of over-the-counter azoles, especially in combination with topical steroids, has led to the development of drug resistance in dermatophytes (5, 6). Accordingly, reports indicate an estimated 19% azole resistance rate among dermatophytes, particularly in certain tropical areas (6). In this sense, novel antifungal compounds with new targets are under investigation. Although the majority of antifungal drug discovery research is driven by the need for better treatment of invasive and systemic fungal diseases, novel compounds may be applicable in treatment of superficial mycosis. Olorofim, a highly potent and selective fungal DHODH inhibitor, is active against clinically relevant molds, except Mucorales. Due to its novel mechanism of action, it is unlikely for olorofim to cause or be affected by cross-resistance to other antifungal classes (14, 17). The olorofim-susceptible fungal species are closely related in their DHODH amino acid sequences, compared to non-susceptible fungi, such as Candida spp, Mucorales spp, and C. neoformans.
Here, we have investigated the antifungal activity of olorofim against three different genera of dermatophytes, Trichophyton, Microsporum, and Epidermophyton. Our results confirmed the high sensitivity of these organisms to the drug, which is in agreement with previous reports (14, 25). The MIC levels (0.015 to 0.062 μg/ml) were in a range similar to those reported for Trichophyton and Microsporum by Astvad et al. (25). In our study, the variations observed in MIC levels may re-emphasize the species-specific differences among dermatophytes (8). The small number of fungal strains used for MIC determinations can be considered as a limitation of the present investigation. The high susceptibility of dermatophytes to olorofim can be attributed to the significant sequence similarity (∼63%) of their DHODH amino acid sequences with that of Aspergillus spp. Based on the report by Oliver et al. 6, Val200 and Met209 are identified as key residues in A. fumigatus sensitivity to olorofim, such that mutation of these two amino acids occupying the same position in Candida albicans DHODH (Phe162 and Val171) to Val162 and Met171 resulted in a recombinant DHODH that was susceptible to olorofim inhibition in in vitro assays, while the original C. albicans DHODH was not. In the case of Met209 in the A. fumigatus sequence, it seems that a conservative replacement of Leu in the dermatophytes studied is tolerated by olorofim, judging by the low MICs observed in these organisms. The remaining six residues, expected to influence olorofim binding of DHODH, are identically conserved between A. fumigatus and the dermatophytes, consistent with the low MIC values observed.
To investigate the in vivo efficacy of olorofim against dermatophytes, a guinea pig model of dermatophytosis was created. Guinea pigs have been widely used as a model for establishment of dermatophytosis as the clinical signs of dermal infection in this animal are comparable to those seen in humans. The guinea pig model has also been used in predicting the efficacy of antifungal preparations, which makes it a valuable tool for the preclinical assessment of new antifungal compounds (26). We used an animal isolate of M. gypseum for infection in this model. Experimental infection of guinea pigs with M. gypseum has been previously described (21, 27, 28). Here, we first made several attempts to infect animals via skin abrasion, however with limited success. Hence, we decided to use corticosteroids to temporally suppress the immune system of the animals and to enhance the chance of infection. The administration of corticosteroids for successful establishment of dermatophytosis has also been previously reported (29). Despite the similarly used method for fungal inoculation, some variation in the clinical signs of infection was observed between the animals. This can be attributed to host factors, such as the varying strength of the immune system, in outbred animals (27, 30). However, clear differences in clinical outcomes in treated animals versus controls were observed (e.g., compare olorofim-treated, Fig. 2 aII; versus control-treated, cI; and immediately prior to olorofim treatment, aI).
