ABSTRACT
Mycobacterium abscessus lung disease is difficult to treat due to intrinsic drug resistance and the persistence of drug-tolerant bacteria. Currently, the standard of care is a multidrug regimen with at least 3 active drugs, preferably including a β-lactam (imipenem or cefoxitin). These regimens are lengthy and toxic and have limited efficacy. The search for more efficacious regimens led us to evaluate bedaquiline, a diarylquinoline licensed for treatment of multidrug-resistant tuberculosis. We performed in vitro time-kill experiments to evaluate the activity of bedaquiline alone and in combination with the first-line drug imipenem against M. abscessus under various conditions. Against actively growing bacteria, bedaquiline was largely bacteriostatic and antagonized the bactericidal activity of imipenem. Contrarily, against nutrient-starved persisters, bedaquiline was bactericidal, while imipenem was not, and bedaquiline drove the activity of the combination. In an intracellular infection model, bedaquiline and imipenem had additive bactericidal effects. Correlations between ATP levels and the bactericidal activity of imipenem and its antagonism by bedaquiline were observed. Interestingly, the presence of Tween 80 in the media affected the activity of both drugs, enhancing the activity of imipenem and reducing that of bedaquiline. Overall, these results show that bedaquiline and imipenem interact differently depending on culture conditions. Previously reported antagonistic effects of bedaquiline on imipenem were limited to conditions with actively multiplying bacteria and/or the presence of Tween 80, whereas the combination was additive or indifferent against nutrient-starved and intracellular M. abscessus, where promising bactericidal activity of the combination suggests it may have a role in future treatment regimens.
KEYWORDS: Mycobacterium abscessus, bedaquiline, imipenem
INTRODUCTION
Lung disease caused by Mycobacterium abscessus infection is difficult to treat. Currently, the recommended treatment for M. abscessus lung infections is a multidrug regimen comprising at least 3 drugs with in vitro activity and preferably including imipenem or cefoxitin (1). This regimen, which can be administered for months to years, results in cure in only about 50% of patients and is plagued by problems with drug toxicity and poor tolerability (2). Thus, there is an urgent need to improve treatment of M. abscessus lung infections. One approach to identify new treatment options is to evaluate drugs that are effective for other mycobacterial infections, with bedaquiline being a good candidate for repurposing against M. abscessus.
Traditional treatment regimens for multidrug-resistant tuberculosis (MDR-TB) were 18–24 months in duration and associated with about 50% cure rates (3), a situation comparable to the current standard of care for M. abscessus lung disease. Bedaquiline—a diarylquinoline approved for treatment of MDR-TB—is a key component of a new, 6-month MDR-TB regimen (bedaquiline-pretomanid-linezolid or “BPaL”) with a demonstrated 90% cure rate (4). Using mouse models, each individual drug in BPaL was shown to contribute to bacterial killing of the regimen, and bedaquiline specifically was found to contribute significantly through its treatment-shortening activity (5). As several studies have demonstrated that bedaquiline has activity against M. abscessus, including previous work by our group showing that bedaquiline was highly bactericidal against non-replicating M. abscessus populations (6, 7), we considered that bedaquiline has potential for both improving and shortening treatment of M. abscessus lung disease.
Understanding how bedaquiline combines with current first-line drugs is an important step in characterizing bedaquiline’s potential as part of a shorter, curative regimen for M. abscessus lung disease. To that end, the objective of this study was to evaluate the activity of bedaquiline-imipenem combinations against M. abscessus. Interestingly, Lindman and Dick recently reported that bedaquiline antagonized imipenem’s in vitro bactericidal activity against actively growing M. abscessus (8). However, Le Moigne et al. did not observe antagonism between these drugs in a mouse model of M. abscessus lung disease (9). To achieve our objectives, and in light of these recent findings, we conducted a series of in vitro studies focused on evaluating the activity of bedaquiline-imipenem combinations across different M. abscessus populations, namely, actively growing, nutrient-starved non-replicating, and intracellular bacteria. Additionally, we generated resistant mutants to address the impact of bedaquiline resistance on the combined drug activity across these different conditions. This systematic evaluation has produced novel data sets that highlight how experimental conditions can impact drug activity and also provides insight into how bedaquiline and imipenem may be used together for treatment of M. abscessus lung infections.
RESULTS
Selection and characterization of bedaquiline-resistant M. abscessus isolates.
We selected and characterized three unique isolates with decreased in vitro susceptibility to clofazimine in the M. abscessus strain ATCC 19977 (wild-type [WT]) background) (Table S1 in the supplemental material). Each of these isolates contained a mutation in MAB_2299c, a gene encoding a TetR-family transcriptional repressor of MmpS-MmpL efflux pump systems that confers cross-resistance to clofazimine and bedaquiline when inactivated (10). Indeed, each of our MAB_2299c mutant isolates had reduced susceptibility to bedaquiline. The two isolates with MAB_2299c mutations that were analyzed (OM4 and OM7) for concurrent mutations in atpE, which encodes the ATP synthase subunit targeted by bedaquiline (11), had no such mutations. None of the isolates had mutations in MAB_4384, another gene reported to be associated with bedaquiline resistance in M. abscessus (12). In cation-adjusted Mueller-Hinton broth (CAMHB), the MIC of bedaquiline increased 4- or 8-fold to 0.25–0.5 μg/ml against the MAB_2299c mutants compared to the wild-type parent strain (MIC 0.0625 μg/ml). Although neither the Clinical and Laboratory Standards Institute (CLSI) nor the manufacturer have defined testing standards for bedaquiline against M. abscessus (13, 14), MICs in this range were previously characterized as “bedaquiline-resistant” for M. abscessus isolates (10, 15). Therefore, we refer to our MAB_2299c mutants as bedaquiline-resistant strains. Isolate OM7, which contained a 150 bp deletion starting at position −28 upstream of the MAB_2299c start codon (Fig. S1), was utilized in experiments described in this report.
Assessment of bedaquiline and imipenem/avibactam against M. abscessus WT and OM7 mutant in nutrient-rich conditions with Tween 80.
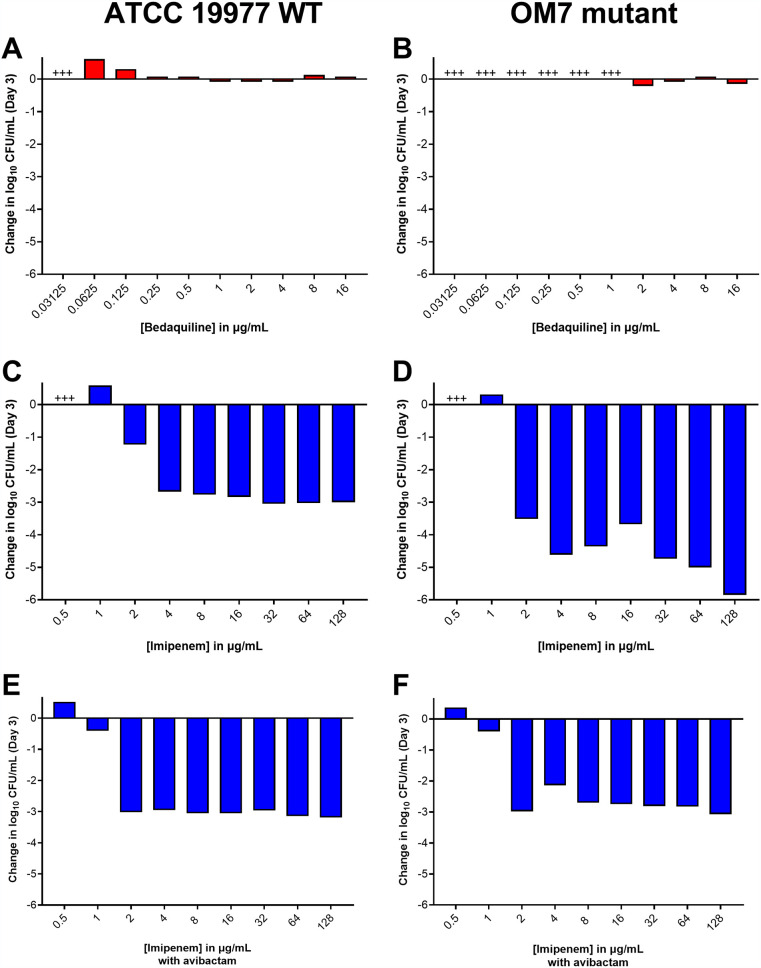
We first evaluated the activity of bedaquiline and imipenem alone against actively multiplying M. abscessus ATCC 19977 WT parent and OM7 mutant populations in CAMHB supplemented with 0.05% (vol/vol) Tween 80, a surfactant included to reduce the impact of mycobacterial clumping on quantification of CFU (16, 17). Bedaquiline alone exerted only bacteriostatic activity, limiting growth at concentrations ≥0.0625 and ≥2 μg/ml against WT and OM7, respectively (Fig. 1A and B; see Table S2 for all CFU data). In contrast, imipenem alone limited growth at 1 μg/ml and had strong bactericidal activity at concentrations ≥2 μg/ml against both strains after 3 days of drug exposure (Fig. 1C and D). We also evaluated the activity of imipenem in combination with avibactam, a β-lactamase inhibitor that enhances the susceptibility of M. abscessus to β-lactams, including imipenem (18, 19). In combination with avibactam at 2 μg/ml, the imipenem concentration needed to inhibit growth decreased 2-fold to 1 μg/ml for both strains (Fig. 1E and F).
FIG 1.
Activity of bedaquiline (A, B), imipenem (C, D), and imipenem with avibactam (E, F) against M. abscessus ATCC 19977 WT (A, C, E) and OM7 mutant (B, D, F) in CAMHB with 0.05% Tween 80. The change in log10 CFU/ml after 3 days of drug exposure relative to Day 0 is presented for each drug/strain set. Avibactam concentration in panels E and F was 2 μg/ml. +++ indicates bacterial overgrowth and clumping that precluded CFU determination. Avibactam alone at concentrations from 0.5 to 64 μg/ml had no anti-M. abscessus activity; bacteria grew/clumped at all avibactam concentrations, precluding CFU determination. The Day 0 bacterial concentrations (in log10 CFU/ml) for each panel were as follows: A, 5.96; B, 6.11; C, 5.85; D, 5.86; E, 5.85; F, 5.86. CFU values are provided in Table S2.
Next, we evaluated the activity of bedaquiline and imipenem/avibactam combinations against actively growing M. abscessus WT and OM7 mutant strains. As previously observed, bedaquiline alone at 0.5 and 1 μg/ml prevented growth of the WT strain and permitted growth of the OM7 mutant, and imipenem/avibactam combinations of 2/2 and 4/2 μg/ml were bactericidal against both bacterial strains (Fig. 2; Table S3). However, the addition of bedaquiline to the imipenem/avibactam combinations abolished this bactericidal activity. Against both strains, the activity of bedaquiline alone appeared concentration-dependent, but this relationship was less clear when combined with imipenem/avibactam. Therefore, we next examined the concentration-ranging activity of bedaquiline when added to imipenem/avibactam combinations against M. abscessus WT and OM7. Against both strains, bedaquiline/imipenem/avibactam combinations were more bactericidal when bedaquiline was included at concentrations ≤0.0156 μg/ml, and this bactericidal activity was bedaquiline concentration-dependent (Fig. S2; Table S4), with bactericidal activity declining with increasing bedaquiline concentration. Overall, the magnitude of killing associated with bedaquiline/imipenem/avibactam combinations was greater against the OM7 mutant than against the WT strain.
