Abstract
Background:
Cerebrovascular disease is a common cause of dementia in older adults, and potentially preventable with early intervention. Oxylipins are produced from the oxidation of long-chain polyunsaturated fatty acids (PUFA) possessing potent vascular effects. Oxylipins generated from the cytochrome P450 pathway are enzymatically converted to diols by soluble epoxide hydrolase (sEH); sEH products have been associated with small vessel ischemic disease. Little is known about oxylipins’ impact on markers of dementia risk.
Objective:
An exploratory examination of the association between omega-6 and omega-3 derived oxylipins, brain MRI, and cognition.
Methods:
Thirty-seven non-demented participants with controlled hypertension (mean age 65.6 years) were enrolled in a dementia prevention study investigating fish oil and lipoic acid on preserving cognitive function. Baseline associations between plasma oxylipins, white matter hyperintensity (WMH), and Trails-B were examined using linear regression. P450-derived diol/epoxide ratio was an indirect measure of sEH activity.
Results:
Omega-6 derived 9-HODE was associated with increased WMH (p = 0.017) and reduced grey matter volume (p = 0.02). Omega-6 P450-derived diol/epoxide ratio 9,10-DiHOME/9,10-EpOME was associated with increased WMH (p = 0.035) and poorer performance on Trails-B (p = 0.05); ratio14,15-DHET/14,15-EET was associated with increased WMH (p = 0.045). Omega-3 P450-derived diol/epoxide ratio 19,20-DiHDPE/19,20-EpDPE was associated with increased WMH (p = 0.04) and poorer performance on Trails-B (p = 0.04). Arachidonic acid was associated with better performance on Trails-B (p = 0.012); Omega-3 derived 16,17-EpDPE was associated with decreased WMH (p = 0.005).
Conclusions:
With the exception of arachidonic acid, it was specific oxylipin products, not their parent PUFAs, that were associated with unfavorable and favorable MRI and cognitive markers of dementia risk.
Keywords: Alzheimer’s disease, cross-sectional studies, fatty acids, humans, oxylipins, vascular dementia
INTRODUCTION
Changes in magnetic resonance imaging (MRI) brain structure and volume are associated with brain aging [1] as well as underlying Alzheimer’s disease (AD) pathology [2, 3] and can be observed before overt dementia is clinically detected [4, 5], even in middle-aged individuals [6]. Specific markers of brain health, including grey matter volume (GM), may be indicative of neuronal reserve, with greater volumes conferring protection against age-related cognitive decline [7].
Cerebrovascular disease (CVD) is a common contributor to cognitive impairment with advancing age, and can be manifested as white matter hyperintensities (WMH) on T2-weighted MRI. Greater WMH burden is associated with cognitive and motor decline, as well as increased risk of conversion to mild cognitive impairment (MCI) and dementia [8–11].
WMHs are widely thought to be indicative of small vessel ischemia, with previous studies showing correlations with reduced in vivo cerebral blood flow [12] and postmortem arteriolosclerosis [13–15], with recent studies linking WMH to AD pathology [13, 16]. While AD is the most common cause of dementia in older individuals, population-based autopsy studies indicate that mixed pathologies are extremely common, with both AD and vascular pathologies being more prevalent than AD pathology alone [17, 18]. Furthermore, it is has been shown that midlife vascular risk factors accelerate structural brain aging, including both WMH progression and decreased brain volume [19]. Accordingly, identifying therapies targeted at modifying vascular risk during midlife, before evidence of cognitive changes, may delay or prevent the onset of clinical AD and other dementias in our growing aging population.
Oxylipins are a class of bioactive lipid metabolites derived from the oxidation of polyunsaturated fatty acids (PUFAs) by cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP450) enzymes [20]. Oxylipins in free form are thought to be bioactive. Less is known about the function of the esterified form; this form has been reported to have cell membrane modulatory functions [20]. Oxylipins derived from omega-6 arachidonic acid (AA) have been well-studied in hypertension as AA P450 derived oxylipin epoxides have the ability to relax vascular smooth muscle with effects in mesenteric, renal, cerebral, and coronary arteries [21, 22]. AA epoxides are quickly hydrolyzed to diols by the enzyme soluble epoxide hydrolase (sEH). This conversion from epoxides to diols transforms the biologic potential of epoxides from protective to adverse [23]. For example, in a postmortem brain study, AA-derived diol levels, not epoxides, were elevated in cerebral microvasculature in patients with vascular cognitive impairment compared to controls [24]. In animal studies, EPA and DHA compete with AA for P450 metabolism to produce their own bioactive oxylipins that are vasodilators and anti-inflammatory [20, 25, 26]. A randomized, double-blind, placebo-controlled study evaluating flaxseeds (containing high omega-3 linolenic acid) in participants with peripheral artery disease (75% hypertensive) showed a significant decrease in systolic and diastolic blood pressure in the flaxseed group compared to the placebo group, the observed change in oxylipins was attributed for the decrease in blood pressure [27, 28]. Little is known about omega-3 or omega-6 oxylipins and their impact on brain structure in non-demented older individuals with vascular risk factors, including hypertension. The objective of this exploratory study was to examine the relationship between omega-6 and omega-3 fatty acid derived oxylipins, MRI markers of brain health, and cognition, in a cohort of younger old adults with hypertension, and therefore, at risk for cognitive impairment.
MATERIAL AND METHODS
Participants
This study focused on a subset of 37 participants who underwent MRI at baseline and were part of a larger pool of hypertensive individuals enrolled in a dementia prevention study investigating fish oil and lipoic acid on preserving cognitive function in those with low blood omega-3 levels (n = 42). Participants were recruited from the Portland, Oregon metropolitan area, and provided written informed consent before enrollment. Inclusion criteria were age 55 years or older, diagnosis of essential hypertension (systolic 90–160 mmHg, diastolic 60–90 mmHg), non-demented (Montreal Cognitive Assessment (MoCA)>25, Clinical Dementia Rating Scale (CDR) = 0), on stable antihypertensive medication for at least four months prior to enrollment, low blood omega-3 status (≤5% of EPA+DHA of total fatty acids), not depressed (Geriatric Depression Scale (GDS) <5) [29], and a general health status that would not interfere with participation. Exclusion criteria were diagnosis of neurodegenerative disease, including AD, health conditions such as cancer diagnosed ≤5 years prior to enrollment (prostate cancer Gleason grade <3 and non-metastatic skin cancers were acceptable), liver disease, history of ventricular fibrillation or tachycardia, major psychiatric disorder, central nervous system diseases (e.g., brain tumor, seizure disorder), insulin-dependent diabetes or uncontrolled diabetes, fish intake > one 6 ounce serving a week less than four months prior to enrollment, omega-3 or lipoic acid supplementation within four months or one month, respectively, of enrollment, use of corticosteroids, neuroleptics, antiparkinsonian agents (low dose Sinemet and dopamine agonist taken for restless leg syndrome was not an exclusion), and/or narcotic analgesic, contraindications to MRI, and enrollment in another treatment study. This study was approved and monitored by the Institutional Review Board of Oregon Health & Science University (OHSU) and was registered with ClinicalTrials.gov, NCT01780974.
Study design
The study involved a cross-sectional analysis of a subset of participants who underwent an MRI brain scan at baseline (n = 37). In brief, participants were enrolled in a 12-month longitudinal clinical trial investigating the effects of fish oil and lipoic acid against placebo on measures of cognitive function. There was an a priori statistical plan for an exploratory analysis on association between oxylipins, MRI, and Trails-B for executive function performance [30].
Preparation of samples and oxylipin calibrators
Participants were fasted during blood draw and EDTA plasma was stored at −80°C until assay to measure free PUFA and oxylipin levels were run. Total plasma PUFAs were measured in absolute values and analyzed by a modification of the methods described by Langerstedt et al. [31] by gas chromatography-mass spectroscopy. Oxylipins were measured using an assay adapted from Dumalo et al. [32] and Quehenberger et al. [33] to measure cytochrome P450 metabolites.