In most studies, the topical or oral treatment of dermatophytosis was undertaken 3–5 days following the infection (31). However, in our study, olorofim (0.1 mg/ml in PEG300) was topically administered daily at a dosage of 10 μg/lesion from the eighth day, postinfection. This starting time of treatment was chosen based on the time at which the skin lesions were clearly visible (i.e., 7 days postinfection). Topical treatment of olorofim was carried out every day for 7 days, at which time the results were compared with the positive and negative controls. The selection of such a short time period was to avoid the self-healing time course of cutaneous dermatophytosis in guinea pigs (32). The drug cured the skin lesions during the first week of treatment, and the skin looked healthy and smooth with no redness, swelling, or scarring. These results indicated that olorofim was highly effective in the treatment of dermatophytosis in the guinea pig model. This may be due to the very low MICs observed for this compound against dermatophytes. We also found that the olorofim dosage of 10 μg/lesion (2.5 μg/cm2) mimics the therapeutic dose of clotrimazole, although other concentrations remain to be examined. The data for clotrimazole indicate that following the application of topical clotrimazole 1% cream, the concentration of clotrimazole would be around 100 μg/cm2 in the stratum corneum, which is much higher than that used for olorofim in our study (33).
In conclusion, we demonstrated the efficacy of olorofim as a novel anti-dermatophytosis agent against various dermatophyte species, in vitro and also against M. gypseum infection in vivo. However, more detailed studies are required to elucidate its efficacy against other dermatophytes, as well as clarifying the drug pharmacokinetics, upon topical administration.
MATERIALS AND METHODS
Ethics.
This study was conducted in accordance with institutional standards and approved by the Ethical Committee of the Pasteur Institute of Iran. (Ethical code: IR.PII.REC.1397.021).
Strains, culture conditions, and antifungal agents.
Dermatophyte strains (n = 6, Table 1) were grown on Sabouraud dextrose agar (SDA, Merck, Germany) slants, supplemented with chloramphenicol (0.005%) and cycloheximide (0.04%), at 28°C for 10–14 days. Aspergillus strains (n =2) were cultivated on SDA plates, at 37°C, for 3–5 days. To collect fresh spores, fungal colonies were gently washed with PBS-Tween 80, and the resulting suspension was filtered through a thin layer of sterilized glass wool to remove the hyphal fragments. The spores were then separated by centrifugation at 1000 × g, for 10 min, and their concentrations were determined using hemocytometer counts. The final concentrations of spores were adjusted at 106/ml to use in MIC assays.
Azole compounds including voriconazole, posaconazole, and clotrimazole were purchased from Sigma-Aldrich, UK. Olorofim was kindly provided by F2g Limited, UK. All compounds were prepared and stored as 5 mg/ml stocks in DMSO at 4°C. Clotrimazole topical cream was provided by Emad Darman Pars pharmaceutical company, Iran.
Antifungal susceptibility testing.
The antifungal susceptibility of dermatophytes was assessed by determining the MIC, based on the M38-E3rd protocol of the Clinical and Laboratory Standards Institute (CLSI), with some modifications (18). Briefly, a total of 104 spores were suspended in 80 μl of RPMI 1640 medium, buffered to pH 7.0 (with MOPS) and seeded onto a 96-well microtiter plate. Then 20 μl of serial 2-fold dilutions of each test compound was added to each well and the plate was incubated at 28°C. The MICs were assessed after 96 h of incubation, at a final compound concentration range of 0.001–10 μg/ml for voriconazole and posaconazole, 0.03-64 μg/ml for clotrimazole, and 0.0001–1 μg/ml for olorofim, MIC endpoints were defined as the lowest concentration of each test compound that resulted in inhibition of growth (80% or more) by visual inspection, compared to the controls.
Sequence analysis.
In order to identify DHODH homologues in different fungi, the amino acid sequence of A. fumigatus DHODH was retrieved from the KEGG database (E.C.1.3.5.2, AFUA_2G11010, XP_755434) and used as a template in a tBLASTn search against available dermatophytes, Candida, and human genomes. The sequences were aligned and formatted by CLC Main Workbench (https://digitalinsights.qiagen.com). Mitochondrial targeting sequences and transmembrane domains of the enzyme were predicted by MitoFates and Phobius servers, respectively (19, 20).
Animal model of infection.