FIG 2.
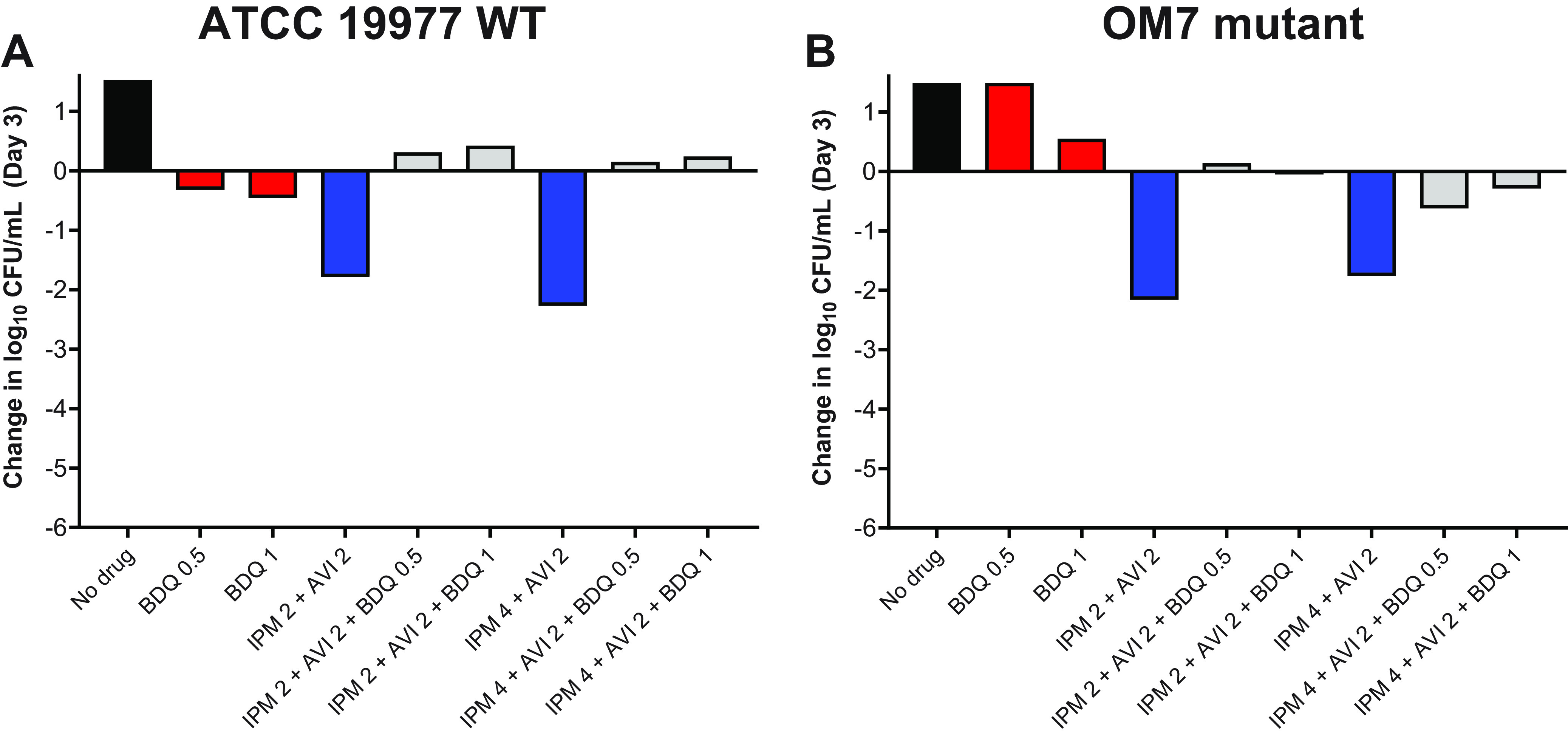
Activity of bedaquiline (BDQ) and imipenem (IPM)/avibactam (AVI) combinations against M. abscessus ATCC 19977 WT (A) and OM7 mutant (B) strains in CAMHB with 0.05% Tween 80. The change in log10 CFU/ml after 3 days of drug exposure relative to Day 0 is presented for each drug/strain set. Black bars represent the no-drug control; red bars indicate BDQ-only samples; blue bars represent IPM/AVI-only samples, and gray bars indicate IPM/AVI plus BDQ samples. The number after each drug abbreviation represents the concentration in μg/ml. The Day 0 bacterial concentrations (in log10 CFU/ml) were 5.57 and 5.76 for WT and OM7 mutant strains, respectively. CFU values are provided in Table S3.
Assessment of bedaquiline combined with other cell wall synthesis inhibitors (and non cell wall synthesis inhibitors) against M. abscessus WT in nutrient-rich conditions with Tween 80.
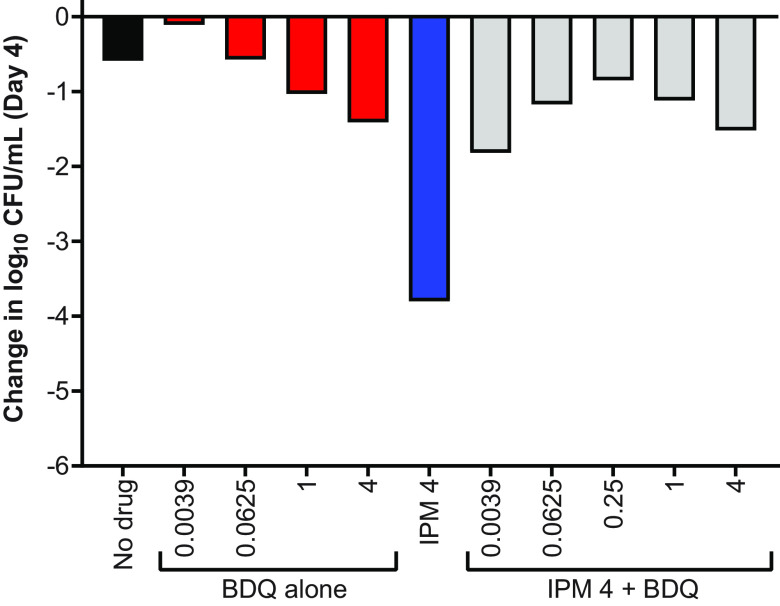
Our data indicated that bedaquiline antagonized the bactericidal activity of imipenem/avibactam against both WT parent and OM7 mutant strains and suggested that this antagonism might only occur at concentrations at which bedaquiline alone was able to limit bacterial growth. Other groups have reported antagonism between bedaquiline and cell wall synthesis inhibitors, including imipenem as well as non-β-lactam inhibitors, against M. abscessus and other mycobacteria (8, 20–22), but these previous studies did not include bedaquiline at sub-inhibitory concentrations. Therefore, we examined the concentration-ranging impact of bedaquiline on the bactericidal activity of two additional cell wall synthesis inhibitors: the β-lactam meropenem and N-4S-methylcyclohexyl-4,6-dimethyl-1H-indole-2-carboxamide, a non-β-lactam MmpL3 inhibitor with demonstrated in vitro activity against M. abscessus (23). We also evaluated the impact of bedaquiline on the bactericidal activity of clarithromycin, a protein synthesis inhibitor. Meropenem, the MmpL3 inhibitor, and clarithromycin were used at concentrations expected to have activity against M. abscessus WT: 16, 2, and 4 μg/ml, respectively (6, 23, 24), and meropenem was used without avibactam to allow for more direct comparisons between the different groups. Because these agents are relatively more stable in aqueous media than imipenem, this experiment was extended out to 8 days. Again, bedaquiline alone had limited activity against actively growing WT bacteria, resulting in bacteriostasis at 0.125 μg/ml and a weak bactericidal effect at concentrations of 1–2 μg/ml (Fig. 3A; see Table S5 for all CFU data from this figure). When combined with meropenem, these bedaquiline concentrations antagonized the bactericidal activity of meropenem over 8 days, while the lower bedaquiline concentrations of 0.0039 and 0.0156 μg/ml that were largely inactive on their own were not antagonistic but rather contributed additional killing compared to meropenem alone at Day 8 (Fig. 3B). When combined with the MmpL3 inhibitor, bedaquiline at concentrations ≥0.0156 μg/ml abrogated the bactericidal activity compared to the MmpL3 inhibitor alone, while bedaquiline added at 0.0039 μg/ml remained highly bactericidal, although to a lesser magnitude than the MmpL3 inhibitor alone (Fig. 3C). In contrast, bedaquiline did not diminish the bactericidal activity of clarithromycin and added activity at concentrations ≥0.125 μg/ml at Day 8 (Fig. 3D).
FIG 3.
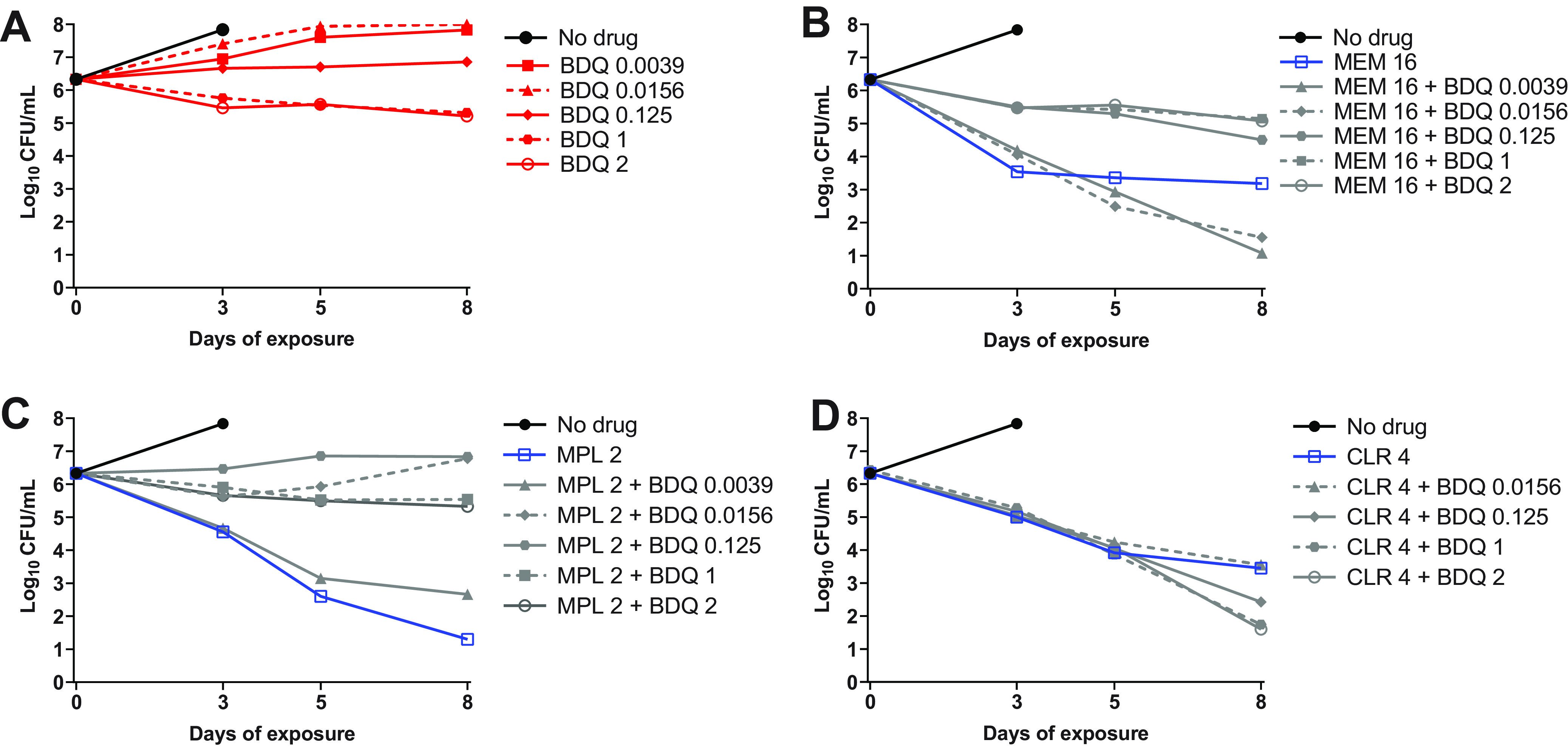
Activity of bedaquiline (BDQ) alone (A), BDQ plus meropenem (MEM) (B), BDQ plus MmpL3 inhibitor (MPL) (C), and BDQ plus clarithromycin (CLR) (D) against M. abscessus ATCC 19977 WT in CAMHB with 0.05% Tween 80. The number after each drug abbreviation represents the concentration in μg/ml. MPL is N-4S-methylcyclohexyl-4,6-dimethyl-1H-indole-2-carboxamide. The bacteria in the no-drug control overgrew and clumped after Day 3, precluding CFU quantification. CFU values are provided in Table S5.