The samples were thawed on ice before 1 ml of plasma was removed and spiked with 10 μl of an anti-oxidant mix consisting of 0.2 mg/ml butylated hydroxytoluene (BHT), 2 mg/ml triphenylphosphine (TPP), and 2 mg/ml indomethacin. Samples were then spiked with an internal standard mix consisting of 1 ng of each of the following: d8-15 HETE, d6-20 HETE, d8 14,15 EET, d11-14,15 DHET, d4-9 HODE, and d4-VI IsoP. 0.3 ml methanol and 0.7 ml of 1 M sodium acetate pH 6 was then added to each sample, followed by a brief vortex, and centrifugation at 2000xg for 5 min. The samples were then applied by gravity to 150 mg Oasis HLB SPE cartridges which had been pre-equilibrated with 5 ml of methanol, followed by 7.5 ml of water. The cartridges were then washed with 7.5 ml of 10% methanol, followed by drying for 25 min at maximum house vacuum of 5–15 in Hg before being eluted with 5 ml of methanol, followed by 4.0 ml of ethyl acetate, 4.0 ml of ethyl acetate:hexane (vol:vol) and finally with 1 ml of hexane. After elution a trap solution consisting of 20 μl of 10% glycerol with 500 μM BHT in ethanol was added to each tube prior to drying. Samples were dried under vacuum at 40°C for 1 h 30 min, with a small residue remaining. The tubes were then washed with 1 ml of hexane, transferred to smaller tubes and re-dried for 15–30 min. Frequently a small residue remains. The samples are then brought up in 100 μl of start solvent which consisted of 30:70 (vol:vol) acetonitrile: water with 0.2 mg/ml TPP, 0.01% BHT, and 0.01% formic acid and placed in sample vials with inserts and analyzed by LC-MS/MS. The injection volume was 20 μl. An un-extracted standard curve was used for these studies.
DHETs, HETEs, and EETs levels were analyzed using a 5500 Q-TRAP hybrid/triple quadrupole linear ion trap mass spectrometer (Applied Biosystems) with electrospray ionization in negative mode. The mass spectrometer was interfaced to a Shimadzu (Columbia, MD) SIL-20AC XR auto-sampler followed by 2 LC-20AD XR LC pumps and analysis on an Applied Biosystems/SCIEX Q5500 instrument (Foster City, CA). The instrument was operated with the following settings: source voltage −4000 kV, GS1 40, GS2 40, CUR 40, TEM 450, and CAD gas HIGH. Compounds were infused individually and instrument parameters optimized for each MRM transition. The gradient mobile phase was delivered at a flow rate of 0.5 ml per min, and consisted of two solvents, A: 0.05% acetic acid in water and B: 0.05% acetic acid in acetonitrile, prepared fresh before every run. Initial concentration of solvent B was 30%; this was held for 0.1 min before being increased to 60% over 5 min, then increased to 65% over 9 min, followed by an increase to 95% over 2 min, held at 95% for 2 min, decreased to start conditions of 30% B over 0.5 min, and then equilibrated for 5.5 min. The Betabasic-18 100 × 2 mm, 3μ column was kept at 40°C using a Shimadzu CTO-20AC column oven. Data were acquired using Analyst 1.6.2 software and analyzed Multi-quant 3.0.1 software. The standard curves were from 0–1000 pg/sample and the limit of quantification was 10 pg per sample except for 19 HETE and 20 HETE where the limit of quantification was 25 pg per sample where the relative standard deviation was less than 20%.
MRI acquisition and analysis
MRI scans were performed at the OHSU Advanced Imaging Research Center (AIRC) with a 3 Tesla Siemens Tim Trio instrument. Imaging sequence protocols were similar to those used in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [34]. The following protocol was used: 1) T1-weighted magnetization prepared rapid gradient echo (MPRAGE): TE = 3.45 ms, TR = 2300 ms, TI = 1200 ms, 1 mm isotropic; full brain coverage, FOV 256 mm × 192 mm, 144 axial slices. 2) 3D Fluid attenuated inversion recovery (FLAIR): TE = 388 ms; TR = 6,000 ms, TI = 2,100 ms; 1 mm slice thickness; in plane resolution 0.488 mm × 0.488 mm; full brain coverage; FOV = 250 mm × 250 mm.
Tissue type segmentation to derive GM volumes was performed using Freesurfer v6.0 [35] followed by manual correction for accuracy. WMH volumes were calculated based on FLAIR intensity and spatial location using an algorithm previously described in Promjunyakul et al. [36]. Briefly, voxels of signal intensity > 45% of the median of voxels in the WM mask were used as seeds for an iterative cluster growing algorithm followed by manual correction.
Statistical analysis
Baseline characteristics were summarized using means and standard deviations for continuous data, and counts and percentages for categorical data. Statistical analysis was carried out using ordinary least-squares linear regression to model the associations between oxylipin levels with MRI volumetrics and Trails-B. Given the small sample size (n = 37), only oxylipins with detectable levels in at least 80% of the participants (30/37) were included for data analysis. P450-derived ratio of diol/epoxide was used as an indirect measure of sEH activity. Age, gender, and parent PUFA were added as covariates in the model to adjust for confounding effects on MRI volumetric measures and Trails-B. Due to instances of non-normal data distribution, parent PUFAs, oxylipins and diol/epoxide ratios, WMH volume, GM volume, and Trails-B measures were log transformed as necessary; models for MRI volumetric measures were also adjusted for intracranial volume. Model integrity and identification of influential outliers was carried out using a combination of visual inspection of the model residuals and formal diagnostics of leverage and Cook’s distance. Although considered, model behavior was sufficient to focus on parametric assessments instead of non-parametric median equivalents. All statistical analyses were done using R 3.3.2 [37].
RESULTS
Patient characteristics
The mean age of participants was 65.6 years (SD 7.1) with 62.2% women. Baseline characteristics are presented in Table 1. Out of 40 enzymatically-derived oxylipins measured detectable levels were observed in 28 (70%) oxylipins at a threshold of at least 80% of participants per oxylipin (Table 2). Linear regression models showed that age had a positive and significant relationship with WMH and Trails-B (respectively) but not on GM volume (Supplementary Table 1).
Table 1.
Baseline Characteristics (n = 37)
| Variable (units) | Mean (SD or %) |
|---|---|
|
| |
| Age (y) | 65.6 (7.1) |
| Female | 23/37 (62.2) |
| Race (White) | 32/37 (86.5) |
| College or greater | 29/37 (78.3) |
| ApoE4 Carrier | 11/35 (29.7) |
| Systolic BP (mmHg) | 132.9 (14.2) |
| Diastolic BP (mmHg) | 73.9 (10.4) |
| Smoker, former | 18/36 (48.6) |
| Geriatric Depression Scale (GDS) | 1.2 (1.3) |
| Grey Matter Volume (cc)* | 476.8 (30.9) |
| White Matter Hyperintensity (cc)* | 5.8 (9.9) |
| Linoleic Acid (mcg/ml) | 387.6 (82.3) |
| Arachidonic Acid (mcg/ml) | 246.6 (65.2) |
| Eicosapentaenoic Acid (mcg/ml) | 26.8 (11.5) |
| Docosahexaenoic Acid (mcg/ml) | 51.9 (22.7) |
Not adjusted for intracranial volume.
Table 2.