Nine albino female guinea pigs (300–350 g each) were purchased from the Laboratory of Animal Sciences, Pasteur Institute of Iran. Animals were housed in groups of three, kept under standard laboratory conditions (room temperature of 18–22°C, relative humidity of 40–50%, and 12h light/day cycle) and provided with food and water ad libitum. The guinea pigs were acclimated for 1 week prior to experimental treatments. To establish dermatophytosis, animals were immunosuppressed via intramuscular injection of prednisolone (10 mg/kg) and subcutaneous injection of hydrocortisone (5 mg/kg), 1 day prior to infection and 3 days thereafter. All guinea pigs were anesthetized, using intraperitoneal injection of ketamine (50 mg/kg), xylazine (5 mg/kg), and acepromazine (1 mg/kg). Once the animals were fully anesthetized, the posterior dorsal areas were gently shaved (∼ 2 × 2 cm) and then abraded with the back of a sterile scalpel blade. The inoculation of fungi was carried out according to previous protocols, with some modifications (21). Briefly, an animal isolate of Microsporum gypseum was grown on SAB agar for 14 days and checked for the presence of micro- and macroconidia. One hundred microliters of this inoculum (108 conidia in PBS/Tween 0.01%) were spread on the abraded area using a sterile pipette tip and left to dry. The inoculated area was surrounded by a thin layer of Vaseline and then dressed with sterile pads and bandaged with non-woven tape (TGMED), for dressing fixation. Each animal was placed on a hot water blanket until full recovery from anesthesia was achieved. For mycological evaluations, surface scrapings were collected from the inoculation sites at 7 days postinfection, and the obtained specimens underwent direct microscopic examination using routine KOH wet mount.
Eight days postinfection, the animals were randomly divided into 3 groups (a–c) and received topical treatments of the following compounds, once daily for 7 days: Group a, Olorofim (100 μl of 0.1 mg/ml in PEG300); Group b, 1% clotrimazole (as positive control, topical cream); and Group c, PEG 300 (100 μl, as negative control).
ACKNOWLEDGMENTS
We gratefully acknowledge F2G Ltd for providing olorofim compound as a gift.
This work was supported by grants from the National Science Foundation (INSF) (no. 92026749) and Pasteur Institute of Iran (no. 1696).
We declare no conflict of interest.
E.M.A. designed the animal model study, performed all the related experiments, and participated in preparation of the first draft of the manuscript. A.S. performed the bioinformatic analyses and assisted in preparation of the first draft of the manuscript. N.P. performed MIC assays. M.N. and M.Z. helped in preparation of fungal cultures and spore inoculations. S.E. assisted in animal study. M.R.-A. was involved in interpretation of MICs. V.K. designed and supervised the whole study and critically revised and finalized the manuscript.
REFERENCES
- 1.Urban K, Chu S, Scheufele C, Giesey RL, Mehrmal S, Uppal P, Delost GR. 2021. The global, regional, and national burden of fungal skin diseases in 195 countries and territories: A cross-sectional analysis from the Global Burden of Disease Study 2017. JAAD Int 2:22–27. 10.1016/j.jdin.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Álvarez-Mosquera I, Hernáez S, Sánchez J, Suárez MD, Cisterna R. 2018. Diagnosis of superficial mycoses by a rapid and effective PCR method from samples of scales, nails and hair. Mycopathologia 183:777–783. 10.1007/s11046-018-0290-5. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Rossi NM, Bitencourt TA, Peres NTA, Lang EAS, Gomes EV, Quaresemin NR, Martins MP, Lopes L, Rossi A. 2018. Dermatophyte resistance to antifungal drugs: mechanisms and prospectus. Front Microbiol 9:1108. 10.3389/fmicb.2018.01108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hay R. 2018. Therapy of skin, hair and nail fungal infections. J Fungi (Basel) 4(3):99. 10.3390/jof4030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khurana A, Sardana K, Chowdhary A. 2019. Antifungal resistance in dermatophytes: recent trends and therapeutic implications. Fungal Genet Biol 132:103255. 10.1016/j.fgb.2019.103255. [DOI] [PubMed] [Google Scholar]