Correlation of ATP levels with bedaquiline/imipenem bactericidal activity against M. abscessus WT in nutrient-rich conditions with Tween 80.
Previous work by Lindman and Dick demonstrated that the bactericidal activity of imipenem correlated with increased M. abscessus ATP production, which was also abrogated with co-exposure to bedaquiline (8). Therefore, we next evaluated the relative bacterial ATP levels associated with bedaquiline and imipenem against actively growing WT bacteria in CAMHB with Tween 80. To reduce study variables, avibactam was omitted. In both biological replicates, we observed that imipenem’s potent bactericidal activity was unchanged or even increased when bedaquiline was added at concentrations of 0.0039 or 0.0078 μg/ml, yet adding bedaquiline at concentrations ≥0.0625 μg/ml nearly eliminated imipenem’s bactericidal activity (Fig. 4A and B; Table S6). The relative levels of bacterial ATP, measured as relative light units (RLU) adjusted by CFU count, correlated with the bactericidal effect; ATP levels increased when bacteria were exposed to bactericidal imipenem/bedaquiline concentrations, and ATP levels decreased as the bedaquiline concentration increased (Fig. 4C and D). RLU data not adjusted by CFU counts are presented in Fig. S3.
FIG 4.
Activity of bedaquiline (BDQ) and imipenem (IPM) against M. abscessus ATCC 19977 WT (A, B) and relative bacterial ATP levels (C, D), in CAMHB with 0.05% Tween 80. The change in log10 CFU/ml after 3 days of drug exposure relative to Day 0 is presented for each biological replicate in panels A and B. CFU-adjusted ATP levels, indicated by relative light units (RLU)/CFU from samples after 3 days of drug exposure, are presented for each biological replicate in panels C and D. Black bars represent the no-drug control; red bars indicate BDQ-only samples; blue bars represent IPM-only samples; and gray bars indicate IPM plus BDQ samples. The number after each drug abbreviation represents the concentration in μg/ml (for gray bars the BDQ concentration in μg/ml is presented under each bar). RLU/ml for all samples (not adjusted for CFU) are presented in Fig. S3. The Day 0 bacterial concentration for panels A and B was 6.25 and 7.03 log10 CFU/ml, respectively. CFU and RLU values are provided in Table S6.
Assessment of bedaquiline and imipenem activity against M. abscessus ATCC 19977 WT and OM7 in nutrient-rich conditions without Tween 80.
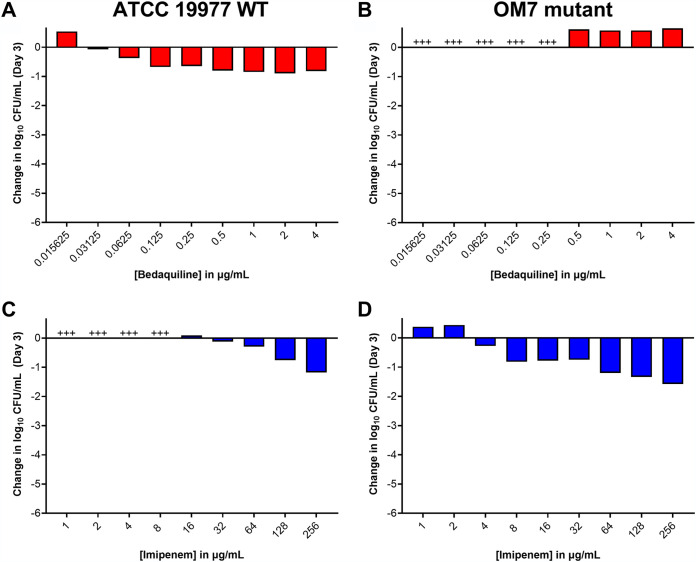
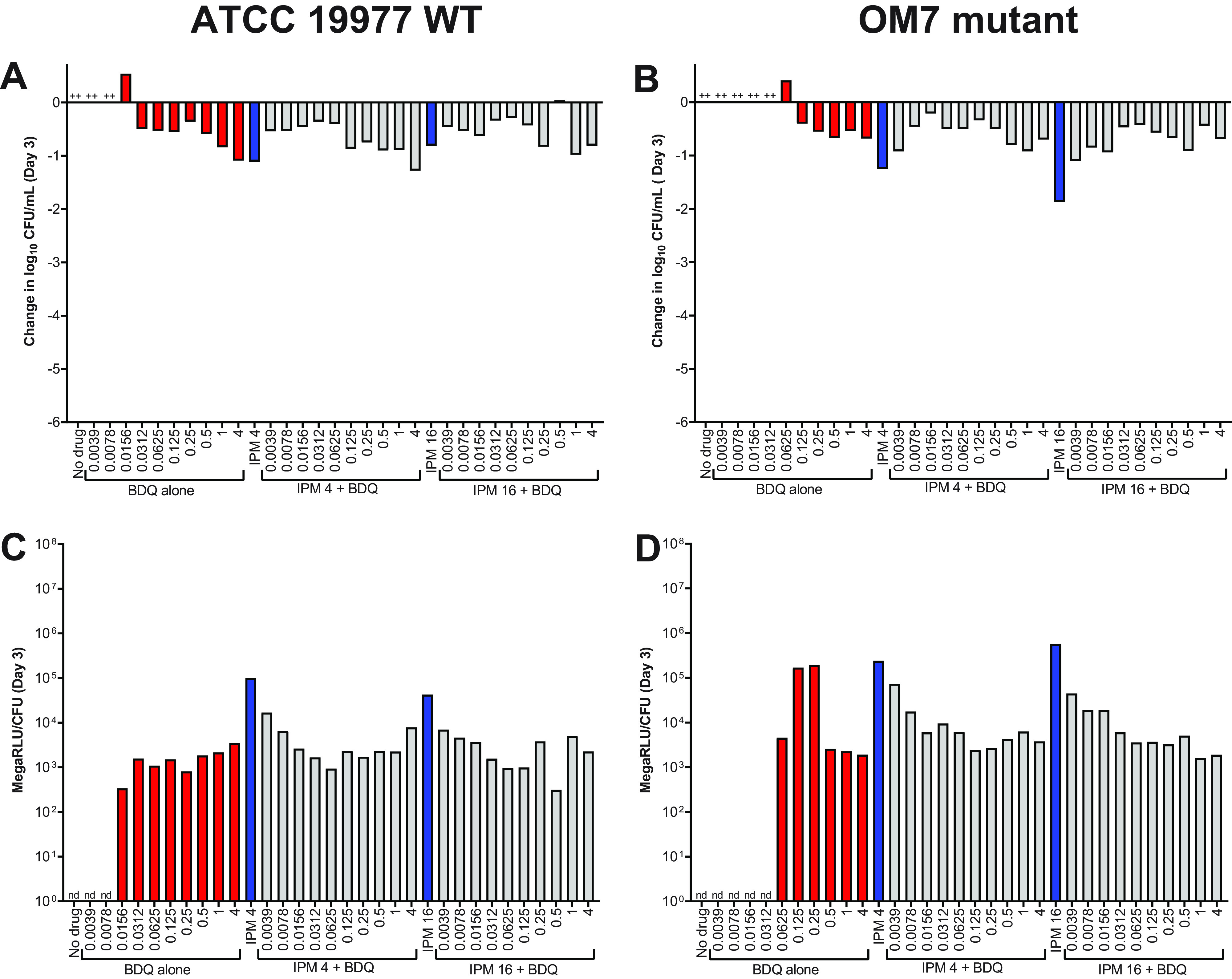
Thus far, the surfactant Tween 80 was present in all experimental conditions. As noted earlier, this was done in an effort to reduce bacterial clumping. However, as bacterial clumping was not always prevented (Fig. 1 and 3; Table S3 and S4), and as surfactants such as Tween 80 can influence cell wall permeability and drug susceptibility (25, 26), we also evaluated the activity of bedaquiline and imipenem against M. abscessus in CAMHB without Tween 80. After 3 days in these assay conditions, bedaquiline completely inhibited the growth of WT bacteria starting at 0.0312 μg/ml and exhibited bactericidal activity (reducing CFU by ∼0.5–0.9 log10 CFU/ml) at concentrations ≥0.0625 μg/ml (Fig. 5A; see all CFU data in Table S7). Bedaquiline only limited growth of the OM7 mutant at concentrations ≥0.5 μg/ml (Fig. 5B). Therefore, against both WT and OM7 populations, bedaquiline was more active (on a μg/ml basis) in CAMHB without Tween 80 than that with Tween 80 (Fig. 1A and B). In contrast, the activity of imipenem was reduced when Tween 80 was omitted from the media. After 3 days of exposure, imipenem inhibited growth of WT and OM7 at 16 and 4 μg/ml, respectively (Fig. 5C and D). Although imipenem was tested up to 256 μg/ml, the magnitude of killing never exceeded 2 log10 CFU/ml against either strain, while in CAMHB with 0.05% Tween 80, CFU reductions exceeded 2 log10 CFU/ml at 2–4 μg/ml imipenem (Fig. 1C and D).
FIG 5.
Activity of bedaquiline (A, B) and imipenem (C ,D) against M. abscessus ATCC 19977 WT (A, C) and OM7 mutant (B, D) in CAMHB without Tween 80. The change in log10 CFU/ml after 3 days of drug exposure relative to Day 0 is presented for each drug/strain set. +++ indicates bacterial growth and clumping that precluded CFU determination. The Day 0 bacterial concentrations (in log10 CFU/ml) for each panel were as follows: A, 5.90; B, 5.08; C, 5.91; D, 6.20. CFU values are provided in Table S7.