PUFA Oxylipin Detected in Cohort (n = 37)
| Abbreviated Oxylipin Name | Oxylipin | PUFA | Enzyme | Percent detected |
|---|---|---|---|---|
|
| ||||
| 11-HETE | 11-hydroxyeicosatetraenoic acid | AA | COX | 97.3% |
| 12 HHTrE | 12-hydroxyheptadecatrienoic acid | AA | COX | 40.5% |
| PGE2 | PGE2 | AA | COX | 40.5% |
| 5-HETE | 5-hydroxyeicosatetraenoic acid | AA | LOX | 97.3% |
| 12-HETE | 12-hydroxyeicosatetraenoic acid | AA | LOX | 97.3% |
| 15-HETE | 15-hydroxy eicosatetraenoic acid | AA | LOX | 97.3% |
| LTB4 | LTB4 | AA | LOX | 0 |
| 14,15-EET | 14(15)-epoxyeicosatrienoic acid | AA | P450 | 97.3% |
| 11,12-EET | 11(12)-epoxy eicosatrienoic acid | AA | P450 | 97.3% |
| 8,9-EET | 8(9)-epoxyeicosatrienoic acid | AA | P450 | 0 |
| 18-HETE | 18-hydroxyeicosatetraenoic acid | AA | P450 | 97.3% |
| 19-HETE | 19-hydroxyeicosatetraenoic acid | AA | P450 | 97.3% |
| 20-HETE | 20-hydroxyeicosatetraenoic acid | AA | P450 | 97.3% |
| 14,15-DHET | 14, 15-dihydroxyeicosatrienoic acid | AA | P450 | 97.3% |
| 11,12-DHET | 11,12-dihydroxyeicosatrienoic acid | AA | P450 | 97.3% |
| 8,9-DHET | 8,9-dihydroxyeicosatrienoic acid | AA | P450 | 97.3% |
| 5,6-DHET | 5,6-dihydroxyeicosatrienoic acid | AA | P450 | 97.3% |
| 17 HdoHE | 17-hydroxydocosahexaenoic acid | DHA | COX | 0 |
| 17 KETO DHA | 17-keto-docosapentaenoic acid | DHA | COX | 0 |
| 7 HdoHE | 7-hydroxydocosahexaenoic acid | DHA | LOX | 97.3% |
| Resolvin D2 | Resolvin D2 | DHA | LOX | 0 |
| 16,17 EpDPE | 16(17)-epoxydocosapentaenoic acid | DHA | P450 | 91.9% |
| 19,20 EpDPE | 19(20)-docosapentaenoic acid | DHA | P450 | 81.0% |
| 19,20 DiHDPE | 19,20-dihydroxydocosapentaenoic acid | DHA | P450 | 97.3% |
| PGE3 | 9-oxo-11,15S-dihydroxy-prosta-5Z,13E,17Z-trien-1-oic acid | EPA | COX | 0 |
| 5 HEPE | 5-hydroxyeicosapentaenoic acid | EPA | LOX | 94.5% |
| 12 HEPE | 12-hydroxyeicosapentaenoic acid | EPA | LOX | 97.3% |
| 15 HEPE | 15-hydroxyeicosapentaenoic acid | EPA | LOX | 94.5% |
| 8,9 EpETE | 8(9)-epoxyeicosatetraenoic acid | EPA | P450 | 0 |
| 11,12 EpETE | 11(12)-epoxyeicosatetraenoic acid | EPA | P450 | 0 |
| 14,15 EpETE | 14(15)-epoxyeicosatetraenoic acid | EPA | P450 | 29.7% |
| 17,18 EpETE | 17(18)-epoxyeicosatetraenoic acid | EPA | P450 | 78.3% |
| 14,15 DiHETE | 14,15-dihydroxyeicosatetraenoic acid | EPA | P450 | 94.6% |
| 17,18 DiHETE | 17,18-dihydroxyeicosatetraenoic acid | EPA | P450 | 97.3% |
| 9-HODE | 9-hydroxyoctadecadienoic acid | LA | LOX | 97.3% |
| 13-HODE | 13-hydroxyoctadecadienoic acid | LA | LOX | 97.3% |
| 9,10 EpOME | 9(10)-epoxyoctadecenoic acid | LA | P450 | 97.3%) |
| 12,13 EpOME | 12(13)epoxyoctadecenoic acid | LA | P450 | 97.3% |
| 12,13-DiHOME | 12,13-dihydroxyoctadecenoic acid | LA | P450 | 97.3% |
| 9,10-DiHOME | 9(10)-dihydroxyoctadecenoic acid | LA | P450 | 97.3% |
AA, arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid; COX, cyclooxygenase; LOX, lipoxygenase; P450, cytochrome P450.
Omega-6 oxylipins, MRI volumetric measures, and Trails-B
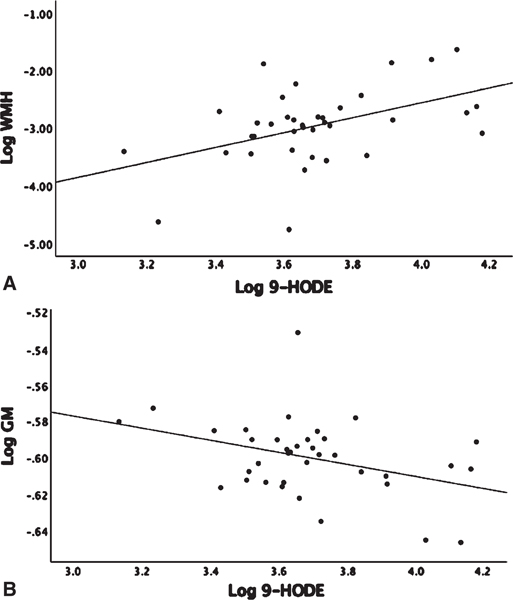
Linear regression analysis adjusted for age and gender showed no significant associations between omega-6 fatty acid levels, AA, and linoleic acid (LA), with WMH or GM volumes (Supplementary Table 2). The LA derived 9-HODE showed a positive association with WMH (β = 1.04, p = 0.017) and a negative association with GM volume (β = −0.034, p = 0.02) (Fig. 1A, B). LA derived 13-HODE showed a positive association with WMH (β = 0.99, p = 0.015) (Supplementary Table 1). There were no detectable levels of LA COX derived oxylipins (Table 2). There were no associations found between individual LA P450 derived oxylipins and either MRI or Trails B; however, associations were found when assessing sEH activity on P450 products that were measured indirectly by diol/epoxide ratios. A higher ratio of LA-derived 9,10-DiHOME-Diol/9,10-EpOME–Epoxide showed a positive association with both WMH (β = 0.67, p = 0.035) and with Trails-B (β = 0.161, p = 0.05) (Fig. 2A, B). AA showed a negative association with Trails-B (β = −0.55, p = 0.012) (Fig. 3), a finding not observed with the parent PUFA LA (Supplementary Table 2). No significant associations were found between AA derived P450, COX or LOX oxylipins, and WMH, GM, or Trails-B (p > 0.20). Assessing sEH activity on P450 products indirectly by diol/epoxide ratios, a higher ratio of AA-derived 11,12-DHET-Diol/11,12-EET-Epoxide and 14,15-DHET-Diol/14,15-EET-Epoxide showed a positive association with WMH (β = 1.06, p = 0.02) and (β = 1.02, p = 0.045) (Supplementary Table 1, Fig. 4).
Fig. 1.
Linear regression analysis corrected for age, gender, and parent fatty acid linoleic acid (LA). 9-HODE is a LA-derived oxylipin. 9-HODE, white matter hyperintensity (WMH) and grey matter volume (GM) were log transformed. WMH and GM were adjusted for intracranial volume before log transforming. A) Association Log 9-HODE and Log WMH (p = 0.017). B) Log 9-HODE and Log GM (p = 0.02).
Fig. 2.
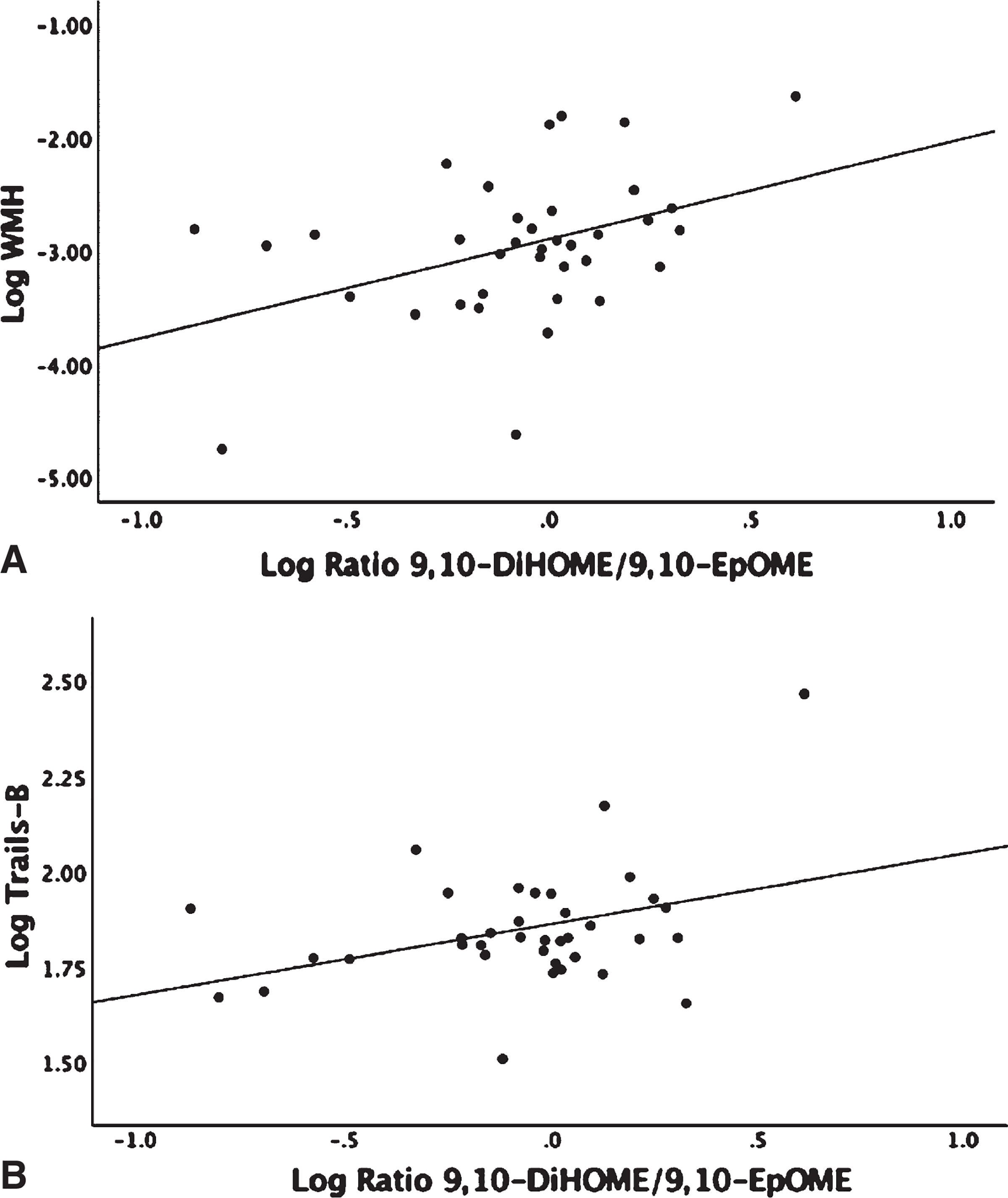
Linear regression analysis corrected for age, gender, and parent fatty acid linoleic acid (LA). 9,10-EpOME is a cytochrome P450 epoxide that is enzymatically transformed to its corresponding diol by sEH to 9,10-DiHOME; the ratio of 9,10-DiHOME/9,10-EpOME is an indirect measure of sEH activity. The ratio of 9,10-DiHOME/9,10-EpOME, white matter hyperintensity (WMH), and Trails-B were log transformed. WMH was adjusted for intracranial volume before log transforming. A) Association Log Ratio 9,10-DiHOME/9,10-EpOME and WMH (p = 0.035). B) Association Log Ratio 9,10-DiHOME/9,10-EpOME and Trails-B (p = 0.05).