- 6.Ghannoum M. 2016. Azole resistance in dermatophytes: Prevalence and mechanism of action. J Am Podiatr Med Assoc 106:79–86. 10.7547/14-109. [DOI] [PubMed] [Google Scholar]
- 7.Fattahi A, Shirvani F, Ayatollahi A, Rezaei-Matehkolaei A, Badali H, Lotfali E, Ghasemi R, Pourpak Z, Firooz A. 2021. Multidrug-resistant Trichophyton mentagrophytes genotype VIII in an Iranian family with generalized dermatophytosis: report of four cases and review of literature. Int J Dermatol 60:686–692. 10.1111/ijd.15226. [DOI] [PubMed] [Google Scholar]
- 8.Nenoff P, Verma SB, Ebert A, Süß A, Fischer E, Auerswald E, Dessoi S, Hofmann W, Schmidt S, Neubert K, Renner R, Sohl S, Hradetzky U, Krusche U, Wenzel H-C, Staginnus A, Schaller J, Müller V, Tauer C, Gebhardt M, Schubert K, Almustafa Z, Stadler R, Fuchs A, Sitaru C, Retzlaff C, Overbeck C, Neumann T, Kerschnitzki A, Krause S, Schaller M, Walker B, Walther T, Köhler L, Albrecht M, Willing U, Monod M, Salamin K, Burmester A, Koch D, Krüger C, Uhrlaß S. 2020. Spread of terbinafine-resistant Trichophyton mentagrophytes Type VIII (India) in Germany—“The Tip of the Iceberg?” J Fungi (Basel) 6:207. 10.3390/jof6040207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh A, Singh P, Meis JF, Chowdhary A. 2021. In vitro activity of the novel antifungal olorofim against dermatophytes and opportunistic moulds including Penicillium and Talaromyces species. J Antimicrob Chemother 76:1229–1233. 10.1093/jac/dkaa562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siopi M, Efstathiou I, Theodoropoulos K, Pournaras S, Meletiadis J. 2021. Molecular epidemiology and antifungal susceptibility of Trichophyton isolates in Greece: emergence of terbinafine-resistant Trichophytonmentagrophytes Type VIII locally and globally. J Fungi (Basel) 7:419. 10.3390/jof7060419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taghipour S, Shamsizadeh F, Pchelin IM, Rezaei-Matehhkolaei A, Zarei Mahmoudabadi A, Valadan R, Ansari S, Katiraee F, Pakshir K, Zomorodian K, Abastabar M. 2020. Emergence of Terbinafine resistant Trichophyton mentagrophytes in Iran, harboring mutations in the squalene epoxidase (SQLE) gene. Infect Drug Resist 13:845–850. 10.2147/IDR.S246025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dogra S, Uprety S. 2016. The menace of chronic and recurrent dermatophytosis in India: is the problem deeper than we perceive? Indian Dermatol Online J 7:73–76. 10.4103/2229-5178.178100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Rossi NM, Peres NTA, Bitencourt TA, Martins MP, Rossi A. 2021. State-of-the-art Dermatophyte infections: epidemiology aspects, pathophysiology, and resistance mechanisms. J Fungi (Basel) 7:629. 10.3390/jof7080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliver JD, Sibley GEM, Beckmann N, Dobb KS, Slater MJ, McEntee L, Du Pre S, Livermore J, Bromley MJ, Wiederhold NP, Hope WW, Kennedy AJ, Law D, Birch M. 2016. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc Natl Acad Sci USA 113:12809–12814. 10.1073/pnas.1608304113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du Pre S, Beckmann N, Almeida MC, Sibley GEM, Law D, Brand AC, Birch M, Read ND, Oliver JD. 2018. Effect of the novel antifungal drug F901318 (Olorofim) on growth and viability of Aspergillus fumigatus. Antimicrob Agents Chemother 62:e00231-18. 10.1128/AAC.00231-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sousa FM, Refojo PN, Pereira MM. 2021. Investigating the amino acid sequences of membrane bound dihydroorotate:quinone oxidoreductases (DHOQOs): structural and functional implications. Biochim Biophys Acta Bioenerg 1862:148321. 10.1016/j.bbabio.2020.148321. [DOI] [PubMed] [Google Scholar]