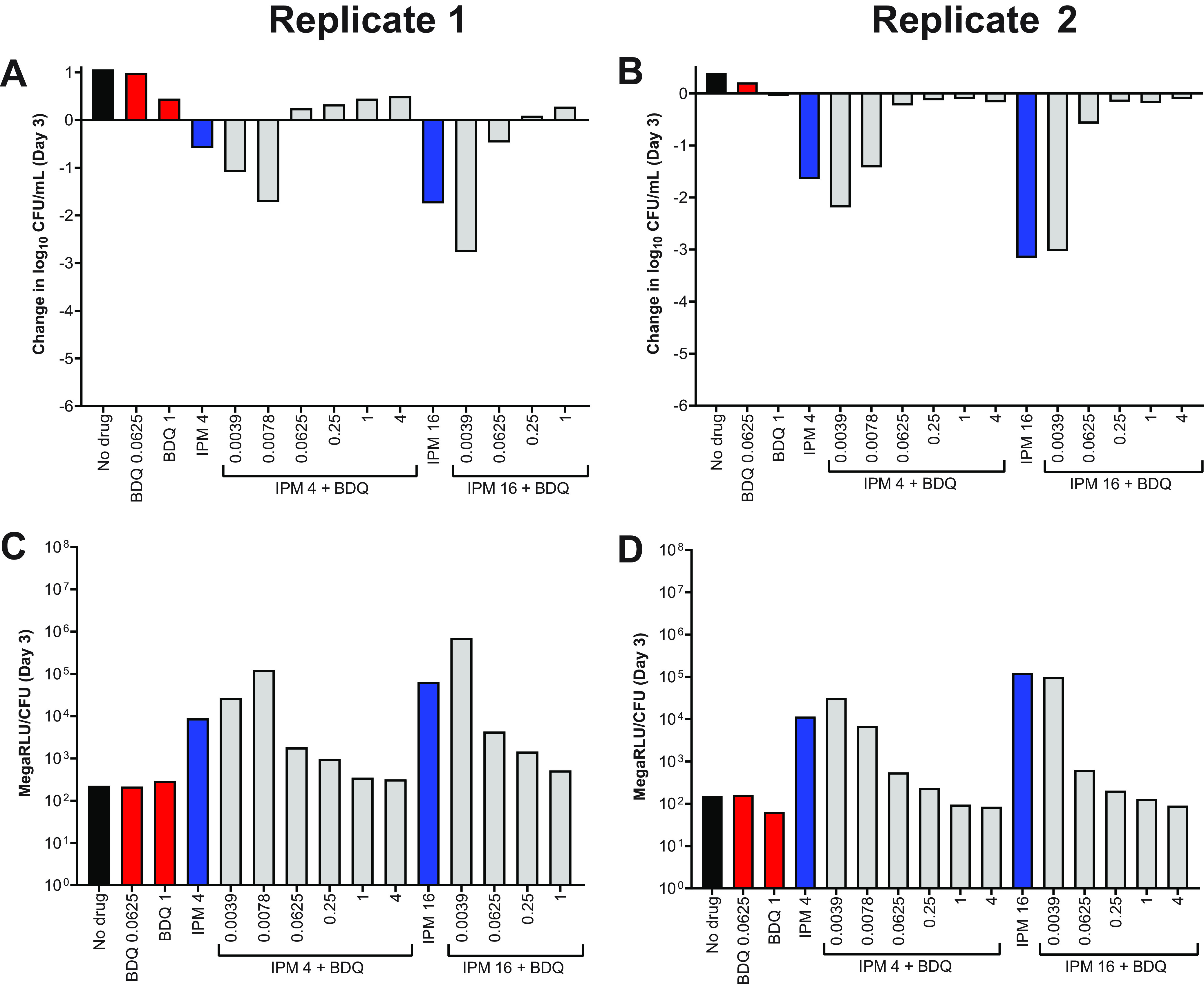
We next assessed the activity of imipenem/bedaquiline combinations in CAMHB without Tween 80. Again, we observed that, relative to activity in Tween 80-containing media, bedaquiline alone had increased killing and imipenem alone had decreased killing in the absence of Tween 80 (Fig. 6A and B (replicate 1); Fig. S4A (replicate 2); see all CFU data in Tables S8–S9). For both WT and OM7 strains, the addition of bedaquiline decreased imipenem’s bactericidal activity, and lower concentrations of bedaquiline combined with imipenem did not increase bactericidal activity, as was observed in Tween 80-containing media (Fig. 4; Fig. S2). In these assay conditions, relative bacterial ATP levels also correlated with bacterial killing (Fig. 6C and D; Fig. S4B). ATP levels, not adjusted by CFU counts, indicate that ATP levels in the samples that overgrew and clumped (precluding CFU quantification) were similar to the ATP levels of the no-drug control samples for both WT and OM7 populations (Fig. S4C and S5; see all RLU data in Tables S8–S9).
FIG 6.
Activity of bedaquiline (BDQ) and imipenem (IPM) against M. abscessus ATCC 19977 WT (A) and OM7 mutant (B), with relative bacterial ATP levels (C, D), in CAMHB without Tween 80. The changes in log10 CFU/ml after 3 days of drug exposure relative to Day 0 are presented in panels A and B; CFU-adjusted ATP levels, indicated by relative light units (RLU)/CFU from samples after 3 days of drug exposure, are presented in panels C and D. Red bars indicate BDQ-only samples; blue bars represent IPM-only samples, and gray bars indicate IPM + BDQ samples. The number after the IPM abbreviation represents the concentration in μg/ml; for red and gray bars, the BDQ concentration in μg/ml is under each bar. ++ indicates that the bacteria overgrew and clumped, precluding CFU quantification. nd indicates not determined (due to lack of CFU count data). RLU/ml for all samples (not adjusted for CFU) are presented in Fig. S5. The Day 0 bacterial concentration for panels A and B was 5.62 and 5.70 log10 CFU/ml, respectively. CFU and RLU values are provided in Table S8.
Evaluation of bedaquiline and imipenem activity against nutrient-starved M. abscessus ATCC 19977 WT and OM7 in PBS without Tween 80.
Regardless of the presence or absence of Tween 80, imipenem alone was clearly more active than BDQ alone against actively growing M. abscessus strains in nutrient-rich media, a finding that aligns with previously published work (6–8). However, we previously reported the converse against nutrient-starved M. abscessus: namely, that bedaquiline is highly bactericidal while imipenem has no observable activity (6). Therefore, we next evaluated the bactericidal activity of imipenem/bedaquiline combinations against M. abscessus WT and OM7 populations that had been nutrient-starved in PBS for 14 days prior to drug exposure. After nutrient starvation, drug activity assays were performed in PBS without Tween 80. As expected, bedaquiline alone had potent, concentration-dependent bactericidal activity against nutrient-starved M. abscessus WT (Fig. 7A, Day 3; Fig. S6A, Day 7). Bedaquiline also exerted concentration-dependent killing against the nutrient-starved OM7 mutant (Fig. 7B; Fig. S6B), although with a much lower magnitude of killing compared to the WT parent strain (see all CFU data in Table S10). Imipenem alone at 4 or 16 μg/ml had no bactericidal activity against either strain, and the bactericidal activity of bedaquiline was unchanged by the addition of imipenem. In these nutrient starvation conditions, bacterial ATP levels adjusted by CFU counts correlated with bactericidal activity for both strains (Fig. 7C and D; Fig. S6C and D). For both WT and OM7, the total ATP levels, not adjusted by CFU, decreased with increasing bedaquiline concentration (Fig. S7 and S8), similar to what was observed in CAMHB without Tween 80 (Fig. S5), although with overall lower bacterial ATP levels in nutrient starvation conditions.
FIG 7.
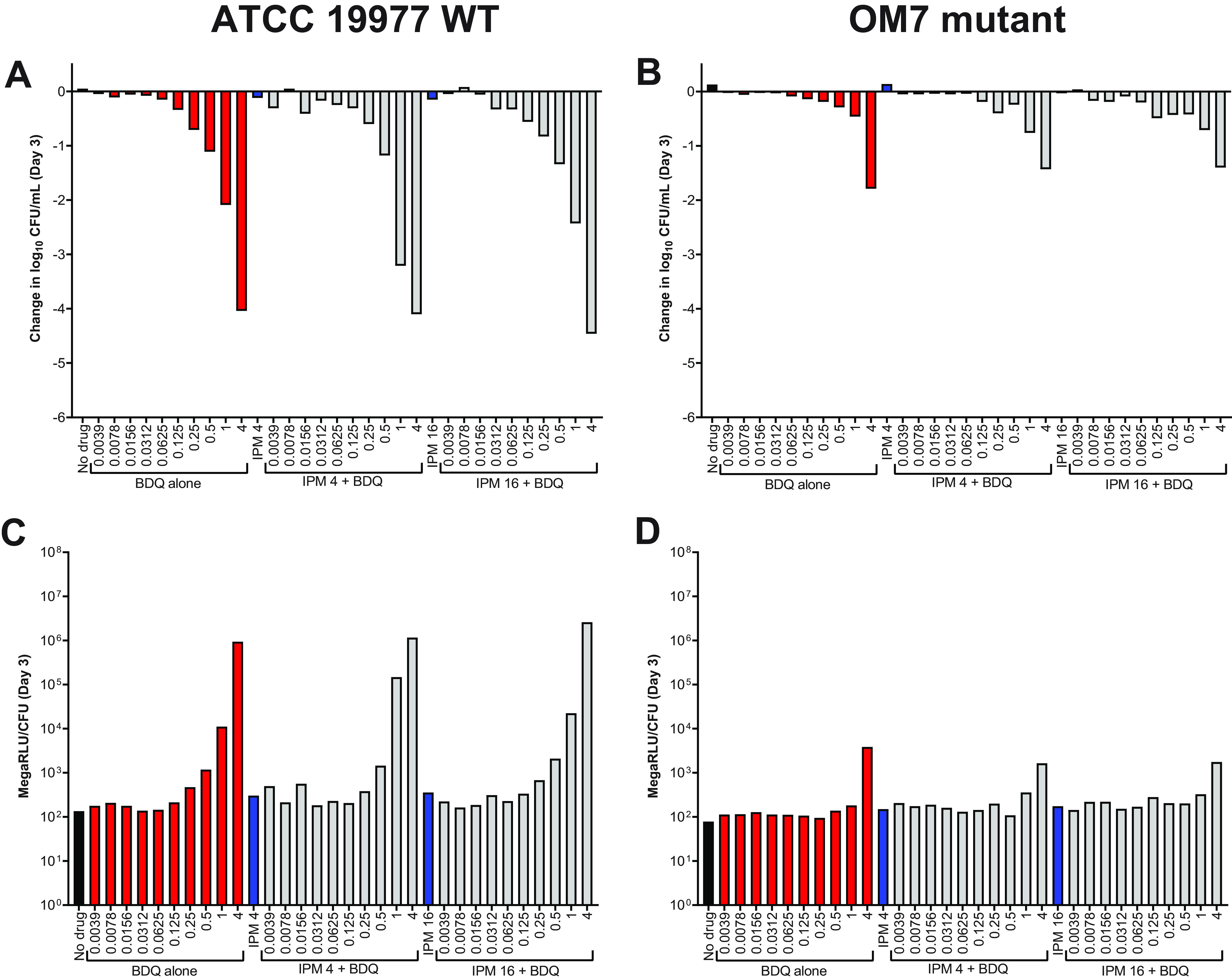
Activity of bedaquiline (BDQ) and imipenem (IPM) against nutrient-starved M. abscessus ATCC 19977 WT (A) and OM7 mutant (B), with relative bacterial ATP levels (C, D), in PBS without Tween 80. Bacteria were nutrient-starved in PBS for 14 days prior to drug exposure. The changes in log10 CFU/ml after 3 days of drug exposure relative to Day 0 are presented in panels A and B; CFU-adjusted ATP levels, indicated by relative light units (RLU)/CFU from samples after 3 days of drug exposure, are presented in panels C and D. Red bars indicate BDQ-only samples; blue bars represent IPM-only samples; and gray bars indicate IPM + BDQ samples. The number after the IPM abbreviation represents the concentration in μg/ml; for red and gray bars, the BDQ concentration in μg/ml is under each bar. Data after 7 days of drug exposure are presented in Fig. S7. RLU/ml (not adjusted for CFU) for WT and OM7 are presented in Fig. S8 and Fig. S9, respectively. The Day 0 bacterial concentration for panels A and B was 6.25 and 6.29 log10 CFU/ml, respectively. CFU and RLU values are provided in Table S9.