Fig. 3.
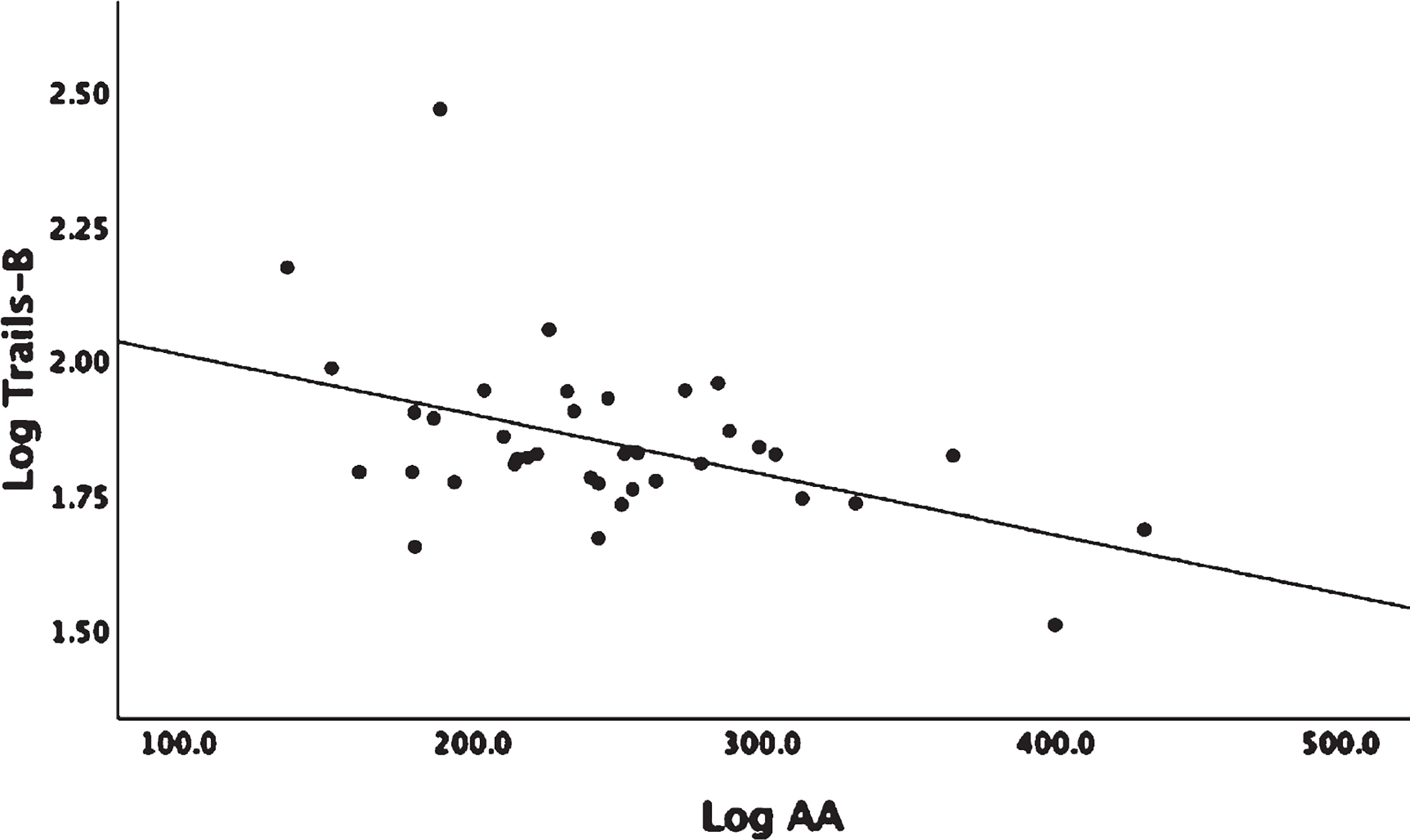
Linear regression analysis corrected for age and gender. Arachidonic acid (AA) is an Omega-6 PUFA. AA and Trails-B were log transformed. Association Log AA and Log Trails-B (p = 0.012).
Fig. 4.
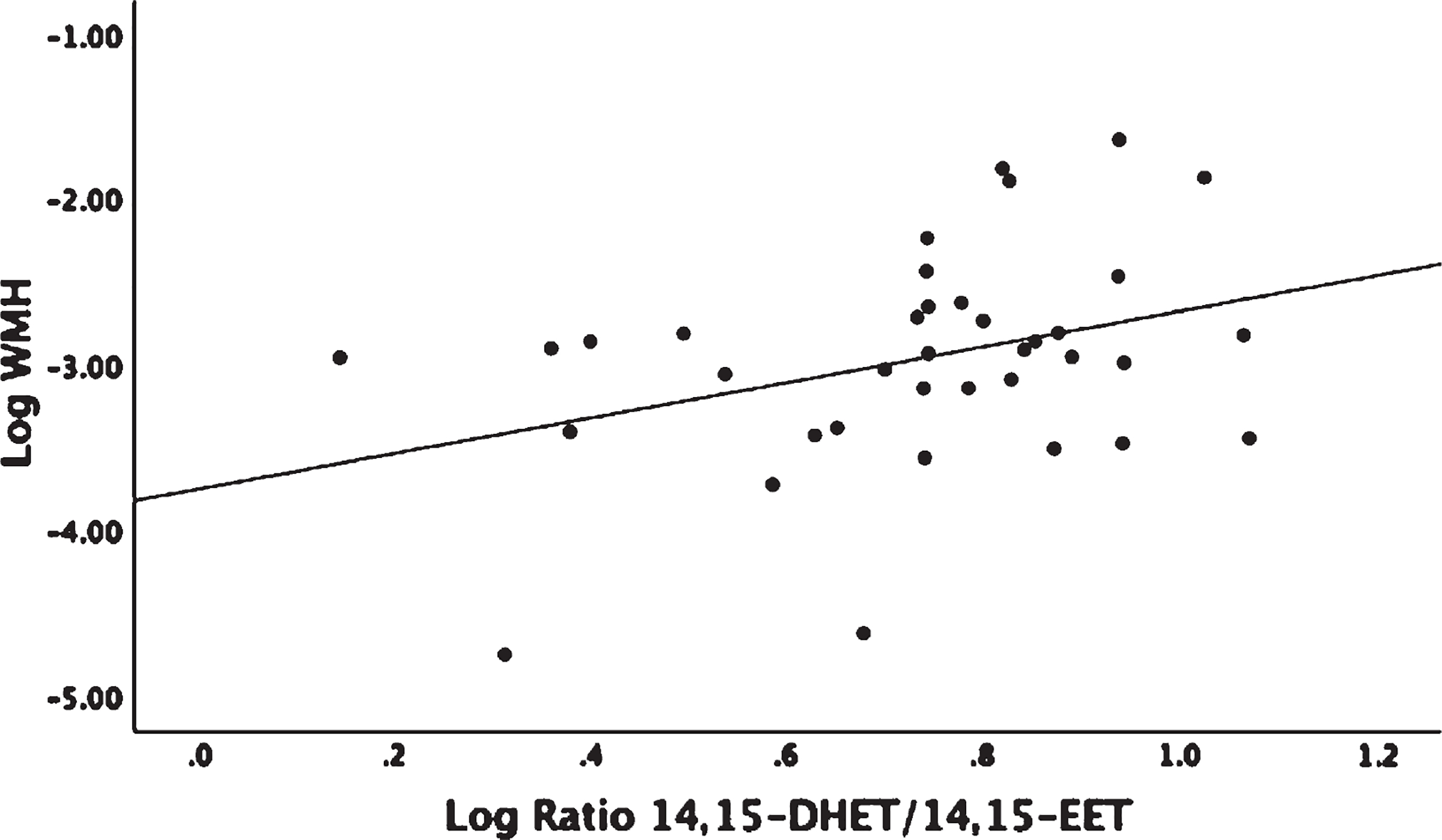
Linear regression analysis corrected for age, gender, and parent fatty acid arachidonic acid (AA). 14,15-EET is a cytochrome P450 Omega-6 derived epoxide that is enzymatically transformed to its corresponding diol by sEH to 14,15-DHET; the ratio of 14,15-DHET/14,15-EET is an indirect measure of sEH activity. The ratio of 14,15-DHET/14,15-EET and white matter hyperintensity (WMH) were log transformed. WMH was adjusted for intracranial volume before log transforming. Association Log Ratio 14,15-DHET/14,15-EET and Log WMH (p = 0.045).