- 17.Van Daele R, Spriet I, Wauters J, Maertens J, Mercier T, Van Hecke S, Brüggemann R. 2019. Antifungal drugs: what brings the future? Med Mycol 57:S328–S343. 10.1093/mmy/myz012. [DOI] [PubMed] [Google Scholar]
- 18.CLSI. 2017. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 3rd ed. Clinical and Laboratory Standards Institute. [Google Scholar]
- 19.Fukasawa Y, Tsuji J, Fu SC, Tomii K, Horton P, Imai K. 2015. MitoFates: improved prediction of mitochondrial targeting sequences and their cleavage sites. Mol Cell Proteomics 14:1113–1126. 10.1074/mcp.M114.043083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Käll L, Krogh A, Sonnhammer EL. 2007. Advantages of combined transmembrane topology and signal peptide prediction—the Phobius web server. Nucleic Acids Res 35:W429–32. 10.1093/nar/gkm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SJ, Han JI, Lee GS, Park MJ, Choi IG, Na KJ, Jeung EB. 2007. Antifungal effect of eugenol and nerolidol against Microsporum gypseum in a guinea pig model. Biol Pharm Bull 30:184–188. 10.1248/bpb.30.184. [DOI] [PubMed] [Google Scholar]
- 22.Lim W, Eadie K, Konings M, Rijnders B, Fahal AH, Oliver JD, Birch M, Verbon A, van de Sande W. 2020. Madurella mycetomatis, the main causative agent of eumycetoma, is highly susceptible to olorofim. J Antimicrob Chemother 75:936–941. 10.1093/jac/dkz529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havlickova B, Czaika VA, Friedrich M. 2008. Epidemiological trends in skin mycoses worldwide. Mycoses 51(Suppl 4):2–15. 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 24.Petrucelli MF, Abreu MH, Cantelli BAM, Segura GG, Nishimura FG, Bitencourt TA, Marins M, Fachin AL. 2020. Epidemiology and diagnostic perspectives of Dermatophytoses. J Fungi (Basel) 6(4):310. 10.3390/jof6040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Astvad KMT, Jørgensen KM, Hare RK, Datcu R, Arendrup MC. 2020. Olorofim susceptibility testing of 1423 Danish mould isolates 2018–2019 confirms uniform and broad-spectrum activity. Antimicrob Agents Chemother 65:e01527-20. 10.1128/AAC.01527-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghannoum MA, Hossain MA, Long L, Mohamed S, Reyes G, Mukherjee PK. 2004. Evaluation of antifungal efficacy in an optimized animal model of Trichophyton mentagrophytes-dermatophytosis. J Chemother 16:139–144. 10.1179/joc.2004.16.2.139. [DOI] [PubMed] [Google Scholar]
- 27.Chittasobhon N, Smith JM. 1979. The production of experimental dermatophyte lesions in guinea pigs. J Invest Dermatol 73:198–201. 10.1111/1523-1747.ep12581683. [DOI] [PubMed] [Google Scholar]
- 28.Weigl E. 1980. Conditioned pathogenicity of Microsporum gypseum biochemical mutants. Mycopathologia 70:3–8. 10.1007/BF00704315. [DOI] [PubMed] [Google Scholar]
- 29.Cambier L, Heinen MP, Mignon B. 2017. Relevant animal models in dermatophyte research. Mycopathologia 182:229–240. 10.1007/s11046-016-0079-3. [DOI] [PubMed] [Google Scholar]
- 30.Wagner DK, Sohnle PG. 1995. Cutaneous defenses against dermatophytes and yeasts. Clin Microbiol Rev 8:317–335. 10.1128/CMR.8.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimamura T, Kubota N, Shibuya K. 2012. Animal model of dermatophytosis. J Biomed Biotechnol 2012:125384. 10.1155/2012/125384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venturini J, Alvares AM, Camargo MR, Marchetti CM, Fraga-Silva TF, Luchini AC, Arruda MS. 2012. Dermatophyte-host relationship of a murine model of experimental invasive dermatophytosis. Microbes Infect 14:1144–1151. 10.1016/j.micinf.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Anonymous. 2001. LOTRISONE, p 2. https://www.accessdata.fda.gov/drugsatfda_docs/label/2001/18827s7s9s20s22lbl.pdf