Comparison of bedaquiline and imipenem activity against nutrient-starved M. abscessus ATCC 19977 WT in PBS with or without Tween 80.
Because the presence of Tween 80 positively impacted the activity of imipenem and negatively impacted the activity of bedaquiline against actively growing M. abscessus, we also evaluated the impact of Tween 80 on the bactericidal activity of these drugs, alone and together, against nutrient-starved bacteria in PBS with 0.05% Tween 80. For logistical reasons, bacteria were nutrient starved in PBS for 20 days prior to drug exposure in this experiment, and bacteria were exposed to drugs for up to 4 days in Tween-containing PBS. Although bedaquiline alone still exerted concentration-dependent bactericidal activity in these conditions (Fig. 8; Fig. S9; see all CFU data in Table S11), the magnitude of the killing was greatly reduced compared to its activity in PBS with Tween 80. In PBS with Tween 80, bedaquiline alone at 1 and 4 μg/ml reduced CFU counts by approximately 1 and 1.5 log10 CFU/ml, respectively, after 4 days of exposure, while bedaquiline alone at 1 and 4 μg/ml reduced them by approximately 2 and 4 log10 CFU/ml, respectively, in 3 days against nutrient-starved WT in PBS without Tween 80 (Fig. 7A). Conversely, imipenem alone reduced CFU counts by >3 log10 CFU/ml against nutrient-starved bacteria in PBS with Tween 80, which was similar to the magnitude of killing observed in CAMHB with Tween 80 (Fig. 1C). When imipenem and bedaquiline were combined, bactericidal activity was reduced compared to imipenem alone; however, the combined killing of imipenem/bedaquiline at higher concentrations of bedaquiline aligned with the bactericidal activity of bedaquiline alone. A head-to-head-comparison in PBS with or without Tween against WT bacteria that were nutrient-starved for 14 days prior to drug exposure confirmed these findings (Fig. S10; Table S12).
FIG 8.
Activity of bedaquiline (BDQ) and imipenem (IPM) against nutrient-starved M. abscessus ATCC 19977 WT in PBS with 0.05% Tween 80. Bacteria were nutrient-starved in PBS for 20 days prior to drug exposure. The changes in log10 CFU/ml after 4 days of drug exposure relative to Day 0 are presented. Red bars indicate BDQ-only samples; blue bars represent IPM-only samples; and gray bars indicate IPM + BDQ samples. The number after the IPM abbreviation represents the concentration in μg/ml; for red and gray bars, the BDQ concentration in μg/ml is under each bar. Data after 1 day of drug exposure, as well as data from a biological replicate, are presented in Fig. S13. The Day 0 bacterial concentration was 6.07 log10 CFU/ml. CFU values are provided in Table S11.
Investigation of bedaquiline and imipenem activity against intracellular M. abscessus ATCC 19977 WT and OM7 mutant.
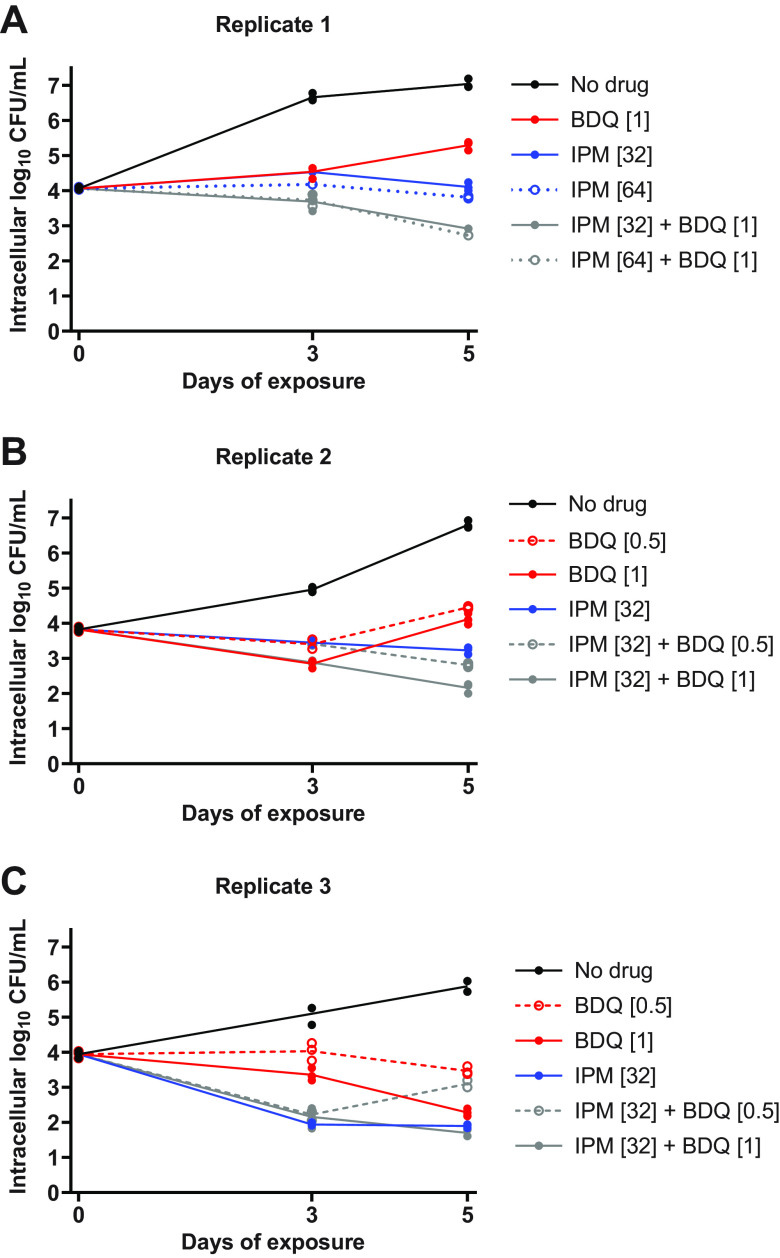
Our in vitro results highlighted the impact that experimental conditions can have on the assessment of drug activity against M. abscessus. In order to better understand which assay conditions, if any, may be predictive of activity in models of higher biological complexity, we next evaluated the activity of bedaquiline and imipenem against intracellular M. abscessus WT during infection of THP-1 monocytic cells. Exposure to bedaquiline alone at concentrations ranging from 0.25 to 16 μg/ml resulted in concentration-dependent activity, ranging from growth-limiting to bacteriostatic to bactericidal, against intracellular M. abscessus (Fig. S11A-C; see all CFU data in Table S13). In contrast, concentration-ranging activity was not clearly observed with imipenem at concentrations ranging from 16 to 64 μg/ml; imipenem at 16–32 μg/ml had similar activity (static or cidal, depending on the replicate) against intracellular M. abscessus, while imipenem at 8 μg/ml was consistently less active and only limited intracellular bacterial growth relative to the no-drug control (Fig. S11D-F). The combination of bedaquiline at 0.5 or 1 μg/ml with imipenem at 32–64 μg/ml exerted bactericidal activity, killing ∼1-2 log10 intracellular CFU/ml over 5 days (Fig. 9). The 2-drug combinations demonstrated equivalent or better activity against intracellular M. abscessus compared to either drug alone, and antagonism was not observed in these assay conditions.
FIG 9.
Activity of bedaquiline (BDQ) and imipenem (IPM) combinations against intracellular (THP-1 cells) M. abscessus ATCC 19977 WT. Data are presented for 3 biological replicates: replicate 1, panel A; replicate 2, panel B; replicate 3, panel C. Each data point represents a technical replicate, and the connecting lines pass through the mean values. The number in brackets after each drug abbreviation represents the concentration in μg/ml. All CFU values, including data from additional BDQ+IPM combinations and a fourth biological replicate, are provided in Table S13.
DISCUSSION
In this series of experiments, we systematically evaluated the in vitro activity of bedaquiline and imipenem against the ATCC 19977 wild-type strain of M. abscessus and an isogenic MAB_2299c mutant in different culture conditions: actively growing bacteria in nutrient-rich media; net non-replicating bacteria under nutrient starvation in PBS; and actively growing intracellular bacteria in THP-1 cells. Overall, we found that the activity of bedaquiline and imipenem, either alone or in combination, was significantly influenced by assay conditions, highlighting current knowledge gaps and limiting broad generalizations with respect to translating in vitro data into predictions of in vivo efficacy.
One consistent finding was that bedaquiline alone had limited or no bactericidal activity against actively multiplying M. abscessus in nutrient-rich media, a finding that aligns with previous reports (6–8, 11). However, even within this limited activity, we observed that the antibacterial effects of bedaquiline were greater (on a μg/ml basis) against bacteria in CAMHB without the surfactant Tween 80 than in CAMHB supplemented with 0.05% Tween 80. We also consistently observed that bedaquiline had strong bactericidal activity against nutrient-starved M. abscessus in the absence of Tween 80, in agreement with our previous report (6), but the magnitude of killing decreased in the presence of Tween 80. Bedaquiline’s activity against intracellular M. abscessus, which was mainly bacteriostatic with limited bacterial killing, aligned best with the activity in CAMHB, with the magnitude of effect (based on the μg/ml bedaquiline added to assay media) falling somewhere between the activity observed in CAMHB with and without Tween 80.
The activity of imipenem was affected by Tween 80 to an even greater extent. In CAMHB without Tween 80, imipenem alone had limited bactericidal activity, a finding that aligns with previous reports (6, 27). In contrast, imipenem alone was highly bactericidal in CAMHB with Tween 80. In agreement with our previous study (6), imipenem alone had no bactericidal activity against nutrient-starved bacteria in the absence of Tween 80; but, in the presence of Tween 80, imipenem had striking bactericidal activity against nutrient-starved M. abscessus, with killing of a similar magnitude as in CAMHB with Tween 80. The largely bacteriostatic or limited bactericidal activity of imipenem against intracellular bacteria, a finding also reported by others (28, 29), aligned best with the activity in CAMHB without Tween 80.