Omega-3-derived oxylipins, MRI volumetric measures, and Trails-B
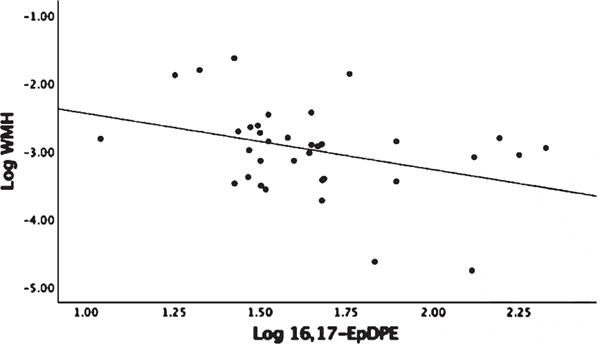
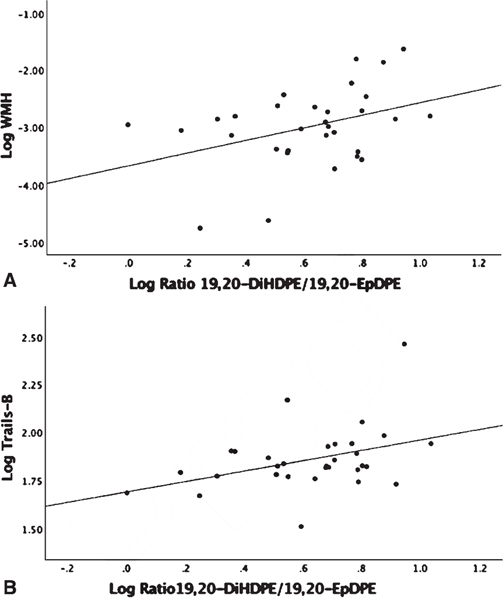
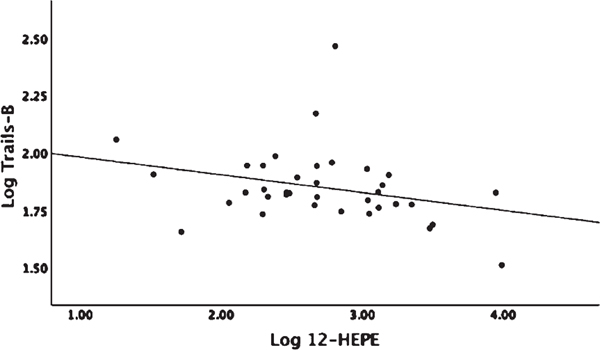
Linear regression analysis adjusted for age and gender showed no significant associations between omega-3 parent fatty acid levels, EPA and DHA, with MRI volumetric measures or Trails-B (Supplemental Table 2). There were no detectable levels of DHA derived COX oxylipins (Table 2) and no associations with MRI variables or Trails-B found with LOX oxylipins (p > 0.20). DHA P450 derived 16,17-EpDPE-Epoxide showed a negative association with WMH (β = −0.984, p = 0.005) (Fig. 5). Assessing P450 products of DHA sEH activity indirectly by diol/epoxide ratios, a higher ratio of 19,20-DiHDPE-Diol/19,20-EpDPE-Epoxide showed a positive association with WMH (β = 1.17, p < 0.04) and with Trails-B (β = 0.258, p < 0.044) (Fig. 6A, B). There were no detectable levels of EPA-derived COX oxylipins and no significant associations found with EPA-derived P450 products or products from sEH activity (Table 2). The EPA-derived LOX oxylipin 12-HEPE showed a negative association with Trails-B (β = −0.083, p < 0.048) (Fig. 7).
Fig. 5.
Linear regression analysis corrected for age, gender, and parent fatty acid docosahexaenoic acid (DHA). 16,17-EpDPE is a cytochrome P450 Omega-3 derived epoxide. 16,17-EpDPE and white matter hyperintensity (WMH) were log transformed. WMH was adjusted for intracranial volume before log transforming. Association Log Ratio 16,17-EpDPE and Log WMH (p = 0.005).
Fig. 6.
Linear regression analysis corrected for age, gender, and parent fatty acid docosahexaenoic acid (DHA). 19,20-EpDPE is a cytochrome P450 Omega-3 derived epoxide that is enzymatically transformed to its corresponding diol by sEH to 19,20-DiHDPE; the ratio of 19,20-DiHDPE/19,20-EpDPE is an indirect measure of sEH activity. The ratio of 19,20-DiHDPE/19,20-EpDPE, white matter hyperintensity (WMH), and Trails-B were log transformed. WMH was adjusted for intracranial volume before log transforming. A) Association Log Ratio 19,20-DiHDPE/19,20-EpDPE and Log WMH (p = 0.04). B) Association Log Ratio 19,20-DiHDPE/19,20-EpDPE and Log Trails-B (p = 0.044).
Fig. 7.
Linear regression analysis corrected for age, gender, and parent fatty acid eicosapentaenoic acid (EPA). 12-HEPE is an EPA-derived LOX oxylipin. 12-HEPE and Trails-B were log transformed. Association Log 12-HEPE and Log Trails-B (p = 0.048).
DISCUSSION
Oxylipins are bioactive metabolites formed from PUFA oxidation, are influenced by diet, and have modulatory effects on vascular function, inflammation, and cell proliferation in animal and human studies [20]. This study showed that with the exception of AA, which was associated with Trails-B, it was specific oxylipin products, not their parent PUFAs, that were associated with unfavorable and favorable MRI and cognitive markers of dementia risk. The results also highlight a pattern of sEH products from both omega-3 and omega-6 PUFAs that were associated with higher WMH and poorer performance on Trails-B. These preliminary findings point to the oxidized metabolites of PUFAs as key mediators to brain changes indicative of cerebrovascular disease in a cohort of cognitively healthy, hypertensive young old.
Oxylipins associated with unfavorable MRI and cognitive markers of dementia risk
LA is the most abundant omega-6 fatty acid in the Western diet because it is found in vegetable oils like safflower and corn oils that are used in fast foods and processed foods. LA is a precursor to circulating bioactive fatty acid metabolites implicated in AD, vascular dementia, and dementia risk groups that include people with atherosclerosis and cardiovascular disease [38–40]. Hydroxyoctadecadienoic acids (HODEs) are derived from the LOX enzymatic oxidation and from non-enzymatic auto-oxidation of LA and have been found in higher concentrations in plasma and red blood cell membranes in people with AD and vascular dementia when compared to age-matched controls [38]. Taha et al. evaluated the effects of increasing dietary LA in rats on changes in plasma and cerebral cortex oxylipin levels; increasing dietary LA resulted in an increase in plasma LA and AA-derived oxylipins with a decrease in EPA and DHA-derived oxylipins. In the cerebral cortex, LA and AA oxylipins increased while omega-3 EPA oxylipins decreased [39]. In addition, cortical 9- and 13- HODE significantly increased 2–3-fold in the highest percent of the LA-fed group compared to the lowest LA-fed group. The authors conclude that lowering dietary LA may be a useful approach to decrease LA derived oxylipins and to increase protective omega-3 EPA and DHA derived oxylipins in disorders like AD [39]. In this study including hypertensive individuals (100% on anti-hypertensive medication) with suboptimal blood levels of DHA and EPA (≤5.0% of total fatty acids), we found that greater LA-derived HODEs (9- and 13-) were associated with increased WMH (Fig. 1A). In addition, increased 9-HODE levels were also associated with reduced gray matter volume (Fig. 1B).