The activity of imipenem-bedaquiline combinations was also highly dependent on assay conditions. Against actively multiplying M. abscessus in CAMHB with Tween 80, the potent bactericidal activity of imipenem was enhanced when bedaquiline was added at low concentrations, ≤0.0078 μg/ml. However, as the concentration of bedaquiline increased above 0.0078 μg/ml, the addition of bedaquiline decreased the bactericidal activity of imipenem until it was no longer evident, although the activity of the imipenem-bedaquiline combination was better than the activity of bedaquiline alone. Lindman and Dick previously reported that inhibitory concentrations of bedaquiline antagonize the in vitro bactericidal activity of imipenem against actively multiplying M. abscessus; however, the media used in their assay was Middlebrook 7H9 broth (BD Difco) supplemented with 0.5% albumin, 0.2% glucose, 0.085% sodium chloride, 0.0003% catalase, 0.2% glycerol, and 0.05% Tween 80 (verified by written correspondence) (8). In CAMHB without Tween 80, activity of the two-drug combination was driven by the activity of bedaquiline. Any antagonism of imipenem by bedaquiline could not be ascertained, as the activity of imipenem alone was more limited in the absence of Tween. Similarly, the bactericidal activity of imipenem-bedaquiline combinations against nutrient-starved M. abscessus in the absence of Tween also appeared to be driven by the activity of bedaquiline. However, in the presence of Tween 80, the addition of bedaquiline drastically antagonized the bactericidal activity of imipenem against nutrient-starved bacteria. Additive activity was observed between imipenem and bedaquiline against intracellular bacteria.
Consideration of the clinical relevance of assay conditions is always important when evaluating antibacterial drugs and regimens in vitro. Unfortunately, for treatment of M. abscessus lung infections, there are no established or validated in vitro assays known to predict in vivo activity, and the situation is further complicated by the lack of data regarding clinical treatment outcomes. However, inclusion of Tween 80 in drug activity assays may lead to conclusions that are clinically misleading. Tween is a non-ionic surfactant used as an emulsifier in drugs and food. It has been known for decades that Tween 80 can impact drug activity against bacteria, and for agents acting on the cell wall, such as imipenem, Tween 80 may increase susceptibility by increasing membrane permeability (25, 26). Interestingly, Tween 80 had the opposite impact on bedaquiline activity against M. abscessus, decreasing bedaquiline’s activity in CAMHB and in nutrient starvation assays. Lounis et al. reported similar findings for bedaquiline against M. tuberculosis, and suggested that Tween 80 may be directly interacting with bedaquiline, thus limiting the amount of free drug in solution (30). We initially included Tween 80 in our assay to reduce bacterial clumping, without appreciating how drastic its impact would be on assay outcomes. In this study, the most biologically complex system used was the intracellular infection assay. Overall, the drug activity against intracellular bacteria seemed to be best predicted by activity in CAMHB without Tween 80, providing further weight to the data from in vitro assays performed without Tween 80.
The impact of Tween 80 on nutrient-starved drug activity assays is further complicated by the presence of oleic acid, a breakdown product of Tween 80 in aqueous solutions and hydrolysis by mycobacterial enzymes (17, 31–33). Oleic acid can serve as a nutrient source for mycobacteria. Due to its known ability to enhance the in vitro growth of M. tuberculosis (17, 31), it is routinely used as a supplement in liquid and solid media for isolating and cultivating mycobacteria. Including Tween 80 in the nutrient-starvation samples during drug exposure likely introduced the nutrient oleic acid into the PBS. Although we did not observe net bacterial growth in these conditions, it is possible that the presence of oleic acid impacted bacterial metabolism such that the bacteria became more susceptible to killing by imipenem. This concept is further supported by data from Berube et al., who reported that imipenem alone had no bactericidal activity against nutrient-starved M. abscessus in PBS with 0.05% tyloxapol, a surfactant that does not provide a nutrient source for the bacteria (34). In this study, we did not have an intracellular or other more biologically complex model of net non-replicating M. abscessus to aid in our translation of drug activity in the presence or absence of Tween 80.
The in vivo activity of imipenem, bedaquiline, and/or imipenem-bedaquiline have been evaluated in different mouse models of M. abscessus lung infection. Story-Roller et al. reported that imipenem (100 mg/kg twice daily) had modest in vivo bactericidal activity against an actively multiplying M. abscessus lung infection in a C3HeB/FeJ mouse model with dexamethasone-induced immunosuppression, with imipenem killing just over 2 log10 CFU/lung over 4 weeks of treatment (35). Le Moigne et al. also evaluated imipenem activity in the lungs of M. abscessus-infected C3HeB/FeJ mice, but without inducing immune suppression, and thus in their model, the bacterial burden in untreated mice decreased over time. The lung bacterial burden in mice that received 2 weeks of imipenem alone (100 mg/kg twice daily) was no different from the lung burden in untreated mice (9). Le Moigne et al. also evaluated the activity of bedaquiline and bedaquiline-imipenem combinations in this mouse model. After 2 weeks of treatment, bedaquiline alone (30 mg/kg once daily) contributed modest bactericidal activity resulting in an approximately 1 log10 CFU/lung reduction compared to the decline observed in untreated mice, and the activity of imipenem-bedaquiline was like that of bedaquiline alone, indicating that the activity of the 2-drug combination was driven more by bedaquiline’s activity than imipenem’s activity (9). Lerat et al. also evaluated bedaquiline alone (25 mg/kg) in an athymic nude mouse model of M. abscessus infection in which the lung bacterial burden in untreated mice decreased over time. After 1 month of treatment, there was no difference in lung bacterial burden between untreated and bedaquiline-treated mice, but after 2 months of treatment, there was a modest decline in lung CFU counts in bedaquiline-treated mice (36). In contrast, Obregón-Henao et al. reported that dosing bedaquiline alone (30 mg/kg once daily for 9 days) resulted in strong bactericidal activity against M. abscessus in the lungs of GKO−/− mice, in which the bacterial counts decreased in untreated mice, and also in the lungs of SCID mice, in which the lung bacterial burden increased in untreated mice (37). The mouse models utilized by Story-Roller et al., Le Moigne et al., and Lerat et al. used M. abscessus strain ATCC 19977, while Obregón-Henao et al. used a different strain in their studies.
How well do the imipenem and bedaquiline activity profiles from our different in vitro and intracellular assays align with the observed in vivo activity in these mouse models of M. abscessus lung infection? Direct comparison between the in vitro and in vivo studies is confounded by several factors. The duration of our in vitro studies, which ranged from 3 to 7 days, was much shorter than most of the treatment durations in mouse models. In addition, understanding the drug exposures in mice is more complicated than in in vitro assays. The imipenem dose administered to mice in the studies described, 100 mg/kg, has been shown to result in a maximum plasma concentration of about 85 μg/ml, but the drug is rapidly metabolized, with a plasma half-life of about 18 min, which leads to the requirement for multiple doses per day to achieve suitable exposures (38). Furthermore, the imipenem concentrations that we utilized for our in vitro combination studies do not fluctuate over time in the manner that they do in mice. The goal is to have the range of concentrations tested be representative of exposures in mice; overall, the imipenem activity data in the mouse models of actively multiplying and declining M. abscessus lung infections would have been best predicted by the in vitro activity observed in assays without Tween 80 and by the intracellular assays.
For bedaquiline, comparison between in vitro and in vivo findings is limited by the nature of bedaquiline metabolism in mice. Compared to humans, mice more rapidly convert bedaquiline into its M2 metabolite, N-desmethyl bedaquiline, which is the dominant species in mouse plasma and lungs (39). Without understanding the activity of the M2 metabolite against M. abscessus, it is impossible to compare in vitro activity to the activity observed in bedaquiline-treated mice. However, as bedaquiline treatment resulted in bactericidal activity in several mouse models (9, 37), it is reasonable to hypothesize that the M2 metabolite does indeed have activity against M. abscessus. It is known that the M2 metabolite contributes approximately 50% of the in vivo activity of bedaquiline in mice infected with M. tuberculosis (39). Keeping all of this in mind, and assuming average plasma concentrations in mice of around 0.2 and 1 μg/ml of BDQ and M2, respectively (39), the nature of the bedaquiline and imipenem-bedaquiline activity in the mouse model reported by Le Moigne et al. seems to align best with in vitro activity observed in the nutrient starvation assay without Tween 80.
Another aspect of the present study was the assessment of bacterial ATP levels in association with imipenem and bedaquiline exposure and bacterial cell death. Similar to Lindman and Dick (8), we found that exposure to bactericidal concentrations of imipenem in CAMHB with Tween 80 was associated with an increase in bacterial ATP levels. This relationship also correlated with the activity of imipenem-bedaquiline combinations in these assay conditions. As observed by Lindman and Dick, exposure to inhibitory concentrations of bedaquiline alone was associated with a modest decline in ATP levels, consistent with bedaquiline’s mechanism of action, and ATP levels decreased in association with the observed antagonism of imipenem’s bactericidal effects when these inhibitory concentrations of bedaquiline were added to imipenem. However, we also expanded on the observations of Lindman and Dick by evaluating the addition of lower, subinhibitory concentrations of bedaquiline to imipenem, which proved to increase, rather than antagonize, the bactericidal effects of imipenem in association with increases in ATP levels. These concentration-dependent effects of bedaquiline when combined with imipenem may shed further light on the relationship between intrabacterial ATP levels and killing by imipenem and other β-lactams described by Lindman and Dick. That the bacteria may respond to sub-inhibitory concentrations of bedaquiline by, at least transiently, increasing ATP production and that this may augment a similar response to imipenem exposure and result in additive effects on both ATP levels and killing further supports their conclusion that increased ATP levels are at least a surrogate for bactericidal effects of imipenem and may be part of the causal pathway. Interestingly, in CAMHB without Tween 80, a condition in which imipenem was not as bactericidal, bacterial ATP levels in imipenem-exposed M. abscessus were not higher than those in the no-drug control samples (Fig. S4 and S5). Just as the bactericidal activity of imipenem-bedaquiline combinations seemed to be driven by bedaquiline in this condition, ATP levels in bacteria exposed to imipenem-bedaquiline decreased as the bedaquiline concentration increased, and overall the ATP levels in bacteria exposed to imipenem-bedaquiline were not different than in bacteria exposed to bedaquiline alone. A similar relationship was observed during drug exposure in nutrient starvation conditions without Tween 80. Because bedaquiline was bactericidal in these conditions, when adjusting the RLU by CFU counts, it may appear that ATP levels are increasing as the bedaquiline concentration increases (Fig. 7). However, the non-CFU-adjusted RLU data indicate that total ATP levels decreased with increasing BDQ concentration (Fig. S7 and S8).
Again, we must ask, what is the biological and/or clinical relevance of these data? Overall, we lean toward the conclusion that assay conditions without Tween 80 are more relevant to drug activity in intracellular and mouse infection models. Lindman and Dick reported that the bactericidal activity of imipenem and cefoxitin against actively multiplying M. abscessus was associated with bacterial ATP bursts, and that the addition of bedaquiline antagonized this activity; however, the assay media used was 7H9 broth with 0.05% Tween 80 (8). Shetty and Dick demonstrated similar bacterial ATP bursts in Mycobacterium bovis BCG exposed to cell wall synthesis inhibitors, including isoniazid, and that both bactericidal activity and ATP levels were decreased when the samples were co-exposed to bedaquiline or other agents affecting ATP production (21), and Zeng et al. confirmed these findings (20). These assays were conducted in Middlebrook 7H9 broth supplemented with 0.05% Tween 80 and DTA medium supplemented with 0.02% Tween 80. There are intriguing biological processes occurring in mycobacterial cells exposed to cell wall synthesis inhibitors and agents that target ATP production, and further dissection of the impact of media conditions on these relationships is needed to understand their impact during in vivo treatment.
The overarching purpose of our work is to provide data that will help inform the design of improved treatment regimens for patients with M. abscessus lung disease. A key issue we sought to address in this work was whether imipenem and bedaquiline could be used together in a treatment regimen. The data from our in vitro (without Tween) and intracellular assays, as well as from the in vivo combination study reported by Le Moigne et al. (9), indicate that the activity of imipenem-bedaquiline combinations may be driven largely by the activity of bedaquiline, without marked antagonism of imipenem. However, even if antagonism of imipenem by bedaquiline does have clinical relevance, it may not necessarily rule out using these drugs together. For example, in tuberculosis treatment, the activity of isoniazid, which is highly bactericidal against actively multiplying M. tuberculosis, is antagonized both in mouse models and in humans, by pyrazinamide, a drug more active against non-replicating bacteria; however, regimens including this combination of drugs have been used successfully for decades (40–43). Furthermore, if we consider that imipenem has a much shorter half-life than bedaquiline and reaches steady state much sooner than bedaquiline, imipenem could exert its activity against actively replicating bacilli without interference or may be augmented by sub-inhibitory concentrations of bedaquiline during the initial stages of treatment. Finally, our results show that mutational disruption of MAB 2299c reduces bacterial susceptibility to bedaquiline across multiple assay conditions but may increase susceptibility to imipenem. If bedaquiline proves to be a drug with treatment-shortening potential in M. abscessus infections, imipenem may be a particularly effective companion agent for preventing emergence of bedaquiline resistance. Ultimately, an imipenem-bedaquiline combination would be part of a larger multidrug regimen, and other optimized companion agents may further enhance the utility of imipenem-bedaquiline for treatment of M. abscessus lung disease.
MATERIALS AND METHODS
Bacteria.
M. abscessus subsp. abscessus strain ATCC 19977 was obtained from the American Type Culture Collection (ATCC) and used in all experiments.
Media.
Bacterial cultures were initiated in standard growth media: Middlebrook 7H9 broth supplemented with 10% (vol/vol) Middlebrook OADC supplement, 0.1% (vol/vol) glycerol, and 0.05% (vol/vol) Tween 80. Drug activity assays in nutrient-rich media were performed using either Middlebrook 7H9 broth with 10% (vol/vol) OADC and 0.1% (vol/vol) glycerol but without Tween; or CAMHB with or without 0.05% Tween 80. Polystyrene petri dishes (100 mm × 15 mm) containing 20 ml 7H11 agar supplemented with 10% (vol/vol) OADC and 0.1% (vol/vol) glycerol were used to determine CFU counts. Difco BBL Mueller-Hinton II broth (cation-adjusted) powder (i.e., CAMHB powder), Difco Middlebrook 7H9 broth powder, Difco Mycobacteria 7H11 agar powder, and BBL Middlebrook OADC enrichment were manufactured by Becton, Dickinson and Company. Glycerol and Tween 80 were purchased from Fisher Scientific.
Drugs.
Imipenem powder was purchased from Biosynth Carbosynth. Bedaquiline and N-4S-methylcyclohexyl-4,6-dimethyl-1H-indole-2-carboxamide were provided by the TB Alliance. Clofazimine, meropenem, and clarithromycin were purchased from Sigma. Drugs were dissolved in either PBS (imipenem and meropenem) or dimethyl sulfoxide (bedaquiline, N-4S-methylcyclohexyl-4,6-dimethyl-1H-indole-2-carboxamide, clofazimine, and clarithromycin), and drug solutions were filter-sterilized.
Selection and characterization of MAB_2299c mutants.
Frozen stock of M. abscessus ATCC 19977 WT was cultured in standard growth media until an optical density at 600 nm (OD600) >1.5 was achieved. Ten-fold dilutions (in PBS) of the bacterial suspension were cultured on 7H11 agar containing the following concentrations of clofazimine: 8 μg/ml, 16 μg/ml, 32 μg/ml, and 64 μg/ml. Individual colonies growing on 7H11 agar containing 8 μg/ml clofazimine were selected, expanded, and stored in growth media at −80°C; these isolates were also subjected to a second round of selection on agar containing clofazimine at 32 μg/ml and 64 μg/ml. Individual colonies were selected, expanded, and stored in growth media. The MICs of both clofazimine and bedaquiline were determined for selected isolates using the broth microdilution method in round-bottom, polystyrene 96-well plates as previously described (24), with assays conducted in CAMHB and Middlebrook 7H9 with 10% (vol/vol) OADC and 0.1% (vol/vol) glycerol, without Tween 80 in either media. The concentration ranges tested were clofazimine 128-0.125 μg/ml and bedaquiline 4-0.0038 μg/ml in CAMHB and 16-0.125 μg/ml in 7H9. MIC was defined as the lowest concentration without visible growth. Isolates with increased MICs at least 4 times higher than the WT parent strain were evaluated by PCR and sequencing for mutations in the following genes: MAB_2299c, MAB_4384, and atpE (MAB_1448). Genomic DNA was prepared from each isolate as previously described (44). Primer sequences and expected amplicon sizes were as follows: MAB_2299c forward: 5′ CGC GTT TCA TCA GGA TCT TT 3′, reverse: 5′ CCT ACG TGG ATG CCA AGG 3′, 862 bp; MAB_4384 forward: 5′ GGC AGG GTC AGC AGA AAT 3′, reverse: 5′ ATG TTG TGT GCG GGG TCT 3′, 840 bp; atpE forward: 5′ TGG ACG AGG ACC ATC ACT AA 3′, reverse: 5′ GAC GGC AGA AGC GAC AC 3′, 375 bp. Purified amplicons were analyzed by Sanger sequencing at GENEWIZ. Sequencing results were compared against the M. abscessus ATCC 19977 published genome, NCBI accession number NC_010397.
Drug activity assays in nutrient-rich media.
Frozen stocks of M. abscessus were cultured in standard growth media until the bacterial suspension reached OD600 ∼1 (approximately 107–108 CFU/ml), at which point the assays were initiated (“Day 0”). Assays were conducted with the indicated media in a total volume of 2.5 ml in 14 ml, round-bottom, polystyrene tubes as previously described (6).
Drug activity assays in nutrient starvation conditions.
Frozen stocks of M. abscessus were cultured in growth media from frozen stock until the bacterial suspension reached OD600 ∼1. The bacterial suspension was subsequently spun (1900 rcf for 10 min), washed, and resuspended three times to the original volume in PBS with 0.05% (vol/vol) Tween 80, as previously described (6). After the third wash, the bacteria were resuspended in PBS with 0.05% (vol/vol) Tween 80 and were incubated at 37°C for the indicated duration (14 or 20 days). Assays were initiated after the indicated duration of nutrient starvation; the bacterial suspensions were diluted in PBS with or without Tween 80 to an OD600 of 0.6 on Day 0 of the assay. Drugs stocks were added to achieve the indicated concentrations. Assays were conducted in a total volume of 2.5 ml in 14 ml, round-bottom, polystyrene tubes as previously described (6).
Relative ATP measurements.
The Promega BacTiter-Glo Microbial Cell Viability Assay was used to measure the relative ATP in assay samples. At each indicated time point, 20 μl of assay sample was removed and incubated with 20 μl of the BacTiter-Glo reagent in clear 1.5 ml snap-cap tubes at room temperature for 5 min. After the incubation, RLU was recorded for each sample using Turner TD-20/20 luminometer.
Intracellular infections and drug activity assays.
Frozen stocks of M. abscessus were cultured in standard growth media. After reaching OD600 ∼1, the culture was spun at 1100 rpm for 7 min to remove any cellular debris or dead cells. For infection, the bacterial suspension was subsequently diluted in Roswell Park Memorial Institute (RPMI) medium with l-glutamine, supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS) to generate a bacterial suspension of ∼5 × 104 cells/ml. THP-1 cells (obtained from ATCC, product number TIB-202) were grown in supplemented RPMI medium to a density of 105 cells/ml, aliquoted into 24-well polystyrene plates (tissue culture-treated, 1 ml cell suspension per well), and activated by incubation with 50 nM phorbol 12-myristate 13-acetate for 24 h at 37°C. After activation, the adherent THP-1 cells were washed with PBS and then infected with 1 ml of the adjusted bacterial suspension to achieve an MOI of 10:1, 1:1, and 1:10 (bacteria: THP-1 cells), respectively. All drug activity assays were conducted at an MOI of 1:10. After 3 h of infection at 37°C, each well was washed three times with 1 ml PBS to remove extracellular bacteria. After the final wash, 1 ml RPMI media containing bedaquiline or imipenem at the indicated concentrations (or no drug) was added to the appropriate wells. Drug-containing media were replaced daily. At each indicated time point, each well was washed three times with PBS, and then the THP-1 cells were lysed with sterile deionized water, and lysates were collected for CFU determination. In each biological replicate, three wells were used for each condition (i.e., triplicate technical replicates). All THP-1 cells were incubated at 37°C and 5% CO2.
Quantitative cultures, CFU counting, and analysis.
In all assays, CFU counts were determined by culturing serial 10-fold dilutions of bacterial suspensions on 7H11 agar. CFU counts were not determined for samples where the bacteria had overgrown, clumped, and fallen out of suspension. Serial dilutions were prepared in PBS, and 0.5 ml of each dilution was cultured on 7H11 agar. After all liquid was absorbed into the agar, the plates were sealed in plastic bags and incubated at 37°C for 5–7 days before reading and recording CFU counts. The dilution that yielded CFU counts between 10 and 120 and closest to 50 was used to determine CFU/ml. The CFU/ml value (x) was log transformed as log10 (x + 1) prior to analysis. All analyses were conducted using GraphPad Prism version 9.