Hypertension is highly associated with white matter lesion burden [41] which is predictive of cognitive decline [42, 43]. Hypertension is also strongly associated with the development of coronary artery disease [44], also a risk factor for dementia, including that due to AD [45]. High HODE levels have been measured in peripheral atherosclerotic plaques in animal and human studies [40, 46] suggesting a role for this oxylipin in both central and peripheral vascular disease.
In a rabbit model of atherosclerosis designed to assess the relationship between a subset of plasma oxylipins and aortic plaque progression, 9-HODE and 13-HODE were two of the five oxylipins with the highest concentration in aortic plaques [40]. In a study investigating the association between mRNA expression of specific LOX enzymes and their products in human carotid atherosclerotic plaques in banked tissue from people with > 70% carotid artery stenosis, the majority of oxylipins measured were LA derived HODEs (77%) followed by AA-derived HETEs (54%); mRNA expression of a specific LOX enzyme was higher in symptomatic carotid lesions compared to asymptomatic lesions, implicating this enzyme’s role in forming atherogenic HODEs and HETEs [46]. Of note, 80–90% of this group were taking anti-platelet, anti-hypertensives and/or statins [46]. Findings from the present study provide preliminary evidence for plasma 9- and 13 HODE as potential biomarkers for dementia risk in people with hypertension and suboptimal EPA and DHA levels. Future studies investigating the effects of lower dietary LA on decreasing corresponding oxylipins in the prevention of vascular cognitive impairment in those at increased risk are warranted.
sEH activity and associations with unfavorable MRI and cognitive markers of dementia
DHA and AA are the most abundant PUFAs in neuronal membranes and essential to brain development in utero and infancy [47]. In older adults, longitudinal populations studies have identified high fish consumption (high dietary EPA and DHA) with a decrease risk of dementia, including that from AD [48, 49]. However, studies evaluating supplemental EPA and DHA in AD have reported null or inconclusive benefit [50, 51]. Long chain PUFAs that include, AA, LA, EPA, and DHA can be transformed to epoxides by cytochrome P450 enzymes, which have previously been shown to be biologically active oxylipins that are primarily anti-inflammatory [52]. PUFA epoxides can undergo further enzymatic transformation by sEH to form diols, which are less biologically active than epoxides and thought to contribute to inflammation by reducing epoxide levels [52].
This study showed a consistent pattern of worse performance on Trails-B and/or greater WMH burden associated with increased sEH activity (measured indirectly by diol/epoxide ratio) for LA, AA, and DHA metabolites (Figs. 2, 4, and 6, respectively). The findings have comparable outcomes with published findings from two studies evaluating the link between sEH activity and vascular cognitive impairment (VCI) [24, 53].
In a postmortem study, 14,15-DHET-Diol was elevated in cortical brain tissue of VCI patients when compared to controls; immunohistochemistry in VCI showed sEH enhancement in microvascular endothelium that was not present in neurons or astrocytes [24]. In addition, VCI patients (mean age at MRI 85.3 years) had higher WMH than age-matched controls. Our study found that a higher ratio of AA derived 14,15-DHET-Diol/14,15-EET-Epoxide (diol/epoxide ratio is an indirect measure of sEH activity) was associated with higher WMH in a non-demented hypertensive cohort with a mean age 20 years younger than reported in the VCI study, suggesting that interventions aimed at the reduction of deleterious PUFA metabolites may be most effective when implemented prior to advanced age and pre-existing cerebrovascular disease.
Yu et al. examined the associated between serum sEH activity (diol/epoxide ratio) and WMH and cognitive performance in patients with subcortical ischemic vascular disease. The LA-derived ratio of serum 9,10- and 12,13- DiHOME-Diol/EpOME-Epoxide was elevated in in subjects with extensive subcortical ischemic vascular disease (n = 29, mean age 71.8 years ± 11.0) compared to age-matched controls (n = 25). The Yu et al. study also observed higher serum levels of the ratio of 12,13-DiHOME-Diol/12,13-EpOME-Epoxide associated with greater periventricular WMH and poorer performance on a composite cognitive score that included measures of psychomotor processing speed/attention/executive function [53].
Our study did not find associations with 12,13-DiHOME-Diol/12,13-EpOME-Epoxide ratio with vascular dementia risk markers. However, this study did find that higher plasma levels of the ratio 9,10-DiHOME-Diol/9,10-EpOME-Epoxide was associated with higher WMH and poorer executive function performance (Trails-B) (Fig. 2A, B). This study and the Yu et al. study observed that sEH metabolites of the omega-6 fatty acid LA in cohorts with vascular risk factors are associated with greater WMH and poorer cognitive performance.
The detrimental effects of sEH activity in this study was not limited to omega-6 PUFAs, an increased ratio of DHA-derived 19,20-DiHDPE-Diol/19,20-EpDPE-Epoxide (used as an indirect measure of sEH activity) was associated with higher WMH and poorer performance on Trails-B (Fig. 6A, B). A study evaluating the effects of sEH products from DHA in diabetic retinopathy found an accumulation of DHA 19,20-DiHDPE-Diol in retinas from a mouse model of diabetic retinopathy, and in retinas of diabetic patients [54]. In the mouse model, this accumulation was found to initiate pericyte loss and dysregulation of endothelial barrier function and sEH inhibitors prevented pericyte loss and abnormal endothelial permeability [54]. Our findings are consistent with previous reports for an association between sEH activity on PUFAs and markers of vascular risk.
Oxylipins associated with favorable MRI and cognitive markers of dementia risk
In this study, higher DHA-derived P450 epoxide16,17-EpDPE was associated with lower WMH (Fig. 5). P450 generated epoxides are primarily anti-inflammatory [52] and the result suggest that 16,17-EpDPE may have a protective effect operating by vascular modulation. DHA epoxides are reported to possess anti-inflammatory, vasodilatory, and anti-cancer effect and are modulated through diet or EPA and DHA supplementation [20].
In an animal model of hypertension, mice were infused with angiotensin II alone (hypertensive group with no treatment), and angiotensin II combined with the following: 19,20-EpDPE-Epoxide alone, soluble epoxide inhibitor (sEH-I) alone, and 19,20-EpDPE-Epoxide combined with sEH-I [55]. The group that received 19,20-EpDPE-Epoxide had a significant reduction in systolic blood pressure compared to the angiotensin II hypertension group, with the group that received the combination of 19,20-EpDPE-Epoxide and sEH-I showing the greatest systolic BP reduction. The authors conclude that 19,20-EpDPE-Epoxide has antihypertensive actions in an angiotensin II model of hypertension with activity that is stabilized by adding a sEH inhibitor that prevents the catalysis of 19,20-EpDPE to its weaker diol form 19,20-DiHDPE-Diol [55].
Higher plasma EPA has been associated with lower incidence of dementia [56] and better performance on global cognitive function tests [57] in two non-demented cohorts, the Three-City Study (France, mean age 74.1–78.3 years) and the Keys to Optimal Cognitive Aging Study (Okinawa, Japan, mean age 84.1 years), respectively. Our findings did not show an association with EPA on markers of dementia risk or cognitive performance. However, we did find that higher levels of an EPA-derived LOX oxylipin, 12-HEPE, was associated with better Trails-B performance (Fig. 7). While there is scant published data on 12-HEPE and no reported studies on associations with cognitive or brain MRI markers of dementia risk, the current finding is in a relatively young-old population (mean age 65.7 ± 7.1 years) and suggests that specific omega-3 fatty acid derived oxylipins may be sensitive and earlier indicators of dementia risk or protection.
An unexpected finding was that higher plasma AA levels were associated with better Trails-B performance (Fig. 3). AA and DHA are essential in infant growth and brain development; during brain development AA functions include mediating neuronal firing, signaling, and helping to maintain hippocampal plasticity [47]. There are very few studies that report the association of AA and cognitive performance in non-demented cohorts. In the Atherosclerosis Risk in Communities study (ARIC, n = 2251, mean age 56.2 years women and 57.74 years men) higher AA levels were associated with a risk of cognitive decline [58]. The ARIC study cohort was not selected for suboptimal blood DHA and EPA and hypertension (54.6% had hypertension in ARIC versus 100% in this study), the study also had a slightly lower mean age (57.7 ± 4.2 years) than this study. Cohort differences may have contributed to the different directions in association with AA and cognitive performance.
Limitations
This study has several limitations. The sample size was relatively small, and results should be interpreted with caution until replicated in a larger cohort. Furthermore, the cross-sectional design limits the interpretation of the results to associative rather than causal inferences. By design, the study included several inclusion and exclusion criteria that decreased confounding outcomes from sample heterogeneity that included a well-characterized cohort of relatively young-old (mean age 65.6 years) that were cognitively intact (CDR = 0, MoCA > 25) and not depressed (GDS < 5), all diagnosed with essential hypertension on antihypertensive therapy, with low blood EPA + DHA levels. In addition, all blood samples were fasted and statistical analysis was adjusted for age, gender, and parent fatty acid to decrease confounding effects from these factors. As the study was exploratory, it was not powered to see significant associations if adjusted for multiple comparisons of the 28 detectable oxylipins used in analysis.
The current study only measured the free and not esterified form of oxylipins, although one small study in healthy men and women (n = 10) measured both forms of oxylipins at baseline and post-omega-3 supplementation over 12 weeks (daily encapsulated fish oil containing 1.1 g EPA and 0.74 gDHA). The ratio of free to esterified at baseline and at 12 weeks remained unchanged, indicating either form may serve as biomarkers for assessing peripheral oxylipin levels [59]. Plasma sEH was not assessed directly, the ratio of P450 generated diol to epoxide was used as proxy measure of sEH activity [53].
Conclusions
These results suggest that in a non-demented group with vascular risks for dementia, oxylipins may serve as sensitive markers of cerebrovascular disease risk before the clinical manifestations of cognitive impairment is evident, when therapeutic intervention is most likely to be effective. The results presented are preliminary and unique, as this is the first study to observe the association between specific DHA and EPA derived oxylipins, 16,17-EpDPE and 12-HEPE that show a protective effect for vascular risk markers. The findings on sEH PUFA products are consistent with other reports showing they are associated with vascular risk markers, specifically markers for microvessel damage, and is one of only a few studies demonstrating these effects in living human subjects. The ability to shift oxylipin profiles through diet and PUFA supplementation warrants further characterization and exploration as potential indicators of vascular dementia risk.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank the Storms Family Foundation and Ken Bado for funding the study. The oxylipin and fatty acid analysis were conducted in the Bioanalytical Shared Resource/Pharmacokinetics Core (BSR/PKcore). The facility is part of the University Shared Resource Program supported in part by Oregon Health and Science University. In addition, support from the National Institute on Aging (R21AG023805, R01AG033613, P30AG080017, R01AG036772) and the National Center of Research Resources (M01RR00334, ULITR000128).
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-1197r1).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-191197.
REFERENCES
- [1].Galluzzi S, Lanni C, Pantoni L, Filippi M, Frisoni GB (2008) White matter lesions in the elderly: Pathophysiological hypothesis on the effect on brain plasticity and reserve. J Neurol Sci 273, 3–9. [DOI] [PubMed] [Google Scholar]
- [2].Silbert LC, Quinn JF, Moore MM, Corbridge E, Ball MJ, Murdoch G, Sexton G, Kaye JA (2003) Changes in premorbid brain volume predict Alzheimer’s disease pathology. Neurology 61, 487–492. [DOI] [PubMed] [Google Scholar]
- [3].Jack CR, Dickson DW, Parisi JE, Xu YC, Cha RH, O’brien PC, Edland SD, Smith GE, Boeve BF, Tangalos EG, Kokmen E, Petersen RC (2002) Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology 58, 750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Andrews KA, Modat M, Macdonald KE, Yeatman T, Cardoso MJ, Leung KK, Barnes J, Villemagne VL, Rowe CC, Fox NC, Ourselin S, Schott JM (2013) Atrophy rates in asymptomatic amyloidosis: Implications for Alzheimer prevention trials. PloS One 8, e58816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chetelat G, Villemagne VL, Villain N, Jones G, Ellis KA, Ames D, Martins RN, Masters CL, Rowe CC (2012) Accelerated cortical atrophy in cognitively normal elderly with high β-amyloid deposition. Neurology 78, 477–484. [DOI] [PubMed] [Google Scholar]
- [6].Mecca AP, Barcelos NM, Wang S, Brück A, Nabulsi N, Planeta-Wilson B, Nadelmann J, Benincasa AL, Ropchan J, Huang Y, Gelernter J, Van Ness PH, Carson RE, van Dyck CH (2018) Cortical β-amyloid burden, gray matter, and memory in adults at varying APOE ε4 risk for Alzheimer’s disease. Neurobiol Aging 61, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Laubach M, Lammers F, Zacharias N, Feinkohl I, Pischon T, Borchers F, Slooter AJ, Kühn S, Spies C, Winterer G, BioCog Consortium (2018) Size matters: Grey matter brain reserve predicts executive functioning in the elderly. Neuropsychologia 119, 172–181. [DOI] [PubMed] [Google Scholar]
- [8].DeCarli C, Murphy DG, Tranh MA, Grady CL, Haxby JV, Gillette JA, Salerno JA, Gonzales-Aviles A, Honvitz B, Rapoport SI, Schapiro MB (1995) The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology 45, 2077–2084. [DOI] [PubMed] [Google Scholar]
- [9].Longstreth WT, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O’Leary D, Fried L (1996) Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: The Cardiovascular Health Study. Stroke 27, 1274–1282. [DOI] [PubMed] [Google Scholar]
- [10].Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA (2008) Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology 71, 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Silbert LC, Dodge HH, Perkins LG, Sherbakov L, Lahna D, Erten-Lyons D, Woltjer R, Shinto L, Kaye JA (2012) Trajectory of white matter hyperintensity burden preceding mild cognitive impairment. Neurology 79, 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Promjunyakul NO, Dodge HH, Lahna D, Boespflug EL, Kaye JA, Rooney WD, Silbert LC (2018) Baseline NAWM structural integrity and CBF predict periventricular WMH expansion over time. Neurology 90, e2119–e2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Erten-Lyons D, Woltjer R, Kaye J, Mattek N, Dodge HH, Green S, Tran H, Howieson DB, Wild K, Silbert LC (2013) Neuropathologic basis of white matter hyperintensity accumulation with advanced age. Neurology 81, 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schneider JA, Arvanitakis Z, Bang W, Bennett DA (2007) Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69, 2197–2204. [DOI] [PubMed] [Google Scholar]
- [15].Gouw AA, Seewann A, Van Der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, Geurts JJ (2011) Heterogeneity of small vessel disease: A systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 82, 126–135. [DOI] [PubMed] [Google Scholar]
- [16].McAleese KE, Walker L, Graham S, Moya EL, Johnson M, Erskine D, Colloby SJ, Dey M, Martin-Ruiz C, Taylor JP, Thomas AJ, McKeith IG, De Carli C, Attems J (2017) Parietal white matter lesions in Alzheimer’s disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol 134, 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM (2015) Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology 85, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schneider JA, Arvanitakis Z, Bang W, Bennett DA (2007) Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69, 2197–2204. [DOI] [PubMed] [Google Scholar]
- [19].Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C (2011) Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77, 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM (2015) Advances in our understanding of oxylipins derived from dietary PUFAs. Adv Nutr 6, 513–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bellien J, Joannides R (2013) Epoxyeicosatrienoic acid pathway in human health and diseases. J Cardiovasc Pharmacol 61, 188–196. [DOI] [PubMed] [Google Scholar]
- [22].Theken KN, Schuck RN, Edin ML, Tran B, Ellis K, Bass A, Lih FB, Tomer KB, Poloyac SM, Wu MC, Hinderliter AL, Zeldin DC, Stouffer GA, Lee CR (2012) Evaluation of cytochrome P450-derived eicosanoids in humans with stable atherosclerotic cardiovascular disease. Atherosclerosis 222, 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang T, Peng R, Guo Y, Shen L, Zhao S, Xu D (2013) The role of 14, 15-dihydroxyeicosatrienoic acid levels in inflammation and its relationship to lipoproteins. Lipids Health Dis 12, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nelson JW, Young JM, Borkar RN, Woltjer RL, Quinn JF, Silbert LC, Grafe MR, Alkayed NJ (2014) Role of soluble epoxide hydrolase in age-related vascular cognitive decline. Prostaglandins Other Lipid Mediat 113, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, Konkel A, von Schacky C, Luft FC, Muller DN, Rothe M, Schunck WH (2010) Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of ω−3 fatty acids. J Biol Chem 285, 32720–32733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Konkel A, Schunck WH (2011) Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim Biophys Acta 1814, 210–222. [DOI] [PubMed] [Google Scholar]