ACKNOWLEDGMENTS
This work was funded by the National Institutes of Health (R21-AI137814 to E.L.N.), the Cystic Fibrosis Foundation (E.L.N.), and by Genentech (research fellowship grant to O.M.). K.E.D. is supported by National Institutes of Health (K24AI150349).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, Huitt GA, Knight SL, Leitman P, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, van Ingen J, Wagner D, Winthrop KL. 2020. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 56:2000535. 10.1183/13993003.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasperbauer SH, De Groote MA. 2015. The treatment of rapidly growing mycobacterial infections. Clin Chest Med 36:67–78. 10.1016/j.ccm.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Eurosurveillance Editorial Team. 2013. WHO publishes global tuberculosis report 2013. Euro Surveill 18(43):20615. [PubMed] [Google Scholar]
- 4.Haley CA, Macias P, Jasuja S, Jones BA, Rowlinson M-C, Jaimon R, Onderko P, Darnall E, Gomez ME, Peloquin C, Ashkin D, Goswami ND. 2021. Novel 6-month treatment for drug-resistant tuberculosis, United States. Emerg Infect Dis 27:332–334. 10.3201/eid2701.203766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tasneen R, Li S-Y, Peloquin CA, Taylor D, Williams KN, Andries K, Mdluli KE, Nuermberger EL. 2011. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother 55:5485–5492. 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J, Ammerman N, Agarwal A, Naji M, Li SY, Nuermberger E. 2021. Differential in vitro activities of individual drugs and bedaquiline-rifabutin combinations against actively multiplying and nutrient-starved Mycobacterium abscessus. Antimicrob Agents Chemother 65:e02179-20. 10.1128/aac.02179-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruth MM, Sangen JJN, Remmers K, Pennings LJ, Svensson E, Aarnoutse RE, Zweijpfenning SMH, Hoefsloot W, Kuipers S, Magis-Escurra C, Wertheim HFL, van Ingen J. 2019. A bedaquiline/clofazimine combination regimen might add activity to the treatment of clinically relevant non-tuberculous mycobacteria. J Antimicrob Chemother 74:935–943. 10.1093/jac/dky526. [DOI] [PubMed] [Google Scholar]
- 8.Lindman M, Dick T. 2019. bedaquiline eliminates bactericidal activity of beta-Lactams against Mycobacterium abscessus. Antimicrob Agents Chemother 63:e00827-19. 10.1128/AAC.00827-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Moigne V, Raynaud C, Moreau F, Dupont C, Nigou J, Neyrolles O, Kremer L., Herrmann J.-L. 2020. Efficacy of bedaquiline, alone or in combination with imipenem, against Mycobacterium abscessus in C3HeB/FeJ mice. Antimicrob Agents Chemother 64:e00114-20. 10.1128/AAC.00114-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richard M, Gutiérrez AV, Viljoen A, Rodriguez-Rincon D, Roquet-Baneres F, Blaise M, Everall I, Parkhill J, Floto RA, Kremer L. 2019. Mutations in the MAB_2299c TetR regulator confer cross-resistance to clofazimine and bedaquiline in Mycobacterium abscessus. Antimicrob Agents Chemother 63:e01316-18. 10.1128/AAC.01316-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupont C, Viljoen A, Thomas S, Roquet-Banères F, Herrmann J-L, Pethe K, Kremer L. 2017. Bedaquiline Inhibits the ATP Synthase in Mycobacterium abscessus and is effective in infected zebrafish. Antimicrob Agents Chemother 61:e01225-17. 10.1128/AAC.01225-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li B, Ye M, Guo Q, Zhang Z, Yang S, Ma W, Yu F, Chu H. 2018. Determination of MIC distribution and mechanisms of decreased susceptibility to bedaquiline among clinical isolates of Mycobacterium abscessus. Antimicrob Agents Chemother 62:e00175-18. 10.1128/AAC.00175-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2018. Susceptibility testing of mycobacteria, nocardia spp., and other aerobic actinomycetes. 3rd ed. CLSI, Pittsburgh, PA. [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2018. Performance standards for susceptibility testing of mycobacteria, nocardia spp., and other aerobic actinomycetes. 1st ed. CLSI, Pittsburgh, PA. [PubMed] [Google Scholar]
- 15.Brown-Elliott BA, Wallace RJ. 2019. In vitro susceptibility testing of bedaquiline against Mycobacterium abscessus complex. Antimicrob Agents Chemother 63:e01919-18. 10.1128/AAC.01919-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortes MA, Nessar R, Singh AK. 2010. Laboratory maintenance of Mycobacterium abscessus. Curr Protoc Microbiol 18:10D.1.1– 10D.1.12. 10.1002/9780471729259.mc10d01s18. [DOI] [PubMed] [Google Scholar]
- 17.Dubos RJ, Middlebrook G. 1948. The effect of wetting agents on the growth of tubercle bacilli. J Exp Med 88:81–88. 10.1084/jem.88.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaushik A, Gupta C, Fisher S, Story-Roller E, Galanis C, Parrish N, Lamichhane G. 2017. Combinations of avibactam and carbapenems exhibit enhanced potencies against drug-resistant Mycobacterium abscessus. Future Microbiol 12:473–480. 10.2217/fmb-2016-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubée V, Bernut A, Cortes M, Lesne T, Dorchene D, Lefebvre AL, Hugonnet JE, Gutmann L, Mainardi JL, Herrmann JL, Gaillard JL, Kremer L, Arthur M. 2015. beta-Lactamase inhibition by avibactam in Mycobacterium abscessus. J Antimicrob Chemother 70:1051–1058. [DOI] [PubMed] [Google Scholar]
- 20.Zeng S, Soetaert K, Ravon F, Vandeput M, Bald D, Kauffmann J-M, Mathys V, Wattiez R, Fontaine V. 2019. Isoniazid bactericidal activity involves electron transport chain perturbation. Antimicrob Agents Chemother 63:e01841-18. 10.1128/AAC.01841-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shetty A, Dick T. 2018. Mycobacterial cell wall synthesis inhibitors cause lethal ATP burst. Front Microbiol 9:1898. 10.3389/fmicb.2018.01898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee BS, Kalia NP, Jin XEF, Hasenoehrl EJ, Berney M, Pethe K. 2019. Inhibitors of energy metabolism interfere with antibiotic-induced death in mycobacteria. J Biol Chem 294:1936–1943. 10.1074/jbc.RA118.005732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franz ND, Belardinelli JM, Kaminski MA, Dunn LC, Calado Nogueira de Moura V, Blaha MA, Truong DD, Li W, Jackson M, North EJ. 2017. Design, synthesis and evaluation of indole-2-carboxamides with pan anti-mycobacterial activity. Bioorg Med Chem 25:3746–3755. 10.1016/j.bmc.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaushik A, Ammerman NC, Lee J, Martins O, Kreiswirth BN, Lamichhane G, Parrish NM, Nuermberger EL. 2019. In vitro activity of the new β-lactamase Inhibitors relebactam and vaborbactam in combination with β-Lactams against Mycobacterium abscessus complex clinical isolates. Antimicrob Agents Chemother 63:e02623-18. 10.1128/AAC.02623-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Youmans AS, Youmans GP. 1948. The effect of “Tween 80” in vitro on the bacteriostatic activity of twenty compounds for Mycobacterium tuberculosis. J Bacteriol 56:245–252. 10.1128/JB.56.2.245-252.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sader HS, Rhomberg PR, Flamm RK, Jones RN. 2012. Use of a surfactant (polysorbate 80) to improve MIC susceptibility testing results for polymyxin B and colistin. Diagn Microbiol Infect Dis 74:412–414. 10.1016/j.diagmicrobio.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Raynaud C, Daher W, Roquet-Banères F, Johansen MD, Stec J, Onajole OK, Ordway D, Kozikowski AP, Kremer L. 2020. Synergistic interactions of indole-2-carboxamides and β-lactam antibiotics against Mycobacterium abscessus. Antimicrob Agents Chemother 64:e02548-19. 10.1128/AAC.02548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Run E, Arthur M, Mainardi JL. 2019. In vitro and intracellular activity of imipenem combined with tedizolid, rifabutin, and avibactam against Mycobacterium abscessus. Antimicrob Agents Chemother 63:e01915-18. 10.1128/AAC.01915-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Run E, Arthur M, Mainardi JL. 2018. In vitro and intracellular activity of imipenem combined with rifabutin and avibactam against Mycobacterium abscessus. Antimicrob Agents Chemother 62:e00623-18. 10.1128/AAC.00623-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lounis N, Vranckx L, Gevers T, Kaniga K, Andries K. 2016. In vitro culture conditions affecting minimal inhibitory concentration of bedaquiline against M. tuberculosis. Med Mal Infect 46:220–225. 10.1016/j.medmal.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Dubos RJ, Middlebrook G. 1947. Media for tubercle bacilli. Am Rev Tuberc 56:334–345. [PubMed] [Google Scholar]
- 32.Parker SK, Curtin KM, Vasil ML. 2007. Purification and characterization of mycobacterial phospholipase A: an activity associated with mycobacterial cutinase. J Bacteriol 189:4153–4160. 10.1128/JB.01909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh P, Rao RN, Reddy JRC, Prasad RBN, Kotturu SK, Ghosh S, Mukhopadhyay S. 2016. PE11, a PE/PPE family protein of Mycobacterium tuberculosis is involved in cell wall remodeling and virulence. Sci Rep 6:21624. 10.1038/srep21624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berube BJ, Castro L, Russell D, Ovechkina Y, Parish T. 2018. Novel screen to assess bactericidal activity of compounds against non-replicating Mycobacterium abscessus. Front Microbiol 9:2417. 10.3389/fmicb.2018.02417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Story-Roller E, Maggioncalda EC, Lamichhane G. 2019. Synergistic efficacy of β-lactam combinations against Mycobacterium abscessus pulmonary infection in mice. Antimicrob Agents Chemother 63:e00614-19. 10.1128/AAC.00614-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lerat I, Cambau E, Roth Dit Bettoni R, Gaillard J-L, Jarlier V, Truffot C, Veziris N. 2014. In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J Infect Dis 209:905–912. 10.1093/infdis/jit614. [DOI] [PubMed] [Google Scholar]
- 37.Obregón-Henao A, Arnett KA, Henao-Tamayo M, Massoudi L, Creissen E, Andries K, Lenaerts AJ, Ordway DJ. 2015. Susceptibility of Mycobacterium abscessus to antimycobacterial drugs in preclinical models. Antimicrob Agents Chemother 59:6904–6912. 10.1128/AAC.00459-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsumoto K, Kurihara Y, Kuroda Y, Hori S, Kizu J. 2016. Pharmacokinetics and brain penetration of carbapenems in mice. J Infect Chemother 22:346–349. 10.1016/j.jiac.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Rouan M-C, Lounis N, Gevers T, Dillen L, Gilissen R, Raoof A, Andries K. 2012. Pharmacokinetics and pharmacodynamics of TMC207 and its N-desmethyl metabolite in a murine model of tuberculosis. Antimicrob Agents Chemother 56:1444–1451. 10.1128/AAC.00720-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mccune RM, Mcdermott W, Tompsett R. 1956. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J Exp Med 104:763–802. 10.1084/jem.104.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Almeida D, Nuermberger E, Tasneen R, Rosenthal I, Tyagi S, Williams K, Peloquin C, Grosset J. 2009. Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis. Antimicrob Agents Chemother 53:4178–4184. 10.1128/AAC.00830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jindani A, Aber VR, Edwards EA, Mitchison DA. 1980. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis 121: 939–949. 10.1164/arrd.1980.121.6.939. [DOI] [PubMed] [Google Scholar]
- 43.Jindani A, Dore CJ, Mitchison DA. 2003. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med 167:1348–1354. 10.1164/rccm.200210-1125OC. [DOI] [PubMed] [Google Scholar]
- 44.Almeida D, Ioerger T, Tyagi S, Li S-Y, Mdluli K, Andries K, Grosset J, Sacchettini J, Nuermberger E. 2016. Mutations in pepQ Confer low-level resistance to bedaquiline and clofazimine in Mycobacterium tuberculosis. Antimicrob Agents Chemother:4590–4599. 10.60.1128/AAC.00753-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.01545-21-s0001.pdf, PDF file, 0.8 MB (811.7KB, pdf)