- [27].Caligiuri SP, Aukema HM, Ravandi A, Guzman R, Dibrov E, Pierce GN (2014) Flaxseed consumption reduces blood pressure in patients with hypertension by altering circulating oxylipins via an α-linolenic acid–induced inhibition of soluble epoxide hydrolase. Hypertension 64, 53–59. [DOI] [PubMed] [Google Scholar]
- [28].Caligiuri SP, Rodriguez-Leyva D, Aukema HM, Ravandi A, Weighell W, Guzman R, Pierce GN (2016) Dietary flaxseed reduces central aortic blood pressure without cardiac involvement but through changes in plasma oxylipins. Hypertension 68, 1031–1038. [DOI] [PubMed] [Google Scholar]
- [29].Sheikh JI, Yesavage JA (1986) Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol 5, 165–173. [Google Scholar]
- [30].Tan ZS, Harris WS, Beiser AS, Au R, Himali JJ, Debette S, Pikula A, DeCarli C, Wolf PA, Vasan RS, Robins SJ, Seshardi RS (2012) Red blood cell omega-3 fatty acid levels and markers of accelerated brain aging. Neurology 78, 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lagerstedt SA, Hinrichs DR, Batt SM, Magera MJ, Rinaldo P, McConnell JP (2001) Quantitative determination of plasma c8–c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol Genet Metab 73, 38–45. [DOI] [PubMed] [Google Scholar]
- [32].Dumlao DS, Buczynski MW, Norris PC, Harkewicz R, Dennis EA (2011) High-throughput lipidomic analysis of fatty acid derived eicosanoids and N-acylethanolamines. Biochim Biophys Acta 1811, 724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Quehenberger O, Armando A, Dumlao D, Stephens DL, Dennis EA (2008) Lipidomics analysis of essential fatty acids in macrophages. Prostaglandins Leukot Essent Fatty Acids 79, 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jack CR Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, L. Whitwell J, Ward C, Dale AM, Felmlee JP, Gunter JL, Hill DL, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler, Weiner MW (2008) The Alzheimer’s disease neuroimaging initiative (ADNI): MRI methods. J Magn Reson Imaging 27, 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9, 179–194. [DOI] [PubMed] [Google Scholar]
- [36].Promjunyakul N, Lahna D, Kaye J, Dodge HH, Erten-Lyons D, Rooney WD, Silbert LC (2015) Characterizing the white matter hyperintensity penumbra with cerebral blood flow measures. Neuroimage Clin 8, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Team RC (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. [Google Scholar]
- [38].Yoshida Y, Yoshikawa A, Kinumi T, Ogawa Y, Saito Y, Ohara K, Yamamoto H, Imai Y, Niki E (2009) Hydroxyoctadecadienoic acid and oxidatively modified peroxiredoxins in the blood of Alzheimer’s disease patients and their potential as biomarkers. Neurobiol Aging 30, 174–185. [DOI] [PubMed] [Google Scholar]
- [39].Taha AY, Hennebelle M, Yang J, Zamora D, Rapoport SI, Hammock BD, Ramsden CE (2018) Regulation of rat plasma and cerebral cortex oxylipin concentrations with increasing levels of dietary linoleic acid. Prostaglandins Leukot Essent Fatty Acids 138, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bojic LA, McLaren DG, Harms AC, Hankemeier T, Dane A, Wang SP, Rosa R, Previs SF, Johns DG, Castro-Perez JM (2016) Quantitative profiling of oxylipins in plasma and atherosclerotic plaques of hypercholesterolemic rabbits. Anal Bioanal Chem 408, 97–105. [DOI] [PubMed] [Google Scholar]
- [41].Kuller LH, Margolis KL, Gaussoin SA, Bryan NR, Kerwin D, Limacher M, Wassertheil-Smoller S, Williamson J, Robinson JG, Women’s Health Initiative Memory Study Research Group (2010) Relationship of hypertension, blood pressure, and blood pressure control with white matter abnormalities in the Women’s Health Initiative Memory Study (WHIMS)—MRI trial. J Clin Hypertens 12, 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Silbert LC, Howieson DB, Dodge H, Kaye JA (2009) Cognitive impairment risk: White matter hyperintensity progression matters. Neurology 73, 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Prasad K, Wiryasaputra L, Ng A, Kandiah N (2011) White matter disease independently predicts progression from mild cognitive impairment to Alzheimer’s disease in a clinic cohort. Dement Geriatr Cogn Disord 31, 431–434. [DOI] [PubMed] [Google Scholar]
- [44].Nakanishi R, Baskaran L, Gransar H, Budoff MJ, Achenbach S, Al-Mallah M, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJ, DeLago A, Hadamitzky M, Hausleiter J, Cury R, Feuchtner G, Kim YJ, Leipsic J, Kaufmann PA, Maffei E, Raff G, Shaw LJ, Villines TC, Dunning A, Marques H, Pontone G, Andreini D, Rubinshtein R, Bax J, Jones E, Hindoyan N, Gomez M, Lin FY, Min JK, Berman DS (2017) Relationship of hypertension to coronary atherosclerosis and cardiac events in patients with coronary computed tomographic angiography. Hypertension 70, 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Serrano-Pozo A, Growdon JH (2019) Is Alzheimer’s disease risk modifiable? J Alzheimers Dis 67, 795–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gertow K, Nobili E, Folkersen L, Newman JW, Pedersen TL, Ekstrand J, Swedenborg J, Kühn H, Wheelock CE, Hansson GK, Hedin U, Haeggstrom JZ, Gabrielsen A (2011) 12-and 15-lipoxygenases in human carotid atherosclerotic lesions: Associations with cerebrovascular symptoms. Atherosclerosis 215, 411–416. [DOI] [PubMed] [Google Scholar]
- [47].Hadley KB, Ryan AS, Forsyth S, Gautier S, Salem N (2016) The essentiality of arachidonic acid in infant development. Nutrients 8, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM (1997) Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol 42, 776–782. [DOI] [PubMed] [Google Scholar]
- [49].Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, Aggarwal N, Schneider J (2003) Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol 60, 940–946. [DOI] [PubMed] [Google Scholar]
- [50].Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, Galvin JE, Emond J, Jack CR, Weiner M, Shinto L, Aisen PS (2010) Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA 304, 1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wu S, Ding Y, Wu F, Li R, Hou J, Mao P (2015) Omega-3 fatty acids intake and risks of dementia and Alzheimer’s disease: A meta-analysis. Neurosci Biobehav Rev 48, 1–9. [DOI] [PubMed] [Google Scholar]
- [52].Wagner KM, McReynolds CB, Schmidt WK, Hammock BD (2017) Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol Ther 180, 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yu D, Hennebelle M, Sahlas DJ, Ramirez J, Gao F, Masellis M, Cogo-Moreira H, Swartz RH, Herrmann N, Chan PC, Pettersen JA, Stuss DT, Black SE, Taha AY, Swardfager W (2019) Soluble epoxide hydrolase-derived linoleic acid oxylipins in serum are associated with periventricular white matter hyperintensities and vascular cognitive impairment. Transl Stroke Res 10, 522–533. [DOI] [PubMed] [Google Scholar]
- [54].Hu J, Dziumbla S, Lin J, Bibli SI, Zukunft S, De Mos J, Awwad K, Frömel T, Jungmann A, Devraj K, Cheng Z, Wang L, Fauser S, Eberhart CG, Sodhi A, Hammock BD, Liebner S, Muller OJ, Glaubitz C, Hammes HP, Popp R, Fleming I (2017) Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy. Nature 552, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ulu A, Lee KS, Miyabe C, Yang J, Hammock BG, Dong H, Hammock BD (2014) An omega-3 epoxide of docosahexaenoic acid lowers blood pressure in angiotensin-II dependent hypertension. J Cardiovasc Pharmacol 64, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Samieri C, Féart C, Letenneur L, Dartigues JF, Pérès K, Auriacombe S, Peuchant E, Delcourt C, Barberger-Gateau P (2008) Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am J Clin Nutr 88, 714–721. [DOI] [PubMed] [Google Scholar]
- [57].Nishihira J, Tokashiki T, Higashiuesato Y, Willcox DC, Mattek N, Shinto L, Ohya Y, Dodge HH (2016) Associations between serum omega-3 fatty acid levels and cognitive functions among community-dwelling octogenarians in Okinawa, Japan: The KOCOA study. J Alzheimers Dis 51, 857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR (2007) Plasma n–3 fatty acids and the risk of cognitive decline in older adults: The Atherosclerosis Risk in Communities Study. Am J Clin Nutr 85, 1103–1111. [DOI] [PubMed] [Google Scholar]
- [59].Schebb NH, Ostermann AI, Yang J, Hammock BD, Hahn A, Schuchardt JP (2014) Comparison of the effects of long-chain omega-3 fatty acid supplementation on plasma levels of free and esterified oxylipins. Prostaglandins Other Lipid Mediat 113, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.